Abstract
Previous studies have shown that members of the family Geobacteraceae that attach to the anodes of sediment fuel cells are directly involved in harvesting electricity by oxidizing organic compounds to carbon dioxide and transferring the electrons to the anode. In order to learn more about this process, microorganisms from the anode surface of a marine sediment fuel cell were enriched and isolated with Fe(III) oxide. Two unique marine isolates were recovered, strains A1T and A2. They are gram-negative, nonmotile rods, with abundant c-type cytochromes. Phylogenetic analysis of the 16S rRNA, recA, gyrB, fusA, rpoB, and nifD genes indicated that strains A1T and A2 represent a unique phylogenetic cluster within the Geobacteraceae. Both strains were able to grow with an electrode serving as the sole electron acceptor and transferred ca. 90% of the electrons available in their organic electron donors to the electrodes. These organisms are the first psychrotolerant members of the Geobacteraceae reported thus far and can grow at temperatures between 4 and 30°C, with an optimum temperature of 22°C. Strains A1T and A2 can utilize a wide range of traditional electron acceptors, including all forms of soluble and insoluble Fe(III) tested, anthraquinone 2,6-disulfonate, and S0. In addition to acetate, both strains can utilize a number of other organic acids, amino acids, long-chain fatty acids, and aromatic compounds to support growth with Fe(III) nitrilotriacetic acid as an electron acceptor. The metabolism of these organisms differs in that only strain A1T can use acetoin, ethanol, and hydrogen as electron donors, whereas only strain A2 can use lactate, propionate, and butyrate. The name Geopsychrobacter electrodiphilus gen. nov., sp. nov., is proposed for strains A1T and A2, with strain A1T (ATCC BAA-880T; DSM 16401T; JCM 12469) as the type strain. Strains A1T and A2 (ATCC BAA-770; JCM 12470) represent the first organisms recovered from anodes that can effectively couple the oxidation of organic compounds to an electrode. Thus, they may serve as important model organisms for further elucidation of the mechanisms of microbe-electrode electron transfer in sediment fuel cells.
Energy can be harvested from aquatic sediments by burying a graphite electrode in anoxic sediments and making an electrical connection between this electrode and a similar electrode in the overlying aerobic water (4, 17, 42, 45). The recovery of electricity from these sediments (4, 17, 42, 45) is analogous to that from previously described biological fuel cells (4, 5, 7, 10-13, 15, 20-23, 27, 36-39, 41, 43). Biological fuel cells use the natural catalytic ability of microorganisms to oxidize a wide variety of substrates, while still producing electrons in a form that can be harvested at an electrode. For example, fuel cells utilizing sulfate-reducing bacteria have been constructed, with the microbially produced hydrogen sulfide serving to shuttle electrons to the electrode surface (10, 15). In addition, Fe(III)-reducing microorganisms such as Shewanella putrefaciens (20, 22), Clostridium butyricum (39), Aeromonas hydrophila (41), Rhodoferax ferrireducens (7), Desulfobulbus propionicusT (16), and several species of Geobacteraceae (4, 5) have been shown to directly transfer electrons from the oxidation of organic compounds to the surface of an electrode without the need for a shuttle.
In sediment fuel cells, natural populations of microorganisms in the anoxic sediments are responsible for electron transfer to the current-harvesting anode. Previous molecular and culturing studies have indicated that a specific group of Fe(III)-reducing microorganisms, the Geobacteraceae, is primarily responsible for electricity production by sediment fuel cells (4, 17, 45). Further analysis of pure cultures of Geobacteraceae has shown that these organisms can conserve energy to support growth by oxidizing organic compounds to carbon dioxide, with an electrode serving as the sole electron acceptor (4, 5). Therefore, a likely explanation for the functioning of sediment fuel cells is that microorganisms in the sediments convert the complex sediment organic matter to fermentation products, most notably acetate, and that Geobacteraceae colonizing the anode oxidize these fermentation products to carbon dioxide, with transfer of electrons to the current-harvesting electrode (4). These electrons then flow to the cathode in the overlying aerobic water, where they react with oxygen (42).
In order to rationally optimize the harvesting of electricity from aquatic sediments, it is necessary to understand the mechanisms of microbe-electrode electron transfer. Ideally, this phenomenon should be studied in microorganisms known to colonize energy-harvesting electrodes in sediments. However, until now, such organisms have not been available. Here we report two strains isolated from the surfaces of electricity-harvesting electrodes incubated in marine sediments. Both isolates represent a novel genus in the Geobacteraceae with the capacity to grow at temperatures (4°C) lower than those previously reported for microorganisms in this family. These organisms can also oxidize a variety of electron donors, with electrodes and Fe(III) serving as the terminal electron acceptors. This is the first report of microorganisms isolated from electrode surfaces that can effectively oxidize organic compounds to carbon dioxide with an electrode serving as the electron acceptor.
MATERIALS AND METHODS
Source of organisms.
A marine sediment fuel cell was constructed in the laboratory with sediments collected from Boston Harbor, Mass., near the World's End peninsula, at a water depth of 5 m, as previously described (4, 17). After incubation and energy harvesting at 15°C for 6 months, the anode was pulled from the sediment and washed with a sterile anaerobic marine medium lacking an electron donor or acceptor and containing Na2S (1.0 mM) as a reducing agent. The anode surface was then scraped with a sterile razor blade into the marine medium, forming an anode suspension that was rapidly transferred to anaerobic tubes as an inoculum for enrichment and isolation.
Isolation.
The culture medium was a slightly modified version of APW medium (9) containing the following: NaCl (340 mM), KCl (10 mM), NH4Cl (5 mM), KH2PO4 (0.7 mM), MgSO4 · 7H2O (0.8 mM), NaHCO3 (24 mM), and trace minerals and vitamins (29). Before NaHCO3 was added, the pH was adjusted to 6.8 with 5 N NaOH. The medium (10 ml) was dispensed into 26-ml anaerobic pressure tubes (Bellco Glass, Inc., Vineland, N.J.) and sparged with oxygen-free N2-CO2 (80:20, vol/vol) for 15 min to remove dissolved oxygen. The tubes were then sealed with thick butyl rubber stoppers and autoclaved. After autoclaving, the medium was supplemented with FeCl2 · 2H2O (1.0 mM) from a 100 mM sterile anoxic stock solution.
The anode suspension (0.2 ml) was inoculated into the medium with poorly crystalline Fe(III) oxide (100 mM) as the electron acceptor (30). Acetate (5 mM) or benzoate (0.5 mM) was provided as the electron donor. The initial inoculum was then transferred in a series of eight 10-fold serial dilutions which were incubated at 15°C in the dark. The highest dilutions that reduced Fe(III) were diluted to extinction an additional three times. The highest dilution that grew after the fourth serial transfer was then transferred in an anaerobic chamber (N2-CO2-H2 [83:10:7, vol/vol]) to solidified medium (2% agar) on plates amended with 0.02% yeast extract, with acetate (5 mM) as the electron donor and Fe(III) pyrophosphate (10 mM) as the electron acceptor. The plates were incubated in the chamber at 22°C. Isolated single colonies were selected from each plate and resuspended in 2 ml of liquid medium with Fe(III) pyrophosphate (10 mM) provided as the electron acceptor and with acetate (5 mM) or benzoate (0.5 mM) as the electron donor. When growth was observed, this 2-ml culture was inoculated into liquid medium, with Fe(III) oxide (100 mM) provided as the electron acceptor and with acetate (5 mM) or benzoate (0.5 mM) as the electron donor, and was incubated at 15°C in the dark.
Evaluation of electron donors and acceptors and temperature optimum.
Alternate electron donors were added to media from concentrated sterile anoxic stock solutions. Hydrogen as an electron donor was provided as an H2-CO2 mixture (80:20, vol/vol, at an overpressure of 101 kPa), with 0.1 mM acetate provided as a carbon source. To evaluate the utilization of alternate electron acceptors, acetate (5 mM) or malate (10 mM) was provided as the donor, and various electron acceptors were added to media from sterile anoxic stock solutions. Growth on the various electron donors and acceptors was considered positive only after four transfers (5% inoculum).
The temperature optimum was evaluated with acetate (5 mM) as the electron donor and Fe(III) oxide (100 mM) as the electron acceptor.
Electrodes and electrode chambers.
Metabolism with an electrode serving as the sole electron acceptor was evaluated in an anoxic dual-chamber fuel cell as previously described (4, 5). The chambers were separated with a cation-selective membrane (Nafion 117; Electrosynthesis, Lancaster, N.Y.), and electrodes were 2.54- by 7.62- by 1.27-cm sticks of unpolished graphite (grade G10; Graphite Engineering and Sales, Greenville, Mich.). An Ag/AgCl reference electrode (BAS, West Lafayette, Ind.) was placed in the anode or working electrode chamber, and electrodes were poised with a model 2049 AMEL Instruments (Milan, Italy) potentiostat. The counterelectrode chamber was continuously flushed with N2-CO2 (80:20) to remove hydrogen created at the counterelectrode.
A PowerLab/4SP unit connected to a Power Macintosh computer collected current and voltage measurements directly from potentiostat outputs every 10 s. The data were logged with Chart 4.0 software (ADInstruments, Mountain View, Calif.) as current (in milliamperes) production over time. Current (in milliamperes) was converted to electrons recovered by using the following conversions: 1 C = 1 A × 1 s, 1 C = 6.24 × 1018 electrons, and 1 mol = 6.02 × 1023 electrons (96,500 C/mol). Total electron recovery was calculated by dividing the number of donor electrons oxidized (determined by high-pressure liquid chromatography) by the number of electrons transferred to the anode to generate current. Background current (current at the working electrode in the absence of cells; ca. 0.03 mA) was determined for each experiment and subtracted from all values before calculation of the total electron recovery.
Analytical techniques.
Organic acids were measured with an HP series 1100 high-pressure liquid chromatograph (Hewlett-Packard, Palo Alto, Calif.) on a Fast Acid Analysis column (Bio-Rad Laboratories, Hercules, Calif.) with an eluent of 5 mM H2SO4 and absorbance detection at 210 nm.
Fe(III) reduction was monitored by measuring the formation of Fe(II) over time with a ferrozine assay in a split-beam, dual-detector spectrophotometer (Spectronic Genosys2; Thermo Electron Corp., Mountain View, Calif.) at an absorbance of 562 nm (33, 34), after a 5-h extraction at room temperature with an anaerobic oxalate solution (197 mM ammonium oxalate and 119 mM oxalic acid or 0.5 N HCl) as previously described (33, 34).
Cell numbers were determined by counting acridine orange-stained cells by fluorescence microscopy on a Nikon Eclipse E600 microscope as previously described (8). Cells were stained by adding a 1-ml aliquot of cells to a 0.1-ml glutaraldehyde solution (final concentration, 2.5%) (Sigma Chemical Company, St. Louis, Mo.). To ensure fixation of the cells, they were vortexed and incubated at room temperature for 5 min. Once the cells were fixed, 5.9 ml of filter-sterilized oxalate solution and 1.7 mM FeCl2 were added to the mixture. Two milliliters of this solution was then mixed with ca. 0.2 ml of a filtered acridine orange solution (final concentration, 0.01%) and incubated at room temperature for 2 min. The sample was then filtered through a black Isopore membrane filter (pore diameter, 0.2 μm; Millipore) and examined under UV light.
Cytochrome detection.
For detection of cytochromes, strains A1T and A2 were grown in the culture medium described above with an electrode poised at 0.522 V (in reference to a standard hydrogen electrode) and with fumarate (10 mM) provided as the electron donor. Whole-cell suspensions were prepared by centrifugation at 16.1 × g for 5 min in an Eppendorf 5415R centrifuge (Brinkman Instruments, Westbury, N.Y.). The pellet was resuspended in 20 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) buffer (pH 7.0). Dithionite-reduced and air-oxidized spectra (350 to 700 nm) from whole-cell suspensions were analyzed on a Shimadzu (Baltimore, Md.) UV2401-PC dual-beam spectrophotometer.
Light and electron microscopy.
Cells were routinely examined by phase-contrast microscopy with a Nikon E600 microscope as previously described (19). For electron microscopy, 10 ml of cells grown on Fe(III) citrate was pelleted by centrifugation for 5 min at 5,000 rpm. The pellet was resuspended in 1 ml of phosphate-buffered saline (PBS) buffer (137 mM NaCl, 1.5 mM KCl, 5.4 mM Na2HPO4, 1.8 mM KH2PO4 [pH 7.4]) and 2% glutaraldehyde (Sigma Chemical Company) and then incubated at room temperature for 3 h. Fixed cells were then pelleted again by centrifugation at 5,000 rpm for 5 min and resuspended in 1 ml of PBS buffer.
In order to prepare Fe(III) oxide-grown cells for transmission electron microscopy, 10 ml of cells was resuspended in 10 ml of PBS buffer and 0.8 ml of glutaraldehyde solution (final concentration, 2.0%), vortexed vigorously, and allowed to sit at room temperature for 5 h. A 20-ml volume of oxalate solution (197 mM ammonium oxalate and 119 mM oxalic acid) was added, and the mixture was incubated at room temperature until poorly crystalline Fe(III) was completely solubilized (∼30 min). The cell solution was then collected on a nitrocellulose filter (pore diameter, 0.2 μm; Millipore) and immobilized in a 2% low-melting-point agarose block (FMC BioProducts, Rockland, Maine). Electron microscopy was performed in the microscopy facility at the University of Massachusetts, Amherst.
DNA extraction and PCR amplification of 16S rRNA and recA genes.
Cells (10 ml) were collected by centrifugation, and genomic DNA was extracted by using the FastDNA spin kit (BIO 101 Inc., Vista, Calif.) according to the manufacturer's instructions.
16S rRNA gene sequences for strains A1T and A2 were amplified with primers 8 forward (25, 26) and 1492 reverse (1, 3) as previously described (18). Gene fragments from recA genes in strains A1T and A2 were amplified with RECAF and RECAR as previously described (18). PCR products were agarose gel purified with the QIAquick gel extraction kit (QIAGEN, Inc., Valencia, Calif.) and ligated into the TOPO TA cloning kit, version K2 (Invitrogen, Carlsbad, Calif.), according to the manufacturers' instructions. Plasmid inserts were then amplified with M13 forward and reverse primers (Invitrogen), and PCR products were prepared for sequencing reactions with a QIAquick PCR purification kit (QIAGEN, Inc.).
Phylogenetic analysis of strains A1T and A2.
The 16S rRNA, recA, gyrB, rpoB, nifD, and fusA gene fragments from strains A1T and A2 were compared to the GenBank nucleotide and protein databases by using BLASTN and BLASTX algorithms (2). Nucleotide and amino acid sequences were manually aligned, and hypervariable regions were masked in the Genetics Computer Group (Madison, Wis.) sequence editor (Wisconsin Package, version 10). Aligned sequences were imported into PAUP 4.0b 4a (44), where phylogenetic trees were inferred. Branching order was determined and compared by using maximum parsimony, maximum likelihood, and distance-based algorithms. Bootstrap values were obtained by maximum parsimony analysis, and 1,100 nucleotide positions and 200, 265, 182, 150, and 176 amino acid positions were considered for 16S rRNA and recA, gyrB, fusA, nifD, and rpoB gene comparisons, respectively. Thermotoga maritima was the outgroup for construction of 16S rRNA and RecA phylogenetic trees.
The similarity matrix program (35) available on the Ribosomal Database Project II website and LFASTA, version 3.2 (40), were used to generate similarity matrices for which 1,100 bp were considered for the 16S rRNA gene and 200 amino acid positions from the translation product of the recA gene fragment were considered.
Nucleotide sequence accession numbers.
The 16S rRNA, recA, fusA, nifD, rpoB, and gyrB gene sequences of strain A1T have been deposited in GenBank (accession numbers AY187303, AY186902, AY188887, AY186941, AY186911, and AY186971).
RESULTS
Morphology.
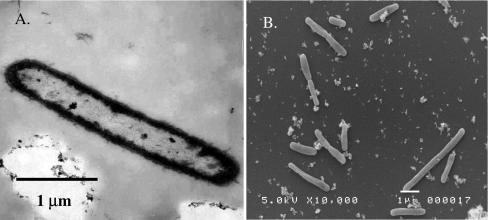
Strains A1T and A2 were isolated from the electrode surface inoculum in enrichments with acetate (5 mM) provided as the electron donor and poorly crystalline Fe(III) oxide (100 mM) as the acceptor. Strain A2 was also recovered on medium with benzoate (0.5 mM) provided as the electron donor and Fe(III) oxide (100 mM) as the acceptor. Both strains are rod shaped, curved, gram negative, and nonsporulating; dimensions are ca. 2.5 to 4 μm by 0.25 μm (Fig. 1). Cells from both strains appeared singly or in chains and formed aggregates when grown with Fe(III) as an electron acceptor. Motility was not observed.
FIG. 1.
(A) Thin-section electron micrograph of a whole cell of strain A1T grown on medium with poorly crystalline Fe(III) oxide (100 mM) provided as the electron acceptor and acetate (10 mM) as the electron donor. (B) Scanning electron micrograph of strain A2 cells grown on medium with Fe(III) citrate (50 mM) provided as the electron acceptor and malate (10 mM) as the electron donor.
Temperature optimum.
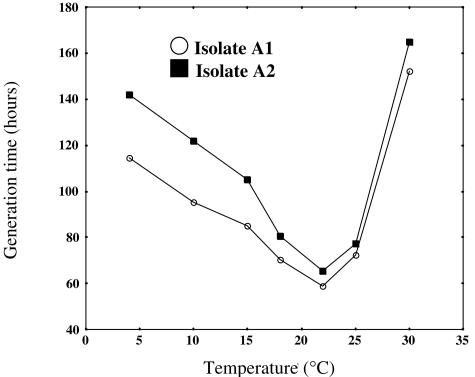
Strains A1T and A2 grew between 4 and 30°C, with an optimum temperature of 22°C, in acetate-amended medium (5 mM) with Fe(III) oxide (100 mM) provided as the electron acceptor (Fig. 2). There was no growth above 30°C, and temperatures below 4°C were not tested.
FIG. 2.
Effects of temperature on growth rates of strains A1T and A2. Cells were grown on medium with Fe(III) oxide (100 mM) provided as the electron acceptor and acetate (5 mM) as the electron donor. All data presented are averages from triplicate incubations.
Electron donors and acceptors utilized.
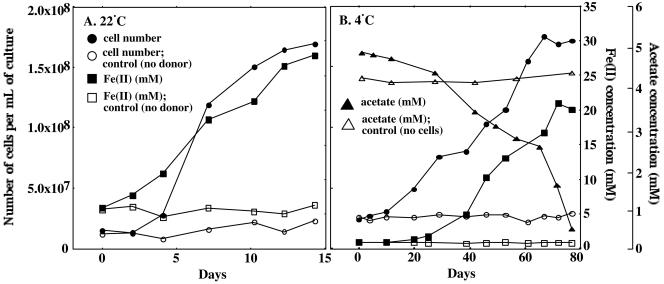
Both strain A1T (Fig. 3) and strain A2 (data not shown) were able to couple the complete oxidation of acetate to CO2 with the reduction of poorly crystalline Fe(III) oxide at temperatures as low as 4°C. The stoichiometry of acetate utilization and Fe(III) reduction indicated that poorly crystalline Fe(III) oxide served as the sole electron acceptor during growth: CH3COO− + 8 Fe3+ + 4H2O → 2HCO3− + 8Fe2+ + 9 H+.
FIG. 3.
Growth of strain A1T on medium with Fe(III) oxide (100 mM) provided as the electron acceptor and acetate (5 mM) as the electron donor. (A) Cultures were incubated at the optimum temperature (22°C). (B) Cultures were incubated at 4°C. All data presented are averages from triplicate incubations.
When acetate was provided as the electron donor, both strains were also able to conserve energy to support growth with colloidal S° (20 g/liter), Mn(IV) oxide (20 mM), Fe(III) pyrophosphate (10 mM), Fe(III) nitrotriacetic acid (NTA) (10 mM), Fe(III) citrate (50 mM), or an electrode poised at +0.52 V (in reference to a standard hydrogen electrode) as the electron acceptor. Both strains could also grow with anthraquinone 2,6,-disulfonate (AQDS; 5 mM) as the electron acceptor when malate (10 mM), but not acetate, was provided as the electron donor. Neither strain was able to utilize sulfate, thiosulfate, sulfite, or fumarate as an electron acceptor.
In addition to using acetate or malate as an electron donor, both strains also grew with several other organic acid donors such as fumarate, citrate, and succinate when Fe(III) NTA (10 mM) was provided as the electron acceptor (Table 1). Strain A1T could utilize more amino acids than strain A2 (i.e., aspartic acid, glycine, and methionine) and could grow when hydrogen (0.1 mM acetate provided as a carbon source), acetoin, or ethanol was provided as the electron donor. Strain A2 was able to couple the oxidation of butyrate to Fe(III) reduction and was the only strain able to utilize lactate or propionate during Fe(III) reduction. Both strains were able to reduce Fe(III) NTA when stearate or benzoate was provided as the electron donor.
TABLE 1.
Growth of strains A1 and A2 on various electron donors with Fe(III) NTA (10 mM) provided as the electron acceptor
| Donor (concn) | Growth of strain:
|
|
|---|---|---|
| A1 | A2 | |
| Aspartic acid (2.5 mM) | + | − |
| Glutamic acid (2.5 mM) | + | + |
| Glycine (2.5 mM) | + | − |
| Alanine (2.5 mM) | + | + |
| Methionine (2.5 mM) | + | − |
| Lysine (2.5 mM) | − | − |
| Histidine (2.5 mM) | − | − |
| Serine (2.5 mM) | − | − |
| Tyrosine (2.5 mM) | − | − |
| Acetate (5 mM) | + | + |
| Succinate (5 mM) | + | + |
| Malate (5 mM) | + | + |
| Formate (5 mM) | − | − |
| Citrate (5 mM) | + | + |
| Butyrate (5 mM) | − | + |
| Valerate (5 mM) | − | − |
| Fumarate (5 mM) | + | + |
| Pyruvate (5 mM) | + | + |
| Lactate (5 mM) | − | + |
| Propionate (5 mM) | − | + |
| Casamino Acids (0.1%) | + | + |
| Peptone (0.1%) | + | + |
| Yeast extract (0.1%) | + | + |
| Acetoin (2 mM) | + | − |
| Methanol (5 mM) | − | − |
| Ethanol (5 mM) | + | − |
| NTA (10 mM) | − | − |
| Hydrogena | + | − |
| Benzoate (0.5 mM) | + | + |
| Stearate (1 mM) | + | + |
Acetate (0.1 mM) was provided as the carbon source during growth on hydrogen.
Cytochrome content.
The dithionite-reduced minus air-oxidized difference spectra of whole-cell suspensions of strains A1T and A2 had absorbance peaks at 420 and 552 nm and a shoulder at 522 nm, indicative of c-type cytochromes. A similar spectrum was obtained with the control, Geobacter sulfurreducens (data not shown).
Electron transfer to graphite electrodes.
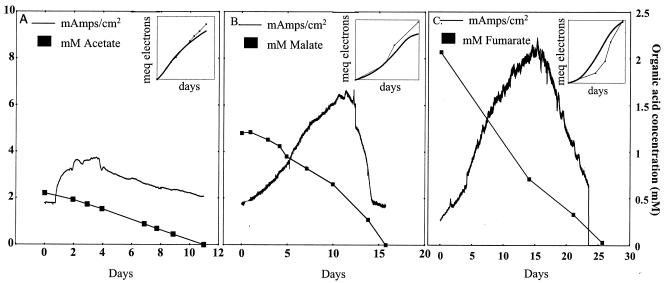
Strains A1T and A2 were able to grow when an electrode was provided as the sole electron acceptor. They both could oxidize several organic acids (acetate, malate, fumarate, and citrate) with electron transfer to an electrode poised at +0.52 V (in reference to a standard hydrogen electrode). When strain A1T was grown with acetate (0.55 mM) provided as the electron donor and a poised electrode as the electron acceptor, 90.2% of the electrons available from the complete oxidation of acetate to CO2 were transferred to the electrode to produce current (maximum current, 3.73 mA/cm2) (Fig. 4A). In addition, current was produced by strain A1T (∼8.89 mA/cm2) when fumarate (2.07 mM) was provided as the electron donor and a poised electrode was the sole electron acceptor (96.3% electron recovery) (Fig. 4C).
FIG. 4.
Current production by strains A1T and A2 when an electrode poised at +0.52 V (in reference to the H2 electrode) served as the sole electron acceptor. (A) Strain A1T; acetate (0.55 mM) was provided as the electron donor. (B) Strain A2; malate (1.2 mM) was provided as the electron donor. (C) Strain A1T, with fumarate (2.07 mM) as the electron donor. Insets compare the number of electrons from the respective organic acid that was oxidized by strain A1T or A2 to the number of electrons transferred to the anode surface over time.
Strain A2 was also able to grow in the anode chamber when organic acids were provided as the fuel. A maximum current of 6.6 mA/cm2 was produced by the complete oxidation of malate (1.2 mM) to CO2 (Fig. 4B), and 85.4% of the electrons available from malate oxidation were recovered as electricity. Significantly higher levels of current were detected when donor concentrations were increased. For example, 121.43 mA/cm2 of current was produced when strain A2 was provided with 10 mM fumarate (data not shown).
Phylogenetic analysis of strains A1T and A2.
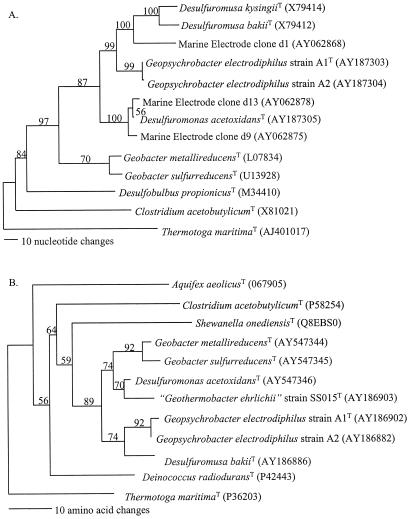
Phylogenetic analysis of the 16S rRNA and recA genes indicated that strains A1T and A2 cluster within the family Geobacteraceae in the δ subdivision of the Proteobacteria (Fig. 5). The 16S rRNA gene sequences from strains A1T and A2 were most similar to that of Malonomonas rubra (95.0% similar) (Table 2). Sequence comparisons between recA, gyrB, rpoB, fusA, and nifD genes from known Geobacteraceae indicated that the gyrB, recA, and nifD genes from strains A1T and A2 are most similar to those of Desulfuromonas palmitatis, while the rpoB and fusA genes are most similar to those of Desulfuromusa succinoxidans (Table 2). Strains A1T and A2 should be considered strains of the same species, and their phylogeny and physiological characteristics warrant the establishment of a new genus and species.
FIG. 5.
Phylogenetic trees constructed by maximum parsimony analysis comparing 16S rRNA and recA gene fragments from strains A1T and A2 to those from other bacterial species and environmental clones. Bootstrap analysis was performed with 100 replicates, and Thermotoga maritima was used as the outgroup. (A) Comparison of 16S rRNA gene sequences; (B) Comparison of RecA amino acid sequences.
TABLE 2.
Nucleotide and amino acid sequence similarities of 16S rRNA, gyrB, recA, rpoB, fusA, and nifD gene fragments from different Geobacteraceae species to the corresponding genes in strain A1T
| Gene | % Sequence similaritya to strain A1T
|
||||
|---|---|---|---|---|---|
| Geothermobacter ehrlichii | Desulfuromonas palmitatis | Malonomonas rubra | Desulfuromusa succinoxidans | Pelobacter acetylenicus | |
| 16S rRNA | 94.1 | 93.5 | 95.3 | 94.0 | 93.2 |
| gyrB | 71.6 | 74.9 | 65.5 | 72.3 | 76.9 |
| recA | 76.8 | 86.5 | 85.3 | 87.4 | 84.4 |
| rpoB | 73.1 | 80.4 | 77.7 | 88.6 | 82.4 |
| fusA | 80.1 | 74.5 | 71.9 | 84.3 | 74.3 |
| nifD | 74.0 | 93.4 | 74.2 | 75.3 | 75.5 |
Nucleotide sequences were compared for the 16S rRNA gene; amino acid sequences were compared for all other genes.
Strains A1T and A2 cluster with the predominant 16S rRNA gene sequences associated with the electrode communities previously detected by molecular techniques (Fig. 5) (17). For example, ca. 65% of the 16S rRNA sequences associated with the current-harvesting electrodes in marine sediment fuel cells (17) are 90 to 97% similar to the 16S rRNA sequences of strains A1T and A2.
DISCUSSION
Strains A1T and A2 are the first microorganisms recovered in pure culture from an energy-harvesting electrode that have 16S rRNA gene sequences similar to those of organisms detected in situ and can quantitatively transfer electrons from oxidation of organic matter to the electrode surface. These strains also expand the known physiological range of Geobacteraceae, most notably growing at temperatures much lower than those previously reported for this family. Thus, strains A1T and A2 provide important pure-culture models for studying the physiology of electron transfer to electrodes in sediment fuel cells and for the growth of Geobacteraceae at the low temperatures often found in sedimentary environments.
Growth at low temperatures.
Many aquatic sediments are perennially cold, and many other sedimentary environments have temperatures that remain at 0 to 8°C throughout the year; i.e., far northern aquifers and permafrost areas (46). Therefore, Fe(III)-reducing organisms capable of growth at such low temperatures have a distinct advantage in cold, Fe(III)-rich subsurface environments. However, only a few dissimilatory Fe(III)-reducing bacteria have previously been reported to grow at these cold temperatures. For example, Fe(III) reduction by psychrotrophic enrichment cultures has been observed at temperatures as low as 1.5°C (46). However, when pyruvate was provided as the electron donor and Fe(III) citrate served as the electron acceptor, cell numbers continued to increase after Fe(III) reduction stopped. These results suggest that metabolic processes other than Fe(III) reduction were occurring in these mixed cultures. Furthermore, no organisms from these enrichment cultures were ever identified, making inferences about their physiologies at cold temperatures difficult. Therefore, in order to truly understand Fe(III) reduction in cold environments, pure isolates must first be characterized.
Growth by several Shewanella species capable of Fe(III) citrate reduction has been observed at temperatures between <0 and 4°C (6, 28). Sulfate-reducing microorganisms capable of growth with sulfate at −1.8°C have also been reported to use Fe(III) citrate as an electron acceptor (24). However, no data on growth with Fe(III) at reduced temperatures were provided for any of these organisms, and they were not reported to use insoluble Fe(III) oxides, the primary form of Fe(III) in most sedimentary environments (31). R. ferrireducens is capable of dissimilatory Fe(III) reduction at temperatures as low as 4°C (14) and can transfer electrons to Fe(III) oxide as well as to electrodes (7). Although R. ferrireducens shows promise for the conversion of sugars and other organic compounds to electricity in contained microbial fuel cells (7), organisms closely related to R. ferrireducens do not appear to be important colonizers of electrodes in sediment fuel cells (4, 17, 45).
Until recently, microorganisms in the family Geobacteraceae were reported to grow at temperatures ranging from 25 to 40°C (31). These temperatures closely corresponded to the temperatures for enrichment and isolation of each organism. More recently, enrichment and isolation at 55°C yielded a thermophilic member of the Geobacteraceae, Geothermobacter ehrlichii, which can grow at temperatures as high as 65°C (19). Strains A1T and A2 are the first members of the Geobacteraceae found to grow at temperatures as low as 4°C. The recovery of these strains with the ability to grow at lower temperatures probably reflects the fact that the temperature for enrichment and isolation was lower than those typically used in previous attempts to recover such organisms.
Relevance to power production from sediment fuel cells.
It is important to study organisms that have been directly isolated from the anode surface in order to fully understand the mechanisms involved in microbial electron transfer to energy-harvesting electrodes. Prior to this study, only two organisms had been isolated from the anode of a microbial fuel cell: C. butyricum (39) and A. hydrophila (41) strains. Both the Clostridium and Aeromonas strains were able to grow in a microbial fuel cell and generate current; however, their energy to support growth could be attributed primarily to fermentative metabolism (39, 41). Molecular and culturing studies have shown that fermentative organisms are not enriched on the surfaces of current-harvesting electrodes (4, 17, 45), suggesting that fermentative microorganisms do not play a significant role in power production by sediment fuel cells.
The available evidence suggests that power production in sediment fuel cells can be attributed primarily to Geobacteraceae that can oxidize acetate and other organic acids to carbon dioxide with an electrode serving as the sole electron acceptor. Analysis of 16S rRNA gene sequences on the surfaces of energy-harvesting electrodes in sediments demonstrated that the electrodes were heavily enriched with Geobacteraceae in comparison to control electrodes not harvesting power (4, 17, 45). For example, approximately 65% of the 16S rRNA gene sequences associated with the active anodes from various sediment fuel cells were most similar to those of bacteria from the family Geobacteraceae that are known to completely oxidize acetate and reduce Fe(III), whereas only ca. 10% of the bacterial sequences were most similar to those of fermentative microorganisms (4, 17, 45). Furthermore, the percentages of fermentative organisms on the active anodes were similar to those found on control electrodes placed in the sediment but not harvesting current, suggesting that fermentation was not associated with energy harvesting (4, 17, 45). In contrast, quantitative PCR indicated that Geobacteraceae were 100-fold more abundant on energy-harvesting electrodes than on control electrodes not harvesting current (4, 17).
Although Geobacteraceae previously available in culture have been shown to effectively transfer electrons to electrodes (4, 5), there is considerable physiological diversity within the Geobacteraceae (31, 32). Therefore, it is preferable to conduct detailed studies on electron transfer to electrodes by the Geobacteraceae with species that have been isolated from electrode surfaces. In order to increase the chances of recovering microorganisms that were actually involved in energy production by the marine sediment fuel cell, enrichment cultures were enriched, isolated, and studied at temperatures representative of sedimentary environments, rather than at the higher temperatures at which many previously described Geobacteraceae have been isolated. Furthermore, Fe(III) oxide was used as the electron acceptor in order to provide an electron acceptor that, like a graphite electrode, was insoluble and would require reduction at the outer cell surface. Acetate, which is likely to be the primary electron donor for electricity production by sediment fuel cells (4), was provided as the electron donor.
This culturing approach appeared to yield organisms that were closely related to microorganisms associated with current-harvesting anodes. Phylogenetic comparisons indicated that the 16S rRNA gene sequences from strains A1T and A2 were 90 to 97% similar to those of the predominant organisms associated with current-harvesting electrodes in marine sediment fuel cells (17). In addition, the facts that strains A1T and A2 could convert ca. 90% of the electrons available in their electron donors to electricity and that they are capable of utilizing acetate as an electron donor for current production suggest that strains A1T and A2 provide good pure culture models for further investigations into the mechanisms of microbial electron transfer to sediment fuel cells.
Further optimization of microbe-electrode electron transfer for applications such as energy harvesting and bioremediation will require a better understanding of the mechanisms of this process. The physiological characteristics of strains A1T and A2 and the fact that they are colonizers of electrodes in sediments suggest that they are good candidates for such further studies.
Description of Geopsychrobacter, gen. nov.
Geopsychrobacter (Gr. n. ge, earth; Gr. adj. psukhros, cold; N.L. masc. n. bacter [from Gr. n. bakterion], a rod; N.L. masc. n. Geopsychrobacter, a rod from cold earth). Cells are curved rods that occur as single cells or in chains and are nonmotile. Cells do not have pili or flagella and do not form spores. Cell wall structure is typical of a gram-negative bacterium. Geopsychrobacter can grow at temperatures as low as 4°C and is a strictly anaerobic chemoorganotroph which can conserve energy to support growth by coupling the oxidation of acetate or malate to the reduction of Fe(III), S°, Mn(IV), or AQDS. Organisms in this genus are also able to directly transfer electrons to a current-harvesting electrode. Reduction of poorly crystalline Fe(III) oxide results in the formation of magnetite. Cells contain abundant c-type cytochromes. The genus Geopsychrobacter belongs to the family Geobacteraceae, in the δ subdivision of the Proteobacteria. The type strain of the genus Geopsychrobacter is Geopsychrobacter electrodiphilus strain A1T.
Habitat.
Strains A1T and A2 were isolated from the surface of an anaerobic electrode in a laboratory-incubated marine sediment fuel cell. Sediments were collected from Boston Harbor, Mass., near the World's End peninsula, at a water depth of 5 m.
Description of Geopsychrobacter electrodiphilus, sp. nov., strain A1T.
Geopsychrobacter electrodiphilus (N.L. n. electrodum, electrode; Gr. adj. philos, loving, friendly; N.L. masc. adj. electrodiphilus, electrode loving). Nonmotile, gram-negative curved rods, approximately 2.5 μm in length and 0.22 μm in diameter. G. electrodiphilus can couple the reduction of Fe(III) to the oxidation of aspartic acid, glutamic acid, glycine, alanine, methionine, acetate, succinate, malate, citrate, fumarate, pyruvate, peptone, tryptone, Casamino Acids, yeast extract, acetoin, ethanol, hydrogen, benzoate, or stearate. No growth was observed when lysine, serine, tyrosine, histidine, formate, butyrate, valerate, lactate, propionate, methanol, or nitrotriacetic acid was provided as the electron donor. This species can utilize Fe(III), AQDS, Mn(IV) oxide, and colloidal S° as electron acceptors and can transfer electrons to a current-harvesting anode. No growth was observed when sulfate, sulfite, thiosulfate, fumarate, or malate was provided as the electron acceptor, with acetate or malate as the electron donor. Growth occurs at temperatures between 4 and 30°C (optimum temperature, approximately 22°C).
This strain has been deposited in the American Type Culture Collection (ATCC BAA-880), the German Collection of Microorganisms and Cell Cultures (DSM 16401), and the Japan Collection of Microorganisms (JCM 12470).
Acknowledgments
This research was supported by the Office of Naval Research (ONR) (grant N00014-00-1-0776), the Defense Advanced Research Projects Agency (DARPA) Defense Sciences Office (DSO) (grant N66001-02-C-8044), and the Office of Science (Biological and Environmental Research), U.S. Department of Energy (cooperative agreement DE-FC02-02ER63446).
REFERENCES
- 1.Achenbach, L., and C. Woese. 1995. 16S and 23S rRNA-like primers, p. 201-203. In F. T. Robb, A. R. Place, K. R. Sowers, H. J. Schreier, S. DasSarma, and E. M. Fleischmann (ed.), Archaea: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Amann, R., B. Binder, R. Olson, S. Chisholm, R. Devereux, and D. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bond, D. R., D. E. Holmes, L. M. Tender, and D. R. Lovley. 2002. Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295:483-485. [DOI] [PubMed] [Google Scholar]
- 5.Bond, D. R., and D. R. Lovley. 2003. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 69:1548-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowman, J. P., S. A. McCammon, D. S. Nichols, J. H. Skerratt, S. M. Rea, D. S. Nichols, and T. A. McMeekin. 1997. Shewanella gelidimarina sp. nov. and Shewanella frigidimarina sp. nov., novel Antarctic species with the ability to produce eicosapentaenoic acid (20:5ω3) and grow anaerobically by dissimilatory Fe(III) reduction. Int. J. Syst. Bacteriol. 47:1040-1047. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhuri, S. K., and D. R. Lovley. 2003. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 21:1229-1232. [DOI] [PubMed] [Google Scholar]
- 8.Coates, J. D., T. B. Councell, D. J. Ellis, and D. R. Lovley. 1998. Carbohydrate-oxidation coupled to Fe(III) reduction, a novel form of anaerobic metabolism. Anaerobe 4:277-282. [DOI] [PubMed] [Google Scholar]
- 9.Coates, J. D., D. J. Lonergan, and D. R. Lovley. 1995. Desulfuromonas palmitatis sp. nov., a long-chain fatty acid oxidizing Fe(III) reducer from marine sediments. Arch. Microbiol. 164:406-413. [PubMed] [Google Scholar]
- 10.Cooney, M. J., E. Roschi, I. W. Marison, C. Comninellis, and U. von Stockar. 1996. Physiologic studies with the sulfate-reducing bacterium Desulfovibrio desulfuricans: evaluation for use in a biofuel cell. Enzyme Microb. Technol. 18:358-365. [DOI] [PubMed] [Google Scholar]
- 11.Emde, R., and B. Schink 1990. Enhanced propionate formation by Propionibacterium freudenreichii subsp. freudenreichii in a three-electrode amperometric culture system. Appl. Environ. Microbiol. 56:2771-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emde, R., and B. Schink. 1990. Oxidation of glycerol, lactate, and propionate by Propionibacterium freudenreichii in a poised-potential amperometric culture system. Arch. Microbiol. 153:506-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emde, R., A. Swain, and B. Schink. 1989. Anaerobic oxidation of glycerol by Escherichia coli in an amperometric poised-potential culture system. Appl. Microbiol. Biotechnol. 32:170-175. [Google Scholar]
- 14.Finneran, K., C. V. Johnsen, and D. R. Lovley. 2003. Rhodoferax ferrireducens sp. nov., a psychrotolerant, facultatively anaerobic bacterium that oxidizes acetate with the reduction of Fe(III). Int. J. Syst. Evol. Microbiol. 53:669-673. [DOI] [PubMed] [Google Scholar]
- 15.Habermann, W., and E. H. Pommer. 1990. Biological fuel cells with sulphide storage capacity. Appl. Microbiol. Biotechnol. 35:128-133. [Google Scholar]
- 16.Holmes, D. E., D. R. Bond, and D. R. Lovley. 2004. Electron transfer by Desulfobulbus propionicus to Fe(III) and graphite electrodes. Appl. Environ. Microbiol. 70:1234-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes, D. E., D. R. Bond, R. A. O'Neil, C. E. Reimers, and D. R. Lovley. Microbial communities associated with electrodes harvesting electricity from a variety of aquatic sediments. Microb. Ecol., in press. [DOI] [PubMed]
- 18.Holmes, D. E., K. P. Nevin, and D. R. Lovley. 2004. Comparison of 16S rRNA, nifD, recA, rpoB, and fusA genes within the family Geobacteraceae. Int. J. Syst. Evol. Microbiol. 54:1591-1599. [DOI] [PubMed] [Google Scholar]
- 19.Kashefi, K., D. E. Holmes, J. A. Baross, and D. R. Lovley. 2003. Thermophily in the Geobacteraceae: Geothermobacter ehrlichii gen. nov., sp. nov., a novel thermophilic member of the Geobacteraceae from the “Bag City” hydrothermal vent. Appl. Environ. Microbiol. 69:2985-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, B. H., H. J. Kim, M. S. Hyun, and D. S. Park. 1999. Direct electrode reaction of Fe(III) reducing bacterium, Shewenella putrefaciens. J. Microbiol. Biotechnol. 9:127-131. [Google Scholar]
- 21.Kim, H. J., M. S. Hyun, I. S. Chang, and B. H. Kim. 1999. A microbial fuel cell type lactate biosensor using a metal-reducing bacterium, Shewanella putrefaciens. J. Microbiol. Biotechnol. 9:365-367. [Google Scholar]
- 22.Kim, H. J., H. S. Park, M. S. Hyun, I. S. Chang, M. Kim, and B. H. Kim. 2002. A mediator-less microbial fuel cell using a metal reducing bacterium, Shewenella putrefaciens. Enzyme Microb. Technol. 30:145-152. [Google Scholar]
- 23.Kim, N., Y. Choi, S. Jung, and S. Kim. 2000. Effect of initial carbon sources on the performance of microbial fuel cells containing Proteus vulgaris. Biotechnol. Bioeng. 70:109-114. [DOI] [PubMed] [Google Scholar]
- 24.Knoblauch, C., K. Sahm, and B. B. Jorgensen. 1999. Psychrophilic sulfate-reducing bacteria isolated from permanently cold Arctic marine sediments: description of Desulfofrigus oceanense gen. nov., sp. nov., Desulfofrigus fragile sp. nov., Desulfofaba gelida gen. nov., sp. nov., Desulfotalea psychrophila gen. nov., sp. nov. and Desulfotalea arctica sp. nov. Int. J. Syst. Bacteriol. 49:1631-1643. [DOI] [PubMed] [Google Scholar]
- 25.Lane, D. L. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. Wiley, Chichester, England.
- 26.Lane, D. L., B. Pace, G. J. Olsen, D. Stahl, M. L. Sogin, and N. R. Pace. 1985. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analysis. Proc. Natl. Acad. Sci. USA 82:6955-6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, J., N. Phung, I. Chang, B. Kim, and H. Sung. 2003. Use of acetate for enrichment of electrochemically active microorganisms and their 16S rDNA analyses. FEMS Microbiol. Lett. 223:185-191. [DOI] [PubMed] [Google Scholar]
- 28.Leonardo, M. R., D. P. Moser, E. Barbieri, C. A. Brantner, B. J. MacGregor, B. J. Paster, E. Stackebrandt, and K. H. Nealson. 1999. Shewanella pealeana sp. nov., a member of the microbial community associated with the accessory nidamental gland of the squid Loligo pealei. Int. J. Syst. Bacteriol. 49:1341-1351. [DOI] [PubMed] [Google Scholar]
- 29.Lovley, D., J. Fraga, J. Coates, and E. Blunt-Harris. 1999. Humics as an electron donor for anaerobic respiration. Environ. Microbiol. 1:89-98. [DOI] [PubMed] [Google Scholar]
- 30.Lovley, D., and E. Phillips. 1986. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 51:683-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lovley, D. R. 2000. Fe(III)- and Mn(IV)-reducing prokaryotes. In M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackebrandt (ed.), The Prokaryotes. Springer-Verlag, Inc., New York, N.Y.
- 32.Lovley, D. R., D. E. Holmes, and K. P. Nevin. 2004. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 49:219-286. [DOI] [PubMed] [Google Scholar]
- 33.Lovley, D. R., and E. J. P. Phillips. 1988. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54:1472-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lovley, D. R., and E. J. P. Phillips. 1987. Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl. Environ. Microbiol. 53:1536-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maidak, B. L., J. R. Cole, T. G. Lilburn, C. T. Parker, P. R. Saxman, R. J. Farris, G. M. Garrity, G. J. Olsen, T. M. Schmidt, and J. M. Tiedje. 2001. The RDP-II (Ribosomal Database Project). Nucleic Acids Res. 29:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park, D. H., B. H. Kim, B. Moore, H. A. O. Hill, M. K. Song, and S. K. Rhee. 1997. Electrode reaction of Desulfovibrio desulfuricans modified with organic conductive compounds. Biotechnol. Tech. 11:145-148. [Google Scholar]
- 37.Park, D. H., M. Laivenieks, M. V. Guettler, M. K. Jain, and J. G. Zeikus. 1999. Microbial utilization of electrically reduced neutral red as the sole electron donor for growth and metabolite production. Appl. Environ. Microbiol. 65:2912-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park, D. H., and J. G. Zeikus. 2000. Electricity generation in microbial fuel cells using neutral red as an electronophore. Appl. Environ. Microbiol. 66:1292-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park, H. S., B. H. Kim, H. S. Kim, H. J. Kim, G. T. Kim, M. Kim, I. S. Chang, Y. K. Park, and H. I. Chang. 2001. A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Clostridium butyricum isolated from a microbial fuel cell. Anaerobe 7:297-306. [Google Scholar]
- 40.Pearson, W. R. 1990. Rapid and sensitive sequence comparisons with FASTP and FASTA. Methods Enzymol. 183:63-98. [DOI] [PubMed] [Google Scholar]
- 41.Pham, C. A., S. J. Jung, N. T. Phung, J. Lee, I. S. Chang, B. H. Kim, H. Yi, and J. Chun. 2003. A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Aeromonas hydrophila, isolated from a microbial fuel cell. FEMS Microbiol. Ecol. 223:129-134. [DOI] [PubMed] [Google Scholar]
- 42.Reimers, C. E., L. M. Tender, S. Fertig, and W. Wang. 2001. Harvesting energy from the marine sediment-water interface. Environ. Sci. Technol. 35:192-195. [DOI] [PubMed] [Google Scholar]
- 43.Roller, S. D., H. P. Bennetto, G. M. Delaney, J. R. Mason, J. L. Stirling, and C. F. Thurston. 1984. Electron-transfer coupling in microbial fuel cells. 1. Comparison of redox-mediator reduction rates and respiratory rates of bacteria. J. Chem. Tech. Biotechnol. 34B:3-12. [Google Scholar]
- 44.Swofford, D. L. 1998. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland, Mass.
- 45.Tender, L. M., C. E. Reimers, H. A. Stecher, D. E. Holmes, D. R. Bond, D. A. Lowy, K. Pilobello, S. J. Fertig, and D. R. Lovley. 2002. Harnessing microbially generated power on the seafloor. Nat. Biotechnol. 20:821-825. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, C., R. D. Stapleton, J. Zhou, A. V. Palumbo, and T. J. Phelps. 1999. Iron reduction by psychrotropic enrichment cultures. FEMS Microbiol. Ecol. 30:367-371. [DOI] [PubMed] [Google Scholar]