Abstract
Objective
To assess factors associated with favorable seizure outcome after surgery for symptomatic epileptic spasms and improve knowledge on pathophysiology of this seizure type.
Methods
Inclusion criteria were: (1) age between 6 months and 15 years at surgery; (2) active epileptic spasms; (3) follow‐up after surgery >1 year.
Results
We retrospectively studied 80 children (aged 1.3 ± 2 years at seizure onset; 5.8 ± 4 years at surgery, 11.7 ± 5.7 years at last follow up). Magnetic resonance imaging (MRI) revealed structural abnormalities in 77/80 patients (96.3%; unilateral in 69: 89.6%). We performed invasive recordings in 24 patients (30%). In 21 patients in whom MRI or histopathology detected a lesion, electrodes exploring it constantly captured initial ictal activity at spasm onset. Fifty‐eight patients (72.5%) underwent unilobar and 22 (27.5%) multilobar or hemispheric procedures. At last follow‐up, 49 patients (61.3%) were in Engel class I. Multivariate logistic models showed completeness of resection of the seizure onset zone (OR = 0.016, 95%CI: 0.002, 0.122) and of the MRI visible lesion (OR = 0.179, 95% CI: 0.032, 0.999) to be significantly associated with Engel class IA outcome. Unfavorable outcome was associated with an older age at surgery, when it reflected a longer duration of epilepsy (OR = 1.383, 95% CI: 0.994,1.926).
Interpretation
Data emerging from invasive recordings and the good seizure outcome following removal of discrete epileptogenic lesions support a focal cortical origin of spasms. In patients with discrete epileptogenic lesions, the pragmatic approach to surgery should follow the same principles applied to focal epilepsy favoring, whenever possible, unilobar, one‐stage resections.
Introduction
Epileptic spasms account for 13–45.5% of new onset epilepsies in infancy1 and represent the most common manifestation of intractable epilepsy in the first 3 years of life.2 Although epilepsy surgery is an established treatment option in children with drug‐resistant seizures,3 literature on surgical treatment of drug‐resistant spasms is limited to small retrospective series with short follow‐ups.4, 5, 6, 7, 8, 9, 10 In two different cohorts, surgery for infantile spasms was performed in 6% of 543 children operated during calendar year 200411 and in 4.4% of 238 children operated between 1990 and 2011.12
Epilepsy surgery for spasms poses considerable challenges as the seizure onset zone is difficult to identify11, 13 and multilobar resections or hemispherotomies are often performed, with high risk of complications and postsurgical deficits.6, 8 These factors, in addition to uncertainty concerning developmental outcome, contribute to delaying surgery.11, 14
We retrospectively analysed clinical features, imaging, noninvasive and invasive EEG findings, surgical procedures and histopathology of 80 children we operated on for drug‐resistant spasms and assessed factors associated with favorable seizure outcome after surgery. We also analyzed how intracranial EEG recordings, beyond their role in guiding the surgical strategy in a subgroup of patients, provided relevant insights into the pathophysiology of spasms.
Methods
Patients
Patients were operated at two Epilepsy Surgery Centers (Children's Hospital Anna Meyer, Florence and “Claudio Munari” Center, Niguarda Hospital, Milan) between January 1997 and June 2014. Inclusion criteria for this study were: (1) age between 6 months and 15 years at the time of surgery; (2) epileptic spasms being a target for surgery; (3) at least 1 year follow‐up after surgery.
We identified 80 patients, evaluated clinical features, imaging, invasive and noninvasive EEG findings, surgical procedures and histopathology (complete information is available in Data S2 Methods and References).
This study was approved by the Pediatric Ethical Committee on Human Experimentation of the Tuscany Region. Informed consent for all procedures was obtained for all patients.
Results
Patients
(Table 1). There were 43 males and 37 females; mean age at surgery was 5.8 ± 4 years. Spasms were the initial seizures in 46 out of 80 patients (57.5%), while the remaining 34 patients (42.5%) had focal seizures at epilepsy onset. In ten patients (12.5%) spasms were the only seizure type, while in the remaining 70 patients (87.5%) focal seizures were, or had been, also present. Age at seizure onset (all seizures) was 1.3 ± 2 years; age at spasm onset was 1.9 ± 3 years. The mean interval from seizure onset (all seizures) to surgery was 4.5 ± 3 years; the mean interval from spasms onset to surgery was 3.9 ± 3 years.
Table 1.
General characteristics, neuroimaging and electroclinical findings of 80 patients operated on for epileptic spasms
| N˚Pts | 80 |
| Sex | 43M/37F |
| Age at seizure onset | 1.3 ± 2 years |
| Age at surgery | 5.8 ± 4 years |
| Epilepsy duration | 4.5 ± 3 years |
| Type of first seizure | 46 Spasms |
| 34 Focal sz | |
| Spasm semiology | 19 Asymmetric |
| 39 Symmetric | |
| 22 Asymmetric + symmetric | |
| Seizure frequency | 76 Daily |
| 4 Weekly | |
| Preoperative cognitive level | 23 Borderline or mild ID |
| 22 Moderate ID | |
| 18 Severe ID | |
| 17 Normal ID | |
| Preoperative neurological deficits | 21 Hemiparesis |
| 3 Visual field defect | |
| Brain MRI | 77 Abnormal (69 unilateral abnormality) |
| 3 Normal | |
| Interictal EEG (scalp) | 24 Diffuse or multifocal |
| 56 Focal | |
| Ictal EEG (scalp) | 45 Diffuse or multifocal |
| 25 Focal predominance | |
| Invasive EEG recordings | 22 SEEG |
| 2 Grids + Depth electrodes | |
| SOZ | |
| Topography | 56 Unilobar |
| 24 Multilobar/hemispheric | |
| SOZ vs. MRI visible lesion | 41 SOZ overlapped with lesion |
| 28 SOZ extending beyond lesion | |
| 8 SOZ within lesion | |
F, female; ID, intellectual disability; IQ, cognitive level scores; L, left; M, male; Pts, patients; R, right; SEEG, stereoelectroencephalography; SOZ, seizure onset zone; Sz, seizure; MRI, magnetic resonance imaging.
All patients were drug‐resistant. Seizures occurred daily in 76 patients (95%) and weekly in the remaining 4 (5%). Spasms were symmetric in 39 patients (48.8%), asymmetric in 19 (23.8%), and both symmetric and asymmetric in the remaining 22 (27.5%).
Twenty‐one patients (26.3%) had hemiparesis and three (3.8%) visual field defects.
Neuroimaging
(Table 1). Preoperative magnetic resonance imaging (MRI) was available for all patients. We identified structural abnormalities in 77 patients (96.3%), which were unilateral in 69 (89.6%; 51 unilobar and 18 multilobar or involving an entire hemisphere).
Scalp video‐EEG recordings
(Table 1). Interictal scalp EEGs were available for analysis in all patients. EEG abnormalities were focal in 56 patients (70%) and diffuse in the remaining 24 (30%). Ictal scalp EEGs recordings of spasms were available for analysis in 70 patients (87.5%). They revealed the EEG counterpart of spasms to be a slow wave with superimposed low voltage fast activity in 60 patients (85.7%) and a slow or sharp wave in the remaining 10 patients (14.3%). In 25 patients (35.7%) ictal activity had an obvious focal predominance.
Invasive recordings
(Table S1). We performed invasive recordings in 24 patients (30%), due to either nonlocalizing electroclinical data on surface recordings (15 patients), proximity of the epileptogenic lesion to eloquent areas (6 patients) or unrevealing MRI (3 patients).
Twenty‐two patients had SEEG (10–15 electrodes with 8–15 contacts each) and two patients had a combined subdural and depth electrodes approach.
During invasive recordings we captured both spasms and focal seizures in 18 patients and only spasms in six.
Intracranial EEGs during spasms demonstrated in 21 children low voltage fast activity, which was either isolated (14 patients), followed (5 patients) or preceded (2 patients) by a slow wave. In three remaining children (including two explored using a combined approach), we observed a slow or sharp wave with superimposed low voltage fast activity. In eight children, focal subclinical fast discharges preceded clinical spasms by several seconds.
Analysis of EEG data revealed that cortical areas involved at spasm onset could either remain constant or vary in subsequent clusters in the same patient (Fig. 1). In all 21 patients in whom MRI or histopathology revealed a lesion, electrodes exploring the structurally abnormal area constantly captured the initial ictal activity at spasm onset (Figs. 1, 2).
Figure 1.
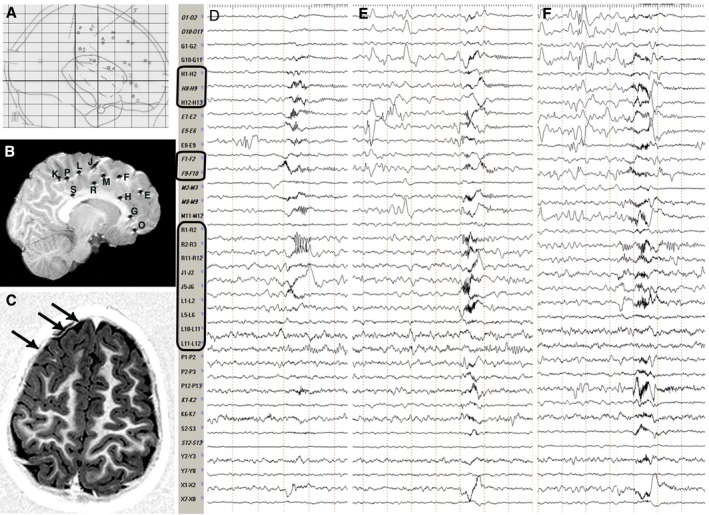
Stereotactic scheme, Post‐implantation and Preoperative MRI, Intracranial Ictal EEG. Patient 7, Table S1. (A) Stereotactic scheme, according to the bicommissural reference system (lateral view), of a right frontal SEEG exploration. Electrodes, labeled by uppercase letters, are indicated with either circles or dotted lines, depending on the orthogonal or oblique trajectory of the implantation. (B) Post‐implantation sagittal T1‐weighted MRI showing the exact position of each electrode. Electrodes appear as either circles or lines depending on the orthogonal or oblique trajectory of the implantation and are labeled as in the stereotactic scheme. (C) Preoperative axial T1‐weighted MRI showing a right frontal anterior area of cortical thickening and abnormal folding consistent with FCD (black arrows). (D,E,F) Intracranial ictal EEG recordings during three clusters of spasms. Electrodes exploring the lesion (i.e. electrodes H, F, R, J and L; black rectangles) are always involved by initial ictal activity at spasm onset. Conversely, no consistent pattern of spread is recognizable. Electrodes are labeled as in the stereotactic scheme and postimplantation MRI. FCD, focal cortical dysplasia; MRI, magnetic resonance imaging.
Figure 2.
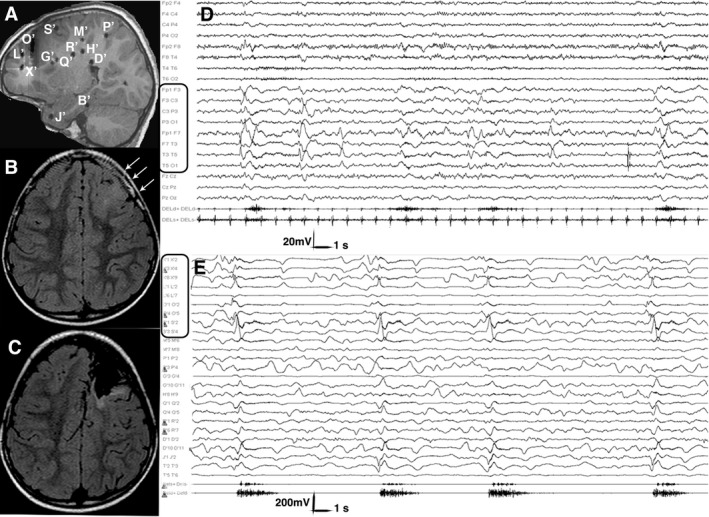
Postimplantation and Preoperative MRI, Scalp and Intracranial Ictal EEG. Patient 13, Table S1. (A) Post‐implantation sagittal T1‐weighted MRI showing the exact position of each electrode. Electrodes, labeled by uppercase letters, appear as either circles or lines, depending on the orthogonal or oblique trajectory. (B) Preoperative axial FLAIR MRI showing a left frontal area of cortical thickening, abnormal folding and increased signal intensity extending from the pole to the rolandic area, consistent with FCD (white arrows). (C) Postoperative axial FLAIR MRI showing the extent of resection. The primary motor area was spared, due to functional constraints. (D) Scalp ictal EEG during a cluster of asymmetric spasms. Ictal activity involves the left fronto‐temporal leads. Electromyogram shows a stronger contraction of the right deltoid during spasms. (E) Intracranial ictal EEG during a cluster of asymmetric spasms. Electrodes exploring the lesion (i.e. electrodes X’, L’, O’ and S’; black rectangles) are involved by initial ictal activity at spasm onset. Electromyogram shows again a stronger contraction of the right deltoid accompanying each spasm. Electrodes are labeled as in the postimplantation MRI. FCD, focal cortical dysplasia; MRI, magnetic resonance imaging.
When comparing different recordings in the same patient or between patients, we could not identify a consistent pattern of ictal spread during spasms. In the 18 children in whom both spasms and focal seizures were recorded, spasms either followed (7 patients), or preceded (6 patients), or occurred independently (11 patients) from focal seizures. In all patients in whom focal seizures were recorded and a lesion was demonstrated by either MRI or histopathology, or both, initial ictal activity always involved the electrodes exploring it. No consistent pattern of spread could be identified when comparing electrographic features of spasms versus focal seizures.
Identification of the SOZ
The SOZ had lobar distribution in 56 patients (70%), and was multilobar or involved the whole hemisphere in 24 patients (30%); it overlapped with the MRI visible lesion in 41 patients (53.2%), extended beyond it in 28 patients (36.4%) and was narrower than it in eight patients (10.4%). MRI was unrevealing in the three remaining patients.
Neuropsychology
(Table 1). Preoperative cognitive level scores (IQ) were available for all children. We observed borderline cognitive skills to mild ID in 23 children (28.8%), moderate ID in 22 (27.5%) and severe ID in 18 (22.5%). Cognitive level was normal in 17 patients (21.3%).
Surgery
(Table 2). We performed 58 unilobar (72.5%) and 22 multilobar or hemispheric surgical procedures (27.5%).
Table 2.
Surgical strategy, postsurgical complications, seizure outcome and histopathology in 80 patients surgically treated for epileptic spasms
| N˚Pts | 80 |
| Type of resection | 28 lesionectomy + corticectomy |
| 15 lesionectomy | |
| 15 lobectomy | |
| 11 corticectomy | |
| 6 lobar disconnection | |
| 4 hemispherotomy | |
| 1 hemispherectomy | |
| Post‐Surgical Complications | 4 subgaleal fluid collection |
| 4 hydrocephalus | |
| 2 pneumonia | |
| 2 anaemia | |
| 1 local infection | |
| 1 sinking of the parietal bone flap | |
| 1 subdural hematoma | |
| Post‐surgical deficits | 13 visual field defect |
| 10 transient hemiparesis | |
| 4 transient worsening of preexisting motor deficit | |
| 2 facial motor deficit | |
| Completeness of resection | 46 complete resection of the MRI visible lesion |
| 46 complete resection of the SOZ | |
| 33 complete resection of the MRI visible lesion and the SOZ | |
| Histopathology | 37 FCD (18 FCD I 10 FCD IIa, 9 FCD IIb) |
| 12 tumors (10 glioneuronal tumors, 1 oligoastrocytoma, 1 pylocitic astrocytoma) | |
| 10 reactive gliosis in patients with suspected FCD on MRI | |
| 9 (postischemic, post‐traumatic or postencephalitis) scars | |
| 8 tubers | |
| 2 HMG | |
| 1 PMG | |
| 1 NA | |
| Postoperative FU | Mean duration: 5.7 ± 4.2 years |
| 1 years: 80 pts | |
| 2 years: 70 pts | |
| 5 years: 52 pts | |
| >5 years: 31 pts | |
| Seizure Outcome (Engel class) | 45 IA |
| 3 IC | |
| 1 ID | |
| 5 II | |
| 9 III | |
| 15 IV | |
| 29 postsurgery AED withdrawal |
AED, antiepileptic drugs; FCD, focal cortical dysplasia; FU, follow‐up; HMG, hemimegalencephaly; NA, not available; PMG, polymicrogyria; Pts, patients, SOZ, seizure onset zone; MRI, magnetic resonance imaging.
Resection of the MRI visible lesion was deemed to be complete in 46 (57.5%) patients as was the resection of the SOZ (46 patients). Resection of both the lesion and the SOZ was complete in 33 (41.2%) patients.
Mean follow‐up after surgery was 5.7 ± 4.2 years. At last follow‐up, 49 patients (61.3%) were in Engel class I, 45 of whom (56.2%) were in Engel class IA category. In 29 patients (36.3%) treatment was withdrawn.
Ten out of 80 patients (12.5%) experienced minor complications after surgery. Six patients (7.5%) required reoperation due to various complications, including sinking of the parietal bone flap after lesionectomy (1), frontal subdural hematoma after lesionectomy + corticectomy (1), hydrocephalus after hemispherotomy (4). There was no surgery‐related mortality. Additional information on surgical procedures and postsurgical complications is summarized in Table 2.
Histopathology
(Table 2). Histopathology was available for 79 patients and revealed focal cortical dysplasia (FCD) in 37 (46.8%), tumors in 12 (15.2%), gliosis in nine (11.4%), tubers in eight (10.1%), hemimegalencephaly in two (2.5%) and polymicrogyria in one (1.3%). In 10 patients (12.7%) in whom, based on MRI, we suspected FCD, histopathology revealed reactive gliosis. In one patient no tissue specimen was available after hemispherotomy.
Statistical analysis
Information on univariate analyses is included in Table S2 and Data S1.
Outcome I: Engel class IA versus classes IB‐IV
(Table 3). The multivariate logistic models showed that completeness of resection of the SOZ (OR = 0.016, 95% CI: 0.002, 0.122) and of the MRI visible lesion (OR = 0.179, 95% CI: 0.032, 0.999) were both significantly associated with Engel class IA outcome.
Table 3.
Multivariate logistic models
| Odds ratio | 95% CI | P‐value | ||
|---|---|---|---|---|
| Outcome class IA vs IIb‐IV | ||||
| Age at surgery | 1.383 | 0.994 | 1.926 | 0.055 |
| SOZ lateralization | 0.196 | 0.030 | 1.265 | 0.087 |
| Unilobar vs Multilobar SOZ | 0.770 | 0.124 | 4.782 | 0.779 |
| Age at seizure onset | 0.847 | 0.414 | 1.733 | 0.649 |
| Age at spasm onset | 0.898 | 0.496 | 1.624 | 0.721 |
| Need of Invasive recordings | 0.851 | 0.122 | 5.921 | 0.871 |
| Complet res. MRI visible lesion | 0.179 | 0.032 | 0.998 | 0.050 |
| Complet res. SOZ | 0.016 | 0.002 | 0.122 | 0.000 |
| Cognitive_0 (normal) ref | 1.000 | |||
| Cognitive_1 (mild ID) | 0.422 | 0.040 | 4.505 | 0.475 |
| Cognitive_2 (moderate ID) | 0.738 | 0.064 | 8.498 | 0.807 |
| Cognitive_3 (severe ID) | 6.850 | 0.566 | 82.959 | 0.131 |
| _cons | 11.530 | 0.760 | 174.810 | 0.078 |
| Outcome class I vs II‐IV | ||||
| Age at surgery | 1.209 | 0.881 | 1.660 | 0.240 |
| SOZ lateralization | 0.089 | 0.008 | 0.969 | 0.047 |
| Unilobar vs Multilobar SOZ | 0.967 | 0.082 | 11.453 | 0.979 |
| Age at seizure onset | 0.482 | 0.117 | 1.983 | 0.312 |
| Age at spasm onset | 0.795 | 0.357 | 1.770 | 0.574 |
| Neurological signs | 3.893 | 0.468 | 32.404 | 0.209 |
| Need for Invasive recordings | 1.182 | 0.095 | 14.696 | 0.897 |
| Complet res. MRI visible lesion | 2.219 | 0.262 | 18.759 | 0.464 |
| Complet. res. SOZ | 0.001 | 0.000 | 0.058 | 0.001 |
| Cognitive_0 (normal) ref. | 1.000 | |||
| Cognitive_1 (mild ID) | 0.112 | 0.004 | 2.970 | 0.191 |
| Cognitive_2 (moderate ID) | 0.310 | 0.017 | 5.769 | 0.433 |
| Cognitive_3 (severe ID) | 0.537 | 0.027 | 10.854 | 0.685 |
| Spasm semiology_0 (sym) ref | 1.000 | |||
| Spasm semiology_1: asym | 0.168 | 0.010 | 2.818 | 0.215 |
| Spasm semiology_2: sym+asym | 11.984 | 0.560 | 256.558 | 0.112 |
| _cons | 34.326 | 0.920 | 1280.798 | 0.056 |
| Post‐surgery AED withdrawal vs continued treatment | ||||
| Age at surgery | 1.122 | 0.972 | 1.296 | 0.115 |
| SOZ lateralization | 1.364 | 0.477 | 3.905 | 0.563 |
| Unilobar vs Multilobar SOZ | 0.687 | 0.212 | 2.224 | 0.531 |
| Complet. res. SOZ | 0.237 | 0.074 | 0.762 | 0.016 |
| Cognitive_0 (normal) ref | 1.000 | |||
| Cognitive_1 (mild ID) | 2.995 | 0.720 | 12.454 | 0.131 |
| Cognitive_2 (moderate ID) | 1.098 | 0.262 | 4.589 | 0.899 |
| Cognitive_3 (severe ID) | 4.760 | 0.800 | 28.341 | 0.086 |
| _cons | 1.130 | 0.190 | 6.720 | 0.894 |
| Population average logistic model for longitudinal seizure outcome | ||||
| Age at surgery | 1.216 | 1.060 | 1.394 | 0.005 |
| SOZ lateralization | 0.718 | 0.286 | 1.801 | 0.480 |
| Unilobar vs Multilobar SOZ | 2.271 | 0.824 | 6.258 | 0.113 |
| 6 months FU ref | 1.000 | |||
| 1 Year FU | 1.144 | 0.443 | 2.955 | 0.782 |
| 2 Years FU | 1.512 | 0.574 | 3.987 | 0.403 |
| 5 Years FU | 1.489 | 0.508 | 4.371 | 0.468 |
| >5 's FU | 1.450 | 0.370 | 5.677 | 0.594 |
| Complet. res. SOZ | 0.019 | 0.005 | 0.067 | 0.000 |
| SOZ_superimp to lesion_0 ref | 1.000 | |||
| SOZ_within lesion_1 (less ext) | 3.414 | 0.795 | 14.671 | 0.099 |
| SOZ_extend. beyond_lesion_2 | 4.994 | 1.803 | 13.830 | 0.002 |
| Age at seizure onset | 0.424 | 0.278 | 0.645 | 0.000 |
| Spam semiology_0 (sym) ref | 1.000 | |||
| Spasm semiology_1: asym | 1.210 | 0.367 | 3.989 | 0.755 |
| Spasm semiology_2: syim+asym | 8.510 | 2.530 | 28.625 | 0.001 |
| _cons | 0.327 | 0.090 | 1.190 | 0.090 |
Variables with P ≤ 0.05 are indicated in bold.
AED, antiepileptic drugs; Asym, asymmetric; Complet, completeness; Extend, extending; ID, intellectual disability; MRI, magnetic resonance imaging; Ref, reference; Res, resection; SOZ, seizure onset zone; Superimp, superimposed; Sym, symmetric.
The sensitivity analysis confirmed the results of multivariate logistic models.
The multivariate logistic models showed that completeness of resection of the SOZ (OR = 0.010, 95% CI: 0.001, 0.113) was associated with favorable seizure outcome after surgery.
The sensitivity analysis confirmed the results of multivariate logistic models.
Outcome III: Post‐surgery AED withdrawal versus continuing treatment
(Table 3). The multivariate logistic models showed a significant association between completeness of the resection of the SOZ and AED withdrawal after surgery (OR = 0.37, 95% CI: 0.074, 0.762).
The sensitivity analysis confirmed the results of the multivariate logistic models.
Longitudinal outcome
(Table 3). Engel class I outcome in relation to follow‐up duration was achieved in 67.5% of the 80 patients with at least 1 year follow‐up, in 62.9% of 70 patients with 2 years, in 59.6% of 52 patients with 5 years and in 58.1% of 31 patients with >5 years.
The population average logistic models showed that completeness of resection of the SOZ (OR = 0.019, 95% CI: 0.005, 0.067) and later age at seizure onset (OR = 0.242, 95% CI: 0.278, 0.645) were associated with Engel class I outcome after surgery. Conversely, older age at surgery (OR = 1.216, 95% CI: 1.060, 1.394), a SOZ extending beyond the MRI visible lesion (OR = 4.994, 95% CI: 1.803,13.830) and co‐occurrence of symmetric and asymmetric spasms (OR = 8.510, 95% CI: 2.530, 28.625) were associated with unfavorable seizure outcome. Comparing subgroups of children of similar ages at seizure onset but operated on 1 year apart revealed unfavorable seizure outcome in those operated later (OR = 1.216, 95% CI: 1.060, 1.394).
There was no significant effect of duration of follow‐up on the risk of seizure recurrence.
Discussion
In this series, 61.3% of 80 children surgically treated for drug‐resistant epileptic spasms were in Engel class I at last follow‐up (mean duration of follow‐up after surgery 5.7 ± 4.2 years, range: 1–18 years). Initial reports on surgery for spasms included a few selected observations demonstrating that removal of a causative lesion could be successful.15, 16 Subsequent series reported average rates of seizure freedom after surgery ranging from 46% to 72%.5, 8, 9, 10, 13, 14, 17, 18 However, a comparison between our own and previous series is hampered by considerable differences in inclusion criteria, presurgical protocols and surgical approaches.
In our series, 70% of patients underwent surgery without invasive recordings (‘one‐stage surgery’) and 72.5% underwent unilobar removal/disconnections. In previous series most patients with spasms achieving seizure freedom received multilobar or hemispheric resections/disconnections.6, 7, 8, 17 Our results suggest that unilobar procedures, which carry lower rates of complications and postsurgical deficits compared to multilobar or hemispheric resections/disconnections,6, 8, 13, 19 are effective in selected patients.
We did not observe a significant increased risk of relapse with longer follow‐ups, which is in contrast with Jonas et al.,8 figures indicating seizure freedom rates after surgery to drop from 62.5% at 6 months to 44% at 5 years. However, in our study, the lower percentage of drug‐free patients at last follow‐up might have partially masked surgical failures.20 In other previous series, small sample size13, 18, 21, 22 or short follow‐ups6, prevented adopting longitudinal population average logistic models.
In our series, completeness of resection of the SOZ and of the MRI visible lesion were the main factors determining favorable seizure outcome, as assessed by multivariate logistic models and sensitivity analysis. Two previous studies on surgical treatment of spasms provided indications that complete removal of the SOZ and of the area of cortical dysplasia generating a ‘leading spike’ were important determinants of favorable seizure outcome.10, 21 However, due to the small sample size, in neither study could multivariate analysis be performed.
In a large series from Detroit,6 detection of an MRI visible lesion was associated per se, with seizure freedom, while the removal of the SOZ or of the lesion, or both, were not analysed as prognostic factors with respect to seizure outcome in multivariate analysis. The different statistical inferences obtained in the Detroit and our own series might also be a reflection of us having included only patients operated on for active spasms, while 43% of the Detroit patients included in the multivariate analysis had no longer spasms at the time of surgery.
Using univariate models we identified several weak unfavorable prognostic factors for seizure freedom, including the need for invasive recordings, severe intellectual disability, hemiparesis, visual field defects and the co‐occurrence of asymmetric and symmetric spasms. These findings are not unexpected. Invasive recordings are often necessary in complex cases in whom the chances of obtaining complete resection of the SOZ and, consequently, of achieving seizure freedom, are low.23 Specifically, we used SEEG in presumed symptomatic cases, in patients whose lesions were close to eloquent areas and when scalp EEG was nonlocalizing. These circumstances make surgical approach more complex as they limit the possibility of accurately planning the resection area and achieving completeness of resection. The need for invasive recordings has progressively decreased over the years and will undoubtedly continue to do so, thanks to advances in functional neuroimaging and improved understanding of prognostic factors.
Concerning the other results of univariate analyses, we confirmed neurological signs, severe intellectual disability and coexistence of symmetric and asymmetric spasms to be unfavorable prognostic factors for long‐term outcome as they are often related to widespread epileptogenicity or diffuse lesions.24, 25, 26, 27 We also found earlier age at seizure onset and longer duration of epilepsy before surgery to be accompanied by lower rates of freedom from disabling seizures after surgery, thus suggesting that early intervention is advised in children with spasms.6, 21
In this series, invasive recordings demonstrated that for both spasms and focal seizures, electrodes exploring the structurally abnormal area constantly captured the initial ictal electrographic changes, thus suggesting a common mechanism of generation of these two seizure types.18, 28 In the ten patients who only had spasms, the surgical strategy we adopted was the same as for children also experiencing focal seizures, as they exhibited MRI visible lesions and EEG findings clearly pointing out to the structural abnormality. Two of the ten children who had only spasms underwent SEEG, during which electrodes exploring the structurally abnormal area recorded the initial ictal activity at spasm onset.
Using longitudinal population average logistic models, we observed that, in order to achieve postoperative drug withdrawal, complete resection of the SOZ was a stronger favorable prognostic factor than the mere resection of the MRI visible lesion. An SOZ extending beyond the lesional area was an unfavorable prognostic factor for Engel class I outcome. As a whole, our statistical results suggest that a careful neurophysiological definition of the SOZ for spasms increases the chances to obtain seizure freedom.
During SEEG, we observed spasms to be regularly accompanied by low voltage fast activity, either isolated or intermingled with a slow or sharp wave, as previously reported.18, 29 An ictal pattern consisting of fast activity, preceded or not by a ‘leading spike’, was described using electrocorticography.5 In our study, the high number of SEEG electrodes we used helped carrying out a dynamic analysis of the spatio‐temporal spread of the ictal discharge from the inner to the outer aspects of the explored areas and precisely localizing initial ictal activity at spasm onset, even when ictal discharges were diffuse on scalp recordings.
Pathophysiology of spasms remains to be fully elucidated. Earliest studies suggested brainstem dysfunction as a trigger for both spasms and the hypsarrhythmic EEG.30, 31, 32 Based on PET studies showing focal cortical hypometabolism33, 34 and electroclinical demonstrations of co‐occurring partial seizures and spasms,35 abnormal functional interactions between brainstem and a focal or diffuse cortical abnormality were hypothesized.33, 36 A predominant role of either the cortical abnormality18, 37, 38 or the subcortical structures39, 40 has always been a divisive argument. Uncertainties on pathophysiology have prompted caution in proposing surgery as a viable therapeutic approach for spasms,30, 40 with subsequent limited surgical referral rates.11, 12 However, in many cases, spasms have a focal cortical origin as suggested by invasive EEG recordings as well as the favorable outcome following removal of discrete epileptogenic lesions.5, 6, 8, 9, 10, 13, 18
Our study has limitations, especially due to its retrospective design, etiologic heterogeneity, the inclusion of children with both spasms and focal seizures and the limited sampling SEEG allows. Certainly, selection of surgical candidates leaves many conditions with epileptic spasms that may have a completely different pathophysiology, especially genetic ones, outside the scope of our study. However, through analysis a large series we confirm that surgery is an effective treatment option for a selected population of children with drug‐resistant spasms and suggest that extended resections and invasive recordings may not be needed to achieve seizure freedom. Major determinants of favorable outcome after surgery, that is, completeness of resection of the MRI visible lesion and of the SOZ and shorter duration of epilepsy, are similar to those reported for symptomatic focal epilepsy. Despite the complex pathophysiology of spasms and the difficulties in identifying the SOZ, in patients with a discrete epileptogenic lesion the pragmatic approach to surgery should follow the same principles applied to focal epilepsy, favoring unilobar, one‐stage resections, thus reducing the risk of facing major surgical complications and postoperative deficits.
Author Contributions
Carmen Barba: study design, invasive EEG and clinical data analysis and drafting of the manuscript. Roberto Mai: invasive EEG and clinical data analysis and revision of the manuscript. Laura Grisotto: statistical analyses and revision of the manuscript. Francesca Gozzo: clinical data analysis. Simona Pellacani: clinical data analysis. Laura Tassi: EEG data analysis and revision of the manuscript. Stefano Francione: EEG data analysis and revision of the manuscript. Flavio Giordano: surgical data analysis and revision of the manuscript. Francesco Cardinale: surgical data analysis. Renzo Guerrini: study concept, clinical data analysis and drafting of the manuscript.
Conflicts Of Interest
The authors report no disclosures relevant to the publication.
Supporting information
Table S1. Invasive recordings: Initial ictal activity at onset of spasms and of focal seizures and pattern of ictal spread.
Table S2. Logistic models: Univariate analyses.
Data S1. Supplementary results.
Data S2. Supplementary methods.
Acknowledgments
This research was supported by a grant from the European Union Seventh. Framework Programme FP7/2013 under the project DESIRE – Development and Epilepsy ‐ Strategies for Innovative Research to improve diagnosis, prevention and treatment in children with difficult to treat Epilepsy (grant agreement 602531 2013–2018) to R.G. and by a grant from the Italian Ministry of Health and Tuscany Region (RF‐2010‐2309954, 2012–2016) ‘Reorganization of cortical function after surgery for lesional epilepsy in children’ to C.B.
References
- 1. Wilmshurst JM, Gaillard WD, Vinayan KP, et al. Summary of recommendations for the management of infantile seizures: task force report for the ILAE commission of pediatrics. Epilepsia 2015;56:1185–1197. [DOI] [PubMed] [Google Scholar]
- 2. Wirrell E, Wong‐Kisiel L, Mandrekar J, Nickels K. Predictors and course of medically intractable epilepsy in young children presenting before 36 months of age: a retrospective, population‐based study. Epilepsia 2012;53:1563–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol 2008;7:525–537. [DOI] [PubMed] [Google Scholar]
- 4. Shields WD, Shewmon DA, Chugani HT, Peacock WJ. Treatment of infantile spasms: medical or surgical? Epilepsia 1992;33(Suppl 4):S26–S31. [DOI] [PubMed] [Google Scholar]
- 5. Asano E, Juhász C, Shah A, et al. Origin and propagation of epileptic spasms delineated on electrocorticography. Epilepsia 2005;46:1086–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chugani HT, Ilyas M, Kumar A, et al. Surgical treatment for refractory epileptic spasms: the detroit series. Epilepsia 2015;56:1941–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kang JW, Rhie SK, Yu R, et al. Seizure outcome of infantile spasms with focal cortical dysplasia. Brain Dev 2013;35:816–820. [DOI] [PubMed] [Google Scholar]
- 8. Jonas R, Asarnow RF, LoPresti C, et al. Surgery for symptomatic infant‐onset epileptic encephalopathy with and without infantile spasms. Neurology 2005;64:746–750. [DOI] [PubMed] [Google Scholar]
- 9. Nariai H, Matsuzaki N, Juhász C, et al. Ictal high‐frequency oscillations at 80–200 Hz coupled with delta phase in epileptic spasms. Epilepsia 2011;52:e130–e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nariai H, Nagasawa T, Juhász C, et al. Statistical mapping of ictal high‐frequency oscillations in epileptic spasms. Epilepsia 2011;52:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harvey AS, Cross JH, Shinnar S, Mathern GW. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia 2008;49:146–155. [DOI] [PubMed] [Google Scholar]
- 12. Lamberink HJ, Boshuisen K, van Rijen PC, et al. Changing profiles of pediatric epilepsy surgery candidates over time: A nationwide single‐center experience from 1990 to 2011. Epilepsia 2015;56:717–725. [DOI] [PubMed] [Google Scholar]
- 13. Moseley BD, Nickels K, Wirrell EC. Surgical outcomes for intractable epilepsy in children with epileptic spasms. J Child Neurol 2012;27:713–720. [DOI] [PubMed] [Google Scholar]
- 14. Gowda S, Salazar F, Bingaman WE, et al. Surgery for catastrophic epilepsy in infants 6 months of age and younger. J Neurosurg Pediatr 2010;5:603–607. [DOI] [PubMed] [Google Scholar]
- 15. Ruggieri V, Caraballo R, Fejerman N. Intracranial tumors and West syndrome. Pediatr Neurol 1989;5:327–329. [DOI] [PubMed] [Google Scholar]
- 16. Uthman BM, Reid SA, Wilder BJ, et al. Outcome for West syndrome following surgical treatment. Epilepsia 1991;32:668–671. [DOI] [PubMed] [Google Scholar]
- 17. Wyllie E, Lachhwani DK, Gupta A, et al. Successful surgery for epilepsy due to early brain lesions despite generalized EEG findings. Neurology 2007;69:389–397. [DOI] [PubMed] [Google Scholar]
- 18. de la Vaissière S, Milh M, Scavarda D, et al. Cortical involvement in focal epilepsies with epileptic spasms. Epilepsy Res 2014;108:1572–1580. [DOI] [PubMed] [Google Scholar]
- 19. Cukiert A, Rydenhag B, Harkness W, et al. Technical aspects of pediatric epilepsy surgery: report of a multicenter, multinational web‐based survey by the ILAE Task Force on Pediatric Epilepsy Surgery. Epilepsia 2016;57:194–200. [DOI] [PubMed] [Google Scholar]
- 20. Boshuisen K, Arzimanoglou A, Cross JH, et al. Timing of antiepileptic drug withdrawal and long‐term seizure outcome after paediatric epilepsy surgery (TimeToStop): a retrospective observational study. Lancet Neurol 2012;11:784–791. [DOI] [PubMed] [Google Scholar]
- 21. Lee YJ, Lee JS, Kang HC, et al. Outcomes of epilepsy surgery in childhood‐onset epileptic encephalopathy. Brain Dev 2014;36:496–504. [DOI] [PubMed] [Google Scholar]
- 22. Kang H‐C, Jung DE, Kim KM, et al. Surgical treatment of two patients with infantile spasms in early infancy. Brain Dev 2006;28:453–457. [DOI] [PubMed] [Google Scholar]
- 23. Tonini C, Beghi E, Berg AT, et al. Predictors of epilepsy surgery outcome: a meta‐analysis. Epilepsy Res 2004;62:75–87. [DOI] [PubMed] [Google Scholar]
- 24. Fusco L, Vigevano F. Ictal clinical electroencephalographic findings of spasms in West syndrome. Epilepsia 1993;34:671–678. [DOI] [PubMed] [Google Scholar]
- 25. Riikonen RS. Favourable prognostic factors with infantile spasms. Eur J Paediatr Neurol 2010;14:13–18. [DOI] [PubMed] [Google Scholar]
- 26. Watanabe K. West syndrome : etiological and prognostic aspects. Brain Dev 1998;20:1–8. [DOI] [PubMed] [Google Scholar]
- 27. Gaily EK, Shewmon DA, Chugani HT, Curran JG. Asymmetric and asynchronous infantile spasms. Epilepsia 1995;36:873–882. [DOI] [PubMed] [Google Scholar]
- 28. Panzica F, Binelli S, Canafoglia L, et al. ICTAL EEG fast activity in West syndrome: from onset to outcome. Epilepsia 2007;48:2101–2110. [DOI] [PubMed] [Google Scholar]
- 29. Ricard‐Mousnier B, Dorfmuller G, Fohlen M, et al. Late‐onset epileptic spasms may be cured by focal cortical resective surgery. Epileptic Disord 2012;14:313–320. [DOI] [PubMed] [Google Scholar]
- 30. Hrachovy RA, Frost JD. Infantile spasms. Pediatr Clin North Am 1989;36:311–329. [DOI] [PubMed] [Google Scholar]
- 31. Satoh J, Mizutani T, Morimatsu Y. Neuropathology of the brainstem in age‐dependent epileptic encephalopathy–especially of cases with infantile spasms. Brain Dev 1986;8:443–449. [DOI] [PubMed] [Google Scholar]
- 32. Neville BG. The origin of infantile spasms: evidence from a case of hydranencephaly. Dev Med Child Neurol 1972;14:644–647. [DOI] [PubMed] [Google Scholar]
- 33. Chugani HT, Shewmon DA, Shields WD, et al. Surgery for intractable infantile spasms: neuroimaging perspectives. Epilepsia 1993;34:764–771. [DOI] [PubMed] [Google Scholar]
- 34. Chugani HT, Shields WD, Shewmon DA, et al. Infantile spasms: I. PET identifies focal cortical dysgenesis in cryptogenic cases for surgical treatment. Ann Neurol 1990;27:406–413. [DOI] [PubMed] [Google Scholar]
- 35. Pachatz C, Fusco L, Vigevano F. Epileptic spasms and partial seizures as a single ictal event. Epilepsia 2003;44:693–700. [DOI] [PubMed] [Google Scholar]
- 36. Shields WD, Shewmon DA, Peacock WJ, et al. Surgery for the treatment of medically intractable infantile spasms: a cautionary case. Epilepsia 1999;40:1305–1308. [DOI] [PubMed] [Google Scholar]
- 37. Kobayashi K, Miya K, Akiyama T, et al. Cortical contribution to scalp EEG gamma rhythms associated with epileptic spasms. Brain Dev 2013;35:762–770. [DOI] [PubMed] [Google Scholar]
- 38. Vigevano F, Fusco L, Pachatz C. Neurophysiology of spasms. Brain Dev 2001;23:467–472. [DOI] [PubMed] [Google Scholar]
- 39. Dulac O. What is West syndrome? Brain Dev 2001;23(7):447–452. [DOI] [PubMed] [Google Scholar]
- 40. Lado FA, Moshé SL. Role of subcortical structures in the pathogenesis of infantile spasms: what are possible subcortical mediators? Int Rev Neurobiol 2002;49:115–140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Invasive recordings: Initial ictal activity at onset of spasms and of focal seizures and pattern of ictal spread.
Table S2. Logistic models: Univariate analyses.
Data S1. Supplementary results.
Data S2. Supplementary methods.


