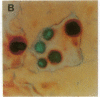
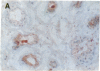
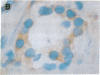
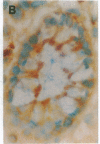
Abstract
The BCL2 protooncogene encodes an inner mitochondrial membrane protein that blocks programmed cell death. BCL2 was isolated from the chromosomal breakpoint of follicular B-cell lymphoma. Transgenic mice that overexpress BCL2 display extended survival of resting B cells. In this study we use a monospecific anti-human BCL2 antibody to define the distribution of BCL2 protein within organized tissues. BCL2 is restricted within germinal centers to the follicular mantle and to portions of the light zone implicated in the selection and maintenance of plasma cells and memory B cells. BCL2 is present in the surviving T cells in the thymic medulla. All hematopoietic lineages that derive from a renewing stem cell also display BCL2. A limited number of nonlymphoid tissues demonstrate BCL2 and can be grouped as (i) glandular epithelium in which hormones or growth factors regulate hyperplasia and involution, (ii) complex differentiating epithelium such as skin and intestine characterized by long-lived stem cells, and (iii) long-lived postmitotic cells such as neurons. Within these tissues that demonstrate apoptotic cell turnover, BCL2 is often topographically restricted to long-lived or proliferating cell zones. BCL2's function as an antidote to apoptosis may confer longevity to progenitor and effector cells in these tissues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakhshi A., Jensen J. P., Goldman P., Wright J. J., McBride O. W., Epstein A. L., Korsmeyer S. J. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985 Jul;41(3):899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- Blackman M., Kappler J., Marrack P. The role of the T cell receptor in positive and negative selection of developing T cells. Science. 1990 Jun 15;248(4961):1335–1341. doi: 10.1126/science.1972592. [DOI] [PubMed] [Google Scholar]
- Chen-Levy Z., Nourse J., Cleary M. L. The bcl-2 candidate proto-oncogene product is a 24-kilodalton integral-membrane protein highly expressed in lymphoid cell lines and lymphomas carrying the t(14;18) translocation. Mol Cell Biol. 1989 Feb;9(2):701–710. doi: 10.1128/mcb.9.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Leblond C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974 Dec;141(4):537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- Cleary M. L., Sklar J. Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7439–7443. doi: 10.1073/pnas.82.21.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Smith S. D., Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986 Oct 10;47(1):19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Cowan W. M., Fawcett J. W., O'Leary D. D., Stanfield B. B. Regressive events in neurogenesis. Science. 1984 Sep 21;225(4668):1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- Ferguson D. J., Anderson T. J. Ultrastructural observations on cell death by apoptosis in the "resting" human breast. Virchows Arch A Pathol Anat Histol. 1981;393(2):193–203. doi: 10.1007/BF00431076. [DOI] [PubMed] [Google Scholar]
- Gallucci B. B., Shulman H. M., Sale G. E., Lerner K. G., Caldwell L. E., Thomas E. D. The ultrastructure of the human epidermis in chronic graft-versus-host disease. Am J Pathol. 1979 Jun;95(3):643–662. [PMC free article] [PubMed] [Google Scholar]
- Goudswaard W. B., Houthoff H. J., Koudstaal J., Zwierstra R. P. Nesidioblastosis and endocrine hyperplasia of the pancreas: a secondary phenomenon. Hum Pathol. 1986 Jan;17(1):46–54. doi: 10.1016/s0046-8177(86)80154-x. [DOI] [PubMed] [Google Scholar]
- Graninger W. B., Seto M., Boutain B., Goldman P., Korsmeyer S. J. Expression of Bcl-2 and Bcl-2-Ig fusion transcripts in normal and neoplastic cells. J Clin Invest. 1987 Nov;80(5):1512–1515. doi: 10.1172/JCI113235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey W. F., Gonatas N. K., Kimura H., Wilson D. B. Identification and quantitation of T lymphocyte subsets found in the spinal cord of the Lewis rat during acute experimental allergic encephalomyelitis. J Immunol. 1983 Dec;131(6):2805–2809. [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Searle J. Deletion of cells by apoptosis during castration-induced involution of the rat prostate. Virchows Arch B Cell Pathol. 1973 Jun 25;13(2):87–102. doi: 10.1007/BF02889300. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longacre T. A., Bartow S. A. A correlative morphologic study of human breast and endometrium in the menstrual cycle. Am J Surg Pathol. 1986 Jun;10(6):382–393. doi: 10.1097/00000478-198606000-00003. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Lees R. K. Programmed death of autoreactive thymocytes. Nature. 1990 Feb 15;343(6259):642–644. doi: 10.1038/343642a0. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C., Gray D. Antigen-driven selection of virgin and memory B cells. Immunol Rev. 1986 Jun;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- Mahmoud I., Colin I., Many M. C., Denef J. F. Direct toxic effect of iodide in excess on iodine-deficient thyroid glands: epithelial necrosis and inflammation associated with lipofuscin accumulation. Exp Mol Pathol. 1986 Jun;44(3):259–271. doi: 10.1016/0014-4800(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Martin D. P., Schmidt R. E., DiStefano P. S., Lowry O. H., Carter J. G., Johnson E. M., Jr Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J Cell Biol. 1988 Mar;106(3):829–844. doi: 10.1083/jcb.106.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. J., Bradley J. G., Cotter T. G. HL-60 cells induced to differentiate towards neutrophils subsequently die via apoptosis. Clin Exp Immunol. 1990 Mar;79(3):448–453. doi: 10.1111/j.1365-2249.1990.tb08110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell T. J., Deane N., Platt F. M., Nunez G., Jaeger U., McKearn J. P., Korsmeyer S. J. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989 Apr 7;57(1):79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Korsmeyer S. J. Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14; 18). Nature. 1991 Jan 17;349(6306):254–256. doi: 10.1038/349254a0. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Nunez G., Platt F. M., Hockenberry D., London L., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2-immunoglobulin transgene expands a resting but responsive immunoglobulin M and D-expressing B-cell population. Mol Cell Biol. 1990 May;10(5):1901–1907. doi: 10.1128/mcb.10.5.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrini M., Silini E., Kozak C., Tsujimoto Y., Croce C. M. Molecular analysis of mbcl-2: structure and expression of the murine gene homologous to the human gene involved in follicular lymphoma. Cell. 1987 May 22;49(4):455–463. doi: 10.1016/0092-8674(87)90448-x. [DOI] [PubMed] [Google Scholar]
- Nuñez G., London L., Hockenbery D., Alexander M., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990 May 1;144(9):3602–3610. [PubMed] [Google Scholar]
- Olson R. L., Gaylor J., Everett M. A. Ultraviolet-induced individual cell keratinization. J Cutan Pathol. 1974;1(3):120–125. doi: 10.1111/j.1600-0560.1974.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Pezzella F., Tse A. G., Cordell J. L., Pulford K. A., Gatter K. C., Mason D. Y. Expression of the bcl-2 oncogene protein is not specific for the 14;18 chromosomal translocation. Am J Pathol. 1990 Aug;137(2):225–232. [PMC free article] [PubMed] [Google Scholar]
- Potten C. S., Allen T. D. Ultrastructure of cell loss in intestinal mucosa. J Ultrastruct Res. 1977 Aug;60(2):272–277. doi: 10.1016/s0022-5320(77)80071-3. [DOI] [PubMed] [Google Scholar]
- Savill J. S., Wyllie A. H., Henson J. E., Walport M. J., Henson P. M., Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989 Mar;83(3):865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto M., Jaeger U., Hockett R. D., Graninger W., Bennett S., Goldman P., Korsmeyer S. J. Alternative promoters and exons, somatic mutation and deregulation of the Bcl-2-Ig fusion gene in lymphoma. EMBO J. 1988 Jan;7(1):123–131. doi: 10.1002/j.1460-2075.1988.tb02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg E., Lubera J. A. Cancer statistics, 1989. CA Cancer J Clin. 1989 Jan-Feb;39(1):3–20. doi: 10.3322/canjclin.39.1.3. [DOI] [PubMed] [Google Scholar]
- Tew J. G., Mandel T. E. Prolonged antigen half-life in the lymphoid follicles of specifically immunized mice. Immunology. 1979 May;37(1):69–76. [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Gorham J., Cossman J., Jaffe E., Croce C. M. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985 Sep 27;229(4720):1390–1393. doi: 10.1126/science.3929382. [DOI] [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Walker N. I., Bennett R. E., Kerr J. F. Cell death by apoptosis during involution of the lactating breast in mice and rats. Am J Anat. 1989 May;185(1):19–32. doi: 10.1002/aja.1001850104. [DOI] [PubMed] [Google Scholar]