Abstract
Objective:
Low-level laser therapy (LLLT) has been promoted for its beneficial effects on tissue healing and pain relief. However, according to the results of in vivo studies, the effectiveness of this modality varies. Our purpose was to assess the putative effects of LLLT on healing using an experimental wound model.
Design and Setting:
We used a randomized, triple-blind, placebo-controlled design with 2 within-subjects factors (wound and time) and 1 between-subjects factor (group). Data were collected in the laboratory setting.
Subjects:
Twenty-two healthy subjects (age = 21 ± 1 years, height = 175.6 ± 9.8 cm, mass = 76.2 ± 14.2 kg).
Measurements:
Two standardized 1.27-cm2 abrasions were induced on the anterior forearm. After wound cleaning, standardized digital photos were recorded. Each subject then received LLLT (8 J/cm2; treatment time = 2 minutes, 5 seconds; pulse rate = 700 Hz) to 1 of the 2 randomly chosen wounds from either a laser or a sham 46-diode cluster head. Subjects reported back to the laboratory on days 2 to 10 to be photographed and receive LLLT and on day 20 to be photographed. Data were analyzed for wound contraction (area), color changes (chromatic red), and luminance.
Results:
A group × wound × time interaction was detected for area measurements. At days 6, 8, and 10, follow-up testing revealed that the laser group had smaller wounds than the sham group for both the treated and the untreated wounds (P < .05). No group × wound × time differences were detected for chromatic red or luminance.
Conclusions:
The LLLT resulted in enhanced healing as measured by wound contraction. The untreated wounds in subjects treated with LLLT contracted more than the wounds in the sham group, so LLLT may produce an indirect healing effect on surrounding tissues. These data indicate that LLLT is an effective modality to facilitate wound contraction of partial-thickness wounds.
Keywords: modalities, experimental wound model
Allied health professionals regularly care for a variety of wounds to the skin, among them abrasions, turf burns, surgical incisions, and, perhaps the most difficult to treat, ulcerations. From acute wound management to augmentation of scar tissue remodeling, the clinician seeks to optimize wound care to promote healing. Although low-level laser therapy (LLLT) has received only specified US Food and Drug Administration clearance, its clinical efficacy for tissue healing has been widely reported.1–5 In vitro data suggest that LLLT facilitates collagen synthesis,6 keratinocyte cell motility,7 and growth factor release8 and transforms fibroblasts to myofibroblasts.9 Many authors of clinical studies have reported the benefits of LLLT on tissue healing, but others have shown no effect.10–13 These conflicting results are likely due to variations in treatment factors and limitations in experimental design, including comparison of heterogeneous clinical wounds, lack of control groups, and limited or no blinding of investigators.14,15
Several researchers have used superficial wounds to assess the putative effects of LLLT on healing.15 Some have used clinical wounds or ulcers of various sizes and depths,1,2,12 and others have developed superficial wound models in animals.10,11,16,17 These different methods have produced varied results and conclusions as to the effectiveness of LLLT. When analyzing healing among wounds, it would be beneficial if the wounds were as alike as possible; therefore, the differences in healing could be attributed to the treatment and not to other factors, such as wound variability. Claus et al18 developed a superficial wound model for use in human subjects that controlled for wound size and depth. This model allows for evaluation of partial-thickness abrasions of controlled size and depth at measurement intervals set by the investigator.
Our purpose was to assess potential changes in healing due to LLLT over time using a human experimental wound model. Healing was measured in terms of wound contraction and changes in chromatic red and luminance. Chromatic red is an indication of wound healing as a wound changes in color from dark red to pale pink over time. Luminance refers to the homogeneity of a wound as the tissue heals and becomes more smooth and consistent.
METHODS
We used a 2 × 2 × 6 factorial design to compare treatment groups (1 group treated with LLLT and 1 group treated with a sham treatment) and treatment wounds (treated wound and untreated wound) across time intervals. The subject, clinician, and investigator examining the wounds were blinded as to which treatment group was the sham. After data analysis, the manufacturer revealed the true treatment head. Measurements used to quantify healing included wound area (pixels), wound color (chromatic red), and wound luminance (L).
Subjects
Volunteers (age = 21 ± 1 years, height = 175.6 ± 9.8 cm, mass = 76.2 ± 14.2 kg) were 22 physically active males (13) and females (9) recruited from a university student population. Subjects were randomly assigned to either the laser group (5 females, 6 males, age = 21 ± 1 years, height = 174.8 ± 11.7 cm, mass = 76.9 ± 16.3 kg) or the sham group (4 females, 7 males, age = 21 ± 1 years, height = 176.4 ± 7.8 cm, mass = 75.5 ± 12.6 kg). Each subject completed a preparticipation questionnaire to determine eligibility for the study. Subjects were excluded if they were taking any medications that would thin the blood or affect normal healing or immune function. Subjects had no history of immune suppression, circulatory dysfunction, or excessive scarring and were not tobacco users. Risks were explained to all subjects, and each subject provided informed consent. Human subject approval was obtained from the university's institutional review board. All subjects complied with treatments and measurements for the entire 20-day period.
Procedures
A superficial wound model was adapted from Claus et al.18 All Occupational Safety and Health Administration guidelines for handling blood and bodily fluids were followed.19 Sandpaper was autoclaved before use, and wound templates were sterilized before contact with the skin. All investigators wore latex gloves during the procedures.
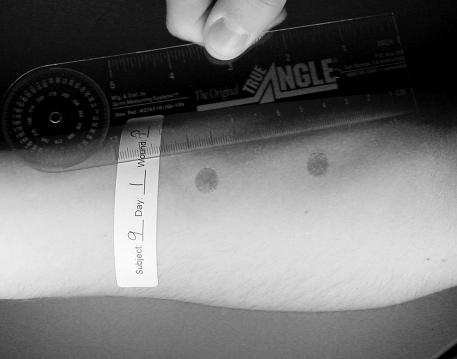
Subjects were prepared to receive two 1.27-cm2 circular abrasions to a central area of the anterior forearm (Figure 1) of the nondominant arm. If needed, the area was shaved with electric hair clippers. A 10-minute ice-massage treatment was first applied to the anterior forearm to help desensitize the area and then an anesthetic cream (EMLA [2.5% lidocaine and 2.5% prilocaine], Astra USA, Inc, Westboro, MA) was applied to areas that were to be abraded. The anterior forearm was then cleaned with a Betadine scrub (Purdue Pharma LLP, Stamford, CT) and allowed to air dry.
Figure 1.
Experimental wounds before treatment on day 1.
A 25 × 10-cm template made of 0.15-mm clear vinyl was affixed to the nondominant arm with Leukotape (BSN-Jobst, Inc, Charlotte, NC) so that two 1.27-cm2 circular holes allowed skin to protrude through the template. The circular holes were spaced 6.35 cm center to center. A 12.7× 20.3-cm piece of 60-grit autoclaved sandpaper was attached to a weighted (2.73-kg) “sanding sled.” The sled measured 24 × 11.5 cm with an attached handle that allowed the sled to be pulled across the template without applying additional downward pressure. The sled was pulled back and forth across the circular cutouts to the beat of a metronome (60 beats/s) for 50 passes. Pilot work provided a determination of the optimal number of passes for appropriate wound depth. We defined the appropriate wound depth as an abrasion that extended partially into the dermis. We were able to determine this depth by observing how many passes were necessary to produce even bleeding from the surface of the wound. The template and sandpaper were removed and discarded using standard Occupational Safety and Health Administration procedures.
Bleeding was controlled by direct pressure with sterile gauze. Wounds were then thoroughly rinsed with hydrogen peroxide and allowed to air dry. Subjects randomly selected cards identifying which of the 2 wounds was to be treated. Subjects were also randomly assigned to 1 of the 2 treatment groups (LLLT or sham) for which identical treatment heads were used, differentiated with a number on the head. Labels were placed on the forearm identifying the subject number, day, and treatment wound (Figure 1). The subject's arm was placed under a lighted copy stand, and a digital camera (Sony DSC-S75, Tokyo, Japan) was mounted 40 cm perpendicular to the subject's forearm. Digital images (1600 × 1200 pixels, JPEG format) were recorded.
Subjects completed a daily questionnaire that permitted them to rate their level of pain on a visual analog scale, help investigators identify signs of infection, track compliance with instructed wound care, and examine perceptions of the laser therapy. These data were used to help identify any problems associated with infection that might put the subject at risk or create outlying data and to objectively examine the blinding procedure. An adhesive sterile bandage was applied to the untreated wound in preparation for treatment of the other wound. The forearm was then covered with neoprene (2-mm thickness); a 5-cm-diameter circular cutout exposed the wound to be treated.
Irradiation was delivered to the exposed wound from a 46-diode cluster head (Omega Excel, Omega Laser Systems, Crawley, UK). The cluster head (surface area = 19.6 cm2) has a single laser (820 nm) in the center surrounded by concentric rings of superluminous diodes of varying wavelengths (Table 1). The average energy density of the treatment was 8 J/cm2 (treatment time = 2 minutes, 5 seconds; pulse rate = 700 Hz). These settings reflect common clinical practice15 and the guidelines of the manufacturer.20 During treatment, the cluster head was centered over the wound and held stationary while contact was maintained with the neoprene template. The neoprene template prevented direct contact between the treatment head and the wound, providing a 2-mm gap between the two. Both the subject and the administrator wore laser safety goggles before, during, and after the treatment. The goggles prevented detection of light from the laser, preventing potential detection of the laser head by either the subject or the administrator. Additionally, the cluster head was placed over the template before the settings were established, preventing any visible light from the laser. Treatment was administered using the same protocol for the sham group.
Table 1.
Technical Specifications of the 46-Diode Cluster Probe20
After treatment, an adhesive sterile bandage was applied over the treated wound. Subjects were instructed to remove the bandages only to shower, reapply new bandages after the shower, and replace both bandages if 1 was removed. For 10 days, subjects returned daily for a photograph, treatment, and assessment session. Subjects returned on day 20 for a final picture and assessment of the wounds. Subjects completed a daily questionnaire before each session. During each session, the wounds were flushed with sterile saline and allowed to air dry. Labels were then applied to specify the day, subject, treatment wound, and group. Digital images were then recorded under the same conditions (distance, lighting, settings) as the original images. The LLLT was administered under the same conditions previously described.
All images were analyzed using image-measurement software (Professional Version; Bersoft Inc, Ottawa, ON, Canada). Each wound was analyzed for area, chromatic red, and luminance. Area was measured to analyze wound contraction as a function of healing using the automatic area-measurement component of the software. This allowed for the wound borders to be defined with the pixels that were within a specific color range of the open or “unhealed” portion of the wound. We defined the wound area through test analysis as the total area that was ±10 pixels (red value) from a cluster of pixels located in the open portion (center) of the wound. The total number of pixels within the color range represented the relative wound area.
Chromatic red and luminance were calculated by recording the mean red, green, and blue values for a standard area across all wounds. Superficial wounds tend to lighten from red to pale pink and become more homogeneous and more consistent in texture as they heal.21 Calculations of chromatic red and luminance help to make these subjective observations objective. Chromatic red decreases and luminance increases as a wound heals, representing these subjective changes. A 2000-pixel circular area was selected from the center of each wound. The software provided mean red (R), green (G), and blue (B) values for each selected area. Additionally, a 2000-pixel area was selected from an area of uninjured skin between the 2 wounds for normalization of all color values. For color normalization, each color value (R, B, and G) from the wound area was divided by the corresponding color value from the uninjured skin area and multiplied by 100. Chromatic red (r) and luminance (L) were calculated for both wounds using the following formulae from Hansen et al22:
Statistical Analysis
We used data from days 1, 4, 6, 8, 10, and 20 in analyses. Mixed-factor, 3-way analyses of variance were computed to detect differences between groups (laser and sham) and wounds (treated and untreated) over time for each dependent variable. Follow-up, 2-way analyses of variance were computed to detect differences between groups and wounds at each time interval. We used the Tukey Honestly Significant Difference test for post hoc comparisons (P < .05 for all tests). Descriptive statistics were computed for questionnaires.
RESULTS
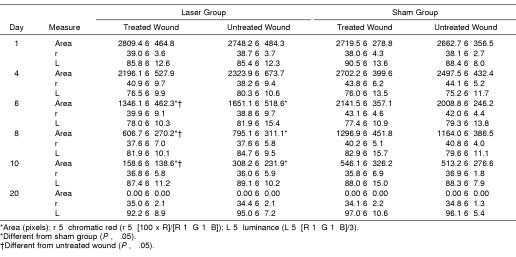
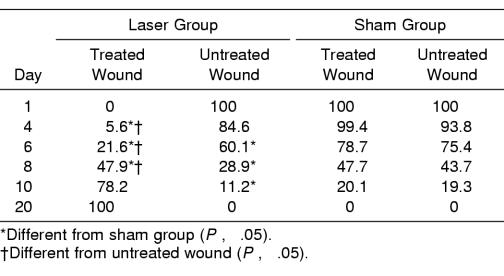
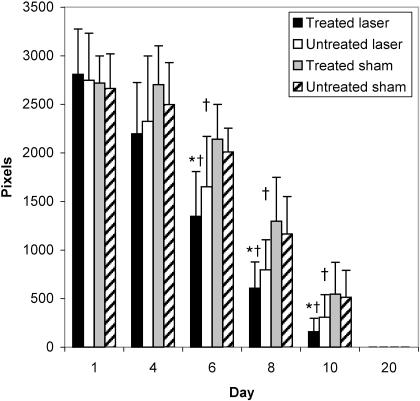
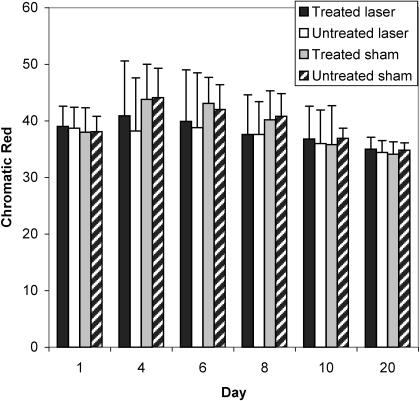
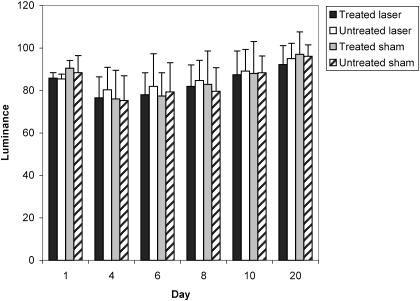
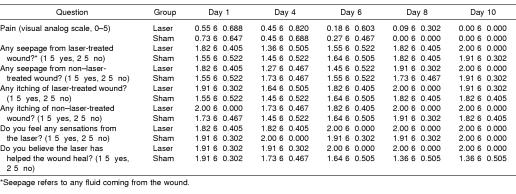
Summary data for measures of area, r, and L are presented in Table 2, and percentage changes from baseline area measurements are presented in Table 3. A group × wound × time interaction (F5,95 = 4.632, P ≤ .001) was detected for area measurements. Follow-up group × wound interactions were detected at days 6 (F1,20 = 15.897, P ≤ .001), 8 (F1,20 = 10.596, P ≤ .004), and 10 (F1,20 = 7.36, P ≤ .013). At days 6, 8, and 10, follow-up testing revealed that the laser group had smaller wounds than the sham group for both the treated and the untreated wounds (P < .05) (Figure 2). Effect size was also calculated for differences identified between groups at days 6 (2.11), 8 (1.46), and 10 (1.18). Further, the treated wound in the laser group was smaller than the untreated wound (P < .05) at days 6, 8, and 10. The effect sizes for differences detected between the 2 wounds in the laser group were 0.53 (day 6), 0.55 (day 8), and 0.69 (day 10). All wounds were completely contracted when the subjects returned for a final assessment on day 20. No differences were detected between wounds within subjects at each time interval (P < .05). No group × wound × time differences were detected for r or L (Figures 3 and 4; P < .05). Descriptive data for the daily questionnaire are presented in Table 4.
Table 2.
Wound Area, Chromatic Red, and Luminance Over Time*
Table 3.
Percentage Change From Day 1 Area Measurement
Figure 2.
Average wound areas (pixels) of all groups over time. *Different from sham group for both treated and untreated wounds (P > .05). †Different from the untreated wound in the laser group.
Figure 3.
Wound chromatic red (r) values of all groups over time. r = (100 × R)/(R + G + B).
Figure 4.
Wound luminance (L) values of all groups over time. L = (R + G + B)/3.
Table 4.
Summary Data From the Daily Questionnaire Completed Before Daily Treatment
DISCUSSION
Our primary finding was that the laser group had greater wound contraction than the sham group. The greatest difference between groups in wound contraction was seen on treatment day 6, when the laser-treated wounds of the study group demonstrated 153% greater wound contraction than the treated wounds of the sham group (see Figure 2). On day 8, the laser group exhibited 55% greater wound contraction and on day 10, 22%. The healing curve correlates with data collected by Bon et al,21 who found that healing after skin-graft removal was difficult to evaluate before day 6 but then progressed until it was considered complete after day 12. Although wounds in both groups were completely healed by day 20, LLLT appeared to facilitate the repair phase of healing, in which the strength of the granulation tissue would be affected.
Numerous case reports and clinical trials with humans have shown impressive wound healing outcomes using LLLT.1–3,5 Further work with animals has also supported the use of LLLT to facilitate wound healing.16,17 Conversely, several groups have shown no advantage in healing with LLLT.10–13 To complicate matters, light absorption may be specific to cell and tissue type,15 reducing the ability to generalize the results of animal data to human wounds. With few controlled clinical trials and different methods and research goals, it is difficult to compare current results with these studies.
In addition to the use of LLLT in the treatment of skin lesions, clinicians and investigators have sought other potential applications for the therapeutic modality in the sports injury setting. The results have been mixed as to the effectiveness of LLLT in treating pain and restoring function in musculoskeletal injury or disease.23–29 Beckerman et al23 concluded that methodologic errors among studies performed using therapeutic laser did not permit adequate conclusions to be drawn relative to the effectiveness of the modality. Absent from most studies were carefully controlled and blinded procedures to clarify and quantify the apparent differences between laser-treated wounds and nonlaser-treated wounds. Furthermore, clinicians often employ a variety of strategies simultaneously, which makes it difficult to measure the effects of a single intervention. The numerous intrinsic and extrinsic factors associated with the inflammatory response and healing make well-designed investigations mandatory before definitive conclusions can be drawn. Our data are a solid beginning to systematic study of this relatively new modality.
The exact mechanism by which LLLT facilitates wound healing is largely unknown. However, several theories may help explain the enhanced wound contraction observed here. In vitro studies have shown an increase in fibroblast proliferation after irradiation,6,30 suggesting that LLLT therapy may facilitate fibroplasia during the repair phase of tissue healing. Our data support this suggestion. We did not observe differences between the LLLT and sham groups until day 6, when wounds would have been well into the repair phase of soft tissue healing. However, it should also be noted that other investigators found no in vitro changes in fibroblast proliferation after LLLT.31,32 The disparity could be due to changes in specific laser irradiation settings, such as wavelength, duration, power, and intensity.15
Facilitated wound contraction may also be supported by work from Pourreau-Schneider et al,9 who reported that laser irradiation transforms fibroblasts into myofibroblasts. Myofibroblasts are directly involved in granulation tissue contraction, and increased numbers could lead to facilitated wound contraction. A myofibroblast is a modified fibroblast with ultrastructural and functional properties of fibroblasts and muscle cells.33 Cytoplasmic fibrils of actomyosin allow for contraction of myofibroblasts, pulling on the borders of the wound and reducing the size during the repair phase of soft tissue healing.33 Because our data provided support that LLLT enhanced wound contraction but did not necessarily enhance other variables (r and L changes) associated with superficial wound healing, myofibroblast stimulation may be a viable explanation.
Our data suggest that LLLT has an indirect healing effect on surrounding tissues. The LLLT was applied to 1 of 2 randomly chosen wounds spaced 5 cm apart. Although we found a slight difference in wound contraction between the 2 wounds in the laser group, both the treated and the untreated wounds showed enhanced healing compared with those in the sham group. These data are supported by Braverman et al,34 who found augmented tensile strength in rabbit wounds treated with LLLT and in control wounds on the contralateral limb. They suggested that LLLT may have caused release of tissue growth factors into circulation, which may have affected surrounding tissues or entire systems. Indirect healing could be a very beneficial effect of this modality in treating tissue damage of large size or at multiple locations. It might also suggest that deeper tissues could be affected by light therapy.
Although we demonstrated enhanced wound contraction using LLLT, our data did not support significant wound healing in terms of changes in r or L. Superficial wounds tend to lighten from red to pale pink and become more homogeneous and consistent in texture as they heal.21 These 2 qualitative changes may be quantified through previously mentioned formulas for r and L.22 In relation to the qualitative changes, r should decrease over time, while L should increase. The fact that we saw no significant effect in r or L could point to the potential lack of sensitivity of these measures, and perhaps these measures are not good indicators of healing. It does not, however, take away from the tissue-contraction data. Fibroblast proliferation and enhanced myofibroblast conversion could explain enhanced wound contraction while not having any measurable effect on r or L.
The questionnaire in this study was used to help identify any signs of infection, ensure compliance in caring for the wounds, and validate the blinding procedures used in this project. Also noteworthy is that the subjects did not perceive that LLLT was effective in either group, even though wounds contracted more in the treatment group. This could be due to the fact that both wounds, treated and untreated, on each subject healed at a faster rate than those in the sham group. Because the treated wound did not heal any faster than the untreated wound, the subjects perceived the treatment to be ineffective.
Evidence exists for the usefulness of LLLT for various conditions, but currently this may be of little consequence in the United States because of Food and Drug Administration guidelines on the use of lasers for the treatment of humans. In 1983, the Food and Drug Administration refused to support sales of LLLT as a wound-treatment device because of a lack of controlled human investigations supporting the therapy.35 Since that time, the Food and Drug Administration has taken the stance that laser devices from individual manufacturers for selective therapeutic benefits may be considered for clearance/ permission to legally market the device. To date, such devices have been cleared for adjunctive use in the temporary relief of neck and shoulder pain of musculoskeletal origin, wrist and hand pain associated with carpal tunnel syndrome, and iliotibial band syndrome pain. The LLLT devices may also be imported for use in the United States for controlled clinical trials with institutional review board approval, as was the case in our study. Further well-designed investigations should ultimately determine if LLLT is a safe and effective modality for specific conditions.
A criticism of many in vivo experiments designed to examine the efficacy of LLLT has been lack of control, poor experimental design, and inadequate specification of treatment settings.15 Although we made every effort to control as many of these variables as possible, a few limitations to this study do exist. The standardized wound model is limited to acute, partial-thickness wounds that extend into the dermis. The healing process may be similar throughout other tissues of the body, but we have collected no direct evidence that similar effects would be observed in wounds of other tissues or in chronic wounds. Our data are also limited to the specific irradiation settings we used. These settings reflect common clinical practice15 and the manufacturer's recommendations and guidelines.20 The cluster probe used in this investigation was a combination of 1 (820-nm) laser and 45 superluminous diodes and represents common clinical practice in that the less expensive semiconductor-based diode lasers are more popular.15 Another limitation to our study was that we did not apply the cluster head directly in contact with the open wound. We used a neoprene template that covered the areas around the wound and on which the rim of the cluster head rested during treatment. This resulted in a 2-mm gap between the cluster head and the wound and some assumed divergence of the laser light and reduction of irradiation intensity to the tissue. Although the wound model used in this study is fairly well standardized, variations among subjects did exist in terms of number of pixels. We assume this variation to be the result of individual differences in tissue elasticity and compliance when the vinyl template was applied to the arm. In other words, the amount of tissue protruding through the holes allowed for slight variation, depending on the subject.
CONCLUSIONS
The LLLT is an effective treatment for enhancing wound contraction of partial-thickness abrasions. It also facilitates wound contraction of untreated wounds on the same arm, suggesting an indirect effect on surrounding tissues. Although our data focused on enhanced contraction of superficial wounds, we believe they are the first step in formulating meaningful conclusions regarding LLLT. Further controlled data are necessary to determine the efficacy of LLLT in facilitating healing and reducing pain associated with musculoskeletal disorders.
REFERENCES
- 1.Chromey PA. The efficacy of carbon dioxide laser surgery for adjunct ulcer therapy. Clin Podiat Med Surg. 1992;9:709–719. [PubMed] [Google Scholar]
- 2.Gogia PP, Hurt BS, Zirn TT. Wound management with whirlpool and infrared cold laser treatment: a clinical report. Phys Ther. 1988;68:1239–1242. [PubMed] [Google Scholar]
- 3.Schindl A, Schindl M, Schindl L. Successful treatment of persistent radiation ulcer by low power laser therapy. J Am Acad Dermatol. 1997;37:646–648. doi: 10.1016/s0190-9622(97)70187-7. [DOI] [PubMed] [Google Scholar]
- 4.Schindl M, Kerschan K, Schindl A, Schon H, Heinzl H, Schindl L. Induction of complete wound healing in recalcitrant ulcers by low-intensity laser irradiation depends on ulcer cause and size. Photodermatol Photoimmunol Photomed. 1999;15:18–21. doi: 10.1111/j.1600-0781.1999.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 5.Sugrue ME, Carolan J, Leen EJ, Feeley TM, Moore DJ, Shanik GD. The use of infrared laser therapy in the treatment of venous ulceration. Ann Vasc Surg. 1990;4:179–181. doi: 10.1007/BF02001375. [DOI] [PubMed] [Google Scholar]
- 6.Abergel RP, Meeker CA, Lam TS, Dwyer RM, Lesavoy MA, Uitto J. Control of connective tissue metabolism by lasers: recent developments and future prospects. J Am Acad Dermatol. 1984;11:1142–1150. doi: 10.1016/s0190-9622(84)80194-2. [DOI] [PubMed] [Google Scholar]
- 7.Haas AF, Isseroff RR, Wheeland RG, Rood PA, Graves PJ. Low-energy helium-neon laser irradiation increases the motility of human keratinocytes. J Invest Dermatol. 1990;94:822–826. doi: 10.1111/1523-1747.ep12874679. [DOI] [PubMed] [Google Scholar]
- 8.Yu W, Naim JO, Lanzafame RJ. The effects of photo-irradiation on the secretion of TGF and PDGF from fibroblasts in vitro. Lasers Surg Med Suppl. 1994;6:8. [Google Scholar]
- 9.Pourreau-Schneider N, Ahmed A, Soudry M, et al. Helium-neon laser treatment transforms fibroblasts into myofibroblasts. Am J Pathol. 1990;137:171–178. [PMC free article] [PubMed] [Google Scholar]
- 10.Allendorf JD, Bessler M, Huang J, et al. Helium-neon laser irradiation at fluences of 1, 2, and 4 J/cm2 failed to accelerate wound healing as assessed by wound contracture rate and tensile strength. Lasers Surg Med. 1997;20:340–345. doi: 10.1002/(sici)1096-9101(1997)20:3<340::aid-lsm13>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 11.Hunter J, Leonard L, Wilson R, Snider G, Dixon J. Effects of low energy laser on wound healing in a porcine model. Lasers Surg Med. 1984;3:285–290. doi: 10.1002/lsm.1900030404. [DOI] [PubMed] [Google Scholar]
- 12.Lundeberg T, Malm M. Low-power HeNe laser treatment of venous leg ulcers. Ann Plastic Surg. 1991;27:537–539. doi: 10.1097/00000637-199112000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Saperia D, Glassberg E, Lyons RF, et al. Demonstration of elevated type I and type III procollagen mRNA levels in cutaneous wounds treated with helium-neon laser: proposed mechanism for enhanced wound healing. Biochem Biophys Res Commun. 1986;138:1123–1128. doi: 10.1016/s0006-291x(86)80399-0. [DOI] [PubMed] [Google Scholar]
- 14.Basford JR. Low-energy laser therapy: controversies and new research findings. Lasers Surg Med. 1989;9:1–5. doi: 10.1002/lsm.1900090103. [DOI] [PubMed] [Google Scholar]
- 15.Baxter GD. Therapeutic Lasers: Theory and Practice. Edinburgh, UK: Churchill Livingstone; 1994. [Google Scholar]
- 16.Dyson M, Young S. Effect of laser therapy on wound contraction and cellularity in mice. Lasers Med Sci. 1986;1:126–130. [Google Scholar]
- 17.Mester E, Jaszsagi-Nagi E. The effect of laser radiation on wound healing and collagen synthesis. Studia Biophys. 1973;35:227–230. [Google Scholar]
- 18.Claus EE, Fusco CF, Ingram T, Ingersoll CD, Edwards JE, Melham TJ. Comparison of the effects of selected dressings on the healing of standardized abrasions. J Athl Train. 1998;33:145–149. [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold BL. A review of selected blood-borne pathogen position statements and federal regulations. J Athl Train. 1995;30:171–176. [PMC free article] [PubMed] [Google Scholar]
- 20.Omega Laser Systems Excel Laser System Manual. 2nd ed. West Sussex, UK: Omega Laser Systems Ltd; 1999. [Google Scholar]
- 21.Bon FX, Briand E, Guichard S, et al. Quantitative and kinetic evolution of wound healing through image analysis. IEEE Trans Med Imaging. 2000;19:767–772. doi: 10.1109/42.875206. [DOI] [PubMed] [Google Scholar]
- 22.Hansen G, Sparrow E, Kokate J, Leland KJ, Iaizzo PA. Wound status evaluation using color image processing. IEEE Trans Med Imaging. 1997;16:78–86. doi: 10.1109/42.552057. [DOI] [PubMed] [Google Scholar]
- 23.Beckerman H, de Bie RA, Bouter LM, De Cuyper HJ, Oostendorp RAB. The efficacy of laser therapy for musculoskeletal and skin disorders: a criteria-based meta-analysis of randomized clinical trials. Phys Ther. 1992;72:483–491. doi: 10.1093/ptj/72.7.483. [DOI] [PubMed] [Google Scholar]
- 24.Brosseau L, Welch V, Wells G, et al. Low level laser therapy for osteoarthritis and rheumatoid arthritis: a meta-analysis. J Rheumatol. 2000;27:1961–1969. [PubMed] [Google Scholar]
- 25.de Bie RA, de Vet HCW, Lenssen TF, van den Wildenberg FAJM, Koostra G, Knipschild PG. Low-level laser therapy in ankle sprains: a randomized clinical trial. Arch Phys Med Rehabil. 1998;79:1415–1420. doi: 10.1016/s0003-9993(98)90237-4. [DOI] [PubMed] [Google Scholar]
- 26.Gam AN, Thorsen H, Lonnberg F. The effect of low-level laser therapy on musculoskeletal pain: a meta-analysis. Pain. 1993;52:63–66. doi: 10.1016/0304-3959(93)90114-5. [DOI] [PubMed] [Google Scholar]
- 27.Gür A, Karakoc M, Nas K, Cevik R, Sarac J, Demir E. Efficacy of low power laser therapy in fibromyalgia: a single-blind, placebo-controlled trial. Lasers Med Sci. 2002;17:57–61. doi: 10.1007/s10103-002-8267-4. [DOI] [PubMed] [Google Scholar]
- 28.Gür A, Karakoc M, Nas K, Cevik R, Sarac J, Ataoglu S. Effects of low power laser and low dose amitriptyline therapy on clinical symptoms and quality of life in fibromyalgia: a single-blind, placebo-controlled trial. Rheumatol Int. 2002;22:188–193. doi: 10.1007/s00296-002-0221-z. [DOI] [PubMed] [Google Scholar]
- 29.Simunovic Z, Ivankovich AD, Depolo A. Wound healing of animal and human body sport and traffic accident injuries using low-level laser therapy treatment: a randomized clinical study of seventy-four patients with control group. J Clin Laser Med Surg. 2000;18:67–73. doi: 10.1089/clm.2000.18.67. [DOI] [PubMed] [Google Scholar]
- 30.Boulton M, Marshall J. He-Ne laser stimulation of human fibroblast proliferation and attachment in vitro. Lasers in Life Sci. 1986;1:125–134. [Google Scholar]
- 31.Colver GB, Priestly GC. Failure of HeNe to affect components of wound healing in vitro. Br J Dermatol. 1989;121:179–186. doi: 10.1111/j.1365-2133.1989.tb01797.x. [DOI] [PubMed] [Google Scholar]
- 32.Hallman HO, Basford JR, O'Brien JF, Cummins LA. Does low-energy helium-neon laser irradiation alter “in vitro” replication of human fibroblasts? Lasers Surg Med. 1988;8:125–129. doi: 10.1002/lsm.1900080206. [DOI] [PubMed] [Google Scholar]
- 33.Spector TD, Axford JS. An Introduction to General Pathology. 4th ed. Edinburgh, UK: Churchill Livingstone; 1999. [Google Scholar]
- 34.Braverman B, McCarthy RJ, Ivankovich AD, Forde DE, Overfield M, Bapna MS. Effect of helium-neon and infrared laser irradiation of wound healing in rabbits. Lasers Surg Med. 1989;9:50–58. doi: 10.1002/lsm.1900090111. [DOI] [PubMed] [Google Scholar]
- 35.Forney R, Mauro T. Using lasers in diabetic wound healing. Diabetes Technol Ther. 1999;1(2):189–192. doi: 10.1089/152091599317404. [DOI] [PubMed] [Google Scholar]