Abstract
Background
Palliation for inoperable malignant distal biliary strictures can be achieved with self-expandable metal stents (SEMS) and plastic stents (PS). This is a meta-analysis to compare PS and SEMS. The aim of the study is to compare clinical outcomes in patients with SEMS and PS.
Methods
Study selection criteria were studied using SEMS and PS for palliation in patients with malignant distal biliary stricture. For data collection and extraction, articles were searched in Ovid journals, Medline, Cochrane database, and Pubmed. Pooled proportions were calculated using both Mantel–Haenszel method and DerSimonian Laird method for statistical analysis.
Results
Initial search identified 1376 reference articles, of which 112 were selected and11 studies (N = 947) were included in this analysis. Pooled analysis showed SEMS patency to be 167.7days (95% CI = 159.2–176.3) compared to 73.3days (95% CI = 69.8–76.9) in PS. SEMS have lower odds of occlusion when compared to PS with an odds ratio of 0.48 (95% CI = 0.34–0.67). SEMS has a lower odds of cholangitis compared to SP, with an odds ratio of 0.46 (95% CI = 0.30–0.69).
Conclusion
SEMS seem to be superior to PS with better patency periods and survival duration. SEMS have lower occlusion rates, re-intervention rates, and cholangitis.
Keywords: Self-expandable metal stents, Plastic stents, Malignant distal biliary stricture
Introduction
Malignant distal biliary obstruction occurs as a result of primary neoplasms of pancreato-biliary tract and other local cancers (gall bladder and liver malignancies) that can compress the biliary tract. The local tumors manifest as strictures occluding the biliary tract.1 The 5 year survival rate of most of these malignancies is less than 5%.2 These malignancies are often unresectable at the time of presentation, thus making palliation with biliary stents a widely accepted management option.3, 4, 5, 6 Biliary obstruction causes jaundice, malabsorption, pruritus, anorexia, or cholangitis.4, 5 Endoscopic retrograde biliary drainage with decompression and placement of biliary stents can relieve obstructive symptoms and improve quality of life.7 Furthermore, biliary stents have lower morbidity than bypass surgery and are often the best method for palliation.8, 9, 10
Biliary stents can broadly be classified into self-expandable metal stents (SEMS) and plastic stents (PS). Biliary stenting has shown to improve the quality of life of these patients and relieve jaundice.7 Bore size of the stent plays a key role in stent patency. Smaller bore size leads to early blockade of stent from accumulation of biliary sludge.5 Diameter of the PS is approximately 10–14 Fr compared to the diameter of the SEMS which is approximately 30 Fr after stent deployment. For a long time, PS have been used for palliation, however due to the short patency period, they had to be changed every few months. It is presumed that PS have significantly shorter patency period compared to uncovered self-expandable metal stents (USEMS).8, 9, 10, 11
Previous retrospective studies, randomized control trials (RCTs), reviews and meta-analyses comparing the efficacy of PS and SEMS showed wide heterogeneity of results.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 On comparison, there have been mixed outcomes in regards to stent patency periods, stent occlusion rates, stent migration, survival benefit, pancreatitis, cholecystitis, and cholangitis. There have also been recent studies published that were not included in the prior meta-analyses. In our meta-analysis, we sought to include all the available studies including RCTs and retrospective cohort studies comparing the efficacy of PS and SEMS in palliation of malignant distal biliary obstruction. Primary outcomes are stent patency periods (number of days the stent is patent) and occlusion rates of PS versus metal stents in managing malignant distal biliary strictures. Secondary outcomes include survival benefit, overall complications, stent migration, pancreatitis, cholangitis, and cholecystitis in both groups.
Material and Methods
Inclusion criteria
Studies using SEMS and PS for palliation in patients with malignant distal biliary stricture/obstruction. Studies comparing the two wings were included in this analysis. Studies should have looked at a minimum of two variables that must include stent patency days and adverse events. Patients in the studies should have had a malignant distal biliary stricture that is either non-resectable (probably due to extensive distant metastasis or vascular invasion) or inoperable (due to other co-morbidities).
Exclusion criteria
Studies without a comparison arm (non-comparison studies) were excluded. Studies performed on biliary strictures in hilar or middle portion of biliary tree were excluded. Studies that looked at patients with prior radiological biliary procedures, prior biliary surgical procedures, and prior biliary stent placement were also excluded. Patients with American Society of Anesthesiologists (ASA) grade 4 or 5, inability to follow up, duodenal obstruction, potentially benign biliary obstruction were all excluded.
Data collection and extraction
Articles were searched in Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Medline, Cumulative Index for Nursing & Allied Health Literature, EMBASE, ACP journal club, Medline nonindexed citations, Ovid journals, old Medline, DARE, International Pharmaceutical Abstracts, and OVID Healthstar. The search was performed for the years 1966 to December 2015. Major gastroenterology journals from the past 3 years were searched manually and relevant abstracts. Study authors for the abstracts included in this analysis were contacted when the required data for the outcome measures could not be determined from the publications. The search terms used were SEMS, PS, malignant distal biliary stricture, patency, occlusion rate, stent migration, mortality, morbidity, complications, systematic review and meta-analysis. Data was extracted and searched by two authors independently (HM and SP) into an abstraction form. Cohen's κ was used to quantify the agreement among the reviewers for the data collected.19 Co-authors have resolved any differences in the study process by mutual agreement.
Definitions
Stent patency is defined as the interval between stent insertion and stent occlusion or stent replacement. Stent occlusion is defined as development of jaundice with biochemical evidence of cholestasis, worsening transaminasemia and/or cholestasis with episodes of fever suggestive of cholangitis. Follow up period for all the studies included in this analysis was either till patient's death or stent occlusion or 12 months after first stent placement; which ever occurred first. Time till death is defined as the number of days the patient was alive since the first biliary stent placement as a palliative attempt.
Quality of the studies
Quality of a study was assessed by many criteria (e.g. blinding of outcome, randomization, concealment of allocation, selection bias of the arms in the study).20, 21 Quality of the study could be assessed in RCTs or prospective studies with a treatment and control arm. In studies with no control arms, there is no clear consensus regarding how to assess them. Hence, the above mentioned criteria cannot be used to assess studies without a control arm.21
Statistical methods
Pooled proportions were calculated in this meta-analysis. Freeman–Tukey variant of the arcsine square root transformed proportion was used to transform individual study proportions (stent patency, re-interventions, stent occlusion, survival benefit, overall complications, and individual complications) into a quantity. Inverse arcsine variance weights and DerSimonian–Laird weights were used to calculate the pooled proportions as the back-transform of the weighted mean of the transformed proportions for fixed and random effects model respectively.22, 23 Pooled estimate and point estimate of individual studies were graphically represented using forest plots. In the Forest plots weight assigned to a particular study was proportional to the width of the point estimates. Cochran's Q test was used to test the heterogeneity among studies based upon inverse variance weights.24 When the p value is above 0.10, null hypothesis was rejected. Null hypothesis assumes that the studies are heterogeneous. Harbord et al. bias indicator25 along with Begg et al. bias indicator26 were used to assess the publication bias and selection bias in the study. Publication bias was also assessed by constructing funnel plots. Diagnostic odds ratio and standard error were used to generate the funnel plots.27, 28
Results
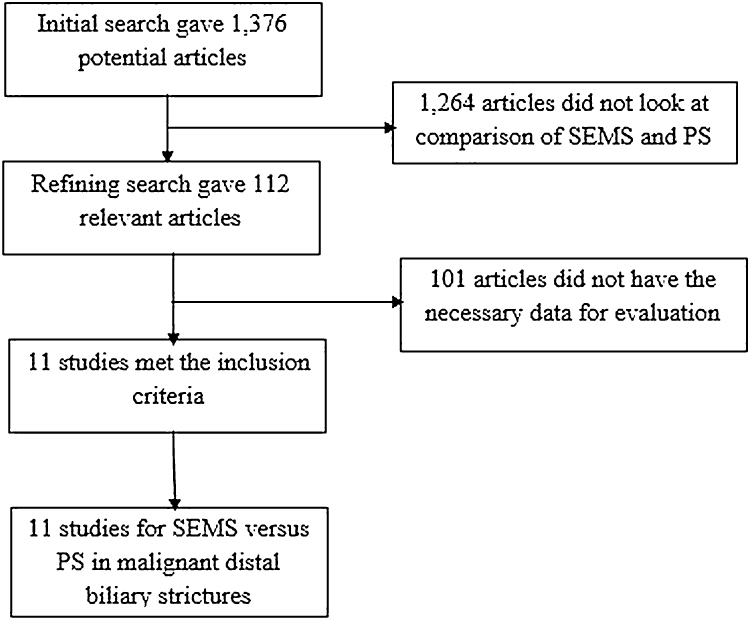
Initial search identified 1376 reference articles, of which 112 articles were selected and reviewed. Eleven studies8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 (N = 947) of SEMS and PS met the inclusion criteria. Data was extracted from these 11 studies. The eleven studies were published as full articles/manuscripts. Search results are displayed in Fig. 1. Random and fixed effect models were used to calculate all the pooled estimates.
Fig. 1.
Flow chart with search results and selection criterion.
Table 1 shows the baseline characteristics of the studies. Of the 11 studies included in the study, seven8, 9, 10, 11, 13, 15, 17 were RCTs. This article looked at various outcomes including stent occlusion rates, survival rates, cholangitis episodes, etc. However, most of the studies did not have information on all the variables studied in this meta-analysis. Studies with pertinent information regarding a particular variable were included in calculated the pooled effect of that particular variable. The pooled effects estimated by random and fixed effect models were similar. For all the pooled accuracy estimates, the p for Chi-squared heterogeneity was >0.10.
Table 1.
Basic characteristics of the included studies.
| No. | Study, year, place | Type of study | Sex: M/F | Total no. of patients in study | No. of patients with SEMS | No. of patients with PS |
|---|---|---|---|---|---|---|
| 1 | Elwir et al., 2013, USA | Retrospective | 50/27 | 114a | 44 | 33 |
| 2 | Moses et al., 2013, USA | Randomized prospective trial | 40/39 | 79 | 39 | 40 |
| 3 | Adams et al., 2012, USA | Retrospective | 27/25 | 52 | – | – |
| 4 | Soderlund et al., 2006, Sweden | Randomized controlled trial | 50/50 | 100 | 49 | 51 |
| 5 | Katsinelos et al., 2006, Greece | Prospective | 24/23 | 47 | 23 | 24 |
| 6 | Kaassis et al., 2003, France | Randomized prospective trial | 53/65 | 118 | 59 | 59 |
| 7 | Prat et al., 1998, France | Randomized prospective trial | 49/52 | 101 | 34 | 67 |
| 8 | Lammer et al., 1996, Austria | Randomized prospective trial | 38/63 | 101 | 52 | 49 |
| 9 | Knyrim et al., 1993, Germany | Randomized controlled trial | - | 55 | 27 | 28 |
| 10 | Davids et al., 1992, Netherlands | Randomized prospective trial | 47/58 | 105 | 49 | 56 |
| 11 | Yoon et al., 2009, South Korea | Retrospective | 62/50 | 112 | 56 | 56 |
This study also included another subset of patients with Double layered stent that was excluded from this meta-analysis.
– Not mentioned in the study.
Patency period, re-intervention rate and stent occlusion rate:
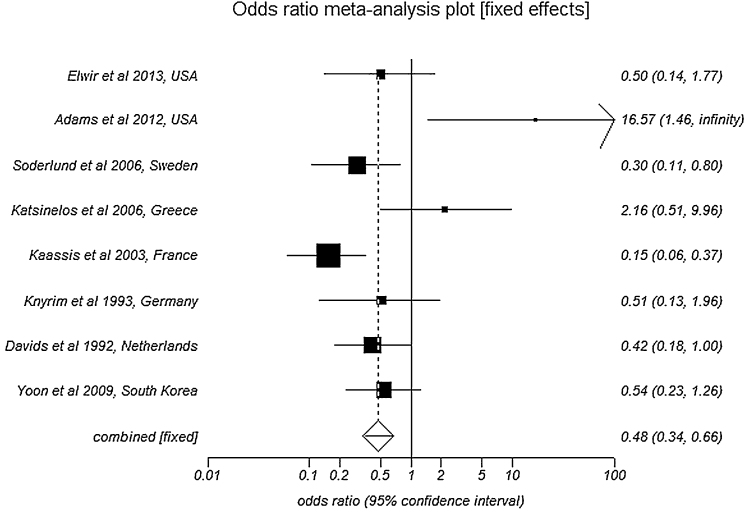
Pooled analysis by fixed effects showed SEMS patency to be 167.7 days (95% CI = 159.2–176.3) compared to 73.3 days (95% CI = 69.8–76.9) in PS. Number of re-interventions per patient in SEMS group was 1.1 (95% CI = 0.9–1.3) compared to 1.7 (95% CI = 1.5–1.9) in PS group. SEMS have significantly lower odds of occlusion when compared to PS with an odds ratio of 0.48 (95% CI = 0.34–0.67). Fig. 2 is a Forest plot showing the pooled estimate of odds ratio and individual study proportions for occlusion rates in SEMS versus PS. The values of bias indicators are as follows: Begg–Mazumdar Kendall's tau = 0.38 (p = 0.12) and Egger: bias = 10.18 (95% CI = 5.77–14.57), p = 0.0005.
Fig. 2.
Forest plot: Individual study proportions and the pooled estimate of odds ratio for occlusion rates in SEMS versus PS (fixed effects).
Mortality
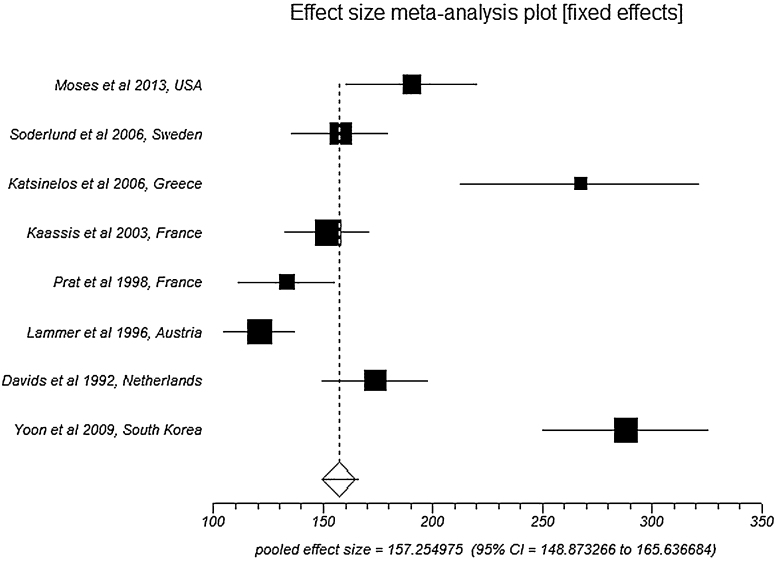
Pooled analysis by fixed effects showed an overall survival/time to death in SEMS group to be 157.3 days (95% CI = 148.9–165.6) compared to 120.6 days (95% CI = 114.3–126.9) in PS group. Fig. 3 is a Forest plot showing the pooled estimate of overall survival/time to death and individual study proportions in SEMS group. Egger: bias = 10.02 (95% CI = 5.14–14.90), p = 0.0024.
Fig. 3.
Forest plot: Individual study proportions and the pooled estimate of overall survival/time to death in SEMS group (fixed effects).
Complications
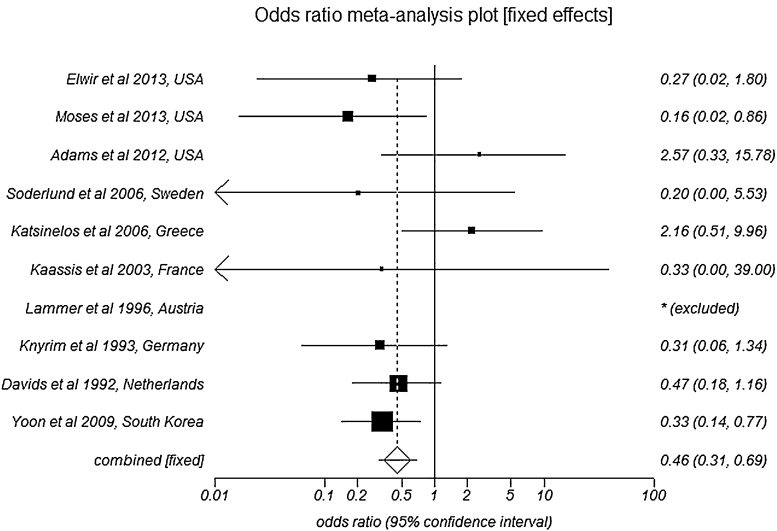
The effect size of pooled complication rate in SEMS group was 3.83 (95% CI = 3.52–4.14) compared to 2.17 (95% CI = 1.91–2.42) in PS group. Pooled data for all the complications is shown in Table 2. Patients with SEMS have lower odds of developing cholangitis as a complication when compared to patients with PS, with an odds ratio of 0.46 (95% CI = 0.30–0.69). Fig. 4 is a Forest plot showing the pooled estimate of odds ratio and individual study proportions for cholangitis in SEMS versus PS. Egger: bias = 0.10 (95% CI = −2.58 to 2.78), p = 0.93 and Horbold–Egger: bias = 0.07 (92.5% CI = −2.29 to 2.43), p = 0.95.
Table 2.
Odds ratio of all complications along with their respective publication bias (SEMS versus PS).
| Variable | Metal versus plastic stents: odds ratio with 95% CI |
|---|---|
| Cholangitis | 0.46 (95% CI = 0.31–0.69) |
| Stent migration | 0.45 (95% CI = 0.15–1.37) |
| Cholecystitis | 1.85 (95% CI = 0.45–7.56) |
| Pancreatitis | 0.80 (95% CI = 0.33–1.96) |
| Occlusion rate | 0.48 (95% CI = 0.34–0.67) |
Fig. 4.
Forest plot: Individual study proportions and the pooled estimate of odds ratio for cholangitis in SEMS versus PS (fixed effects).
Subgroup analysis of RCTs
A subgroup analysis was performed on RCTs only. Seven RCTs8, 9, 10, 11, 13, 15, 17 were included in this subgroup analysis. Total number of patients in this subgroup (N) was 659, with 45% males. Median patient age was 74 years. Pooled analysis by fixed effects showed SEMS patency to be 165.07 days (95% CI = 155.34–174.80) compared to 86.79 days (95% CI = 81.71–91.87) in PS. Number of re-interventions per patient in SEMS group was 0.78 (95% CI = 0.56–0.99) compared to 1.27 (95% CI = 1.04–1.49) in PS group. SEMS have significantly lower odds of occlusion when compared to PS with an odds ratio of 0.29 (95% CI = 0.19–0.46). Pooled analysis by fixed effects showed an overall survival/time to death in SEMS group to be 147.15 days (95% CI = 138.77–155.54) compared to 111.84 days (95% CI = 105.24–118.43) in PS group. Odds ratio for pooled adverse events in SEMS versus PS group was 0.84 (95% CI = 0.44–1.60). Patients with SEMS have lower odds of developing cholangitis as a complication when compared to patients with PS, with an odds ratio of 0.34 (95% CI = 0.17–0.68).
Discussion
Biliary obstruction is found in most patients with malignancy of the distal bile-duct system.10, 15, 16 However, disease is usually detected in late stages because most patients do not have early symptoms.12, 29 Surgical resection offers the greatest chance of cure for distal common bile malignancy.12 Yet, five-year survival rates remain less than 10% and the prognosis is extremely poor.29, 30 Metastatic or locally advanced disease cannot be cured in greater than 80% of patients.10, 15, 16 As mentioned above, decompression of biliary system using a biliary stent has proved beneficial in these patients.
It is a popular opinion that ERCP with SEMS insertion remains an invaluable palliative modality in inoperable malignant biliary strictures and patients in whom surgery has been delayed due to neo-adjuvant therapy.31 Few studies compared unilateral stenting versus bilateral stenting. Hong et al.32, 33 mentioned that unilateral stenting had higher successful stent insertion compared to bilateral stenting. Puli et al.34 showed that bilateral plastic stenting is comparable to unilateral plastic stenting in regards to adverse events in managing patients with malignant hilar biliary strictures.
Adams et al.14 compared the efficacy metal versus PS in pancreatic cancer patients undergoing neo-adjuvant therapy. Their study concluded that metal stents are superior to PS in terms of complication rate, rate of hospitalization for stent related complications and first quartile estimate of time to stent complications. Elwir et al.12 retrospectively compared Double layered stents (DLS) with metal stents and PS. Their analysis showed that DLS is comparable to SEMS but superior to PS.
Cost effectiveness of PS versus SEMS is another area of interest that many studies tried to evaluate. Yoon et al.18 showed that metals stents offer better palliation compared to PS without significant increase in cost. In this study there was no statistical difference in the mean cost of relief of jaundice in both the groups. Soderlund et al.15 showed that cost effectiveness is equal in both groups. Three RCTs8, 35, 36 however showed that SEMS are more cost effective compared to PS in palliation of malignant biliary strictures. This benefit is thought to be from longer patency, less re-intervention rates and fewer complications and hospitalizations in the SEMS group. In our meta-analysis, we were not able to derive any conclusions in regards to cost effectiveness. This is due to the non-uniformity of cost effectiveness criteria used in the aforementioned studies. Kaassis et al.17 mentioned that the overall hospital stay (in days) of patient receiving palliation for malignant distal biliary strictures was less in SEMS group (80 days) compared to PS group (246 days). This could be from relatively less occlusions, increased patency period.
There are a few limitations for this study. Different type of stents with slightly variable bore size have been used in these studies. Few studies used trial stents that were not used in another study. Most of the studies used PS with bore size 10 Fr, however two studies – Prat et al.9 and Knyrim et al.8 used 11.5 Fr PS. Lammer et al.11 used 12 Fr PS. Covered metal stents were used in Soderlund et al.15 and partially covered SEMS were used in Moses et al.13 The type of malignancy causing the biliary obstruction, stage of the malignancy, presence of metastasis could have all influenced the results. Retrospective cohort studies were included in this meta-analysis along with RCTs. Strengths of this meta-analysis include the high quality methodology of statistical analysis, high quality methodology used in individual studies, large number of studies compared to prior meta-analyses, large pooled patient population (N = 947) and homogeneity of data among the individual studies. This study analyzed the efficacy of stents only in distal biliary strictures.
Based on the results of this meta-analysis, which in-turn is supported by the conclusions from prior studies, we can conclude that SEMS have increased patency period and survival benefit compared to PS. Re-intervention rate, occlusion rate and cholangitis is comparatively low in SEMS. Overall complication rate is marginally higher in SEMS compared to PS. Although this might be due to various individual complications like increased tumor ingrowth in SEMS, it is worth mentioning that there could be a contribution from longer patency of SEMS. More events (complications) tend to accumulate over the prolonged patency duration and SEMS have a longer patency period compared to PS. Due to the limited data available on the cost benefit of SEMS and PS, we were not able to derive any meaningful conclusions in this regard. Based on the results of our study and systematic review of this topic, our opinion is that; in a patient with unresectable malignant distal biliary obstruction with a longer expected survival period, SEMS are superior to PS. This being said, in-order to choose one stent over the other, every patient case must be individualized based on the overall cost of the stents, anticipated survival period, anticipation of stent retrieval, anticipation of re-interventions.
It is a common observation that studies with positive results that are statistically significant tend to be cited and published. Due to the inherent nature of statistical analysis, larger studies may show smaller treatment effects compared to smaller studies. The summary estimates may be effected by the selection bias and publication bias. Shape of the funnel plot could be effected by the bias, and bias is usually estimated with Egger bias indicators. In this systematic review and meta-analysis, Harbord et al.25 and Begg et al.26 bias indicators was used to estimate bias. No statistically significant bias was noted based of these indicators. As mentioned earlier, funnel plots were used to estimate publication bias. The studies included in this analysis did not have a statistically significant publication bias based on the funnel plots.
Conclusions
SEMS seem to have lower odds of occlusion and cholangitis when compared to PS. SEMS had marginally lower re-intervention rate and are patent twice as long as PS in patients with malignant distal CBD strictures. SEMS might be associated with longer overall survival periods compared to PS. Metal stents seems to have marginally higher percentage of overall adverse events compared to PS. Although there were slight differences in the occurrence of stent migration, pancreatitis and cholecystitis in both the groups, the results were not statistically significant. Based on these conclusions, it is reasonable to prefer using SEMS over a PS in a patient with malignant distal biliary stricture.
Conflicts of interest
The authors have none to declare.
References
- 1.Jemal A., Siegel R., Ward E. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Ries L.A.G., Harkins D., Krapcho M. National Cancer Institute; Bethesda: 2005. SEER Cancer Statistics Review, 1975–2003. Available at: http://seer.cancer.gov/csr/1975_2003/ Accessed 27.12.14. [Google Scholar]
- 3.Shepherd H.A., Royle G., Ross A.P. Endoscopic biliary endoprosthesis in the palliation of malignant obstruction of the distal common bile duct: a randomized trial. Br J Surg. 1988;75(12):1166–1168. doi: 10.1002/bjs.1800751207. [DOI] [PubMed] [Google Scholar]
- 4.Smith A.C., Dowsett J.F., Russell R.C. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bile duct obstruction. Lancet. 1994;344(8938):1655–1660. doi: 10.1016/s0140-6736(94)90455-3. [DOI] [PubMed] [Google Scholar]
- 5.Speer A.G., Cotton P.B., Russell R.C. Randomised trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet. 1987;2(8550):57–62. doi: 10.1016/s0140-6736(87)92733-4. [DOI] [PubMed] [Google Scholar]
- 6.Andersen J.R., Sørensen S.M., Kruse A. Randomised trial of endoscopic endoprosthesis versus operative bypass in malignant obstructive jaundice. Gut. 1989;30(8):1132–1135. doi: 10.1136/gut.30.8.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abraham N.S., Barkun J.S., Barkun A.N. Palliation of malignant biliary obstruction: a prospective trial examining impact on quality of life. Gastrointest Endosc. 2002;56(6):835–841. doi: 10.1067/mge.2002.129868. [DOI] [PubMed] [Google Scholar]
- 8.Knyrim K., Wagner H.J., Pausch J., Vakil N. A prospective, randomized, controlled trial of metal stents for malignant obstruction of the common bile duct. Endoscopy. 1993;25(3):207–212. doi: 10.1055/s-2007-1010294. [DOI] [PubMed] [Google Scholar]
- 9.Prat F., Chapat O., Ducot B. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest Endosc. 1998;47(1):1–7. doi: 10.1016/s0016-5107(98)70291-3. [DOI] [PubMed] [Google Scholar]
- 10.Davids P.H., Groen A.K., Rauws E.A., Tytgat G.N., Huibregtse K. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340(8834-8835):1488–1492. doi: 10.1016/0140-6736(92)92752-2. [DOI] [PubMed] [Google Scholar]
- 11.Lammer J., Hausegger K.A., Flückiger F. Common bile duct obstruction due to malignancy: treatment with plastic versus metal stents. Radiology. 1996;201(1):167–172. doi: 10.1148/radiology.201.1.8816539. [DOI] [PubMed] [Google Scholar]
- 12.Elwir S., Sharzehi K., Veith J. Biliary stenting in patients with malignant biliary obstruction: comparison of double layer, plastic and metal stents. Dig Dis Sci. 2013;58(7):2088–2092. doi: 10.1007/s10620-013-2607-z. [DOI] [PubMed] [Google Scholar]
- 13.Moses P.L., Alnaamani K.M., Barkun A.N. Randomized trial in malignant biliary obstruction: plastic vs partially covered metal stents. World J Gastroenterol. 2013;19(46):8638–8646. doi: 10.3748/wjg.v19.i46.8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams M.A., Anderson M.A., Myles J.D., Khalatbari S., Scheiman J.M. Self-expanding metal stents (SEMS) provide superior outcomes compared to plastic stents for pancreatic cancer patients undergoing neoadjuvant therapy. J Gastrointest Oncol. 2012;3(4):309–313. doi: 10.3978/j.issn.2078-6891.2011.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soderlund C., Linder S. Covered metal versus plastic stents for malignant common bile duct stenosis: a prospective, randomized, controlled trial. Gastrointest Endosc. 2006;63(7):986–995. doi: 10.1016/j.gie.2005.11.052. [DOI] [PubMed] [Google Scholar]
- 16.Katsinelos P., Paikos D., Kountouras J. Tannenbaum and metal stents in the palliative treatment of malignant distal bile duct obstruction: a comparative study of patency and cost effectiveness. Surg Endosc. 2006;20(10):1587–1593. doi: 10.1007/s00464-005-0778-1. Epub 07.08.06. [DOI] [PubMed] [Google Scholar]
- 17.Kaassis M., Boyer J., Dumas R. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest Endosc. 2003;57(2):178–182. doi: 10.1067/mge.2003.66. [DOI] [PubMed] [Google Scholar]
- 18.Yoon W.J., Ryu J.K., Yang K.Y. A comparison of metal and plastic stents for the relief of jaundice in unresectable malignant biliary obstruction in Korea: an emphasis on cost-effective in a country with a low ERCP cost. Gastrointest endosc. 2009;70(2):284–288. doi: 10.1016/j.gie.2008.12.241. [DOI] [PubMed] [Google Scholar]
- 19.Brennan P., Silman A. Statistical methods for assessing observer variability in clinical measures. BMJ. 1992;304:1491–1494. doi: 10.1136/bmj.304.6840.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jadad A.R., Moore R.A., Carroll D. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 21.Stroup D.F., Berlin J.A., Morton S.C. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-Analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 22.Stuart A., Ord J.K. 6th ed. Edward Arnold; London: 1994. Kendall's Advanced Theory of Statistics. [Google Scholar]
- 23.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clinical Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Deeks J.J. Systematic reviews of evaluations of diagnostic and screening tests. In: Egger M., Smith G.D., Altman D.G., editors. Systematic Reviews in Health Care. Meta-Analysis in Context. BMJ Books; London: 2001. [Google Scholar]
- 25.Harbord R.M., Egger M., Sterne J.A.C. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2005;25(20):3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 26.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 27.Sterne J.A.C., Egger M., Davey-Smith G. Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sterne J.A.C., Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 29.Raju R.P., Jaganmohan S.R., Ross W.A. Optimum palliation of inoperable hilar cholangiocarcinoma: comparative assessment of the efficacy of plastic and self-expanding metal stents. Dig Dis Sci. 2011;56(5):1557–1564. doi: 10.1007/s10620-010-1550-5. [DOI] [PubMed] [Google Scholar]
- 30.Liberato M.J., Canena J.M. Endoscopic stenting for hilar cholangiocarcinoma: efficacy of unilateral and bilateral placement of plastic and metal stents in a retrospective review of 480 patients. BMC Gastroenterol. 2012;12:103. doi: 10.1186/1471-230X-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozarek R. Role of preoperative palliation of jaundice in pancreatic cancer. J Hepatobiliary Pancreat Sci. 2013 doi: 10.1007/s00534-013-0612-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Hong W., Sun X., Zhu Q. Endoscopic stenting for malignant hilar biliary obstruction: should it be metal or plastic and unilateral or bilateral? Eur J Gastroenterol Hepatol. 2013;25(9):1105–1112. doi: 10.1097/MEG.0b013e328360b9ec. [DOI] [PubMed] [Google Scholar]
- 33.Hong W.D., Chen X.W., Wu W.Z. Metal versus plastic stents for malignant biliary obstruction: an update meta-analysis. Clin Res Hepatol Gastroenterol. 2013;37(5):496–500. doi: 10.1016/j.clinre.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Puli S.R., Kalva N., Pamulaparthy S.R. Bilateral and unilateral stenting for malignant hilar obstruction: a systematic review and meta-analysis. Indian J Gastroenterol. 2013;32(6):355–362. doi: 10.1007/s12664-013-0413-3. [DOI] [PubMed] [Google Scholar]
- 35.Mukai T., Yasuda I., Nakashima M. Metallic stents are more efficacious than plastic stents in unresectable malignant hilar biliary strictures: a randomized controlled trial. J Hepatobiliary Pancreat Sci. 2013;20(2):214–222. doi: 10.1007/s00534-012-0508-8. [DOI] [PubMed] [Google Scholar]
- 36.Wagner H.J., Knyrim K., Vakil N., Klose K.J. Plastic endoprostheses versus metal stents in the palliative treatment of malignant hilar biliary obstruction. A prospective and randomized trial. Endoscopy. 1993;25(3):213–218. doi: 10.1055/s-2007-1010295. [DOI] [PubMed] [Google Scholar]