Abstract
Parietal cell loss represents the initial step in the sequential progression toward gastric adenocarcinoma. In the setting of chronic inflammation, the expansion of the mucosal response to parietal cell loss characterizes a crucial transition en route to gastric dysplasia. Here, we detail methods for using the selective estrogen receptor modulator tamoxifen as a novel tool to rapidly and reversibly induce parietal cell loss in mice in order to study the mechanisms that underlie these pre-neoplastic events.
Keywords: Tamoxifen, Parietal cell loss, Metaplasia, Oxyntic atrophy, Spasmolytic polypeptide-expressing metaplasia (SPEM)
1 Introduction
Gastric adenocarcinoma remains one of the leading causes of cancer-related deaths worldwide [1]. The sequence of events leading to the development of gastric dysplasia and neoplasia begins with the loss of acid-secreting parietal cells, a process known as oxyntic atrophy, followed by the expansion of pre-neoplastic changes in the setting of chronic inflammation [2]. The early mucosal response to oxyntic atrophy includes reorganization of the gastric unit, characterized initially by an increased proliferation of gastric progenitor cells and the reprogramming of post-mitotic chief cells at the base of the gastric gland into a proliferating population of metaplastic cells [3]. Overall, the pattern of gastric unit reorganization that characterizes the response to oxyntic atrophy is known as spasmolytic polypeptide-expressing metaplasia (SPEM), as the metaplastic chief cells express spasmolytic polypeptide (also known as trefoil factor family 2; TFF2). SPEM can either be a transient alteration in the gastric landscape, followed by repair and restoration of normal architecture, or it can represent a crucial pre-neoplastic event en route to gastric dysplasia in the setting of chronic inflammation. The study of the mechanisms underlying the development and evolution of SPEM has been accelerated by recently developed tools [4–6] which rapidly induce SPEM in animal models of gastric dysplasia. Here, we describe the discovery and use of the selective estrogen receptor modulator, tamoxifen, as a model for studying SPEM.
In addition to its widespread therapeutic use as hormonal therapy, tamoxifen has recently found a role in conditional gene targeting in the mouse [7]. Notably, the development of a ligand-dependent Cre-ER recombinase, in which the Cre enzyme is fused to a mutated hormone-binding domain of the estrogen receptor, has allowed for the use of tamoxifen to modulate gene expression in a spatiotemporal fashion [8]. As a result, tamoxifen now serves as a tool for regulating tissue-specific Cre activity.
However, the use of tamoxifen for induction of the Cre-ER recombinase led to a serendipitous discovery in the mouse stomach that has broadened its role beyond the Cre-ER system and implicated tamoxifen as a unique agent for studying the early events following oxyntic atrophy [9, 10]. Serial intra-peritoneal injections of various strains of wild-type mice with tamoxifen induced apoptosis in the vast majority of parietal cells, metaplastic changes in the chief cells at the bases of the gastric glands, and an increased proliferation of gastric progenitor cells, changes characteristic of and consistent with SPEM. This effect is reproducible [11], estrogen-independent, and reversible, with a normalization of gastric histology within weeks of tamoxifen discontinuation [10]. The tamoxifen administration protocol described below therefore offers a unique method for reproducing oxyntic atrophy and dissecting early pre-neoplastic events leading to gastric dysplasia.
2 Materials
2.1 Preparation of Tamoxifen Stock
2.2 Mouse Injection
Insulin syringe with needle (0.5 mL, 27 G × 0.5 in.).
Balance.
Alcohol wipes.
3 Methods
Carry out all procedures at room temperature unless otherwise specified.
The following protocol corresponds to a tamoxifen solution dissolved in 10 % ethanol and 90 % sunflower seed oil (see Note 3).
3.1 Preparation of Tamoxifen Stock
Weigh mice (see Note 4).
Weigh out 25 mg of dry tamoxifen and place it in a 1.5 mL Eppendorf tube (see Note 5).
Slowly add 100 μL of 100 % ethanol, trying to keep the tamoxifen at the bottom of the tube. Do not shake, mix, or pipet.
Measure 900 μL of sterile sunflower seed oil in a separate 1.5 mL Eppendorf tube.
Sonicate the tamoxifen/ethanol mixture in the Eppendorf tube at 40 % amplitude in 20-s pulses until the tamoxifen is completely dissolved (see Note 6).
Immediately combine the tamoxifen/ethanol mixture with the sunflower seed oil. Cap and vortex the solution to ensure adequate mixing (see Note 7).
The tamoxifen mixture can be stored at 4 °C for up to 3 days or at −20 °C indefinitely (see Note 8). Allow the mixture to warm to room temperature prior to injection.
3.2 Tamoxifen Treatment
Using the insulin syringe needle, measure out the appropriate amount of the tamoxifen mixture so as to inject 5 mg tamoxifen for every 20 g mouse body weight (see Note 9).
Sanitize the injection site by wiping the mouse abdomen with an alcohol wipe. Intra-peritoneally inject the vehicle (10 % ethanol/90 % sunflower seed oil) or tamoxifen mixture (see Note 10).
Repeat the injection for 3 consecutive days using the same tamoxifen stock, stored at 4 °C.
Mouse stomachs can be harvested at any time following the first injection or thereafter, and tissue can be processed accordingly (Figs. 1 and 2, and see Note 11).
Fig. 1.
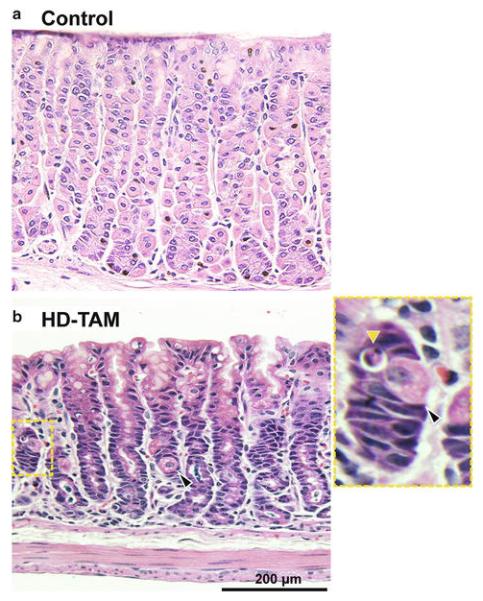
Tamoxifen treatment results in acute parietal cell loss. Representative hematoxylin and eosin stain of gastric corpus from wild-type C57BL/6 mice after intra-peritoneal injection with 3 days of either vehicle (a; Control) or 5 mg/20 g body weight tamoxifen (b; HD-TAM). Note the relative decrease in parietal cells (black arrowhead) compared to the vehicle-treated mouse. An apoptotic body (yellow arrowhead) adjacent to a dying parietal cell is highlighted (inset)
Fig. 2.
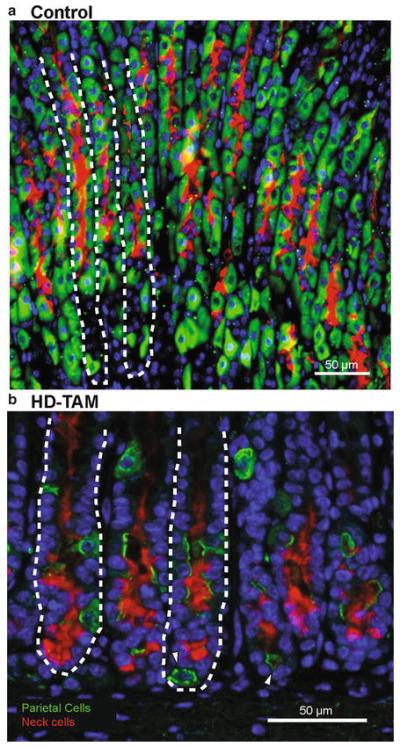
Tamoxifen treatment causes acute parietal cell loss and alters the GSII expression pattern in gastric units. (a) Representative immunostain of the gastric corpus of a mouse intra-peritoneally injected with vehicle alone (Control) for 3 days demonstrates normal-appearing gastric units, highlighted by abundant parietal cells (stained with H+/K+ ATPase; green) and neck cells (stained with GSII; red). Nuclei are stained with Hoescht (blue), and representative gastric units are highlighted by dashed lines. (b) A representative immunostain of the gastric corpus from a mouse intra-peritoneally injected with 5 mg/20 g body weight tamoxifen for 3 days (HD-TAM) shows an acute loss of parietal cells, as demonstrated by the relative paucity of VEGFb-staining cells (green). Fragments of parietal cells are highlighted by the white arrowheads. In addition, note the shift in GSII expression (red) toward the bases of glands in tamoxifen-treated mice compared to the vehicle-treated controls. Nuclei are stained with Hoescht (blue), and representative gastric units are highlighted by dashed lines
Table 1.
Characteristics of various inducers of SPEM
| Agent | Route of administration | Time to onset of SPEM |
Inflammatory response? |
Reversibility |
|---|---|---|---|---|
| Helicobacter pylori a | Oral gavage | Months | Yes | No |
| Helicobacter felis a | Oral gavage | Months | Yes | No |
| DMP-777 | Oral gavage | 10-14 days | No | Yes |
| L-635 | Oral gavage | 3 days | Yes | Yes |
| Tamoxifen | Intra-peritoneal, oral gavage b |
3 days | Scant | Yes |
See text and associated references for more details
Reported in C57BL/6 mice and Mongolian gerbils
Other forms of tamoxifen administration have been described and are referenced in the text
Acknowledgments
The protocol described here is based on previous studies from our lab, all performed according to protocols approved by the Washington University School of Medicine Animal Studies Committee. We acknowledge the Advanced Imaging and Tissue Analysis Core of the Washington University Digestive Disease Core Center (DDRCC) for histological preparation.
This work was supported by grants from the National Institutes of Health to Jason C. Mills (R01 DK094989, 2P30 DK052574) and to Jose B. Saenz (T32 DK007130-42).
4 Notes
The source of tamoxifen has no appreciable effect on the ability to induce parietal cell loss. Tamoxifen stocks from three separate commercial suppliers, Sigma (St. Louis, MO), Cayman Chemical Company (Ann Arbor, MI), and Toronto Research Company (Toronto, Canada), have demonstrated similar efficacy [10]. In addition, parietal cell toxicity is specific to tamoxifen and not a general toxic effect of selective estrogen receptor modulators, as treatment with raloxifene, a member of the estrogen receptor modulator family with pro- and anti-estrogenic effects, had no appreciable toxicity at a comparable dose [10].
To sterilize the sunflower seed oil, heat an appropriate amount in an Erlenmeyer flask on a hot plate to 85–90 °C for 15–20 min. Do not boil. Allow the flask to cool and store 40-mL aliquots at room temperature. Alternatively, the sunflower seed oil can be autoclaved prior to use.
The free base form of tamoxifen and one of its commonly used active metabolites, 4-hydroxytamoxifen (see Note 12), are largely insoluble in water. The original formulation for intraperitoneal injection was found to be soluble in 60 % ethanol [12], and its solubility has since been optimized in a sunflower seed oil/ethanol mixture (see Note 13). Tamoxifen citrate, an oral formulation that has been developed for administering tamoxifen to mice via chow ([13]; see Note 10), is soluble in water at 0.3 mg/L at 20 °C. Tamoxifen-free base powder should be stored at −20 °C in the dark.
Our experience has shown that three different wild-type mouse strains ( C57BL/6, BALB/c, and FVB/N; all purchased from the Jackson Laboratory) have similar gastric mucosal responses to tamoxifen treatment [10]. In our limited experience with the strain, BALB/c mice are particularly sensitive to tamoxifen treatment, with mice commonly dying of unknown causes during treatment. Mice are typically used at 6–8 weeks of age, but SPEM is effectively induced in mice as old as 6 months of age. The effects on older mice are less obvious, potentially due to increased body fat causing changes in tamoxifen metabolism and distribution.
An injection dose of 5 mg/20 g mouse weight over 3 days results in a dramatic phenotype, with >90 % loss of parietal cells, a significant increase in gastric progenitor cells, and morphologic changes in the chief cells at the bases of glands in the gastric corpus, histologic changes consistent with the induction of SPEM ([10], Figs. 1b and 2b). However, we have previously shown that tamoxifen injections at lower doses (≤1 mg/20 g body weight) can be used for efficient, inducible Cre-mediated recombination in the context of the Cre-ERT/loxP system, without the development of SPEM [9]. It is thus possible to obtain specific recombination of floxed alleles in tamoxifen-inducible Cre lines in a dose-dependent manner, while avoiding the stomach-altering effects seen at higher tamoxifen doses. Interestingly, though this has not been formally tested, SPEM induction by tamoxifen seems to have an all-or-none response, where no detectable damage can be seen at ≤1 mg/20 g mouse body weight, but ≥3 mg/20 g mouse body weight causes near complete SPEM, without an intermediate phenotype.
Make sure to wear protective headphones when using the sonicator.
Vortex the solution for at least 20 s. Allow the solution to sit at room temperature for several minutes. Proper mixing is crucial, and the mixture should be homogeneous. If it looks cloudy or layered, discard the mixture and start over.
No appreciable decline in the ability of tamoxifen to induce SPEM has been seen for tamoxifen mixtures stored at 4 °C over the duration of injections. Similarly, the tamoxifen stock can be stored at −20 °C until further use. Our lab, however, makes a fresh tamoxifen stock prior to each treatment regimen and uses this stock for the duration of the treatment.
Given the viscosity of the tamoxifen mixture, aspiration into the syringe can take 10–15 s.
Various tamoxifen formulations and numerous modes of tamoxifen administration have been reported. We focus here on intra-peritoneal administration, which we use most commonly, though we also observe SPEM induction with oral gavage. It is worth noting that other methods, in addition to oral gavage [14], for inducing Cre recombinase activity via tamoxifen have been used, including via drinking water [15], chow [13, 16], and subcutaneous implantation [17]. In that respect, we can only attest that oral gavage and intra-peritoneal administration of tamoxifen cause SPEM and have not tested the effects of other modes of tamoxifen administration.
The effects of tamoxifen on the mouse stomach can be seen within 12–24 h of the first intra-peritoneal injection [10]. Our laboratory nomenclature designates the first day of tamoxifen injection as day 0 (D0), with the last day of injection corresponding to day 2 (D2). A recent report found that a single intra-peritoneal injection at 4 mg/25 g mouse body weight induced a 57 % loss of parietal cells in the gastric corpus [11]. In our experience, the peak effect (i.e., maximal parietal cell loss, see Figs. 1b and 2b) is seen at 1 day following the third tamoxifen injection (D3). We have also achieved ≥90 % loss of parietal cells at D3 even after a single injection of tamoxifen at 5 mg/20 g mouse body weight. The single-injection protocol, however, shows more variability between mice than the 3-day injection protocol. A recovery of the gastric epithelium and a return to normal histology are seen within 14–21 days [10]. Like previously described pharmacologic induction of SPEM (see Note 14), the effects of tamoxifen on the mouse stomach are transient. Many studies using tamoxifen-inducible Cre lines wait at least 2 weeks prior to assessing recombination, by which point parietal cells have largely recovered. This may explain how tamoxifen-induced parietal cell loss is often missed by investigators using tamoxifen to induce gene recombination in the stomach.
Tamoxifen is a prodrug that is hepatically metabolized to produce two predominant active metabolites, 4-hydroxytamoxifen and N-desmethyl-4-hydroxytamoxifen [18]. Multiple studies have used 4-hydroxytamoxifen for induction of the Cre-ERT2 system. It is worth noting that 4-hydroxytamoxifen has shown higher affinity for the estrogen receptor than tamoxifen in vitro [19] and a greater inhibitory effect on proliferation of normal human breast cells as well as breast cancer cell lines in culture [20–22]. Differential effects between tamoxifen and 4-hydroxytamoxifen have also been reported in apoptosis of human mammary epithelial cells [23] and uterine gene expression in rats [24]. In our limited experience, intra-peritoneally administered 4-hydroxytamoxifen, at commonly used doses for Cre recombinase induction, induces less SPEM in mice compared to similar doses of tamoxifen.
It has been speculated that the observed effects of intraperitoneal tamoxifen administration could be unrelated to the tamoxifen itself and rather an effect of the ethanol solvent on parietal cells. Though the effect of ethanol on parietal cell membranes and H+/K+ ATPase function has been reported [25], our experience has shown that intra-peritoneal injection of mice with ethanol does not induce substantial parietal cell loss. On the other hand, excluding ethanol as a solvent results in poor solubility of tamoxifen. Oral gavage or intra-peritoneal injection of the resulting suspension, rather than solution, might cause suboptimal absorption and less substantial and/or consistent SPEM induction. Differences in solubilization methods may also explain why some investigators using tamoxifen for Cre recombinase induction may not have observed SPEM in their control mice.
Previous methods for inducing oxyntic atrophy and SPEM have been described, varying in their mechanism of action, onset of effect, and degree of inflammation (Table 1). Chronic infection of mice with Helicobacter felis [26] or of Mongolian gerbils with Helicobacter pylori [27] results in the emergence of SPEM within months of infection. In contrast to these chronic infectious models, pharmacologic induction of SPEM has provided a more rapid and reversible means for achieving the same result. The neutrophil elastase inhibitor DMP-777 has been shown to cause a rapid loss of parietal cells in rats and mice within 3–4 days of daily dosing [4, 5]. Treatment with this parietal cell-specific apical membrane protonophore leads to the emergence of SPEM within 7–10 days, in the absence of inflammation. Like tamoxifen [10], the effects on parietal cell loss can be mitigated by pretreatment with omeprazole. More recently, a related variant of DMP-777, known as L-635, was found to produce a more rapid onset of SPEM in mice within 3 days of treatment [6]. The mechanism of action of L-635 is similar to that of DMP-777, though, unlike DMP-777, the onset of SPEM is accompanied by an exuberant inflammatory response.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods, and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Ernst PB, Peura DA, Crowe SE. The translation of Helicobacter pylori basic research to patient care. Gastroenterology. 2006;130:188–206. doi: 10.1053/j.gastro.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 3.Goldenring JR, Nam KT, Mills JC. The origin of pre-neoplastic metaplasia in the stomach: chief cells emerge from the Mist. Exp Cell Res. 2011;317:2759–2764. doi: 10.1016/j.yexcr.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldenring JR, Ray GS, Coffey RJ, et al. Reversible drug-induced oxyntic atrophy in rats. Gastroenterology. 2000;118:1080–1093. doi: 10.1016/s0016-5085(00)70361-1. [DOI] [PubMed] [Google Scholar]
- 5.Nomura S, Yamaguchi H, Ogawa M, et al. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Gastrointest Liver Physiol. 2005;288:G362–G375. doi: 10.1152/ajpgi.00160.2004. [DOI] [PubMed] [Google Scholar]
- 6.Nam KT, Lee HJ, Sousa JF, et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders TL. Inducible transgenic mouse models. Methods Mol Biol. 2011;693:103–115. doi: 10.1007/978-1-60761-974-1_7. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 9.Huh WJ, Mysorekar IU, Mills JC. Inducible activation of Cre recombinase in adult mice causes gastric epithelial atrophy, metaplasia, and regenerative changes in the absence of “floxed” alleles. Am J Physiolo Gastrointest Liver Physiol. 2010;299:G368–G380. doi: 10.1152/ajpgi.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huh WJ, Khurana SS, Geahlen JH, et al. Tamoxifen induces rapid, reversible atrophy, and metaplasia in the mouse stomach. Gastroenterology. 2012;142:21–24. doi: 10.1053/j.gastro.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sigal M, Rothenberg ME, Logan CY, et al. Helicobacter pylori activates and expands Lgr5+ stem cells through direct colonization of the gastric glands. Gastroenterology. 2015;148(7):1392–404.e21. doi: 10.1053/j.gastro.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 12.Sohal D, Nghiem M, Crackower MA, et al. Temporally regulated and tissue-specific gene manipulations in the adult and embryoinc heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89:20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 13.Casanova E, Fehsenfeld S, Lemberger T, et al. ER-based double iCre fusion protein allows partial recombination in forebrain. Genesis. 2002;34:208–214. doi: 10.1002/gene.10153. [DOI] [PubMed] [Google Scholar]
- 14.Park EJ, Sun X, Nichol P, et al. System for tamoxifen-inducible expression of Crerecombinase from the Foxa2 locus in mice. Dev Dyn. 2008;237:447–453. doi: 10.1002/dvdy.21415. [DOI] [PubMed] [Google Scholar]
- 15.Jones ME, Kondo M, Zhuang Y. A tamoxifen inducible knock-in allele for investigation of E2A function. BMC Dev Biol. 2009;9:51. doi: 10.1186/1471-213X-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiermayer C, Conrad M, Schneider M, et al. Optimization of spatiotemporal gene inactivation in mouse heart by oral application of tamoxifen citrate. Genesis. 2007;45:11–16. doi: 10.1002/dvg.20244. [DOI] [PubMed] [Google Scholar]
- 17.Sheh A, Ge Z, Parry NM, et al. 17β-estradiol and tamoxifen prevent gastric cancer by modulating leukocyte recruitment and oncogenic pathways in Helicobacter pylori-infected INS-GAS male mice. Cancer Prev Res (Phila) 2011;4:1426–1435. doi: 10.1158/1940-6207.CAPR-11-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poon GK, Walter B, Lonning PE, et al. Identification of tamoxifen metabolites in human HepG2 cell line, human liver homogenate, and patients on long-term therapy for breast cancer. Drug Metab Dispos. 1995;23:377–382. [PubMed] [Google Scholar]
- 19.Robertson DW, Katzenellenbogen JA, Hayes JR, Katzenellenbogen BS. Antiestrogen basicity – activity relationships: a comparison of the estrogen receptor binding and antiuterotrophic potencies of several analogues of (Z)-1,2-diphenyl-1-[4-[2-(dimethylamino)ethoxy] ephenyl]-1-butene (tamoxifen, Nolvadex) having altered basicity. J Med Chem. 1982;25:167–171. doi: 10.1021/jm00344a015. [DOI] [PubMed] [Google Scholar]
- 20.Malet C, Gompel A, Spritzer P, et al. Tamoxifen and hydroxytamoxifen isomers versus estradiol effects on normal human breast cells in culture. Cancer Res. 1988;48:7193–7199. [PubMed] [Google Scholar]
- 21.Coezy E, Borgna JL, Rochefort H. Tamoxifen and metabolites in MCF7 cells: correlation between binding to estrogen receptor and inhibition of cell growth. Cancer Res. 1982;42:317–323. [PubMed] [Google Scholar]
- 22.Vignon F, Bouton MM, Rochefort H. Antiestrogens inhibit the mitogenic effect of growth factors on breast cancer cells in the total absence of estrogens. Biochem Biophys Res Commun. 1987;146:1502–1508. doi: 10.1016/0006-291x(87)90819-9. [DOI] [PubMed] [Google Scholar]
- 23.Dietze EC, Caldwell LE, Grupin SL, et al. Tamoxifen but not 4-hydroxytamoxifen initiates apoptosis in p53(-) normal human mammary epithelial cells by inducing mitochondrial depolarization. J Biol Chem. 2001;276:5384–5394. doi: 10.1074/jbc.M007915200. [DOI] [PubMed] [Google Scholar]
- 24.Reed CA, Berndtson AK, Nephew KP. Dose-dependent effects of 4-hydroxytamoxifen, the active metabolite of tamoxifen, on estrogen receptor-alpha expression in the rat uterus. Anticancer Drugs. 2005;16:559–567. doi: 10.1097/00001813-200506000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Mazzeo AR, Nandi J, Levine RA. Effects of ethanol on parietal cell membrane phospholipids and proton pump function. Am J Physiol. 1988;254:G57–G64. doi: 10.1152/ajpgi.1988.254.1.G57. [DOI] [PubMed] [Google Scholar]
- 26.Wang TC, Goldenring JR, Dangler C, et al. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology. 1998;114:675–689. doi: 10.1016/s0016-5085(98)70581-5. [DOI] [PubMed] [Google Scholar]
- 27.Yoshizawa N, Takenaka Y, Yamaguchi H, et al. Emergence of spasmolytic polypeptide-expressing metaplasia in Mongolian gerbils infected with Helicobacter pylori. Lab Invest. 2007;87:1265–1276. doi: 10.1038/labinvest.3700682. [DOI] [PubMed] [Google Scholar]


