Abstract
AIM
To detect the expression of trefoil factors (TFFs) and TWIST1 in colorectal cancer (CRC) and analyze their correlation with metastasis and survival.
METHODS
This study examined the expression of TFF1, TFF3 and TWIST1 in a total of 75 tumor samples, 47 matched normal samples (15 cm from the lesion margin), 30 metastatic lymph nodes, and 10 liver metastatic cancer samples from patients with CRC. The relationship was then analyzed between the protein expression and different clinical records. TFF1, TFF3, TWIST1,E-cadherin, vimentin and β-catenin mRNA and protein expression levels were measured in colon cancer cell lines with different metastatic potentials (HIEC, HT29, SW620, and LoVo cells), and the correlation of the expression levels with epithelial-mesenchymal transition (EMT) was discussed.
RESULTS
It was found that 66.7% (50/75), 78.7% (59/75) and 54.7% (41/75) of tumor tissue samples exhibited positive staining for TFF1, TFF3 and TWIST1 and so did 27.3% (13/47), 100% (47/47) and 17% (8/47) of adjacent normal colorectal tissues. Compared with adjacent normal tissues, significant differences were found in the expression of all three proteins in different cancerous tissues (P < 0.05). Higher expression of TFF3 and TWIST1 was significantly correlated with lymph node metastasis (P = 0.034, P = 0.000), advanced stage (P = 0.031, P = 0.003), and poorer survival (P = 0.042 for the TFF3 group, P = 0.003 for the TWIST1 group). The expression of TFF3 and TWIST1 in cancer cell lines was higher than that in HIEC (a normal human intestinal epithelial cell line)(P < 0.05), and the expression intensity demonstrated a tendency to rise with increased metastatic potential both at the protein and mRNA levels. However, TFF1 expression demonstrated the opposite tendency. It was also observed that the expression of E-cadherin and β-catenin tended to decrease while that of vimentin, TWIST1 and Snail tended to rise with the increase in metastatic potential.
CONCLUSION
The expression of TFF3 and TWIST1 might be associated with the survival of patients with CRC after curative resection and might be pivotal predictors of disease progression. TFF3 may be correlated to the invasiveness of CRC.
Keywords: Colorectal cancer, Trefoil factors, TWIST1, Survival, Metastasis
Core tip: The expression of trefoil factors (TFFs) and TWIST1 in colorectal cancer (CRC) and their roles in metastasis and survival are unclear. This study involved the preliminary examination of the expression of TFF1, TFF3 and TWIST1 in CRC tissues and different metastasis samples from patients and cell lines. This study also analyzed the relationship between the expression of these proteins and metastatic potential and survival. It can be concluded that the expression of TFF3 and TWIST1 in CRC might be associated with patient survival after curative resection and may play an active role in disease progression. Finally, TFF3 may be correlated to the invasiveness of the CRC.
INTRODUCTION
Colorectal cancer is one of the most common cancers and a principal cause of death worldwide. The morbidity and mortality rates of CRC continue to increase, but the etiology is still not clear. In 2012, 1361000 new cases of CRC were reported globally, of which 253000 (accounting for 18.6%) were in China; notably, CRC has become the third most common malignancy in China[1]. Approximately one quarter of patients have metastases when CRC is initially diagnosed and nearly half ultimately develop metastases; thus, the 5-year survival rate is less than 10%[2-3]. In order to improve the prognosis of CRC, the most important considerations are the selection of high-risk patients with the help of the biomarkers and the intervention in the metastases.
The trefoil factors (TFFs) are three-loop structures that function as secretory peptides. They contain a highly conserved motif of cysteine disulfide bonds, which endows them with significant functional stability[4-6]. TFF1 is predominantly expressed in the stomach and colon, TFF2 expression is primarily localized in the stomach, and TFF3 expression is commonly observed in the intestine[7,8]. Although TFFs have been shown to protect the gastrointestinal tract against mucosal damage[9,10], recent evidence from experimental and clinical studies indicates a pivotal role of TFFs in oncogenic transformation, growth, and metastasis of human solid tumors[11-19]. TFF1, also called “estrogen-inducible pS2 protein”, can be isolated from human breast cancer and has been determined to have prognostic significance in breast cancer patients[20]. TFF3 has indicated both pro-invasive and pro-angiogenic activities in cells derived from several common human solid tumors[15,17,21-25]. TFF1 and TFF3 have been found to be co-expressed particularly in tumors of the human mammary gland[12,26], where they each regulate the expression of the other in a positive feedback loop[27]. Moreover, serum levels of TFFs in patients with several cancers have been reported as useful biomarkers for the prediction of the presence of cancer[28-30]. The exact role of TFFs in the progression of CRC and their prognostic value have not yet been extensively expounded. Despite that TFFs are primarily secreted by the epithelium of the gastrointestinal tract, there are no any consensus about if TFFs are oncogenic or anti-oncogenic[31].
TWIST1 is a highly conserved basic helix-loop-helix (bHLH) protein and transcription factor[32]. It is involved in embryonic development in vertebrates through its regulation of epithelial-mesenchymal transition (EMT) during neural crest migration[33,34]. The EMT program is also activated in a wide variety of cancer cells as they leave the primary tumor and colonize distant organs and form metastases[35,36]. TWIST1 overexpression induces EMT and E-cadherin repression, suggesting that TWIST1 promotes metastasis by inducing EMT[37,38]. Zhu et al[39] reported that TFF3 continuously up-regulates the expression of TWIST1 in HT29 cell line, which indicates the possible involvement of TFF3 in the process of EMT.
In this study, we detected the expression of TFF1, TFF3 and TWIST1 in different CRC tissues and cell lines with different metastatic potential to identify the relationship between the protein expression and metastatic potential and survival of CRC.
MATERIALS AND METHODS
This study included a total of 75 tumor samples, 47 matched normal controls (tissue located 15 cm away from the lesion margin), 30 metastatic lymph nodes, and 10 liver metastatic cancer samples from patients with CRC. Among the 75 patients studied, 48 (64%) were men and 27 (36%) were women with a median age of 56 years (range, 24 to 86 years). Fifteen (20%) cases had stage I disease, 27 (36%) had stage II disease, 27 (36%) had stage III disease, and 6 (8%) had stage IV disease (Table 1). CRC tissues were obtained from treatment-naive patients who underwent surgery at the Fourth Military Medical University Hospital of Xi Jing, between 2007 and 2008. The clinicopathologic variables and survival data were obtained from the medical records, and the disease stages of the patients were classified according to the 2010 UICC colorectal cancer TNM staging system. The data were analyzed anonymously and reported. No patients received any interventions, and personal information was not revealed.
Table 1.
TFF1, TFF3, and TWIST1 expression in different colorectal cancer tissues n (%)
| Histology | n |
TFF1 |
TFF3 |
TWIST1 |
|||||||||
| - | + | ++ | +++ | - | + | ++ | +++ | - | + | ++ | +++ | ||
| CRC | 75 | 25 (41) | 19 (35.8) | 23 (67.6) | 8 (57.1) | 16 (72.7) | 34 (55.7) | 21 (38.9) | 4 (16) | 34 (34.7) | 18 (56.3) | 16 (64) | 7 (100) |
| MNT | 47 | 34 (55.7) | 12 (22.6) | 1 (2.9) | 0 | 0 | 8 (13.1) | 22 (40.7) | 17 (68) | 39 (41.9) | 7 (21.9) | 1 (4.3) | 0 |
| MLN | 30 | 2 (3.3) | 17 (32.1) | 8 (23.5) | 3 (21.4) | 6 (27.3) | 14 (23) | 8 (14.8) | 2 (8) | 20 (21.7) | 5 (15.6) | 5 (21.7) | 0 |
| Liver M | 10 | 0 | 5 (9.4) | 2 (5.9) | 3 (21.4) | 0 | 5 (8.2) | 3 (5.6) | 2 (8.0) | 5 (5.4) | 2 (6.3) | 3 (13) | 0 |
| Overall | P = 0.000 | P = 0.000 | P = 0.000 | ||||||||||
CRC: Colorectal cancer; MNT: Matched normal tissue; MLN: Metastatic lymph node; Liver M: Liver metastasis; TFF: Trefoil factor.
Immunohistochemistry
Immunohistochemical staining was performed using the streptavidin-biotin peroxidase complex with a commercially available SP-kit (Beijing ZhongshanJinqiao Biotechnology Limited Company, China, SP-9000). A rabbit anti-human TFF1 monoclonal antibody (Abcam-Epitomics, United States, 2801-1 dilution: 1:100), a rabbit anti-human TFF3 monoclonal antibody (Abcam-Epitomics, United States, 2816-1 dilution: 1:200), and a mouse anti-human TWIST1 monoclonal antibody (Abcam, United States, ab135180 dilution: 1:100) were used. As a negative control, PBS was used in place of the primary antibody. After deparaffinization and rehydration, the sections were heated in a microwave oven for 10 min at 100 °C in 10 mmol/L citrate buffer (pH 6.0). The sections were then incubated sequentially with fresh 3% hydrogen peroxide in PBS, 10% normal goat serum, primary antibody, biotinylated goat anti-rabbit IgG, and streptavidin-peroxidase; the slides were washed with PBS three times before each step. The visualization of the sites of peroxidase binding was achieved with diaminobenzidine. The sections were counterstained with hematoxylin. All slides were interpreted by two independent observers in a blinded fashion.
Evaluation of immunostaining
The immunohistochemistry results were evaluated according to a histoscore that combines the intensity of the immunoreaction with the scope of the positive staining area. The intensity (I) of staining was scored as 0 (no staining), 1 (weak), 2 (moderate), or 3 (strong). The density (D) of staining was scored as 1 (less than 10%), 2 (10%-50%), 3 (50%-80%), or 4 (80%-100%) according to the percentage of positively stained regions in relation to the total cancer area. The final immunohistochemical staining scores (0-12) for each tumor were the product of I × D. Samples with I × D ≤ 4 were considered weakly positive; those with I × D ≥ 4 were considered strongly positive.
Cell culture
All cell culture media were purchased from HyClone, Thermo. Three human colon carcinoma cell lines with different invasive potentials (HT29, SW620, and LoVo) and an immortalized human epithelial cell line (HIEC) were gifts from the laboratory of Xi Jing, Digestive Disease Hospital (Fourth Military Medical University). All cell lines were maintained in RPMI 1640 growth medium supplemented with 10% heat-inactivated fetal bovine serum, 1% penicillin, and 1% streptomycin. HIEC cells were also supplemented with human insulin (0.1 U/mL).
Western blot assay
The four colorectal cancer cell lines were harvested separately in ice-cold PBS. Total protein was extracted, separated by SDS-PAGE, and transferred onto PVDF membranes. The membranes were blocked with 5% (w/v) dried skimmed milk powder in Tris-buffered saline (blocking solution) for 1 h at room temperature. Then, the membranes were probed with primary antibodies against TFF1 (1/500 dilution), TFF3 (1/300 dilution), and TWIST1 (1/500) in blocking solution overnight at 4 °C. After a TBST wash, the membranes were incubated with the corresponding secondary antibody (1/2000 dilution) in blocking solution for 1 h at room temperature. Immunoreactive proteins were detected using enhanced chemiluminescence.
Quantitative real-time RT-PCR assays
The mRNA levels of target genes in colon cancer cell lines were compared with those in the immortalized human normal intestinal epithelial cell line (HIEC). Quantitative real-time RT-PCR (qRT-PCR) was performed as previously described[14]; Brilliant SYBR Green QRT-PCR Master Mix as part of a 2-Step kit (Stratagene, La Jolla, CA, United States) was used. The PCR amplification was performed using a Bio-Red Real Time PCR system. The primers used for qRT-PCR were as follows: GAPDH forward (F): 5′-AGCCTTCTCCATGGTGGTGAA-3′, GAPDH reverse (R): 5′-ATCACCATCTTCCAGGAGCGA-3′; TFF1 (F): 5′-AATAAGGGCTGCTGTTTCG-3′, TFF1 (R): 5′-ACTCCTCTTCTGGAGGGAC-3′; TFF3 (F): 5′-CTGCTGCTTTGACTCCAGGAT-3′, TFF3 (R): 5′-CAGCTGGAGGTGCCTCAGAA-3′; TWIST1 (F): 5′-CATGTCCGCGTCCCACTAG-3′, TWIST1-(R): 5′-TGTCCATTTTCTCCTTCTCTGG-3′; E-cadherin (F): 5′-GAGTGCCAACTGGACCATTCAGTA-3′, E-cadherin (R): 5′-AGTCACCCACCTCTAAGGCCATC-3′; vimentin (F): 5′-CAGGCAAAGCAGGAGTCCAC-3′; vimentin (R): 5′-GCAGCTTCAACGGCAAAGTTC-3′; β-catenin (F): 5′-TGAGTGTCATGAAGTGCACAGGAG-3′, β-catenin (R): 5′-AACAGGCTGATGGTGCCAGAG-3′; snail (F): 5′-CGCGCTCTTTCCTCGTCA-3′, snail (R): 5′-TCCCAGATGAGCATTGGCAG-3′. The Ct (threshold cycle) values of the target gene amplifications were normalized to those of the GAPDH control. All reactions were performed in triplicate in a 25-μL reaction volume. The PCR amplification program consisted of 30 s of an initial denaturation at 95 °C followed by 40 cycles of PCR at 95 °C for 5 s, and then 60 °C for 30 s. Standard curves were drawn and the relative amount of target gene mRNA was normalized to that of GAPDH. Specificity was verified by melt curve analysis. The comparative CT method was used to calculate the relative quantification of gene expression.
RESULTS
Expression of TFFs and TWIST1 in normal, primary tumor, and metastatic tissues
The positive expression of TFF1 and TFF3 was found in the cytoplasm and cytomembrane (Figure 1A), while TWIST1 staining was observed in both the cytoplasm and nuclei of the cells (Figure 1A). TFF1 was mainly expressed in CRC cells with varying intensity and was only occasionally expressed or was negative in matched normal tissues (Figure 1B). TFF3 was distributed diffusely and was expressed as fine granules in most goblet cells in normal tissues, but its expression was decreased in the cytoplasm of CRC cells (Figure 1A and B). We found selective expression of TWIST1 in cancerous tissues but scarcely in their normal counterparts (Figure 1A and B).
Figure 1.
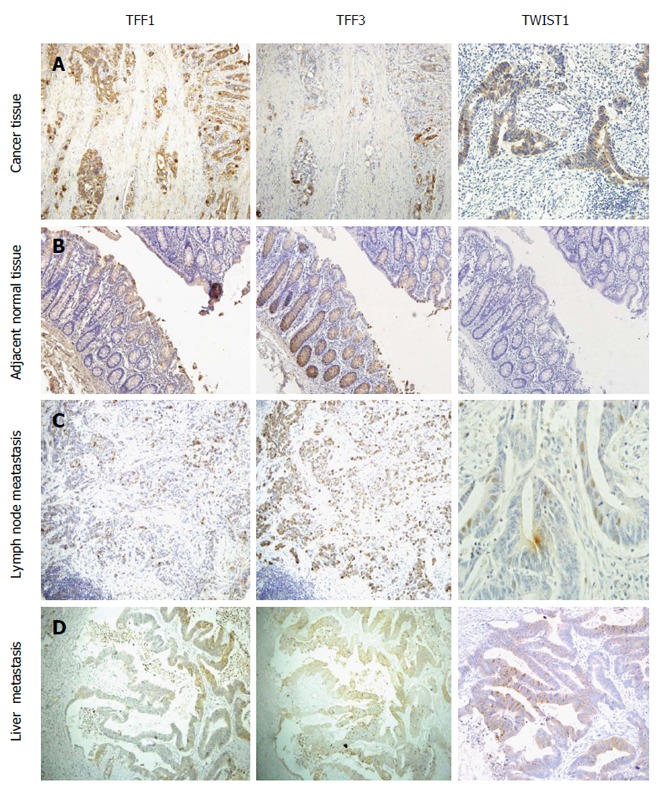
TFF1, TFF3 and TWIST1 expression trends in colorectal cancer (A), adjacent normal tissue (B), metastatic lymph node (C), and liver metastasis (D). Compared with normal mucosal tissue, colorectal cancer tissues expressed significantly higher TFF1 and TWIST1 and lower TFF3. Compared to primary colorectal cancer tissue, there were no statistical differences in TFF3 and TWIST1 expression in metastatic lymph node or liver metastatic tissue. But disparity in TFF1 expression between metastatic lymph node and primary colorectal cancer had statistical significance.
Among the 50 tissues that were positive for TFF1, 19 demonstrated weak staining (38%), 23 demonstrated moderate staining (46%), and 8 demonstrated strong staining (16%) (Table 1). Among the 59 patients with TFF3-positive cancer, 34 showed weak staining (57.6%), 21 showed moderate staining (35.6%), and 4 showed strong staining (6.8%) (data not shown). Of the 41 patients with TWIST1-positive cancer, 18 showed weak staining (43.9%), 16 showed moderate staining (39%), and 7 showed strong staining (17.1%) (Table 1).
Results from the assessment of TFF1, TFF3 and TWIST1 expression in primary tumor tissues, metastatic lymph nodes, and liver metastatic tissues are presented in Table 1. It was shown that 66.7% (50/75), 78.7% (59/75), and 54.7% (41/75) of samples exhibited positive staining for TFF1, TFF3 and TWIST1, respectively. In addition, 27.3% (13/47), 100% (47/47), and 17% (8/47) of normal tissues adjacent to colorectal CRC tissues showed positive staining for TFF1, TFF3 and TWIST1, respectively. Compared with normal mucosal tissues, primary CRC tissues expressed significantly higher levels of TFF1 and TWIST1 but a lower level of TFF3 (Table 1). No significant difference was observed in TFF3 or TWIST1 expression between primary CRC tissues and metastatic lymph nodes or liver metastatic tissues (P = 0.879, P = 0.105 for TFF3 and P = 0.08, P = 0.780 for TWIST1). However, the disparity in the expression of TFF1 between metastatic lymph nodes and primary colorectal cancer was statistically significant (P = 0.01, P = 0.03) (Table 2). The correlation analysis between the expression of TFF1 and TFF3 proteins also showed a negative correlation in CRC (P = 0.007, r = -0.312; Table 3). We also found that the expression levels of TFF3 were stronger in mucinous and signet-ring cell cancers than in other cancer types, which might illustrate that the more mucus that is secreted, the stronger the TFF3 expression.
Table 2.
Comparison between low and high expression of TFF1, TFF3, and TWIST1 in different colorectal cancer tissues n (%)
| Histology | n |
TFF1 |
TFF3 |
TWIST1 |
||||||
| Low | High | P value | Low | High | P value | Low | High | P value | ||
| CRC | 75 | 44 (38.6) | 31 (64.6) | 0.000 | 50 (60.2) | 25 (31.6) | 0.000 | 52 (40) | 23 (71.9) | 0.001 |
| MNT | 47 | 46 (40.4) | 1 (2.1) | 0.000 | 8 (9.6) | 39 (49.4) | 0.000 | 46 (35.4) | 1 (3.1) | 0.0001 |
| MLN | 30 | 19 (16.7) | 11 (22.9) | 0.659 | 20 (24.1) | 10 (12.7) | 0.818 | 25 (19.2) | 5 (15.6) | 0.142 |
| Liver M | 10 | 5 (4.4) | 5 (10.4) | 0.602 | 5 (6) | 5 (6.3) | 0.494 | 7 (5.4) | 3 (9.4) | 0.966 |
CRC: Colorectal cancer; MNT: Matched normal tissue; MLN: Metastatic lymph node; Liver M: Liver metastasis.
Table 3.
Correlation among TFF1, TFF3 and TWIST1expression n (%)
| Protein expression |
TFF1 |
TFF3 |
||||
| Low | High | P value | Low | High | P value | |
| TWIST1 | 0.297 | 0.623 | ||||
| Low | 32 (72.7) | 19 (61.3) | 31 (66) | 20 (71.4) | ||
| High | 12 (27.3) | 12 (38.7) | 16 (34) | 8 (28.6) | ||
| TFF3 | ||||||
| Low | 22 (46.8) | 25 (53.2) | 0.007 | |||
| High | 22 (78.6) | 6 (21.4) | ||||
Expression of TFF1, TFF3 and TWIST1 in relation to clinicopathological features
The results illustrating a correlation between TFF1, TFF3, and TWIST1 expression and clinicopathological variables are presented in Table 4. Higher expression of TFF3 and TWIST1 was significantly correlated with lymph node metastasis (P = 0.034, P = 0.000) and advanced stage (P = 0.031, P = 0.003). In contrast, TFF1 expression was correlated with differentiation (P = 0.029).
Table 4.
Correlation between TFF1, TFF3, and TWIST1 expression and clinicopathological Characteristics of colorectal cancers n (%)
| Characteristic n = 75 |
TFF1 |
TFF3 |
TWIST1 |
||||||
| Low | High | P value | Low | High | P value | Low | High | P value | |
| Age (yr) | 0.299 | 0.368 | 0.840 | ||||||
| ≤ 60 | 28 (63.6) | 17 (54.8) | 27 (57.4) | 18 (64.3) | 31 (60.8) | 14 (58.3) | |||
| > 60 | 16 (36.4) | 14 (45.2) | 20 (42.6) | 10 (35.7) | 20 (39.2) | 10 (41.7) | |||
| Gender | 0.369 | 0.146 | 0.134 | ||||||
| Male | 30 (68.2) | 18 (58.1) | 33 (70.2) | 15 (53.6) | 30 (58.8) | 18 (75.0) | |||
| Female | 14 (31.8) | 13 (41.9) | 14 (29.8) | 13 (46.4) | 21 (41.2) | 6 (25.0) | |||
| Histology | 0.089 | 0.475 | 0.397 | ||||||
| Adenocarcinoma | 42 (95.5) | 26 (83.9) | 42 (89.4) | 26 (92.9) | 47 (92.2) | 21 (87.5) | |||
| Mucinous | 2 (4.5) | 5 (16.1) | 5 (10.6) | 2 (7.1) | 4 (7.8) | 3 (12.5) | |||
| Differentiation | 0.029 | 0.136 | 0.234 | ||||||
| Well | 10 (22.7) | 12 (38.7) | 15 (31.9) | 7 (25.0) | 18 (35.3) | 4 (16.7) | |||
| Moderate | 16 (36.4) | 15 (48.4) | 22 (46.8) | 9 (32.1) | 20 (39.2) | 11 (45.8) | |||
| Poor | 18 (40.9) | 4 (12.9) | 10 (21.3) | 12 (42.9) | 13 (25.5) | 9 (37.5) | |||
| Location | 0.802 | 0.411 | 0.847 | ||||||
| Colon | 31 (70.5) | 21 (67.7) | 31 (66.0) | 21 (75) | 35 (68.6) | 17 (70.8) | |||
| Rectum | 13 (29.5) | 10 (32.3) | 16 (34.0) | 7 (25) | 16 (31.4) | 7 (30.7) | |||
| T | 0.545 | 0.651 | 0.246 | ||||||
| 1 | 2 (4.5) | 0 | 1 (2.1) | 1 (3.6) | 1 (2.0) | 1 (4.2) | |||
| 2 | 10 (22.7) | 9 (29.0) | 14 (29.8) | 5 (17.9) | 16 (31.4) | 3 (12.5) | |||
| 3 | 13 (29.5) | 7 (22.6) | 11 (23.4) | 9 (32.1) | 11 (21.6) | 9 (37.5) | |||
| 4 | 19 (43.2) | 15 (48.4) | 21 (44.7) | 13 (46.4) | 23 (45.1) | 11 (45.8) | |||
| N | 0.143 | 0.034 | 0.000 | ||||||
| No | 26 (59.1) | 13 (41.9) | 20 (42.6) | 19 (67.9) | 34 (66.7) | 5 (20.8) | |||
| Yes | 18 (40.9) | 18 (58.1) | 27 (57.4) | 9 (32.1) | 17 (33.3) | 19 (79.2) | |||
| M | 0.484 | 0.267 | 0.288 | ||||||
| No | 41 (93.2) | 28 (90.3) | 42 (89.4) | 27 (96.4) | 48 (94.1) | 21 (87.5) | |||
| Yes | 3 (6.8) | 3 (9.7) | 5 (10.6) | 1 (3.6) | 3 (5.9) | 3 (12.5) | |||
| Staging | 0.917 | 0.031 | 0.003 | ||||||
| I | 9 (20.5) | 6 (19.4) | 11 (23.4) | 4 (14.3) | 13 (25.5) | 2 (8.3) | |||
| II | 17 (38.6) | 10 (32.3) | 11 (23.4) | 16 (57.1) | 23 (45.1) | 4 (16.7) | |||
| III | 15 (34.1) | 12 (38.7) | 20 (42.6) | 7 (25.0) | 12 (23.5) | 15 (62.5) | |||
| IV | 3 (6.8) | 3 (9.7) | 5 (10.6) | 1 (3.6) | 3 (5.9) | 3 (12.5) | |||
CRC: Colorectal cancer; MNT: Matched normal tissue; MLN: Metastatic lymph node; Liver M: Liver metastasis.
Relationship between TFF1, TFF3 and TWIST1 expression and patient's survival
The survival analysis indicated that the 3- and 5-year survival rates of the 75 patients were 71% (55 patients) and 54% (43 patients), respectively. The overall survival of the patients with higher TFF3 or TWIST1 expression levels was significantly less than that of the patients with lower TFF3 or TWIST1 expression levels (P = 0.042 for the TFF3 group; P = 0.003 for the TWIST1 group; Figure 2A and B). TFF1 expression demonstrated no correlation with patient survival (P = 0.952; Figure 2D). In contrast, higher expression of TFF3 and TWIST1 was correlated with an even worse overall survival (Figure 2C). Furthermore, a univariate analysis showed that the survival rate had a close relationship with age, infiltration depth, lymph node metastasis, distant metastasis, TFF3 expression, TWIST1 expression, and TNM stage (P < 0.05). A multivariate analysis demonstrated that only age, tumor stage, and TFF3 expression were correlated with survival (Table 5).
Figure 2.
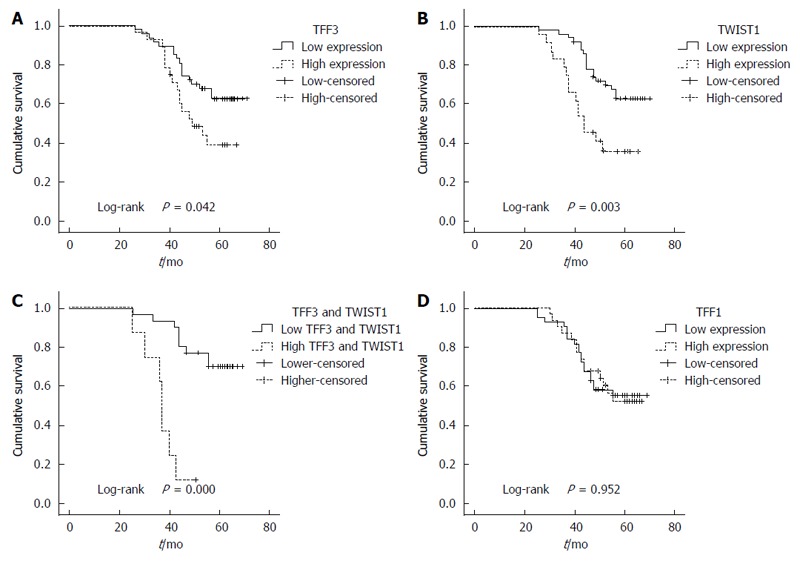
Correlation between protein expression in colorectal cancer and overall survival. The survival analysis indicated that the overall survival of the patients with higher TFF3 or TWIST1 expression was significantly less than that of the patients with lower TFF3 or TWIST1 expression (A and B); Higher expression of the TFF3 and TWIST1 correlated with even worse overall survival (C); TFF1 expression had no correlation with the survival (D).
Table 5.
Significant predictive factors for cancer-specific survival in COX proportional-hazard analysis
| Prognostic factor | Univariate HR (95%CI) | P value | Multivariate HR (95%CI) | P value |
| Age | 0.404 (0.189-0.864) | 0.019 | 0.262 (0.106-0.647) | 0.004 |
| Gender | 1.137 (0.565-2.287) | 0.719 | 1.065 (0.450-2.519) | 0.887 |
| Histopathological type | 0.526 (0.126-2.193) | 0.378 | 0.380 (0.080-1.809) | 0.224 |
| Tumor differentiation | 0.939 (0.611-1.445) | 0.775 | 0.701 (0.395-1.246) | 0.226 |
| T | 1.556 (1.030-2.350) | 0.036 | 0.979 (0.578-1.657) | 0.937 |
| N | 2.961 (1.954-4.485) | 0.000 | 1.668 (0.716-3.889) | 0.236 |
| M | 12.519 (4.593-34.120) | 0.000 | 2.728 (0.672-11.064) | 0.160 |
| Stage | 4.507 (2.706-7.507) | 0.000 | 2.718 (1.003-7.368) | 0.039 |
| TFF1 | 1.805 (0.929-3.509) | 0.081 | 1.539 (0.661-3.579) | 0.317 |
| TFF3 | 0.382 (0.178-0.819) | 0.013 | 0.224 (0.085-0.593) | 0.003 |
| TWIST1 | 2.819 (1.441-5.515) | 0.002 | 1.904 (0.847-4.278) | 0.119 |
Expression of TFF1 and TFF3 proteins and mRNAs demonstrates the opposite tendency and is associated with EMT in vitro
Based on the immunohistochemistry results, we determined gene expression in colon cancer cell lines with different metastatic potentials. Our results showed that the expression levels of TFF3 and TWIST1 in cancer cell lines were higher than those in the normal human intestinal epithelial cell line (P < 0.05). Moreover, the expression intensities were inclined to rise both at the protein and mRNA levels with the increase in metastatic potential. However, TFF1 expression demonstrated the opposite tendency (Figure 3).
Figure 3.
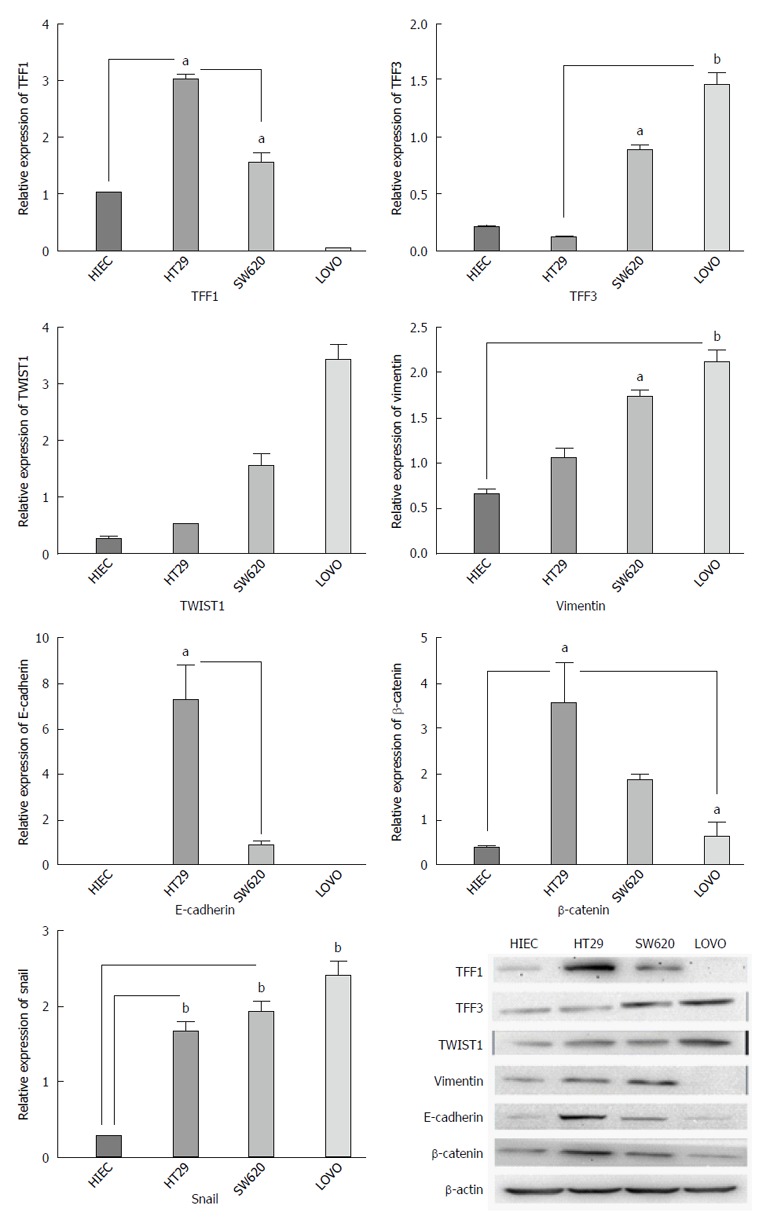
The mRNA and protein expression of TFF1, TFF3, TWIST1, E-cadherin, vimentin, and β-catenin in colon cancer cell lines with different invasion potentials. The expression of TFF3 and TWIST1 in cancer cell lines was higher than that in normal human intestinal epithelial cell line, and the expression intensity was inclined to rise with the increase in metastatic potential both at protein and mRNA levels. But TFF1 expression had the opposite tendency. The TFF1 protein and mRNA expression decreased and TWIST1 and TFF3 expression increased gradually with the increase in metastatic potential of cell lines. E-cadherin and β-catenin expression tended to decrease, while vimentin and TWIST1 expression was inclined to rise with the increase in metastatic potential. aP < 0.05, bP < 0.01.
Based on the above-mentioned outcome, we determined whether TFF1 and TFF3 expression was related to EMT. We investigated E-cadherin, vimentin, β-catenin and Snail, which are classic markers and critical transcription factors of EMT in different cell lines. It was observed that the expression levels of TFF1 protein and mRNA were decreased and that the expression levels of TWIST1 and TFF3 increased gradually with the increase in the metastatic potential of the cell lines. It was also observed that E-cadherin and β-catenin expression tended to decrease, while that of vimentin, TWIST1 and Snail tended to increase with the increase in metastatic potential (Figure 3).
DISCUSSION
Although TFFs display a number of beneficial effects in terms of cytoprotection and restitution, these proteins may lead to adverse outcomes when they are over-expressed in different tumor tissues. TFF1 protein was expressed in 89% of CRCs but not in normal mucosa[40], and in another study, the expression of TFF1 was found to be focally present in 60% of primary CRCs[7]. Our data demonstrated that the TFF1 expression rate was 66.7%. The adjacent normal tissue also exhibited a positive expression rate of 27.3%, which is different to that mentioned above. TFF1 also tended to be increased in adjacent normal tissues, primary cancer tissues, and lymph node metastases successively, which may explain the oncogenic or pro-invasive features of TFF1. Reports on TFF3 and TWIST1 expression in CRC are rare, and the present data showed that their rates of positive expression were 78.7% and 54.7%, respectively, in cancer tissues, while adjacent normal tissues showed positive expression rates of 100% and 17%, respectively. TFF1 and TFF3 protein expression also showed a negative correlation in CRC, which suggests the possibility of mutual regulation among TFF family members. TFF1 protein expression is increased in gastric carcinoma with nodal metastases compared with carcinomas that lack such metastases[7,41]. Our study revealed no statistically significant association between TFF1 expression and clinicopathological features except for differentiation. Im et al[41] reported a much higher frequency of TFF1 expression in undifferentiated and diffuse types of gastric cancer compared with differentiated and intestinal types of gastric cancer. Contrary to this, TFF1 expression in CRC showed a higher frequency in well or moderately differentiated cases than in poorly differentiated cases. No correlation was observed between TFF1 expression and survival.
With regard to the clinical significance of TFF3 and TWIST1 expression, we found that higher TFF3 or TWIST1 expression was significantly correlated with lymph node metastasis and advanced stage. Additionally, we demonstrated that patients with higher expression of TFF3 or TWIST1 had a lower survival rate than those with lower expression of TFF3 or TWIST1. Therefore, it could be concluded that TFF3 and TWIST1 can be considered prognostic factors that may be linked to poor survival of patients with CRC.
TFF1 and TFF3 have been observed to be potent mitogens in both normal and cancer cells. The overexpression of TFF1 and TFF3 mRNAs has been found in all cases of ductal cancer in situ, lobular cancer in situ, invasive lobular cancer and in 21 of 24 invasive ductal cancers tested[42]. The migration and invasiveness of human gastric cancer cells are stimulated by TFF1 in a P13K-dependent manner[43]. TFF3 also induces a migratory and invasive phenotype in human colon cancer cells[22]. TWIST1 has been confirmed to contribute to metastasis through EMT regulation[44]. The inhibition of TWIST1 expression in highly metastatic mammary carcinoma cells specifically suppresses their ability to migrate from the mammary gland to the lung in a mouse model of breast cancer[45]. As for TFF3 and TWIST1 expression, although no significant differences were observed among primary cancers, metastatic lymph nodes and liver metastatic tissues, statistically significant differences were observed among colon cancer cell lines with different invasion potentials by qRT-PCR and Western blot assays.
TFF3, along with TFF1, has been used as a marker for the detection of disseminated breast cancer cells[46]. The decrease in TFF1 mRNA expression and the increase in TFF3 mRNA expression with increasing malignancy in cell lines may suggest opposite functions of these proteins in different types of colon cancer. The decrease in expression of epithelial markers (E-cadherin and β-catenin) and the increase in expression of mesenchymal markers (vimentin, TWIST1, and Snail) indicate that TFF3 may have some correlation with EMT. Further experiments including overexpression or RNA interference assays by transfection are needed to probe this potential correlation.
The expression levels of TFF3 and TWIST1 in CRC might be associated with patient survival after curative resection and are independent predictors of disease progression. In addition, simultaneous positive expression of TFF3 and TWIST1 induces a much worse survival rate. Therefore, it is assumed that TFF3 and TWIST1 expression plays a vital role in the development of CRC. Finally, TFF3 may be correlated to the invasiveness of CRC.
COMMENTS
Background
Despite that TFFs are primarily secreted by the epithelium of the gastrointestinal tract, their role in the progression of colorectal cancer (CRC) and their prognostic value have not been extensively expounded. TWIST1 is a key transcription factor that regulates epithelial-mesenchymal transition (EMT).
Research frontiers
Recent evidence indicates a pivotal role of TFFs in the oncogenic transformation, growth and metastasis of human solid tumors.TFF1 and TFF3 are found to co-express notably in the tumors of the human mammary gland and co-regulate each other in a positive feedback loop. The studies also show that TFF3 continuously up-regulates the expression of TWIST1 in HT29 cell line, which hints the involvement of TFFs in the process of EMT.
Innovations and breakthroughs
Reports on TFFs and TWIST1 expressions in CRC and correlations with metastasis and survival are quite rare. This research findings suggest that The expressions of TFF3 and TWIST1 are associated with CRC patients’ survival after a curative resection and pivotal predictors of disease progression. TFF3 may be associated with the invasiveness of the CRC.
Applications
This research outcomes indicate that TFF3 and TWIST1 expression plays a vital role in the development of CRC and these two proteins might be important markers for disease progression and prognosis of CRC. The correlation of TFF3 with the EMT process may hint a potential target of CRC treatment.
Terminology
EMT is a process by which epithelial cells lose their cell polarity and cell-cell adhesion, and gain migratory and invasive properties to become mesenchymal stem cells; these are multipotent stromal cells that can differentiate into a variety of cell types. EMT is essential for numerous developmental processes including mesoderm formation and neural tube formation. EMT has also been shown to occur in wound healing, in organ fibrosis, and in the initiation of metastasis for cancer progression.
Peer-review
The studies reported in this manuscript are descriptive/correlative in nature and are well organized. Each discussion presented is intriguing and has some guiding significance for clinical practice and future research.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study design and procedures described below were approved by the Ethics Committee of the Affiliated Tumor Hospital of Xinjiang Medical University (No. W-201401).
Informed consent statement: All patients provided informed written consent prior to their participation.
Conflict-of-interest statement: No conflict of interest exists in the submission of this manuscript.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 29, 2016
First decision: September 28, 2016
Article in press: December 2, 2016
P- Reviewer: Bhawal UK S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Cai SJ, Peng JJ. Colorectal cancer epidemiology and prevention strategies [CSCO2014: China¡¯s Progress of Clinical Oncology] People’s Medical Publishing House: 2014. pp. 294–301. [Google Scholar]
- 2.Li S, Wang J, Lu Y, Fan D. Screening and early diagnosis of colorectal cancer in China: a 12 year retrospect (1994-2006) J Cancer Res Clin Oncol. 2007;133:679–686. doi: 10.1007/s00432-007-0231-z. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, et al. SEER Cancer Statistics Review, 1975-2008, National Cancer Institute. Bethesda: 2011. [Google Scholar]
- 4.Thim L. A new family of growth factor-like peptides. ‘Trefoil’ disulphide loop structures as a common feature in breast cancer associated peptide (pS2), pancreatic spasmolytic polypeptide (PSP), and frog skin peptides (spasmolysins) FEBS Lett. 1989;250:85–90. doi: 10.1016/0014-5793(89)80690-8. [DOI] [PubMed] [Google Scholar]
- 5.Sands BE, Podolsky DK. The trefoil peptide family. Annu Rev Physiol. 1996;58:253–273. doi: 10.1146/annurev.ph.58.030196.001345. [DOI] [PubMed] [Google Scholar]
- 6.Wright NA, Poulsom R, Stamp G, Van Noorden S, Sarraf C, Elia G, Ahnen D, Jeffery R, Longcroft J, Pike C. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Gastroenterology. 1993;104:12–20. doi: 10.1016/0016-5085(93)90830-6. [DOI] [PubMed] [Google Scholar]
- 7.Regalo G, Wright NA, Machado JC. Trefoil factors: from ulceration to neoplasia. Cell Mol Life Sci. 2005;62:2910–2915. doi: 10.1007/s00018-005-5478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madsen J, Nielsen O, Tornøe I, Thim L, Holmskov U. Tissue localization of human trefoil factors 1, 2, and 3. J Histochem Cytochem. 2007;55:505–513. doi: 10.1369/jhc.6A7100.2007. [DOI] [PubMed] [Google Scholar]
- 9.Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4:721–732. doi: 10.1038/nrm1203. [DOI] [PubMed] [Google Scholar]
- 10.Hernández C, Santamatilde E, McCreath KJ, Cervera AM, Díez I, Ortiz-Masiá D, Martínez N, Calatayud S, Esplugues JV, Barrachina MD. Induction of trefoil factor (TFF)1, TFF2 and TFF3 by hypoxia is mediated by hypoxia inducible factor-1: implications for gastric mucosal healing. Br J Pharmacol. 2009;156:262–272. doi: 10.1111/j.1476-5381.2008.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.May FE, Westley BR. Trefoil proteins: their role in normal and malignant cells. J Pathol. 1997;183:4–7. doi: 10.1002/(SICI)1096-9896(199709)183:1<4::AID-PATH1099>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Poulsom R, Hanby AM, Lalani EN, Hauser F, Hoffmann W, Stamp GW. Intestinal trefoil factor (TFF 3) and pS2 (TFF 1), but not spasmolytic polypeptide (TFF 2) mRNAs are co-expressed in normal, hyperplastic, and neoplastic human breast epithelium. J Pathol. 1997;183:30–38. doi: 10.1002/(SICI)1096-9896(199709)183:1<30::AID-PATH1085>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 13.Henry JA, Bennett MK, Piggott NH, Levett DL, May FE, Westley BR. Expression of the pNR-2/pS2 protein in diverse human epithelial tumours. Br J Cancer. 1991;64:677–682. doi: 10.1038/bjc.1991.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan VY, Chan MW, Leung WK, Leung PS, Sung JJ, Chan FK. Intestinal trefoil factor promotes invasion in non-tumorigenic Rat-2 fibroblast cell. Regul Pept. 2005;127:87–94. doi: 10.1016/j.regpep.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Yio X, Zhang JY, Babyatsky M, Chen A, Lin J, Fan QX, Werther JL, Itzkowitz S. Trefoil factor family-3 is associated with aggressive behavior of colon cancer cells. Clin Exp Metastasis. 2005;22:157–165. doi: 10.1007/s10585-005-6615-z. [DOI] [PubMed] [Google Scholar]
- 16.Dhar DK, Wang TC, Tabara H, Tonomoto Y, Maruyama R, Tachibana M, Kubota H, Nagasue N. Expression of trefoil factor family members correlates with patient prognosis and neoangiogenesis. Clin Cancer Res. 2005;11:6472–6478. doi: 10.1158/1078-0432.CCR-05-0671. [DOI] [PubMed] [Google Scholar]
- 17.Babyatsky M, Lin J, Yio X, Chen A, Zhang JY, Zheng Y, Twyman C, Bao X, Schwartz M, Thung S, et al. Trefoil factor-3 expression in human colon cancer liver metastasis. Clin Exp Metastasis. 2009;26:143–151. doi: 10.1007/s10585-008-9224-9. [DOI] [PubMed] [Google Scholar]
- 18.Efstathiou JA, Noda M, Rowan A, Dixon C, Chinery R, Jawhari A, Hattori T, Wright NA, Bodmer WF, Pignatelli M. Intestinal trefoil factor controls the expression of the adenomatous polyposis coli-catenin and the E-cadherin-catenin complexes in human colon carcinoma cells. Proc Natl Acad Sci USA. 1998;95:3122–3127. doi: 10.1073/pnas.95.6.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taupin D, Ooi K, Yeomans N, Giraud A. Conserved expression of intestinal trefoil factor in the human colonic adenoma-carcinoma sequence. Lab Invest. 1996;75:25–32. [PubMed] [Google Scholar]
- 20.Tuna B, Sökmen S, Sarioğlu S, Füzün M, Küpelioğlu A, Ellidokuz H. PS2 and HSP70 expression in rectal adenocarcinomas: an immunohistochemical investigation of 45 cases. Appl Immunohistochem Mol Morphol. 2006;14:31–36. doi: 10.1097/01.pai.0000141544.28862.35. [DOI] [PubMed] [Google Scholar]
- 21.Williams R, Elia G, Stamp GWH. Characterization of monoclonal antibodies raised to C-terminal peptides of pS2: a major trefoil peptide and motility factor expressed in adenocarcinomas and regions of mucosal injury. Hum Pathol. 1996;12:1259–1266. doi: 10.1016/s0046-8177(96)90334-2. [DOI] [PubMed] [Google Scholar]
- 22.Rivat C, Rodrigues S, Bruyneel E, Piétu G, Robert A, Redeuilh G, Bracke M, Gespach C, Attoub S. Implication of STAT3 signaling in human colonic cancer cells during intestinal trefoil factor 3 (TFF3) -- and vascular endothelial growth factor-mediated cellular invasion and tumor growth. Cancer Res. 2005;65:195–202. [PubMed] [Google Scholar]
- 23.Chan MW, Chan VY, Leung WK, Chan KK, To KF, Sung JJ, Chan FK. Anti-sense trefoil factor family-3 (intestinal trefoil factor) inhibits cell growth and induces chemosensitivity to adriamycin in human gastric cancer cells. Life Sci. 2005;76:2581–2592. doi: 10.1016/j.lfs.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Taupin DR, Kinoshita K, Podolsky DK. Intestinal trefoil factor confers colonic epithelial resistance to apoptosis. Proc Natl Acad Sci USA. 2000;97:799–804. doi: 10.1073/pnas.97.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchino H, Kataoka H, Itoh H, Koono M. Expression of intestinal trefoil factor mRNA is downregulated during progression of colorectal carcinomas. J Clin Pathol. 1997;50:932–934. doi: 10.1136/jcp.50.11.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taupin D, Wu DC, Jeon WK, Devaney K, Wang TC, Podolsky DK. The trefoil gene family are coordinately expressed immediate-early genes: EGF receptor- and MAP kinase-dependent interregulation. J Clin Invest. 1999;103:R31–R38. doi: 10.1172/JCI3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vestergaard EM, Borre M, Poulsen SS, Nexø E, Tørring N. Plasma levels of trefoil factors are increased in patients with advanced prostate cancer. Clin Cancer Res. 2006;12:807–812. doi: 10.1158/1078-0432.CCR-05-1545. [DOI] [PubMed] [Google Scholar]
- 28.Kaise M, Miwa J, Tashiro J, Ohmoto Y, Morimoto S, Kato M, Urashima M, Ikegami M, Tajiri H. The combination of serum trefoil factor 3 and pepsinogen testing is a valid non-endoscopic biomarker for predicting the presence of gastric cancer: a new marker for gastric cancer risk. J Gastroenterol. 2011;46:736–745. doi: 10.1007/s00535-011-0396-8. [DOI] [PubMed] [Google Scholar]
- 29.Aikou S, Ohmoto Y, Gunji T, Matsuhashi N, Ohtsu H, Miura H, Kubota K, Yamagata Y, Seto Y, Nakajima A, et al. Tests for serum levels of trefoil factor family proteins can improve gastric cancer screening. Gastroenterology. 2011;141:837–845.e1-7. doi: 10.1053/j.gastro.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qu Y, Yang Y, Ma D, Xiao W. Increased trefoil factor 3 levels in the serum of patients with three major histological subtypes of lung cancer. Oncol Rep. 2012;27:1277–1283. doi: 10.3892/or.2012.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perry JK, Kannan N, Grandison PM, Mitchell MD, Lobie PE. Are trefoil factors oncogenic? Trends Endocrinol Metab. 2008;19:74–81. doi: 10.1016/j.tem.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Hebrok M, Wertz K, Füchtbauer EM. M-twist is an inhibitor of muscle differentiation. Dev Biol. 1994;165:537–544. doi: 10.1006/dbio.1994.1273. [DOI] [PubMed] [Google Scholar]
- 33.Chen ZF, Behringer RR. twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 1995;9:686–699. doi: 10.1101/gad.9.6.686. [DOI] [PubMed] [Google Scholar]
- 34.Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E, et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511:246–250. doi: 10.1038/nature13305. [DOI] [PubMed] [Google Scholar]
- 36.Burns TF, Dobromilskaya I, Murphy SC, Gajula RP, Thiyagarajan S, Chatley SN, Aziz K, Cho YJ, Tran PT, Rudin CM. Inhibition of TWIST1 leads to activation of oncogene-induced senescence in oncogene-driven non-small cell lung cancer. Mol Cancer Res. 2013;11:329–338. doi: 10.1158/1541-7786.MCR-12-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morel AP, Hinkal GW, Thomas C, Fauvet F, Courtois-Cox S, Wierinckx A, Devouassoux-Shisheboran M, Treilleux I, Tissier A, Gras B, et al. EMT inducers catalyze malignant transformation of mammary epithelial cells and drive tumorigenesis towards claudin-low tumors in transgenic mice. PLoS Genet. 2012;8:e1002723. doi: 10.1371/journal.pgen.1002723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22:725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu YQ, Tan XD. TFF3 modulates NF-{kappa}B and a novel negative regulatory molecule of NF-{kappa}B in intestinal epithelial cells via a mechanism distinct from TNF-{alpha} Am J Physiol Cell Physiol. 2005;289:C1085–C1093. doi: 10.1152/ajpcell.00185.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Häckel C, Falkenberg B, Günther T, Lippert H, Roessner A. The pS2 protein in colorectal carcinomas and metastases. Pathol Res Pract. 1998;194:171–176. doi: 10.1016/S0344-0338(98)80018-6. [DOI] [PubMed] [Google Scholar]
- 41.Im S, Yoo C, Jung JH, Choi HJ, Yoo J, Kang CS. Reduced expression of TFF1 and increased expression of TFF3 in gastric cancer: correlation with clinicopathological parameters and prognosis. Int J Med Sci. 2013;10:133–140. doi: 10.7150/ijms.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emami S, Rodrigues S, Rodrigue CM, Le Floch N, Rivat C, Attoub S, Bruyneel E, Gespach C. Trefoil factor family (TFF) peptides and cancer progression. Peptides. 2004;25:885–898. doi: 10.1016/j.peptides.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 43.Yio X, Diamond M, Zhang JY, Weinstein H, Wang LH, Werther L, Itzkowitz S. Trefoil factor family-1 mutations enhance gastric cancer cell invasion through distinct signaling pathways. Gastroenterology. 2006;130:1696–1706. doi: 10.1053/j.gastro.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 44.Vernon AE, LaBonne C. Tumor metastasis: a new twist on epithelial-mesenchymal transitions. Curr Biol. 2004;14:R719–R721. doi: 10.1016/j.cub.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 45.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Lacroix M. Significance, detection and markers of disseminated breast cancer cells. Endocr Relat Cancer. 2006;13:1033–1067. doi: 10.1677/ERC-06-0001. [DOI] [PubMed] [Google Scholar]


