Abstract
Objective:
To determine a balance recovery timeline after a functional exertion protocol using the Balance Error Scoring System (BESS).
Design and Setting:
Five subject groups (4 test, 1 control) were tested 3 times during 1 session: once before the exertion protocol (pretest) and twice after the exertion protocol (posttest I and posttest II). Posttest I occurred at staggered intervals of 0, 5, 10, and 15 minutes, depending on experimental group assignment, and posttest II occurred at 20 minutes.
Subjects:
One hundred subjects (80 test, 20 control) volunteered to participate in this study. None of the subjects had a balance disorder, mild head injury, or lower extremity injury in the 6 months before testing.
Measurements:
We assessed balance using the BESS, assigning a score for each stance-surface condition.
Results:
We found a significant decrease in BESS performance after the exertion protocol in all test groups, with exertion having the greatest effect on the tandem and single-leg stance conditions. All subjects recovered by posttest II, which was administered 20 minutes after cessation of the exertion protocol.
Conclusions:
Athletic trainers need to be aware of the effect of exertion when administering the BESS after physical activity. Athletic trainers can expect the BESS performance of healthy athletes to return to baseline levels within 20 minutes of rest.
Keywords: postural stability, fatigue, recuperation, concussion
Sport-related concussions are a serious problem that can have potentially catastrophic complications if improperly managed. Proper management requires a comprehensive assessment that allows health care professionals to make safe return-to-play decisions. Currently, concussion assessment relies mainly on the athlete's willingness to share subjective symptoms.1 However, athletes often fear they will be withheld from activity if they divulge such information.1 Hence, objective assessment tools are needed to thoroughly evaluate the extent of the injury and prevent premature return to competition, a decision that can potentially lead to devastating complications, including second-impact syndrome.2,3
The Balance Error Scoring System (BESS) was developed as an objective sideline assessment tool for the evaluation of postural-stability deficits after concussion.4 The BESS test battery consists of 6 testing conditions that use 3 stances (double leg, single leg, tandem) on 2 surfaces (firm, foam). Although the BESS has been shown to be reliable and valid in both healthy4 and head-injured subjects,5 these assessments were established in controlled laboratory environments, with the subjects at relative rest. Because sport-related concussions most often occur during physical activity, athletes who sustain a concussion are not at rest and will likely be under some level of physical stress, if not fatigued. This is an important consideration, because it is well established that balance ability decreases after exertion.6–12 Recent researchers13,14 have demonstrated an increase in BESS scores in healthy subjects after exertion.
Although the effect of exertion on postural-stability measures is evident, the time needed to recover from exertion and regain postural stability consistent with baseline measures has yet to be clearly established. The limited literature available shows decreased balance immediately after exertion but no deficits as early as 20 minutes after exertion.8,9,12,15 However, across these studies, no measurements were taken between 13 and 20 minutes after exertion, leaving unclear when balance recovery actually occurs.
For any assessment tool to be used effectively in evaluating deficits after a concussion, other factors that affect performance need to be considered and controlled.16 The objective of the BESS is to establish baseline norms for comparison after injury. After a concussion, the BESS can be used to observe increased error scores that might indicate residual symptoms from the injury. In addition, the use of controls in many investigations allows for comparison of balance ability with a normal sample. Because exertion also increases error scores,13,14 the BESS cannot be used to accurately assess balance deficits secondary to a concussion unless a recovery timeline after exertion is established. Therefore, the purpose of our study was to determine a balance recovery timeline in college-age individuals after a functional exertion protocol. We hypothesized that BESS error scores would increase after exertion and return to baseline within 20 minutes.
METHODS
One hundred recreationally active college students volunteered to participate in this study. We defined recreationally active as individuals who participated in physical activity on a regular basis for at least 30 minutes 4 times a week. We excluded subjects who had a diagnosed vestibular disorder or had experienced a head injury or lower extremity injury within the 6 months before testing through self-report. Before participating in this study, all subjects read and signed an informed consent form approved by the university's Institutional Review Board for the Protection of Human Subjects.
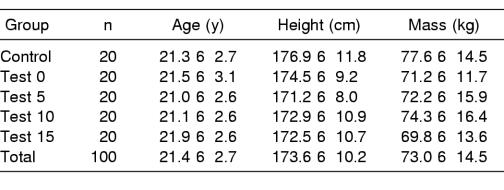
Before testing, we obtained demographic information from the subjects (Table 1). We placed each subject into 1 of 5 groups (control, test 0, test 5, test 10, and test 15) by random assignment. The names of the test groups denote the number of minutes after the end of the exertion protocol before administering BESS posttest I. The subjects self-stretched as needed before testing to prevent injuries during balance testing and the exertion protocol. We provided subjects 1 practice trial per BESS condition immediately before testing to familiarize them with the surfaces and stances used in the BESS test battery.
Table 1.
Demographic Data (Mean ± SD)
We tested each subject 2 times, once before a 20-minute intervention (pretest) and twice after the intervention (posttest I, posttest II). For the control subjects, the intervention was a 20-minute period of complete rest. The testing timeline for the control group consisted of the pretest, 20 minutes of complete rest, posttest I immediately after the rest period, and posttest II 20 minutes after the end of the rest period. For the exertion subjects (test groups), the intervention was a 20-minute exertion protocol. The timeline for the 4 test groups consisted of the pretest followed by the 20-minute exertion protocol or posttest I immediately after the exertion protocol (test 0) or 5 minutes (test 5), 10 minutes (test 10), or 15 minutes (test 15) after the end of the exertion protocol, with posttest II occurring at the same time for all test groups (20 minutes after the end of the exertion protocol).
Exertion Protocol
We used a 7-station exertion protocol designed by Wilkins et al13 to simulate exertion during athletic activity. Station 1 was a 5-minute moderate jog at the subject's self-selected pace. Station 2 was 3 minutes of sprints up and down the length of a basketball court. Station 3 was 2 minutes of push-ups. Station 4 was 2 minutes of sit-ups. Station 5 was 3 minutes of 12-in (30.48-cm) step-ups. Station 6 was another 3 minutes of sprints. Station 7 was a 2-minute run, during which subjects were instructed to maintain the fastest pace they could for the entire 2 minutes. Subjects were given verbal feedback in an attempt to maintain a high level of exertion throughout the entire exertion protocol.
Ratings of Perceived Exertion
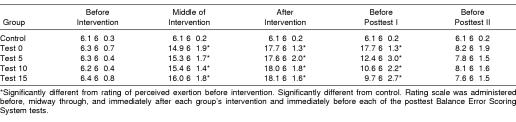
We used the Borg 15-point scale to measure each subject's Rating of Perceived Exertion (RPE)17 to try to ensure adequate exertion was achieved. Adequate exertion was deemed to be achieved with an RPE score of 15; previous authors who used the RPE scale and a similar population have found that this RPE level correlated to 75% to 90% maximum oxygen consumption.18,19 For all subjects, we monitored RPE scores immediately before the intervention (after the pretest), once during the middle of the intervention, immediately after the intervention, immediately before posttest I, and immediately before posttest II. The control group rested during their intervention. Test 0 performed posttest I immediately after the exertion protocol, and test 5, test 10, and test 15 performed posttest I 5, 10, and 15 minutes, respectively, after the end of the exertion protocol. All subjects performed posttest II 20 minutes after the end of their respective interventions.
Balance Assessment
We used the BESS to assess balance under 3 testing stances (double leg, single leg, tandem) on 2 surfaces. In the double-leg stance, participants stood with their feet together. In the single-leg stance, participants stood on the nondominant leg (dominance determined by which leg they would prefer to kick a ball), with the contralateral leg positioned in approximately 30° of hip flexion and 90° of knee flexion and the foot held approximately 6 in (15.24 cm) off the ground. In the tandem stance, participants stood with the dominant foot in front of the nondominant foot in heel-to-toe fashion. Each stance was performed on a firm surface and on a medium-density (60 kg/ m3) foam block, with a load deflection of 80 to 90 kg/m3 (Exertools, Inc, Novato, CA).
One 20-second trial of each test condition was performed in the following order: double firm, single firm, tandem firm, double foam, single foam, and tandem foam. We chose this testing order because it progressively challenges the sensory systems.20 Before testing, we instructed subjects on BESS performance and gave a practice session for each condition. For each test condition, we asked the subjects to keep their eyes closed and their hands on their iliac crests while maintaining the appropriate stance. We instructed the subjects that if at any time they fell out of position, they were to return to the test position as quickly as possible, keeping their eyes open until they regained balance. As the subjects performed each 20-second trial, we observed and recorded the number of errors each subject made (Table 2). We stood approximately 10 ft (3.05 m) from the subject to observe the eyes, hips, and feet at the same time. One examiner (T.M.S.) scored all subjects and all trials. We confirmed scoring consistency before data collection through intratester reliability using a video camera. Thirty-six subjects were videotaped performing the BESS, and the scores of the live and videotaped conditions were used to determine the intratester reliability. Our intratester reliability intraclass correlation coefficients (ICCs [3, 1]) on 36 subjects ranged from 0.63 to 0.82, and the SEM ranged from 0.62 to 0.93 errors. The ICC of 0.82 and the SEM of 0.62 were found on the double-firm condition. The ICC of 0.63 and the SEM of 0.93 were found on the double-foam condition.
Table 2.
Balance Error Scoring System Errors
Statistical Analysis
Our dependent variables were the BESS error scores and RPE scores, and our independent variables were group, time, and BESS condition. We entered and analyzed the data using SPSS statistical software (version 10.0; SPSS Inc, Chicago, IL). To assess recovery from exertion, we used a 2-within (time [3], BESS condition [6]), 1-between (group [5]) repeated-measures analysis of variance comparing pretest, posttest I, and posttest II scores among all groups and conditions. To ensure all groups were equally exerted, we used a 1-within (RPE time [5]), 1-between (group [5]) repeated-measures analysis of variance comparing the RPE scores among the groups across time. We further examined significant differences with the Tukey Honestly Significant Difference post hoc analysis. We set our α level a priori at .05.
RESULTS
Analysis of the RPE scores revealed significant time (F4,380 = 915.6, P < .001) and group (F4,95 = 145.5, P < .001) main effects and a time-by-group interaction (F16,380 = 82.5, P < .001). All exertion groups reported a higher perceived exertion than the control group halfway through the exertion, at the end of the exertion, and before the first posttest (Table 3). Across all exertion groups, the mean RPE score was 6.3 ± 0.6 before exertion (indicating “no exertion at all”), 17.8 ± 1.7 (indicating a “very” to “very, very hard” level of exertion) immediately after the exertion protocol, and 7.9 ± 1.6 (a “very” to “very, very light” level of exertion) 20 minutes after the exertion protocol. Further analysis revealed no differences among the exertion groups, either during or immediately after the exertion protocol, indicating that our exertion protocol was equally effective in all exertion groups.
Table 3.
Rating of Perceived Exertion Scores for Each Time Point by Group (Mean ± SD)
Analysis of BESS scores with the groups combined revealed significant time (F2,190 = 242.2, P < .001) and BESS condition (F5,475 = 905.2, P < .001) main effects and a time-by-BESS condition interaction (F10,950 = 10.3, P < .001). Across time, scores at posttest I were significantly greater than at both the pretest and posttest II. Across BESS conditions, participants had more errors with the single-foam condition than with all other conditions; more errors with single firm and tandem foam compared with double firm, double foam, and tandem firm; and more errors with tandem firm than double firm and double foam. The order of errors from greatest to least was single foam > single firm = tandem foam > tandem firm > double foam = double firm. Time had an effect on all conditions except double firm and double foam, which remained unchanged with exertion.
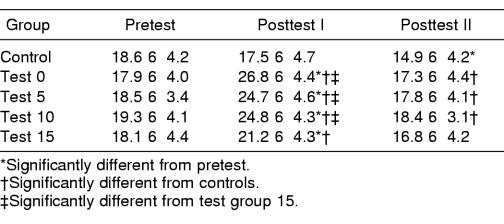
Analysis of the BESS scores among groups revealed a significant difference in groups on BESS performance by time (F8,190 = 19.1, P < .001) and a time-by-BESS condition interaction (F40,950 = 1.6, P = .014). Posttest I scores for all the exertion groups were higher than pretest scores and were also higher than the posttest I score of the control group (Table 4). Although the posttest I score of test group 15 was still elevated over the pretest score, it was significantly lower than the posttest I scores for test groups 0, 5, and 10. After 20 minutes of rest, posttest II scores for all exertion groups were no different than their pretest scores. However, the posttest II scores for the control group were significantly lower than the pretest scores. The effect of exertion on conditions across time was similar across all groups, except for the control group.
Table 4.
Total Balance Error Scoring System Error Scores for Each Time Point by Group (Mean ± SD)
DISCUSSION
Our primary finding was that exertion adversely affected balance, as measured through the BESS clinical assessment tool, with balance recovery (ie, return to pretest score) occurring within 20 minutes after exercise ceased. These findings agree with previous studies6–14 that showed balance detriments after fatiguing exercise. These findings also agree with previous studies8,9,12,15 that placed balance recovery at approximately 20 minutes. A secondary finding of this study was that the effect of exertion on balance and balance recovery was task specific. Although total BESS scores increased at posttest I and decreased by posttest II, notable differences were seen with individual conditions. Essentially, exertion had little or no effect on the double-stance conditions (double firm and double foam) and had the greatest effect on the more challenging tandem and single-leg conditions. These findings are similar to those of Crowell et al,14 who noted that fatigue affected the conditions that were the most difficult.
Balance is a complex task that requires intact information from the somatosensory, visual, and vestibular systems and an intact central nervous system to maintain upright stance.21–23 If inaccurate information is provided by any of the 3 sensory systems because of local fatigue and compensation is inadequate, balance is disturbed. Similarly, if the central nervous system is suppressed through central fatigue, balance is also disturbed. Researchers who perform balance studies have examined both local and central fatigue mechanisms and have produced different results.
Most authors who study local fatigue recovery have examined the recovery of muscle contractility,24 firing rate,25,26 and muscle force.27 These studies24–27 have placed recovery of muscle activity at 1 to 3 minutes after fatigue. Because we, along with previous investigators,8,9,12,15 found balance deficits persisting beyond 3 minutes of recovery, local fatigue mechanisms may not be the primary factor in causing muscle impairments after fatiguing exercise.28–30 Instead, central fatigue mechanisms might have a more extensive effect on maintaining balance after multijoint exercise.
Other researchers have examined the amount of time required to fully recover maximal muscle force, body strength, and muscular endurance.28–31 Baker et al28 determined that approximately 75% of maximal anterior tibialis muscle force is recovered within 15 minutes after a 15- to 20-minute fatigue protocol of progressive maximal contractions. Häkkinen31 determined that 80% to 90% of maximal body strength, as measured by a 1-repetition maximal squat, is regained within the first hour of recovery, with full recovery occurring 2 days after total body fatigue. Funderburk et al29 and Yates et al30 determined that 80% to 90% of muscular endurance is recovered within 20 minutes after intermittent isometric contractions to fatigue, with full endurance not recovering until several hours after activity. Because maintaining a stable posture does not require full muscular strength or endurance, it seems reasonable that balance recovery should occur within 20 minutes, once most muscular strength and endurance are regained.8 This conjecture is supported by our data, with a lack of balance detriments observed after 20 minutes of recovery.
Specific to balance ability, central or total body fatigue studies are limited in number, with most authors only examining balance after various periods of rest or recovery as a secondary purpose. Balance deficits were seen after five9 and ten12 minutes of recovery but not after 20 minutes or more.9,12,15 We found only one study specifically dedicated to determining the time course of balance recovery after fatigue. Nardone et al8 studied postural-sway deviations after a 25-minute treadmill run. Sway measures were still elevated after 13 minutes of recovery but had returned to baseline after 23 minutes. However, because no measures were taken between 13 and 23 minutes, an exact timeline was not established.
We attempted to determine a more precise time course of balance recovery after exertion by testing balance at 5-minute intervals from 0 to 20 minutes. We found balance deficits at 0, 5, 10, and 15 minutes after exertion, with deficits improving by 15 minutes and resolving by 20 minutes. Our balance recovery timeline is consistent with the previous recovery literature,8,9,12,15 which is clinically important for 2 reasons. First, our results confirm previous computerized recovery studies using a field test. Second, our results provide evidence as to how and when the BESS should be used as a sideline assessment tool.
Previous investigators have used expensive computerized equipment to examine sway values and center-of-pressure changes after various fatigue protocols.7–10,12,15 By producing similar results, our study suggests that the BESS is an appropriate clinical measure of balance. In addition, previous authors4 found moderate correlations between the BESS and the long force-platform sway measures of the NeuroCom Smart Balance Master (NeuroCom International, Inc, Clackamas, OR), indicating the former can be used on the sideline as a substitute for computerized equipment or when such equipment is otherwise unavailable.
The BESS is commonly used as a sideline assessment tool for mild head injuries.4,5 However, an assessment tool is ineffective if factors that affect its use are not identified and accounted for. Because our study, along with previous research, shows a decrease in BESS performance after exertion,13,14 administering the BESS immediately after a concussive injury (as prescribed) could yield false-positive findings. In the case of an athlete with a concussion, who would be kept out of the event for at least 15 to 20 minutes before a return-to-play decision was made, waiting to perform the BESS near the end of this observation period would elicit a score less likely to be confounded by exertion and more representative of the athlete's postconcussion postural-stability status. By understanding when BESS performance returns to pre-exertion levels in healthy individuals, health care professionals can be reasonably confident that performance detriments lasting beyond 20 minutes of leaving the practice or game can be attributed to the suspected concussion rather than to exertion.
Another important finding of our study was the presence of a practice effect in the control group, which was apparently not present in any of the exertion groups. The practice effect in the control group is consistent with other research showing improved scores with repeat testing in healthy subjects.13,32,33 Whether or not exertion subjects demonstrated a practice effect is beyond the scope of this study. We did not have a test group 20 in our design; therefore, we are limited in speculating whether that group would have demonstrated a practice effect. However, the previous literature shows a lack of a learning effect after exertion compared with control subjects, suggesting that exertion may interfere with or impair the learning of a task.11,34,35 Nevertheless, the fact remains that exerted healthy individuals returned to pretest scores within 20 minutes of rest, whereas exerted individuals with mild head injuries and balance disturbances likely would not.2,3,5,36,37
One limitation of our study was the lack of an objective exertion protocol. Our goal was to induce central fatigue in our subjects to mimic the central fatigue felt during sports in which an athlete might be at risk for a concussion (eg, soccer, football). However, no gold standard is available to quantify central fatigue in the same way electromyography is used to quantify peripheral fatigue. In peripheral fatigue studies, a subject is often considered fatigued when contraction force falls below 50% of the maximal volitional isometric contraction, whether it occurs after 2 minutes or after 20 minutes. However, with central fatigue, no such cutoff exists, forcing us to use a standard, timed protocol. We attempted to quantify levels of fatigue by using the RPE scale, a clinical measurement tool that can easily be administered on the sidelines of athletic events. Yet various factors can influence RPE scores, including level of fitness, psychological state, and environmental conditions among others. We attempted to control for some of these factors (testing all subjects indoors, for example), but factors such as psychological state could not be controlled. The extent to which such internal factors have affected our results is beyond the scope of this initial study into the issue of fatigue recovery and thus cannot be assessed.
Another limitation of our study was that only one specific exertion protocol was studied; hence, our recovery findings are limited to activities that create similar levels of exertion. It is possible that lower exertion levels may show earlier recovery and more intense exercise bouts may lengthen the recovery timeline. Different sports have different metabolic demands and, thus, athletes experience different levels of exertion; for example, levels of exertion among football players obviously differ from those of soccer or lacrosse players. We attempted to incorporate aspects of both fatigue mechanisms, anaerobic and aerobic, to try to mimic both extremes and enhance our applicability. However, more research is needed to evaluate the time course of balance deficits after either strictly aerobic or strictly anaerobic exercise and apply those results to individual sports.
An interesting post hoc finding from our study is a significant positive correlation (r = 0.542, P = .01) between the subject's posttest BESS score and the corresponding RPE score. This suggests that level of exertion may be a factor that affects balance deficits and recovery, and an RPE score might be a better indication of recovery than a specified time interval. Because heart-rate measures also demonstrate a strong positive correlation with RPE scores,17 one could postulate that heart rate or other physiologic measures might also be better indications of recovery than a specified time interval. This suggestion is supported by research that shows no balance deficits after heart-rate measures had returned to baseline values.10,38 Our findings could also indicate that highly trained athletes recover faster after exertion. More research is needed to effectively determine recovery from exertion so that valid BESS measurements can be obtained in different athletic populations. Our study represents an initial step in this line of inquiry and opens important directions for future research, which will build on the current findings through examination of the intensity and time course of various levels and types of exertion.
Clinical Significance
Sideline concussion assessments have evolved from simple subjective questioning to the multifaceted approach that is advocated today. A comprehensive approach should include subjective questioning along with neuropsychological and balance testing.3,39–42 Health care professionals who choose to use the BESS should understand how and when to use the BESS as an effective assessment tool. Based on our findings, clinicians should wait at least 20 minutes after physical activity ceases to allow the athlete to return to a resting state and yield measures consistent with baseline testing. Waiting 20 minutes ensures a more comparable result with baseline testing conditions and increases the likelihood that any postural deficits noted can be attributed to potential concussive symptoms. Hence, our findings lend further justification for the classic recommendation of waiting 20 minutes after a suspected concussion to observe the presence of delayed signs and symptoms before returning an athlete to activity.
REFERENCES
- 1.Cantu RC. Second impact syndrome: immediate management. Physician Sportsmed. 1992;20(9):55–58. 66. [Google Scholar]
- 2.Guskiewicz KM, Riemann BL, Perrin DH, Nashner LM. Alternative approaches to the assessment of mild head injury in athletes. Med Sci Sports Exerc. 1997;29:S213–S221. doi: 10.1097/00005768-199707001-00003. [DOI] [PubMed] [Google Scholar]
- 3.Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36:263–273. [PMC free article] [PubMed] [Google Scholar]
- 4.Riemann BL, Guskiewicz KM, Shields EW. Relationship between clinical and forceplate measure of postural stability. J Sport Rehabil. 1999;8:71–82. [Google Scholar]
- 5.Riemann BL, Guskiewicz KM. Effects of mild head injury on postural stability as measured through clinical balance testing. J Athl Train. 2000;35:19–25. [PMC free article] [PubMed] [Google Scholar]
- 6.Derave W, Tombeux N, Cottyn J, Pannier JL, De Clercq D. Treadmill exercise negatively affects visual contribution to static postural stability. Intl J Sports Med. 2002;23:44–49. doi: 10.1055/s-2002-19374. [DOI] [PubMed] [Google Scholar]
- 7.Lepers R, Bigard AX, Diard JP, Gouteyron JF, Guezennec CY. Posture control after prolonged exercise. Eur J Appl Physiol Occup Physiol. 1997;76:55–61. doi: 10.1007/s004210050212. [DOI] [PubMed] [Google Scholar]
- 8.Nardone A, Tarantola J, Galante M, Schieppati M. Time course of stabilometric changes after a strenous treadmill exercise. Arch Phys Med Rehabil. 1998;79:920–924. doi: 10.1016/s0003-9993(98)90088-0. [DOI] [PubMed] [Google Scholar]
- 9.Nardone A, Tarantola J, Giordano A, Schieppati M. Fatigue effects on body balance. Electroencephal Clin Neurophysiol. 1997;105:309–320. doi: 10.1016/s0924-980x(97)00040-4. [DOI] [PubMed] [Google Scholar]
- 10.Seliga R, Bhattacharya A, Succop P, Wixkstrom R, Smith D, Willeke K. Effect of work load and respirator wear on postural stability, heart rate, and perceived exertion. Am Ind Hyg Assoc J. 1991;52:417–422. doi: 10.1080/15298669191364965. [DOI] [PubMed] [Google Scholar]
- 11.Thomas JR, Cotten DJ, Spieth WR, Abraham NL. Effects of fatigue on stabilometer performance and learning of males and females. Med Sci Sports. 1975;7:203–206. [PubMed] [Google Scholar]
- 12.Yaggie JA, McGregor S, Armstrong CW. The effects of isokinetic fatigue on balance and the ranges of postural control [abstract] Med Sci Sports Exerc. 1999;31:S284. [Google Scholar]
- 13.Wilkins JC, Valovich TC, Perrin DH, Gansneder BM. Performance on the Balance Error Scoring System decreases following exertion. J Athl Train. 2004;39:156–161. [PMC free article] [PubMed] [Google Scholar]
- 14.Crowell DH, Guskiewicz KM, Prentice WE, Onate JA. The effect of fatigue on postural stability and neuropsychological function [abstract] J Athl Train. 2001;36:S-33. [Google Scholar]
- 15.Derave W, De Clercq D, Bouckaert J, Pannier JL. The influence of exercise and dehydration on postural stability. Ergonomics. 1998;41:782–789. doi: 10.1080/001401398186630. [DOI] [PubMed] [Google Scholar]
- 16.Guskiewicz KM, Perrin DH. Research and clinical applications of assessing balance. J Sport Rehabil. 1996;5:45–63. [Google Scholar]
- 17.Borg G. Borg's Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- 18.Robertson RJ, Moyna NM, Sward KL, Millich NB, Goss FL, Thompson PD. Gender comparison of RPE at absolute and relative physiological criteria. Med Sci Sports Exerc. 2000;32:2120–2129. doi: 10.1097/00005768-200012000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Moyna NM, Robertson RJ, Meckes CL, Peoples JA, Millich NB, Thompson PD. Intermodal comparison of energy expenditure at exercise intensities corresponding to the perceptual preference range. Med Sci Sports Exerc. 2001;33:1404–1410. doi: 10.1097/00005768-200108000-00025. [DOI] [PubMed] [Google Scholar]
- 20.Goldie PA, Bach TM, Evans OM. Force platform measures for evaluating postural control: reliability and validity. Arch Phys Med Rehabil. 1989;70:510–517. [PubMed] [Google Scholar]
- 21.Diener HC, Dichgans J. On the role of vestibular, visual and somatosensory information for dynamic postural control in humans. Prog Brain Res. 1988;76:253–262. doi: 10.1016/s0079-6123(08)64512-4. [DOI] [PubMed] [Google Scholar]
- 22.Horak FB, Nashner LM, Diener HC. Postural strategies associated with somatosensory and vestibular loss. Exp Brain Res. 1990;82:167–177. doi: 10.1007/BF00230848. [DOI] [PubMed] [Google Scholar]
- 23.Shumway-Cook A, Horak FB. Assessing the influence of sensory interaction of balance: suggestions from the field. Phys Ther. 1986;66:1548–1550. doi: 10.1093/ptj/66.10.1548. [DOI] [PubMed] [Google Scholar]
- 24.Häkkinen K, Komi PV. Electromyographic and mechanical characteristics of human skeletal muscle during fatigue under voluntary and reflex conditions. Electroencephalogr Clin Neurophysiol. 1983;55:436–444. doi: 10.1016/0013-4694(83)90132-3. [DOI] [PubMed] [Google Scholar]
- 25.Bigland-Ritchie BR, Dawson NJ, Johansson RS, Lippold OCJ. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol. 1986;379:451–459. doi: 10.1113/jphysiol.1986.sp016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woods JJ, Furbush F, Bigland-Ritchie B. Evidence for a fatigue-induced reflex inhibition of motoneuron firing rates. J Neurophysiol. 1987;58:125–137. doi: 10.1152/jn.1987.58.1.125. [DOI] [PubMed] [Google Scholar]
- 27.Fulco CS, Rock PB, Muza SR, et al. Slower fatigue and faster recovery of the adductor pollicis muscle in women matched for strength with men. Acta Physiol Scand. 1999;167:233–239. doi: 10.1046/j.1365-201x.1999.00613.x. [DOI] [PubMed] [Google Scholar]
- 28.Baker AJ, Kostov KG, Miller RG, Weiner MW. Slow force recovery after long-duration exercise: metabolic and activation factors in muscle fatigue. J Appl Physiol. 1993;74:2294–2300. doi: 10.1152/jappl.1993.74.5.2294. [DOI] [PubMed] [Google Scholar]
- 29.Funderburk CF, Hipskind SG, Welton RC, Lind AR. Development of and recovery from fatigue induced by static effort at various tensions. J Appl Physiol. 1974;37:392–396. doi: 10.1152/jappl.1974.37.3.392. [DOI] [PubMed] [Google Scholar]
- 30.Yates JW, Kearney JT, Noland MP, Felts WM. Recovery of dynamic muscular endurance. Eur J Appl Physiol Occup Physiol. 1987;56:662–667. doi: 10.1007/BF00424807. [DOI] [PubMed] [Google Scholar]
- 31.Häkkinen K. Neuromuscular fatigue and recovery in male and female athletes during heavy resistance exercise. Int J Sports Med. 1993;14:53–59. doi: 10.1055/s-2007-1021146. [DOI] [PubMed] [Google Scholar]
- 32.Valovich TC, Perrin DH, Gansneder BM. Repeat administration elicits a practice effect with the Balance Error Scoring System but not with the Standardized Assessment of Concussion in high school athletes. J Athl Train. 2003;38:51–56. [PMC free article] [PubMed] [Google Scholar]
- 33.Mancuso JJ, Guskiewicz KM, Onate JA, Ross SE. An investigation of the learning effect for the Balance Error Scoring System and its clinical implications [abstract] J Athl Train. 2002;37:S-10. [Google Scholar]
- 34.Adlerton AK, Moritz U. Does calf-muscle fatigue affect standing balance? Scand J Med Sci Sports. 1996;6:211–215. doi: 10.1111/j.1600-0838.1996.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 35.Marks R, Quinney HA. Effect of fatiguing maximal isokinetic quadriceps contractions on ability to estimate knee-position. Percept Mot Skills. 1993;77:1195–1202. doi: 10.2466/pms.1993.77.3f.1195. [DOI] [PubMed] [Google Scholar]
- 36.Guskiewicz KM, Perrin DH, Gansneder BM. Effect of mild head injury on postural stability in athletes. J Athl Train. 1996;31:300–306. [PMC free article] [PubMed] [Google Scholar]
- 37.Lehmann JF, Boswell S, Price R, et al. Quantitative evaluation of sway as an indicator of functional balance in post-traumatic brain injury. Arch Phys Med Rehabil. 1990;71:955–962. [PubMed] [Google Scholar]
- 38.Jeong BY. Respiration effect on standing balance. Arch Phys Med Rehabil. 1991;72:642–645. [PubMed] [Google Scholar]
- 39.Guskiewicz KM, Weaver NL, Padua DA, Garrett WE., Jr Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28:643–650. doi: 10.1177/03635465000280050401. [DOI] [PubMed] [Google Scholar]
- 40.Barr WB. Methodologic issues in neuropsychological testing. J Athl Train. 2001;36:297–302. [PMC free article] [PubMed] [Google Scholar]
- 41.McCrea M. Standardized mental status testing on the sideline after sport-related concussion. J Athl Train. 2001;36:274–279. [PMC free article] [PubMed] [Google Scholar]
- 42.Oliaro S, Anderson SJ, Hooker D. Management of cerebral concussion in sports: the athletic trainer's perspective. J Athl Train. 2001;36:257–262. [PMC free article] [PubMed] [Google Scholar]