Abstract
Shiga toxins Stx1 and Stx2 play a prominent role in the pathogenesis of Shiga toxin-producing Escherichia coli (STEC) infections. Several variants of the stx2 gene, encoding Stx2, have been described. In this study, we developed a PCR-restriction fragment length polymorphism system for typing stx2 genes of STEC strains. The typing system discriminates eight described variants and allows the identification of new stx2 variants and STEC isolates carrying multiple stx2 genes. A phylogenetic tree, based on the nucleotide sequences of the toxin-encoding genes, demonstrates that stx2 sequences with the same PvuII HaeIII HincII AccI type generally cluster together.
Shiga toxin-producing Escherichia coli (STEC) strains produce toxins with a biological activity similar to those produced by Shigella dysenteriae type I. Shiga toxins (Stx) are cytotoxic on cultured Vero cells and are therefore also called verocytotoxins (18). Most STEC strains induce the formation of attaching-effacing lesions in intestinal epithelial cells by means of gene products of the pathogenicity island locus of enterocyte effacement (21, 33, 39) with genes coding, for example, for intimin (EaeA) (48) and the translocated intimin receptor (Tir) (17). Furthermore, STEC may carry a 60-MDa plasmid that codes for an enterohemolysin and carries regulatory sequences (3, 11, 37, 42). Enterohemorrhagic E. coli (EHEC) strains are defined as a subset of STEC that cause clinical disorders in humans. A great diversity of EHEC serotypes exists, although serotype EHEC O157:H7 is the best studied and is frequently associated with hemolytic-uremic syndrome (HUS) (4, 12). Epidemiological investigations indicate that cattle are the principal reservoirs of EHEC (43, 47).
STEC strains may produce two types of Shiga toxins, i.e., those that are antigenically similar to the Shiga toxin produced by S. dysenteriae (Stx1) and those that differ (Stx2) (16, 25, 40). Severe clinical symptoms were more frequently associated with E. coli strains producing Stx2 than with those producing Stx1 (4, 12, 20, 28). Both types of Shiga toxins are encoded by stx genes on temperate bacteriophages (26, 40). While stx1 is rather conserved, many variants of stx2, showing different toxicities for cultured cells and/or animals, have been described (14, 16, 23, 29, 31, 32, 34, 38). Among them, Stx2 and Stx2c are the most prominent in human clinical isolates. Variants Stx2vha (14) and Stx2vhb (14) can be activated by intestinal mucus and are therefore classified as activatable variants, also termed Stx2d variant toxins (22). Shiga toxins are composed of a single enzymatic 32-kDa A subunit, which is the active component of the toxin, and a pentamer of 7.7-kDa B subunits (7, 25). The B subunit is responsible for binding the toxin to the host cell receptor (13, 15, 27).
Molecular typing of STEC can be performed in different ways. Pulsed-field gel electrophoresis (PFGE) is an accurate and reproducible reference method (10, 45, 46) for the molecular typing of STEC strains. DNA sequence-based methods like multilocus sequence typing are also used as epidemiological tools. Another approach for typing STEC strains is to consider polymorphisms in virulence genes. A number of multiplex PCR assays detecting virulence-associated genes have been described (5, 6, 8, 24, 30, 44). Since Stx2 is the key virulence factor (4, 35), several investigators have developed PCR-restriction fragment length polymorphism (RFLP) assays that allow the rapid identification of known and new stx2 variants (1, 24, 34, 41, 49). However, these assays discriminate only a limited number of described variants. In addition, the designation of the toxin subtypes is insufficient to account for all the differences on the sequence level.
In the present study, we describe a PCR-RFLP typing system which is based on the use of four restriction enzymes (PvuII, HaeIII, HincII, and AccI), defining variants of stx2. This PCR-RFLP typing system includes a rational classification (PHIA) that was applied to 36 bovine and 27 human STEC strains of different origins. New stx2 variants were found, and the corresponding genes were cloned, sequenced, and analyzed. The PCR-RFLP assay was validated with Scottish E. coli O157 isolates from three different outbreaks.
Analysis of known stx2 sequences.
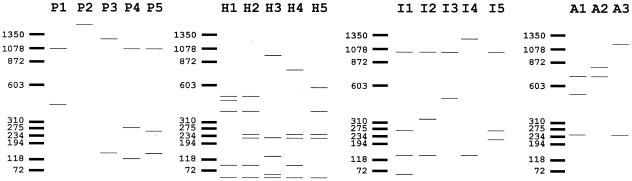
The sequences of 10 known stx2 variants (Table 1) were compared in order to select primers in conserved regions that would amplify the complete Stx2-encoding region. Primers oli320b (5′-GGTCACTGGTTCGAATCCAGTAC-3′; accession number X07865) and oli321(5′-GGGATCCTGAATTGTGACACAGATTACACTTGTTAC-3′; accession number X07865) were chosen. Subsequently, the 1.4-kb stx2 fragment was explored, by use of the VectorNTI 5.0 software, for restriction enzymes that, upon digestion, would result in profiles specific to each stx2 variant. Our analysis suggested that the combination of the profiles obtained using enzymes PvuII, HaeIII, HincII, and AccI, also used in previous studies (1, 34), would be discriminatory for 8 out of the 10 stx2 variants. The individual profiles thus obtained are schematically represented in Fig. 1.
TABLE 1.
Described stx2 variants and their restriction profilea
| Reference strain or plasmid | Accession no. | Stx2 variant | PHIA pattern | Reference |
|---|---|---|---|---|
| C600(933W) | X07865 | Stx2 | 1-1-1-1 | 16 |
| E32511 | M59432 | Stx2c | 1-2-2-1 | 38 |
| 7279 | X61283 | Stx2vhc | 3-2-3-1 | 23 |
| 343 | NA | Stx2vha | 2-2-2-2 | 14 |
| 544 | NA | Stx2vhb | 3-2-3-1 | 14 |
| pJCP520 | X65949 | Stx2OX3a | 4-3-4-1 | 31 |
| pJCP521 | L11079 | Stx2OX3b | 1-4-2-1 | 32 |
| pJCP522 | L11078 | Stx2O111 | 4-3-4-1 | 29 |
| pJCP523 | Z37725 | Stx2O48 | 5-1-5-1 | 29 |
| EH250 | AF043627 | Stx2O118 | 4-5-4-3 | 34 |
NA, not available.
FIG. 1.
Graphical representation of restriction profiles of known Stx2 variants. P1 to P5 are the profiles obtained with PvuII, H1 to H5 are the profiles obtained with HaeIII, I1 to I5 are the profiles obtained with HincII, and A1 to A3 are the profiles obtained with AccI.
PCR-RFLP analysis of the corresponding reference strains confirmed the predicted profiles. PCR amplification was performed with total bacterial DNA (genomic DNA buffer set; QIAGEN) in a 50-μl reaction mixture with 1 U of ExTaq DNA polymerase (Takara) in a Perkin-Elmer apparatus under the following conditions: an initial denaturation at 94°C for 50 s, followed by 10 cycles of 94°C for 10 s, 55°C for 30 s and 68°C for 2 min. During the next 20 cycles, the elongation time at 68°C was increased 15 s each cycle. Denaturation and annealing times remained constant. PCR products were purified with the Qiaquick PCR purification kit (QIAGEN). One microgram of the purified PCR product was digested with the enzymes PvuII, HaeIII, HincII, and AccI. Restriction fragments were separated by agarose gel electrophoresis on a 3% Nusieve 3:1 agarose gel (BioWhittaker Molecular Applications).
The typing scheme described here represents a rational nomenclature for Stx2 variants (Table 1). Three methods for the further subtyping of the stx2 gene by PCR-RFLP were previously described (1, 34, 41). The stx2 subtyping scheme designed by Tyler et al. (41) includes identification of Stx2 and variants Stx2vha and Stx2vhb and consists of PCR amplification of the B subunit sequence and digestion with endonucleases HaeIII, RsaI, and NciI. Piérard et al. (34) extended Tyler's typing system by including three new primers, allowing amplification of variants stx2-O118, stx2-OX3a, and stx2-O111, carried by some less-virulent STEC strains. By use of the PCR-RFLP system described by Bastian et al. (1), with the primer set of Lin et al. (19) and restriction endonucleases HincII and AccI, four different restriction profiles were obtained among variants Stx2, Stx2c, Stx2vhb, Stx2vha, Stx2OX3b, Stx2OX3a, and Stx2O111. This system did not discriminate between variants Stx2c, Stx2OX3b, and Stx2vha, whereas these variants showed different PHIA profiles. The PHIA typing system shows higher discrimination due to the larger PCR fragment and the combination of four enzymes (Fig. 2). In spite of the higher discrimination, variants Stx2vhb and Stx2vhc shared the same PHIA profile (3-2-3-1) but could be further distinguished by use of EcoRV that cuts only the stx2-vhb sequence, producing fragments of 258 bp and 1,160 bp. Variants Stx2OX3a and Stx2O111 both have PHIA profile 4-3-4-1, since they differ at only four positions in the nucleotide sequence. Variants Stx2e and Stx2f were not included in this study because of their rare occurrence among human cases and their considerable sequence divergence.
FIG. 2.
Graphical representation of the 1.4-kb fragment indicating the positions (between brackets) of the relevant restriction sites for the different stx2 variants.
stx2 subtyping of bovine STEC strains and human EHEC strains from sporadic cases.
The stx2 genes of 36 bovine and 27 human STEC strains, randomly selected among Belgian isolates, were typed with the PHIA system. Among the bovine STEC strains, the most frequently observed variant profiles were 1-2-2-1 and 1-1-1-1 and, to lesser extents, 1-4-2-1, 3-2-3-1, and 2-2-2-2 (Table 2). For human EHEC strains of serogroup O157, PHIA profiles 1-1-1-1, 1-2-2-1, and 1-4-2-1 were found, which were also detected among the bovine isolates of this serogroup (Table 2). For the human strains of the other serogroups studied, PHIA profile 1-1-1-1 was found for all except one isolate, which showed profile 1-4-2-1. PHIA profiles of the related strains, either the bovine STEC strains EBC32, EBC34, and EBC35 isolated on the same farm at different times and from different sources (animal or environmental) or the human EHEC strains EH123 and EH125, originating from the same family, were the same. In none of the E. coli strains studied could we detect variant Stx2OX3a, Stx2O111, or Stx2O118. This result confirms the findings of Piérard et al. (34), who did not detect these stx2 variant gene products in isolates of the most-virulent E. coli serotypes, i.e., O157, O26, O103, O111, and O145. Also, these variants were not detected during other studies of human O157 isolates and non-O157 STEC strains (24, 36). Friedrich et al. (9) did not find these variants in isolates associated with HUS. In contrast to other studies (24, 36), there was no indication of a clear association between PHIA type and serogroup.
TABLE 2.
Distribution of PHIA variants among bovine and human STEC isolates with a single stx2 copya
| PHIA pattern | Serogroupa | No. of isolates with indicated pattern
|
|
|---|---|---|---|
| Human | Bovine | ||
| 1-1-1-1 | O26 | 1 | 0 |
| O103 | 1 | 0 | |
| O111 | 1 | 1 | |
| O145 | 1 | 0 | |
| O157 | 10 | 6 | |
| — | 3 | 2 | |
| 1-2-2-1 | O103 | 0 | 1 |
| O157 | 7 | 9 | |
| — | 0 | 2 | |
| 1-4-2-1 | O157 | 2 | 3 |
| — | 1 | 0 | |
| 3-2-3-1 | O113 | 0 | 1 |
| O157 | 0 | 1 | |
| 2-2-2-2 | O103 | 0 | 1 |
| — | 0 | 2 | |
| 2-1-1-1 | O103 | 0 | 2b |
| 2-1-2-1 | O128 | 0 | 1b |
| 3-1-5-1 | O103 | 0 | 1b |
—, no agglutination with antisera to any of the following serogroups: O26, O103, O111, O113, O145, and O157.
Cloned and sequenced.
Among the bovine STEC strains, four showed profiles differing from those found in the reference strains, i.e., 2-1-1-1 (two strains), 2-1-2-1, and 3-1-5-1. The stx2 genes of the corresponding STEC strains were cloned in the expression vector pBADMycHisC (Invitrogen) to facilitate future toxicity studies. After amplification with primers oli320b and oli321H (5′-GGGAAGCTTTGAATTGTGACACAGATTACACTTGTTAC-3′), the purified PCR product (Qiaquick PCR purification kit; QIAGEN) was digested with HindIII and cloned (Ready-To-Go T4 DNA ligase kit; Amersham Biosciences) between the filled-in NcoI site and the HindIII site of pBADMycHisC. Clones were given a pEHEC number and sequenced. The PHIA patterns obtained for the clones after PCR-RFLP and sequence analysis confirmed the PHIA patterns of the corresponding STEC strains (Table 2).
STEC strains may contain more than one copy of stx2 or stx2 variants (1, 2, 14, 38). Among the selection of bovine STEC isolates, we identified three STEC strains showing a complex restriction profile (Table 3). The stx2 genes of these isolates were cloned in the SmaI site of pUC18 or pUC19 (Sureclone ligation kit; Amersham Biosciences). Transformants were given a pVTEC number and sequenced. After PCR-RFLP analysis, different PHIA patterns were found among the respective clones. If these profiles were superimposed, the result corresponded with the PHIA pattern obtained for the STEC strain (Table 3). Among the clones, two new stx2 variants were identified, i.e., 2-2-2-1 and 2-6-6-3. The latter variant has a restriction profile for HaeIII and HincII that did not correspond with any of the reference strains. Consequently, in addition to new combinations of existing profiles, new restriction profiles for one or more endonucleases were identified.
TABLE 3.
Complex PHIA profiles for bovine STEC isolates and their corresponding stx2 clonesa
| Strain | STEC isolate
|
stx2 clone
|
||
|---|---|---|---|---|
| PHIA pattern | Serogroup | Clone | PHIA pattern | |
| EBC229 | 1+2+3-1-1+5-1 | O103 | pVTEC2 | 1-1-1-1 |
| pVTEC3 | 3-1-5-1 | |||
| pVTEC16 | 2-1-1-1 | |||
| EBC258 | 1+2-1+6-1+6-1+3 | —a | pVTEC1 | 1-1-1-1 |
| pVTEC15 | 2-6-6-3 | |||
| EBC281 | 1+2-2-2-1 | O103 | pVTEC7 | 2-2-2-1 |
| pVTEC9 | 1-2-2-1 | |||
—, no agglutination with antisera to any of the following serogroups: O26, O103, O111, O113, O145, and O157.
In our study, more variation in PHIA types was observed among the bovine isolates than among the human isolates for which no complex or new restriction profiles were found. PHIA types from human EHEC isolates were a subset of those found in the bovine STEC population. In this study, variants Stx2vha (2-2-2-2) and Stx2vhb (3-2-3-1) were found only in bovine STEC, although they are associated with HUS according to the literature.
These preliminary results may indicate that not all bovine STEC strains are pathogenic for humans. This hypothesis is supported by the data of an ongoing study on Belgian STEC and EHEC isolates which were typed with the PHIA system (L. De Baets et al., unpublished data).
stx2 subtyping of STEC strains from outbreaks.
To validate our PCR-RFLP analysis, we analyzed Scottish isolates associated with three different E. coli O157 outbreaks (Table 4). In addition, three random Scottish STEC isolates were typed. Isolates were from a clinical, animal, or environmental origin. The PHIA type was the same for all strains within one outbreak, as was their PFGE pattern (results not shown). As shown in Table 4, the PHIA profiles of strains from outbreaks one and two (1-1+2-1+2-1) were combinations of two simple profiles. The stx2 genes of one strain from both outbreaks were cloned in pBADMycHisC and subsequently analyzed by means of PCR-RFLP. Two different PHIA patterns (1-1-1-1 and 1-2-2-1) were found among these clones. When the two profiles were superimposed, the result corresponded to the PHIA pattern obtained for the STEC isolate. Within outbreak three, variant 1-1-1-1 was found for all strains. Random isolates showed the PHIA profiles 1-2-2-1 (two times) and 1-1-1-1. Since related strains show the same PHIA type, we conclude that the PHIA system is an efficient tool for epidemiological research on STEC infections. The typing method can be used to compare STEC strains and to identify the source of infection.
TABLE 4.
PHIA pattern of related Scottish E. coli O157 isolates from outbreaks
| Outbreaka | PHIA pattern | Clone | PHIA pattern |
|---|---|---|---|
| 1 (n = 9) | 1-1+2-1+2-1 | pEHEC398 | 1-1-1-1 |
| pEHEC399 | 1-2-2-1 | ||
| 2 (n = 3) | 1-1+2-1+2-1 | pEHEC410 | 1-1-1-1 |
| pEHEC414 | 1-2-2-1 | ||
| 3 (n = 11) | 1-1-1-1 | pEHEC412 | 1-1-1-1 |
n, number of isolates.
DNA sequence analysis and phylogenetic comparison of stx2 sequences.
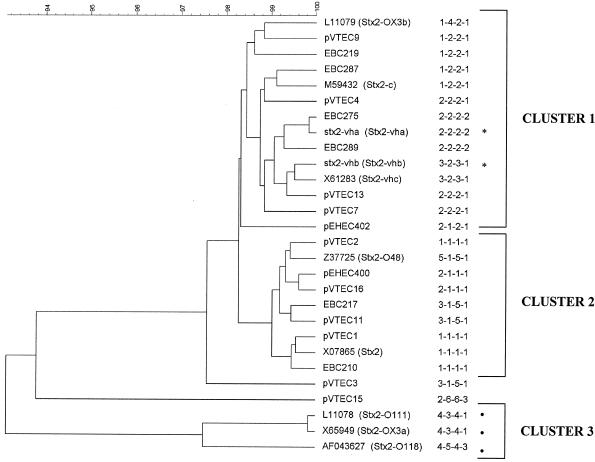
Ten described stx2 sequences and 18 sequences determined in this study were selected to construct a phylogenetic tree by use of the Bionumerics 3.0 (Applied Maths) software. For bovine STEC strains EBC210, EBC217, EBC219, EBC275, EBC287, and EBC289, the stx2 PCR fragments were directly sequenced. pVTEC and pEHEC clones were sequenced as described above. Nucleotide sequences were aligned from the start codon of stx2a to the stop codon of stx2b (Fig. 3). None of the stx2 sequences are identical at the nucleotide level. Nevertheless, in the phylogenetic tree, stx2 sequences of the same PHIA type generally clustered together. Two main clusters that shared 98% similarity were identified. Moreover, Stx2 variants with a specific phenotypic characteristic were located in the same cluster. The activatable variants (Stx2vha and Stx2vhb) are located in the first cluster. The amino acid sequences allowing activation by intestinal mucus (22) are also present in four other variants (EBC275, Stx2vhc, pVTEC 13, and pEHEC 402), which were grouped in the first cluster. Stx2, which cannot be activated (22), is located in the second cluster. A third cluster showing less than 94% sequence similarity contained the Stx2 variant toxins Stx2OX3a, Stx2O111, and Stx2O118, which showed low cytotoxicity for Vero cells (29). Together with the indications for a reduced toxicity, these data support the classification of these variants as a different Stx2 subtype. The new variant designated by PHIA profile 2-6-6-3 (pVTEC 15) also showed more sequence divergence. Our results suggest that stx2 sequences and PHIA types with the same phenotypic characteristics are grouped together in the phylogenetic tree. Future toxicity experiments using cell cultures with members of different clusters (PHIA types) of the phylogenetic tree may further elucidate the association with their cytotoxicity.
FIG. 3.
Phylogenetic tree (unweighted pair group method with arithmetic mean) of stx2 sequences analyzed in this study. The sequences start at the ATG start codon of the A-subunit gene and end at the stop codon of the B-subunit gene. Accession numbers AY443043, AY443044, AY443045, AY443046, AY443047, AY443048, AY443049, AY443050, AY443051, AY443052, AY443053, AY443054, AY443055, AY443056, AY443057, AY443058, AY443059, AY443060, respectively, were given to the stx2 sequences of the following isolates or clones: EBC219, pVTEC9, EBC287, pVTEC4, EBC275, pVTEC7, EBC289, pVTEC13, pVTEC2, pEHEC400, pVTEC16, EBC217, pVTEC11, pVTEC1, EBC210, pEHEC402, pVTEC3, and pVTEC15. *, activatable by intestinal mucus; •, low cytotoxicity for cultured Vero cells.
Acknowledgments
This work was supported by contract research grant S-6115 from the Federal Public Service Health, Food Chain Security and Environment (Brussels, Belgium), and by the FWO-Vlaanderen (grant WOAL241) and was cofinanced by the “Fonds voor de Gezondheid en de Kwaliteit van Dieren en Dierlijke Producten” and the Research Council of the Vrije Universiteit Brussel.
We thank James Paton for kindly providing plasmids 343 and 544. We thank Pam Taylor for the PFGE typing of the Scottish isolates. We also gratefully acknowledge the technical assistance of Hilde Paesen, Heidi Vander Veken, Nele Buys, and Nancy De Backer.
REFERENCES
- 1.Bastian, S. N., I. Carle, and F. Grimont. 1998. Comparison of 14 PCR systems for the detection and subtyping of stx genes in Shiga-toxin-producing Escherichia coli. Res. Microbiol. 149:457-472. [DOI] [PubMed] [Google Scholar]
- 2.Bertin, Y., K. Boukhors, N. Pradel, V. Livrelli, and C. Martin. 2001. Stx2 subtyping of Shiga toxin-producing Escherichia coli isolated from cattle in France: detection of a new Stx2 subtype and correlation with additional virulence factors. J. Clin. Microbiol. 39:3060-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutin, L., M. A. Montenegro, I. Ørskov, F. Ørskov, J. Prada, S. Zimmermann, and R. Stephan. 1989. Close association of verotoxin (Shiga-like toxin) production with enterohemolysin production in strains of Escherichia coli. J. Clin. Microbiol. 27:2559-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boerlin, P., S. A. McEwen, F. Boerlin-Petzold, J. B. Wilson, R. P. Johnson, and C. L. Gyles. 1999. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 37:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.China, B., F. Goffaux, V. Pirson, and J. Mainil. 1999. Comparison of eae, tir, espA and espB genes of bovine and human attaching and effacing Escherichia coli by multiplex polymerase chain reaction. FEMS Microbiol. Lett. 178:177-182. [DOI] [PubMed] [Google Scholar]
- 6.China, B., V. Pirson, and J. Mainil. 1996. Typing of bovine attaching and effacing Escherichia coli by multiplex in vitro amplification of virulence-associated genes. Appl. Environ. Microbiol. 62:3462-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donohue-Rolfe, A., M. Jacewicz, and G. T. Keusch. 1989. Isolation and characterization of functional Shiga toxin subunits and renatured holotoxin. Mol. Microbiol. 3:1231-1236. [DOI] [PubMed] [Google Scholar]
- 8.Fratamico, P. M., S. K. Sackitey, M. Wiedmann, and M. Y. Deng. 1995. Detection of Escherichia coli O157:H7 by multiplex PCR. J. Clin. Microbiol. 33:2188-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedrich, A. W., M. Bielaszewska, W. L. Zhang, M. Pulz, T. Kuczius, A. Ammon, and H. Karch. 2002. Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J. Infect. Dis. 185:74-84. [DOI] [PubMed] [Google Scholar]
- 10.Gautom, R. K. 1997. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J. Clin. Microbiol. 35:2977-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gómez-Duarte, O. G., and J. B. Kaper. 1995. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect. Immun. 63:1767-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic Escherichia coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-98. [DOI] [PubMed] [Google Scholar]
- 13.Hagnerelle, X., C. Plisson, O. Lambert, S. Marco, J. L. Rigaud, L. Johannes, and D. Levy. 2002. Two-dimensional structures of the Shiga toxin B-subunit and of a chimera bound to the glycolipid receptor Gb3. J. Struct. Biol. 139:113-121. [DOI] [PubMed] [Google Scholar]
- 14.Ito, H., A. Terai, H. Kurazono, Y. Takeda, and M. Nishibuchi. 1990. Cloning and nucleotide sequencing of Vero toxin 2 variant genes from Escherichia coli O91:H21 isolated from a patient with the hemolytic uremic syndrome. Microb. Pathog. 8:47-60. [DOI] [PubMed] [Google Scholar]
- 15.Jacewicz, M. S., M. Mobassaleh, S. K. Gross, K. A. Balasubramanian, P. F. Daniel, S. Raghavan, R. H. McCluer, and G. T. Keusch. 1994. Pathogenesis of Shigella diarrhea. XVII. A mammalian cell membrane glycolipid, Gb3, is required but not sufficient to confer sensitivity to Shiga toxin. J. Infect. Dis. 169:538-546. [DOI] [PubMed] [Google Scholar]
- 16.Jackson, N., R. J. Neill, A. D. O'Brien, R. K. Holmes, and J. W. Newland. 1987. Nucleotide sequence analysis and comparison of the structural genes for Shiga-like toxin I and Shiga-like toxin II encoded by bacteriophages from Escherichia coli 933. FEMS Microbiol. Lett. 44:109-114. [Google Scholar]
- 17.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic Escherichia coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 18.Konowalchuk, J., J. I. Speirs, and S. Stavric. 1977. Vero response to a cytotoxin of Escherichia coli. Infect. Immun. 18:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin, Z., H. Kurazono, S. Yamasaki, and Y. Takeda. 1993. Detection of various variant verotoxin genes in Escherichia coli by polymerase chain reaction. Microbiol. Immunol. 37:543-548. [DOI] [PubMed] [Google Scholar]
- 20.Louise, C. B., and T. G. Obrig. 1995. Specific interaction of Escherichia coli O157:H7-derived Shiga-like toxin II with human renal endothelial cells. J. Infect. Dis. 172:1397-1401. [DOI] [PubMed] [Google Scholar]
- 21.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melton-Celsa, A. R., S. C. Darnell, and A. D. O'Brien. 1996. Activation of Shiga-like toxins by mouse and human intestinal mucus correlates with virulence of enterohemorrhagic Escherichia coli O91:H21 isolates in orally infected, streptomycin-treated mice. Infect. Immun. 64:1569-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer, T., H. Karch, J. Hacker, H. Bocklage, and J. Heesemann. 1992. Cloning and sequencing of a Shiga-like toxin II-related gene from Escherichia coli O157:H7 strain 7279. Zentbl. Bakteriol. 276:176-188. [DOI] [PubMed] [Google Scholar]
- 24.Nakao, H., K. Kimura, H. Murakami, T. Maruyama, and T. Takeda. 2002. Subtyping of Shiga toxin 2 variants in human-derived Shiga toxin-producing Escherichia coli strains isolated in Japan. FEMS Immunol. Med. Microbiol. 34:289-297. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien, A. D., and R. K. Holmes. 1987. Shiga and Shiga-like toxins. Microbiol. Rev. 51:206-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien, A. D., L. R. M. Marques, C. F. Kerry, J. W. Newland, and R. K. Holmes. 1989. Shiga-like toxin converting phage of enterohemorrhagic Escherichia coli strain 933. Microb. Pathog. 6:381-390. [DOI] [PubMed] [Google Scholar]
- 27.Obrig, T. G. 1997. Shiga toxin mode of action in Escherichia coli O157:H7 disease. Front. Biosci. 2:d635-d642. [DOI] [PubMed] [Google Scholar]
- 28.Ostroff, S. M., P. I. Tarr, M. A. Neill, J. H. Lewis, N. Hargrett-Bean, and J. M. Kobayashi. 1989. Toxin genotypes and plasmid profiles as determinants of systemic sequelae in Escherichia coli O157:H7 infections. J. Infect. Dis. 160:994-998. [DOI] [PubMed] [Google Scholar]
- 29.Paton, A. W., A. J. Bourne, P. A. Manning, and J. C. Paton. 1995. Comparative toxicity and virulence of Escherichia coli clones expressing variant and chimeric Shiga-like toxin type II operons. Infect. Immun. 63:2450-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paton, A. W., and J. C. Paton. 1998. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic Escherichia coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 36:598-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paton, A. W., J. C. Paton, M. W. Heuzenroeder, P. N. Goldwater, and P. A. Manning. 1992. Cloning and nucleotide sequence of a variant Shiga-like toxin II gene from Escherichia coli OX3:H21 isolated from a case of sudden infant death syndrome. Microb. Pathog. 13:225-236. [DOI] [PubMed] [Google Scholar]
- 32.Paton, A. W., J. C. Paton, and P. A. Manning. 1993. Polymerase chain reaction amplification, cloning and sequencing of variant Escherichia coli Shiga-like toxin type II operons. Microb. Pathog. 15:77-82. [DOI] [PubMed] [Google Scholar]
- 33.Perna, N. T., G. F. Mayhew, G. Pósfai, S. Elliott, M. S. Donnenberg, J. B. Kaper, and F. R. Blattner. 1998. Molecular evolution of a pathogenicity island from enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 66:3810-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piérard, D., G. Muyldermans, L. Moriau, D. Stevens, and S. Lauwers. 1998. Identification of new verocytotoxin type 2 variant B-subunit genes in human and animal Escherichia coli isolates. J. Clin. Microbiol. 36:3317-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritchie, J. M., P. L. Wagner, D. W. K. Acheson, and M. K. Waldor. 2003. Comparison of Shiga toxin production by hemolytic-uremic syndrome-associated and bovine-associated Shiga toxin-producing Escherichia coli isolates. Appl. Environ. Microbiol. 69:1059-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russmann, H., H. Schmidt, A. Caprioli, and H. Karch. 1994. Highly conserved B-subunit genes of Shiga-like toxin II variants found in Escherichia coli O157 strains. FEMS Microbiol. Lett. 118:335-340. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt, H., L. Beutin, and H. Karch. 1995. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect. Immun. 63:1055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitt, C. K., M. L. McKee, and A. D. O'Brien. 1991. Two copies of Shiga-like toxin II-related genes common in enterohemorrhagic Escherichia coli strains are responsible for the antigenic heterogeneity of the O157:H−strain E32511. Infect. Immun 59:1065-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sperandio, V., J. B. Kaper, M. R. Bortolini, B. C. Neves, R. Keller, and L. R. Trabulsi. 1998. Characterization of the locus of enterocyte effacement (LEE) in different enteropathogenic Escherichia coli (EPEC) and Shiga-toxin producing Escherichia coli (STEC) serotypes. FEMS Microbiol. Lett. 164:133-139. [DOI] [PubMed] [Google Scholar]
- 40.Strockbine, N. A., L. R. M. Marques, J. W. Newland, H. W. Smith, R. K. Holmes, and A. D. O'Brien. 1986. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect. Immun. 53:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tyler, S. D., W. M. Johnson, H. Lior, G. Wang, and K. R. Rozee. 1991. Identification of verotoxin type 2 variant B subunit genes in Escherichia coli by the polymerase chain reaction and restriction fragment length polymorphism analysis. J. Clin. Microbiol. 29:1339-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tzipori, S., H. Karch, K. I. Wachsmuth, R. M. Robins-Browne, A. D. O'Brien, H. Lior, M. L. Cohen, J. Smithers, and M. M. Levine. 1987. Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect. Immun. 55:3117-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valcour, J. E., P. Michel, S. A. McEwen, and J. B. Wilson. 2002. Associations between indicators of livestock farming intensity and incidence of human Shiga toxin-producing Escherichia coli infection. Emerg. Infect. Dis. 8:252-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, G., C. G. Clark, and F. G. Rodgers. 2002. Detection in Escherichia coli of the genes encoding the major virulence factors, the genes defining the O157:H7 serotype, and components of the type 2 Shiga toxin family by multiplex PCR. J. Clin. Microbiol. 40:3613-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welinder-Olsson, C., M. Badenfors, T. Cheasty, E. Kjellin, and B. Kaijser. 2002. Genetic profiling of enterohemorrhagic Escherichia coli strains in relation to clonality and clinical signs of infection. J. Clin. Microbiol. 40:959-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welinder-Olsson, C., E. Kjellin, M. Badenfors, and B. Kaijser. 2000. Improved microbiological techniques using the polymerase chain reaction and pulsed-field gel electrophoresis for diagnosis and follow-up of enterohaemorrhagic Escherichia coli infection. Eur. J. Clin. Microbiol. Infect. Dis. 19:843-851. [DOI] [PubMed] [Google Scholar]
- 47.Whipp, S. C., M. A. Rasmussen, and W. C. Cray, Jr. 1994. Animals as a source of Escherichia coli pathogenic for human beings. J. Am. Vet. Med. Assoc. 204:1168-1175. [PubMed] [Google Scholar]
- 48.Yu, J., and J. B. Kaper. 1992. Cloning and characterization of the eae gene of enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 6:411-417. [DOI] [PubMed] [Google Scholar]
- 49.Ziebell, K. A., S. C. Read, R. P. Johnson, and C. L. Gyles. 2002. Evaluation of PCR and PCR-RFLP protocols for identifying Shiga toxins. Res. Microbiol. 153:289-300. [DOI] [PubMed] [Google Scholar]