Abstract
AIM
To investigate whether gut microbiota metabolite sodium butyrate (NaB) is an effective substance for attenuating non-alcoholic fatty liver disease (NAFLD) and the internal mechanisms.
METHODS
Male C57BL/6J mice were divided into three groups, normal control were fed standard chow and model group were fed a high-fat diet (HFD) for 16 wk, the intervention group were fed HFD for 16 wk and treated with NaB for 8 wk. Gut microbiota from each group were detected at baseline and at 16 wk, liver histology were evaluated and gastrointestinal barrier indicator such as zonula occluden-1 (ZO-1) were detected by immunohistochemistry and realtime-PCR, further serum or liver endotoxin were determined by ELISA and inflammation- or metabolism-associated genes were quantified by real-time PCR.
RESULTS
NaB corrected the HFD-induced gut microbiota imbalance in mice, while it considerably elevated the abundances of the beneficial bacteria Christensenellaceae, Blautia and Lactobacillus. These bacteria can produce butyric acid in what seems like a virtuous circle. And butyrate restored HFD induced intestinal mucosa damage, increased the expression of ZO-1 in small intestine, further decreased the levels of gut endotoxin in serum and liver compared with HF group. Endotoxin-associated genes such as TLR4 and Myd88, pro-inflammation genes such as MCP-1, TNF-α, IL-1, IL-2, IL-6 and IFN-γ in liver or epididymal fat were obviously downregulated after NaB intervention. Liver inflammation and fat accumulation were ameliorated, the levels of TG and cholesterol in liver were decreased after NaB intervention, NAS score was significantly decreased, metabolic indices such as FBG and HOMA-IR and liver function indicators ALT and AST were improved compared with HF group.
CONCLUSION
NaB may restore the dysbiosis of gut microbiota to attenuate steatohepatitis, which is suggested to be a potential gut microbiota modulator and therapeutic substance for NAFLD.
Keywords: Non-alcoholic fatty liver disease, Sodium butyrate, Gut microbiota, Gastrointestinal barrier, Endotoxin
Core tip: Non-alcoholic fatty liver disease (NAFLD) is a global epidemic metabolic health crisis that lacks effective therapeutic strategies. We found that NaB could correct the high-fat diet (HFD)-induced gut microbiota imbalance in mice, while it considerably elevated the abundances of the beneficial bacteria. These bacteria can produce butyric acid in what seems like a virtuous circle. And butyrate restored HFD induced intestinal mucosa damage, improved tight junction structure, reduced gut endotoxin into liver, leading to attenuate HFD induced liver inflammation and lipid accumulation, which may be a potential gut microbiota modulator and therapeutic substance for NAFLD.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is an emerging public health problem with an increasing incidence and prevalence globally[1,2]. NAFLD is a clinical-histological syndrome that is characterized histopathologically by predominantly macrovesicular steatosis with varying amounts of inflammation, cytological ballooning and fibrosis, and it is associated with significant morbidity and mortality. Few diagnostic and therapeutic strategies for patients with NAFLD are established[3].
The gut microbiota plays a pivotal role in the development and progression of NAFLD, although the underlying mechanisms remain largely uninvestigated[4-6]. Recent evidence has revealed that not only the gut microbiota themselves but also the bacterial metabolites are important for regulating the body’s life activities and metabolism. These include short-chain fatty acids (SCFAs)[7], which have fewer than 7 carbon atoms and are mainly produced by the fermentation of gut microbiota. SCFAs have been suggested to play a key role in ameliorating obesity, hypertension, and dyslipidemia.
The SCFA butyrate has multiple beneficial effects in mammals[8], such as regulation of the secretion of gut hormones[9], inhibition of the production of pro-inflammatory factors[10,11], and even inhibition of the growth of pernicious bacteria in the gut[12] as well as its beneficial role for barrier function in the gastrointestinal tract[13], which is great associated with pathogenetic mechanism of NAFLD development. However, many apparently contradictory results demonstrate the complexity of the interactions among the gut microbiota, butyrate concentration and host energy metabolism[14], the further interactions among NAFLD, gut microbiota and its metabolites still need more investigations to clarify[15].
We hypothesized that sodium butyrate (NaB) supplementation alone would attenuate high-fat diet (HFD)-induced steatohepatitis in mice via modulation of gut microbiota. Our results showed that NaB treatment protected mice against HFD-induced liver fat accumulation and inflammation, improved gut microbiota dysbiosis induced by HFD and attenuated gut microbiota-derived endotoxin-induced liver injury. Based on these findings, we propose that NaB may play an important role in relieving steatohepatitis and may be a useful therapeutic approach in the management of NAFLD.
MATERIALS AND METHODS
Animal experiments
Specified pathogen-free (SPF) male C57BL/6 mice (SLAC laboratory animal co., LTD, Shanghai, China) were housed in a controlled environment (23 °C, 12 h daylight cycle, lights off at 18.00 h). The mice were acclimatized for 7 d after arrival with free access to water and a standard chow diet. The mice were then assigned randomly to three groups: control, model (HF) and intervention group (HF + NaB) (n = 15/group, 5 mice per cage). Mice from the control group were fed a standard diet. The HF group and HF + NaB group were fed a high-lard-fat and high-cholesterol diet (88% standard diet, 10% lard and 2% cholesterol). Body weight and food consumption were recorded weekly. Eight weeks after initiation of the experimental diets, we sacrificed 3 randomly selected mice from every group to assess liver damage. The intervention group was underwent daily intragastric administration with NaB at 200 mg/kg body weight (Sigma-Aldrich, United States), while the HF group received the same amount of normal saline once per day for 8 wk. After 8 wk of intervention, the mice were fasted for 12 h and blood or tissue samples were collected. All animals were euthanized by pentobarbital sodium for tissue collection.
All animal experiments were approved by the Institutional Animal Care and Use Committee of Xinhua hospital affiliated to Shanghai Jiao Tong University School of Medicine and were conducted in accordance with the National Research Council Guide for Care and Use of Laboratory Animals.
Fecal sample collection from mice
Fecal samples were collected immediately upon defecation from each mouse at baseline and at 16 wk and stored at -80 °C. Fecal DNA was extracted from fecal samples using the E.Z.N.A Soil DNA Kit (Omega Bio-tek, Norcross, GA, United States) according to the manufacturer’s protocols. The V4-V5 region of the bacteria 16S ribosomal RNA gene was amplified by PCR. Amplicons were extracted from 2% agarose gels and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, United States) according to the manufacturer’s instructions and quantified using QuantiFluor™ -ST (Promega, United States). Purified amplicons were pooled at equimolar concentrations and paired-end sequenced (2 × 250) on an Illumina MiSeq platform according to standard protocols. Processing of the sequencing data and bioinformatics analysis were conducted by Majorbio in Shanghai[16]. These sequences were clustered into operational taxonomic units (OTUs) with a 97% sequence identity using mothur (furthest neighbor method) and chopseq (Majorbio). Rarefaction analysis was performed using mothur and plot-rarefaction (Majorbio). From these analyses, the Shannon diversities and Chao1 richness estimations were calculated using mothur. The unweighted UniFrac distance was used to quantify differences in community composition. Principal component analysis (PCA)[17] and nonmetric multidimensional scaling (NMDS) diagrams[18] were generated using the R package vegan to demonstrate the clustering of different samples. The hierarchical cluster analysis was performed using MVSP 3.1 software (Majorbio)[19].
Serum assays
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), glucose in plasma were then measured using an automated analyzer (Sysmex CHEMIX-180, Japan). Insulin (Rat/Mouse Insulin ELISA Kit, Merck-Millipore) in serum were measured by enzyme-linked immunosorbent assay. Mouse endotoxin concentrations in serum and liver homogenate were measured by enzyme-linked immunosorbent assay (Mouse ET ELISA Kit, Trust Specialty Zeal). Samples and standards were processed according to the manufacturer’s instructions.
Tissue histological analysis
Liver tissue was fixed in 4% paraformaldehyde, frozen in O.C.T, or snap-frozen in liquid nitrogen and stored at -80 °C. The small intestine was either fixed in 4% paraformaldehyde or snap-frozen in liquid nitrogen and stored at -80 °C. The small intestinal morphometric analysis was performed in 30 villi from each animal. The height of the villi comprises from extension of the crypt-villus junction up to top of the villi. The images were captured by an optical microscope (Leica DMI3000B, United States). The image analysis software Image-Pro Plus version 4.5.0.29 (Media Cybernetics, Silver Spring, MD, United States) was used for morphometric measurements in recorded images through the determination of villus height in µm. Epididymal fat was snap-frozen in liquid nitrogen and stored at -80 °C. Paraformaldehyde-fixed paraffin sections of the liver and small intestine were stained with hematoxylin-eosin for pathological analysis or Masson’s trichrome for fibrosis, and the nonalcoholic fatty liver activity score (NAS) was assessed. Frozen sections were stained with Oil Red O to detect lipids. For zonula occluden-1 (ZO-1) (Abcam, United States) staining, paraffin-embedded sections were used. Horseradish peroxidase-conjugated secondary antibody was applied, and the reaction was visualized using 3,3’-diaminobenzidine tetrahydrochloride.
Triglyceride and cholesterol evaluation in liver
Intrahepatic triglycerides (TGs) and cholesterol were measured using a triglyceride assay kit or cholesterol assay kit (Applygen Technologies Inc., Beijing, China). Samples and standards were then processed according to the manufacturer’s instructions. The final concentrations of triglycerides and cholesterol were corrected for protein content.
Real-time quantitative polymerase chain reaction
Total RNA was extracted from liver and small intestine using TRIzol (D9108B, Takara, Dalian, China) and reverse-transcribed into cDNA using PrimeScript RT master mix (RR036A, Takara, Dalian, China). Real-time quantitative polymerase chain reaction (qPCR) was performed with an Applied Biosystems 7500 Real-time PCR system using the SYBR Premix Ex Taq (Tli RNase H Plus) (RR420A, Takara, Dalian, China). Primers of the target genes were synthesized by Sangon Biotech (Shanghai, China). The primer sequences are listed in Supplementary Table 1. The primer specificity was confirmed by dissociation curves using 7500 system SDS software. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (B661304, Sangon Biotech) was used as the internal control.
Statistical analysis
Data are expressed as the means ± SEM. Comparisons were performed using one-way analysis of variance (ANOVA) in GraphPad Prism 5. Post-hoc Student-Newman-Keuls analyses were performed when > 2 groups were present. P < 0.05 was considered statistically significant. The statistical methods of this study were reviewed by GuangYu Chen from Clinical Epidemiology Center, Shanghai Jiaotong University.
RESULTS
NaB treatment improved liver indices and metabolism in HFD-fed mice
After 16 wk of the HF diet, all of the mice in the HF group gained much more body weight than those in the control group. The liver specimens were larger and paler in the HF group than the control group (Figure 1A). However, the HF + NaB group displayed a significantly improved whole body and liver profile than the HF group (Figure 1A).
Figure 1.
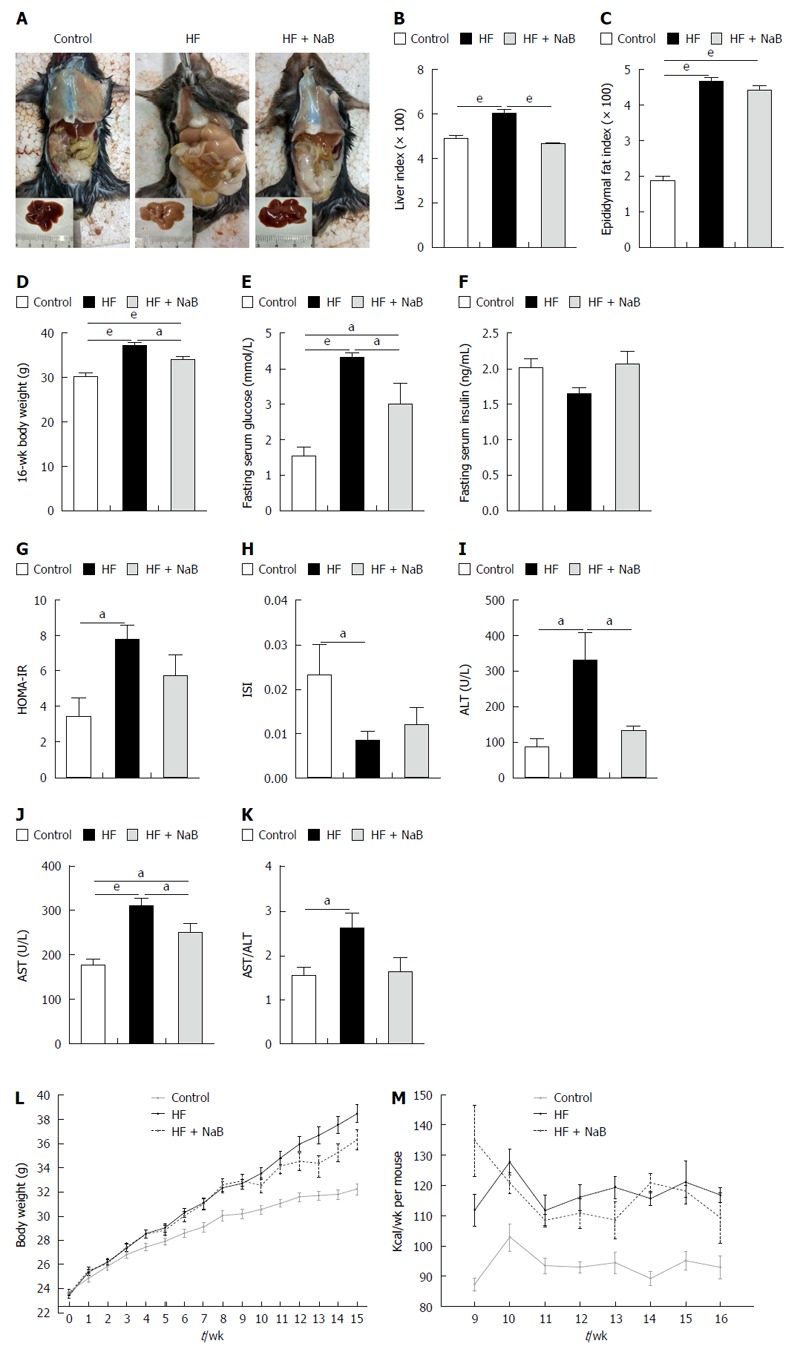
Sodium butyrate attenuates high-fat diet-induced obesity, liver injury and metabolic disturbance. A: Gross appearances of mice in the control, HF and HF + NaB groups; B: Liver index = liver weight/body weight × 100; C: Epididymal fat index = epididymal fat weight/body weight × 100; D: Body weight at 16 wk; E-H: Fasting serum glucose, fasting serum insulin, HOMA-IR and ISI of the three groups; I-K: Liver aminotransferases represent liver function; L: Body weight changes; M: Energy intake per mouse per week. Data represent means ± SEM. (n = 12 mice per group), aP < 0.05, bP < 0.01 and eP < 0.001.
The HF group displayed significantly higher liver and epididymal fat indices, elevated fasting blood glucose, a higher homeostasis model assessment of insulin resistance (HOMA-IR), and a lower insulin sensitivity index (ISI) than the control group (Figure 1). The NaB intervention attenuated HF diet-induced weight gain without reductions in energy intake, accompanied by opposite changes in HOMA-IR, ISI, fasting blood glucose, and especially liver indices (resulting in a 22% reduction, equivalent to the control group) (Figure 1). However, the NaB intervention had no effect on the epididymal fat index or insulin (Figure 1). Both ALT and AST, the specific markers of liver function, were significantly increased in the HF group compared with those in the control and were significantly decreased by the NaB intervention compared with those in the HF group (Figure 1).
Effects of NaB intervention on the tight junction of the small intestine and morphometry of the villi
Integrated tight junctions of the small intestine are associated with systemic inflammation. HE staining of the small intestine revealed greater damage to the intestinal mucosa in the HF group than the control group, which was repaired to a certain degree by NaB intervention (Figure 2A). The HF group displayed a reduction of the villus height of the small intestine of 20%, compared to the control group, while NaB intervention significantly attenuated the reduction of the villus height induced by HFD (Figure 2B). Immunohistochemistry for ZO-1, a tight junction marker, revealed that ZO-1 was more abundant after NaB intervention than in the HF group (Figure 2C). Next, we measured the mRNA levels of ZO-1 in the small intestine. Although there was no significant difference between the control and the HF group, ZO-1 expression was significantly increased in the HF + NaB group compared with either the control or the HF group (Figure 2D).
Figure 2.
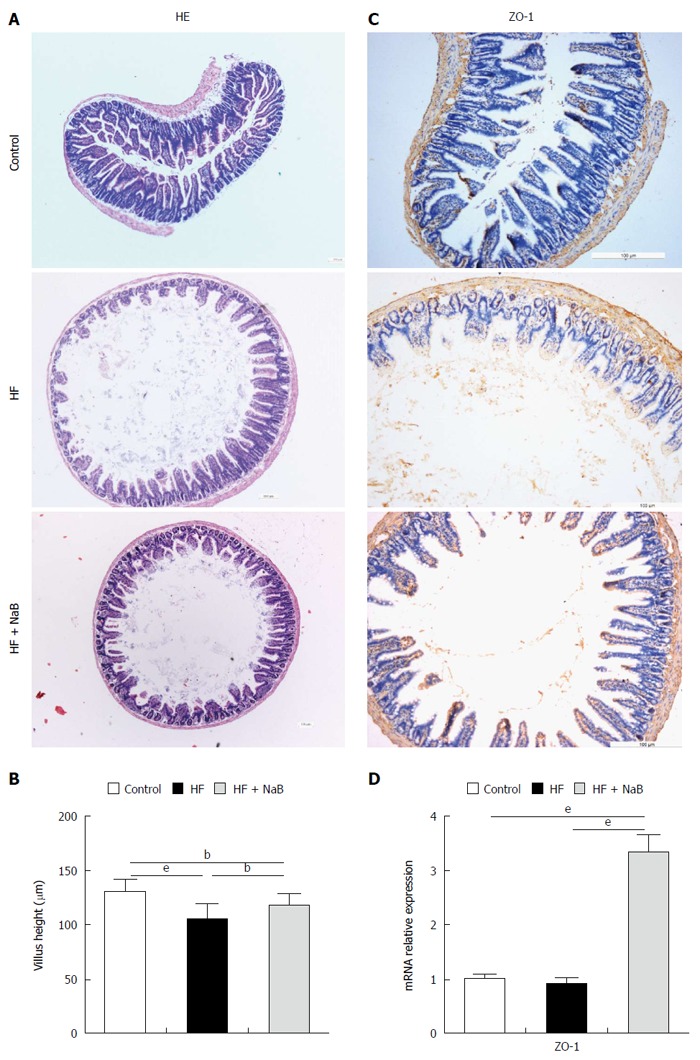
Beneficial effects of sodium butyrate on the small intestine. A: HE staining of the small intestine showing that NaB ameliorated HFD-induced mucosal damage; B: Morphometric analysis of villus; C: Immunohistochemistry for ZO-1 showing that NaB increased ZO-1 expression, indicating improvement of the tight junctions of the small intestine; D: ZO-1 mRNA expression in the small intestine. Data represent means ± SEM (n = 12 mice per group), aP < 0.05, bP < 0.01 and eP < 0.001.
Gut microbiota variations in response to diet or NaB intervention
To elucidate the effects of diet and NaB on the composition of the microbiota, we conducted Illumina MiSeq sequencing of bacterial 16S rRNA at baseline and at 16 wk after treatment. The quality of the sequencing, which included microbial richness, biodiversity, and rarefaction curves, met the requirements for subsequent analysis.
Sixteen weeks of HFD feeding induced significant changes in the gut microbial community at the phylum level compared with the control, with increased abundances of Bacteroidetes (63.1% vs 52.9%) and decreased abundances of Actinobacteria (0.04% vs 0.15%), Tenericutes (0.09% vs 1.42%) and Firmicutes (35.8% vs 44.6%). In addition, the ratio of Firmicutes to Bacteroidetes was lower in the HF group compared with the control (0.572 vs 0.851). However, NaB intervention mitigated the HFD-induced decrease in Actinobacteria and Tenericutes, enhanced the HFD-induced decreases in Firmicutes and the ratio of Firmicutes to Bacteroidetes, and enhanced the HFD-induced increases in Bacteroidetes and Proteobacteria (Supplementary Table 2).
At the genus level, Alistipes, Christensenellaceae_uncultured, Enterorhabdus, Lactobacillus, Parabacteroides, Parasutterella, Rikenella, Ruminococcaceae_incertae_sedis, Ruminococcaceae_unclassified, and Ruminococcaceae_uncultured were reduced in the HF group compared with the control (Figure 3). NaB intervention reversed the HFD-induced changes in Christensenellaceae_uncultured, Parabacteroides, Parasutterella and Lactobacillus (Figure 3). Furthermore, NaB treatment significantly increased Blautia compared with both the HF group and the control group (Figure 3).
Figure 3.
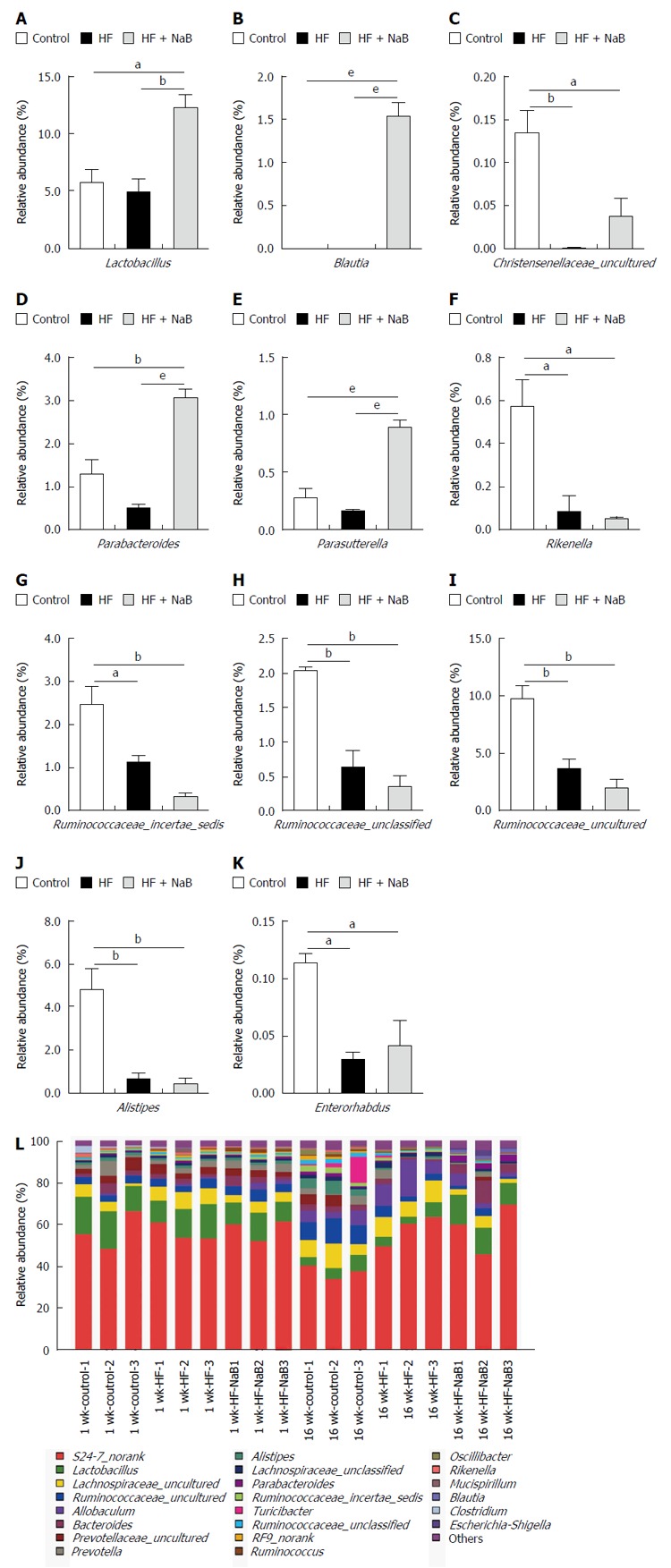
Influences of sodium butyrate on gut microbiota at the genus level. A-K: Alistipes, Christensenellaceae_uncultured, Enterorhabdus, Lactobacillus, Parabacteroides, Parasutterella, Rikenella, Ruminococcaceae_incertae_sedis, Ruminococcaceae_unclassified, and Ruminococcaceae_uncultured in the HF group were lower than the control. NaB intervention reversed the changes in Christensenellaceae_uncultured, Parabacteroides, Parasutterella and Lactobacillus. NaB treatment significantly increased Blautia; L: Relative read abundance of different bacterial genera within the different communities. Sequences that could not be classified into any known group were assigned as “norank”. Data represent means ± SEM. aP < 0.05, bP < 0.01 and eP < 0.001.
The NMDS analysis based on the Bray-Curtis distance clearly separated the 16 wk-HF group from the 16 wk-control, with the 16 wk-HF + NaB group positioned between them (Figure 4A). Thus, NaB intervention shifted the overall composition of the HFD-disrupted gut microbiota toward that of the control mice. PCA based on the OTU abundance was performed to provide an overview of the gut microbiota composition of 6 animal groups at baseline and at the end of the trial. There were no detectable differences in microbiota composition among the different groups before the intervention (Figure 4B). PC1, accounting for 68.24% of the total variance, predominantly reflected age-related changes in the composition of the gut microbiota because PC1 clearly separated samples obtained at baseline from those obtained at 16 wk PC2, accounting for 14.48% of the total variance, separated the 16 wk-control from the 16 wk-HF and 16 wk-HF + NaB groups, indicating that PC2 reflects the effect of diet (Figure 4B). Furthermore, the hierarchical cluster analysis showed that the 1 w-control, 1 wk-HF, 1 wk-HF + NaB, 16 wk-control and 16 wk-HF + NaB communities grouped together and then clustered in order with the 16 wk-HF communities (Figure 4C), which was consistent with the results of both the NMDS and the PCA analysis.
Figure 4.
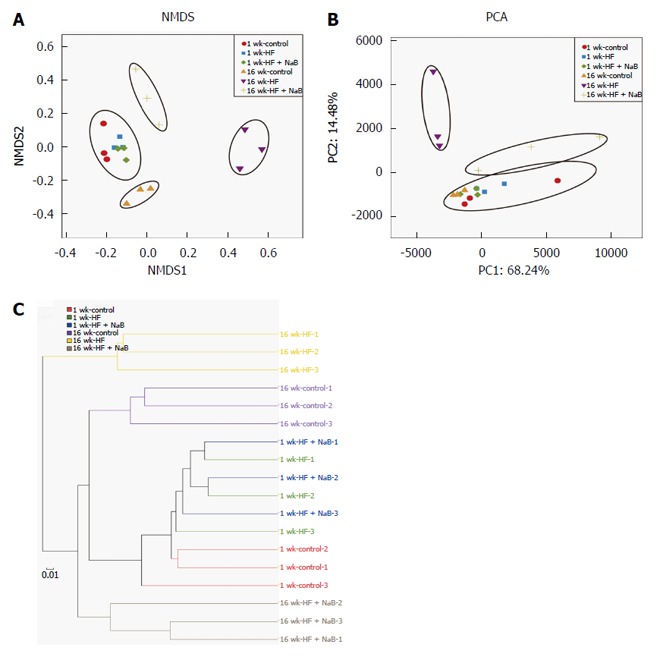
Effects of sodium butyrate on the overall structure of the gut microbiota. A: Nonmetric multidimensional scaling (NMDS) showing the difference in bacterial communities according to the Bray-Curtis distance; B: Scatter plot of the principal component analysis (PCA) score showing the similarity of the 18 bacterial communities based on the Unifrac distance. Principal components (PCs) 1 and 2 explained 68.24% and 14.48% of the variance, respectively; C: Hierarchical cluster analysis showing that the 1 wk groups and 16 wk-control communities grouped together, and then clustered in order with the 16 wk-HF + NaB groups, finally clustering with the 16 wk-HF groups.
Effects of NaB on inflammation and the metabolism of epididymal fat
To evaluate fat inflammation, mRNA expression of MCP-1 was detected in epididymal fat in the HF group, and the results showed that it was almost 3 times higher than that in the control. The mRNA levels of TNF-α were 1.5 times higher in the HF group than in the control. NaB intervention significantly reduced MCP-1 and TNF-α expression to the level of the control (Figure 5A). In addition, we found that both PPAR-α (Peroxisome Proliferator Activated Receptor-α) and PPAR-γ expression, either in the HF or the HF + NaB group, were significantly reduced compared with the control (Figure 5B).
Figure 5.
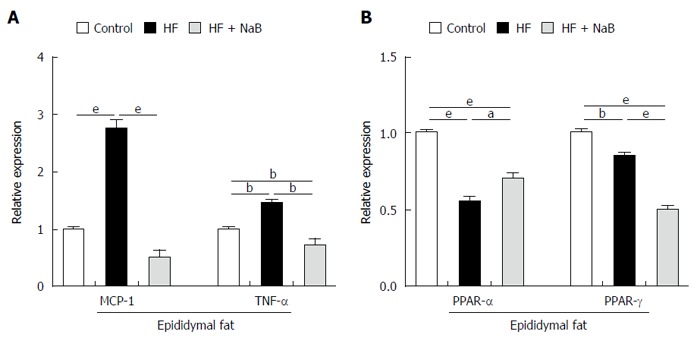
Sodium butyrate improves inflammation and lipid metabolism in epididymal fat. A: Gene expression levels of MCP-1 and TNF-α in epididymal fat; B: Gene expression levels of PPAR-α and PPAR-γ in epididymal fat. Data represent means ± SEM (n = 12 mice per group), aP < 0.05, bP < 0.01 and eP < 0.001.
NaB ameliorated inflammation and fat accumulation in the liver of HFD-fed mice
After 16 wk, the end point, the HF group had higher NAS (5.67 ± 0.225 vs 0.20 ± 0.145 and 4.42 ± 0.358), steatosis (3.00 vs 0 and 2.17 ± 0.112), inflammation (0.75 ± 0.217 vs 0.06 ± 0.066 and 0.50 ± 0.150), and ballooning scores than the control and HF + NaB groups (1.92 ± 0.083 vs 0.13 ± 0.091 and 1.75 ± 0.131) (Table 1). Although HE staining directly demonstrated increased fat accumulation in the liver in the HF group compared with the control or HF + NaB groups, oil red O staining provided a much more indicative view of the fat accumulation (Figure 6A). As confirmation, intrahepatic TGs were 4 times and almost 1.33 times higher in the HF group and HF + NaB group, respectively (Figure 6B). Intrahepatic cholesterol was 10 times and almost 3.33 higher in the HF group and HF + NaB group (Figure 6C). Masson staining was conducted to assess liver fibrosis, but no positive results were found, which indicated that the liver had not progressed to fibrosis at 16 wk (Figure 6D). Although collagen deposition and liver fibrosis were not detected by the naked eye, fibrosis-associated gene expression was significantly enhanced in the liver in the HF group, indicating a tendency toward future fibrosis. Further, both serum and liver endotoxin in HF group were increased compared with control, while significantly decreased after NaB intervention (Figure 6E and F). TGF-β1, α-SMA, Smad7, and Smad2 were increased > 200% by HFD feeding. Notably, NaB intervention reduced these mRNAs to the levels observed in the control group (Figure 6G).
Table 1.
Liver nonalcoholic fatty liver activity score including steatosis, ballooning, and lobular inflammation
| Group | n | Steatosis | Ballooning | Lobular inflammation | NAS |
| Control | 12 | - | 0.13 ± 0.091 | 0.06 ± 0.066 | 0.20 ± 0.145 |
| HF | 12 | 3.00b | 1.92 ± 0.083b | 0.75 ± 0.217b | 5.67 ± 0.225b |
| HF + NaB | 12 | 2.17 ± 0.112bc | 1.75 ± 0.131b | 0.50 ± 0.150b | 4.42 ± 0.358bd |
aP < 0.05,
P < 0.01 vs control;
P < 0.05,
P < 0.01 vs HF. NAS: Nonalcoholic fatty liver activity score.
Figure 6.
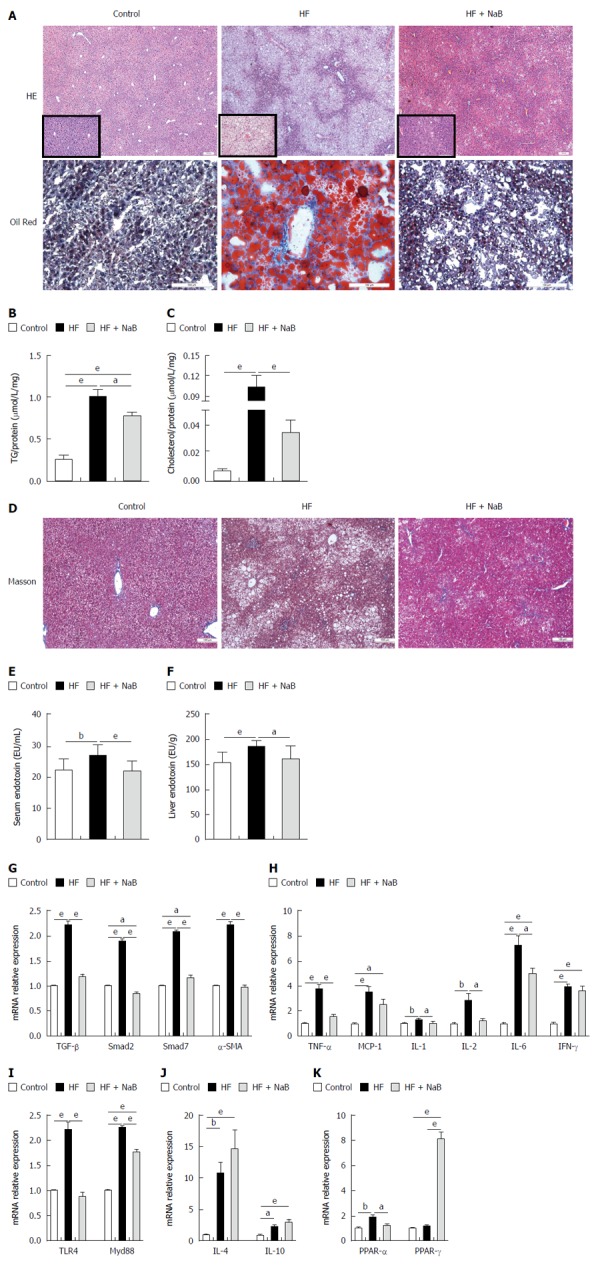
Sodium butyrate improves inflammation and lipid metabolism in liver. A: HE and oil red O staining; B: TG concentration; C: Cholesterol concentration; D: Masson staining; E, F: The levels of serum and liver endotoxin; G: Fibrosis-associated gene expression of TGF-β, Smad2, Smad7 and α-SMA; H: Pro-inflammation-associated gene expression; I: Endotoxin-associated gene expressions; J: Anti-inflammation-associated gene expression; K: Lipid metabolism-associated PPAR-α and PPAR-γ gene expression. Gene expression levels are expressed as values relative to the control group. Data represent means ± SEM (n = 12 mice per group), aP < 0.05, bP < 0.01 and eP < 0.001.
Pro-inflammatory cytokine mRNA expression was widely enhanced in the liver in the HF group, such as MCP-1, TNF-α, IL-1, IL-2, IL-6 and IFN-γ; endotoxin-associated TLR4 and Myd88 were significantly increased compared with the control group, while anti-inflammatory cytokines, such as IL-4, IL-10, were also unexpectedly enhanced in the liver in the HF group (Figure 6H-J). In contrast, these pro-inflammatory cytokine and endotoxin-associated gene mRNAs were all significantly reduced, and anti-inflammatory cytokine mRNAs were enhanced by NaB intervention compared with HF alone (Figure 6H-J). These results indicated that NaB promoted the maintenance of liver homeostasis.
To investigate lipid metabolism in the liver, we measured two vital lipid-associated transcription factors, PPAR-α and PPAR-γ. PPAR-α was significantly increased in the HF group compared with the control but was similar between the HF + NaB group and the control. PPAR-γ was similar between the HF and control groups but was significantly increased in the HF + NaB group compared with either the control or the HF group (Figure 6K).
DISCUSSION
NAFLD is the most common cause of liver disease worldwide, and its prevalence has increased in parallel with that of obesity. In recent years, the importance of the gut microbiota for health has been widely acknowledged. Accumulating data support the pivotal role of the gut microbiota or its metabolites in NAFLD development and progression[5]. We speculated that therapeutic targeting of the gut microbiota or its metabolites may be applied for the treatment of NAFLD. The new findings of this study are that NaB intervention significantly improved the gut microbiota in HFD-fed mice, enhanced intestinal mucosal barrier then reduced gut endotoxin induced systemic inflammation, finally attenuated liver histological damage induced by HFD.
Previous study[20] found a greater proportion of Ruminococcaceae in healthy subjects compared with patients with NASH. The physiological function of Ruminococcaceae is to produce SCFAs, including butyrate[21-23], and another study revealed that the butyrate concentration in feces in HFD-fed mice was significantly lower than that in the control[24]. These findings were consistent with two studies conducted in diabetes patients who lacked butyrate-producing bacteria compared with the control[25,26]. SCFAs exert multiple beneficial effects on mammalian metabolism[6,7]. Dietary administration of SCFAs protected mice against diet-induced obesity and insulin resistance, and another study found a significant reduction of butyrate-producing bacteria in feces with lower rather than bacterial gene counts[27]. One clinical study showed that propionate stimulated the release of gut hormones from human colonic cells and that inulin-propionate ester supplementation significantly reduced weight gain, intra-abdominal adipose tissue distribution, and intrahepatocellular lipid content and prevented the deterioration in insulin sensitivity[28]. And previous studies have demonstrated beneficial effects of butyrate on animal models of steatohepatitis[29,30]. But there are also many apparently contradictory results[14]. Some studies have shown that obese ob/ob mice and obese human subjects have increased amounts of cecal and fecal SCFAs[31,32].
Our investigation revealed that NaB intervention effectively decreased HFD-induced weight gain and serum glucose despite no reductions in energy intake, which was consistent with the findings of Henagan et al[33]. Both HOMA-IR and ISI decreased but not significantly in response to NaB treatment compared with HF alone. The serum insulin and epididymal fat index were not altered by NaB intervention. These findings are not completely concordant with the study conducted by Gao, although the methodological differences between our study and theirs should not be ignored[34]. A recent cytology study revealed that butyrate enhanced adipogenesis and lipid accumulation in adipocytes, reduced lipolysis, and induced adiponectin expression, resulting in the activation of downstream target genes such as AMPK[8]. This cell-level research may explain why butyrate had no effect on the epididymal fat index. The decrease in body weight may be associated with the ability of butyrate to promote energy expenditure and induce mitochondrial activity[34]. Furthermore, PPAR-α mainly acts on fatty acid oxidation, whereas PPAR-γ regulates lipid homeostasis and insulin sensitivity, and PPAR-γ agonist can improve liver histology[35], both of the two nuclear factors were obviously changed after NaB intervention which suggested that NaB triggered lipid metabolism of the body.
The gut microbiota is intimately related to healthy function of intestinal mucosal barrier which exhibits an inseparable relationship with metabolic health, including NAFLD[5]. We performed assays to detect any influence of NaB on the gut microbiota. According to the NMDS and PCA results, NaB treatment reversed the changes in the overall gut microbiota composition induced by HFD, resulting in a pattern more similar to the control. This phenomenon reflected the integrally bifidogenic effect of NaB. Recent studies on the gut microbiota suggest that it is better to focus on the genus or species level than the phylum level to investigate connections between gut microbiota and diseases[36]. Our study revealed that HFD induced dysbiosis of the gut microbiota by decreasing the proportions of Alistipes, Christensenellaceae and Lactobacillus, all of which are linked to a healthy state[36]. NaB treatment significantly increased the abundance of Christensenellaceae_uncultured, Blautia and Lactobacillus. Christensenellaceae is related to a low body mass index in humans and reduced weight gain in mice and is more likely to reduce body weight[37]. Lactobacillus is a probiotic bacterium with numerous beneficial effects on body metabolism and human health, including NAFLD[38,39]. Lactobacillus produces lactate, which can increase butyrate production in feces[27], and it increases butyrate uptake in intestinal epithelial cells, which is essential for intracellular effects such as the promotion of gut hormone secretion and colonic mucosal integrity, as well as the inhibition of inflammation[40]. Blautia, another genus that is affected by NaB, is a beneficial bacterium that is negatively correlated with metabolic syndromes. Its main biological function is to produce butyrate[23]. Blautia contributes to an increase in the butyrate concentration in feces, which may further enhance intestinal health. These bifidogenic effects may be partially attributed to the NaB-induced decrease in the gut pH to a more suitable level for the growth of beneficial but not harmful bacteria[41], another study suggested that butyrate could enhances antibacterial effects via immunity regulation[42]. Thus, these effects of NaB seem to form a virtuous circle, promoting beneficial effects on the body.
Our study demonstrated that HF diet disrupted gut microbiota then further impaired intestinal mucosal barrier, visually, NaB repaired the damage to the intestinal mucosa and strengthened the intestinal tight junctions. Previous studies indicated that microbial butyrate may contribute to the restoration of the tight junction barrier via up-regulating the protein level of ZO-1, which was attributed to its histone deacetylase inhibition[13,43]. It decreased the intestinal permeability to reduce the escape of pathogen-associated molecular patterns into the blood; thus, we comfirmed that no matter serum or liver endotoxin derived from gut microbiota was significantly decreased compared with HF group, Toll-like receptor 4 (TLR4), a receptor of lipopolysaccharide (LPS) or endotoxin, and its downstream protein Myd88 were significantly decreased in the liver after NaB treatment. TLR activation leads to the translocation of NF-κB into the nucleus and the induction of pro-inflammatory gene transcription, such as TNF-α, IL-1β, and IL-6[44], which is a classic pathway participated in the progression of NAFLD[5]. This may represent a pivotal LPS-associated mechanism to attenuate liver inflammation after NaB intervention. These beneficial effects were greatly associated with the improvement of the intrahepatic environment, such as, many pro- inflammatory factors in the liver (MCP-1, TNF-α, IL-1, IL-2, IL-6, IFN-γ) and epididymal fat (MCP-1, TNF-α), were as expected decreased and anti-inflammatory factors in the liver (IL-4, IL-10) were significantly increased after NaB treatment. The changes of these immune factors were partially inseparable from the immunoregulation of NaB via inhibiting histone acetylation enzymes or G protein-coupled receptors pathway[10-12,45-49], helping correct an unbalanced physiological environment induced by HFD, and significantly improved liver histology by attenuating inflammation and fat accumulation.
Our study also had some limitations. First, the role of butyrate in maintaining intestinal homeostasis is undoubted which is confirmed by our study[13,50], meanwhile, gut microbiota has an indispensable role in the human body[51,52], and the challenge is to find out whether these changes in gut microbiota composition are the cause or the consequence of disorder[53]. Further researches on this aspect may uncover the precise relationship between gut microbiota and NAFLD. Second, this study was conducted in an animal model. Further studies should be performed in human subjects.
In conclusion, we demonstrated the application of NaB to attenuate HFD-induced steatohepatitis in mice. NaB beneficially regulated the gut microbiota and enhanced gastrointestinal health to improve whole body metabolism. This study was limited to animals, and whether it will work on clinical populations remains a significant challenge, however, the unpleasant taste and odor of NaB make it extremely difficult to administrate orally[29]. Thus, new formulations of butyrate with a better palatability, which can be easily administered orally, are needed. Overall, it opens potential avenues for intervention strategies for the treatment of NAFLD. Urgent attention should be devoted to the gut microbiota and its metabolites, which could produce novel therapeutic targets.
COMMENTS
Background
Non-alcoholic fatty liver disease (NAFLD) is an emerging public health problem with an increasing incidence that lacks effective therapeutic strategies. Previous data demonstrates that the gut microbiota and its metabolites such as butyrate play a pivotal role in the development and progression of NAFLD, however, the underlying mechanisms and interactions among NAFLD, gut microbiota and its metabolites still need more investigations to clarify.
Research frontiers
Sodium butyrate (NaB) is one of gut metabolite which exerts multiple beneficial effects on mammalian metabolism. The research hotspot lies in that NaB could significantly improve the overall structure of gut microbiota, correct the high-fat diet (HFD)-induced gut microbiota dysbiosis in mice, meanwhile it considerably elevated the abundances of the beneficial bacteria. These bacteria can produce butyric acid in what seems like a virtuous circle.
Innovations and breakthroughs
To elucidate the effects of HFD and NaB on the composition of the microbiota and further clarify the effect of gut metabolite sodium butyrate (NaB) on NAFLD, Illumina MiSeq sequencing of bacterial 16S rRNA from gut microbiota was conducted and analysed, and further metabolism indices, liver and small intestine histologies were evaluated. Inflammation- or metabolism-associated genes in the liver and epididymal fat tissue were detected, furthermore, the serum and intrahepatic levels of endotoxin, intrahepatic triglyceride and cholesterol were measured. This study found that NaB significantly corrected the gut microbiota dysbiosis induced by HFD, restored intestinal mucosa damage, improved tight junction structure, finally reduced gut endotoxin into liver and attenuated HFD induced steatohepatitis.
Applications
The results suggested that gut metabolite NaB had the ability to reverse HFD-induced dysbiosis of gut microbiota and finally attenuated HFD-induced steatohepatitis which indicated the potential therapeutic approach in the treatment of NAFLD.
Terminology
Short chain fatty acids, also referred to as volatile fatty acids, are mainly produced by the fermentation of gut microbiota and have fewer than 6 carbon atoms, including formic acid, acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid and isovaleric acid.
Peer-review
The study of Zhou et al is an interesting study describing sodium butyrate attenuation of high-fat diet-induced steatohepatitis in mice. The authors investigated its mechanisms and concluded that the effects were possibly by improving gut microbiota and gastrointestinal barrier.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Xinhua Hospital Ethics Committee Affiliated to Shanghai Jiaotong University School of Medicine.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of Xinhua hospital affiliated to Shanghai Jiao Tong University School of Medicine.
Conflict-of-interest statement: The authors declare no competing financial interests.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at fattyliver2004@126.com. Participants gave informed consent for data sharing.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 12, 2016
First decision: October 20, 2016
Article in press: November 16, 2016
P- Reviewer: Cardoso CRL S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Wang FS, Fan JG, Zhang Z, Gao B, Wang H-Y. The global burden of liver disease: The major impact of China. Hepatology. 2014;60:2099–2108. doi: 10.1002/hep.27406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rinella ME. Nonalcoholic Fatty Liver Disease. Jama. 2015;31:2263. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 4.Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gkolfakis P, Dimitriadis G, Triantafyllou K. Gut microbiota and non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2015;14:572–581. doi: 10.1016/s1499-3872(15)60026-1. [DOI] [PubMed] [Google Scholar]
- 6.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 7.Hartstra AV, Bouter KE, Bäckhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38:159–165. doi: 10.2337/dc14-0769. [DOI] [PubMed] [Google Scholar]
- 8.Yan H, Ajuwon KM. Mechanism of Butyrate Stimulation of Triglyceride Storage and Adipokine Expression during Adipogenic Differentiation of Porcine Stromovascular Cells. PLoS One. 2015;10:e0145940. doi: 10.1371/journal.pone.0145940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: Gut microbiota: the neglected endocrine organ. Mol Endocrinol. 2014;28:1221–1238. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JS, Lee EJ, Lee JC, Kim WK, Kim HS. Anti-inflammatory effects of short chain fatty acids in IFN-gamma-stimulated RAW 264.7 murine macrophage cells: involvement of NF-kappaB and ERK signaling pathways. Int Immunopharmacol. 2007;7:70–77. doi: 10.1016/j.intimp.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Zhang WH, Jiang Y, Zhu QF, Gao F, Dai SF, Chen J, Zhou GH. Sodium butyrate maintains growth performance by regulating the immune response in broiler chickens. Br Poult Sci. 2011;52:292–301. doi: 10.1080/00071668.2011.578121. [DOI] [PubMed] [Google Scholar]
- 12.Wen ZS, Lu JJ, Zou XT. Effects of sodium buty-rate on the intestinal morphology and DNA-binding activity of intestinal nuclear factor-kappa B in wean-ling pigs. Dongwu Yixue Jinzhan. 2012;11:814–821. [Google Scholar]
- 13.Plöger S, Stumpff F, Penner GB, Schulzke JD, Gäbel G, Martens H, Shen Z, Günzel D, Aschenbach JR. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci. 2012;1258:52–59. doi: 10.1111/j.1749-6632.2012.06553.x. [DOI] [PubMed] [Google Scholar]
- 14.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan JG, Jia JD, Li YM, Wang BY, Lu LG, Shi JP, Chan LY. Guidelines for the diagnosis and management of nonalcoholic fatty liver disease: update 2010: (published in Chinese on Chinese Journal of Hepatology 2010; 18: 163-166) J Dig Dis. 2011;12:38–44. doi: 10.1111/j.1751-2980.2010.00476.x. [DOI] [PubMed] [Google Scholar]
- 16.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Sheng HF, He Y, Wu JY, Jiang YX, Tam NF, Zhou HW. Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of illumina tags. Appl Environ Microbiol. 2012;78:8264–8271. doi: 10.1128/AEM.01821-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noval Rivas M, Burton OT, Wise P, Zhang YQ, Hobson SA, Garcia Lloret M, Chehoud C, Kuczynski J, DeSantis T, Warrington J, et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J Allergy Clin Immunol. 2013;131:201–212. doi: 10.1016/j.jaci.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang XT, Peng X, Deng GH, Sheng HF, Wang Y, Zhou HW, Tam NF. Illumina sequencing of 16S rRNA tag revealed spatial variations of bacterial communities in a mangrove wetland. Microb Ecol. 2013;66:96–104. doi: 10.1007/s00248-013-0238-8. [DOI] [PubMed] [Google Scholar]
- 20.Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, Smith S, Greenwood R, Sikaroodi M, Lam V, Crotty P, et al. Fecal Microbiome and Volatile Organic Compound Metabolome in Obese Humans With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2013;11:868–875.e863. doi: 10.1016/j.cgh.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 21.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Guo Z, Xue Z, Sun Z, Zhang M, Wang L, Wang G, Wang F, Xu J, Cao H, et al. A phylo-functional core of gut microbiota in healthy young Chinese cohorts across lifestyles, geography and ethnicities. ISME J. 2015;9:1979–1990. doi: 10.1038/ismej.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berni Canani R, Sangwan N, Stefka AT, Nocerino R, Paparo L, Aitoro R, Calignano A, Khan AA, Gilbert JA, Nagler CR. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 2016;10:742–750. doi: 10.1038/ismej.2015.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Tang H, Zhang C, Zhao Y, Derrien M, Rocher E, van-Hylckama Vlieg JE, Strissel K, Zhao L, Obin M, et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015;9:1–15. doi: 10.1038/ismej.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 26.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 27.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 28.Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SE, MacDougall K, Preston T, Tedford C, Finlayson GS, et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64:1744–1754. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattace Raso G, Simeoli R, Russo R, Iacono A, Santoro A, Paciello O, Ferrante MC, Canani RB, Calignano A, Meli R. Effects of sodium butyrate and its synthetic amide derivative on liver inflammation and glucose tolerance in an animal model of steatosis induced by high fat diet. PLoS One. 2013;8:e68626. doi: 10.1371/journal.pone.0068626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin CJ, Sellmann C, Engstler AJ, Ziegenhardt D, Bergheim I. Supplementation of sodium butyrate protects mice from the development of non-alcoholic steatohepatitis (NASH) Br J Nutr. 2015;114:1745–1755. doi: 10.1017/S0007114515003621. [DOI] [PubMed] [Google Scholar]
- 31.Nieuwdorp M, Gilijamse PW, Pai N, Kaplan LM. Role of the microbiome in energy regulation and metabolism. Gastroenterology. 2014;146:1525–1533. doi: 10.1053/j.gastro.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity. 2010;18:190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 33.Henagan TM, Stefanska B, Fang Z, Navard AM, Ye J, Lenard NR, Devarshi PP. Sodium butyrate epigenetically modulates high-fat diet-induced skeletal muscle mitochondrial adaptation, obesity and insulin resistance through nucleosome positioning. British J Pharmacol. 2015;172:2782–2798. doi: 10.1111/bph.13058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gastaldelli A, Harrison SA, Belfort-Aguilar R, Hardies LJ, Balas B, Schenker S, Cusi K. Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis. Hepatology. 2009;50:1087–1093. doi: 10.1002/hep.23116. [DOI] [PubMed] [Google Scholar]
- 36.Stenman LK, Burcelin R, Lahtinen S. Establishing a causal link between gut microbes, body weight gain and glucose metabolism in humans - towards treatment with probiotics. Benef Microbes. 2015 doi: 10.3920/BM2015.0069. Epub ahead of print: 1-12. [DOI] [PubMed] [Google Scholar]
- 37.Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, et al. Human genetics shape the gut microbiome. Cell. 2014;159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nova E, Pérez de Heredia F, Gómez-Martínez S, Marcos A. The Role of Probiotics on the Microbiota: Effect on Obesity. Nutr Clin Pract. 2016;31:387–400. doi: 10.1177/0884533615620350. [DOI] [PubMed] [Google Scholar]
- 39.Mishra AK, Dubey V, Ghosh AR. Obesity: An overview of possible role(s) of gut hormones, lipid sensing and gut microbiota. Metabolism. 2016;65:48–65. doi: 10.1016/j.metabol.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 40.Kumar A, Alrefai WA, Borthakur A, Dudeja PK. Lactobacillus acidophilus counteracts enteropathogenic E. coli-induced inhibition of butyrate uptake in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2015;309:G602–G607. doi: 10.1152/ajpgi.00186.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guilloteau P, Martin L, Eeckhaut V, Ducatelle R, Zabielski R, Van Immerseel F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr Res Rev. 2010;23:366–384. doi: 10.1017/S0954422410000247. [DOI] [PubMed] [Google Scholar]
- 42.Fernando MR, Saxena A, Reyes JL, McKay DM. Butyrate enhances antibacterial effects while suppressing other features of alternative activation in IL-4-induced macrophages. Am J Physiol Gastrointest Liver Physiol. 2016;310:G822–G831. doi: 10.1152/ajpgi.00440.2015. [DOI] [PubMed] [Google Scholar]
- 43.Bordin M, D’Atri F, Guillemot L, Citi S. Histone deacetylase inhibitors up-regulate the expression of tight junction proteins. Mol Cancer Res. 2004;2:692–701. [PubMed] [Google Scholar]
- 44.Zhang G, Ghosh S. Molecular mechanisms of NF-kappaB activation induced by bacterial lipopolysaccharide through Toll-like receptors. J Endotoxin Res. 2000;6:453–457. doi: 10.1179/096805100101532414. [DOI] [PubMed] [Google Scholar]
- 45.Kim CH, Park J, Kim M. Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Immune Netw. 2014;14:277–288. doi: 10.4110/in.2014.14.6.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8:80–93. doi: 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Millard A, Mertes P, Ittelet D, Villard F, Jeannesson P, Bernard J. Butyrate affects differentiation, maturation and function of human monocyte-derived dendritic cells and macrophages. Clin Exper Immunol. 2002;130:245–255. doi: 10.1046/j.0009-9104.2002.01977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vinolo MA, Ferguson GJ, Kulkarni S, Damoulakis G, Anderson K, Bohlooly-Y M, Stephens L, Hawkins PT, Curi R. SCFAs induce mouse neutrophil chemotaxis through the GPR43 receptor. PLoS One. 2011;6:e21205. doi: 10.1371/journal.pone.0021205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leonel AJ, Alvarez-Leite JI. Butyrate: implications for intestinal function. Curr Opin Clin Nutr Metab Care. 2012;15:474–479. doi: 10.1097/MCO.0b013e32835665fa. [DOI] [PubMed] [Google Scholar]
- 51.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO reports. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sommer F, Bäckhed F. The gut microbiota--masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 53.Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Frontiers Microbiol. 2016:7. doi: 10.3389/fmicb.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]


