Abstract
2-Mercaptobenzothiazole, which is mainly used in the rubber industry as a vulcanization accelerator, is very toxic and is considered to be recalcitrant. We show here for the first time that it can be biotransformed and partially mineralized by a pure-culture bacterial strain of Rhodococcus rhodochrous. Three metabolites, among four detected, were identified.
2-Mercaptobenzothiazole (MBT) is the most important and most widely used member of the benzothiazole (BT) family. MBT is typically a rubber additive (16, 17), but it has also other applications, such as inhibiting biocorrosion in cooling systems or in paper manufacturing (4). The annual MBT production in Western Europe is estimated to be excess of 40,000 tons.
Direct discharges of MBT occur in effluents from factories producing and using MBT. Indirect sources of environmental contamination are mainly leachates from landfills where MBT is deposited and from rubber products (18). MBT can also be found in tannery wastewater (20). Finally, BTs have been found in urban runoff, in residential and highway road dust, and in urban particulate matter, most probably as a result of vehicle tire wear (18). The U.S. Environmental Protection Agency estimated that over 500 tons of MBT may be lost annually to the environment (22). Its toxicity towards microorganisms (3, 6, 7, 9), its allergenicity, resulting in serious dermatoses (11), and its potential mutagenic effects (12) make its presence in the environment of great concern.
Information on the environmental fate of BT in the literature is scarce, and information on its biodegradative pathways is scarcer still. Several studies were first carried out in laboratories or in pilot-scale activated sludge systems in order to remove such a compound (5, 20, 21). These studies show that MBT is rather recalcitrant and is not completely mineralized when its concentration reaches a certain threshold. The metabolites observed under these conditions were benzothiazolylsulfonate and 2-methylthiobenzothiazole (MTBT) (10, 20), the disulfide derivative of MBT (5).
Only a few bacterial isolates have been shown to biotransform MBT. Drotar et al. (10) observed MBT-methylating activity in crude extracts of a variety of soil and water isolates, including a Corynebacterium sp., a Pseudomonas sp., and Escherichia coli, yielding the more stable methylated product MTBT, which accumulated in the medium.
This paper reports studies of the biotransformation of MBT by Rhodococcus rhodochrous OBT18, isolated by De Wever et al. (8) from activated sludge from a wastewater treatment plant of an MBT-producing factory (Bayer, Antwerp, Belgium). This strain was previously shown to degrade BT, 2-hydroxybenzothiazole (OBT) (2, 8), and 2-aminobenzothiazole (ABT) (13). The main objective of this work was to identify the structure of the metabolites formed in order to establish the metabolic pathway of MBT for this strain. Because BT structures are difficult to analyze (due to the presence of N and S heteroatoms) and the metabolism of BT is almost unknown, various powerful analytical tools must be used, such as two-dimensional (2D) nuclear magnetic resonance (NMR) spectroscopies (1H-15N gradient heteronuclear multiple-bond correlation [gHMBC], 1H-13C heteronuclear single-quantum coherence [HSQC], 1H-13C heteronuclear multiple-bond correlations [HMBC], and 1H-1H total correlation spectroscopy [TOCSY]) and mass spectrometry (MS) methods (electrospray identification [ESI]-MS and tandem MS [MS/MS]). Because of the sensitivity limits of these techniques (especially 2D NMR), rather high (i.e., millimolar) concentrations of metabolites are needed. For this reason, Rhodococcus cells were grown in a rich medium (Trypticase soy broth) to get large amounts of biomass, and then resting cells were used for the incubations. Also, the experiment was performed with water instead of buffer (K2HPO4, 1 g/liter; KH2PO4, 1 g/liter; FeCl3 · 6H2O, 4 g/liter; and MgSO4 · 7H2O, 40 g/liter; pH 6.7) because salts (especially phosphates) are not convenient for some MS analyses. However, the same metabolites were detected when the kinetics of MBT degradation were observed under both conditions. Although growing cells might behave differently in the environment, information about potential metabolites is important, especially because they can accumulate and be toxic.
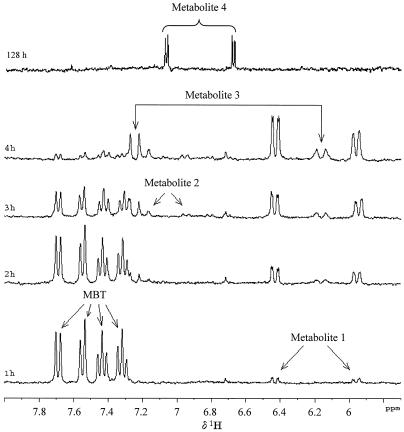
Trypticase soy broth-grown resting cells of R. rhodochrous were incubated in distilled water with 1.5 mM MBT as previously described (14). The kinetics of degradation were monitored by in situ 1H NMR (2). 1H NMR signals corresponding to MBT (δ ppm, 7.32, 7.44, 7.55, and 7.69) decreased with time (Fig. 1), and new signals in the aromatic region appeared: after 1 h of incubation, two new doublets (δ ppm, 6.43 and 5.96) were present (indicating metabolite 1). These signals increased with time. A second metabolite, giving signals resonating at 6.95, 7.16, and 7.41 ppm, was detected after 3 h (metabolite 2). These signals disappeared with time. At 2 h, other signals were observed (indicating metabolite 3), resonating at 7.25 and 6.16 ppm as doublets. After 21 h, metabolites 1 to 3 disappeared, and a new metabolite was detected (metabolite 4; indicated by two doublets resonating at 6.68 and 7.09 ppm) and was still present after 128 h (Fig. 1). This result showed that MBT was biotransformed but not completely mineralized after 128 h. The total organic carbon (TOC) measurement was used to evaluate the formation of CO2, and the results showed that about 30% of MBT was completely mineralized after 128 h. The time course of this TOC measurement showed that this mineralization process was not stopped after 128 h. Further evidence of MBT mineralization was given by the release of NH4+ and SO42−. After 48 h of incubation, these ions were found in 0.2 and 0.7 M concentrations, respectively, per mole of MBT. The stoichiometry was not fully balanced, which may be due to the fact that the form in which N and S are released is unknown, but at least it confirms that part of MBT was mineralized.
FIG. 1.
In situ 1H NMR spectra of samples taken at 1, 2, 3, 4, and 128 h of incubation of 1.5 mM MBT with resting cells of R. rhodochrous OBT18.
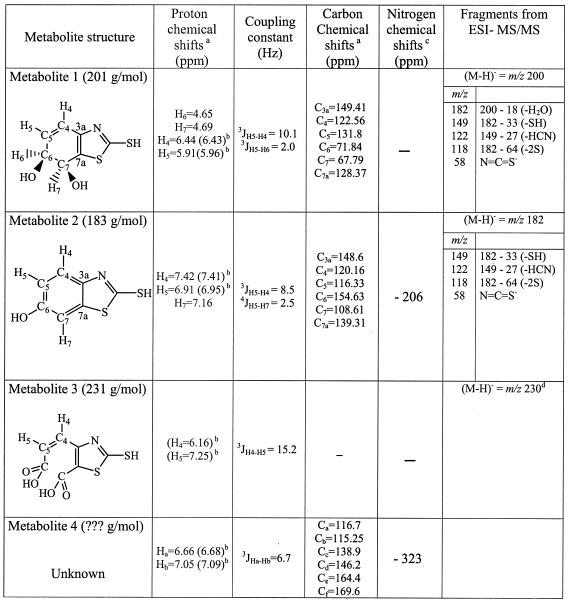
The structures of these unknown metabolites were established by using more sophisticated 2D NMR experiments and MS. In particular, 1H-1H TOCSY NMR experiments were performed with a sample obtained after 4 h of incubation to detect protons hidden by the presence of a massive amount of H2O in 1D NMR spectra. 1H-13C HSQC and 1H-13C HMBC experiments were carried out as previously described (13, 14). To perform these experiments, for which a higher metabolite concentration is required, a quantitative assay was carried out. R. rhodochrous (55 g [wet weight]) was incubated in each of 11 Erlenmeyer flasks containing 100 ml of water and 1.5 mM MBT. The biodegradation kinetics were monitored by NMR in order to stop the experiment at the times when the maximum concentrations of the different metabolites were achieved (4 h for metabolites 1 to 3, and 128 h for metabolite 4). The incubation medium was centrifuged, and the supernatant was freeze-dried and dissolved in D2O. Selected samples were analyzed by liquid chromatography (LC)-ESI-MS according to a method described elsewhere (19). Structure elucidation was based on product ion spectra generated by collision-induced dissociation in the negative-ion mode.
Metabolite 1.
1H and 13C NMR data for metabolite 1 are consistent with the structure of a cis-dihydrodiol of MBT (Table 1). LC-MS/MS data for the major compound detected in the sample taken after 3.5 h of incubation are also listed in Table 1 and coincide with this structure. From the molecular anion m/z 200, a loss of water (−18 atomic mass units) is readily observed, as is the case for for all fragment ions generated from metabolite 2.
TABLE 1.
NMR and LC-ESI-MS data for metabolites 1 to 4 resulting from MBT transformation by R. rhodochrous
Chemical shifts determined from 1H-1H TOCSY, 1H-13C HSQC, and HMBC experiments performed with freeze-dried samples resuspended in D2O. -, not measured.
1H chemical shifts measured from in situ 1D 1H NMR spectra.
Chemical shifts determined from 1H-15N gHMBC performed with samples dissolved in methanol. —, not measured.
Determined by ESI-MS with samples (at 4 h) directly introduced into the source (MS engine 59 987 A, 10 μl/min, negative-ion mode, 4.5 kV, 220°C; quadrupole analyzer; Hewlett-Packard Co.).
Metabolite 2.
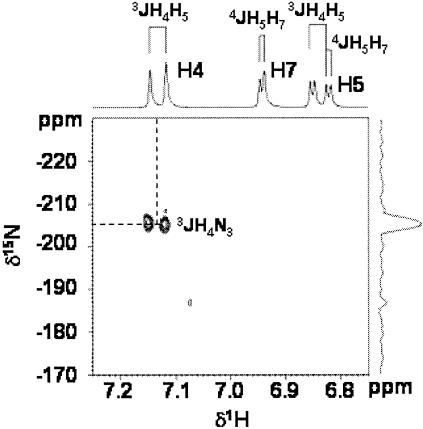
Both the multiplicity of the 1H signals and the 13C chemical shifts of metabolite 2 (Table 1) are in accordance with an aromatic ring structure substituted in position 5 or 6, similar to those previously observed for 6-OH derivatives of OBT and ABT (2, 13). To confirm this structure, metabolite 2 was isolated and purified as previously described for 6-OH-OBT (2). The mass spectrum (chemical ionization, CH4) of the isolated compound presented a peak at m/z 182 (M+H)+, confirming that the metabolite formed was a hydroxylated derivative. The proposed structure was also confirmed by LC-MS/MS experiments performed with the sample taken at 5.5 h. All fragment ions generated from the molecular anion (m/z 182) correspond to those found for MBT (19), except for the presence of 18 additional atomic mass units in the hydroxyl moiety. The fragment anion m/z 58 confirms that the heteroaromatic ring system was unaltered. Finally, the 1H-15N gHMBC spectrum of the purified metabolite 2, recorded as previously described (2), showed only one correlation between the endocyclic 15N (−206 ppm) and the doublet at 7.16 ppm, clearly indicating that the OH substituent was on the benzene ring at position 6 of MBT (Fig. 2 and Table 1).
FIG. 2.
1H-15N gHMBC spectrum of purified 6-hydroxy-MBT recorded in methanol; the experiment was set up for assumed 3J (delay, 80 ms) long-range coupling. The spots visible on the 2D spectrum correspond to the correlations between N3 and H4 (3JH4N3). The presence of a proton at position 5 of the benzene ring demonstrates that the hydroxyl group is at position 6.
Metabolite 3.
Unfortunately, no 13C data could be obtained for metabolite 3, because this metabolite was lost during the freeze-drying process necessary to get a concentrated sample for the 2D 1H-13C NMR experiment. Nevertheless, 1H NMR data (Table 1) recall the pattern obtained for the diacid derivative of BT (14). This hypothetical structure was confirmed by the detection of the anion m/z 230 (M-H)− when ESI-MS was performed with samples taken after 4 h of incubation and directly injected into the source (Table 1).
Metabolite 4.
Only partial structural information could be obtained for metabolite 4 (Table 1). These data showed that this metabolite contains two protons and at least six carbons that are likely to belong to the initial aromatic part of MBT. In addition, 1H-15N gHMBC experiments clearly showed the presence of a nitrogen atom resonating at −323 ppm that could correspond to the nitrogen of the thiazole ring. It can be concluded that part of the MBT structure is conserved.
Very few cases of MBT transformation by pure-culture bacterial strains have been reported to date (10); Corynebacterium spp., Pseudomonas spp., and E. coli isolates were shown to transform MBT into MTBT, but this methylated metabolite accumulated in the medium without further transformation. We describe here a new case of MBT transformation by an isolate from the genus Rhodococcus. Both the type of bacteria and the type of metabolism are very different from previously described cases.
In this work, we found the following information about MBT metabolism. First, we showed that, although MBT is known to be extremely recalcitrant, it can be biotransformed into four metabolites and that 30% of MBT was completely mineralized under our experimental conditions. The transformation of MBT clearly results from enzymatic processes, as no degradation of MBT was observed in control experiments in which no cells, either live or heat-killed, were used. Although the experiments reported here were not performed with growing cells, the results may give indications about processes that could occur in the environment. To our knowledge, this is the first report showing that MBT is potentially degradable by a pure culture and that the enzymatic equipment of R. rhodochrous OBT18 could operate under some environmental conditions. Also, it was shown that 6-OH-MBT is less toxic than MBT, according to the standardized Microtox test performed as previously described (15). For an exposure time of 15 min, the 50% effective concentrations were 9.5 μM for 6-OH-MBT and 1.5 μM for MBT.
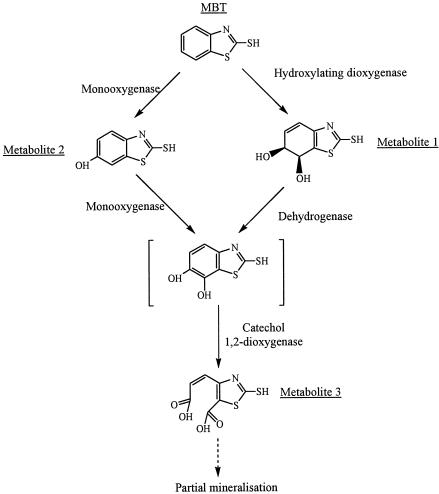
Second, the identification of metabolites 1 to 3 allows a more detailed description of the metabolic pathway of MBT in R. rhodochrous (Fig. 3). These metabolite structures are completely different than those previously described (5, 10, 20, 21). Metabolite 1, a cis-dihydrodiol derivative of MBT, results from the activity of a hydroxylating dioxygenase (1). This is the first report of such an enzyme in BT metabolism. Although a catechol structure could not be detected under our experimental conditions, the presence of the diacid derivative of MBT (metabolite 3) indicates the activity of a catechol 1,2-dioxygenase. We have already shown the involvement of such an enzyme in Rhodococcus pyridinivorans PA in the degradation of BT (14). As the structure of metabolite 4 was not fully identified, we could not integrate it into a plausible metabolic pathway.
FIG. 3.
Proposed metabolic pathway of MBT in R. rhodochrous OBT18.
Finally, the formation of 6-OH-MBT (metabolite 2) shows that the hydroxylation of the benzene ring at position 6 is a general mechanism involved in the biotransformation of BT derivatives. 6-OH-MBT could be formed by the action of a monooxygenase (Fig. 3). This hydroxylation was previously shown to operate in ABT in R. rhodochrous (13), in BT and OBT in Rhodococcus erythropolis, R. rhodochrous, and R. pyridinivorans PA (2, 14), and on N-[2-benzothiazolyl]-N,N′-dimethylurea (methabenzthiazuron) in Aspergillus niger (15) and Cunninghamella echinulata (23).
Acknowledgments
N. Haroune is a recipient of a grant from the French Ministère de l'Education Nationale et de la Recherche.
We thank Gilles Mailhot for the TOC measurements and F. Bonnemoy for the Microtox assays.
REFERENCES
- 1.Bertini, I., M. A. Cremonini, S. Ferreti, I. Lozzi, C. Luchinat, and M. S. Viezzoli. 1996. Arene hydroxylases: metalloenzymes catalyzing dioxygenation of aromatic compounds. Coordin. Chem. Rev. 151:145-160. [Google Scholar]
- 2.Besse, P., B. Combourieu, G. Boyse, M. Sancelme, H. De Wever, and A. M. Delort. 2001. Long-range 1H-15N heteronuclear shift correlation at natural abundance: a tool to study benzothiazole biodegradation by two Rhodococcus strains. Appl. Environ. Microbiol. 67:1412-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bujdakova, H., T. Kuchta, E. Sidoova, and A. Gvozdjakova. 1993. Anti-Candida activity of four antifungal benzothiazoles. FEMS Microbiol. Lett. 112:329-334. [DOI] [PubMed] [Google Scholar]
- 4.Chudoba, J., F. Tucek, and K. Zeis. 1977. Biochemischer Abbau von Benzothiazolderivaten. Acta Hydrochim. Hydrobiol. 5:485-498. [Google Scholar]
- 5.De Wever, H., and H. Verachtert. 1994. 2-Mercaptobenzothiazole degradation in laboratory fed-batch systems. Appl. Environ. Biotechnol. 42:623-630. [DOI] [PubMed] [Google Scholar]
- 6.De Wever, H., and H. Verachtert. 1997. Biodegradation and toxicity of benzothiazoles. Water Res. 31:2673-2684. [Google Scholar]
- 7.De Wever, H., K. De Moor, and H. Verachtert. 1994. Toxicity of 2-mercaptobenzothiazole towards bacterial growth and respiration. Appl. Microbiol. Biotechnol. 42:631-635. [DOI] [PubMed] [Google Scholar]
- 8.De Wever, H., K. Vereecken, A. Stolz, and H. Verachtert. 1998. Initial transformations in the biodegradation of benzothiazoles by Rhodococcus isolates. Appl. Environ. Microbiol. 64:3270-3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Wever, H., S. Van Der Neste, and H. Verachtert. 1997. Inhibitory effect of 2-mercaptobenzothiazole on microbial growth in a variety of trophic conditions. Environ. Toxicol. Chem. 16:843-848. [Google Scholar]
- 10.Drotar, A., G. A. Burton, Jr., J. E. Tavernier, and R. Fall. 1987. Widespread occurrence of bacterial thiol methyltransferases and the biogenic emission of methylated sulfur gases. Appl. Environ. Microbiol. 53:1626-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geier, J., W. Uter, A. Schnuch, J. Brasch, et al. 2002. Diagnostic screening for contact allergy to mercaptobenzothiazole derivatives. Am. J. Contact Dermat. 13:66-70. [PubMed] [Google Scholar]
- 12.Gold, L. S., T. H. Slone, B. R. Stern, and L. Bernstein. 1993. Comparison of target organs of carcinogenicity for mutagenic and non-mutagenic chemicals. Mutat. Res. 286:75-100. [DOI] [PubMed] [Google Scholar]
- 13.Haroune, N., B. Combourieu, P. Besse, M. Sancelme, and A. M. Delort. 2001. 1H NMR: a tool to study the fate of pollutants in the environment. C. R. Acad. Sci. 4:759-763. [Google Scholar]
- 14.Haroune, N., B. Combourieu, P. Besse, M. Sancelme, T. Reemtsma, A. Kloepfer, A. Diab, J. S. Knapp, S. Baumberg, and A. M. Delort. 2002. Benzothiazole degradation by Rhodococcus pyridinovorans [sic] strain PA: evidence of a catechol 1,2-dioxygenase activity. Appl. Environ. Microbiol. 68:6114-6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malouki, M. A., G. Giry, P. Besse, B. Combourieu, M. Sancelme, F. Bonnemoy, C. Richard, and A.-M. Delort. 2003. Sequential bio- and photo-transformation of herbicide methabenzthiazuron in water. Environ. Toxicol. Chem. 22:2013-2019. [PubMed] [Google Scholar]
- 16.Morgan, B., and W. J. McGill. 2000. Benzothiazole-accelerated sulfur-vulcanization. I. 2-Mercaptobenzothiazole as accelerator for 2,3-dimethyl-2-butene. J. Appl. Polym. Sci. 76:1377-1385. [Google Scholar]
- 17.Nonaka, S., H. Kanda, S. Matsunaga, and M. Kawasaki. 2002. Vulcanizable rubber composition with good storage stability and their sponge rubbers. Japan Kokai Tokkyo Koho patent JKXXAF JP 2002256121 A2 200220911. (In Japanese.)
- 18.Reddy, C. M., and J. G. Quinn. 1997. Environmental chemistry of benzothiazoles derived from rubber. Environ. Sci. Technol. 31:2847-2853. [Google Scholar]
- 19.Reemtsma, T. 2000. Determination of 2-substituted benzothiazoles of industrial use from water by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 14:1612-1618. [DOI] [PubMed] [Google Scholar]
- 20.Reemtsma, T., O. Fiehn, G. Kalnowski, and M. Jekel. 1995. Microbial transformations and biological effects of fungicide-derived benzothiazoles determined in industrial wastewater. Environ. Sci. Technol. 29:478-485. [DOI] [PubMed] [Google Scholar]
- 21.Repkina, V. I., S. A. Dokudovskaya, R. A. Umrikhina, and V. A. Samokhina. 1983. Maximum permissible concentrations of benzothiazole and 2-mercaptobenzothiazole during biochemical treatment of wastewaters. Khim. Prom-st'. 10:598-599. [Google Scholar]
- 22.Vogt, S. F. 1988. 2-Mercaptobenzothiazole; final test rule. Fed. Regist. 53:34514-34521. [Google Scholar]
- 23.Wallnoefer, P., G. Tillmanns, R. Thomas, C. Wünsche, J. Kurz, and H. J. Jarczyk. 1976. Mikrobieller Abbau des Herbizids Methabenzthiazuron und Identifizierung der Metaboliten. Chemosphere 5:377-382. [Google Scholar]