Abstract
Wolbachia is an intracellular endosymbiont infecting most arthropod and some filarial nematode species that is vertically transmitted through the maternal lineage. Due to this primary mechanism of transmission, most studies have focused on Wolbachia interactions with the host germline. However, over the last decade many studies have emerged highlighting the prominence of Wolbachia in somatic tissues, implicating somatic tissue tropism as an important aspect of the life history of this endosymbiont. Here, we review our current understanding of Wolbachia–host interactions at both the cellular and organismal level, with a focus on Wolbachia in somatic tissues.
Keywords: cytoskeleton, endosymbiont, horizontal, insect, invasion, migration, nematode, pathogen resistance, somatic, transfer, virus, Wolbachia
1. Introduction
Wolbachia is an intracellular bacterium found primarily in arthropods and filarial nematodes. In insects, Wolbachia is abundant in both the male and female germlines, though it is vertically transmitted exclusively through the female germline. In filarial nematodes, Wolbachia is present only in the female germline, facilitating efficient mitochondria‐like maternal transmission. In the majority of hosts, Wolbachia exists as an endosymbiont. That is, it maintains a neutral relationship with its host. In most arthropods, this relationship is facultative, whereas in filarial nematodes, Wolbachia maintains a fixed obligate relationship with its host. Furthermore, depending on both intrinsic and extrinsic factors, Wolbachia can act as a mutualist, commensalist, or pathogen. Because Wolbachia is primarily transmitted through the maternal germline, it maintains an extraordinary ability to influence host reproduction to favor proliferation by infected females. Wolbachia biology has recently enjoyed increased interest because of two crucial findings: it is a major cause of pathogenicity associated with parasitic filarial nematodes and it has the ability to reduce the titer of dengue virus and other mosquito‐borne human pathogens when infecting the vector species (Eleftherianos, Atri, Accetta, & Castillo, 2013; Taylor, 2003).
Wolbachia was first described by Cowdry (1923) and Hertig and Wolbach (1924) as a gram‐negative, intracellular, Rickettsiae‐like bacteria concentrated in the germline and somatic tissues of a broad array of insects and other arthropods. In his 1936 publication, Marshall Hertig honored his mentor Simeon B. Wolbach with the statement, “The name Wolbachia pipientis is proposed for the rickettsia of Culex pipiens” (Hertig 2013). In the 1970s Wolbachia received renewed attention with the classic publication by Yen and Barr demonstrating that a form of reproductive incompatibility (cytoplasmic incompatibility; CI) among mosquito isolates was due to the presence of this maternally inherited, antibiotic curable, rickettsia‐like organism (Yen & Barr, 1973). In infected populations, CI results in infected females maintaining a selective advantage over uninfected females through increased egg hatch rates. Briefly, infected females mated with infected or uninfected males produce viable embryos. In contrast, in unidirectional CI uninfected females mated with infected males produce inviable embryos. Furthermore, in bidirectional CI, mating of males and females infected with different strains of Wolbachia also results in the production of inviable embryos (Werren, 1997). Later, additional mechanisms by which Wolbachia favor proliferation of infected females in mixed‐infected populations were discovered. These include feminization of genetically male offspring, parthenogenesis by infected females, and male killing of infected males (Louis & Nigro, 1989; Saridaki & Bourtzis, 2010; Werren, Baldo, & Clarke, 2008; Yen & Barr, 1973). Of these mechanisms CI is the most prevalent and well‐studied.
Perhaps because CI operates with the combined effects of Wolbachia in the male and female germlines, and Wolbachia is transmitted through the latter, much of the work on Wolbachia has focused on its interaction with the host germline. However, work over the past decade has reinforced original observations that, in addition to localization to the germline, a conserved feature of Wolbachia infection is localization to somatic tissue. Equally significant, these studies have begun to shed light on the functional importance of tissue‐specific somatic localization of Wolbachia. Here, we review the current state of knowledge regarding the somatic aspects of Wolbachia infection and the functional consequences for both host and endosymbiont.
2. An Overview of Distribution in Somatic Tissues
In the original description of Wolbachia, the authors describe not only a concentration of “rodlike organisms in the reproductive tissue but also in the somatic tissue” (Hertig & Wolbach, 1924). Since these initial descriptions, numerous researchers have documented the presence of Wolbachia in a variety of somatic tissues (Table 1). In fact, the few examples in the literature where Wolbachia is restricted to the reproductive tissues, such as certain strains of the mosquito Aedes albopictus and female Glossina morsitans tsetse flies (Dobson et al., 1999), appear to be the exception rather than the rule. PCR and fluorescent cytological approaches have been used to assay for the presence of Wolbachia, with both techniques revealing a broad distribution in specific somatic cells and tissues (Table 1). Most of these data come from studies in either Drosophila or mosquitos. However, similar distribution patterns have been observed in numerous other insect and nematode species. Table 1 and Figure 1 depict the documented cellular and tissue distribution for these and other organisms.
Table 1.
Wolbachia distribution in somatic tissues
| Organism | Species | Somatic Tissues | References |
|---|---|---|---|
| Fruit Fly | D. melanogaster (adult) | Central brain (intra & extracellular), retina, optic lobe, ganglia, somatic cyst cells, somatic stem cells | Albertson et al., 2013; Casper‐Lindley et al., 2011; Strunov et al., 2013; Toomey et al., 2013; Veneti et al., 2003; |
| D. simulans (adult) | Head, muscle, midgut, malpighian tubules, wings, hemolymph | Dobson et al., 1999; Osborne et al. 2009 | |
| D. melanogaster (larva) | Nerves, malpighian tubules, salivary glands, trachea, fat body, proventriculus | Clark et al., 2005; | |
| D. simulans (larva) | Brain, salivary gland, midgut, fat body | Dobson et al., 1999; | |
| Mosquito | Ae. albopictus | Salivary glands, some strains no somatic tissue | Dobson et al., 1999; Zouache et al., 2009; |
| An. gambiae (wMelPop) | Brain, sensory organs, mouthparts, hemocytes, fat body, abdomen | Hughes, Koga et al., 2011; | |
| C. pipiens | Head, malpighian tubules, wings, hemolymph | Dobson et al., 1999; | |
| C. cautella | Head, muscles, midgut, malpighian tubules, wings, hemolymph | Dobson et al., 1999; | |
| C. tarsalis | Head, muscle, ganglia, fat body, ovary follicles | Dodson et al., 2014; | |
| Nematode | B. malayi | Hypodermal chords, excretory canal, pseudocoelom | Fischer et al., 2011; Landmann et al., 2010; |
| M. perforate | Epithelial gonad, intestinal wall | Ferri et al., 2011; | |
| C. japonica | Epithelial gonad | Ferri et al., 2011; | |
| O. flexuosa | Hypodermis, median chords, intestine | McNulty et al., 2013; | |
| Tsetse Fly | G. austeni | Head, salivary gland, milk gland, fat body | Cheng et al., 2000; |
| G. brevipalpis | No somatic tissue | Cheng et al., 2000; | |
| G. morsitans | No somatic tissue | Cheng et al., 2000; | |
| Bed Bug | C. lectularius | Bacteriome, mesospermalage | Hosokawa et al., 2010; |
| Leafcutter Ant | A. octospinosus | Foregut, midgut, feces, muscle, thorax | Andersen et al., 2012; |
| Kissing Bug | R. pallescens | Salivary glands, intestine | Espino et al., 2009; |
| Termite | C. subarquatus | Head, salivary glands, thorax, legs | Roy et al., 2015 |
Figure 1.
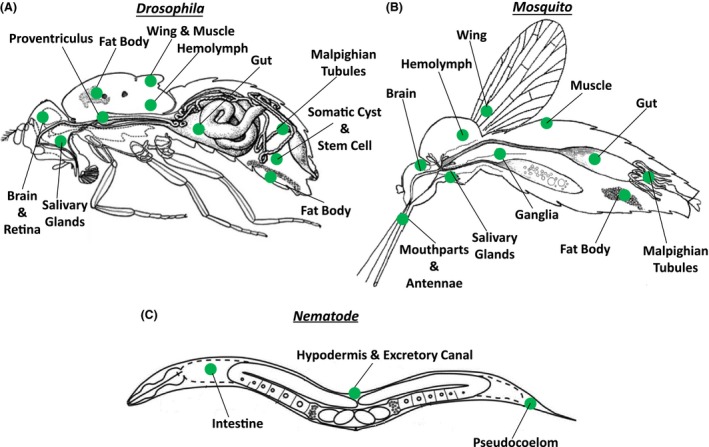
Wolbachia distribution in somatic tissues. Wolbachia has been detected by PCR and fluorescent cytology in various somatic tissues of numerous (A) fly, (B) mosquito, and (C) filarial nematode species, as indicated in green
In brief, Wolbachia is prevalent in tissues of the nervous system in Drosophila and other flies (Albertson et al., 2013; Casper‐Lindley et al., 2011; Dobson et al., 1999; Mitsuhashi, Saiki, Wei, Kawakita, & Sato, 2002; Moreira et al., 2009; Osborne, Leong, O'Neill, & Johnson, 2009; Strunov & Kiseleva, 2014). In Drosophila, the distribution of the pathogenic Wolbachia strain, wMelPop, in the nervous system of adults is temperature dependent, with increased temperature favoring the expansion of Wolbachia from the central brain to peripheral areas such as the optic lobe and retina (Strunov, Kiseleva, & Gottlieb, 2013). These data suggest that temperature may be a possible determinant of Wolbachia replication in somatic tissues. In addition to the nervous system, Wolbachia is also present in digestive and metabolic tissues such as the fat body, gut, salivary glands, hemocytes, and malpighian tubules of various arthropod species where it may play a role in regulating host immunity and bioenergetics (Andersen, Boye, Nash, & Boomsma, 2012; Chevalier et al., 2011; Dobson et al., 1999; Faria & Sucena, 2013; Hughes, Koga, Xue, Fukatsu, & Rasgon, 2011; Hughes et al., 2011; Ponton et al., 2015; Zouache et al., 2009). Wolbachia has been further documented in muscle and wing tissue of some species (Andersen et al., 2012; Cheng et al., 2000; Dobson et al., 1999; Dodson et al., 2014; Frydman, Li, Robson, & Wieschaus, 2006; Min & Benzer, 1997), though the significance of this remains largely unknown. In filarial nematodes, the somatic distribution of Wolbachia is restricted to the lateral chords (hypodermis), excretory canal, and the intestine (Ferri et al., 2011; Fischer, Beatty, Jiang, Weil, & Fischer, 2011; Landmann et al., 2012).
The repeated observation of Wolbachia in specific somatic tissues suggests that somatic tissue tropism is not incidental, but rather a key aspect of Wolbachia biology. For instance, somatic localization of Wolbachia may be evolutionarily maintained because it aids horizontal transmission within and between species, thus serving as a mechanism to increase the genetic diversity of Wolbachia. Additionally, somatic Wolbachia may confer advantageous phenotypes in the host that enhance its germline transmission. Below, we further explore the mechanisms and functional significance of the somatic localization patterns of Wolbachia.
3. Segregation Patterns During oogenesis and Early Embryogenesis Influence Tissue Distribution Later in Development
3.1. Arthropods
As in many insect species, the Drosophila egg chamber consists of a syncytium of 15 nurse cells and an oocyte, all connected through cytoplasmic bridges (Spradling, 1993). During maturation, nurse cell cytoplasm is pumped into the oocyte. Importantly, specific determinants essential for anterior‐posterior (AP) axis formation are also transported from the nurse cells to the specific regions of the maturing oocyte. Localization of these AP axis and germline determinants requires microtubules, microtubule‐based motor proteins and association with posterior cortical cytoskeletal elements (Chang et al., 2011). Meanwhile, efficient transmission of most Wolbachia strains from one generation to the next requires that the bacteria concentrate at the posterior pole of the mature oocyte, as this is the future site of the germline (Kose & Karr, 1995). Thus, Wolbachia must migrate from the nurse cells to the posterior pole, navigating the constantly changing and tumultuous environment of the developing oocyte due to cytoplasmic streaming (Monteith et al., 2016). However, some strains, such as Wolbachia Riverside (wRi) of D. simulans incorporate into the pole cells independently of posterior concentration by maintaining a high titer throughout the entire oocyte (Serbus & Sullivan, 2007; Veneti, Clark, Karr, Savakis, & Bourtzis, 2004), whereas others (wNo, wMa, wKi) maintain a predominantly anterior localization (Veneti et al. 2004). These differences may ultimately contribute to differential somatic localization in adult flies.
Functional studies in Drosophila demonstrate that Wolbachia movement through the nurse cells to the anterior pole of the oocyte relies on the minus‐end directed motor protein dynein (Ferree et al., 2005). At this point in oogenesis the oocyte microtubules switch orientations such that transport to the posterior pole requires plus‐end directed microtubule movement. It has been difficult to attribute a functional significance of this dramatic switch in microtubule orientation, as well as cytoplasmic streaming. It may be that these are defense mechanisms preventing germline transmission of microbial invaders. Accordingly, Wolbachia rely on the plus‐end directed motor protein for transport and concentration at the posterior pole (Serbus & Sullivan, 2007). Finally, stable association with the posterior cortex requires key germ plasm and AP axis components such as Staufen and Oskar (Serbus & Sullivan, 2007). Thus, germline transmission of Wolbachia requires a sophisticated developmentally controlled association with dynein, kinesin, and finally conserved posterior determinants. Phylogenetic analyses of Wolbachia that vary in their niche tropism demonstrate that Wolbachia‐encoded factors are required for the posterior concentration (Toomey, Panaram, Fast, Beatty, & Frydman, 2013). One possibility is that Wolbachia expresses a developmentally programmed set of surface proteins that facilitates sequential engagement with host dynein, kinesin, and finally pole plasm determinants.
In all insect species examined, there is also a significant fraction of Wolbachia that is not associated with the posterior cortex but remains dispersed throughout the oocyte, as shown in Figure 2 (Veneti et al., 2004). During the syncytial divisions following fertilization, these bacteria concentrate at the centrosomes and undergo cell‐cycle regulated movements along the spindle and astral microtubules associated with the dividing syncytial nuclei (Albertson, Casper‐Lindley, Jian, Tram, & Sullivan, 2009; Kose & Karr, 1995). As in the oocyte, it is likely this movement relies on the microtubule‐based motor proteins dynein and kinesin (Ferree et al., 2005). The functional significance of these movements is unclear. One possibility is that it serves to distribute Wolbachia throughout the embryo such that they will fate map to numerous developmental lineages. Thus, as with the oocyte, the final distribution of the Wolbachia throughout the cellularized embryo prior to gastrulation is determined by a combination of host and Wolbachia factors.
Figure 2.
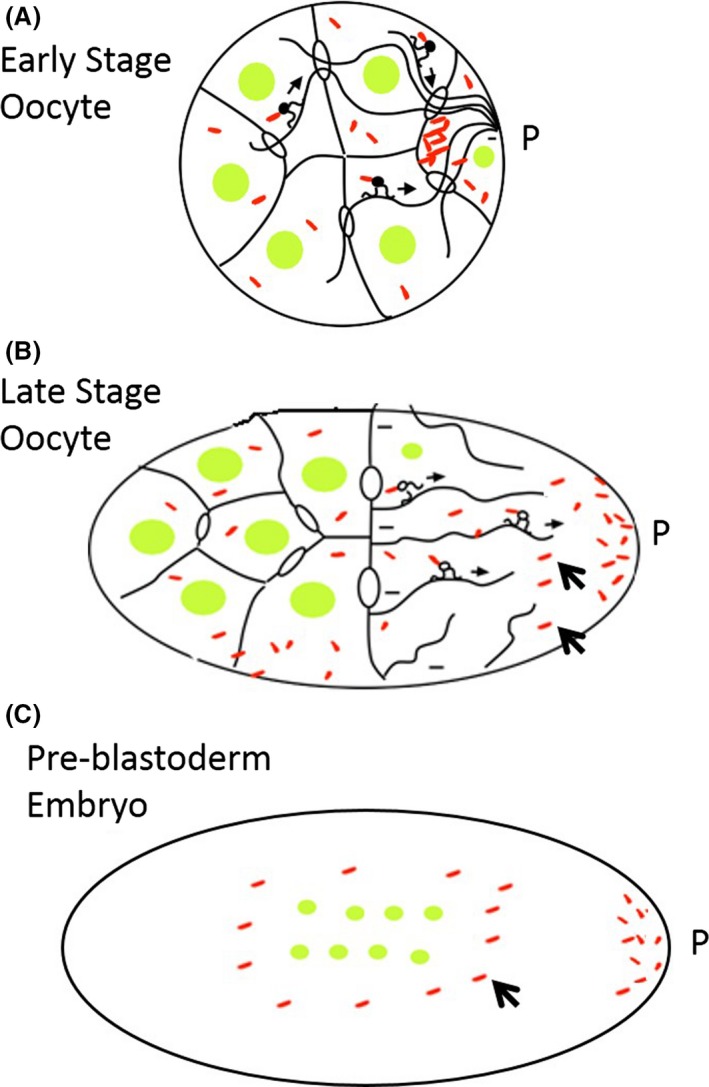
Wolbachia localization in somatic and germline cells during host development. The posterior localization of Wolbachia in the (A,B) developing oocyte and (C) embryo embryo relies on interactions with host microtubules, motor proteins, and posterior determinants. Wolbachia that localize to the posterior pole (P) are incorporated into the germline. However, a fraction of Wolbachia remains dispersed throughout the developing oocyte and embryo (arrowheads) and fate map to somatic tissues. Host nuclei=green, Wolbachia=red
Examination of Drosophila larva reveals that, as embryonic development progresses, Wolbachia also concentrates in the embryonic and larval epithelial‐derived neuroblast stem cells (Albertson et al., 2009). In contrast to the symmetric segregation of Wolbachia in the syncytial divisions, Wolbachia in the neuronal lineage exhibits a highly asymmetric segregation pattern (Albertson et al., 2009). The dividing neuroblast produces a self‐renewing neuroblast daughter cell and a daughter cell that will differentiate into larval neurons. Wolbachia almost exclusively segregates with the neuroblasts with only a few bacteria localizing to the cells that will differentiate into larval neurons. This asymmetric localization and segregation is largely dependent on the robust astral microtubules associated with the self‐renewing neuroblast cell. Larval neuroblast cells undergo a period of quiescence and ultimately divide and differentiate into the cells that will become the adult central nervous system (Homem & Knoblich, 2012). Thus, the asymmetric neuroblast localization during the larval divisions ensures their eventual localization to the adult brain (Albertson et al., 2013).
Unfortunately, we know little about Wolbachia localization during the pupal stages. However, numerous studies that have examined its cellular and tissue distribution in the adult stages. These are described in section 2 (2) as well as Table 1 and Figure 1.
3.2. Filarial nematodes
As with arthropods, Wolbachia is inherited primarily through the female germline in filarial nematodes (Kozek, 1977). In insects, axis determination and the site of germline formation is established during oogenesis. In filarial nematodes an asymmetric MTOC is also present before fertilization, in contrast to the model nematode Caenorhabditis elegans. Posterior localization of Wolbachia in both Drosophila and filarial nematodes relies on microtubules and motor proteins. During the establishment of polarity in the filarial nematode Brugia malayi, Wolbachia is associated with high levels of dynein, and dynein is required for their posterior localization (Landmann et al., 2014). Therefore, in both insects and filarial nematodes, microtubules and motor proteins are required for Wolbachia posterior enrichment. In addition, maintenance of Wolbachia at the posterior pole in B. malayi relies on posterior determinants, as in insects. The equivalence of the embryonic lineages between the model nematode C. elegans and B. malayi facilitates lineage tracing of Wolbachia in the latter. Such analysis revealed that during the initial embryonic divisions Wolbachia segregates with a precursor lineage to the germline and lateral chords (Caragata, Real, Zalucki, & McGraw, 2011; Fischer et al., 2011; Landmann, Foster, Slatko, & Sullivan, 2010). However, when this lineage diverges at the 12‐cell stage, Wolbachia segregates exclusively with the lateral chord lineages, leaving the germline lineage devoid of Wolbachia. This pattern of segregation is conserved in four filarial nematode species (B. malayi, L. sigmondontis, D. immitis, O. japonica), suggesting that it relies on conserved signaling factors associated with these species and perhaps others (Landmann et al., 2012). During the subsequent L3 and L4 larval stages, the hypodermal chords become syncytial through a process of cell fusion. Following this, Wolbachia proliferate extensively and spread anteriorly to fill the chord. In order to infect the germline, Wolbachia then migrate from the chord into the germline, crossing multiple plasma membranes. Images demonstrate that Wolbachia achieves this in female worms through the depolymerization of actin‐based microfilaments at the point of somatic‐germline cell contact (Landmann et al., 2012). Surprisingly, Wolbachia does not invade the germline in male nematodes, indicating Wolbachia is responding to signaling molecules specific to the female germline. Thus, in the late larva and adult males, Wolbachia is exclusively localized in the hypodermal lineage, whereas in females, Wolbachia resides in the hypodermal and germline lineages(Fischer et al., 2011; Landmann et al., 2012).
4. Molecular Mechanisms of Migration and Invasion of Somatic Cells
The studies described above indicate that in insects and nematodes the adult somatic distribution of Wolbachia is largely determined by a combination of symmetric and asymmetric segregation patterns during the mitotic divisions and cell‐to‐cell migration (Albertson et al., 2009; Landmann et al., 2010, 2012). With respect to the segregation patterns in both systems, it is clear that microtubules play a key role. Live imaging of the syncytial cortical divisions in Drosophila reveal Wolbachia maintains a tight association with the centrosome during interphase, but once the cell enters mitosis, Wolbachia undergoes extensive movement along pole to pole and astral microtubules (Albertson et al., 2009; Kose & Karr, 1995). Based on studies in the oocyte, this is likely to be driven by the microtubule‐based motor proteins dynein and kinesin (Ferree et al., 2005). This movement facilitates the even distribution of Wolbachia to daughter nuclei and serves to distribute them throughout the embryo. This is similar to what occurs in B. malayi, where Wolbachia moves along the astral and spindle microtubules during mitosis, facilitating their migration (Landmann et al., 2010). Wolbachia also relies on cortical microtubules and dynein to localize to the posterior cortex in B. malayi (Landmann et al., 2014).
The structural mechanisms by which Wolbachia engages host motor proteins and how this is regulated remain unknown. Sequence analysis reveals the Wolbachia genome contains several outer membrane proteins (WSPs, Wolbachia surface proteins) and these are likely to play a role in interacting with host cytoskeleton (Wu et al., 2004). However, electron microscopy has revealed that Wolbachia is encompassed by a host membrane (Callaini, Riparbelli, & Dallai, 1994; Fischer, Beatty, Weil, & Fischer, 2014), perhaps derived from the endoplasmic reticulum or golgi, making it difficult for WSPs to interact directly with the motor proteins. Nonetheless, some biochemical evidence indicates that WSPs directly bind host actin and Wolbachia interactions with host actin appear necessary for efficient migration of Wolbachia in the developing oocyte, as maternal transmission efficiency is greatly reduced in flies encoding cytoskeletal mutations (Melnikow et al., 2013; Newton, Savytskyy, & Sheehan, 2015). Wolbachia also encodes sec (Wu et al., 2004) and type IV secretion genes (Rances, Voronin, Tran‐Van, & Mavingui, 2008), suggesting the possibility that secreted effector proteins are used to interact with the host cytoskeleton. Notably, Salmonella, another intracellular bacterium that is also encompassed by a host membrane, utilizes an array of effector proteins to manipulate the cytoskeleton and achieve proper intracellular localization of the vacuole within which it resides. (LaRock, Chaudhary, & Miller, 2015).
Ultimately, interactions with host organelles, including the cytoskeleton, are used by intracellular bacteria in order to support replication and cell exit and entry. For instance, Chlamydia manipulates cytoskeletal Rab proteins in the host to recruit Golgi ministacks to the bacterial inclusion membrane in order to obtain lipids for cellular growth in human cells (Al‐Zeer et al., 2014; Heuer et al., 2009). Furthermore, Neisseria utilizes the host endocytic pathway for invasion through clathrin coated pits (Harvey, Jennings, Campbell, Williams, & Apicella, 2001). Since the ability of intracellular bacteria to manipulate host cells is in many cases conserved, the possibility that Wolbachia engage in similar processes to invade and persist in somatic cells should be further studied. For example, Wolbachia has been observed extracellularly in both the hemolymph of insects and pseudocoelomic cavity of filarial nematodes (Fischer et al., 2011, 2014). This localization indicates that Wolbachia is exocytosed and cell‐to‐cell transmission may occur through endocytosis. Accordingly, Wolbachia resides inside Golgi‐related vesicles near the host cell membrane in the Drosophila embryo (Cho, Kim, & Lee, 2011). In further support of an endocytosis hypothesis, free Wolbachia is able to invade uninfected germline tissues of Anopheles mosquitoes when the two are cocultured ex vivo (Hughes, Pike, Xue, & Rasgon, 2012). In these experiments, Wolbachia more efficiently invades tissues of their native hosts as opposed to those of more divergent ones. This suggests that Wolbachia enter cells through a receptor‐mediated mechanism that can be affected by polymorphisms in specific proteins that arise during speciation. These potential mechanisms are of particular importance to the finding that Wolbachia localizes to the somatic niche cells of the female germline in many Drosophila species (Fast et al., 2011; Toomey et al., 2013). Studies in which Wolbachia bacteria are injected into the adult abdomen demonstrate that Wolbachia can hone to these regions through migration (Frydman et al., 2006). How they achieve this remains unclear, as they must traverse a number of membrane and extracellular matrix barriers. However, receptor‐mediated endocytosis into specific cell types after movement through the hemolymph is a plausible route. Despite these intriguing lines of evidence, the role of the endocytic pathway in Wolbachia infection remains largely unexplored.
5. Horizontal Transmission of Infection
The discordance between Wolbachia and host phylogenies suggests that on evolutionary time scales horizontal transmission of Wolbachia between species has occurred numerous times. This conclusion is supported through studies demonstrating a strong linkage disequilibrium between mitochondrial and Wolbachia genomes in a number of species (Gómez‐Valero et al., 2004; Heath, Butcher, Whitfield, & Hubbard, 1999; Morrow, Frommer, Shearman, & Riegler, 2014; Schuler et al., 2013; Vavre, Fleury, Lepetit, Fouillet, & Boulétreau, 1999; Werren, Zhang, & Guo, 1995; Zhang, Han, & Hong, 2013). Such phylogenetic analyses provide clues to the most plausible routes of horizontal transmission. Horizontal transmission appears to take place within and between species through both direct and indirect interactions. For example, intraspecies horizontal transmission in organisms such as fruit flies and spiders likely happens through direct contact or the environment, given the ecological roles of these organisms do not allow for a vectored mechanism (Baldo et al., 2008; Haine, Pickup, & Cook, 2005). Likewise, interspecies horizontal transfer in intertidal amphipod crustaceans (Cordaux et al., 2001) and butterflies sharing the same habitat probably occurs through the environment (Dyson, Kamath, & Hurst, 2002). In plant‐feeding pumpkin arthropods, Wolbachia transfer appears to be linked to feeding on particular leaf substrates (Sintupachee, Milne, Poonchaisri, Baimai, & Kittayapong, 2006), suggesting that transfer can occur through ingestion. A similar link exists between predatory mirid bugs and their prey, leafhoppers (Kittayapong, Jamnongluk, Thipaksorn, Milne, & Sindhusake, 2003), whereas in mycophagous Diptera, the mushroom habitat appears to play a role in horizontal transmission (Stahlhut et al., 2010).
Whether horizontal transmission is a common occurrence on shorter time scales remains uncertain, though studies tracing Wolbachia movement among bee populations suggest it is an infrequent event (Gerth, Rothe, & Bleidorn, 2013). Analyses of cannibalistic terrestrial isopods have demonstrated new infections in various organs after ingestion of an infected individual by an uninfected one (Le Clec'h et al., 2013). Similarly, mixing experiments in the laboratory have shown that mites can transmit Wolbachia infection between Drosophila by feeding on infected corpses and subsequently being ingested by uninfected flies (Brown & Lloyd, 2015). In colonies of Cubitermes termites, the exchange of salivary secretions, also known as trophallaxys, appears to facilitate intraspecies transfer of Wolbachia between individuals of different castes (Roy, Girondot, & Harry, 2015). Thus, a similar route may be involved in other social insects. For example, in Acromyrmex ants, Wolbachia is present in the fat body, hemolymph, and feces, suggesting the potential for fecal‐oral transmission (Frost, Pollock, Smith, & Hughes, 2014). Interestingly, sequencing and FISH experiments have shown that parasitoid wasps are capable of horizontally acquiring new Wolbachia infections during larval development inside an infected host (Ahmed et al., 2015). Parasitoid wasps can also transmit their own vertically acquired Wolbachia to other coinfecting parasitoid species that may be occupying the same space during development inside a host (Huigens, de Almeida, Boons, Luck, & Stouthamer, 2004). In these examples, Wolbachia transmission is likely independent of the germline, relying solely on somatic tissues.
The mechanisms and routes of horizontal transmission are largely unexplored. However, some insight into these issues is provided by experimental transfer in the laboratory. Early experiments in Drosophila provided proof‐of‐principle that Wolbachia from one organism was capable of stably infecting another by localizing to the germline. That is, Wolbachia extracted from the cytoplasm of an infected Drosophila egg could be injected into an uninfected embryo and yield germline infection (Boyle, O'Neill, Robertson, & Karr, 1993). Experiments of a similar nature have since been conducted from adult to adult, and adult to immature stage insects of other species with varying degrees of success (Grenier et al., 1998; Kageyama, Narita, & Noda, 2008; Pigeault et al., 2014; Van Meer & Stouthamer, 1999). Though infection intensity appears to decline over time, in some cases stable germline infection can be achieved through injection (Grenier et al., 1998; Van Meer & Stouthamer, 1999). For example, Wolbachia injected into the abdomen of Drosophila can migrate to the germline (Frydman et al., 2006). Thus, one possible mechanism for natural horizontal transmission is through contact of an uninfected wounded individual with infected hemolymph from a wounded Wolbachia host, as has been demonstrated in woodlice (Rigaud & Juchault, 1995). Interestingly, experimental transfer of Wolbachia between related host species can in some cases be virulent and affect reproductive fitness (Le Clec'h et al., 2012; McGraw, Merritt, Droller, & O'Neill, 2002).
Given the diversity of interactions that appear to mediate horizontal transmission, it is likely that the phenomenon also occurs in other, yet undiscovered, Wolbachia hosts. It is particularly intriguing that a species barrier to horizontal transmission appears to exist, but that this can in some cases be overcome both in nature as described above, but also in the laboratory. For instance, the establishment of Drosophila‐derived Wolbachia infections in mosquito cell cultures has facilitated cross‐species transinfection in vivo (Dobson, Marsland, Veneti, Bourtzis, & O'Neill, 2002; McMeniman et al., 2008). It is not clear whether the species barrier is regulated by host or bacterial genes, as the molecular mechanisms governing horizontal transmission of infection have yet to be discovered and a variety of factors are possibly involved. Most prominently, the ability of Wolbachia to occupy and move through host somatic tissues such as the gut, and perhaps even the extracellular environment such as the hemolymph, are likely key components in horizontal transmission. This area remains relatively unexplored and future advances in understanding Wolbachia transit and invasion at the cellular level may yield greater understanding of the conditions required for horizontal transmission. In particular, studies that trace Wolbachia migration to somatic tissues after introduction through various routes are needed.
6. Extracellular Survival and Routes of Transmission
The ability of Wolbachia to transfer horizontally between organisms suggests that the bacterium is capable of surviving in an extracellular environment, though this idea is somewhat controversial. In the laboratory, Wolbachia has been isolated from both, infected cell cultures and tissues (Gamston & Rasgon, 2007; Rasgon, Gamston, & Ren, 2006). While Wolbachia obtained in this manner can be maintained in cell‐free medium and retain viability for at least a week, no replication is apparent. Nonetheless, these results indicate that Wolbachia is able to survive at least for a limited time outside of host cells. However, the fact that Wolbachia lack the ability to synthesize many essential lipids (Wu et al., 2004) and amino acids (Caragata, Rancès, O'Neill, & McGraw, 2014) is likely a major factor limiting the extent of extracellular survival.
Studies in vivo demonstrating the presence of Wolbachia in the hemolymph of both larvae and adults of Drosophila and mosquitoes provide further support for the idea that Wolbachia can survive extracellularly (Dobson et al., 1999; Frydman et al., 2006). Furthermore, when Wolbachia is injected into the abdomen of an uninfected Drosophila host, it is capable of surviving and migrating through the hemolymph to reach the germline (Frydman et al., 2006). From the hemolymph, Wolbachia may be able to also enter somatic tissues. For example, in the bedbug Cimex lectularius, Wolbachia is found in the mesospermalage, a hemocyte‐containing organ used to receive sperm during traumatic insemination (Hosokawa, Koga, Kikuchi, Meng, & Fukatsu, 2010). More importantly, contact with the infected hemolymph of wounded hosts can be a natural mechanism for horizontal transmission, as demonstrated by hemolymph transfer experiments (Rigaud & Juchault, 1995). Meanwhile, in the nematode B. malayi, extracellular Wolbachia are found in the pseuodocoelom, indicating that perhaps pseudoscoelomic fluid serves as a route for Wolbachia transfer between germline and somatic tissues (Fischer et al., 2014), similar to hemolymph in insects.
In addition to surviving in the hemolymph, Wolbachia has been observed extracellularly in various other important host tissues where it can exert both beneficial and harmful effects with respect to the host. For instance, while Wolbachia has been shown to concentrate in the central brain and optic lobe with little detriment (Albertson et al., 2013), studies show that some virulent Wolbachia strains can exit these cells, perhaps through cell lysis, and invade the extracellular space in the brain, causing pathogenesis (Min & Benzer, 1997; Strunov & Kiseleva, 2014).
A nutrient‐based symbiotic relationship may exist between extracellular Wolbachia and other hosts. For instance, in leaf‐cutter ants of the genus Acromyrmex, Wolbachia is observed extracellularly in the foregut, midgut lumen, and fecal fluid (Andersen et al., 2012; Frost et al., 2014; Sapountzis et al., 2015). Wolbachia is also found in the digestive tract of Drosophila (Clark, Anderson, Cande, & Karr, 2005; Ponton et al., 2015) and likely in triatomine bugs which excrete Wolbachia in their feces (Espino et al., 2009). These gut bacteria may provide essential metabolic pathways lacking from the insects, thereby controlling various aspects of host physiology and life history, and perhaps contributing to pathogen resistance.
Furthermore, in C. lectularius, Wolbachia resides within a highly specialized organ called the bacteriome (Hosokawa et al., 2010). The bacteriome is composed of bacteriocytes, a cell type similar to fat cells. These are maternally transmitted and serve primarily to protect endosymbiotic bacteria in exchange for nutrients. In this case, it appears Wolbachia may also be acting as a nutritional mutualist. Indeed, removal of endogenous Wolbachia from these bedbugs reduced host growth and reproductive fitness through a mechanism dependent on biotin synthesis (Nikoh et al., 2014).
Infection in extracellular compartments and the tissues discussed above may not only be important for horizontal transmission, but may also explain the various effects of Wolbachia on host physiology that appear to be independent of the germline. Across diverse taxa, the gut is a key tissue for regulating immunity, metabolism, and longevity. Likewise, the brain regulates these and other central processes while also controlling behavior. Thus, it is possible that the digestive tract is not only a route for Wolbachia transfer between hosts, but also, along with the brain, involved in the functional consequences of Wolbachia infection that are discussed below.
7. The Functions of Somatic Infection
In the mature oocyte, Wolbachia concentrates at the posterior pole facilitating its incorporation into the germline of the developing host embryo. In Drosophila and other insects, however, a large fraction of Wolbachia is also positioned anteriorly resulting in a distribution throughout the length of the embryos (Ferree et al., 2005; Serbus & Sullivan, 2007; Veneti et al., 2004). This Wolbachia fraction is not incorporated into the germline and fate maps to the somatic cells of the developing insect. In filarial nematodes, Wolbachia segregate to the posterior pole after fertilization and through asymmetric segregation all of the Wolbachia concentrate in the somatic hypodermal chords, leaving the germline uninfected. In females, a subset of these hypodermal Wolbachia invades the neighboring germline stem cells through cell‐to‐cell transfer. Strikingly in males invasion of the germline does not occur indicating this process relies on female germline‐specific signals (Landmann et al., 2010). Thus, unlike in insects where Wolbachia is distributed in most, if not all tissues of the adult, in filarial nematodes the only somatic tissue in which Wolbachia is consistently observed is the hypodermis.
In insects, the concentration of Wolbachia in the central nervous system, gut, and fat bodies, is particularly intriguing as these tissues direct many facets of insect behavior and physiology. The somatic distribution of Wolbachia may be viewed as a consequence of the fact that many Wolbachia fail to localize to the posterior pole and these bacteria are passively included into the newly formed somatic cells with little functional consequences. Alternatively, the dual somatic‐germline localization of Wolbachia may have evolved through positive selection in which somatically localized bacteria influence host cell biology and physiology such that vertical or horizontal transmission is enhanced. Below, we summarize evidence supporting the latter interpretation.
7.1. Effects on host behavior
There are many examples illustrating that vertically transmitted endosymbionts influence host behavior (Goodacre & Martin, 2012). Presumably these behavior modifications have evolved to enhance transmission of the endosymbiont. Over the past decade, a number of publications demonstrate that Wolbachia also has profound effects on insect behavior. This is likely a consequence of Wolbachia localization in the central nervous system and fat bodies, as they are hormone sources and influence physiology and behavior (Albertson et al., 2013; Arrese & Soulages, 2010; Nassel, 1993). A number of studies in Drosophila and spider mites have found that Wolbachia infection alters mating preference, duration, and frequency, as well as oviposition substrate preference (Goodacre & Martin, 2012; Koukou et al., 2006; Miller, Ehrman, & Schneider, 2010; Panteleev et al., 2007; Vala, Egas, Breeuwer, & Sabelis, 2004). However, a more recent study found no effect of Wolbachia infection on mating preference (Arbuthnott, Levin, & Promislow, 2016). Thus, the effect of Wolbachia on mating may be highly strain and host dependent.
In addition to mating behavior, feeding patterns appear to change during infection, as blood feeding success is reduced in Wolbachia‐infected mosquitoes (Turley, Moreira, O'Neill, & McGraw, 2009). While in this particular case, reduced feeding is not associated with reduced olfaction, other studies have found that Wolbachia can reduce host responsiveness to olfactory food cues (Peng, Nielsen, Cunningham, & McGraw, 2008). Changes in locomotor activity, also induced by Wolbachia infection in Drosophila, may contribute to apparent behavioral alterations (Caragata et al., 2011; Evans et al., 2009). While the mechanisms that underlie the phenomenon of behavioral change are undetermined, Wolbachia likely gain from altering essential host behaviors. Most prominently, changes in reproductive behavior may drive the spread of infection through populations by favoring the production of infected females. Similarly, changes in feeding behavior could confer a fitness advantage for infected individuals. For instance, in mosquitoes blood feeding is a costly behavior that can reduce fitness (Murdock, Moller‐Jacobs, & Thomas, 2013).
Many conclusions on the effects of Wolbachia on insect behavior must be treated with caution because the unaffected control insects are often obtained through antibiotic‐based curing of Wolbachia. Antibiotic treatment is certain to have profound effects on the composition of the gut and other host microbe populations (Broderick & Lemaitre, 2012). In addition, antibiotic treatment of Drosophila not infected with Wolbachia has dramatic long‐term effects on behavior and physiology, including mitochondrial function and lifespan (Albertson et al., 2013; Ballard & Melvin, 2007). Significantly, these effects persist many generations after the exposure to antibiotics (Albertson et al., 2013). Therefore, it is difficult to attribute the changes in behavior specifically to the loss of Wolbachia, despite the fact that most researchers attempt to control for this by curing several generations in advance of experimental manipulation. Given these issues, multiple generations of backcrossing is the preferred method of creating uninfected controls from infected insect lines when possible.
7.2. Effects on host metabolism
Wolbachia localization to the fat body, a key endocrine tissue in insects (Arrese & Soulages, 2010), has been observed on numerous occasions. The Wolbachia genome encodes an array of proteins that may be involved in regulating metabolism (Darby et al., 2012). This includes several facilitators of cation membrane transport that provide essential cofactors for enzymes in the respiratory chain. Furthermore, in filarial nematodes, Wolbachia can directly influence the expression of host enzymes involved in glucose and glycogen metabolism (Voronin et al., 2016). Therefore, it is unsurprising that Wolbachia increases the basal metabolic rate of infected mosquitoes as measured by the production of carbon dioxide (Evans et al., 2009). In Drosophila, Wolbachia also influence host iron‐utilization, whereas in C. lectularius Wolbachia appear to play a role in the synthesis of B vitamins (Brownlie et al., 2009; Hosokawa et al., 2010). These experiments suggest that Wolbachia not only affects macronutrient metabolism, but also the provisioning of mineral micronutrients and cofactors. In addition, some behavioral effects of Wolbachia in Drosophila may be explained by alterations in hormone biosynthesis pathways. For example, wMelPop may increase aggressive male behavior through control of octopamine synthesis (Rohrscheib et al., 2015). While these interesting effects on metabolism have not yet been explained, an increase in insulin signaling is one possible source of Wolbachia's effects on host metabolism (Ikeya, Broughton, Alic, Grandison, & Partridge, 2009). Another possibility is that Wolbachia may affect mitochondrial mass or activity directly (Ballard & Melvin, 2007). Intriguingly, Wolbachia‐mediated metabolic alterations are suggestive of gainful manipulation of host physiology. Host diet in Drosophila, perhaps acting through the insulin signaling pathway, has been shown to regulate Wolbachia titer (Serbus et al., 2015). Therefore, it would not be surprising to discover that Wolbachia, like many other invasive bacteria, has the ability to modulate the metabolism of its host to increase its own transmission.
7.3. Cell autonomous and non‐autonomous effects on pathogen resistance
Wolbachia in infected flies and mosquitoes has the ability to confer resistance against a wide array of viral, bacterial, parasitic, and fungal pathogens (Eleftherianos et al., 2013). This property allows pathogen‐infected hosts to survive and continue to reproduce in a situation where uninfected hosts would not survive, thus providing a great evolutionary advantage for Wolbachia and its host. In mosquitoes, Wolbachia provides resistance against the malaria parasite Plasmodium (Kambris et al., 2010) and the filarial nematode B. pahangi (Kambris, Cook, Phuc, & Sinkins, 2009) as well as protection from the bacterium Erwinia caratova (Kambris et al., 2009) and the dengue and chikungunya viruses (Moreira et al., 2009). In Drosophila, Wolbachia infection imparts resistance against various positive‐sense single‐stranded RNA viruses such as: Drosophila C virus, noravirus, and cricket paralysis virus (Hedges, Brownlie, O'Neill, & Johnson, 2008; Rainey et al., 2016; Teixeira, Ferreira, & Ashburner, 2008) and against the entomopathogenic fungus Beauveria bassiana (Panteleev et al., 2007). However, Wolbachia protection does not include all infections. For instance, the titer of the intracellular bacteria Salmonella typhimurium and Listeria monocytogenes is not affected by Wolbachia in Drosophila, though it should be noted that these pathogens do not naturally infect flies (Rottschaefer & Lazzaro, 2012). Therefore, it is possible that Wolbachia may confer protection against intracellular bacteria that can naturally colozine arthropods.
Pathogen resistance imparted on the host by Wolbachia has been observed on numerous occasions and has been reviewed elsewhere (Eleftherianos et al., 2013). However, information regarding the conditions necessary for this phenotype, as well as mechanistic insight is still lacking (Rainey, Shah, Kohl, & Dietrich, 2014). One proposed mechanism is the priming of the immune response by Wolbachia that subsequently hastens pathogen removal upon infection. However, there is conflicting evidence for this claim and establishing a concrete link between Wolbachia and host immunity will greatly further understanding of the pathogen resistance phenotype (Bourtzis, Pettigrew, & O'Neill, 2000; Moreira et al., 2009; Rances et al., 2013; Wong, Hedges, Brownlie, & Johnson, 2011; Ye, Woolfit, Rances, O' Neill, & McGraw, 2013). Alternatively, some have suggested that the synthesis of reactive oxygen/nitrogen species and cholesterol is involved (Caragata et al., 2013; Pan et al., 2011; Wong, Brownlie, & Johnson, 2015). There is also some evidence that increased host cell autophagy driven by Wolbachia infection plays a role in viral resistance (Le Clec'h et al., 2012). Each of these mechanisms would require Wolbachia‐mediated effects on somatic tissues and cells that regulate the host response to infection, such as the gut, fat body, and hemocytes. The particular cells and tissues involved in each case are not fully known. In Drosophila, Wolbachia titer in the head, gut, and malpighian tubules is correlated with antiviral protection (Osborne et al., 2009). Furthermore, the emergence of fluorescence‐based assays for the detection of both Wolbachia and viruses have recently allowed for experiments that map their distribution and localization in whole insects (Kliot & Ghanim, 2015). In several tissues, such as the midgut and salivary glands, Wolbachia and dengue virus co‐localize. In such cases, it would appear that the effects of Wolbachia on dengue virus are cell autonomous, or restricted to the Wolbachia‐infected cells. However, viruses may also be impacted in a non‐autonomous manner due to Wolbachia in tissues where viruses are not present, such as Malpighian tubules and fat bodies that may control reactive oxygen species and cholesterol synthesis as mentioned above. Further studies of a similar nature should eventually facilitate greater understanding of the interactions between Wolbachia and pathogens in somatic cells.
7.4. Effects on stress resistance and longevity
As most mutualists and parasites, Wolbachia undoubtedly benefits from the health and longevity of its host. Therefore, it is not surprising that Wolbachia influences host responses to cellular stress and damage as well as lifespan. In insects, Wolbachia induces the production of host reactive oxygen species (ROS) (Pan et al. 2011; Wong et al., 2015). Perhaps because Wolbachia must persist in this oxidative intracellular environment without causing damage to the host, infection also upregulates host antioxidant genes (Brennan, Haukedal, Earle, Keddie, & Harris, 2012; Brennan, Keddie, Braig, & Harris, 2008). Wolbachia also reduces oxidative stress by regulating host iron homeostasis. Iron is a highly toxic precursor to ROS and the expression of Wolbachia bacterioferretin reduces labile iron concentrations, which in turn prevents toxicity (Kremer et al., 2009). Intriguingly, while Wolbachia protects against iron toxicity, resistance to lead is decreased during infection (Wang et al., 2012), suggesting that protection from heavy metals is restricted.
Reduced iron toxicity is associated with the inhibition of apoptosis in the wasp Asobara tabida (Kremer et al., 2009). In this organism, Wolbachia is required for proper oogenesis, and oocytes fail to mature when it is removed due to extensive apoptosis (Miller et al., 2010; Pannebakker et al., 2007). As mitochondria‐derived ROS are also involved in modulating apoptosis, the ability of Wolbachia to regulate responses to these stressors may have far reaching consequences for host lifespan and reproduction.
Whether Wolbachia modulates apoptosis from host germline or somatic tissues is unclear. Apoptosis in the wasp oocyte is likely due to Wolbachia in the same tissues. On the other hand, the loss of Wolbachia in filarial nematodes through antibiotic therapy also induces apoptosis in both the adult germline and somatic cells of the embryo (Landmann, Voronin, Sullivan, & Taylor, 2011). Since Wolbachia does not reside in the male germline of nematodes, this effect must be mediated by somatic Wolbachia. Significantly, apoptosis is upregulated in cells not infected with Wolbachia demonstrating that this effect is not cell autonomous. A greater understanding of the means by which Wolbachia regulate apoptosis is still necessary. Though some studies have suggested that WSPs are directly able to inhibit apoptosis (Bazzocchi et al., 2007), this mechanism does not account for apoptosis in tissues not infected with Wolbachia.
Ultimately the impact of Wolbachia on oxidative stress and apoptosis may affect host lifespan and longevity. For example, the removal of Wolbachia can decrease Drosophila lifespan (Alexandrov et al., 2007). Interestingly, a virulent strain of Wolbachia (wMelPop) in insect hosts can be pathogenic and induce apoptosis in a variety of tissues ultimately leading to death and reduced lifespan (Kambris et al., 2009; McMeniman et al., 2009; Min & Benzer, 1997; Strunov & Kiseleva, 2014; Zhukova & Kiseleva, 2012). Though such effects appear counterintuitive given Wolbachia gains from increased host fitness, perhaps pathogenicity and decreased lifespan contribute to the life history of Wolbachia in other ways.
7.5. Somatic routes of germline infection
The discordance between Wolbachia and host insect phylogenies strongly argues for multiple horizontal transmission events over evolutionary timescales. Insight into possible mechanisms and routes of transmission have come from experiments in which Wolbachia injected into the abdomen is able to reach the germline through the somatic stem cells (Frydman et al., 2006), suggesting that this localization during natural infection serves to facilitate reaching of the germline for vertical transmission. Indeed, from the somatic stem cell niche, Wolbachia is supplied to the somatic stem cell, which can then divide and transmit Wolbachia to follicle cells (Toomey et al., 2013). From infected follicle cells, Wolbachia may then transfer to the developing oocyte (Toomey et al., 2013).
Studies of oocytes isolated from wild caught Drosophila suggest that somatic to germline transmission of Wolbachia may be a common occurrence (Casper‐Lindley et al., 2011). Egg chambers isolated from infected females were discovered in which Wolbachia was absent from the early, but not the late stage chambers. These uninfected chambers are likely a consequence of an occasional failure of the Wolbachia in the germline stem cell to segregate during mitosis into the daughter cell that will become a nascent egg chamber. However, later in oogenesis, these chambers become infected. The most likely route of infection is from the Wolbachia‐infected follicle cells that encompass each egg chamber. Infection via these somatically derived follicle cells may have evolved as a backup mechanism to ensure the observed high rates of Wolbachia vertical transmission.
Perhaps the strongest support for a somatic to germline route of Wolbachia infection comes from the Wolbachia lineage studies in B. malayi described above in section 3. This analysis revealed that Wolbachia exclusively segregates to the lineage that forms the lateral chords. Here, it proliferates and completely fills the chords. At this point in larval development, no Wolbachia is present in the germline. Germline infection requires cell‐to‐cell transfer, or exiting the hypodermal chord cells and entering the adjacent germline cells. This mechanism of transfer remains unexplored, although cellular analysis suggests this involves Wolbachia‐mediated microfilament deploymerization at the point of entry.
8. Conclusions
While Wolbachia are most prevalent in the host germline and primarily studied for their effects on these tissues, the studies described in this review demonstrate that Wolbachia is consistently found both intra and extracellularly in important somatic tissues such as the nervous system, fat body, and gut of their arthropod hosts, and in hypodermal chords in the nematode hosts. Wolbachia distribution to these somatic tissues is primarily regulated by segregation patterns during embryonic development. However, active invasion of somatic tissues during development and adulthood is also involved. This mechanism not only regulates somatic distribution, but may be involved in the horizontal spread of infection, which appears to play an important ecological role in the transmission and diversification of Wolbachia. The presence of Wolbachia in somatic tissues may also explain many phenotypic alterations observed in infected hosts, such as: behavioral change, resistance to pathogenic infection, shifts in metabolism, and changes in longevity.
The effects that somatic Wolbachia has on the host germline suggest that invasion of the soma and somatic localization may have evolved as an altruistic mechanism to facilitate vertical transmission. That is, by not entering the germline, somatic Wolbachia are essentially sacrificed, as they will not be inherited by the next generation. However, in doing so, they can produce many of the phenotypes described above that increase the transmission of their sister Wolbachia, thus benefiting the species as a whole. Whether Wolbachia originated as a germline endosymbiont that invaded the soma resulting in these advantageous phenotypes or as a somatic endosymbiont that invaded the germline for vertical transmission remains unresolved. There are cases of Wolbachia existing exclusively in the germline (tsetse fly), but also exclusively in somatic tissues (male nematodes). In addition, invasion of both somatic and germline tissues has been documented, further obscuring the origins of Wolbachia.
Regardless of their origin, understanding the mechanisms by which somatic Wolbachia exert their effects has broad implications in the biomedical and agricultural fields, as the use of Wolbachia to manipulate the physiology of insect crop pests and vectors of human pathogens shows great potential to reduce disease and economic burden. However, studies examining Wolbachia invasion and interactions with host somatic cells at a mechanistic level are lacking. Some experiments suggest that Wolbachia manipulation of the host cytoskeleton and motor proteins plays an important role in cell invasion, but other aspects of host cell biology, such as the endocytic pathway may be involved as well. Thus, cell‐based studies of Wolbachia invasion that trace migration to specific somatic cells after introduction through various routes are sorely needed. Similarly, transmission studies focusing on transfer of Wolbachia between hosts under a variety of conditions will be helpful to fully determining the prevalent routes of horizontal transmission in nature. More importantly, studies directly mapping host phenotypes to Wolbachia in somatic tissues would greatly aid efforts to use this extraordinary endosymbiont for the public good.
Funding Information
This study was funded by the National Institute of General Medical Sciences, (Grant / Award Number: ‘GM104486‘).
Conflicts of Interest
The authors declare no conflicts of interest.
Pietri, J. E. , DeBruhl, H. and Sullivan, W. (2016), The rich somatic life of Wolbachia . MicrobiologyOpen, 5:923–936. doi: 10.1002/mbo3.390
References
- Ahmed, M. Z. , Li, S.‐J. , Xue, X. , Yin, X.‐J. , Ren, S.‐X. , Jiggins, F. M. , … Qiu, B.‐L. (2015). The intracellular bacterium wolbachia uses parasitoid wasps as phoretic vectors for efficient horizontal transmission. PLoS Pathogens, 10, e1004672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson, R. , Casper‐Lindley, C. , Jian, C. , Tram, U. , & Sullivan, W. (2009). Symmetric and asymmetric mitotic segregation patterns influence wolbachia distribution in host somatic tissue. Journal of Cell Science, 122, 4570–4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson, R. , Tan, V. , Leads, R. R. , Reyes, M. , Sullivan, W. , & Casper‐Lindley, C. (2013). Mapping wolbachia distributions in the adult Drosophila brain. Cellular Microbiology, 15, 1527–1544. [DOI] [PubMed] [Google Scholar]
- Aleksandrov, I. D. , Aleksandrova, M. V. , Goriacheva, I. I. , Roshchina, N. V. , Shaĭkevich, E. V. , & Zakharov, I. A. (2007). Removing endosymbiotic Wolbachia specifically decreases lifespan of females and competitiveness in a laboratory strain of Drosophila melanogaster . Russian Journal of Genetics, 43, 1147–1152. [PubMed] [Google Scholar]
- Al‐Zeer, M. A. , Al‐Younes, H. M. , Kerr, M. , Abu‐Lubad, M. , Gonzalez, E. , Brinkmann, V. , & Meyer, T. F. (2014). Chlamydia trachomatis remodels stable microtubules to coordinate golgi stack recruitment to the chlamydial inclusion surface. Molecular Microbiology, 94, 1285–1297. [DOI] [PubMed] [Google Scholar]
- Andersen, S. B. , Boye, N. , Nash, D. R. , & Boomsma, J. J. (2012). Dynamic Wolbachia prevalence in acromyrmex leaf‐cutting ants: Potential for a nutritional symbiosis. Journal of Evolutionary Biology, 25, 1340–1350. [DOI] [PubMed] [Google Scholar]
- Arbuthnott, D. , Levin, T. C. , & Promislow, D. E. (2016). The Impacts of Wolbachia and the microbiome on mate choice in Drosophila melanogaster . Journal of Evolutionary Biology, 29, 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese, E. L. , & Soulages, J. L. (2010). Insect fat body: Energy, metabolism, and regulation. Annual Review of Entomology, 55, 207–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo, L. , Ayoub, N. A. , Hayashi, C. Y. , Russell, J. A. , Stahlhut, J. K. , & Werren, J. H. (2008). Insight into the routes of Wolbachia invasion: High levels of horizontal transfer in the spider genus Agelenopsis revealed by wolbachia strain and mitochondrial DNA diversity. Molecular Ecology, 17, 557–569. [DOI] [PubMed] [Google Scholar]
- Ballard, J. W. O. , & Melvin, R. G. (2007). Tetracycline treatment influences mitochondrial metabolism and MtDNA density two generations after treatment in Drosophila . Insect Molecular Biology, 16, 799–802. [DOI] [PubMed] [Google Scholar]
- Bazzocchi, C. , Comazzi, S. , Santoni, R. , Bandi, C. , Genchi, C. , & Mortarino, M. (2007). Wolbachia surface protein (WSP) inhibits apoptosis in human neutrophils. Parasite Immunology, 29, 73–79. [DOI] [PubMed] [Google Scholar]
- Bourtzis, K. , Pettigrew, M. M. , & O'Neill, S. L. (2000). Wolbachia neither induces nor suppresses transcripts encoding antimicrobial peptides. Insect Molecular Biology, 9, 635–639. [DOI] [PubMed] [Google Scholar]
- Boyle, L. , O'Neill, S. L. , Robertson, H. M. , & Karr, T. L. (1993). Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila . Science (New York, N.Y.), 260, 1796–1799. [DOI] [PubMed] [Google Scholar]
- Brennan, L. J. , Haukedal, J. A. , Earle, J. C. , Keddie, B. , & Harris, H. L. (2012). Disruption of redox homeostasis leads to oxidative DNA damage in spermatocytes of Wolbachia‐infected Drosophila simulans . Insect Molecular Biology, 21, 510–520. [DOI] [PubMed] [Google Scholar]
- Brennan, L. J. , Keddie, B. A. , Braig, H. R. , & Harris, H. L. (2008). The endosymbiont Wolbachia pipientis induces the expression of host antioxidant proteins in an Aedes albopictus cell line. PLoS One, 10, 1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick, N. , & Lemaitre, B . 2012. “Gut‐associated microbes of Drosophila melanogaster .”Gut Microbes, 3,307–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, A. N. , & Lloyd, V. K. (2015). Evidence for horizontal transfer of Wolbachia by a Drosophila mite. Experimental & Applied Acarology, 66, 301–311. [DOI] [PubMed] [Google Scholar]
- Brownlie, J. C. , Cass, B. N. , Riegler, M. , Witsenburg, J. J. , Iturbe‐Ormaetxe, I. , McGraw, E. A. , & O'Neill, S. L. (2009). Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathogens, 5, e1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaini, G. , Riparbelli, M. G. , & Dallai, R. (1994). The distribution of cytoplasmic bacteria in the early Drosophila embryo is Mediated by astral microtubules. Journal of Cell Science, 107, 673–682. [DOI] [PubMed] [Google Scholar]
- Caragata, E. P. , Rancès, E. , O'Neill, S. L. , & McGraw, E. A. (2014). Competition for amino acids between Wolbachia and the mosquito host, Aedes aegypti . Microbial Ecology, 67, 205–218. [DOI] [PubMed] [Google Scholar]
- Caragata, E. P. , Real, K. M. , Zalucki, M. P. , & McGraw, E. A. (2011). Wolbachia infection increases recapture rate of field‐released Drosophila melanogaster . Symbiosis, 54, 55–60. [Google Scholar]
- Caragata, E. P. , Rances, E. , Hedges, L. M. , Gofton, A. W. , Johnson, K. N. , O'Neill, S. L. , & McGraw, E. A. (2013). Dietary cholesterol modulates pathogen blocking by Wolbachia . PLoS Pathogens, 9, e1003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper‐Lindley, C. , Kimura, S. , Saxton, D. S. , Essaw, Y. , Simpson, I. , Tan, V. , & Sullivan, W. (2011). Rapid fluorescence‐based screening for Wolbachia endosymbionts in Drosophila germ line and somatic tissues. Applied and Environmental Microbiology, 77, 4788–4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, C. W. , Nashchekin, D. , Wheatley, L. , Irion, U. , Dahlgaard, K. , Montague, T. G. , … St Johnston, D. (2011). Anterior‐posterior axis specification in Drosophila oocytes: Identification of novel bicoid and oskar mRNA localization factors. Genetics, 188, 883–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Q. , Ruel, T. D. , Zhou, W. , Moloo, S. K. , Majiwa, P. , O'Neill, S. L. , & Aksoy, S. (2000). Tissue Distribution and prevalence of Wolbachia infections in tsetse flies, glossina Spp. Medical and Veterinary Entomology, 14, 44–50. [DOI] [PubMed] [Google Scholar]
- Chevalier, F. , Herbinière‐Gaboreau, J. , Bertaux, J. , Raimond, M. , Morel, F. , Bouchon, D. , … Braquart‐Varnier, C. (2011). The immune cellular effectors of terrestrial isopod Armadillidium vulgare: Meeting with their invaders, Wolbachia . PLoS ONE, 6, e18531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, K. , Kim, G. , & Lee, O. (2011). Wolbachia bacteria reside in host golgi‐related vesicles whose position is regulated by polarity proteins. PLoS ONE, 6, e22703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, M. E. , Anderson, C. L. , Cande, J. , & Karr, T. L. (2005). Widespread prevalence of Wolbachia in laboratory stocks and the implications for Drosophila research. Genetics, 170, 1667–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordaux, R. , Michel‐Salzat, A. , & Bouchon, D. (2001). Wolbachia infection in crustaceans: Novel hosts and potential routes for horizontal transmission. Journal of Evolutionary Biology, 14, 237–243. [Google Scholar]
- Cowdry, E. V. (1923). The distribution of rickettsia in the tissues of insects and arachnids. The Journal of Experimental Medicine, 37, 431–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby, A. C. , Armstrong, S. D. , Bah, G. S. , Kaur, G. , Hughes, M. A. , Kay, S. M. , … Makepeace, B. L. (2012). Analysis of gene expression from the Wolbachia genome of a filarial nematode supports both metabolic and defensive roles within the symbiosis. Genome Research, 22, 2467–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson, S. L. , Marsland, E. J. , Veneti, Z. , Bourtzis, K. , & O'Neill, S. L. (2002). Characterization of Wolbachia host cell range via the in vitro establishment of infections. Applied and Environmental Microbiology, 68, 656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson, S. L. , Bourtzis, K. , Braig, H. R. , Jones, B. F. , Zhou, W. , Rousset, F. , & O'Neill, S. L. (1999). Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochemistry and Molecular Biology, 29, 153–160. [DOI] [PubMed] [Google Scholar]
- Dodson, B. L. , Hughes, G. L. , Paul, O. , Matacchiero, A. C. , Kramer, L. D. , & Rasgon, J. L. (2014). Wolbachia enhances West Nile Virus (WNV) infection in the mosquito culex tarsalis. PLoS Neglected Tropical Diseases, 8, e2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson, E. A. , Kamath, M. K. , & Hurst, G. D. (2002). Wolbachia infection associated with all‐female broods in Hypolimnas bolina (Lepidoptera: Nymphalidae): Evidence for horizontal transmission of a butterfly male killer. Heredity, 88, 166–171. [DOI] [PubMed] [Google Scholar]
- Eleftherianos, I. , Atri, J. , Accetta, J. , & Castillo, J. C. (2013). Endosymbiotic bacteria in insects: Guardians of the immune system? Frontiers in Physiology, 4, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espino, C. I. , Gómez, T. , González, G. , do Santos, M. F. B. , Solano, J. , Sousa, O. , … Osuna, A. (2009). Detection of Wolbachia bacteria in multiple organs and feces of the triatomine Insect rhodnius pallescens (Hemiptera, Reduviidae). Applied and Environmental Microbiology, 75, 547–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, O. , Caragata, E. P. , McMeniman, C. J. , Woolfit, M. , Green, D. C. , Williams, C. R. , … McGraw, E. A. (2009). Increased locomotor activity and metabolism of Aedes aegypti infected with a life‐shortening strain of Wolbachia pipientis. Journal of Experimental Biology, 212, 1436–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria, V. G. , & Sucena, E. (2013). Wolbachia in the malpighian tubules: Evolutionary dead‐end or adaptation?. Journal of Experimental Zoology Part B, Molecular and Developmental Evolution, 320, 195–199. [DOI] [PubMed] [Google Scholar]
- Fast, E. M. , Toomey, M. E. , Panaram, K. , Desjardins, D. , Kolaczyk, E. D. , & Frydman, H. M. (2011). Wolbachia enhance Drosophila stem cell proliferation and target the germline stem cell niche. Science, 334, 990–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferree, Patrick , Frydman, H. M. , Li, J. M. , Cao, J. , Wieschaus, E. , & Sullivan, W. (2005). Wolbachia utilizes host microtobules and dynein for anterior localization in the Drosophila oocyte. PLoS Pathogens, 1, e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri, E. , (2011). New insights into the evolution of Wolbachia infections in filarial nematodes inferred from a large range of screened species. PLoS ONE, 6, e20843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, K. , Beatty, W. L. , Jiang, D. , Weil, G. J. , & Fischer, P. U. (2011). Tissue and stage‐specific distribution of Wolbachia in Brugia malayi . PLoS Neglected Tropical Diseases, 5, e1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, K. , Beatty, W. L. , Weil, G. , & Fischer, P. U. (2014). High pressure freezing/freeze substitution fixation improves the ultrastructural assessment of Wolbachia endosymbiont‐filarial nematode host interaction. PLoS ONE, 9, e86383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost, C. L. , Pollock, S. W. , Smith, J. E. Hughes, W. (2014). Wolbachia in the flesh: Symbiont intensities in germ‐line and somatic tissues challenge the conventional view of Wolbachia transmission routes. PLoS ONE, 9, e95122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydman, H. M. , Li, J. , Robson, D. N. , & Wieschaus, E. (2006). Somatic stem cell niche tropism in Wolbachia . Nature, 441, 509–512. [DOI] [PubMed] [Google Scholar]
- Gamston, C. , & Rasgon, J. (2007). Maintaining Wolbachia in cell‐free medium. Journal of Visualized Experiments : JoVE, 5, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerth, M. , Rothe, J. , & Bleidorn, C. (2013). Tracing horizontal Wolbachia movements among bees: A combined approach using multilocus sequence typing data and host phylogeny. Molecular Ecology, 22, 6149–6162. [DOI] [PubMed] [Google Scholar]
- Gómez‐Valero, L. , Soriano‐Navarro, M. , Pérez‐Brocal, V. , Heddi, A. , Moya, A. , García‐Verdugo, J. M. , & Latorre, A. (2004). Coexistence of Wolbachia with Buchnera aphidicola and a secondary symbiont in the aphid cinara cedri. Journal of Bacteriology, 186, 6626–6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodacre, S. L. , & Martin, O.Y . 2012. Modification of insect and arachnid behaviors by verticall transmitted endosymbionts: Infections as drivers of behavioral change and evolutionary novely. Insects, 246–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier, S. , Bernard, P. , Heddi, A. , Lassabliere, F. , Jager, C. , Louis, C. , & Khatchadourian, C. (1998). Successful horizontal transfer of Wolbachia symbionts between trichogramma wasps. Proceedings of the Royal Society B: Biological Sciences, 265, 1441–1445. [Google Scholar]
- Haine, E. R. , Pickup, N. J. , & Cook, J. M. (2005). Horizontal transmission of Wolbachia in a Drosophila community. Ecological Entomology, 30, 464–472. [Google Scholar]
- Harvey, H. A. , Jennings, M. P. , Campbell, C. A. , Williams, R. , & Apicella, M. A. (2001). Receptor‐Mediated endocytosis of neisseria gonorrhoeae into primary human urethral epithelial cells: The role of the asialoglycoprotein receptor. Molecular Microbiology, 42, 659–672. [DOI] [PubMed] [Google Scholar]
- Heath, B. D. , Butcher, R. D. , Whitfield, W. G. , & Hubbard, S. F. (1999). Horizontal transfer of Wolbachia between phylogenetically distant insect species by a naturally occurring mechanism. Current Biology : CB, 9, 313–316. [DOI] [PubMed] [Google Scholar]
- Hedges, L. M. , Brownlie, J. C. , O'Neill, S. L. , & Johnson, K. N. (2008). Wolbachia and virus protection in insects. Science, 322, 702. [DOI] [PubMed] [Google Scholar]
- Hertig, M. (1936). The Rickettsia, Wolbachia pipientis (gen. et sp.n.) and associated inclusions of the mosquito, culex pipiens. Parasitology, 28, 453. [Google Scholar]
- Hertig, M. , & Wolbach, S. B. (1924). Studies on rickettsia‐like micro‐organisms in insects. The Journal of Medical Research, 44, 329–74.7. [PMC free article] [PubMed] [Google Scholar]
- Heuer, D. , Rejman, L A. , Machuy, N. , Karlas, A. , Wehrens, A. , Siedler, F. , … Meyer, T. F. (2009). Chlamydia causes fragmentation of the golgi compartment to ensure reproduction. Nature, 457, 731–735. [DOI] [PubMed] [Google Scholar]
- Homem, C. C. , & Knoblich, J. A. (2012). Drosophila neuroblasts: A model for stem cell biology. Development, 139, 4297–4310. [DOI] [PubMed] [Google Scholar]
- Hosokawa, T. , Koga, R. , Kikuchi, Y. , Meng, X. , & Fukatsu, T. (2010). Wolbachia as a bacteriocyte‐associated nutritional mutualist. Proceedings of the National Academy of Sciences of the United States of America, 107, 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, G. L. , Koga, R. , Xue, P. , Fukatsu, T. , & Rasgon, J. L. (2011). Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae . PLoS Pathogens, 7, e1002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, G. L. , Pike, A. D. , Xue, P. , & Rasgon, R. L. (2012). Invasion of Wolbachia into anopheles and other insect germlines in an ex vivo organ culture system. PLoS ONE, 7, e36277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, G. L. , Ren, X. , Ramirez, J. L. , Sakamoto, J. M. , Bailey, J. A. , Jedlicka, A. E. , & Rasgon, J. L. (2011). Wolbachia infections in Anopheles gambiae Cells: Transcriptomic characterization of a novel host‐symbiont interaction. PLoS Pathogens, 7, e1001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huigens, M. E. , de Almeida, R. P. , Boons, P. , Luck, R. F. , & Stouthamer, R. (2004). Natural interspecific and intraspecific horizontal transfer of parthenogenesis‐inducing Wolbachia in trichogramma wasps. Proceedings Biological sciences/The Royal Society, 271, 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeya, T. , Broughton, S. , Alic, N. , Grandison, R. , & Partridge, L. (2009). The endosymbiont Wolbachia increases insulin/igf‐like signalling in drosophila. Proceedings of the Royal Society B: Biological Sciences, 276, 3799–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama, D. , Narita, S. , & Noda, H. (2008). Transfection of feminizing Wolbachia endosymbionts of the butterfly, Eurema hecabe, into the cell culture and various immature stages of the silkmoth, Bombyx mori . Microbial Ecology, 56, 733–741. [DOI] [PubMed] [Google Scholar]
- Kambris, Z. , Cook, P. E. , Phuc, H. K. , & Sinkins, S. P. (2009). Immune activation by life‐shortening Wolbachia and reduced filarial competence in mosquitoes. Science, 326, 134–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambris, Z. , Blagborough, A. M. , Pinto, S. B. , Blagrove, M. S. , Godfray, H. C. , Sinden, R. E. , & Sinkins, S. P. (2010). Wolbachia stimulates immune gene expression and inhibits plasmodium development in Anopheles gambiae . PLoS Pathogens, 6, e1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittayapong, P. , Jamnongluk, W. , Thipaksorn, A. , Milne, J. R. , & Sindhusake, C. (2003). Wolbachia Infection complexity among insects in the tropical rice‐field community. Molecular Ecology, 12, 1049–1060. [DOI] [PubMed] [Google Scholar]
- Kliot, A. , & Ghanim, M. (2015). Fluorescent in situ hybridization for the localization of viruses, bacteria, and other microoganisms in insect and plant tissues. Methods, 15, 30171–30177. [DOI] [PubMed] [Google Scholar]
- Kose, H. , & Karr, T. L. (1995). Organization of Wolbachia pipientis in the Drosophila fertilized egg and embryo revealed by an anti‐Wolbachia monoclonal antibody. Mechanisms of Development, 51, 275–288. [DOI] [PubMed] [Google Scholar]
- Koukou, K. , Pavlikaki, H. , Kilias, G. , Werren, J. H. , Bourtzis, K. , & Alahiotis, S. N. (2006). Influence of antibiotic treatment and Wolbachia curing on sexual isolation among Drosophila melanogaster cage populations. Evolution, 60, 87–96. [PubMed] [Google Scholar]
- Kozek, W. J. (1977). Transovarially transmitted intracellular microorganisms in adult and larval stages of Brugia malayi . Journal of Parasitology, 63, 992–1000. [PubMed] [Google Scholar]
- Kremer, N. , Voronin, D. , Charif, D. , Mavingui, P. , Mollereau, B. , & Vavre, F. (2009). Wolbachia interferes with ferretin expression and iron metabolism in insects. PLoS Pathogens, doi:10.1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann, F. , Foster, J. M. , Slatko, B. , & Sullivan, W. (2010). Asymmetric Wolbachia segregation during Early Brugia malayi embryogenesis determines its distribution in adult host tissues. PLoS Neglected Tropical Diseases, 4, e758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann, F. , Voronin, D. , Sullivan, S. , & Taylor, M. J. (2011). Anti‐Filarial activity of antibiotic therapy is due to extensive apoptosis after Wolbachia depletion from filarial nematodes. PLoS Pathogens, 7, e1002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann, F. , Bain, O. , Martin, C. , Uni, S. , Taylor, M. J. , & Sullivan, W. (2012). Both asymmetric mitotic segregation and cell‐to‐cell invasion are required for stable germline transmission of Wolbachia in filarial nematodes. Biology Open, 1, 536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann, F. , Foster, J. M. , Michalski, M. L. , Slatko, B. E. , & Sullivan, W. (2014). Co‐evolution between an endosymbiont and its nematode host: Wolbachia asymmetric posterior localization and Ap polarity establishment. PLoS Neglected Tropical Diseases, 8, e3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRock, D. L. , Chaudhary, A. , & Miller, S. I. (2015). Salmonellae interactions with host processes. Nature Reviews. Microbiology, 13, 191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Clec'h, W. , Braquart‐Varnier, C. , Raimond, M. , Ferdy, J.‐B. , Bouchon, D. , & Sicard, M. (2012). High virulence of Wolbachia after host switching: When autophagy hurts. PLoS Pathogens, 8, e1002844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Clec'h, W. , Chevalier, F. D. , Genty, L. , Bertaux, J. , Bouchon, D. , & Sicard, M. (2013). Cannibalism and predation as paths for horizontal passage of Wolbachia between terrestrial isopods. PLoS ONE, 8, e60232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis, C. , & Nigro, L. (1989). Ultrastructural evidence of Wolbachia rickettsiales in Drosophila simulans and their relationships with unidirectional cross‐incompatibility. Journal of Invertebrate Pathology, 54, 39–44. [Google Scholar]
- McGraw, E. A. , Merritt, D. J. , Droller, J. N. , & O'Neill, S. L. (2002). Wolbachia density and virulence attenuation after transfer into a novel host. Proceedings of the National Academy of Sciences of the United States of America, 99, 2918–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMeniman, C. J. , Lane, A. M. , Fong, A. W. , Voronin, D. A. , Iturbe‐Ormaetxe, I. , Yamada, R. , McGraw, E. A. , & O'Neill, S. L. (2008). Host adaptation of a Wolbachia strain after long‐term serial passage in mosquito cell lines. Applied and Environmental Microbiology, 74, 6963–6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMeniman, C. J. , Lane, R. V. , Cass, B. N. , Fong, A. W. , Sidhu, M. , Wang, Y. F. , & O'Neill, S. L. (2009). Stable introduction of a life‐shortening Wolbachia infection into the mosquito Aedes aegypti . Science (New York, N.Y.), 323, 141–144. [DOI] [PubMed] [Google Scholar]
- McNulty, S. N. , Fischer, K. , Curtis, K. C. , Weil, G. J. , Brattig, N. W. , & Fischer, P. U. (2013). Localization of Wolbachia‐like gene transcripts and peptides in adult onchocerca flexuosa worms indicates tissue specific expression. Parasites & vectors, 6, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnikow, E. , Xu, S. , Liu, J. , Bell, A. J. , Ghedin, E. , Unnasch, T. R. , & Lustigman, S. (2013). A Potential role for the interaction of Wolbachia surface proteins with the Brugia malayi glycolytic enzymes and cytoskeleton in maintenance of endosymbiosis. PLoS Neglected Tropical Diseases, 7, e2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, W. J. , Ehrman, L. , & Schneider, D. (2010). Infectious speciation revisited: Impact of symbiont‐depletion on female fitness and mating behavior of Drosophila paulistorum. Plos Pathogens, doi:10.1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, K. T. , & Benzer, S. (1997). Wolbachia, normally a symbiont of drosophila, can be virulent, causing degeneration and early death. Proceedings of the National Academy of Sciences of the United States of America, 94, 10792–10796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi, W. , Saiki, T. , Wei, W. , Kawakita, H. , & Sato, M. (2002). Two novel strains of Wolbachia coexisting in both species of mulberry leafhoppers. Insect Molecular Biology, 11, 577–584. [DOI] [PubMed] [Google Scholar]
- Monteith, C. E. , Brunner, M. E. , Djagaeva, I. , Bielecki, A. M. , Deutsch, J. M. , & Saxton, W. M. (2016). A Mechanism for cytosplasmic streaming: kinisen‐driven alignment of microtubules and fast fluid flows. Biophysical Journal, 110, 2053–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira, L. A. , Iturbe‐Ormaetxe, I. , Jeffery, J. A. , Lu, G. , Pyke, A. T. , Hedges, L. M. , … Scott, L. (2009). A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and plasmodium. Cell, 139, 1268–1278. [DOI] [PubMed] [Google Scholar]
- Morrow, J. L. , Frommer, M. , Shearman, D. , & Riegler, M. (2014). Tropical tephritid fruit fly community with high incidence of shared Wolbachia strains as platform for horizontal transmission of endosymbionts. Environmental Microbiology, 16, 3622–3637. [DOI] [PubMed] [Google Scholar]
- Murdock, C. C. , Moller‐Jacobs, L. L. , & Thomas, M. B. (2013). Complex environmental drivers of immunity and resistance in malaria mosquitoes. Proceedings, Biological Sciences/The Royal Society, 280, 20132030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassel, D. (1993). Neuropeptides in the insect brain: A review. Cell and Tissue Research, 273, 1–29. [DOI] [PubMed] [Google Scholar]
- Newton, I. L. G. , Savytskyy, O. , & Sheehan, K. B. (2015). Wolbachia utilize host actin for efficient maternal transmission in Drosophila melanogaster . PLoS Pathogens, 11, e1004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoh, N. , Hosokawa, T. , Moriyama, M. , Oshima, K. , Hattori, M. , & Fukatsu, T. (2014). Evolutionary origin of insect‐Wolbachia nutritional mutualism. Proceedings of the National Academy of Sciences, 111, 10257–10262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne, S. E. , Leong, Y. S. , O'Neill, S. L. , & Johnson, K. (2009). Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans . PLoS Pathogens, 5, e1000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, X. , Zhou, G. , Wu, J. , Bian, G. , Lu, P. , Raikhel, A. S. , & Xi, Z. (2011). Wolbachia induces reactive oxygen species (ros)‐dependent activation of the toll pathway to control dengue virus in the mosquito Aedes aegypti . Proceedings of the National Academy of Sciences, 109, E23–E31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannebakker, B. A. , Loppin, B. , Elemans, C. P. H. , Humblot, L. , & Vavre, F. (2007). Parasitic inhibition of cell death facilitates symbiosis. Proceedings of the National Academy of Sciences of the United States of America, 104, 213–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panteleev, D. , Goriacheva, I. , Andrianov, B. V. , Reznik, N. L. , Lazebnyi, O. E. , & Kulikov, A. M. (2007). The Endosymbiotic bacterium Wolbachia enhances the nonspecific resistance to insect pathogens and alters behavior of Drosophila melanogaster . Genetika, 43, 1277–1280. [PubMed] [Google Scholar]
- Peng, Y. , Nielsen, J. E. , Cunningham, J. P. , & McGraw, E. A. (2008). Wolbachia infection alters olfactory‐cued locomotion in Drosophila spp. Applied and Environmental Microbiology, 74, 3943–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigeault, R. , Braquart‐Varnier, C. , Marcadé, I. , Mappa, G. , Mottin, E. , & Sicard, M. (2014). Modulation of host immunity and reproduction by horizontally acquired Wolbachia . Journal of Insect Physiology, 70, 125–133. [DOI] [PubMed] [Google Scholar]
- Ponton, F. , Wilson, K. , Holmes, A. , Raubenheimer, D. , Robinson, K. L. , & Simpson, S. J. (2015). Macronutrients mediate the functional relationship between Drosophila and Wolbachia . Proceedings. Biological Sciences/ The Royal Society, 282, 20142029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey, S. , Shah, P. , Kohl, A. , & Dietrich, I. . (2014). Understanding the Wolbachia‐mediated inhibition of arboviruses in mosquitoes: progress and challenges. The Journal of General Virology, 95, 517–530. [DOI] [PubMed] [Google Scholar]
- Rainey, S. , Martinez, J. , McFarlane, M. , Juneja, P. , Sarkies, P. , Lulla, A. , … Kohl, A. (2016). Wolbachia blocks viral genome replication early in infection without a transcriptional response by the endosymbiont or host small RNA pathways. PLoS Pathogens, doi:10.1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rances, E. , Voronin, D. , Tran‐Van, V. , & Mavingui, P. (2008). Genetic and functional characterization of the type iv secretion system in Wolbachia . Journal of Bacteriology, 190, 5020–5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rances, E. , Johnson, T. K. , Popovici, J. , Iturbe‐Ormaetxe, I. , Zakir, T. , Warr, C. G. , & O'Neill, S. L. (2013). The Toll and Imd pathways are not required for Wolbachia‐mediated dengue virus interference. Journal of Virology, 87, 11945–11949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon, J. L. , Gamston, C. E. , & Ren, X. (2006). Survival of Wolbachia pipientis in cell‐free medium. Applied and Environmental Microbiology, 72, 6934–6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud, T. , & Juchault, P. (1995). Success and failure of horizontal transfers of feminizing Wolbachia endosymbionts in woodlice. Journal of Evolutionary Biology, 8, 249–255. [Google Scholar]
- Rohrscheib, C. E. , Bondy, E. , Josh, P. , Riegler, M. , Eyles, D. , van Swinderen, B. , … Brownlie, J. C. (2015). Wolbachia influences the production of octopamine and affects Drosophila male aggression. Applied and Environmental Microbiology, 81, 4573–4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschaefer, S. M. , & Lazzaro, B. P. (2012). No effect of Wolbachia on resistance to intracellular infection by pathogenic bacteria in Drosophila melanogaster . PLoS ONE, 7, e40500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, V. , Girondot, M. , & Harry, M. (2015). The distribution of Wolbachia in cubitermes (termitidae, termitinae) castes and colonies: A modelling approach. PLoS ONE, 10, e0116070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapountzis, P. , Zhukova, M. , Hansen, L. H. , Sørensen, S. J. , Schiøtt, M. , & Boomsma, J. (2015). Acromyrmex leaf‐cutting ants have simple gut microbiota with nitrogen‐fixing potential. Applied and Environmental Microbiology, 81, 5527–5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saridaki, A. , & Bourtzis, K. (2010). Wolbachia: More than just a bug in insects genitals. Current Opinion in Microbiology, 13, 67–72. [DOI] [PubMed] [Google Scholar]
- Schuler, H. , Bertheau, C. , Egan, S. P. , Feder, J. L. , Riegler, M. , Schlick‐Steiner, B. C. , … Stauffer, C. (2013). Evidence for a recent horizontal transmission and spatial spread of Wolbachia from endemic rhagoletis cerasi (Diptera: Tephritidae) to invasive rhagoletis cingulata in Europe. Molecular Ecology, 22, 4101–4111. [DOI] [PubMed] [Google Scholar]
- Serbus, L. R. , & Sullivan, W. (2007). A cellular basis for Wolbachia recruitment to the host germline. PLoS Pathogens, 3, e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbus, L. R. , White, P. M. , Silva, J. P. , Rabe, A. , Teixeira, L. , & Albertson, R. (2015). The impact of host diet on Wolbachia titer in drosophila. PLoS Pathogens, 11, e1004777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sintupachee, S. , Milne, J. R. , Poonchaisri, S. , Baimai, V. , & Kittayapong, P. (2006). Closely related Wolbachia strains within the pumpkin arthropod community and the potential for horizontal transmission via the plant. Microbial Ecology, 51, 294–301. [DOI] [PubMed] [Google Scholar]
- Spradling, A. C. (1993). Developmental genetics of oogenesis. The Development of Drosophila malanogaster, 1, 1–70. [Google Scholar]
- Stahlhut, J. K. , Desjardins, C. A. , Clark, M. E. , Baldo, L. , Russell, J. A. , Werren, J. H. , & Jaenike, J. (2010). The Mushroom habitat as an ecological arena for global exchange of Wolbachia . Molecular Ecology, 19, 1940–1952. [DOI] [PubMed] [Google Scholar]
- Strunov, A. , & Kiseleva, E. (2014). Drosophila melanogaster brain invasion: Pathogenic Wolbachia in central nervous system of the fly. Insect Science, 23, 253–264. [DOI] [PubMed] [Google Scholar]
- Strunov, A. , Kiseleva, E. , & Gottlieb, Y. (2013). Spatial and temporal distribution of pathogenic Wolbachia strain wmelpop in Drosophila melanogaster central nervous system under different temperature conditions. Journal of Invertebrate Pathology, 114, 22–30. [DOI] [PubMed] [Google Scholar]
- Taylor, M. J. (2003). Wolbachia in the inflammatory pathogenesis of human filariasis. Annals of the New York Academy of Sciences, 990, 444–449. [DOI] [PubMed] [Google Scholar]
- Teixeira, L. , Ferreira, A. , & Ashburner, M. (2008). The Bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster . PLoS Biology DOI, 10, 1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomey, M. E. , Panaram, K. , Fast, E. M. , Beatty, C. , & Frydman, H. M. (2013). Evolutionarily conserved Wolbachia‐encoded factors control pattern of stem‐cell niche tropism in Drosophila ovaries and favor infection. Proceedings of the National Academy of Sciences of the United States of America, 110, 10788–10793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley, A. P. , Moreira, L. A. , O'Neill, S. L. , & McGraw, E. A. (2009). Wolbachia infection reduces blood‐feeding success in the dengue fever mosquito, Aedes aegypti edited by J. G. Valenzuela. PLoS Neglected Tropical Diseases, 3, e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vala, F. , Egas, M. , Breeuwer, J. , & Sabelis, M. W. (2004). Wolbachia Affects oviposition and mating behaviour of its spider mite host. Journal of Evolutionary Biology, 17, 692–700. [DOI] [PubMed] [Google Scholar]
- Van Meer, M. , & Stouthamer, R. (1999). Cross‐order transfer of Wolbachia from muscidifurax uniraptor (Hymenoptera: Pteromalidae) to Drosophila simulans (Diptera: Drosophilidae). Heredity, 82, 163–169. [DOI] [PubMed] [Google Scholar]
- Vavre, F. , Fleury, F. , Lepetit, D. , Fouillet, P. , & Boulétreau, M. (1999). Phylogenetic evidence for horizontal transmission of Wolbachia in host‐parasitoid associations. Molecular Biology and Evolution, 16, 1711–1723. [DOI] [PubMed] [Google Scholar]
- Veneti, Z. , Clark, M. E. , Karr, T. L. , Savakis, C. , & Bourtzis, K. (2004). Heads or Tails: host‐parasite interactions in the drosophila‐Wolbachia system. Applied and Environmental Microbiology, 70, 5366–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veneti, Z. , Clark, M. E. , Zabalou, S. , Karr, T. L. , Savakis, C. , & Bourtzis, K. (2003). Cytoplasmic incompatibility and sperm cyst infection in different drosophila‐Wolbachia associations. Genetics, 164, 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronin, D. , Bachu, S. , Shlossman, M. , Unnasch, T. R. , Ghedin, E. , & Lustigman, S. (2016). Glucose and glycogen metabolism in Brugia malayi is associated with Wolbachia symbiont fitness. PLoS ONE, 11, e0153812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Zhou, C. , He, Z. , Wang, Z.‐G. , Wang, J.‐L. , & Wang, Y.‐F. (2012). Wolbachia infection decreased the resistance of Drosophila to lead. PLoS ONE, 7, doi:10.1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren, J. H. (1997). Biology of Wolbachia . Annual Review of Entomology, 42, 587–609. [DOI] [PubMed] [Google Scholar]
- Werren, J. H. , Baldo, L. , & Clarke, M. E. (2008). Wolbachia: Master manipulators of invertebrate biology. Nature Reviews Microbiology, 6, 741–751. [DOI] [PubMed] [Google Scholar]
- Werren, J. H. , Zhang, W. , & Guo, L. R. (1995). Evolution and phylogeny of Wolbachia: Reproductive parasites of arthropods. Proceedings. Biological Sciences/The Royal Society, 261, 55–63. [DOI] [PubMed] [Google Scholar]
- Wong, Z. S. , Brownlie, J. C. , & Johnson, K. N. (2015). Oxidative stress correlates with Wolbachia‐mediated antiviral protection in wolbachia‐Drosophila associations. Applied and Environment Microbiology, 81, 3001–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, Z. S. , Hedges, L. M. , Brownlie, J. C. , & Johnson, K. N. (2011). Wolbachia‐mediated antibacterial protection and immune gene regulation in drosophila. PLoS ONE, 6, e25430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, M. , Sun, L. V. , Vamathevan, J. , Riegler, M. , Deboy, R. , Brownlie, J. C. , McGraw, E. A. , … Eisen, J. A. (2004). Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: A streamlined genome overrun by mobile genetic elements. PLoS Biology, 2, e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, Y. H. , Woolfit, M. , Rances, E. , O' Neill, S. L. , McGraw, E. A. (2013). Wolbachia‐associated bacterial protection in the mosquito Aedes aegypti . PLoS Neglected Tropical Diseases, 7, e2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen, J. H. , & Barr, A. R. (1973). The etiological agent of cytoplasmic incompatibility in Culex pipiens . Journal of invertebrate pathology, 22, 242–250. [DOI] [PubMed] [Google Scholar]
- Zhang, K. , Han, X. , & Hong, X. (2013). Various infection status and molecular evidence for horizontal transmission and recombination of Wolbachia and cardinium among rice planthoppers and related species. Insect Science, 20, 329–344. [DOI] [PubMed] [Google Scholar]
- Zhukova, M. V. , & Kiseleva, E. (2012). The virulent Wolbachia strain wmelpop increases the frequency of apoptosis in the female germline cells of Drosophila melanogaster . BMC Microbiology, 12, S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouache, K. , Voronin, D. , Tran‐Van, V. , Mousson, L. , Failloux, A.‐B. , & Mavingui, P. (2009). Persistent Wolbachia and cultivable bacteria infection in the reproductive and somatic tissues of the mosquito vector Aedes albopictus . PLoS ONE, 4, e6388. [DOI] [PMC free article] [PubMed] [Google Scholar]


