Abstract
Background
Current research investigating the role of THBS2 and LECT-2 in atherogenesis is very limited. Therefore, we designed this study to demonstrate the role of THBS-2 and LECT-2 in atherosclerosis at the tissue level in fresh specimens.
Material/Methods
A total of 32 patients who underwent coronary bypass surgery were enrolled. Aortic wall punch biopsies were obtained at the site of proximal aortosaphenous bypass graft anastomosis. A specimen of left internal mammarian artery (LiMA) was taken from the segment just proximal to its anastomosis. The aortic tissue is representive of the atherosclerotic tisue, and LiMA tissue is representative of the non-atherosclerotic area. The specimens were painted with CD68 for macrophage, and THBS-2 and LECT-2 antibodies for immunohistochemical staining.
Results
Aortic THBS-2 levels were significantly lower, whereas aortic LECT-2 levels were significantly higher when compare to LiMA (14.4±9.9 (5–30) and 36.9±13.0 (5–60) p: 0.0001 and 20.3±15.0 (5–60) and 20.8±13,8 (10–30) p: 0.0001, respectively). CD68+ and monocyte level correlated significantly with AHA atherosclerosis grade (p=0.01, r=0.45 and p=0.001, r=0.56, Spearman’s test). CD68+ level correlated significantly with LECT-2 levels in atherosclerotic aortic tissue (p=0.026, r=0.392, Spearman’s test), whereas aortic TSBN-2 levels were not.
Conclusions
The present study has taken the first steps to highlight new markers in atherosclerosis by using immunohistochemical method. The study results suggest that the tissue levels of THBS2 and LECT-2 may correlate with the stage of atherosclerosis.
MeSH Keywords: Aorta; Atherosclerosis; Chemotaxis, Leukocyte; Immunohistochemistry; Thrombospondins
Background
Atherosclerosis is a chronic low-grade inflammatory disease, in which inflammatory mediators and cells play crucial roles, however, several mechanisms underlying the pathogenesis of atherosclerosis remain unexplained.
Inflammatory cell activation is critical to the onset of clinical events in atherosclerosis. Monocytes are key players in the atherosclerotic process as monocyte accumulation is considered to be the first step in this process [1]. There is some evidence in the current literature suggesting that thrombospondin-2 (THBS-2) plays an important role in atherosclerosis by regulating cell-to-matrix interaction, neovascularization, and extracelular matrix (ECM) proliferation [2–4]. Moreover, leukocyte cell-derived chemotaxin-2 (LECT-2) is a hepatokine that is related to insulin resistance and obesity; and acts as a chemoattractant for leukocytes [5,6]. Current research investigating the role of THBS2 and LECT-2 in atherogenesis is very limited [7]. Therefore, we designed this study to demonstrate the role of THBS-2 and LECT-2 in atherosclerosis at the tissue level in fresh specimens.
Material and Methods
Patients and tissue sampling
A total of 32 patients who underwent coronary bypass surgery were enrolled in this study. Aortic wall punch biopsies were obtained at the site of proximal aortosaphenous bypass graft anastomosis. A specimen of left internal mammarian artery (LiMA) was taken from the segment just proximal to its anastomosis. The aortic tissue is representative of the atherosclerotic tisue and LiMA tissue is representative of the non-atherosclerotic area.
This study was approved by the local ethics committee. Informed consent was obtained from each patient.
Blood sampling
Hemoglobin and WBC count were measured as part of the automated complete blood count (CBC) using a Sysmex XT-1800i (USA) hematology analyzer. Patients with a WBC count >12,000 cells/μL or <4,000 cells/μL, and body temperatures greater than 38°C were excluded from the study to eliminate co-esisting subclinical inflammatory states.
Immunohistochemisty and histopathology
Aortic tissue with visible atherosclerotic lesion was selected, sampled, and submitted for histological evaluation according to American Heart Association (AHA) histological criteria [8]. During histological classification, lesions were designated by Roman numerals representing lesion grade. Clinically silent atherosclerotic plaques were included in this study, while ruptures and healed plaques (type VI) were not included.
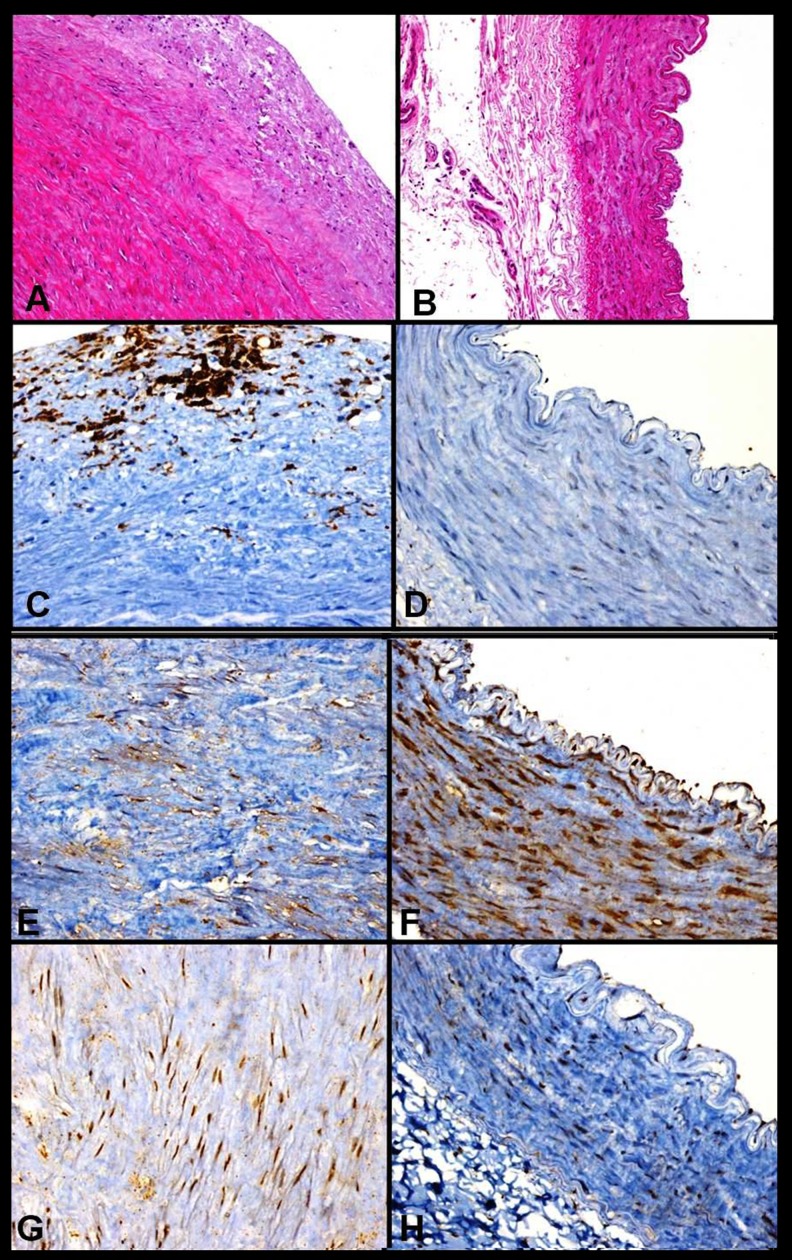
In the present study, 32 aortic tissue samples were embedded in paraffin blocks. For paraffin sections, aortic specimens were fixed in 10% formalin for one day, processed through graded alcohols and xylene, and embedded in paraffin. Tissue sections were then stained with haematoxylin and eosin (H&E) or immunostained (Figure 1). The specimens were painted with CD68 for macrophage, and THBS-2 (Thermo Fisher Scientific, TSP) and LECT-2 (Abcam) antibodies for immunohistochemical staining.
Figure 1.
(A) Hematoxylin and Eosin stained aortic tissue ×200. (B) Hematoxylin and Eosin stained LiMA tissue ×200. (C) Immunohistochemical staining of aortic tissue with anti-CD68 antibodies ×200. (D) Immunohistochemical staining of LiMA tissue with anti-CD68 antibodies ×200. (E) Immunohistochemical staining of aortic tissue with anti-TSBN-2 antibodies ×200. (F) Immunohistochemical staining of LiMA tissue with anti-TSBN-2 antibodies ×200. (G) Immunohistochemical staining of aortic tissue with anti-LECT-2 antibodies ×200. (H) Immunohistochemical staining of LiMA tissue with anti-LECT-2 antibodies ×200.
THBS-2 immunohistochemical staining showed myocyte and endothelial immunoreactivity in LiMA tissue (Figure 1). However, aortic punches showed positive staining in myoctes. LECT-2 immunohistochemical staining of aortic tissue showed positive staining in myocte but LiMA tissue showed myocyte and endothelial immunoreactivity. Aortic and LiMA THBS-2 and LECT-2 levels were measured by semiquantitative immunoreactivity method.
Statistical analyses
Continuous variables were expressed as the mean ± standard deviation (SD) or median (interquartile range), when appropriate. Categorical variables were expressed as percentages. The chi-square test was used to compare categorical variables. Spearman correlation coefficient was used to determine nonparametric measures of statistical dependence between two variables. To compare related variables (aorta and LiMA), Wilcoxon signed ranks test was used. Immunohistochemical and histopathological examinations were performed by two separate pathologist who were blinded to patient charactersitics. The intraclass correlation coefficient and the lower and upper limits the of the 95% CI were determined for interobserver variability. The Bland-Altman method for comparing paired measurements on aortic and LiMA THBS-2 and LECT-2 was used to determine interobserver agreement.
Two-tailed p values of less than 0.05 were considered to indicate statistical significance. All statistical analyses were performed using SPSS 15.0 software (SPSS Inc., Chicago, IL, USA).
Results
Detailed characteristics of the study population are shown in Table 1 (mean age, 58±9 years; 77% of patients were male). The median AHA atherosclerosis grade was 2 (range, 0–5) in aortic tissue. Histological classifications of aortic specimens used in this study are shown in Table 2.
Table 1.
Baseline characteristics of group.
| Variables | (n: 32) | Variables | (n: 32) |
|---|---|---|---|
| Patients characteristics | Laboratory | ||
| Age | 58±9 (40–74) | Creatinin mg/dl | 0.8±0.2 |
| Female/Male | 6/26 | e-GFR ml/min | 100±22 |
| Bmi kg/m2 | 29.2±0.4 | LDL mg/dl | 136±40 |
| Diabetes % | 15/32 | HDL mh/dl | 44±8 |
| Hypertension % | 13/32 | Hb gr/dl | 14±1.7 |
| Hyperlipidemia % | 7/32 | Wbc ×103 | 8.3±2.4 |
| Smoking | 18/32 | Neutrophil ×103 | 5.2±1.8 |
| Admission medication | Lymphocyte ×103 | 2.3±0.8 | |
| Aspirin % | 14/32 | Monocyte ×103 | 0.7±0.3 |
| Beta blocker % | 9/32 | Platelet ×103 | 221±81 |
| Digitals | 0/32 | ||
| Ace/ARB % | 9/32 | ||
| Statin % | 6/32 | ||
| Diuretics % | 0/32 | ||
| Clopidogrel % | 0/32 | ||
| CaCB % | 2/32 |
ACE – angiotensin converting enzyme; ARB – angiotensin reseptor blocker; BMI – body mass index; e-GFR – estimated glomerular filtration rate; NLR – neutrophil to lymphocyte ratio; CaCB – Ca channel blocker; MLR – monocyte to lymphocyte ratio; Wbc – white blood cell. Results are expressed as mean ±SD or frequency (with in group percentage) and median (Interquartile range).
Table 2.
Histological classification of aortic specimen used in the study.
| Specimen containing | AHA classification | (n=32) |
|---|---|---|
| Adaptive intimal thickening | Type I | 13 |
| Intimal xanthoma | Type II | 12 |
| Pathological intimal thickening | Type III | 2 |
| Early fibroatheroma and Late fibroatheroma | Type IV/V | 5 |
Aortic THBS-2 levels were significantly lower, whereas aortic LECT-2 levels were significantly higher when compare to LiMA (14.4±9.9 (5–30) and 36.9±13.0 (5–60), p: 0.0001 and 20.3±15.0 (5–60) and 20.8±13.8 (10–30) p: 0.0001, respectively) (Table 3).
Table 3.
CD68+,TBSN-2 and LECT-2 results between Aort and Lima.
| (n=32) | Aort | Lima | p |
|---|---|---|---|
| CD68+ | (0–3) (1–2) | (0–1) (0–0) | 0.0001* |
| TSBN-2 | 14.4±9.9 (5–30) | 36.9±13.0 (5–60) | 0.0001# |
| Interobserver agrement (CI) | 12.1 (10.6–13.6) | 37.6 (32.7–44.7) | 0.817–0.402 |
| LECT-2 | 40.6±13.7 (30–50) | 20.8±13.8 (10–30) | 0.0001# |
| Interobserver agrement (CI) | 44.5 (42.0–47.0) | 17.2 (14.5–20.2) | 0.255–0.305 |
Results are expressed as mean ±SD and min and maximal value (Interquartile range). CI – confidence interval.
Wilcoxon Signed Ranks and
Chi-square-test are used.
CD68+ and monocyte level correlated significantly with AHA atherosclerosis grade (p=0.01, r=0.45 and p=0.001, r=0.56, Spearman’s test) (Table 4). Table 4 shows Spearman correlation coefficients among all variables and AHA atherosclerosis grade.
Table 4.
Correlation analyses of variables.
| N: 32 | Aterosclerosis | CD68 | Monocyte | |
|---|---|---|---|---|
| Aterosclerosis | CC | |||
| p | ||||
| CD68 | CC | .448 (**) | ||
| p | 0.01 | |||
| Monocyte | CC | .562 (**) | .389 (*) | |
| p | .001 | .028 | ||
| LDL | CC | .281 | −.074 | .335 |
| p | .119 | .688 | .065 | |
| HDL | CC | −.505 (**) | −.434 (**) | −.225 |
| p | .004 | .015 | .224 | |
| TBSN-2 | CC | −0.171 | 0.026 | −.467 (**) |
| p | 0.350 | 0.888 | 0.007 | |
| LECT-2 | CC | 0.225 | 0.392 | 0.091 |
| p | 0.216 | 0.026 | 0.622 |
Correlation is significant at the 0.01 level (2-tailed);
Correlation is significant at the 0.05 level (2-tailed).
CC – correlation co-efficient; TBSN-2 – thrombospondin-2; LECT-2 – leukocyte cell-derived chemotaxin-2 Spearman coleration test is used.
CD68+ level correlated significantly with LECT-2 levels in atherosclerotic aortic tissue (p=0.026, r=0.392, Spearman’s test) whereas aortic TSBN-2 levels were not.
Discussion
Human aortic atherosclerosis was evaluated in the present study. The results revealed a clear relationship between plaque morphology and CD68+ cell infiltration in proximal aortic atherosclerotic lesions. It also showed a clear relationship between THBS-2 and LECT-2 levels and atherosclerosis. This prospective observation indicated that THBS-2 and LECT-2 levels were strongly associated with aortic atherosclerosis when compare with LiMA representing non-atherosclerotic tissue.
It is well-known that atherosclerosis is a chronic inflammatory disease [1–9]. Histological classification of aortic tissue according to the modified AHA classification system defines plaque morphology and complexity [8]. Monocytes play a pivotal role in the atherosclerotic process, and monocyte accumulation in the intima of the vessel is the first step in atherogenesis [1]. Macrophage colony-stimulating factor stimulates the monocytes and differentiates them into macrophages after entering plaque [10]. CD68+ infiltration reflects plaque activation; high levels of CD68+ infiltration are seen in high-grade atherosclerosis. In the present study, CD68+ infiltration was associated with aortic atherosclerosis grade, and in fresh aortic punches, CD68+ levels were significantly higher when compared with LiMA. The results from this study confirmed those from previous studies that evaluated aortic atherosclerosis in cadaveric tissues [11], in which HDL cholesterol was inversely correlated with AHA grade [12].
Thrombospondin is included a family of extracellular matrix glycoproteins. THBS-2 acts as a modulater for cell to matrix interactions and interactor for cell to surface receptors, cytokines, growth factors, proteases, and structural proteins [13]. TBSN-2 is adhesive glycoprotein that mediates cell-to-cell and cell-to-matrix interactions and can bind to fibrinogen, fibronectin, laminin, and type V collagen. Wang et al. suggested that THBS-2 influences atherosclerosis by regulating matrix metalloproteinase-2, which is associated with vulnerability of atherosclerotic plaque. This is because of THBS-2-null fibroblasts produced higher levels of this protein, which has been shown to be lower in atherosclerotic patients than the non-atherosclerotic group [4,14]. Additionally, in the THBS-2 absent mice, vascular and bleeding problems have been seen [15]. Finally, Wang et al. have shown that THBS-2 genetic variants are associated with coronary atherosclerosis in Chinese population [2]. Presents study results for THBS-2 validate the findings from these prior studies.
Leukocyte cell-derived chemotaxin 2 (LECT-2), a hepatokine, was first identified as a neutrophil chemotactic factor especially expressed in liver and it can be found in the bloodstream. It has been related to skeletal muscle insulin resistance and obesity [6,16]. LECT-2 plays a role in macrophage activation via CD209 pathway [17]. Hwang et al. suggested that LECT-2 regulates all stages of atherosclerosis by inducing pro-inflammatory cytokines, as described by Stein et al. [7,18]. In our study, LECT-2 levels were strongly associated with aortic atherosclerosis and CD68+ levels correlated significantly with LECT-2 levels in atherosclerotic tissue when compare with LiMA that represents non-atherosclerotic tissue; these findings supports findings of previous studies.
Study limitations
There are two main limitations to this study. First, the study has relatively a small sample size. Second, multiple histopathological examinations of aortic tissue were not performed. Additional studies using different techniques and inflammatory markers can provide further valuable information.
Conclusions
The mechanism underlying atherosclerosis is still unclear. The present study has taken the first steps to highlight new markers in atherosclerosis by using immunohistochemical method. Second, results of this data suggest that the tissue levels of THBS2 and LECT-2 may correlate with the stage of atherosclerosis. However, further in vivo and clinical studies are needed to understand the exact role of THBS-2 and LECT-2 in pathogenesis of atherosclerosis
Footnotes
Declaration of interest
The authors report no conflicts of interest.
Source of support: Bezmialem Vakif University, Department of Scientific Research Project
References
- 1.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Boekholdt SM, Trip MD, Peters RJ, et al. Thrombospondin-2 polymorphism is associated with a reduced risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2002;22(12):e24–27. doi: 10.1161/01.atv.0000046235.22451.66. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Fu W, Xie F, et al. Common polymorphisms in ITGA2, PON1 and THBS2 are associated with coronary atherosclerosis in a candidate gene association study of the Chinese Han population. J Hum Genet. 2010;55(8):490–94. doi: 10.1038/jhg.2010.53. [DOI] [PubMed] [Google Scholar]
- 4.Yang Z, Kyriakides TR, Bornstein P. Matricellular proteins as modulators of cellmatrix interactions: adhesive defect in thrombospondin 2-null fibroblasts is a consequence of increased levels of matrix metalloproteinase-2. Mol Biol Cell. 2000;11:3353–64. doi: 10.1091/mbc.11.10.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu XJ, Chen J, Yu CH, et al. LECT2 protects mice against bacterial sepsis by activating macrophages via the CD209a receptor. J Exp Med. 2013;210:5–13. doi: 10.1084/jem.20121466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lan F, Misu H, Chikamoto K, et al. LECT2 functions as a hepatokine that links obesity to skeletal muscle insulin resistance. Diabetes. 2014;63:1649–64. doi: 10.2337/db13-0728. [DOI] [PubMed] [Google Scholar]
- 7.Hwang HJ, Jung TW, Hong HC, et al. LECT2 induces atherosclerotic inflammatory reaction via CD209 receptor-mediated JNK phosphorylation in human endothelial cells. Metabolism. 2015;64(9):1175–82. doi: 10.1016/j.metabol.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Stary HC. Natural history and histological classification of atherosclerotic lesions an update. Arterioscler Thromb Vasc Biol. 2000;20:1177–78. doi: 10.1161/01.atv.20.5.1177. [DOI] [PubMed] [Google Scholar]
- 9.Libby P, Ridker PM, Maseri A. Inflammation and atheroscklerosis. Circulation. 2002;105(9):1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 10.Waldo SW, Li Y, Buono C, et al. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am J Pathol. 2008;172(4):1112–26. doi: 10.2353/ajpath.2008.070513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carbone F, Mach F, Montecucco F. Update on the role of neutrophils in atherosclerotic plaque vulnerability. Curr Drug Targets. 2015;16(4):321–33. doi: 10.2174/1389450115666141110093013. [DOI] [PubMed] [Google Scholar]
- 12.Homma S, Ishii T, Malcom GT, et al. Histopathological modifications of early atherosclerotic lesions by risk factors – findings in PDAY subjects. Atherosclerosis. 2001;156(2):389–99. doi: 10.1016/s0021-9150(00)00669-9. [DOI] [PubMed] [Google Scholar]
- 13.Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130:503–6. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noji Y, Kajinami K, Kawashiri MA, et al. Circulating matrix metalloproteinases and their inhibitors in premature coronary atherosclerosis. Clin Chem Lab Med. 2001;39:380–84. doi: 10.1515/CCLM.2001.060. [DOI] [PubMed] [Google Scholar]
- 15.Kyriakides TR, Zhu Y-H, Smith LT, et al. Micethat lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol. 1998;140:419–30. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamagoe S, Yamakawa Y, Matsuo Y, et al. Purification and primary amino acid sequence of a novel neutrophil chemotactic factor LECT2. Immunol Lett. 1996;52:9–13. doi: 10.1016/0165-2478(96)02572-2. [DOI] [PubMed] [Google Scholar]
- 17.Lu XJ, Chen J, Yu CH, et al. LECT2 protects mice against bacterial sepsis by activating macrophages via the CD209a receptor. J Exp Med. 2013;210:5–13. doi: 10.1084/jem.20121466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein S, Matter CM. Protective roles of SIRT1 in atherosclerosis. Cell Cycle. 2011;10:640–47. doi: 10.4161/cc.10.4.14863. [DOI] [PubMed] [Google Scholar]