Abstract
Dyes are recalcitrant compounds that resist conventional biological treatments. The degradation of three textile dyes (Indigo, RBBR and Sulphur Black), and the dye-containing liquid effluent and solid waste from the Municipal Treatment Station, Americana, São Paulo, Brazil, by the cyanobacteria Anabaena flos-aquae UTCC64, Phormidium autumnale UTEX1580 and Synechococcus sp. PCC7942 was evaluated. The dye degradation efficiency of the cyanobacteria was compared with anaerobic and anaerobic–aerobic systems in terms of discolouration and toxicity evaluations. The discoloration was evaluated by absorption spectroscopy. Toxicity was measured using the organisms Hydra attenuata, the alga Selenastrum capricornutum and lettuce seeds. The three cyanobacteria showed the potential to remediate textile effluent by removing the colour and reducing the toxicity. However, the growth of cyanobacteria on sludge was slow and discoloration was not efficient. The cyanobacteria P. autumnale UTEX1580 was the only strain that completely degraded the indigo dye. An evaluation of the mutagenicity potential was performed by use of the micronucleus assay using Allium sp. No mutagenicity was observed after the treatment. Two metabolites were produced during the degradation, anthranilic acid and isatin, but toxicity did not increase after the treatment. The cyanobacteria showed the ability to degrade the dyes present in a textile effluent; therefore, they can be used in a tertiary treatment of effluents with recalcitrant compounds.
Keywords: Dyes, Toxicity, Discoloration, Cyanobacteria, Degradation
Introduction
Synthetic dyes are recalcitrant molecules that constitute the main residue found in the effluent of the textile dyeing industry. Acute toxicity tests showed that most textile dyes are not particularly toxic.1 Nevertheless, their persistence and resulting long exposure time is of particular concern for the discharge of waste dye effluent, since these substances may exhibit chronic effects such as mutagenic damage and carcinogenicity towards biota.2, 3 Several authors reported that dye discharges from textile processing plants were the major contributors to the mutagenic activity found in Brazilian rivers.4, 5
In some textile dyeing operations, as much as 15% of the dyes used do not attach to the fibres, so they are lost to wastewater,6 and the resulting coloured effluents can represent a serious water pollution problem due to their colour content and toxic components. The usual effluent treatment involves biological systems like activated sludge; however, conventional treatment has not efficiently removed the effluent dye due to the recalcitrant nature of the dyes and the diverse composition of the effluent.7 A tertiary treatment of effluents is recommended for the removal of nutrients and recalcitrant substances, which is done mainly by several algae species. Studies have shown the ability of some algae to degrade dyes,8, 9, 10, 11 making tertiary treatment a viable possibility for the efficient degradation of these compounds. Vyayakuma and Manoharau12 studied the degradation by indigenous cyanobacterium species of a textile effluent containing the dyes remazol and venyl sulfone and observed not only colour removal, but also reduction in the levels of the inorganic compounds such as nitrites, phosphates, ammonia, calcium and magnesium. The main cyanobacterium strains reported to be responsible for the removal of nutrients and chemicals from the industrial effluents were Westiellopsis sp., Lynghya sp., Oscillatoria sp. and Chlorella sp.12, 13, 14, 15
The environmental problem caused by hazardous dyes is particularly important in the Municipal Treatment Station of Americana, SP, Brazil, which is responsible for the treatment of effluents from 43 textile plants as well as city sewage. Periodic releases of liquid effluent from this treatment station were carried to the Piracicaba River, a domestic water source for cities downstream. The biological treatment employed is efficient for BOD removal, but the colour persists after the treatment, making this treatment insufficient to remove the contaminants from the effluent. In the present study, three strains of cyanobacteria, Anabaena flos-aquae UTCC64, Phormidium autumnale UTEX1580 and Synechococcus sp. PCC7942 were evaluated for their ability to degrade three different dyes: RBBR (Remazol Brilliant Blue R), indigo and sulphur black, and also the dye-containing liquid effluent and solid waste from the Municipal Treatment Station of Americana (SP, Brazil) containing those dyes.
Materials and methods
Cyanobacterial strains
The cyanobacteria were filamentous heterocystous A. flos-aquae UTCC64, the filamentous non-heterocystous P. autumnale UTEX1580, and unicellular Synechococcus sp. PCC7942, all from the Culture Collection of the Centro de Energia Nuclear na Agricultura – CENA/USP. These strains were chosen based on a study by Fiore and Trevors16 on the bioremediation of metals by the cyanobacterium, in which the three selected strains had the highest detoxification capability. The organisms were maintained in flasks containing 50 mL of AA medium17 for A. flos-aquae UTCC64, or BG-11 medium18 for P. autumnale UTEX1580 and Synechococcus PCC7942; 1 mL from these seven day cultures was used to inoculate the different treatments.
Textile waste sampling
Liquid effluent was sampled (3 L) after biological treatment (biological filters) and the sludge also was sampled (3 kg) after aerobic-anaerobic treatment in the Municipal Treatment Station of Americana, SP, Brazil. Samples were kept at 4 °C until use. Both of these residues still contained a high amount of organics after the treatment, and remained strongly-coloured.
Dye discoloration by cyanobacteria
The synthetic dyes RBBR [(Remazol Brilliant Blue R) purchased from Sigma (Sigma–Aldrich, St. Louis, MO, USA)], indigo and sulphur black (Bayer, São Paulo, SP, Brazil), were added at a concentration of 0.02% (m/v) to Erlenmeyer flasks containing 50 mL of AA or BG-11 culture media. Each flask was inoculated with 1 mL of the cyanobacterium culture medium, in triplicate. The effluent and the solid waste, which were diluted to a concentration of 10% in an AA or a BG-11 media, were treated in the same way as the dyes. All of the treated flasks were incubated for 14 days at 25 °C with constant fluorescent illumination (4000 ± 10% lux). Control experiments were conducted in light and dark conditions in the absence of microorganisms to evaluate the effect of photodegradation. The discoloration was evaluated by absorption spectroscopy. The absorption maximum for each dye was 680 nm (indigo), 595 nm (RBBR) and 454 nm (sulphur black). The samples were centrifuged at 10,000 × g for five min and the colour reduction was based on a standard curve comparing dye concentration in the treatments in relation to non-inoculated control flasks.
An anaerobic–aerobic degradation system was also evaluated for dye discoloration in the liquid wastewater. The anaerobic conditions were obtained by completely filling the triplicate 150 mL penicillin bottles with the effluent, and sealing them with rubber tops in a CO2 atmosphere. The bottles were kept for 15 days at 28 °C in the dark. Then, the bottles were opened and 50 mL of effluent was transferred to sterilized Erlenmeyer flasks and re-enriched with 1 mL of inoculum obtained from a fresh effluent. This inoculum was prepared through centrifuging (2000 × g) 100 mL of fresh effluent and resuspending the pellets in salt solution (0.85% NaCl). The flasks were incubated aerobically for 15 days at 28 °C.
Toxicity evaluation of the treated effluent and sludge
The toxicity of the effluent and sludge was evaluated using the bioindicator organisms Hydra attenuata, the green algae Selenastrum capricornutum and root growth of Lactuca sativa seedlings. These organisms are maintained at CENA/USP and bioassays were conducted as described by Trottier et al.,19 Blaise et al.,20 and Dutka,21 respectively. The 50% inhibitory concentrations (IC50) were determined for each sample using the program EcoTox-Statistics Version 1.1.
Biodegradation of the indigo dye by Phormidium in the bioreactor
The biodegradation of indigo dye by P. autumnale UTEX1580, the strain that showed the highest percentage of indigo discoloration in the previous tests, was further studied in a 15 cm wide, 60 cm high, 6 L bioreactor. In preparation for the inoculation, P. autunmnale was grown previously in 500 mL of a BG-11 medium for 14 days. The bioreactor received constant aeration and illumination by four vertically placed lamps. The indigo dye was added to a concentration of 0.02% in the BG-11 culture medium. The control was kept in the dark at 4 °C. Samples were collected on days 2, 4, 7, 14, 15, 16, 17, 18 and 19 for absorption spectrophotometry analyses (583 and 680 nm) and toxicity bioassays with Hydra attenuatta. Biodegradation was monitored by mass spectrometry analysis using a hybrid quadrupole time-of-flight (Q-TOF) high resolution (7000) and high accuracy (5 ppm) Q-TOF mass spectrometer (Micromass, Manchester, UK) equipped with an electrospray ion source (ESI). The conditions for the positive ESI were: nitrogen gas desolvation at 180 °C, capillary held at a potential of 3.5 kV and a cone voltage 25 kV. Growth cultures were centrifuged at 10,000 × g for 10 min and the supernatant was mixed with 0.5 mL of methanol: formic acid 1% (50:50, v/v) and introduced into the ion source at 5 μL min−1 with a syringe pump. During the final incubation period (19 days), the cyanobacteria-incubated medium was assessed for its mutagenicity potential using the Allium sp. micronucleus assay as described by Fernandes et al.22 and toxicity by the Hydra bioassay.
Results
Dye discoloration by cyanobacteria
The Cyanobacterium Phormidium was able to decolorize the indigo dye extensively (91%), but was not able to decolorize the sulphur black and RBBR dyes. Low discoloration was produced by Synechococcus for all three dyes and Anabaena was able to degrade the indigo, but the discoloration percentage was lower than for Phormidium (Table 1). The sulphur black was decolorized by Anabaena, which produced only a partial degradation. The three strains tested were able to remove the effluent colour, but for Synechococcus a decrease in absorption was not detected because the absorption measures were affected by the green colour of the cultures (Table 1). Phormidium caused more colour removal than Anabaena and, for Synechococcus, visual discoloration seemed higher than the methodology was able to detect.
Table 1.
Percentage of effluent and sludge discoloration by cyanobacteria after 14 days incubation at 25 °C.
| Treatment | Indigo | RBBR | Black sulphur | Effluent | Sludge |
|---|---|---|---|---|---|
| Dark control | 0 ± 0.001 | 0 ± 0.001 | 0 ± 0.001 | 0 ± 0.001 | 0 ± 0.001 |
| Light control | 56.14 ± 0.057 | 4.0 ± 0.001 | 29.41 ± 0.002 | 12.90 ± 0.006 | 39.28 ± 0.006 |
| Anabaena sp. | 71.92 ± 0.057 | 0 ± 0.001 | 42.12 ± 0.006 | 23.87 ± 0 | 8.33 ± 0.001 |
| Phormidium sp. | 91.22 ± 0.057 | 10.62 ± 0.011 | 0 ± 0.001 | 28.38 ± 0.001 | 0 ± 0.002 |
| Synechococcus sp. | 0 ± 0.002 | 11.53 ± 0.009 | 0 ± 0.002 | 1.29 ± 0 | 0 ± 0.002 |
± Standard deviation.
For the anaerobic–aerobic system, 17% and 33% discoloration was obtained after anaerobic treatment and the combined anaerobic–aerobic one, respectively. The anaerobic degradation resulted in colour removal, but produced an effluent with high turbidity, which was removed by aerobic degradation. These compounds require the presence of a combination of anaerobic and aerobic conditions for complete degradation, since they are very complex and recalcitrant due to their xenobiotic character.
Toxicity tests on the effluent
The cyanobacterium treatment was able to reduce the effluent toxicity and showed degradation of the compounds present in the effluent. There was growth inhibition of the organism Selenastrum in the light and dark controls; however, there was no growth inhibition in the effluent treated by the cyanobacterium strains.
In the lettuce seeds test, toxicity was reduced after the treatment too, as demonstrated by a lack of root growth inhibition after the cyanobacterium treatment. The IC50 determination (concentration that inhibited 50% of the organism growth) was possible only for the dark control (IC50 = 95%), whereas other treatments showed less than 50% inhibition at the maximal concentration (Table 2).
Table 2.
Percentage concentration corresponding to NOEC and LOEC for Hydra and IC50 for the lettuce seeds tests conducted with the effluent and sludge that had been incubated with cyanobacteria for 14 days at 25 °C.
| Treatments |
Hydra test |
Seeds test | ||||
|---|---|---|---|---|---|---|
| NOECa |
LOECb |
IC50c |
||||
| Effluent | Sludge | Effluent | Sludge | Effluent | Sludge | |
| Dark control | 6.25 | 6.25 | 12.5 | 3.12 | 94.98 | 17.28 |
| Light control | 12.5 | 6.25 | 25 | 3.12 | 0 | 51.52 |
| Anabaena sp. | 25 | 6.25 | 50 | 3.12 | 0 | 55.37 |
| Phormidium sp. | 50 | 12.5 | n.e.d | 6.25 | 0 | 6.23 |
| Synechococcus sp. | 25 | 12.5 | 50 | 6.25 | 0 | 23.89 |
NOEC, no observed effect concentration.
LOEC, low observed effect concentration.
IC50, concentration that caused 50% inhibition of the test organisms.
n.e., no effect.
In the Hydra test, a higher LOEC and NOEC were observed after treatment with the three strains. Since LOEC is defined as the low observed effect concentration and the NOEC is defined as the no observed effect concentration, the cyanobacterium treatment results in a toxicity reduction for the organisms (Table 2).
In the sludge, the Phormidium treatment caused an increase in the toxicity (Table 2), whereas the Anabaena presented the highest toxicity reduction in the lettuce seeds test. The slight toxicity reduction shown in the sludge by Phormidium and Synechococcus in the Hydra test (Table 2) means that although the algal growth was slow, some degradation did occur. The sludge detoxification was lower than that of the effluent, demonstrating that this residue is more resistant to the degradation and that the cyanobacteria strain with more efficiency in sludge detoxification is different than the most effective one for the effluent, since Anabaena showed more sludge detoxification than Phormidium.
There was more toxicity in the dark control than in the light one suggesting that photodegradation contributed to the detoxification process in such conditions.
Toxicity tests for the anaerobic–aerobic system
For the Hydra test, there was no reduction in toxicity after anaerobic treatment in comparison with the control. However, a complete detoxification occurred after anaerobic–aerobic treatment, showing the toxic compounds were removed only after anaerobic–aerobic treatment.
The algal test demonstrated the same results; no toxicity reduction after anaerobic treatment and complete detoxification after the anaerobic–aerobic one (Table 3). No toxicity was observed in the lettuce seeds test for any of the treatments, not even for the effluent control.
Table 3.
Percentage concentration corresponding to NOEC and LOEC for the Hydra toxicity test and the IC50 for the Algal test after anaerobic and anaerobic–aerobic treatment.
| Treatment |
Hydra test |
Algal test | |
|---|---|---|---|
| NOECa | LOECb | EC50c | |
| Control | 12.5 | 25 | 73.35 |
| Anaerobic | 12.5 | 25 | 70.39 |
| Anaerobic–aerobic | 50 | 100 | 0 |
NOEC, no observed effect concentration.
LOEC, low observed effect concentration.
IC50, concentration that caused 50% effect on the test organisms.
Biodegradation of the indigo dye in a bioreactor
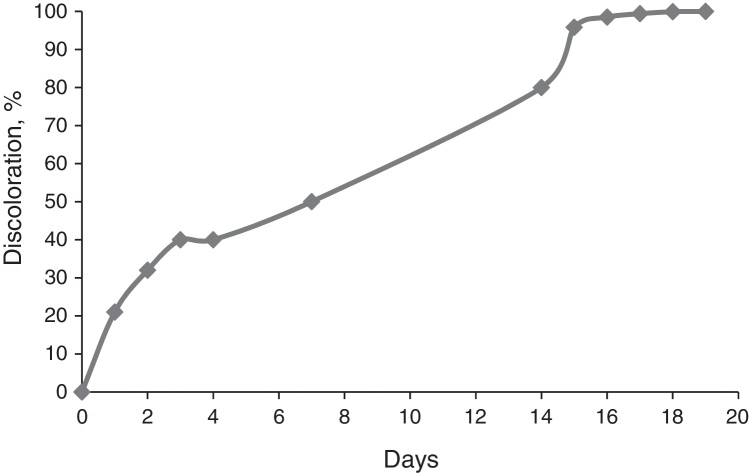
The indigo dye biodegradation by Phormidium was demonstrated by complete discoloration after 19 days of incubation. Colour removal was enhanced after 14 days, showing that a period of acclimatization was necessary. After 19 days there was no more colour left. The cultured cells adhered to the bioreactor wall, leaving a biofilm formation that adsorbed some of the indigo dye. Dye discoloration is shown in Fig. 1.
Fig. 1.
Percentage of indigo discoloration by Phormidium in the bioreactor. Standard deviation: 0.05.
Metabolite analysis
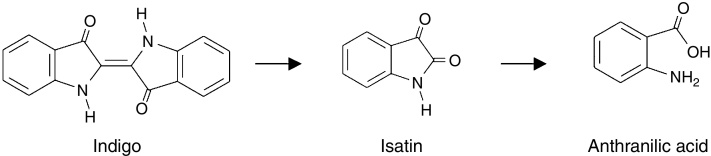
The parental indigo molecule was detected only until the 18th day, showing that the dye was completely degraded by Phormidium and that the degradation occurred over the same period that a complete discoloration was observed in the bioreactor. After the 17th day of incubation, two metabolites appeared and were identified as anthranilic acid (m/z 399) and isatin or indole-2,3-dione (m/z 421); therefore, the degradation of the indigo molecule was not complete. In that study, both of the metabolites were detected until the 19th day, which means they were not fully degraded during the assessment period.
In accord with Campos et al.,23 the first step of indigo degradation is oxidative cleavage of the central bond, which produces isatin as a metabolite. Isatin might undergo hydrolysis and decarboxylation to produce anthranilic acid (Fig. 2).
Fig. 2.
Degradation pathway of the indigo dye by Phormidium.
The Hydra bioassay
No toxic effects were detected in the Hydra bioassay from the control indigo dye, or after the dye treatment by Phormidium. Analyses performed during the incubation period showed that no toxic products were formed during the period studied. The presence of the metabolites isatin and anthranilic acid did not result in an increase in toxicity, suggesting that the Phormidium treatment of indigo is a plausible treatment.
The micronucleus assay
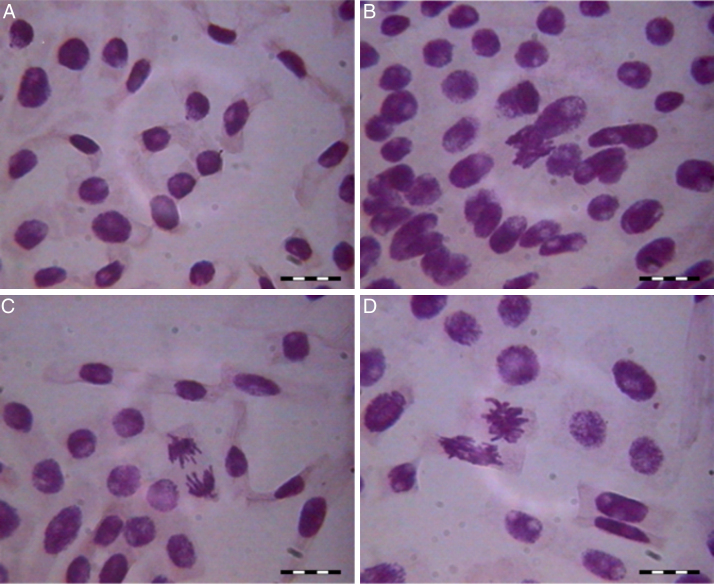
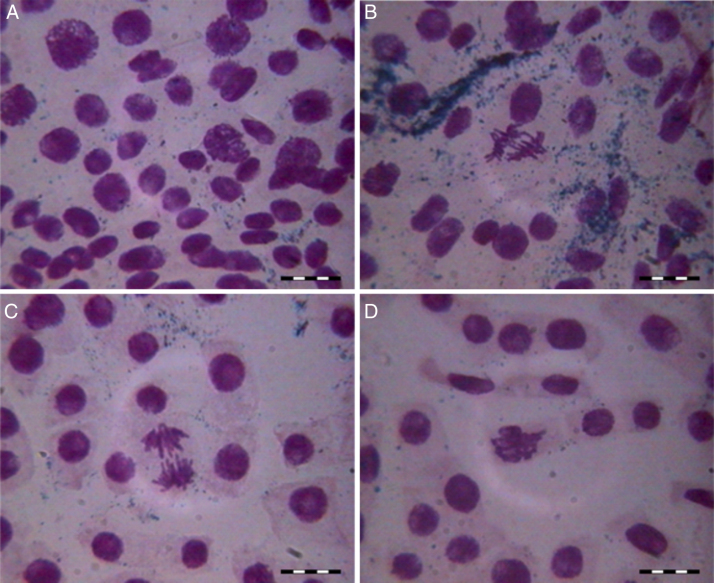
No mutagenicity effect was observed in the micronucleus assay for the indigo control or following the cyanobacterium treatment; the rate of micronucleus formation was the same for both of the treatments and for the water control. Micronuclei can spontaneously form due to an unequal distribution of genetic material during the cell cycle.24 However, there was an increase in some chromosomal aberrations, such as adherences, bridges, polyploidy cells and late chromosomes in the indigo control and following cyanobacterium treatment compared with the mineral water control (Fig. 3, Fig. 4).
Fig. 3.
Mutagenicity signs in the indigo control: (A) micronucleous; (B) adherence; (C) late chromosomes; (D) bridge.
Fig. 4.
Mutagenicity signs following the indigo dye Phormidium treatment: (A) polyploidy; (B) bridge; (C) late chromosomes; (D) adherence.
These abnormalities constitute signs of mutagenicity that indicate an initial stage of DNA damage in the presence of the dye, but that did not result in mutagenicity at the tested dye concentration as demonstrated by no increase in micronucleus formation.
The frequency of cell division for the indigo and cyanobacterium treated medium was higher than for the mineral water, representing an increase in root growth probably because of the high amount of nitrogen and phosphorus present in the BG-11 medium.
Discussion
Cyanobacteria-induced discoloration and detoxification of the effluent was observed for each of the three cyanobacteria in this study. These results showed that cyanobacteria were able to reduce the pollutant load of the effluent. The degradation of dyes by cyanobacteria is species-dependent, since P. autumnale UTEX 1580 more efficiently degraded the indigo dye and A. flos-aquae UTCC64 was superior in the case of the sulphur black. A. flos-aquae UTCC64 was the most efficient strain for sludge decolouration and detoxification. However decolouration of the sludge was not significant, probably because this residue contained more insoluble hydrophobic compounds than did the effluent. A combination of more than one method might improve the degradability of this dye-containing waste, since photodegradation was detected in the dye control.
It seems that filamentous species as P. autumnale UTEX 1580 can break down the dye more efficiently, since the dye penetrates the filaments, which increases the contact between the microorganism and the compound. Although no experiments were performed specifically to investigate the biosynthetic mechanisms of indigo degradation using cyanobacteria, it seems main mechanism must be enzyme-mediated, since in the control no metabolites were formed.
Phormidium sp. was able to degrade several dyes of different chemical classes. In a study by Shah et al.,9 the cyanobacterium Phormidium valderianum discoloured up to 90% of Acid Red, Acid Red 119 and Direct Black 155 dyes. When the pH was strongly alkaline, an increase in discoloration occurred, which is a very common condition in textile effluents due to the addition of NaOH during the dyeing process. Another strain, P. ceylanicum, also was found to degrade Acid Red 97 and FF Sky Blue dyes by up to 80% after 26 days.10 Silva-Stenico et al.25 observed greater than 50% degradation of the indigo dye and six other structurally different dyes by the Phormidium sp. and two another cyanobacterium strains, Synechococcus sp. and Leptolyrigbya sp. Dye decolourization by Anabaena was studied by Queiroz and Stefanelli26 for Blue Drin dye, and they showed 81% colour removal.
The structure of the compound also appears to be a very important factor. In spite of the fact that the three dyes are very recalcitrant, the dyes sulphur black and Remazol Brilliant Blue have very uncommon molecular structures, which confer a high xenobiotic character and greater resistance to degradation.27, 28 In a study by Jinqi and Houtian,8 dye discoloration was correlated with the molecule structure and the recalcitrance was increased for dyes containing radicals such as nitro and sulphur groups, as is the case for sulphur black and Remazol Brilliant Blue.
Degradation of indigo dye by P. autumnale UTEX 1580 resulted in the formation of two metabolites, isatin and anthranilic acid, which arise after the disappearance of indigo from culture media showing that the degradation was incomplete. Toxicity did not increase with the formation of these metabolites, making the treatment of indigo by P. autumnale UTEX 1580 a plausible treatment. The mutagenicity test with Allium cepa also did not demonstrate a mutagenic effect after the indigo degradation.
These metabolites were not degraded during the period of assessment by P. autumnale UTEX 1580 in a pure culture. However, these metabolites are less complex than the parental indigo molecule and its degradation in the environment by other microorganisms may occur more readily. In a tertiary treatment, the presence of mixed algae populations might cause the removal of these metabolites in a possible sequential degradation process by a consortium of algae strains for complete degradation of the indigo dye.
The three cyanobacteria strains evaluated, showed potential to remediate textile effluent and reduce the pollutant load. However, the cyanobacteria treatment of sludge was less efficient than for the effluent, probably due to the high complexicity of the sludge. The cyanobacterium P. autumnale UTEX 1580 completely degraded the indigo dye into secondary metabolites, which were not toxic. Further studies using a mixed culture of algae, instead of a pure culture, should be performed to achieve complete degradation. Algae can be used for removal of toxic dyes in a tertiary treatment using facultative lagoons, mainly to improve the effluent quality when these recalcitrant compounds are not removed in the secondary treatment.
Conflicts of interest
The authors declare no conflicts of interest.
Associate Editor: Valeria Maia de Oliveira
References
- 1.Cripps C., Bumpus J.A., Aust S.D. Biodegradation of azo and heterocyclic dyes by Phanerochaete chrysosporium. Appl Environ Microbiol. 1990;56:1114–1118. doi: 10.1128/aem.56.4.1114-1118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pearce C.J., Lloyd J.R., Guthrie J.T. The removal of color from textile waste-water using whole bacterial cells. A review. Dyes Pigments. 2003;58:179–186. [Google Scholar]
- 3.Bae J.S., Freeman H.S. Aquatic toxicity evaluation of new direct dyes to the Daphnia magna. Dyes Pigments. 2007;73:81–85. [Google Scholar]
- 4.Coelho M.C.L.S., Coimbrão C.A., Valent G.U., Sato M.I.Z., Targa H. Mutagenicity evaluation of industrial effluent by Ames assay. Environ Mol Mutagen. 1992;19(S20):199–211. [Google Scholar]
- 5.Umbuzeiro G.A., Freeman H.S., Warren S.H., Kummrow F., Claxton L.D. The contribution of azodyes to the mutagenic activity of the Cristais River. Chemosphere. 2005;60:55–64. doi: 10.1016/j.chemosphere.2004.11.100. [DOI] [PubMed] [Google Scholar]
- 6.O’Neill C., Hawkes F.R., Hawkes D.L., Lourenço N.D., Pinheiro H.M., Delle W. Colour in textile effluents-sources, measurement, discharge contents and stimulation: a review. J Chem Technol Biotechnol. 1999;74:1009–1018. [Google Scholar]
- 7.Ralanapongleko K., Phetsom J. Decolorization by conventional treatment is inefficient. Int J Chem Eng Appl. 2014;5:26–30. [Google Scholar]
- 8.Jinqi L., Houtian L. Degradation of azo dyes by algae. Environ Pollut. 1992;75:273–278. doi: 10.1016/0269-7491(92)90127-v. [DOI] [PubMed] [Google Scholar]
- 9.Shah V., Garg N., Madamwar D. An integrated process of textile dye removal and hydrogen evolution using cyanobacterium, Phormidium valderianum. World J Microbiol Biotechnol. 2001;17:499–504. [Google Scholar]
- 10.Parikh A., Madamwar D. Textile dye decolorization using cyanobacteria. Biotechnol Lett. 2005;27:323–336. doi: 10.1007/s10529-005-0691-7. [DOI] [PubMed] [Google Scholar]
- 11.Omar H.H. Algal decolorization and degradation of monoazo and diazo dyes. Pak J Biol Sci. 2008;11:1310–1316. doi: 10.3923/pjbs.2008.1310.1316. [DOI] [PubMed] [Google Scholar]
- 12.Vyayakumar S., Manoharau C. Treatment of dye industry effluent using free and immobilized cyanobacteria. J Biorem Biodeg. 2012;3:1–6. [Google Scholar]
- 13.Henciya S., Shankar M., Maliga P. Decolorization of textile dye effluent by marine cyanobacterium Linghya sp. BDU 9001 with coir pith. Int J Environ Sci. 2013;3:1909–1918. [Google Scholar]
- 14.Murali S., Henciya S., Maliga P. Bioremediation of tannery effluent using fresh water cyanobacterium Oscillatoria annae with coir pith. Int J Environ Sci. 2013;3:1881–1890. [Google Scholar]
- 15.Jaysudha S., Sampathkumar P. Nutrient removal from tannery effluent by free and immobilized cells of marine microalgae Chlorella salina. Int J Environ Biol. 2014;4:21–26. [Google Scholar]
- 16.Fiore M.F., Trevors J.T. Cell composition and metal tolerance in cyanobacteria. Biometals. 1994;7:83–103. [Google Scholar]
- 17.Allen M.M., Arnon D.I. Studies on nitrogen-fixing blue-green algae. I – Growth and nitrogen fixation by Anabaena cylindrica Lemm. Plant Physiol. 1955;30:366–372. doi: 10.1104/pp.30.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen M.B. Simple conditions for growth of unicellular blue-green algae on plates. J Appl Phycol. 1968;4:1–4. doi: 10.1111/j.1529-8817.1968.tb04667.x. [DOI] [PubMed] [Google Scholar]
- 19.Trottier S., Blaise C., Kusin T., Johnson E.M. Acute toxicity assessment of aqueous samples using a microplate-based Hydra attenuatta assay. Tech Methods. 1997;12:265–271. [Google Scholar]
- 20.Blaise C., Forget G., Trottier S. Toxicity screening of aqueous samples using a cost-effective 72-H exposure Selenastrum capricornutum assay. Tech Methods. 2000;15:352–359. [Google Scholar]
- 21.Dutka B. National Water Research Institute, CCIW, Environment Canada; Ontario: 1989. Short-term root elongation toxicity bioassay. Methods for toxicological analysis of waters, wastewaters and sediments. [Google Scholar]
- 22.Fernandes T.C.C., Mazzeo D.E.C., Marin-Morales M.A. Mechanisms of micronuclei formation in polyploidizated cells of Allium cepa exposed to trifluralin herbicide. Pestic Biochem Physiol. 2007;88:252–259. [Google Scholar]
- 23.Campos R., Kandelbauer A., Robra K.H.A., Cavaco-Paulo A., Gübitz G.M. Indigo degradation with purified laccases from Trametes hirsuta and Sclerotium rolfsii. J Biotechnol. 2001;89:131–139. doi: 10.1016/s0168-1656(01)00303-0. [DOI] [PubMed] [Google Scholar]
- 24.Khanna N., Sharma S. Allium cepa root chromosomal aberration assay: a review. Indian J Pharm Biol Res. 2013;1:105–119. [Google Scholar]
- 25.Silva-Stenico M.E., Vieira F.D.P., Genuário D.B., Silva C.S.P., Moraes L.A.B., Fiore M.F. Decolorization of textile dyes by cyanobacteria. J Braz Chem Soc. 2012;23:1863–1870. [Google Scholar]
- 26.Queiroz B.P.V., Stefanelli T. Biodegradation de corantes Têxteis por Anabaena flos-aquae. Eng Amb. 2011;8:026–035. [Google Scholar]
- 27.Nguyen T.A., Juang R.S. Treatment of waters and wastewaters containing sulfur dyes: a review. Chem Eng J. 2013;219:109–117. [Google Scholar]
- 28.Padmanaban V.C., Prakash S.S., Sherildar P., Jacob J.P., Nelliparambil K. Biodegradation of antraquinone based compounds: review. Int J Adv Res Eng Technol. 2013;4:74–83. [Google Scholar]