Abstract
The galK gene, encoding galactokinase of the Leloir pathway, was insertionally inactivated in Streptococcus mutans UA159. The galK knockout strain displayed only marginal growth on galactose, but growth on glucose or lactose was not affected. In strain UA159, the sugar phosphotransferase system (PTS) for lactose and the PTS for galactose were induced by growth in lactose and galactose, although galactose PTS activity was very low, suggesting that S. mutans does not have a galactose-specific PTS and that the lactose PTS may transport galactose, albeit poorly. To determine if the galactose growth defect of the galK mutant could be overcome by enhancing lactose PTS activity, the gene encoding a putative repressor of the operon for lactose PTS and phospho-β-galactosidase, lacR, was insertionally inactivated. A galK and lacR mutant still could not grow on galactose, although the strain had constitutively elevated lactose PTS activity. The glucose PTS activity of lacR mutants grown in glucose was lower than in the wild-type strain, revealing an influence of LacR or the lactose PTS on the regulation of the glucose PTS. Mutation of the lacA gene of the tagatose pathway caused impaired growth in lactose and galactose, suggesting that galactose can only be efficiently utilized when both the Leloir and tagatose pathways are functional. A mutation of the permease in the multiple sugar metabolism operon did not affect growth on galactose. Thus, the galactose permease of S. mutans is not present in the gal, lac, or msm operons.
Streptococcus mutans is considered the primary etiological agent of dental caries. Its ability to metabolize a variety of sugars to generate acids is directly related to its cariogenicity. S. mutans can metabolize galactose by two distinct pathways: tagatose 6-phosphate and Leloir. In the tagatose pathway, the galactose moiety of lactose, and possibly galactose alone, can be transported and phosphorylated by a lactose-specific (lacEF) phosphoenolpyruvate:phosphotransferase system (PTS). The resultant galactose 6-phosphate generated by an intracellular 6-phospho-β-galactosidase (lacG) is converted into tagatose 6-phosphate, to tagatose 1,6-bisphosphate, and then to glyceraldehyde 3-phosphate and dihydroxyacetone by the enzymes galactose 6-phosphate isomerase (lacAB), tagatose 6-phosphate kinase (lacC), and tagatose 1,6-bisphospahte aldolase (lacD), respectively. In S. mutans, the genes encoding the tagatose 6-phosphate pathway are arranged as part of the lac operon (15). In the Leloir pathway, galactose enters the cell via an as-yet-unidentified permease, where it is phosphorylated by galactokinase (galK) to yield galactose 1-phosphate, which is then converted into glucose 1-phosphate by hexose1-phosphate uridyltransferase (galT) and UDP-glucose epimerase (galE). The resulting glucose 1-phosphate can enter the glycolytic pathway.
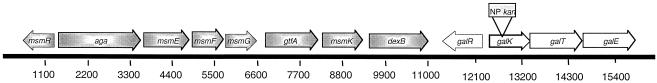
The genes encoding the Leloir pathway are arranged as an operon (galKTE) (4) located downstream of the multiple sugar metabolism (msm) operon (Fig. 1). The msm operon is responsible for the transport and metabolism of melibiose, raffinose, and isomaltosaccharides; the first two of these sugars contain a galactose moiety (22). S. mutans strains that had chromosomal deletions of 18 kbp encompassing the entire msm and gal operons were no longer able to grow in galactose as the sole carbohydrate source (20), although it was not clear if the defect was due to a failure to metabolize galactose or to transport it. In Lactococcus lactis, the Leloir pathway is essential for growth on galactose, and strains that were deficient in UDP-glucose epimerase displayed reduced growth rates in glucose and in maltose and formed longer chains than the wild-type strain (12), probably because of the role of the UDP sugars in cell wall biosynthesis. In Streptococcus salivarius, galactose can be catabolized only by the Leloir pathway because it lacks a lactose-galactose PTS (10), and lactose is catabolized by β-galactosidase. The ability of S. mutans to control and coordinate the catabolism of galactose and galactose-containing sugars is undoubtedly related to its ability to compete effectively with other species in the mouth and to induce dental caries in humans.
FIG. 1.
The arrangement of the gal and msm operons in S. mutans UA159. The location of the insertion of a nonpolar Km (NP kan) cassette within the galK gene to generate the strain JAM2 is indicated.
As part of another study, we attempted to modify S. mutans in a way that would reduce the flow of carbohydrate through the glycolytic pathway to study the effects on global gene expression. To accomplish this modification, we inactivated the galK gene to force carbohydrate metabolism through the tagatose pathway. Unexpectedly, we discovered that recombinant GalK-deficient strains could no longer grow on galactose. Thus, we initiated the present investigation into the utilization of galactose to better understand how this common sugar is assimilated by S. mutans.
MATERIALS AND METHODS
Bacterial strains and growth media.
Escherichia coli strains were cultured in Luria-Bertani (LB) broth supplemented, when necessary, with 100 μg of ampicillin ml−1, 40 μg of kanamycin (KM) ml−1, or 5 μg of erythromycin (EM) ml−1. The S. mutans strains listed in Table 1 were grown in brain heart infusion broth at 37°C in a 5% CO2 atmosphere, and antibiotics were added, when required, at concentrations of 10 μg ml−1 for EM and 1 mg ml−1 for KM. When S. mutans strains were to be assessed for growth in different sugars or assayed for enzymatic activity, cells were grown in tryptone-vitamin (TV) base medium (8) supplemented with the desired carbohydrates.
TABLE 1.
S. mutans strains used in this study
| Strain | Relevant genotype | Description | Source |
|---|---|---|---|
| UA159 | galK+lacR+lacA+msmE+ | Wild type | University of Alabama |
| JAM2 | galK deficient | galK::kan | This study |
| JAM19 | lacR deficient | lacR::erm | This study |
| JAM18 | galK and lacR deficient | galK::kan lacR::erm | This study |
| JAM20 | msmE deficient | msmE::erm | This study |
| JAM21 | lacA deficient | lacA::erm | This study |
DNA manipulations.
Chromosomal DNA was isolated from S. mutans as previously described (9). Plasmid DNA was isolated from E. coli by using a QIAprep Spin Miniprep kit (QIAGEN, Chatsworth, Calif.). Restriction and DNA-modifying enzymes were obtained from Invitrogen (Gaithersburg, Md.), New England BioLabs (Beverly, Mass.), or MBI Fermentas (Amherst, N.Y.). PCRs were carried out with 100 ng of chromosomal DNA by using Taq DNA polymerase (Invitrogen, Carlsbad, Calif.). DNA was introduced into S. mutans by natural transformation and was introduced into E. coli by electroporation. Southern blot analyses were carried out at high stringency.
Construction of a galK mutant.
A 900-bp fragment containing the 5′ portion of the galK gene (1.173 kbp), which encodes galactokinase of the Leloir pathway, was amplified from S. mutans UA159 chromosomal DNA by recombinant PCR (14) to introduce SphI and SacI restriction sites at the 5′ and 3′ ends, respectively, and a BamHI site at the center of the galK structural gene. Briefly, the primary PCRs used primers designated galKS37SphI (5′-TTACTCACGCATGCGGAAGAGAAG-3′) and galKAS500BamHI (5′-GATTCCTGAATTGGATCCGATAAAGTCA-3′), generating a 500-bp product containing engineered SphI and BamHI sites. The second set of primers, galKS500BamHI (5′-TGACTTTATCGGATCCAATTCAGGAATC-3′) and galKAS920SacI (5′-AGAAACATGCGAGCTCTTGACCAGC-3′), generated a 420-bp product containing BamHI and SacI sites. Equimolar quantities of each amplicon (50 ng) were mixed and used as templates in another PCR with the primers galKS37SphI and galKAS920SacI to generate a 900-bp fragment of galK. The final product was digested with SacI and SphI and cloned into a SacI- and SphI-digested pGEM5Zf(+) (Promega, Madison, Wis.) to generate pJA7. To inactivate the galK gene, a BamHI fragment containing a promoterless, nonpolar KM resistance marker (16) was introduced into pJA7 at the unique internal BamHI site, located 500 bp downstream of the galK start codon. The resulting plasmid, pJA8, was used to inactivate the galK gene of S. mutans UA159 by allelic exchange. The mutant was designated JAM2.
Construction of lacR, lacA, and msmE mutants.
In order to have derepressed expression of the lactose PTS, a lacR-deficient strain was constructed by using the PCR ligation mutagenesis strategy (17). The 5′ portion of lacR and upstream sequences were amplified by PCR with a pair of primers designated lacRS1 (5′-CACAAAACTCATTAATAAGC-3′) and lacRAS350EcoRI (5′-CATGGGACATTGAATTCTATTGAAAA-3′), generating a 350-bp product which was subsequently digested with EcoRI. The 3′ portion of the gene was amplified with the primers lacRS352HindIII (5′-GAAAAGCAAAGCTTACAAATAGAAG-3′) and lacRAS710 (5′-CTTGGAAACTTGATGTCAT-3′), which generated another 350-bp product that was digested with HindIII. Each digested fragment was ligated to an EM resistance cassette that was also digested with EcoRI and HindIII. The resulting ligation was used to transform S. mutans UA159 and JAM2 to generate JAM19 and JAM18, respectively.
The lacA gene (426 bp), coding for galactose 6-phosphate isomerase of the tagatose 6-phosphate pathway, was inactivated by an EM resistance cassette that was inserted 120 bp downstream of the start codon. Briefly, a 420-bp fragment consisting of the 5′ region and the first 120 bp of the lacA gene was amplified with the primers lacA-F200 (5′-CGTTAGATAACAAAAAGTGA) and lacA-R620HindIII (5′-GTACAACGTCAAGCTTTGAATCTAC), in which a HindIII site was created in the 3′ portion of the amplicon. Another 660-bp fragment containing the 3′ end of lacA and sequences downstream of the structural gene was amplified with the primers lacA-F620EcoRI (5′-GTAGATTCAGAATTCGCAGTTGTAC) and lacA-R1280 (5′-CGCCAAAGCTAACGACAT), in which an EcoRI site was generated in the 5′ end of the amplicon. PCR products were digested with HindIII or EcoRI and ligated to an erm cassette previously digested with both enzymes. This ligation was then used to transform S. mutans, and the resulting strain was designated JAM21.
To inactivate msmE, a 500-bp PCR product of the 5′ end of the gene and flanking region was obtained with msmES100 (5′-CAGTATGGTTCCTCTTATC-3′) and msmE-ASEcoRI660 (5′-CAGACCGACGAATTCTAGTAATCC-3′) primers, in which an EcoRI restriction site was artificially created. Another pair of primers, msmE-SHindIII660 (5′-GGATTACTAAAGCTTGTCGGTCTG-3′) and msmEAS1430 (5′-GCAAATGCCACAAGACCA-3′), was used to amplify the 770-bp 3′ region of the gene, in which a HindIII artificial site was also generated. The PCR products were digested with EcoRI or HindIII and ligated to an erm cassette that was also previously digested with both enzymes. This ligation was used to transform S. mutans UA159, and the resulting mutant was designated JAM20.
PTS assays.
S. mutans strains were grown to an optical density at 600 nm of 0.6 in TV medium supplemented with 0.5% (wt/vol) of glucose, lactose, or galactose, and PTS activity for each sugar was determined as described by LeBlanc et al. (18). Briefly, 50 ml of late-exponential-phase cells was harvested, washed twice with 0.1 M sodium-potassium phosphate buffer [pH 7.2], and resuspended in 5 ml of the same buffer. This cell suspension was permeabilized with 250 μl of toluene-acetone (1:9), and 10 to 50 μl of permeabilized cells was used in the assays. The assay, which contains 0.1 mM NADH, 10 U of lactic acid dehydrogenase, 5 mM phosphoenolpyruvate, and 10 mM sugar of interest, measures the oxidation of NADH in a phosphoenolpyruvate-dependent manner.
RT-PCR.
To compare the transcriptional levels of lacF (EIIAlac) and galK (galactokinase) in cells grown in glucose, lactose, or galactose (when possible), cells were grown to an optical density at 600 nm of 0.6 in 50 ml of TV medium supplemented with 0.2% of each of the sugars. The cells were harvested, washed twice in 10 mM phosphate buffer [pH 7.4], and resuspended in a 500-μl solution of 10 mM Tris and 1 mM EDTA. RNA from samples was extracted as described by Burne et al. (8), and 1 μg of RNA was used to generate cDNA by using an iScript cDNA synthesis kit (Bio-Rad, Hercules, Calif.) as recommended by the supplier. Ten percent of the cDNA reaction was used in PCRs with primer pairs specific to lacF or galK, and 5 μl of each of the 50-μl PCR products was separated in 0.8% agarose gels.
RESULTS AND DISCUSSION
The arrangement of the gal operon and flanking regions.
The sequence of galK of S. mutans UA159 was obtained from thecomplete genome sequence available at http://www.genome.ou.edu/smutans.html (3). The organization of the gal operon is shown in Fig. 1. as detailed previously by Ajdić et al. (4). A gene coding for a regulatory protein, galR, is located 5′ of galK and transcribed in the opposite orientation (Fig. 1). In L. lactis, the gal gene cluster consists of galPMKTE, where galP encodes a galactose permease and GalM is thought to catalyze the interconversion of the α and β anomers of galactose (5, 7, 12). In Lactobacillus casei, the organization of the Leloir pathway genes is galKETRM, with the permease being located elsewhere in the genome (6). Interestingly, S. mutans UA159 does not possess apparent galP or galM homologues, perhaps explaining at least in part why this organism grows much more slowly on galactose than on other mono- and disaccharides (see below).
Characteristics of galK- and lacA-deficient strains.
To evaluate the contribution of galactokinase to the metabolism of galactose by S. mutans, a nonpolar KM resistance marker was inserted 500 bp downstream of the galK start codon and the insertion was confirmed by Southern blot analysis. The growth of JAM2, the galK-deficient strain, represented by the doubling time in the presence of each sugar tested, is shown in Table 2. JAM2 displayed dramatically impaired growth in 0.5 or 2% galactose, reaching a final optical density similar to that of organisms grown in TV base medium with no added sugar. It should be noted that a galK-deficient strain of S. salivarius and a galE mutant strain of L. lactis displayed slow growth in glucose (10, 12). However, in the case of S. mutans, the doubling times of the GalK-deficient strain in glucose or lactose did not differ from those of the wild-type strain.
TABLE 2.
Doubling time of S. mutans strains in TV broth supplemented with 0.5% (wt/vol) of glucose, lactose, or galactosea
| Strain | Doubling time for growth in:
|
||
|---|---|---|---|
| Glucose | Lactose | Galactose | |
| UA159 | 71.0 ± 8.9 | 71.8 ± 5.7 | 156.5 ± 21.9 |
| JAM2 | 71.6 ± 5.7 | 77.0 ± 7.6 | Impaired |
| JAM18 | 75.2 ± 8.1 | 73.7 ± 7.2 | Impaired |
| JAM19 | 76.4 ± 11.6 | 73.0 ± 6.5 | 154.5 ± 6.4 |
| JAM20 | 67.0 ± 8.2 | 70.3 ± 1.5 | 132.6 ± 5.0 |
Doubling times are given in minutes.
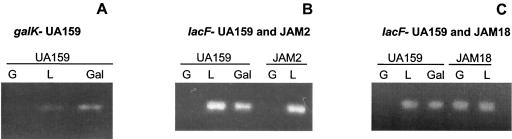
In S. salivarius and Streptococcus thermophilus, the tagatose pathway is not present and galactose is excreted into the supernatant fluid when the cells are grown in the presence of lactose (10). To verify that the galactose moiety originating from lactose could be metabolized via the tagatose pathway, JAM2 was grown overnight in TV supplemented with 10 mM lactose, and the supernatant was recovered, filter sterilized, and then mixed with an equal amount of fresh 2× TV base medium. The reconstituted medium was inoculated with the wild-type strain, and growth was monitored for 24 h. S. mutans UA159 was not able to grow in the spent medium, indicating that the galactose moiety from lactose hydrolysis can indeed be metabolized by the tagatose pathway (data not shown). The observation that lactose can induce the lactose PTS in the galK-deficient background (Fig. 2B) reinforces the idea that lactose metabolism does not need the Leloir pathway.
FIG. 2.
RT-PCR analysis of galK and lacF expression in the wild-type strain UA159, JAM2, and JAM18 grown in glucose (G), lactose (L) or, when possible, galactose (Gal). Panel A shows the expression of galK in UA159 grown in G, L, or Gal. Panel B indicates that the lacF gene is induced or derepressed by lactose or galactose for UA159 and by lactose for JAM2. Panel C shows the expression of lacF in UA159 and JAM18, a galK and lacR mutant strain.
A polar mutation was also introduced in the first gene of the lac operon. Interestingly, the lacA-deficient mutant (JAM21) also displayed impaired growth in 0.5% lactose or 0.5% galactose (data not shown). The finding that both lacA and galK mutants have impaired growth in galactose means that the efficient metabolism of galactose depends on the possession of functional Leloir and tagatose pathways. We propose that the efficient expression of the Leloir pathway depends on components of the tagatose pathway, perhaps mediated by the EII enzymes. Such regulation may be used to sense levels of available galactose to avoid detrimental effects on cell wall biogenesis. It should also be noted that, in a minority of cases, individual isolates of JAM21 would begin to grow in galactose or lactose after 24 to 48 h of incubation. Since the Em insertion was stable, our hypothesis is that spontaneous mutations in either the gal or lac pathway allowed for the reactivated expression of the genes necessary for the catabolism of these sugars. Studies are planned to explore the intriguing and potentially complex interplay between these two catabolic pathways.
Sugar transport.
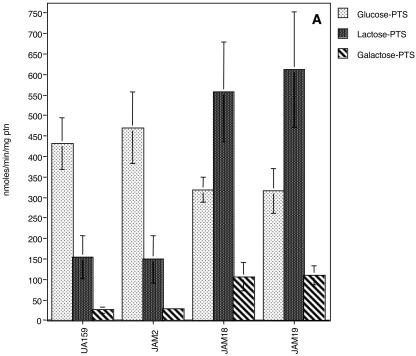
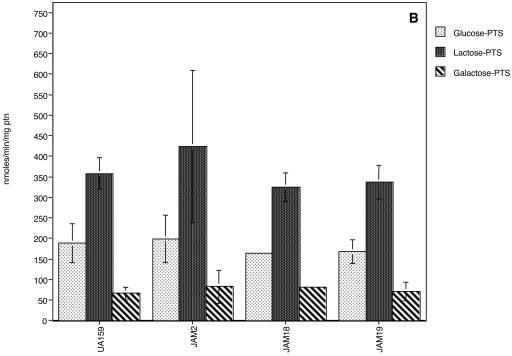
PTS assays demonstrated that JAM2 had wild-type levels of glucose, lactose, and galactose PTS activities (Fig. 3), although galactose PTS activity was very low compared with other PTS activities. For S. salivarius, glucose PTS activity in a galK-deficient strain was not altered (10) and the slower growth in glucose of S. salivarius lacking GalK was attributed to other functions of the Leloir pathway (11, 12), which may include providing intermediates for cell wall synthesis. Glucose PTS activity reached higher levels when cells were grown in glucose (Fig. 3A), confirming earlier observations that S. mutans possesses a constitutive and an inducible glucose PTS (1, 19). Growth in galactose did not dramatically enhance galactose-specific PTS activity in the wild-type strain, in contrast to what was observed for cells grown in lactose (Fig. 3B and C). When compared with cells grown in glucose (Fig. 3A), however, cells grown in galactose had high levels of lactose PTS activity (Fig. 3C), perhaps reflecting the capacity for the induction of the lactose PTS by galactose or its repression by glucose. Notably, there were no detectable catabolite response element sequences (13), which are targets for catabolite repression via CcpA, in the promoter region of the S. mutans lac operon. Also of interest was the fact that glucose PTS activity reached higher values in cells grown in galactose than in glucose (Fig. 3A and C). We speculate that this finding is not necessarily due to a direct effect of galactose on glucose PTS expression or activity but instead may represent the enhanced expression of the glucose PTS due to the slower growth of the organisms and a need to scavenge for more readily metabolizable sugars. Indeed, the glucose PTS of S. mutans has been shown to be more highly expressed at lower growth rates and under carbohydrate-limiting conditions (23).
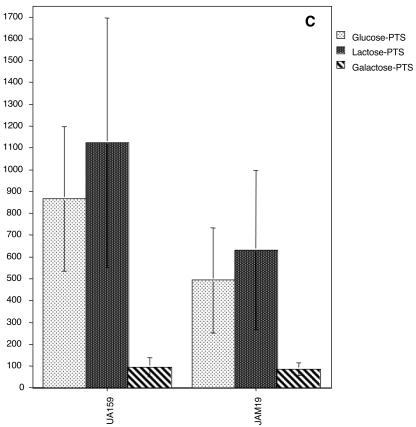
FIG. 3.
Sugar-specific PTS activity of UA159, JAM2, JAM18, and JAM19 grown in glucose (A), lactose (B), or galactose (C). The values are the means ± standard deviations of results from at least three independent experiments.
By using reverse transcription (RT)-PCR to obtain semiquantitative information on the levels of galK and lacF mRNA (Fig. 2), it was found that no galK transcripts could be detected in cells grown in glucose (Fig. 2A). An induction or derepression of galK expression could be observed in the presence of lactose or galactose, confirming the earlier observation of Ajdić et al. (4) that the gal operon of S. mutans may be under the control of carbohydrate catabolite repression. In L. lactis, the expression of galK was also repressed in the presence of glucose, and galactose is required for galK expression (12). It should be noted that a sequence (CGGGACGTAATCA) with similarity to a catabolite response element was found 75 bp downstream of the galK start codon, suggesting that CcpA-dependent carbohydrate catabolite repression could be involved in the expression of this operon.
A possible role for the lactose PTS in galactose uptake.
As indicated above, we proposed that galactose might be transported via the lactose PTS, albeit at a rate insufficient to support growth. To determine whether enhancing the expression of the lactose PTS could enhance galactose transport and allow for growth in galactose, the gene encoding a putative repressor of the genes for the lactose PTS and phospho-β-galactosidase, lacR, was insertionally inactivated by an EM resistance marker. RT-PCR analysis revealed that the absence of a functional lacR gene resulted in constitutive expression of the lacF gene (EIIALac) when cells were grown in glucose (Fig. 2C), demonstrating that the lacR gene product is in fact a negative regulator for the lactose PTS, as originally suggested by Rosey and Stewart (21). The expression of galK in the lacR mutants was still tightly controlled when cells were grown in glucose, suggesting that LacR does not play any role in the regulation of the gal operon (data not shown). This result is not surprising, since the gal operon apparently has its own regulatory protein, GalR, which in the absence of galactose will bind to the intergenic region between galK and galR genes, negatively regulating the transcription of both genes (2).
The doubling times of both lacR mutants, JAM18 and JAM19, in glucose or lactose were not significantly different than those observed for the wild-type strain (Table 2). Also, growth in 0.5% galactose still could not be achieved for the double mutant (galK and lacR) and the growth rate of JAM19 in galactose was not enhanced (Table 2), indicating that the growth block in the GalK-deficient strain occurs because it lacks galactokinase, not because of insufficient PTS-driven transport of galactose. Lactose and galactose PTS levels of JAM19 and JAM18 in cells grown in glucose were significantly higher than those observed for UA159 and JAM2, which have a functional lacR gene (Fig. 3A). It is also of note that when JAM19 (galK and lacR deficient) and JAM18 (lacR deficient) were grown in glucose, lower glucose PTS activity was observed than in the parental strain (Fig. 3A), suggesting a role for lacR or for the lactose PTS in the regulation of the expression or activity of the glucose PTS. Therefore, unlike L. casei (6), S. mutans does not seem to have a galactose-specific PTS. Based on increases in both lactose and galactose PTS activity in the strains lacking LacR, it seems that the lactose PTS can transport galactose, albeit with an affinity or at a rate that is not sufficient to sustain growth.
msmE is not involved in galactose uptake.
A permease for transport of galactose that could fuel the Leloir pathway has yet to be identified for S. mutans. The msm operon has been shown to encode an ABC-type transporter capable of internalizing galactosyl-containing sugars. To determine whether the msm operon could be involved in the uptake of unphosphorylated galactose, the msmE gene, encoding the sugar-binding domain of the porter, was insertionally inactivated by an EM resistance cassette as described above. Disruption of this gene did not lead to impaired growth in galactose (Table 2), suggesting that the galactose permease is located elsewhere in the genome. Using the galP sequence of L. lactis, we performed a BLAST search of the S. mutans genome and were unable to find any open reading frames sharing significant similarity.
Summary.
S. mutans exhibits remarkable versatility in sugar metabolism. A wide range of carbohydrates can be catabolized by this organism; there are apparent overlaps, redundancies, and coordinated regulation across a wide range of sugar catabolism systems; and virulence gene expression is highly responsive to carbohydrate source and availability. We demonstrate that S. mutans can efficiently metabolize galactose in monosaccharide form only when both the Leloir and tagatose pathways are functional. We demonstrate that the tagatose pathway is responsible predominantly for the utilization of the phosphorylated galactose moiety that comes from the breakdown of lactose 6-phosphate. We also provide evidence that the lactose PTS may transport galactose but at such low affinity that growth on galactose in the absence of GalK cannot be supported. Using RT-PCR, we observe that the gal operon is tightly controlled and we confirm the observation that it is repressed in cells growing on glucose (4). It also appears that there is considerable cooperation between the gal and lac systems, since a lacA-deficient strain was also unable to grow in galactose. An apparent interaction between the expression of the lac operon and glucose uptake via the PTS is also evident. The multiple sugar metabolism operon is apparently not a factor in the transport of galactose alone in S. mutans, so a true galactose permease in S. mutans remains to be characterized.
Acknowledgments
This work was supported by NIDCR grants DE12236 and DE13239.
REFERENCES
- 1.Abranches, J., Y.-Y. M. Chen, and R. A. Burne. 2003. Characterization of Streptococcus mutans strains deficient in EIIABMan of the sugar phosphotransferase system. Appl. Environ. Microbiol. 69:4760-4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajdić, D., and J. J. Ferretti. 1998. Transcriptional regulation of the Streptococcus mutans gal operon by the GalR repressor. J. Bacteriol. 180:5727-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajdić, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 99:14434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ajdić, D., I. C. Sutcliffe, R. R. Russell, and J. J. Ferretti. 1996. Organization and nucleotide sequence of the Streptococcus mutans galactose operon. Gene 180:137-144. [DOI] [PubMed] [Google Scholar]
- 5.Beebe, J. A., A. Arabshahi, J. G. Clifton, D. Ringe, G. A. Petsko, and P. A. Frey. 2003. Galactose mutarotase: pH dependence of enzymatic mutarotation. Biochemistry 42:4414-4420. [DOI] [PubMed] [Google Scholar]
- 6.Bettenbrock, K., and C. A. Alpert. 1998. The gal genes for the Leloir pathway of Lactobacillus casei 64H. Appl. Environ. Microbiol. 64:2013-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouffard, G. G., K. E. Rudd, and S. L. Adhya. 1994. Dependence of lactose metabolism upon mutarotase encoded in the gal operon in Escherichia coli. J. Mol. Biol. 244:269-278. [DOI] [PubMed] [Google Scholar]
- 8.Burne, R. A., R. G. Quivey, Jr., and R. E. Marquis. 1999. Physiologic homeostasis and stress responses in oral biofilms. Methods Enzymol. 310:441-460. [DOI] [PubMed] [Google Scholar]
- 9.Burne, R. A., K. Schilling, W. H. Bowen, and R. E. Yasbin. 1987. Expression, purification, and characterization of an exo-β-d- fructosidase of Streptococcus mutans. J. Bacteriol. 169:4507-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, Y.-Y. M., M. J. Betzenhauser, J. A. Snyder, and R. A. Burne. 2002. Pathways for lactose/galactose catabolism by Streptococcus salivarius. FEMS Microbiol. Lett. 209:75-79. [DOI] [PubMed] [Google Scholar]
- 11.Delcour, J., T. Ferain, M. Deghorain, E. Palumbo, and P. Hols. 1999. The biosynthesis and functionality of the cell-wall of lactic acid bacteria. Antonie Leeuwenhoek 76:159-184. [PubMed] [Google Scholar]
- 12.Grossiord, B. P., E. J. Luesink, E. E. Vaughan, A. Arnaud, and W. M. de Vos. 2003. Characterization, expression, and mutation of the Lactococcus lactis galPMKTE genes, involved in galactose utilization via the Leloir pathway. J. Bacteriol. 185:870-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hueck, C. J., and W. Hillen. 1995. Catabolite repression in Bacillus subtilis: a global regulatory mechanism for the gram-positive bacteria? Mol. Microbiol. 15:395-401. [DOI] [PubMed] [Google Scholar]
- 14.Innis, M. A., D. H. Gelfand, J. J. Sninsky, and T. J. White. 1990. PCR protocols: a guide to methods and applications, 1st ed. Academic Press, San Diego, Calif.
- 15.Jagusztyn-Krynicka, E. K., J. B. Hansen, V. L. Crow, T. D. Thomas, A. L. Honeyman, and R. Curtiss III. 1992. Streptococcus mutans serotype c tagatose 6-phosphate pathway gene cluster. J. Bacteriol. 174:6152-6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kremer, B. H., M. van der Kraan, P. J. Crowley, I. R. Hamilton, L. J. Brady, and A. S. Bleiweis. 2001. Characterization of the sat operon in Streptococcus mutans: evidence for a role of Ffh in acid tolerance. J. Bacteriol. 183:2543-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau, P. C., C. K. Sung, J. H. Lee, D. A. Morrison, and D. G. Cvitkovitch. 2002. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J. Microbiol. Methods 49:193-205. [DOI] [PubMed] [Google Scholar]
- 18.LeBlanc, D. J., V. L. Crow, L. N. Lee, and C. F. Garon. 1979. Influence of the lactose plasmid on the metabolism of galactose by Streptococcus lactis. J. Bacteriol. 137:878-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neron, S., and C. Vadeboncoeur. 1987. Two functionally different glucose phosphotransferase transport systems in Streptococcus mutans and Streptococcus sobrinus. Oral Microbiol. Immunol. 2:171-177. [DOI] [PubMed] [Google Scholar]
- 20.Robinson, W. G., L. A. Old, D. S. Shah, and R. R. Russell. 2003. Chromosomal insertions and deletions in Streptococcus mutans. Caries Res. 37:148-156. [DOI] [PubMed] [Google Scholar]
- 21.Rosey, E. L., and G. C. Stewart. 1992. Nucleotide and deduced amino acid sequences of the lacR, lacABCD, and lacFE genes encoding the repressor, tagatose 6-phosphate gene cluster, and sugar-specific phosphotransferase system components of the lactose operon of Streptococcus mutans. J. Bacteriol. 174:6159-6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell, R. R., J. Aduse-Opoku, I. C. Sutcliffe, L. Tao, and J. J. Ferretti. 1992. A binding protein-dependent transport system in Streptococcus mutans responsible for multiple sugar metabolism. J. Biol. Chem. 267:4631-4637. [PubMed] [Google Scholar]
- 23.Vadeboncoeur, C., L. Thibault, S. Neron, H. Halvorson, and I. R. Hamilton. 1987. Effect of growth conditions on levels of components of the phosphoenolpyruvate:sugar phosphotransferase system in Streptococcus mutans and Streptococcus sobrinus grown in continuous culture. J. Bacteriol. 169:5686-5691. [DOI] [PMC free article] [PubMed] [Google Scholar]