Abstract
Pseudomonas putida strain AJ and Ochrobactrum strain TD were isolated from hazardous waste sites based on their ability to use vinyl chloride (VC) as the sole source of carbon and energy under aerobic conditions. Strains AJ and TD also use ethene and ethylene oxide as growth substrates. Strain AJ contained a linear megaplasmid (approximately 260 kb) when grown on VC or ethene, but it contained no circular plasmids. While strain AJ was growing on ethylene oxide, it was observed to contain a 100-kb linear plasmid, and its ability to use VC as a substrate was retained. The linear plasmids in strain AJ were cured, and the ability of strain AJ to consume VC, ethene, and ethylene oxide was lost following growth on a rich substrate (Luria-Bertani broth) through at least three transfers. Strain TD contained three linear plasmids, ranging in size from approximately 90 kb to 320 kb, when growing on VC or ethene. As with strain AJ, the linear plasmids in strain TD were cured following growth on Luria-Bertani broth and its ability to consume VC and ethene was lost. Further analysis of these linear plasmids may help reveal the pathway for VC biodegradation in strains AJ and TD and explain why this process occurs at many but not all sites where groundwater is contaminated with chloroethenes. Metabolism of VC and ethene by strains AJ and TD is initiated by an alkene monooxygenase. Their yields during growth on VC (0.15 to 0.20 mg of total suspended solids per mg of VC) are similar to the yields reported for other isolates (i.e., Mycobacterium sp., Nocardioides sp., and Pseudomonas sp.).
Millions of tons of vinyl chloride (VC) are produced each year, primarily for the manufacture of polyvinyl chloride (25). However, the occurrence of VC in groundwater is typically not a consequence of direct releases to the environment. VC contamination of groundwater results mainly from the transformation of other chlorinated aliphatic compounds, including the reductive dechlorination of polychlorinated ethenes and the dehydrohalogenation of 1,2-dichloroethane (41). It has recently been demonstrated that VC is also formed naturally in soils, presumptively during oxidative reactions involving humic substances, chloride ions, and an oxidant (23). This process may have started as long as 400 million years ago (23), so it seems reasonable to expect that biodegradation processes also developed long ago.
Reduction of VC to ethene is typically the rate-limiting step in the overall reduction of chlorinated ethenes, which can lead to the accumulation of VC in groundwater (13, 31). The comparatively low rate of VC reduction may be related to this reaction being cometabolic in some strains of Dehalococcoides, although other strains have recently been shown to be capable of respiring with VC (9, 19). Oxidative acetogenesis of VC has also been documented for anaerobic sediments (2), although the extent of this process at most locations is not yet known.
In locations where anaerobic groundwater transitions to aerobic conditions, VC that migrates from the anaerobic zone may be subject to aerobic biodegradation. Several strains of Pseudomonas sp. and Mycobacterium sp., along with one Nocardioides sp., have been isolated from soil, river water, groundwater, and activated sludge based on their ability to use VC as the sole source of carbon and energy under aerobic conditions (5, 18, 39, 40). While aerobic biodegradation of VC is frequently reported in field studies (8, 12), it is by no means a universal process. Coleman et al. (5) reported a lack of aerobic VC biodegradation activity in 11 of the 31 samples tested from chlorinated-ethene-contaminated sites. Madl (28) observed no aerobic VC biodegradation activity in three of the six sediment samples tested from an area downgradient of a landfill contaminated with chloroethenes.
A better understanding of aerobic VC metabolism is needed to help predict when this process will or will not occur in the environment. Coleman and Spain (7) recently demonstrated that a four-component monooxygenase initiates the oxidation of ethene and VC in Mycobacterium sp. strain JS60. The ethylene oxide and VC epoxide that are formed then react with an epoxyalkane:coenzyme M transferase. The gene for this transferase (JS60 EaCoMT) hybridized to linear megaplasmids in strain JS60 and five other Mycobacterium strains grown on VC (6). We hypothesized that the genes conferring the VC+ and ethene-positive phenotype in isolates other than Mycobacterium spp. are also carried on a plasmid. While we could have tested the VC-positive (VC+) and ethene-positive (ethene+) Pseudomonas strains that were isolated previously (39, 40), they were derived from activated sludge rather than actual hazardous waste sites; in addition, strain MF1 grows very slowly. We therefore obtained two new isolates that grow on VC and ethene from locations that have groundwater contaminated with chlorinated ethenes. Both cultures also grow on ethylene oxide. The VC+ and ethene+ phenotypes in these isolates are associated with the presence of linear megaplasmids.
(Some preliminary results of this study were presented at the 103rd General Meeting of the American Society for Microbiology, Washington, D.C., 2003.)
MATERIALS AND METHODS
Chemicals and media.
VC gas (99.5%) was purchased from Fluka, ethene (99.9%) was purchased from Matheson, and ethylene oxide (99.5%) was purchased from Sigma-Aldrich. All other chemicals used were of reagent grade. Strains AJ and TD were grown in the minimal salts medium (MSM) described by Hartmans et al. (18), but the amount of (NH4)2SO4 was reduced to 0.67 g/liter. No vitamins or other complex growth factors were added to the MSM. Mycobacterium sp. strain JS60 was grown in the MSM described by Coleman et al. (4).
Analytical methods.
The total amount of VC, ethene, and ethylene oxide present in serum bottles was determined by gas chromatographic analysis of headspace samples, as previously described (39). The amounts for VC and ethene were converted to aqueous phase concentrations by using Henry's law constants of 0.925 for VC and 7.24 for ethene ([mol · m−3 gas concentration]/[mol · m−3 aqueous concentration]) at 23°C (15). The presence of VC epoxide was tested based on matching retention times on a gas chromatograph with chemically synthesized authentic material, in addition to a colorimetric procedure involving a reaction with 4-(4-nitrobenzyl)pyridine in ethylene glycol, as previously described (39). The chloride ion was measured by using an ion-selective electrode (Orion) connected to a pH millivolt meter (Corning) (39). Chemical oxygen demand was determined with a Hach Company kit (Loveland, Colo.) (range, 5 to 150 mg/liter).
Microcosms and enrichment cultures.
Experiments involving VC or ethene were performed with 70- or 160-ml serum bottles capped with grey butyl rubber septa (Wheaton Scientific Products, Millville, N.J.). Previous studies demonstrated that minimal losses of VC and ethene occur with these septa (39). Teflon-faced red rubber septa or grey butyl rubber septa were used during experiments with ethylene oxide.
The two isolates obtained during this study were developed with inocula from different locations. The first source was a former lagoon site in Sacramento, Calif. (8). Reductive dechlorination of chlorinated ethenes and ethanes was documented in the anaerobic source area, along with apparent oxidation of the daughter products (including VC) in the downgradient aerobic region. A sediment and groundwater sample from monitoring well 3037 in the aerobic portion of the plume was used to set up microcosms (14). An enrichment culture was developed by repeatedly supplying VC as the only source of carbon and energy and then transferring an aliquot to MSM. The sediment-free culture was further enriched by repeated additions of VC as the sole substrate for 8 months and periodically diluting the enrichment with fresh MSM.
The second source of inoculum was the sanitary landfill at the Department of Energy's Savannah River Site near Aiken, S.C. First flush groundwater from monitoring well LFW 67D was used to set up the microcosms; sediment was not available. This well was chosen because it is downgradient of the groundwater that flows beneath the landfill, it has a 20-year history of VC contamination, and it is likely aerated by horizontal sparging wells that were installed to control the movement of chlorinated contaminants (3). The pH of the groundwater was adjusted from 5.04 to 7.02 by using dibasic potassium phosphate. One set of microcosms received ethene (50 μM, aqueous phase) as the sole substrate, and a second set received only VC (50 μM, aqueous phase). The set with VC showed no significant activity, even after 450 days of incubation. A low rate of ethene utilization occurred relative to killed controls. The contents of these bottles were concentrated by centrifugation and resuspended in MSM. The rate of ethene utilization increased substantially, but that of VC utilization did not increase. The ethene-grown enrichment culture became the source from which an isolate was obtained with the ability to grow on VC (see below).
Enrichment cultures were maintained by adjusting the pH periodically to 7.2 ± 0.1 by using 8 M NaOH and supplying oxygen by purging the headspace with air or oxygen after VC or ethene was consumed. The enrichments were incubated at room temperature (23°C) in an inverted position on a gyratory shaker (100 to 150 rpm).
Pure cultures.
Three pure cultures capable of growing on VC as the sole source of carbon and energy were used in this study. An isolate from the Sacramento site was obtained by streaking an aliquot of the enrichment culture on trypticase soy agar, incubating for 26 to 38 h at 22°C, and transferring individual white colonies to serum bottles containing MSM with VC as the sole substrate. VC consumption began after 50 to 70 days. The isolate was assigned the strain designation AJ.
An isolate from the SRS ethene enrichment culture was also obtained by streaking an aliquot on trypticase soy agar and incubating for 18 to 22 h at 22°C. Individual white colonies were transferred to MSM, and ethene was supplied as the sole substrate. Ethene consumption began after approximately 25 days and was maintained for several months. Samples of the pure culture were then provided with VC as the sole source of carbon and energy. The culture began using VC immediately and has been maintained on VC as the sole substrate ever since. The isolate was assigned the strain designation TD. Strains AJ and TD were identified based on the sequence of their 16S rRNA gene as previously described (39), with minor modifications (11).
The third pure culture used in this study, Mycobacterium sp. strain JS60, was obtained from the Air Force Research Laboratory at Tyndall Air Force Base. Strain JS60 was isolated from an industrial site contaminated with chlorinated ethenes (5).
Plasmid analysis.
The presence of large linear plasmids in strains AJ and TD following growth on VC and ethene, as well as in strain AJ following growth on ethylene oxide, was evaluated by using a modified procedure for preparation of high-molecular-weight bacterial DNA embedded in agarose plugs as previously described (34), with the following modifications. Cells were centrifuged (10 min at 10,000 rpm in a Sorvall Evolution RC centrifuge) and resuspended in MSM (1 ml) to an optical density at 600 nm of 15 to 300, depending on the isolate and substrate used. The resuspended cell solution (1 ml) was warmed (45°C, 3 to 4 min) and embedded into 1 ml of 1.2% low-melting-temperature agarose containing 25% sucrose (45°C). After solidifying, the plugs were removed from the molds, agitated in a NaCl (200 mM)-Tris-Cl (10 mM, pH 7.2)-EDTA (100 mM at pH 8.0) solution, removed from the NaCl-Tris-Cl-EDTA solution, agitated with a bacterial cell lysis solution followed by a proteinase K solution, and then cut and placed into a 1% agarose gel.
DNA was separated by using a contour-clamped homogeneous electric field (CHEF) system (CHEF DR-III; Bio-Rad) at 1 s to 50 s linear ramp, 6 V/cm, and 14°C in 0.5× Tris-borate-EDTA buffer for 18 h. Concatemers of λ DNA (Lambda Ladder PFG Marker; New England BioLabs) were used as molecular markers. To determine if the plasmids were linear or circular, the pulse times were changed: one gel was run at initial and final switch times of 30 s; a second gel was run at initial and final switch times of 90 s (33).
The presence of linear plasmids was also evaluated following growth of strains AJ and TD on Luria-Bertani (LB) broth. Cells were transferred to LB broth and grown to a maximum optical density. An aliquot was transferred to fresh LB broth, and the process was repeated through at least two more growth cycles before samples were checked for plasmids. Samples were also centrifuged (10,000 rpm in a Sorvall Evolution RC centrifuge, 10 min), washed three times in MSM, resuspended in MSM, and evaluated for their ability to use VC and ethene. LB broth-grown strain AJ was also tested for its ability to resume growth on ethylene oxide. In addition to LB broth, strain AJ was grown through at least three cycles on acetate, ethylene glycol, glyoxylate, glycolate, ethanolamine, and glycolaldehyde. These cells were evaluated for linear plasmids and the ability to resume growth on VC.
The presence of circular plasmids in strain AJ following growth on VC was evaluated. Circular plasmids were isolated as previously described (32) and separated on a 1% gel at 1 to 50 s linear ramp, 6 V/cm, and 14°C in 0.5× Tris-borate-EDTA buffer for 18 h. Pseudomonas aeruginosa pME290 (ATCC 37412), containing a 6.8-kb circular plasmid, and an Escherichia coli clone, containing a 138-kb circular plasmid (a large bee genomic insert carried by a bacterial artificial chromosome vector) (38), served as positive controls.
Growth experiments.
The ability of the isolates to grow on various substrates was evaluated based on an increase in optical density (600 nm; Milton Roy Spec 20D spectrophotometer). For volatile compounds (VC, ethene, ethylene oxide, and glycolaldehyde), experiments were conducted with sealed serum bottles. Repeated additions of substrate were made (starting at 0.040 to 0.10 mmol/bottle and proceeding with higher doses until a total of 0.94 to 1.3 mmol/bottle was consumed), along with a sufficient amount of oxygen. Growth on nonvolatile substrates (glyoxylate, glycolate, ethylene glycol, ethanolamine, acetate, and chloroacetate) in shake flasks was evaluated. For glyoxylate, glycolate, ethylene glycol, ethanolamine, and acetate, 10 to 20 mM concentrations were added (1.0 to 2.0 mmol/bottle). To avoid substrate toxicity, initial concentrations of 1 mM (0.1 mmol/bottle) were used for glycolaldehyde and chloroacetate. Substrate consumption was monitored based on changes in chemical oxygen demand (since consumption of 1 mM was not enough to noticeably increase optical density); more substrate was added when less than 10% of the initial amount remained.
The observed yields for strains AJ and TD were calculated based on the increase in total suspended solids (TSS) following consumption of repeated additions of VC. TSS was determined according to standard methods (17).
Kinetics of VC utilization.
Monod kinetic parameters for the utilization of VC by strain AJ were determined as previously described (16, 39). Culture for the kinetic experiments was obtained from a 2.3-liter reactor operated in a semicontinuous batch mode at a 36-day retention time. After several retention times, the concentration of biomass stabilized at 100 mg of TSS per liter. Batch depletion experiments were set up with samples from the reactor. VC depletion curves were evaluated to determine the maximum specific VC utilization rate (k) and the half-saturation coefficient (KS), taking biomass growth into account (16). The initial VC concentration was varied from 6 to 25 μM, in order to encompass the maximum utilization rate and the region in which the half-saturation value becomes important. k and KS were determined from the batch depletion data by a weighted, nonlinear least-squares method (39). The effect of mass transfer was evaluated by incorporating a mass transfer coefficient for VC (KLa = 34.5 h−1) into the Monod equation and comparing the solutions for k and KS to those without mass transfer (39).
Nucleotide sequence accession number.
The sequences for strains AJ and TD were deposited into GenBank with accession numbers AY391278 and AY623625, respectively.
RESULTS
Identification of strains TD and AJ.
Strain TD was isolated from an enrichment culture developed with groundwater in an area near the sanitary landfill at the Department of Energy's Savannah River Site in S.C., where the groundwater is contaminated with chlorinated ethenes from the landfill leachate (3). The enrichment was grown on ethene as the sole source of carbon and energy; little or no activity had occurred initially with VC alone. However, once the isolate was obtained and grown on ethene, it rapidly transitioned to VC as the sole substrate. Strain TD is a gram-negative motile rod. Based on the sequence of its 16S rRNA gene (1,449 bases), strain TD shares greater than 99.8% identity (by using GenBank) with 19 Ochrobactrum strains, including 4FB9 (accession no. AF229875), which is capable of growing on 4-fluorobenzoate as the sole source of carbon and energy (36).
Strain AJ was isolated from an enrichment culture developed with sediment and groundwater from a hazardous waste site in California that is contaminated with chlorinated ethenes (14). The isolate was initially grown in MSM with VC as its sole source of carbon and energy and with oxygen as the terminal electron acceptor, through numerous transfers. Strain AJ also uses ethene and ethylene oxide as sole sources of carbon and energy. It is gram negative, rod shaped, and motile. Based on the sequence of its 16S rRNA gene (1,496 bases), the closest match to strain AJ (by using GenBank) is Pseudomonas putida. Strain AJ shares 99.8% identity with P. putida ATCC 17527 (accession no. AJ249451).
Presence of linear plasmids.
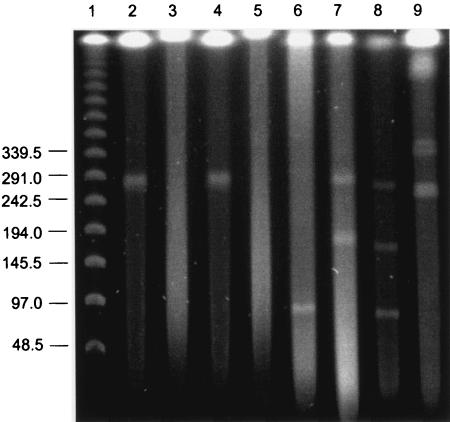
Strain AJ contains a linear megaplasmid (approximately 260 kb) when grown on VC or ethene as the sole source of carbon and energy (Fig. 1, lanes 2 and 4). Changing the CHEF gel pulse times did not alter the movement of the plasmid, confirming that it is linear (data not shown). When the cells were transferred to LB broth and grown through at least three cycles on this rich substrate (in the absence of VC or ethene), the linear plasmid was no longer present (Fig. 1, lanes 3 and 5). A shorter period of growth on LB broth did not reliably cure the plasmids. Two microbes containing circular plasmids (P. aeruginosa pME290 and an E. coli bee bacterial artificial chromosome clone) were used to demonstrate that circular plasmids are not separated from CHEF gel plugs (not shown in Fig. 1) when an extraction procedure for high-molecular-weight DNA (34) is followed. Mycobacterium sp. strain JS60 was used as a positive control since it contains a linear megaplasmid when grown on VC (6) or ethene (T. E. Mattes and J. M. Gossett, personal communication) (Fig. 1, lane 9).
FIG. 1.
Evaluation of linear plasmids under different growth conditions. Lane 1, λ ladder; lane 2, strain AJ grown on VC; lane 3, strain AJ grown on LB broth following growth on VC; lane 4, strain AJ grown on ethene; lane 5, strain AJ grown on LB broth following growth on ethene; lane 6, strain AJ grown on ethylene oxide; lane 7, strain TD grown on VC; lane 8, strain TD grown on ethene; and lane 9, Mycobacterium sp. strain JS60 grown on ethene.
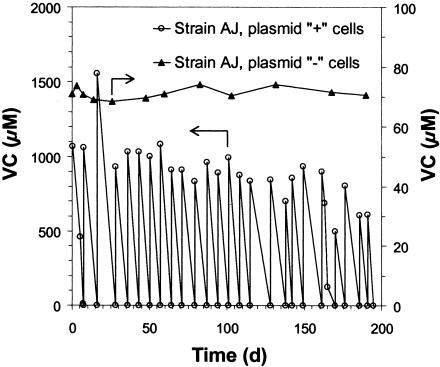
To confirm that the presence of the linear plasmid is required for the VC+ phenotype, the LB broth-grown cells (lacking the plasmid, according to the results shown in Fig. 1) were concentrated, washed, and placed back into MSM with VC as the sole substrate. Even after 190 days of incubation, VC utilization did not resume (Fig. 2). When strain AJ was growing on VC, trace amounts of VC epoxide were occasionally detected during headspace analysis. Following growth of strain AJ on LB broth, there was no transient accumulation of VC epoxide or ethylene oxide, suggesting that the alkene monooxygenase that was presumptively needed to initiate aerobic catabolism of VC and ethene (see below) was also absent or not induced. LB broth-grown cells cured of the linear megaplasmids also lost the ability to use ethylene oxide as the sole substrate.
FIG. 2.
VC consumption by strain AJ containing a 260-kb linear megaplasmid (see Fig. 1, lane 2) and the lack of VC consumption by strain AJ after the plasmid was cured by culturing on LB broth (see Fig. 1, lane 3).
The linear megaplasmid present in VC-grown cells was also cured when strain AJ was grown through three transfers on acetate, ethanolamine, ethylene glycol, glyoxylate, glycolate, and glycolaldehyde (each a potential downstream intermediate in aerobic catabolism of VC and/or ethene). As was the case with LB broth, when strain AJ was grown on these simple substrates and then returned to MSM and VC as the sole substrate, the use of VC did not resume, even after several months of incubation.
Antibiotic resistance in strain AJ with and without the linear megaplasmid was evaluated by plating cells on LB agar containing ampicillin (100 and 200 mg/liter), kanamycin (50 and 100 mg/liter), and chloramphenicol (25 and 50 mg/liter). The VC-grown cells containing the plasmid exhibited no inhibition. When the linear megaplasmid was cured following growth on LB broth, there was still no inhibition, indicating that the genes for resistance to these antibiotics are not carried exclusively on the plasmid.
VC-grown strain AJ switched to ethylene oxide as the sole source of carbon and energy without a lag. During growth on ethylene oxide, a smaller linear plasmid (approximately 100 kb) was present (Fig. 1, lane 6). Following growth of strain AJ on ethylene oxide for several months, samples were returned to VC as the sole substrate and the use of VC began after a lag of approximately 10 days. An analysis of these cells (i.e., cells grown on VC, and then ethylene oxide, and then VC again) indicated that the single 100-kb linear plasmid present during growth on ethylene oxide was retained. With cultures that were switched from ethylene oxide to ethene, the cells did not retain the single 100-kb plasmid. These cells contained two linear plasmids, approximately 30 and 45 kb in size. At this point, the use of ethene as the sole source of carbon and energy became erratic; in several serum bottles, the use of ethene ceased entirely, indicating that the extended incubation of strain AJ on ethylene oxide resulted in the loss of one or more genes needed for the sustained use of ethene as the sole substrate.
The 260-kb linear plasmid initially present in strain AJ was retained following growth on VC for more than 6 months. Two small linear plasmids (approximately 100 kb and 80 kb) and one larger one (approximately 390 kb) appeared in strain AJ after cultivation on VC for more than 1 year. Long-term incubation of strain AJ on ethene and ethylene oxide as sole substrates also led to changes in the linear plasmid arrangement. Linear plasmids of approximately 200 kb and 300 kb appeared in cells grown on ethene. In addition to the 100-kb plasmid, three other plasmids (approximately 210 kb, 230 kb, and 320 kb) appeared in cells grown on ethylene oxide. In spite of these changes, the ability of strain AJ to use VC and ethene as growth substrates was retained during nearly 3 years of incubation. The only instability occurred when ethylene oxide-grown cells were switched to ethene and ethene utilization faltered after several weeks.
As with strain AJ, the ethene+ and VC+ phenotypes in strain TD are associated with linear plasmids. VC-grown strain TD contained two linear plasmids, approximately 190 and 260 kb in size (Fig. 1, lane 7). Ethene-grown strain TD contained three linear plasmids, approximately 90, 175, and 260 kb in size (Fig. 1, lane 8). When samples of strain TD were grown on LB broth through at least three transfers, the plasmids were cured and the resulting cells no longer had the ability to use VC or ethene as the sole source of carbon and energy. As with strain AJ, there was no accumulation of VC epoxide or ethylene oxide when LB broth-grown strain TD was provided with VC or ethene.
Characteristics of strains AJ and TD.
The observed yields for strains AJ and TD grown on VC were 0.196 ± 0.037 and 0.147 ± 0.010 mg of TSS per mg of VC, respectively. These values are similar to those reported for other VC-grown isolates (5, 39) (TSS is assumed to contain 50% protein). Simultaneous nonlinear fitting of data from four batch depletion experiments resulted in the following values for the Monod kinetic parameters for strain AJ: KS = 2.36 ± 0.054 μM and k = 1.41 ± 0.18 μmol VC per mg of TSS per day. The incorporation of a mass transfer coefficient for VC into the Monod equation did not change the resulting values for KS and k. KS is within the range of previously reported values (5, 39, 40), while k is an order of magnitude lower than the values for several Mycobacterium strains and Nocardioides sp. strain JS614, but similar to the value for another Pseudomonas isolate (40). Differences among the k values may be partly attributable to differences in the conditions used to grow the cultures prior to determining the kinetic parameters (39). The maximum growth rate for strain AJ (calculated from k and the yield, as described previously [39]) is 0.017 day−1. The extent of VC dechlorination by strain AJ was assessed based on triplicate measurements of chloride release: 1.009 ± 0.054 mol Cl− per mol of VC consumed, which is very close to the stoichiometric amount expected.
The involvement of a monooxygenase in VC catabolism was evaluated with strains AJ and TD. For each isolate, two sets of duplicate serum bottles received VC (60 μM and 150 μM for strains AJ and TD, respectively); one set also received acetylene (5% headspace concentration). All of the VC was consumed in less than 2 days in the set without acetylene, while less than 10% of the VC was consumed in the set with acetylene. When acetylene and VC were purged from the headspace and VC was added again, VC consumption resumed, indicating that the effect was reversible. Strains AJ and TD are not able to use VC or ethene as a substrate in the absence of oxygen. Strains AJ and TD is capable of growth on ethylene oxide by using nitrate as a terminal electron acceptor, indicating that oxygen is not used as a reactant in the catabolic pathway beyond ethylene oxide.
The ability of strain AJ to resume VC utilization following a period of starvation was evaluated. The resumption of VC utilization was a concern, because several of the aerobic isolates capable of using VC as a growth substrate lose this ability after starvation for only 1 day or less (5, 18). However, with strain AJ, VC metabolism resumed even after 60 days without exposure to VC (or any other substrates).
In addition to VC, ethene, and ethylene oxide, strains AJ and TD grow on glycolate, glyoxylate, acetate, ethanolamine, and ethylene glycol. Strain AJ also grew on glycolaldehyde but was unable to use chloroacetate as a substrate, even after 53 days of incubation.
DISCUSSION
This study demonstrated that the metabolism of VC as the sole source of carbon and energy under aerobic conditions by two isolates obtained from two distinct hazardous waste sites is conferred by linear megaplasmids. In addition, linear megaplasmids are required for strains AJ and TD in the use of ethene as a growth substrate and for strain AJ in the use of ethylene oxide. Linear plasmids have been found in both gram-positive and gram-negative bacteria (20), although no previous reports were found that specifically identify linear plasmids in Pseudomonas putida or Ochrobactrum spp. Jumas-Bilak et al. (21) found two extrachromosomal replicons (approximately 100 and 150 kb) in Ochrobactrum anthropi ATCC 49188T (21), although pulse times for the CHEF gel were not varied in order to confirm their linearity. O. anthropi LMG 3301 also contains two extrachromosomal replicons (approximately 50 and <50 kb) that may be linear plasmids (21), but their physical confirmation was not defined. In several strains of Rhodococcus, linear plasmids carry the genes for isopropyl benzene and trichloroethylene catabolism (10), isopropylcatechol 2,3-dioxygenase (24), an alkene monooxygenase (35), and polychlorinated biphenyl degradation (29). Linear plasmids have also been found in strains of Xanthobacter for biodegradation of 1,2-dichloroethane (37) and propylene (26), in strains of Streptomyces for mercury resistance (33), and in Nocardia opaca for hydrogen autotrophy (22). Mycobacterium sp. strain E-1-57, isolated on ethene, contains two linear plasmids (260 and 340 kb); however, a direct link between their presence and ethene metabolism was not established (35).
Strain TD appears to be the first Ochrobactrum sp. reported that uses VC and ethene as growth substrates. Most of the microbes isolated thus far with this ability are Mycobacterium spp. (5, 18), although several strains of Pseudomonas spp. (39, 40) and one Nocardioides sp. (5) have also been reported. Strain AJ appears to be the first P. putida isolate that grows on VC. Several of the Ochrobactrum strains that share more than 99.8% identity with strain TD include four O. anthropi, which are perhaps best known as opportunistic human pathogens. However, several Ochrobactrum isolates are known for their ability to biodegrade halogenated organic compounds, including 4-fluorobenzoate (36) and atrazine (27).
VC catabolism in strains AJ and TD appears to be initiated by an alkene monooxygenase, based on the inhibition of VC utilization by acetylene, a known inhibitor of monooxygenases (1), the occasional detection of trace amounts of VC epoxide during growth on VC, and a lack of VC consumption in the absence of oxygen. Other isolates capable of growth on VC also use an alkene monooxygenase (5, 7, 18, 39, 40). A large plasmid (approximately 310 kb) in VC-grown Nocardioides sp. strain JS614 carries the genes for an alkene monooxygenase (30). The monooxygenase and coenzyme M genes used by Xanthobacter sp. strain Py2 for propylene metabolism are located on a linear megaplasmid (26). The genes for a monooxygenase and an epoxyalkane:coenzyme M transferase involved in catabolism of VC by Mycobacterium sp. strain JS60 are found on linear megaplasmids (7).
In strains AJ and TD, the pathway for VC and ethene metabolism beyond their respective epoxides is not yet known. Strain AJ grows on several substrates that are potential downstream intermediates, including ethylene glycol, glycolaldehyde, glycolate, glyoxylate, ethanolamine, and acetate. However, growth of strain AJ on these compounds results in loss of the plasmid and the ability to use VC as a substrate. This result may indicate that the genes conferring the initial steps in VC catabolism are encoded on the linear plasmid but that degradation of the predicted intermediate products involves chromosomal genes. Alternatively, the pathway could proceed through other intermediates altogether, e.g., via a reaction with an epoxyalkane:coenzyme M transferase (6, 7). Regardless of the pathway, one would expect that only a few reactions are needed to transform VC epoxide into a compound that can be degraded with enzymes that are not carried on the plasmid. If only a few genes are needed, the question is raised as to why the linear plasmids are so large. Further analysis of these linear plasmids may reveal interesting functions peripherally related to VC, ethene, and ethylene oxide metabolism. For example, given the high degree of reactivity of epoxides with nucleic acids, genes for DNA repair may play a key role in the maintenance of the plasmids.
Acknowledgments
The Department of Energy's Savannah River Site and the South Carolina Universities Research and Education Foundation provided partial funding for this project through a graduate student internship. This research was also supported in part by a grant from the U.S. Environmental Protection Agency.
We thank Jim C. Spain for providing a sample of Mycobacterium sp. strain JS60. The Clemson University Genomics Institute generously provided access to their facilities for several aspects of this work.
REFERENCES
- 1.Bedard, C., and R. Knowles. 1989. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol. Rev. 53:68-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley, P. M., and F. H. Chapelle. 2000. Acetogenic microbial degradation of vinyl chloride. Environ. Sci. Technol. 34:2761-2763. [Google Scholar]
- 3.Brigmon, R. L. 2001. Methanotrophic bacteria: use in bioremediation, p. 1936-1944. In G. B. Bitton (ed.), Encyclopedia of environmental microbiology. John Wiley & Sons, New York, N.Y.
- 4.Coleman, N. V., T. E. Mattes, J. M. Gossett, and J. C. Spain. 2002. Biodegradation of cis-dichloroethene as the sole carbon source by a β-proteobacterium. Appl. Environ. Microbiol. 68:2726-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman, N. V., T. E. Mattes, J. M. Gossett, and J. C. Spain. 2002. Phylogenetic and kinetic diversity of aerobic vinyl chloride-assimilating bacteria from contaminated sites. Appl. Environ. Microbiol. 68:6162-6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman, N. V., and J. C. Spain. 2003. Distribution of the coenzyme M pathway of epoxide metabolism among ethene- and vinyl chloride-degrading Mycobacterium strains. Appl. Environ. Microbiol. 69:6041-6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman, N. V., and J. C. Spain. 2003. Epoxyalkane:coenzyme M transferase in the ethene and vinyl chloride biodegradation pathways of Mycobacterium strain JS60. J. Bacteriol. 185:5536-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox, E., E. Edwards, L. Lehmicke, and D. Major. 1995. Intrinsic biodegradation of trichloroethene and trichloroethane in a sequential anaerobic-aerobic aquifer, p. 223-232. In R. E. Hinchee, J. T. Wilson, and D. C. Downey (ed.), Intrinsic bioremediation. Battelle Press, Columbus, Ohio.
- 9.Cupples, A. M., A. M. Spormann, and P. L. McCarty. 2003. Growth of a Dehalococcoides-like microorganism on vinyl chloride and cis-dichloroethene as electron acceptors as determined by competitive PCR. Appl. Environ. Microbiol. 69:953-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dabrock, B., M. Keβeler, B. Averhoff, and G. Gottschalk. 1994. Identification and characterization of a transmissible linear plasmid from Rhodococcus erythropolis BD2 that encodes isopropylbenzene and trichloroethene catabolism. Appl. Environ. Microbiol. 60:853-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danko, A. S. 2004. Use of vinyl chloride as a growth substrate by aerobic bacteria: pathway analysis and involvement of linear mega plasmids. Ph.D. thesis. Clemson University, Clemson, S.C.
- 12.Edwards, E. A., and E. E. Cox. 1997. Field and laboratory studies of sequential anaerobic-aerobic chlorinated solvent biodegradation, p. 261-265. In B. C. Alleman and A. Leeson (ed.), In situ and on-site bioremediation, vol. 3. Battelle Press, New Orleans, La. [Google Scholar]
- 13.Fennell, D. E., A. B. Carroll, J. M. Gossett, and S. H. Zinder. 2001. Assessment of indigenous reductive dechlorination potential at a TCE-contaminated site using microcosms, polymerase chain reaction analysis, and site data. Environ. Sci. Technol. 35:1830-1839. [DOI] [PubMed] [Google Scholar]
- 14.Freedman, D. L., A. S. Danko, and M. F. Verce. 2001. Substrate interactions during aerobic biodegradation of methane, ethene, vinyl chloride and 1,2-dichloroethenes. Water Sci. Technol. 43:333-340. [PubMed] [Google Scholar]
- 15.Freedman, D. L., and S. D. Herz. 1996. Use of ethylene and ethane as primary substrates for aerobic cometabolism of vinyl chloride. Water Environ. Res. 68:320-328. [Google Scholar]
- 16.Freedman, D. L., M. H. Swamy, N. C. Bell, and M. F. Verce. 2004. Biodegradation of chloromethane under nitrate-reducing and aerobic conditions by Pseudomonas aeruginosa strain NB1. Appl. Environ. Microbiol. 70:4629-4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenberg, A. E., L. S. Clesceri, and A. D. Eaton (ed.). 1992. Standard methods for the examination of water and wastewater, 18th ed. American Public Health Association, American Water Works Association, and Water Environment Federation, Washington, D.C.
- 18.Hartmans, S., and J. A. M. de Bont. 1992. Aerobic vinyl chloride metabolism in Mycobacterium aurum L1. Appl. Environ. Microbiol. 58:1220-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He, J., K. M. Ritalahti, M. R. Aiello, and F. E. Löffler. 2003. Complete detoxification of vinyl chloride by an anaerobic enrichment culture and identification of the reductively dechlorinating population as a Dehalococcoides species. Appl. Environ. Microbiol. 69:996-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinnebusch, J., and K. Tilly. 1993. Linear plasmids and chromosomes in bacteria. Mol. Microbiol. 10:917-922. [DOI] [PubMed] [Google Scholar]
- 21.Jumas-Bilak, E., S. Michaux-Charachon, G. Bourg, M. Ramuz, and A. Allardet-Servent. 1998. Unconventional genomic organization in the alpha subgroup of the Proteobacteria. J. Bacteriol. 180:2749-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalkus, J., M. Reh, and H. G. Schlegel. 1990. Hydrogen autotrophy of Nocardia opaca strains is encoded by linear megaplasmids. J. Gen. Microbiol. 136:1145-1151. [DOI] [PubMed] [Google Scholar]
- 23.Keppler, F., R. Borchers, J. Parcht, S. Rheinberger, and H. F. Scholer. 2002. Natural formation of vinyl chloride in the terrestrial environment. Environ. Sci. Technol. 36:2479-2483. [DOI] [PubMed] [Google Scholar]
- 24.Kesseler, M., E. R. Dabbs, B. Averhoff, and G. Gottschalk. 1996. Studies on the isopropylbenzene 2,3-dioxygenase and the 3-isopropylcatechol 2,3-dioxygenase genes encoded by the linear plasmid of Rhodococcus erythropolis BD2. Microbiology 142:3241-3251. [DOI] [PubMed] [Google Scholar]
- 25.Kielhorn, J., C. Melber, U. Wahnschaffe, A. Aitio, and I. Mangelsdorf. 2000. Vinyl chloride: still a cause for concern. Environ. Health Perspect. 108:579-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krum, J. G., and S. A. Ensign. 2001. Evidence that a linear megaplasmid encodes enzymes of aliphatic alkene and epoxide metabolism and coenzyme M (2-mercaptoethanesulfonate) biosynthesis in Xanthobacter strain Py2. J. Bacteriol. 183:2172-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laura, D., G. de Socio, R. Frassanito, and D. Rotilio. 1996. Effects of atrazine on Ochrobactrum anthropi membrane fatty acids. Appl. Environ. Microbiol. 62:2644-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madl, M. D. 2002. Aerobic biodegradation of ethene, cis-1,2-dichloroethene and vinyl chloride in sediments at the Savannah River Site sanitary landfill. M.S. thesis. Clemson University, Clemson, S.C.
- 29.Masai, E., K. Sugiyama, N. Iwashita, S. Shimizu, J. E. Haushild, T. Hatta, K. Kimbara, K. Yano, and M. Fukuda. 1997. The bphDEF meta-cleavage pathway genes involved in biphenyl/polychlorinated biphenyl degradation are located on a linear plasmid and separated from the initial bphACB genes in Rhodococcus sp. strain RHA1. Gene 187:141-149. [DOI] [PubMed] [Google Scholar]
- 30.Mattes, T. E., N. V. Coleman, J. C. Spain, and J. M. Gossett. 2003. Evidence that vinyl chloride monooxygenase genes are encoded by a megaplasmid in Nocardioides strain JS614, abstr. Q-061, p. 525. Abstracts of the 103rd General Meeting, American Society of Microbiology, Washington, D.C.
- 31.Maymó-Gatell, X., I. Nijenhuis, and S. H. Zinder. 2001. Reductive dechlorination of cis-1,2-dichloroethene and vinyl chloride by “Dehalococcoides ethenogenes.” Environ. Sci. Technol. 35:516-521. [DOI] [PubMed] [Google Scholar]
- 32.O'Sullivan, D. J., and T. R. Klaenhammer. 1993. Rapid mini-prep isolation of high quality plasmid DNA from Lactococcus and Lactobacillus spp. Appl. Environ. Microbiol. 59:2730-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravel, J., H. Schrempf, and R. T. Hill. 1998. Mercury resistance is encoded by transferable giant linear plasmids in two Chesapeake Bay Streptomyces strains. Appl. Environ. Microbiol. 64:3383-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riethman, H., B. Birren, and A. Gnirke. 1997. Preparing HMW bacterial DNA embedded in agarose plugs, p. 136-138. In B. Birren, E. D. Green, S. Klapholz, R. M. Myers, and J. Roskams (ed.), Genome analysis: a laboratory manual, vol. 1: analyzing DNA. Cold Spring Harbor Laboratory Press, Plainview, N.Y. [Google Scholar]
- 35.Saeki, H., M. Akira, B. Keizo, B. Averhoff, and G. Gottschalk. 1999. Degradation of trichloroethene by a linear-plasmid-encoded alkene monooxygenase in Rhodococcus corallinus (Nocardia corallina) B-276. Microbiology 145:1721-1730. [DOI] [PubMed] [Google Scholar]
- 36.Song, B., N. J. Palleroni, and M. M. Haggblom. 2000. Isolation and characterization of diverse halobenzoate-degrading denitrifying bacteria from soils and sediments. Appl. Environ. Microbiol. 66:3446-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tardif, G., C. W. Greer, D. Labbe, and P. C. K. Lau. 1991. Involvement of a large plasmid in the degradation of 1,2-dichloroethane by Xanthobacter autotrophicus. Appl. Environ. Microbiol. 57:1853-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomkins, J. P., M. Luo, G. C. Fang, D. Main, J. L. Goicoechea, M. Atkins, D. A. Frisch, R. E. Page, E. Guzmán-Novoa, Y. Yu, G. Hunt, and R. A. Wing. 2002. New genomic resources for the honey bee (Apis mellifera L.): development of a deep-coverage BAC library and a preliminary STC database. Genet. Mol. Res. 1:306-316. [PubMed] [Google Scholar]
- 39.Verce, M. F., R. L. Ulrich, and D. L. Freedman. 2000. Characterization of an isolate that uses vinyl chloride as a growth substrate under aerobic conditions. Appl. Environ. Microbiol. 66:3535-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verce, M. F., R. L. Ulrich, and D. L. Freedman. 2001. Transition from cometabolic to growth-linked biodegradation of vinyl chloride by a Pseudomonas sp. isolated on ethene. Environ. Sci. Technol. 35:4242-4251. [DOI] [PubMed] [Google Scholar]
- 41.Vogel, T. M., C. S. Criddle, and P. L. McCarty. 1987. Transformations of halogenated aliphatic compounds. Environ. Sci. Technol. 21:722-736. [DOI] [PubMed] [Google Scholar]