Abstract
Four strains of euryhaline bacteria belonging to the genus Halomonas were tested for their response to a range of temperatures (2, 13, and 30°C), hydrostatic pressures (0.1, 7.5, 15, 25, 35, 45, and 55 MPa), and salinities (4, 11, and 17% total salts). The isolates were psychrotolerant, halophilic to moderately halophilic, and piezotolerant, growing fastest at 30°C, 0.1 MPa, and 4% total salts. Little or no growth occurred at the highest hydrostatic pressures tested, an effect that was more pronounced with decreasing temperatures. Growth curves suggested that the Halomonas strains tested would grow well in cool to warm hydrothermal-vent and associated subseafloor habitats, but poorly or not at all under cold deep-sea conditions. The intermediate salinity tested enhanced growth under certain high-hydrostatic-pressure and low-temperature conditions, highlighting a synergistic effect on growth for these combined stresses. Phospholipid profiles obtained at 30°C indicated that hydrostatic pressure exerted the dominant control on the degree of lipid saturation, although elevated salinity slightly mitigated the increased degree of lipid unsaturation caused by increased hydrostatic pressure. Profiles of cytosolic and membrane proteins of Halomonas axialensis and H. hydrothermalis performed at 30°C under various salinities and hydrostatic pressure conditions indicated several hydrostatic pressure and salinity effects, including proteins whose expression was induced by either an elevated salinity or hydrostatic pressure, but not by a combination of the two. The interplay between salinity and hydrostatic pressure on microbial growth and physiology suggests that adaptations to hydrostatic pressure and possibly other stresses may partially explain the euryhaline phenotype of members of the genus Halomonas living in deep-sea environments.
Euryhaline bacteria, which can grow over an extremely wide salt range, are nearly ubiquitous in marine environments and are often cultured from deep-sea sediments and hydrothermal vents (32, 52, 70, 76). These microorganisms are also abundant; bacteria capable of growth on media with 17% total salts, including primarily members of the genera Halomonas and Marinobacter, were found to comprise a remarkably high percentage (up to >10%) of the total microbial community in hydrothermal-vent habitats and the overlying water column in the North and South Pacific ocean (32). Several recently characterized Halomonas strains isolated from low-temperature hydrothermal fluids, hydrothermal plumes, and sulfide rock, including those from 1:50 to 1:500 fluid dilution enrichments, were found to have a minimum growth temperature of −1 to 2°C at 0.1 MPa and 4% total salts (33), similar to Antarctic Halomonas isolates (38, 58, 76). This low minimum temperature for growth closely matches the temperature of the deep sea below 1,500 m (and shallower towards the poles) and leaves open the question of whether Halomonas strains collected from the deep sea, despite their numerical significance, are able to grow in situ in deep regions of the ocean outside of cool to warm hydrothermal-vent habitats.
Piezotolerant microorganisms grow more slowly as hydrostatic pressure increases above the sea surface pressure of 0.1 MPa, whereas the growth rate of piezophilic microorganisms is fastest at hydrostatic pressures of >0.1 MPa (15, 82). For both groups of microorganisms, hydrostatic pressure and temperature act synergistically, such that low temperatures and high hydrostatic pressures have analogous effects on cellular proteins and membranes (2, 13, 14, 83). Hydrostatic pressures equivalent to mid-ocean-ridge or abyssal depths have been shown to increase the cardinal growth temperatures of hyperthermophiles isolated from hydrothermal vents (7, 26, 28, 55), the optimum growth temperature of a mesophilic Pseudomonas strain obtained from deep-sea sediments (31), and the ability of psychropiezophiles isolated from the cold deep sea to tolerate (or prefer [16]) slightly warmer temperatures (81, 83). Stated another way, the higher the hydrostatic pressure, the higher the temperature allowed (Tmax) or required (Tmin) to permit growth. Given that the minimum temperature for growth typically increases with increasing hydrostatic pressures, we hypothesized that Halomonas spp. with a Tmin of −1 to 2°C at 0.1 MPa would not be able to multiply in cold deep-sea habitats.
Cardinal growth temperatures and salinities may also vary with respect to each other, just as lag times and maximum cell yields may also depend on the temperature-salinity regimen. Growth curves have shown that the optimum and maximum salt concentrations for growth of the moderate halophiles Halomonas halophila and Vibrio anguillarum (reclassified as Listonella anguillarum [35]) increased with increasing temperatures (22, 57). Similarly, “Pseudomonas halosaccharolytica” (proposed name) grew faster with elevated salinities at elevated temperatures (51). Growth curves of a facultatively halophilic coccoid bacterium isolated from salted mackerel (ultimately classified as Marinococcus halophilus [23, 49]) likewise showed that the Topt was 35°C with 1.0 M NaCl but that the Tmax with 0.1 M NaCl was only 30°C (48). This salinity-temperature relationship, however, is sometimes observed only for a single cardinal growth parameter or not at all (8, 21, 29, 47, 75, 78).
Moderately halophilic and euryhaline bacteria isolated from marine environments provide an opportunity to examine the combined effects of salinity, hydrostatic pressure, and temperature on microbial growth, phospholipid profiles, and protein expression. Very few studies examining the interactions of all three of these stresses have been performed thus far. Euryhaline and moderately halophilic bacteria, including Salinisphaera shabanensis and Halanaerobium spp., have been isolated from the brine-seawater interface above the Shaban and Kebrit brine deeps (22 to 25°C), at 1,325 and 1,466-m seawater depths, respectively, in the Red Sea (1, 17). At 30°C, S. shabanensis grew with 1 to 28% NaCl at 0.1 MPa and with 5 to 20% NaCl at 15 MPa, but it did not grow at ≥20 MPa (1). At 0.1 MPa, this bacterium accumulated up to 4 M concentrations of compatible solutes under salt stress (17), although the production of compatible solutes under hydrostatic pressure has not yet been reported. Physiological experiments monitoring compatible solutes in the less haloversatile psychropiezophile Photobacterium profundum strain SS9 revealed an additive effect of hydrostatic pressure and salinity on the production of compatible solutes whereby the combination of the two stresses resulted in a disproportionate increase in the accumulation of these compounds (37).
The present set of experiments utilized four Halomonas strains, three of which were isolated from deep-sea hydrothermal vents and one of which was isolated from surface seawater, and explored several facets of the synchronous effects of temperature, hydrostatic pressure, and salinity on microbial growth. The selected parameters were modeled in part on the conditions found at deep-sea hydrothermal vents and the surrounding cold deep sea in order to delineate the marine habitats that (assuming adequate nutrients and energy sources) permit the growth of Halomonas species. H. axialensis and H. hydrothermalis, both of which were isolated from hydrothermal vents, were further investigated by the use of lipid profiles and protein expression patterns because preliminary experiments at a near-optimal temperature and salinity indicated a surprisingly robust growth response at abyssal hydrostatic pressures.
MATERIALS AND METHODS
Growth curves.
Three deep-sea Halomonas strains that were isolated from hydrothermal vents, namely H. axialensis ATCC BAA-802T, H. meridiana strain Slthf1 (ATCC BAA-801T), and H. hydrothermalis ATCC BAA-800T, and one Halomonas strain isolated from surface seawater on the Hawaiian coast, H. pacifica ATCC 27122 (3), were grown under a range of conditions of temperature (2, 13, and 30°C), hydrostatic pressure (0.1, 7.5, 15, 25, 35, 45, and 55 MPa), and salinity (4, 11, and 17% total salts). A growth medium (4% total salts, pH 8.0) described by Kaye and Baross (32) was augmented with NaCl to achieve higher salt concentrations. Cells were inoculated into fresh medium and kept at the relevant incubation temperature for 3 to 12.5 h (for 30°C experiments), 22.25 to 27.5 h (for 13°C experiments), or 2 to 5 days (for 2°C experiments) to allow cells to acclimate to the temperature and salinity conditions before pressurization. The inoculated medium was then used to completely fill 4.6-ml polyethylene transfer pipettes (Samco Scientific) (9) that were sealed by melting the tips in a Bunsen burner flame and crimping them with needle-nosed pliers. The pipettes were placed into stainless steel pressure vessels (Tem-Pres Division, LECO Corp.), topped off with distilled water at the appropriate temperature, manually pressurized with an Enerpac 11-400 pump outfitted with a quick-connect coupling device (84), using distilled water as the hydraulic fluid, and placed into an incubator. The hydrostatic pressure inside the vessels was checked after final equilibration to the incubation temperature and again before depressurization. The vessels remained within ±1 MPa of the target hydrostatic pressure at 7.5 and 15 MPa and within ±3 MPa of the target hydrostatic pressure at ≥25 MPa. The vessels were sequentially sacrificed at five time intervals and slowly depressurized (30 to 60 s), and a triplicate set of pipettes was harvested for each time point and each organism. Growth was assessed at 0.1 MPa by using the same general protocol, with pipettes that were submerged in distilled water.
Growth at 13 and 30°C was monitored by measuring the optical density at 600 nm with a Lambda UV/VIS spectrophotometer and that at 2°C was monitored by direct counts after filtering and staining of the cells with DAPI (4′,6-diamidino-2-phenylindole) (56). The growth rate was calculated by taking the slope of the log of the exponential portion of the growth curve. If the exponential portion of the growth curve could not be clearly ascertained, a region of maximal slope on a log plot from the beginning of the growth curve was used. For growth curves measured by optical density, growth rates were converted into increases in cell numbers over time by calculating the ratios of growth rates determined simultaneously by cell counts and optical turbidities at 20°C, 4% total salts, and 0.1 MPa. The 95% confidence intervals of the slope of the regression through the data points were also calculated.
PLFA analysis.
H. axialensis and H. hydrothermalis were grown at 30°C and 0.1 or 45 MPa in 23-ml transfer pipettes (Samco Scientific) in medium with either 4 or 11% total salts. The cultures were grown in pressure vessels as described above and were harvested during early- to mid-exponential-phase growth. The cells were centrifuged at 10,000 × g at 26 to 29°C immediately after decompression; the pellets were resuspended in 50 mM Tris buffer (pH 8.0) and frozen at −69°C. A subsample of each pellet was sent to Microbial Insights Inc. (Rockford, Tenn.) for standard phospholipid fatty acid (PLFA) analysis by a modified Bligh and Dyer method (79). Lipids were extracted in a one-phase chloroform-methanol buffer solution, recovered and dissolved in chloroform, and separated into neutral, glyco-, and polar lipid pools. Polar lipids were additionally transesterified by exposure to mild alkali. PLFA were identified and quantified by gas chromatography-mass spectrometry (limit of detection, 7 pmol).
Protein analysis.
Subsamples of the same cell pellets used for PLFA analysis were delivered to Kendrick Laboratories Inc. (Madison, Wis.) for fractionation into cytosolic and membrane-bound proteins and subsequent one-dimensional gel electrophoresis. The samples were lysed with an osmotic lysis buffer containing nuclease and protease inhibitors and then centrifuged for 30 min, after which the supernatants were removed and the entire procedure was repeated with the addition of 100 μl of fresh lysis buffer and 100 μl of deionized water. A volume of 100 μl of sodium dodecyl sulfate (SDS) boiling buffer without β-mercaptoethanol was added to the remaining pellet, which was mixed by vortexing and held in a boiling water bath for 5 min. The protein concentration for each fraction was then determined with a bicinchoninic acid assay (68). The samples were then lyophilized, resuspended in a buffer solution comprised of 5.0% SDS, 10% glycerol, 5% β-mercaptoethanol, and 63 mM Tris (pH 6.8) to achieve a final concentration of 5 mg of protein ml−1, and placed in a boiling water bath for 5 min. SDS slab gel electrophoresis (34, 50) was run for each protein fraction in triplicate by use of a 10% acrylamide slab gel (125 mm long by 150 mm wide by 0.75 mm thick) overlaid with a 25-mm-long stacking gel at 15 mA for 3.5 h or until the bromophenol blue front migrated to the end of the slab gel. Gels were stained with Coomassie blue, destained in 10% acetic acid until the background clarified, and dried between cellophane sheets. Lastly, the gels were digitized with a laser densitometer (Molecular Dynamics), and the stain density of individual protein bands was quantified as a fraction of the total stain density per lane by the use of Nonlinear Dynamics 1D Advanced software (version 5.0). Triplicate runs were used to ensure the consistency of protein band patterns and to calculate standard deviations of band intensities. The images provided here were obtained by rerunning gels with a single lane devoted to each sample and each protein fraction.
RESULTS
Growth curves.
In previous studies, H. axialensis, H. meridiana strain Slthf1, H. hydrothermalis, and H. pacifica exhibited psychrotolerant and euryhaline growth at 0.1 MPa (Table 1). The three strains isolated from hydrothermal vents were able to grow at temperatures as low as −1 to 2°C and grew significantly better at 2°C than H. pacifica. The previously reported Tmin at 0.1 MPa for H. pacifica was 4°C, although slight growth was found at 2°C with the growth medium employed in this study and a previous study (32).
TABLE 1.
Source of Halomonas strains used in hydrostatic pressure experiments and their basic growth features at sea surface pressure
| Strain (reference) | Sample characteristics
|
Growth temperature (°C) at 0.1 MPa and 4% total salts
|
Growth salinity (% total salts) at 0.1 MPa and 30°C
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample type | Depth (m) | Temp (°C) | Tmin | Topt | Tmax | Smin | Sopt | Smax | |
| H. axialensis (32, 33) | Low-temperature hydrothermal fluida | 1,533 | 27 | −1 | 30 | 35 | 0.5 | 4 | 24 |
| H. meridiana strain Slthf1 (32,33) | Low-temperature hydrothermal fluida | 2,580 | 9 | −1 | 20-35 | 40 | 0.5 | 2-7 | 22 |
| H. hydrothermalis (32,33) | Low-temperature hydrothermal fluida | 2,580 | 9 | 2 | 30 | 40 | 0.5 | 4-7 | 22 |
| H. pacifica (3, 38) | Tropical seawater | 0 | NDb | 2-4c | NDb | 45 | 0 | 0.5-3 | 20 |
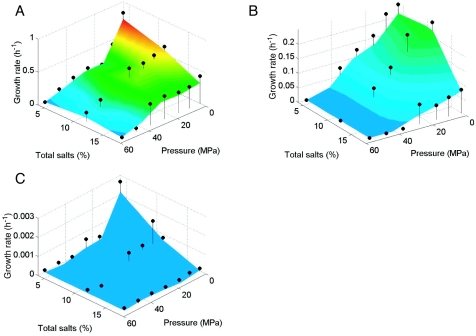
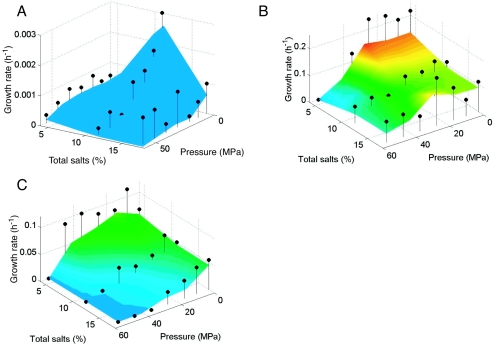
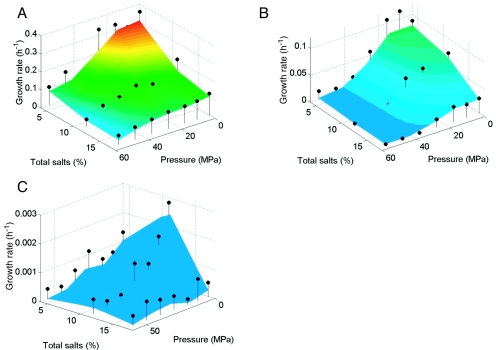
Temperature-hydrostatic pressure-salinity growth curves (Fig. 1 to 3) showed that temperature had a dominant effect on the growth rate, whereby the fastest growth (0.17 to 0.74 h−1) occurred at 30°C for each Halomonas strain, regardless of the tested combination of hydrostatic pressure and salinity. (Note that the color coding on the growth plots was reset for each bacterium such that, for example, red indicates the fastest growth for each organism even though the corresponding growth rate value is different for each strain.) In addition, increased hydrostatic pressures generally resulted in decreased growth rates for each organism at any given salinity and temperature combination, indicating piezotolerant growth for each strain. Growth was very slow or did not occur above 25 MPa at 2°C at all salinities tested. Similarly, decreased temperatures generally resulted in decreased maximum hydrostatic pressures for growth. For example, at 30°C and 4% total salts, the highest hydrostatic pressures that permitted growth for H. axialensis (Fig. 1A) and H. meridiana strain Slthf1 (Fig. 2A) were 45 and >55 MPa, respectively, but at 2°C these values dropped to 25 and 45 MPa, respectively (Fig. 1C and 2C).
FIG. 1.
Growth of H. axialensis at 30°C (A), 13°C (B), and 2°C (C), with 4, 11, and 17% total salts, and under 0.1, 7.5, 15, 25, 35, 45, and 55 MPa of hydrostatic pressure. Warm colors (red) indicate faster growth. The 95% confidence intervals are approximated by the distance from the contoured plot surface to the black dots. Note the differences in the z-axis scale among Fig. 1 to 3.
FIG. 3.
Growth of H. hydrothermalis at 2°C (A) and growth of H. pacifica at 30°C (B) and 13°C (C). Other features are as described for Fig. 1.
FIG. 2.
Growth of H. meridiana strain Slthf1 at 30°C (A), 13°C (B), and 2°C (C). Other features are as described for Fig. 1.
The response to salinity in combination with different temperatures and hydrostatic pressures provided some unexpected growth patterns. At 0.1 MPa, H. axialensis grew fastest with 4% total salts versus higher salinities at both 30°C (Fig. 1A) and 2°C (Fig. 1C), but it grew equally well with 4 and 11% total salts at 13°C (Fig. 1B). Growth was favored in 11% total salts at 30°C at the higher hydrostatic pressures of 7.5 to 55 MPa (Fig. 1A). Salt-enhanced growth was not seen at 13°C (Fig. 1B) or 2°C (Fig. 1C). At 13°C, the growth rates were roughly equal at 4 and 11% total salts between 7.5 and 55 MPa (Fig. 1B), whereas at 2°C the growth rates declined with increasing hydrostatic pressures and/or salinities (Fig. 1C).
H. meridiana strain Slthf1 showed a consistently piezotolerant and psychrotolerant response at 30°C (Fig. 2A) and 13°C (Fig. 2B), with the fastest growth always occurring with 4% total salts. In contrast, its growth at 2°C was markedly enhanced with 11% total salts over the hydrostatic pressure range that yielded growth (0.1 to >35 MPa) (Fig. 2C). Similarly, culturing with 11% total salts increased the growth rate of H. hydrothermalis at 2°C over the hydrostatic pressure range of 0.1 to >35 MPa (Fig. 3A). The growth curves of H. hydrothermalis at 13 and 30°C had large 95% confidence intervals, masking any growth trends (data not shown).
H. pacifica also showed salinity-enhanced growth, but only at 30°C and >45 MPa (Fig. 3B). Growth was faster with 11 and 17% total salts under these conditions than with 4% total salts. (The local peak in growth rate observed at 30°C, 25 MPa, and 17% total salts was associated with a large 95% confidence interval.) At 13°C, the growth rate decreased with both increasing salinities and hydrostatic pressures (Fig. 3C). No significant growth occurred at 2°C (data not shown).
Salt-enhanced growth with increased hydrostatic pressures and low temperatures did not appear to correlate with whether the organism was halophilic (H. axialensis and H. pacifica) or moderately halophilic (H. meridiana strain Slthf1 and H. hydrothermalis).
PLFA analysis.
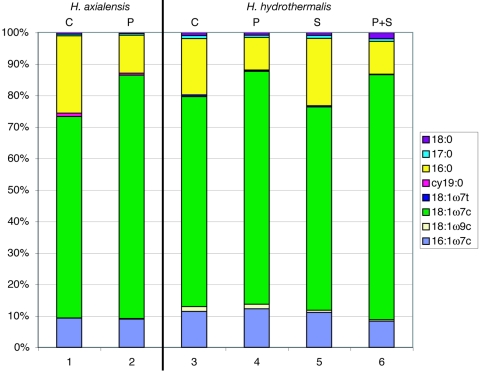
Under the conditions tested (30°C, 0.1 or 45 MPa, 4 or 11% total salts), the predominant phospholipids for both strains examined, H. axialensis and H. hydrothermalis, were 18:1ω7c, 16:0, and 16:1ω7c, comprising 94.2 to 96.3% of the total (Fig. 4; Table 2). H. hydrothermalis contained slightly more of the minor components 18:1ω9c and 18:1ω7t than H. axialensis, and these lipids decreased in concentration with increased salinity in H. hydrothermalis. H. axialensis contained slightly more cy19:0 (0.6 to 1.0%) than H. hydrothermalis (0.0 to 0.2%). At 0.1 MPa, the degree of lipid saturation in H. hydrothermalis increased with increased salinity, from 20.0 to 23.4%. The monounsaturated fatty acids (MUFA) 18:1ω7c and 16:1ω7c comprised 71.4 to 76.7% and 84.5 to 85.7% of the total at 0.1 and 45 MPa, respectively, while the saturated fatty acid 16:0 decreased in concentration, from 17.5 to 24.1% to 10.3 to 11.8%, simultaneously as the hydrostatic pressure and the proportion of MUFA increased. The medium used to grow the cells contained negligible quantities of lipids (data not shown).
FIG. 4.
Phospholipid fatty acid profiles of H. axialensis (columns 1 and 2) and H. hydrothermalis (columns 3 to 6) grown at 30°C under different conditions of hydrostatic pressure and salinity. C, growth under control conditions (4% total salts and 0.1 MPa); P, growth with elevated hydrostatic pressure (45 MPa and 4% total salts); S, growth with elevated salinity (11% total salts and 0.1 MPa); P+S, growth with both elevated hydrostatic pressure and salinity (45 MPa and 11% total salts). Only lipids that comprised ≥0.5% of the total are shown.
TABLE 2.
PLFA profiles of H. axialensis and H. hydrothermalis grown at 30°C under different salinity and hydrostatic pressure conditions
| PLFA category and identitya | Total PLFA (%) at indicated pressureb
|
|||||
|---|---|---|---|---|---|---|
|
H. axialensis
|
H. hydrothermalis
|
|||||
| 4% total salts
|
4% total salts
|
11% total salts
|
||||
| 0.1 MPa (1) | 45 MPa (2) | 0.1 MPa (3) | 45 MPa (4) | 0.1 MPa (5) | 45 MPa (6) | |
| Terminally branched saturates | ||||||
| i15:0 | 0.0 | 0.4 | 0.1 | 0.0 | 0.0 | 0.0 |
| i17:0 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 |
| a17:0 | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 | 0.4 |
| Monoenoics | ||||||
| 16:1ω9c | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| 16:1ω7c | 9.1 | 8.8 | 11.2 | 12.2 | 10.9 | 8.2 |
| 16:1ω7t | 0.1 | 0.1 | 0.1 | 0.0 | 0.1 | 0.0 |
| 16:1ω5c | 0.1 | 0.2 | 0.1 | 0.0 | 0.1 | 0.1 |
| 17:1ω6c | 0.1 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| cy17:0 | 0.4 | 0.2 | 0.2 | 0.2 | 0.2 | 0.4 |
| 18:1ω9c | 0.1 | 0.2 | 1.5 | 1.4 | 0.7 | 0.4 |
| 18:1ω7c | 62.8 | 75.7 | 65.5 | 73.5 | 63.5 | 76.7 |
| 18:1ω7t | 0.0 | 0.0 | 0.5 | 0.3 | 0.4 | 0.0 |
| 18:1ω5c | 0.1 | 0.2 | 0.1 | 0.0 | 0.2 | 0.4 |
| cy19:0 | 1.0 | 0.6 | 0.0 | 0.2 | 0.0 | 0.2 |
| Normal saturates | ||||||
| 14:0 | 0.4 | 0.2 | 0.3 | 0.0 | 0.3 | 0.0 |
| 15:0 | 0.2 | 0.2 | 0.2 | 0.0 | 0.2 | 0.0 |
| 16:0 | 24.1 | 11.8 | 17.5 | 10.3 | 21.1 | 10.3 |
| 17:0 | 0.6 | 0.5 | 1.0 | 0.7 | 0.9 | 0.9 |
| 18:0 | 0.5 | 0.4 | 1.0 | 0.9 | 1.0 | 2.0 |
| 20:1ω7c | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.2 |
| Polyenoic | ||||||
| 18:2ω6 | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 |
| Totals | ||||||
| Terminally branched saturates | 0.4 | 0.8 | 0.6 | 0.3 | 0.3 | 0.4 |
| Monoenoics | 73.8 | 86.2 | 79.4 | 87.8 | 76.2 | 86.4 |
| Normal saturates | 25.8 | 13.1 | 20.0 | 11.9 | 23.4 | 13.2 |
| Polyenoic | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 |
The lipid a15:0 was not detected. No branched monoenoic or mid-chain branched lipids were detected.
Corresponding columns in Fig. 4 are noted in parentheses after the pressures.
Protein patterns.
The cytosolic and membrane protein fractions of the same samples of H. axialensis and H. hydrothermalis as those analyzed for lipids contained 1.2 to 6.7 and 0.94 to 14.5 mg of protein ml−1, respectively. Forty micrograms of protein was run in each gel lane in triplicate, with computerized band matching (data not shown), and the results are summarized in Fig. 5.
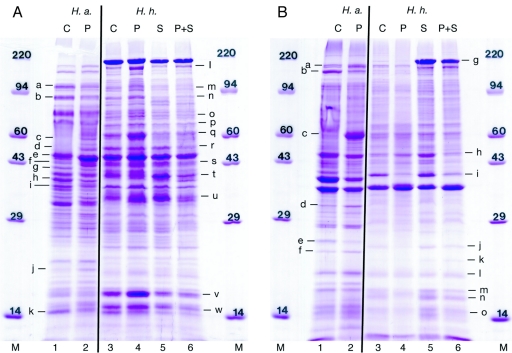
FIG. 5.
Cytosolic (A) and membrane (B) protein patterns of H. axialensis (H.a.; lanes 1 and 2) and H. hydrothermalis (H.h.; lanes 3 to 6) obtained at 30°C. Lanes M, molecular weight markers; other lanes are numbered identically to the columns in Fig. 4. Protein bands with changes in intensity are noted with letters.
When H. axialensis was grown at 30°C with 4% total salts at both 0.1 and 45 MPa, the overall protein patterns were similar within the respective cytosolic and membrane protein fractions (Fig. 5), although a suite of proteins was slightly induced or inhibited by hydrostatic pressure (Table 3). Two proteins were significantly induced by hydrostatic pressure and are considered hydrostatic-pressure-induced proteins (PIP; cytosolic band f and membrane band c [Table 3]).
TABLE 3.
Impact of elevated hydrostatic pressure (45 versus 0.1 MPa) at 30°C on proteins of H. axialensis
| Protein fraction | Protein
|
Effect of pressurea | Commentb | |
|---|---|---|---|---|
| Band label | Approximate mass (kDa) | |||
| Cytosolic (see Fig. 5A) | a | 100 | −− | P repression |
| b | 90 | −− | P repression | |
| c | 60 | + | P enhancement | |
| d | 50 | + | P enhancement | |
| e | 43 | + | P enhancement | |
| f | 42 | +++ | PIP | |
| g | 41 | −− | P repression | |
| h | 38 | − | P repression | |
| i | 36 | − | P repression | |
| j | 20 | + | P enhancement | |
| k | 15 | − | P repression | |
| Membrane (see Fig. 5B) | a | 160 | ++ | P enhancement |
| b | 150 | −− | P repression | |
| c | 60 | +++ | PIP | |
| d | 33 | + | P enhancement | |
| e | 26 | − | P repression | |
| f | 25 | + | P enhancement | |
The symbols indicate the relative degrees of change in the intensities of protein bands.
P, pressure.
Overall, the protein profiles within each protein fraction for H. hydrothermalis at 30°C were also similar to each other (Fig. 5) whether the strain was grown at approximate seawater salinity (4% total salts) or an elevated salinity (11% total salts) and at a sea surface (0.1 MPa) or deep-sea (45 MPa) hydrostatic pressure. However, comparisons of individual bands did reveal a variety of interactions between salinity and hydrostatic pressure (Table 4). Some proteins were induced only by elevated salinity, including strongly enhanced salt-induced proteins (SIP) (membrane bands g and k), and some proteins were PIP (cytosolic bands l, m, n, o, p, v, and w). However, in several instances hydrostatic pressure and salinity had opposing effects—mostly on membrane protein production (cytosolic band q and membrane bands j, l, m, n, and o)—resulting in no apparent net effect on protein expression with the combination of hydrostatic pressure and salinity. Similarly, the induction of certain cytosolic proteins by hydrostatic pressure was mitigated by an elevated salinity (cytosolic bands l, m, n, o, p, v, and w). The expression of other cytosolic proteins was inhibited only with a combination of elevated salinity and hydrostatic pressure (cytosolic bands r, s, and t). The production of one membrane protein was significantly inhibited by hydrostatic pressure but was enhanced with an increased salt concentration; when the two stresses were jointly imposed, the inhibition by hydrostatic pressure was retained as the dominant control (membrane band i). Intriguingly, some proteins that were induced by either elevated salinity or hydrostatic pressure were not induced by the combination of the two (cytosolic band u and membrane band h).
TABLE 4.
Impact of elevated hydrostatic pressure (45 versus 0.1 MPa) and salinity (11 versus 4% total salts) at 30°C on proteins of H. hydrothermalis
| Protein fraction | Protein
|
Effect of stressora
|
Comment | |||
|---|---|---|---|---|---|---|
| Band label | Approximate mass (kDa) | P | S | P and S | ||
| Cytosolic (see Fig. 5A) | l | 190 | + | S cancels P enhancement | ||
| m | 100 | + | S cancels P enhancement | |||
| n | 90 | + | S cancels P enhancement | |||
| o | 70 | + | S cancels P enhancement | |||
| p | 65 | + | S cancels P enhancement | |||
| q | 60 | + | − | S and P cancel each other | ||
| r | 50 | − | Repression by combination of S and P | |||
| s | 43 | − | Repression by combination of S and P | |||
| t | 40 | − | Repression by combination of S and P | |||
| u | 35 | + | + | S and P combined cancel protein induction by either stress alone | ||
| v | 16 | + | S cancels P enhancement | |||
| w | 15 | + | S cancels P enhancement | |||
| Membrane (see Fig. 5B) | g | 200 | +++ | +++ | SIP onlyb | |
| h | 47 | + | ++ | S and P combined cancel protein induction by either stress alone | ||
| i | 40 | −− | + | −− | P repression dominates over S enhancement | |
| j | 25 | − | + | S and P cancel each other | ||
| k | 23 | + | + | SIP onlyb | ||
| l | 21 | − | + | S and P cancel each other | ||
| m | 19 | − | + | S and P cancel each other | ||
| n | 18 | − | + | S and P cancel each other | ||
| o | 15 | − | + | S and P cancel each other | ||
The symbols indicate the relative degrees of change in the intensities of protein bands; a lack of a symbol indicates no significant change. P, pressure; S, salinity.
SIP, salt-induced protein.
DISCUSSION
The Halomonas strains tested were psychrotolerant and piezotolerant, as they were able to grow only slowly or undetectably under cold deep-sea conditions (2°C and >15 MPa) with the medium employed. The growth rates for all of the Halomonas strains were higher under the cool to warm conditions that characterize deep-sea low-temperature hydrothermal fluids and associated subseafloor environments (13 to 30°C, >15 MPa) than at 2°C. Halomonas spp. are therefore candidates as subseafloor heterotrophs, and their growth may be restricted to these habitats in the deep sea. (Cool deep basins, such as the Mediterranean Sea, which is 10 to 15°C at its depth, may also be conducive to the growth of Halomonas spp.) Indeed, molecular phylogenetic analyses of low-temperature hydrothermal fluids and nearby deep seawater at Axial Seamount on the Juan de Fuca Ridge in the northeast Pacific Ocean indicated that H. axialensis is a subseafloor resident atop this volcano (J. Z. Kaye and J. A. Baross, unpublished data). These growth rate data additionally suggest that certain Halomonas spp. will grow or thrive in cold, cool, and warm hypersaline deep-sea environments, such as the brine-seawater interface found above brine pools located in the Gulf of Mexico (65, 66), the eastern Mediterranean Sea (5, 6, 11, 12, 19, 30, 39, 64), and the Red Sea (10, 24). In fact, H. aquamarina was one of only three taxa cultured from the brine-seawater interface above the Urania Basin at an ∼3,500-m depth in the eastern Mediterranean Sea (62). The brine pools themselves are anoxic, however, and anaerobic growth at elevated salinities and hydrostatic pressures was not tested in this study.
An elevated salinity appeared to compensate partially for the depression of the growth rate caused by low temperatures. Halomonas isolates from hydrothermal vents showed increasingly halophilic responses at low temperatures and >7.5 MPa. It is possible that an enhancement of growth at low temperatures by increased salinity would be seen in H. pacifica if the cells were tested at a slightly warmer temperature of a few degrees above its Tmin of 2 to 4°C (32, 38). Low temperature is analogous to elevated hydrostatic pressure in certain effects on proteins and membranes (2), and indeed the same salt compensation effect was seen at 30°C, but only at >7.5 MPa for H. axialensis and >45 MPa for H. pacifica.
Mechanistically, hydrostatic and osmotic pressures exert opposing influences on protein hydration and thus tend to cancel the deleterious effects of each other (37, 80). Hydrostatic pressure favors hydrated protein surfaces due to volume considerations, but osmotic pressure due to solutes like trimethylamine N-oxide, glutamate, and betaine favors folded proteins via preferential exclusion and the minimization of bound water (37, 61, 80). It is important to bear in mind that within Halomonas cells the concentration of compatible solutes, not salt, increases in response to osmotic stress induced by salt ions or organic solutes (20, 76).
Antagonistic interactions between hydrostatic pressure and increased solute concentrations have been observed in a variety of disparate experiments. An elevated salinity was previously shown to increase the maximum hydrostatic pressure for growth in the psychrophiles Moritella marina (54, 73) and Streptococcus faecalis (reclassified as Enterococcus faecalis [63]) (36) and to decrease the piezosensitivity of Escherichia coli to an extremely high hydrostatic pressure of 270 MPa (25). Likewise, 15% NaCl (compared with 3% NaCl) dramatically enhanced the survival of a halophilic euryhaline marine isolate of Micrococcus roseus (reclassified as Kocuria rosea [69]) under a very high hydrostatic pressure of 138 MPa (72). Osmotic pressure (created with low concentrations of ethanol) also mitigated the hydrostatic-pressure-induced inhibition of cell division in E. coli, enabling a reversion of cell morphology from an elongated form to the canonical rod shape (71). Additional in vitro studies showed similar counterbalances between hydrostatic and osmotic pressures. The dissociation of the Arc repressor protein from DNA, its substrate, decreased linearly with increased glycerol concentrations (53). Hydrostatic pressure was also seen to reverse the effects of osmotic pressure (created with organic compounds) on DNA site selection by the restriction endonucleases EcoRI, BamHI, PvuII, and EcoRV, highlighting the opposing interplay that these two stresses have on the hydration of proteins and protein-substrate complexes (59, 60). A salt-induced enhancement of growth with elevated hydrostatic pressures (at cold and warm temperatures) is consistent with the opposing influences of salinity and hydrostatic pressure observed in a variety of systems.
In this study, hydrostatic pressure exerted the dominant control on phospholipid profiles. Consistent with all previous hydrostatic pressure studies, the degree of lipid saturation decreased with increased hydrostatic pressure (2, 82), presumably as a means to maintain membrane fluidity. Consistent with previous studies of Halomonas strains and other moderately halophilic and euryhaline species (41, 42, 44, 51, 74, 77), the proportion of MUFA decreased in concentration (and the proportion of saturated fatty acids concomitantly increased in concentration) with increased salinity, as was observed with H. hydrothermalis at 0.1 MPa and 4 or 11% total salts. An elevated salinity slightly decreased the degree to which hydrostatic pressure caused an increase in membrane unsaturation. Cyclopropane fatty acids (CFA) were in very low abundance (0.0 to 1.0%), and changes in their proportions were not apparent, as previously documented with Halomonas and other moderately halophilic and euryhaline bacteria (27, 41, 42, 45, 51, 74). While changes in the proportion of CFA with salinity are not always observed among Halomonas spp. (46), the CFA cy17:0 and cy19:0 typically comprise a much larger proportion of the phospholipids present (up to 37%) (18, 40, 67). It should be noted as well that lipid profiles vary significantly with the growth phase and between members of the family Halomonadaceae (4, 43). In this study, cells were grown to early to mid-exponential phase, whereas for the other lipid analyses cited, cells were usually harvested during the late exponential or stationary phase. This difference may explain the very low concentration of CFA observed in this experiment.
The cytosolic and membrane protein patterns determined at 30°C showed an overarching consistency at low and high hydrostatic pressures and salinities, indicating that cellular functions are generally consistent between warm, shallow and deep, marine, and hypersaline habitats. On a finer scale, however, the protein patterns revealed a variety of hydrostatic pressure and hydrostatic pressure-salinity effects. H. axialensis was grown at 0.1 and 45 MPa, and several proteins were stimulated (including two PIP) or repressed by hydrostatic pressure. H. hydrothermalis was grown under four hydrostatic pressure-salinity regimens, and while some proteins appeared to be salt or hydrostatic pressure specific and likely performed osmoregulatory or hydrostatic pressure adaptation functions, frequently the combination of elevated salinity and hydrostatic pressure illustrated that the two stresses mitigate the effects of the other. Salinity and hydrostatic pressure were thus competitive, not synergistic, for these proteins. These proteins may be involved in the adaptation of phospholipid membranes given that salinity and hydrostatic pressure exerted opposing influences on lipid saturation. A link between a salt- or hydrostatic-pressure-induced general stress response and/or salt- or hydrostatic-pressure-adapted growth is conceivable given the behavior of the interaction of these stresses. The augmentation of the production of compatible solutes in Photobacterium profundum strain SS9 by the combination of increased salinity and hydrostatic pressure (37) highlights one aspect of this connection. If there is a link, it is possible that the euryhaline phenotype of deep-sea Halomonas spp. and other bacteria may partially reflect an adaptation for growth in pressurized, in addition to hypersaline, marine environments. The protein patterns also specifically indicate that deep-sea brine-seawater interface environments may not induce the expression of certain genes during growth. Both the protein expression and growth rate data thus suggest that deep-sea hypersaline regions, in addition to cool and warm hydrothermal-vent environments, are amenable to the growth and proliferation of members of the genus Halomonas.
Acknowledgments
We sincerely appreciate the laboratory assistance of Joe Hoover, Sheryl Bolton, Robin Froisland, Steve Vance, and Mike Hudson and the programming talent of Susanne Menden-Deuer. We also thank Jody Deming and Jim Holden for discussions and manuscript suggestions.
Support was provided by a Washington Sea Grant (NA 76RG0119) and the NASA Astrobiology Institute at the Carnegie Geophysical Laboratory, Washington, D.C.
REFERENCES
- 1.Antunes, A., W. Eder, P. Fareleira, H. Santos, and R. Huber. 2003. Salinisphaera shabanensis gen. nov., sp. nov., a novel, moderately halophilic bacterium from the brine-seawater interface of the Shaban Deep, Red Sea. Extremophiles 7:29-34. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett, D. H. 2002. Pressure effects on in vivo microbial processes. Biochim. Biophys. Acta 1595:367-381. [DOI] [PubMed] [Google Scholar]
- 3.Baumann, L., R. D. Bowditch, and P. Baumann. 1983. Description of Deleya gen. nov. created to accommodate the marine species Alcaligenes aestus, A. pacificus, A. cupidus, A. venustus, and Pseudomonas marina. Int. J. Syst. Bacteriol. 33:793-802. [Google Scholar]
- 4.Bertone, S., M. Giacomini, C. Ruggiero, C. Piccarolo, and L. Calegari. 1996. Automated systems for identification of heterotrophic marine bacteria on the basis of their fatty acid composition. Appl. Environ. Microbiol. 62:2122-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boldrin, A., and S. Rabitti. 1990. Hydrography of the brines in the Bannock and Tyro anoxic basins (eastern Mediterranean). Mar. Chem. 31:21-33. [Google Scholar]
- 6.Camerlenghi, A. 1990. Anoxic basins of the eastern Mediterranean: geological framework. Mar. Chem. 31:1-19. [Google Scholar]
- 7.Canganella, F., J. M. Gonzalez, M. Yanagibayashi, C. Kato, and K. Horikoshi. 1997. Pressure and temperature effects on growth and viability of the hyperthermophilic archaeon Thermococcus peptonophilus. Arch. Microbiol. 168:1-7. [DOI] [PubMed] [Google Scholar]
- 8.Chan, K.-Y., and O. C. Leung. 1979. Nutrition and growth of the moderately halophilic bacteria Micrococcus morrhuae K-17 and Micrococcus luteus K-15. Microbios 25:71-84. [PubMed] [Google Scholar]
- 9.Chi, E., and D. H. Bartlett. 1993. Use of a reporter gene to follow high-pressure signal transduction in the deep-sea bacterium Photobacterium sp. strain SS9. J. Bacteriol. 175:7533-7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Degens, E. T., and D. A. Ross (ed.). 1969. Hot brines and recent heavy metal deposits in the Red Sea. Springer-Verlag, New York, N.Y.
- 11.de Lange, G. J., G. Catalano, G. P. Klinkhammer, and G. W. Luther III. 1990. The interface between oxic seawater and the anoxic Bannock brine; its sharpness and the consequences for the redox-related cycling of Mn and Ba. Mar. Chem. 31:205-217. [Google Scholar]
- 12.de Lange, G. J., J. J. Middelburg, C. H. van der Weijden, G. Catalano, G. W. Luther III, D. J. Hydes, J. R. W. Woittiez, and G. P. Klinkhammer. 1990. Composition of anoxic hypersaline brines in the Tyro and Bannock basins, eastern Mediterranean. Mar. Chem. 31:63-88. [Google Scholar]
- 13.DeLong, E. F., and A. A. Yayanos. 1985. Adaptation of the membrane lipids of a deep-sea bacterium to changes in hydrostatic pressure. Science 228:1101-1103. [DOI] [PubMed] [Google Scholar]
- 14.DeLong, E. F., and A. A. Yayanos. 1986. Biochemical function and ecological significance of novel bacterial lipids in deep-sea procaryotes. Appl. Environ. Microbiol. 51:730-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deming, J. W. 1986. Ecological strategies of barophilic bacteria in the deep ocean. Microbiol. Sci. 3:205-211. [PubMed] [Google Scholar]
- 16.Deming, J. W., L. K. Somers, W. L. Straube, D. G. Swartz, and M. T. MacDonell. 1988. Isolation of an obligately barophilic bacterium and description of a new genus, Colwellia gen. nov. Syst. Appl. Microbiol. 10:152-160. [Google Scholar]
- 17.Eder, W., L. L. Jahnke, M. Schmidt, and R. Huber. 2001. Microbial diversity of the brine-seawater interface of the Kebrit Deep, Red Sea, studied via 16S rRNA gene sequences and cultivation methods. Appl. Environ. Microbiol. 67:3077-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franzmann, P. D., and B. J. Tindall. 1990. A chemotaxonomic study of members of the family Halomonadaceae. Syst. Appl. Microbiol. 13:142-147. [Google Scholar]
- 19.Fusi, N., G. Aloisi de Larderel, A. Borello, O. Amelio, D. Castradori, A. Negri, B. Rimoldi, R. Sanvoisin, P. Tarbini, and M. B. Cita. 1996. Marine geology of the Medriff corridor, Mediterranean Ridge. Island Arc 5:420-439. [Google Scholar]
- 20.Galinski, E. A. 1995. Osmoadaptation in bacteria. Adv. Microb. Physiol. 37:273-328. [PubMed] [Google Scholar]
- 21.Goldman, M., R. H. Deibel, and C. F. Niven, Jr. 1963. Interrelationship between temperature and sodium chloride on growth of lactic acid bacteria isolated from meat-curing brines. J. Bacteriol. 85:1017-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guérin-Faublée, V., L. Rosso, M. Vigneulle, and J.-P. Flandrois. 1995. The effect of incubation temperature and sodium chloride concentration on the growth kinetics of Vibrio anguillarum and Vibrio anguillarum-related organisms. J. Appl. Bacteriol. 78:621-629. [DOI] [PubMed] [Google Scholar]
- 23.Hao, M. V., M. Kocur, and K. Komagata. 1984. Marinococcus gen. nov., a new genus for motile cocci with meso-diaminopimelic acid in the cell wall; and Marinococcus albus sp. nov. and Marinococcus halophilus (Novitsky and Kushner) comb. nov. J. Gen. Appl. Microbiol. 30:449-459. [Google Scholar]
- 24.Hartmann, M., J. C. Scholten, P. Stoffers, and F. Wehner. 1998. Hydrographic structure of brine-filled deeps in the Red Sea—new results from the Shaban, Kebrit, Atlantis II, and Discovery Deep. Mar. Geol. 144:311-330. [Google Scholar]
- 25.Hauben, K. J. A., K. Bernaerts, and C. W. Michiels. 1998. Protective effect of calcium on inactivation of Escherichia coli by high hydrostatic pressure. J. Appl. Microbiol. 85:678-684. [DOI] [PubMed] [Google Scholar]
- 26.Hei, D. J., and D. S. Clark. 1994. Pressure stabilization of proteins from extreme thermophiles. Appl. Environ. Microbiol. 60:932-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiramatsu, T., Y. Ohno, H. Hara, I. Yano, and M. Masui. 1980. Effects of NaCl concentration on the envelope components in a moderately halophilic bacterium, Pseudomonas halosaccharolytica, p. 189-200. In H. Morisita and M. Masui (ed.), Saline environment: physiological and biochemical adaptation in halophilic microorganisms. Nakanishi Printing, Kyoto, Japan.
- 28.Holden, J. F., and J. A. Baross. 1995. Enhanced thermotolerance by hydrostatic pressure in the deep-sea hyperthermophile Pyrococcus strain ES4. FEMS Microbiol. Ecol. 18:27-34. [Google Scholar]
- 29.Ishida, Y. 1970. Growth behavior of halobacteria in relation to concentration of NaCl and temperature of environments. Bull. Jpn. Soc. Sci. Fish. 36:397-401. [Google Scholar]
- 30.Jongsma, D., A. R. Fortuin, W. Huson, S. R. Troelstra, G. T. Klaver, J. M. Peters, D. van Harten, G. J. de Lange, and L. ten Haven. 1983. Discovery of an anoxic basin within the Strabo Trench, eastern Mediterranean. Nature 305:795-797. [Google Scholar]
- 31.Kaneko, H., H. Takami, A. Inoue, and K. Horikoshi. 2000. Effects of hydrostatic pressure and temperature on growth and lipid composition of the inner membrane of barotolerant Pseudomonas sp. BT1 isolated from the deep-sea. Biosci. Biotechnol. Biochem. 64:72-79. [DOI] [PubMed] [Google Scholar]
- 32.Kaye, J. Z., and J. A. Baross. 2000. High incidence of halotolerant bacteria in Pacific hydrothermal-vent and pelagic environments. FEMS Microbiol. Ecol. 32:249-260. [DOI] [PubMed] [Google Scholar]
- 33.Kaye, J. Z., M. C. Márquez, A. Ventosa, and J. A. Baross. 2004. Halomonas neptunia sp. nov., Halomonas sulfidaeris sp. nov., Halomonas axialensis sp. nov., and Halomonas hydrothermalis sp. nov.: halophilic bacteria isolated from deep-sea hydrothermal-vent environments. Int. J. Syst. E vol. Microbiol. 54:499-511. [DOI] [PubMed] [Google Scholar]
- 34.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 35.MacDonell, M. T., and R. R. Colwell. 1985. Phylogeny of the Vibrionaceae, and recommendation for two new genera, Listonella and Shewanella. Syst. Appl. Microbiol. 6:171-182. [Google Scholar]
- 36.Marquis, R. E., and C. E. ZoBell. 1971. Magnesium and calcium ions enhance barotolerance of streptococci. Arch. Microbiol. 79:80-92. [DOI] [PubMed] [Google Scholar]
- 37.Martin, D. D., D. H. Bartlett, and M. F. Roberts. 2002. Solute accumulation in the deep-sea bacterium Photobacterium profundum. Extremophiles 6:507-514. [DOI] [PubMed] [Google Scholar]
- 38.Mata, J. A., J. Martínez-Cánovas, E. Quesada, and V. Béjar. 2002. A detailed phenotypic characterisation of the type strains of Halomonas species. Syst. Appl. Microbiol. 25:360-375. [DOI] [PubMed] [Google Scholar]
- 39.MEDRIFF Consortium. 1995. Three brine lakes discovered in the seafloor of the eastern Mediterranean. Eos Trans. AGU 76:313, 318. [Google Scholar]
- 40.Mergaert, J., A. Verhelst, M. C. Cnockaert, T.-L. Tan, and J. Swings. 2001. Characterization of facultative oligotrophic bacteria from polar seas by analysis of their fatty acids and 16S rDNA sequences. Syst. Appl. Microbiol. 24:98-107. [DOI] [PubMed] [Google Scholar]
- 41.Monteoliva-Sanchez, M., M. R. Ferrer, A. Ramos-Cormenzana, E. Quesada, and M. Monteoliva. 1988. Cellular fatty acid composition of Deleya halophila: effect of growth temperature and salt concentration. J. Gen. Microbiol. 134:199-203. [Google Scholar]
- 42.Monteoliva-Sanchez, M., and A. Ramos-Cormenzana. 1986. Effect of growth temperature and salt concentration on the fatty acid composition of Flavobacterium halmephilum CCM2831. FEMS Microbiol. Lett. 33:51-54. [Google Scholar]
- 43.Monteoliva-Sanchez, M., and A. Ramos-Cormenzana. 1987. Cellular fatty acid composition in moderately halophilic gram-negative rods. J. Appl. Bacteriol. 62:361-366. [Google Scholar]
- 44.Monteoliva-Sanchez, M., and A. Ramos-Cormenzana. 1987. Cellular fatty acid composition of Planococcus halophilus NRCC 14033 as affected by growth temperature and salt concentration. Curr. Microbiol. 15:133-136. [Google Scholar]
- 45.Monteoliva-Sanchez, M., A. Ramos-Cormenzana, and N. J. Russell. 1993. The effect of salinity and compatible solutes on the biosynthesis of cyclopropane fatty acids in Pseudomonas halosaccharolytica. J. Gen. Microbiol. 139:1877-1884. [Google Scholar]
- 46.Naganuma, T., and K. Horikoshi. 1993. Extremely halotolerant Halomonas sp. isolated from a tar ball and its cellular fatty acid composition. J. Mar. Biotechnol. 1:151-157. [Google Scholar]
- 47.Nichols, D. S., J. Olley, H. Garda, R. R. Brenner, and T. A. McMeekin. 2000. Effect of temperature and salinity stress on growth and lipid composition of Shewanella gelidimarina. Appl. Environ. Microbiol. 66:2422-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Novitsky, T. J., and D. J. Kushner. 1975. Influence of temperature and salt concentration on the growth of a facultatively halophilic “Micrococcus” sp. Can. J. Microbiol. 21:107-110. [DOI] [PubMed] [Google Scholar]
- 49.Novitsky, T. J., and D. J. Kushner. 1976. Planococcus halophilus sp. nov., a facultatively halophilic coccus. Int. J. Syst. Bacteriol. 26:53-57. [Google Scholar]
- 50.O'Farrell, P. H. 1975. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 250:4007-4021. [PMC free article] [PubMed] [Google Scholar]
- 51.Ohno, Y., I. Yano, and M. Masui. 1979. Effect of NaCl concentration and temperature on the phospholipid and fatty acid compositions of a moderately halophilic bacterium, Pseudomonas halosaccharolytica. J. Biochem. 85:413-421. [DOI] [PubMed] [Google Scholar]
- 52.Okamoto, T., K. Fujioka, and T. Naganuma. 2001. Phylogenetic similarity of aerobic gram-negative halophilic bacteria from a deep-sea hydrothermal mound and Antarctic habitats. Polar Biosci. 14:1-9. [Google Scholar]
- 53.Oliveira, A. C., L. P. Gaspar, A. T. Da Poian, and J. L. Silva. 1994. Arc repressor will not denature under pressure in the absence of water. J. Mol. Biol. 240:184-187. [DOI] [PubMed] [Google Scholar]
- 54.Palmer, D. S., and L. J. Albright. 1970. Salinity effects on the maximum hydrostatic pressure for growth of the marine psychrophilic bacterium, Vibrio marinus. Limnol. Oceanogr. 15:343-347. [Google Scholar]
- 55.Pledger, R. J., B. C. Crump, and J. A. Baross. 1994. A barophilic response by two hyperthermophilic, hydrothermal vent Archaea: an upward shift in the optimal temperature and acceleration of growth rate at supra-optimal temperatures by elevated pressure. FEMS Microbiol. Ecol. 14:233-241. [Google Scholar]
- 56.Porter, K. G., and Y. S. Feig. 1980. The use of DAPI for identifying and counting microflora. Limnol. Oceanogr. 25:943-948. [Google Scholar]
- 57.Quesada, E., V. Bejar, M. J. Valderrama, and A. Ramos-Cormenzana. 1987. Growth characteristics and salt requirements of Deleya halophila in a defined medium. Curr. Microbiol. 16:21-25. [Google Scholar]
- 58.Reddy, G. S. N., P. U. M. Raghavan, N. B. Sarita, J. S. S. Prakash, N. Nagesh, D. Delille, and S. Shivaji. 2003. Halomonas glaciei sp. nov. isolated from fast ice of Adelie Land, Antarctica. Extremophiles 7:55-61. [DOI] [PubMed] [Google Scholar]
- 59.Robinson, C. R., and S. G. Sligar. 1994. Hydrostatic pressure reverses osmotic pressure effects on the specificity of EcoRI-DNA interactions. Biochemistry 33:3787-3793. [DOI] [PubMed] [Google Scholar]
- 60.Robinson, C. R., and S. G. Sligar. 1995. Heterogeneity in molecular recognition by restriction endonucleases: osmotic and hydrostatic pressure effects on BamHI, Pvu II, and EcoRV specificity. Proc. Natl. Acad. Sci. USA 92:3444-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robinson, C. R., and S. G. Sligar. 1995. Hydrostatic and osmotic pressure as tools to study macromolecular recognition. Methods Enzymol. 259:395-427. [DOI] [PubMed] [Google Scholar]
- 62.Sass, A. M., H. Sass, M. J. L. Coolen, H. Cypionka, and J. Overmann. 2001. Microbial communities in the chemocline of a hypersaline deep-sea basin (Urania Basin, Mediterranean Sea). Appl. Environ. Microbiol. 67:5392-5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schleifer, K. H., and R. Kilpper-Bälz. 1984. Transfer of Streptococcus faecalis and Streptococcus faecium to the genus Enterococcus nom. rev. as Enterococcus faecalis comb. nov. and Enterococcus faecium comb. nov. Int. J. Syst. Bacteriol. 34:31-34. [Google Scholar]
- 64.Scientific Staff of Cruise Bannock 1984-12. 1985. Gypsum precipitation from cold brines in an anoxic basin in the eastern Mediterranean. Nature 314:152-154. [Google Scholar]
- 65.Sheu, D. D. 1990. The anoxic Orca Basin (Gulf of Mexico): geochemistry of brines and sediments. Rev. Aquat. Sci. 2:491-507. [Google Scholar]
- 66.Shokes, R. F., P. K. Trabant, B. J. Presley, and D. F. Reid. 1977. Anoxic, hypersaline basin in the northern Gulf of Mexico. Science 196:1443-1446. [DOI] [PubMed] [Google Scholar]
- 67.Skerratt, J. H., P. D. Nichols, C. A. Mancuso, S. R. James, S. J. Dobson, T. A. McMeekin, and H. Burton. 1991. The phospholipid ester-linked fatty acid composition of members of the family Halomonadaceae and genus Flavobacterium: a chemotaxonomic guide. Syst. Appl. Microbiol. 14:8-13. [Google Scholar]
- 68.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 69.Stackebrandt, E., C. Koch, O. Gvozdiak, and P. Schumann. 1995. Taxonomic dissection of the genus Micrococcus: Kocuria gen. nov., Nesterenkonia gen. nov., Kytococcus gen. nov., Dermacoccus gen. nov., and Micrococcus Cohn 1872 gen. emend. Int. J. Syst. Bacteriol. 45:682-692. [DOI] [PubMed] [Google Scholar]
- 70.Takami, H., K. Kobata, T. Nagahama, H. Kobayashi, A. Inoue, and K. Horikoshi. 1999. Biodiversity in deep-sea sites located near the south part of Japan. Extremophiles 3:97-102. [DOI] [PubMed] [Google Scholar]
- 71.Tamura, K., T. Shimizu, and H. Kourai. 1992. Effects of ethanol on the growth and elongation of Escherichia coli under high pressures up to 40 MPa. FEMS Microbiol. Lett. 99:321-324. [DOI] [PubMed] [Google Scholar]
- 72.Tanaka, T., J. G. Burgess, and P. C. Wright. 2001. High-pressure adaptation by salt stress in a moderately halophilic bacterium obtained from open seawater. Appl. Microbiol. Biotechnol. 57:200-204. [DOI] [PubMed] [Google Scholar]
- 73.Urakawa, H., K. Kita-Tsukamoto, S. E. Steven, K. Ohwada, and R. R. Colwell. 1998. A proposal to transfer Vibrio marinus (Russell 1891) to a new genus, Moritella gen. nov., as Moritella marina comb. nov. FEMS Microbiol. Lett. 165:373-378. [DOI] [PubMed] [Google Scholar]
- 74.Valderrama, M. J., M. Monteoliva-Sanchez, E. Quesada, and A. Ramos-Cormenzana. 1998. Influence of salt concentration on the cellular fatty acid composition of the moderately halophilic bacterium Halomonas salina. Res. Microbiol. 149:675-679. [DOI] [PubMed] [Google Scholar]
- 75.Valderrama, M. J., E. Quesada, V. Béjar, A. Ventosa, M. C. Gutierrez, F. Ruíz-Berraquero, and A. Ramos-Cormenzana. 1991. Deleya salina sp. nov., a moderately halophilic Gram-negative bacterium. Int. J. Syst. Bacteriol. 41:377-384. [Google Scholar]
- 76.Ventosa, A., J. J. Nieto, and A. Oren. 1998. Biology of moderately halophilic aerobic bacteria. Microbiol. Mol. Biol. Rev. 62:504-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vreeland, R. H., S. L. Daigle, S. T. Fields, D. J. Hart, and E. L. Martin. 1991. Physiology of Halomonas elongata in different NaCl concentrations, p. 233-241. In F. Rodríguez-Valera (ed.), General and applied aspects of halophilic microorganisms. Plenum Press, New York, N.Y.
- 78.Vreeland, R. H., and E. L. Martin. 1980. Growth characteristics, effects of temperature, and ion specificity of the halotolerant bacterium Halomonas elongata. Can. J. Microbiol. 26:746-752. [Google Scholar]
- 79.White, D. C., W. M. Davis, J. S. Nickels, J. D. King, and R. J. Bobbie. 1979. Determination of the sedimentary microbial biomass by extractable lipid phosphate. Oecologia 40:51-62. [DOI] [PubMed] [Google Scholar]
- 80.Yancey, P. H., W. R. Blake, and J. Conley. 2002. Unusual organic osmolytes in deep-sea animals: adaptations to hydrostatic pressure and other perturbants. Comp. Biochem. Physiol. A 133:667-676. [DOI] [PubMed] [Google Scholar]
- 81.Yayanos, A. A. 1986. Evolution and ecological implications of the properties of deep-sea barophilic bacteria. Proc. Natl. Acad. Sci. USA 83:9542-9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yayanos, A. A. 1995. Microbiology to 10,500 meters in the deep sea. Annu. Rev. Microbiol. 49:777-805. [DOI] [PubMed] [Google Scholar]
- 83.Yayanos, A. A., and E. F. DeLong. 1987. Deep-sea bacterial fitness to environmental temperatures and pressures, p. 17-32. In H. W. Jannasch, R. E. Marquis, and A. M. Zimmerman (ed.), Current perspectives in high pressure biology. Academic Press, London, United Kingdom.
- 84.Yayanos, A. A., and R. Van Boxtel. 1982. Coupling device for quick high-pressure connections to 100 MPa. Rev. Sci. Instrum. 53:704-705. [Google Scholar]