Abstract
Evidence has accumulated that adult hematopoietic tissues and other organs contain a population of dormant stem cells (SCs) that are more primitive than other, already restricted, monopotent tissue-committed stem cells (TCSCs). These observations raise several questions, such as the developmental origin of these cells, their true pluripotent or multipotent nature, which surface markers they express, how they can be efficiently isolated from adult tissues, and what role they play in the adult organism. The phenotype of these cells and expression of some genes characteristic of embryonic SCs (ESCs), epiblast SCs (EPiSCs), and primordial germ cells (PGCs) suggests their early-embryonic deposition in developing tissues as precursors of adult SCs. In this review we will critically discuss all these questions and the concept that small dormant stem cells related to migratory PGCs, described as very small embryonic-like stem cells (VSELs), are deposited during embryogenesis in bone marrow and other organs as a backup population for adult tissue committed stem cells (TCSCs) and are involved in several processes related to tissue or organ rejuvenation, aging, and cancerogenesis. The most recent results on successful ex vivo expansion of human VSELs in chemically defined media free from feeder-layer cells opens up new and exciting possibilities for their application in regenerative medicine.
Keywords: VSELs, HSCs, Igf2, H19, RasGRF1, imprinted genes
Introduction
With the advent of better discovery tools, such as cell sorting and high-resolution microscopic imaging, it has become possible to identify and subsequently purify from adult tissues various populations of stem cells (SCs), which possess the ability to self-renew. However, SCs are quite heterogenous and follow a developmental hierarchy. Moreover, evidence has accumulated that in several organs, including bone marrow (BM), it may reside a dormant population of very rare, primitive, and quiescent SCs among more numerous tissue-committed stem cells (TCSCs). 1–20 It has been demonstrated that some of these SCs possess trans-germ layer differentiation potential.
It is widely accepted that SCs have a distinct morphology (i.e., small size and with a lymphocyte-like appearance), express a distinct panel of surface markers (i.e., CD133+, CD34+, CD44+, Lin−), show low accumulation of selected metabolic fluorochromes (e.g., Rhodamine 123, Pyronin Y, or Hoe3342), and display differences in activity of certain enzymes (e.g., aldehyde dehydrogenase [ALDH]). All of these traits are helpful in SC identification as well as in purification strategies 21–25.
An intriguing question has also been raised: Do already lineage-committed TCSCs residing in adult tissues show plasticity and trans-dedifferentiate into cells from other lineages? 26–29. This concept is based on the hypothesis that SCs already committed to a given tissue, for example, hematopoietic stem cells (HSCs), can trans-dedifferentiate and become SCs for different types of tissues—for example, cardiac or liver SCs. However, this hypothesis has been challenged, since it has been hard to reproduce some of the initially published (and perhaps optimistic) reports showing robust trans-dedifferentiation of one type of TCSC into SCs for other tissues 30, 31. Instead, several other alternative explanations have been proposed to explain the involvement of SCs in tissue repair, including i) cell fusion 32, ii) the involvement of SC paracrine effects by soluble factors and SC-derived extracellular microvesicles 33, 34, or iii) the presence of rare pluripotent or multipotent SCs in adult tissues that are developmental precursors for various types of TCSCs 35.
Examples of such published reports of SCs with broader pan-germ-layer differentiation potential include i) mesenchymal stem cells (MSCs) 14, 36, 37, ii) multipotent adult progenitor cells (MAPCs) 6, 38, iii) marrow-isolated adult multilineage inducible (MIAMI) cells 4, iv) multipotent adult stem cells (MASCs) 3, v) elutriation-derived (Fr25/Lin−) stem cells (ELH SCs) 39–41, vi) spore-like stem cells 19, vii) pluripotent Sca-1+CD45−c-kit− cells 20, viii) multilineage-differentiating, stress-enduring stem cells (Muse SCs) 13, 15, 42, and ix) very small embryonic-like stem cells (VSELs), described by our group 1, 43–45. All of these stem cell types were identified by employing different direct or indirect isolation protocols and identification techniques. The similarity in expression of certain early-development genes in these cells suggests that they are related to each other, that they may represent similar, overlapping populations of primitive SCs that reside in adult tissues, and that they are endowed with broader “cross-germ-layer” differentiation potential.
In this review we will focus on VSELs, as these cells (~4–5 μm in diameter as measured in murine bone marrow and ~5–6 μm in human bone marrow or umbilical cord blood) have been highly purified and well characterized at the molecular level 46–48. We propose that VSELs represent the most primitive population of quiescent SCs residing in adult tissues and resemble in some of their properties other primitive SCs described by other investigators (e.g., ELH SCs). The small size of these cells and the paucity of mitochondria are signs of their quiescence and low metabolic activity 49. These very rare cells are isolated from adult tissues (e.g., bone marrow [BM], umbilical cord blood [UCB], and mobilized peripheral blood [mPB]) by multiparameter cell sorting (Figure 1), and several groups that carefully followed the original protocol for their isolation (published by us in Current Protocols of Cytometry 50 or contacted us for help) successfully identified these small cells in postnatal tissues 51–54. BM-purified VSELs have been demonstrated by other investigators to be precursors of HSCs 55, 56, MSCs 57, 58, endothelial SCs 59, 60, lung alveolar epithelial cells 61, 62, and cardiomyocytes 63, 64. At the same time, VSELs isolated from gonads have been proposed to be precursors of male and female gametes 18, 65–67.
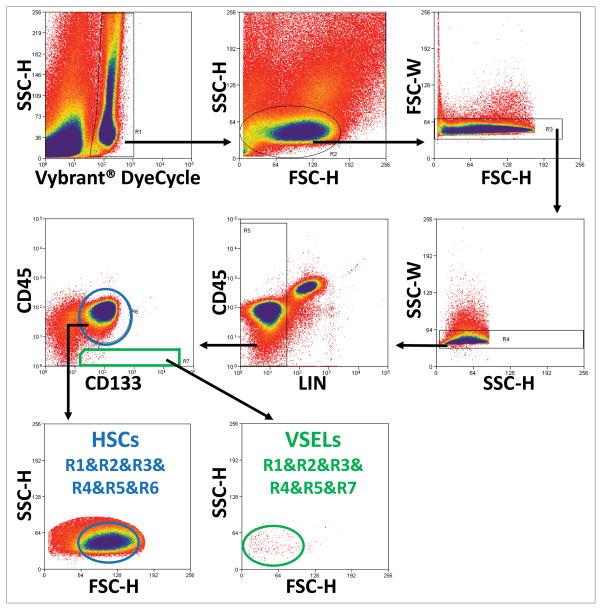
Figure 1. Identification and isolation of VSELs and HSCs from human umbilical cord blood by employing FACS sorter.
To develop a more efficient and less time consuming method for purifying VSELs from UCB, we developed a three-step isolation strategy based on (1) removing erythrocytes by hypotonic lysis, (2) immunomagnetic separation of CD133+ cells, and (3) FACS-based isolation of small CD133+Lin−CD45− cells. Region R1 shows events exhibiting DNA content. Nucleated cells included in R2 are visualized based on their FSC and SSC characteristics. R3 and R4 are employed to exclude doublets. Region R5 presents Lin− cells, which are analyzed on next dot-plot based on CD133 vs. CD45 expression and clasified as VSELs enclosed in R7 (CD133+Lin−CD45−), and HSCs enclosed in R6 (CD133+Lin−CD45+). Small and agranular VSELs from region R7 are presented on cytogram based on FSC vs. SSC characteristics (green ellipse), compared to bigger HSCs from region R6 (blue ellipse).
What is of particular interest, we have recently developed an efficient protocol to expand these highly quiescent cells isolated from human and murine hematopoietic tissues in chemically defined media, without support of third-party feeder-layer cells or transduction by vectors encoding pluripotency-promoting factors. This newest development opens up new and exciting possibilities for VSEL application in regenerative medicine.
The hierarchy of the stem cell compartment in embryonic and postnatal tissues—do we have a definitive model?
The most accepted, but unfortunately oversimplified, view of the BM stem cell compartment is based on the assumption that it consists of HSCs, MSCs, and endothelial progenitor cells (EPCs). However, this old and dogmatic view has been challenged by several reports that suggest the presence of other more primitive SCs in BM tissue 2–4, 6, 13, 14, 19, 20, 38, 42, 68. SCs possess the unique property of symmetric self-renewal or asymmetric division, and evidence indicates that they are not all equal from a hierarchical point of view. Some of them may be endowed with broader differentiation potential across germ layers 69–75. Thus, the hierarchy of the SC compartment needs to be revisited, as many of the early-development SCs may be left unaccounted for in a simplified hierarchy.
From a developmental point of view the most primitive SC are the fertilized oocyte (zygote) and the first blastomeres in the morula, as these cells are able to give rise to both embryo and placenta. Such SCs are called totipotent. By contrast, embryonic stem cells (ESCs) isolated from the inner cell mass (ICM) of the blastocyst differentiate into embryonic tissues only, losing the ability to form the placenta and are therefore called pluripotent stem cells (PSCs). After implantation of the blastocyst into the uterus, ICM-derived PSCs of the blastocyst form the epiblast, and epiblast stem cells (EpiSCs) subsequently give rise to all three germ layers (meso-, ecto-, and endoderm), including primordial germ cells (PGCs). Pluripotent EpiSCs gradually loss their pluripotency by giving rise to multipotent SCs, which are specific for one of the germ layers, and finally monopotent TCSCs, for example, SCs for epidermis, intestinal epithelium, liver, skeletal muscle, or lympho-hematopoiesis. TCSCs in adult organisms reside in stem cell niches (e.g., in the basal layer of epidermis and hair budge [epidermal SCs], the bottom of intestinal crypts [intestinal SCs], around Herring ducts in liver [oval SCs], around muscle fibers [satellite SCs], and in endosteal and vascular niches of the BM [HSCs]). In heart, SCs are believed to be located in the atrial appendages 76–81.
While considering the hierarchy of the SC compartment and their developmental specification, beginning with PSCs and ending with TCSCs, an important question emerges: Is the differentiation process of PSCs or multipotent PSCs during embryogenesis complete, or perhaps do some of these early-development SCs survive beyond embryogenesis into adulthood and remain as a “hibernated" or a “quiescent backup” population of TCSCs? This possibility is supported by the presence in adult BM, UCB, and mPB of VSELs and other rare SC populations that express embryonic, epiblast, and PGC markers that overlap with VSELs. Examples of such cells detected in adult tissues are listed in Table 1.
Table 1.
Examples of published reports by other groups of investigators on SCs in adult non-gonadal tissues that have germline potential and/or express embryonic stem cell markers (e.g., Oct-4, SSEA, or MvH).
| Cells name as originally described in the literature | References |
|---|---|
| Bone marrow and peripheral blood-derived oocytes. It was proposed that BM and peripheral blood could be a source of oocytes that can repopulate the gonads. | 72 |
| Stem cells with germline potential from newborn mouse skin. Oct-4+ cells isolated by FACS from Oct-4–GFP mice that were able to give rise in vitro and in vivo to early oocytes. | 69 |
| Porcine multipotent stem/stromal cells. Oct-3/4+, Nanog+, Sox-2+ cells isolated from porcine skin and adipose tissue that, like the cells from newborn mouse skin cited above, were able to differentiate into oocyte-like cells. | 70 |
| SSEA-1+ murine BM cells. Isolated from murine BM by anti-SSEA-1 immunomagnetic beads. In the presence of BMP4 (bone morphogenic factor 4), Oct-4+Stella+Mvh+ cells differentiated into gamete precursors. | 71 |
| BM-derived putative germ cells. Oct-4+Mvh+Dazl+Stella+ cells present in BM, which may affect recurrence of oogenesis in mice sterilized by chemotherapy. | 72, 73 |
| BM-derived male germ cells. Oct-4+, Mvh+, Stella+ cells isolated as Stra8–GFP cells from BM from Stra8–GFP transgenic mice. These cells expressed several molecular markers of spermatogonial stem cells and spermatogonia. | 74 |
| BM-derived precursors of male germ cells. GFP+ transgenic chicken Oct-4+SSEA-1/3/4+ BM cells after injection into chicken testes gave rise to functional sperm. | 75 |
One of the intriguing puzzles in mammalian development is the fact that PGCs are the first population of SCs specified before gastrulation in the proximal epiblast—a precursor of the entire embryo proper 82, 83. These Blimp+ cells, which emerge in the proximal epiblast close to the extraembryonic endoderm in response to bone morphogen protein 4 (BMP-4) signaling, migrate out of the embryo into extraembryonic tissue and then make a turn and reenter the embryo proper through the primitive streak at the beginning of gastrulation. On the way to the genital ridges, which is their final destination, the PGCs are amplified in number and, what is worthwhile to explore, certain cells closely related to PGCs (e.g., EpiSCs) may be deposited in adult tissues as a population of dormant SCs that are endowed with broad, cross-germ-layer differentiation potential 69–75, 84, 85.
What is also an intriguing hypothesis is that the developmental migration of PGCs from the epiblast over the extraembryonic tissues and back to the gonadal ridges may be related to the developmental origin of HSCs and EPCs. First, at the time when PGCs migrate through the extraembryonic tissues, the first hemangioblasts, which are precursors of primitive HSCs and EPCs, emerge at the bottom of the yolk sac 86. Later on, when the PGCs migrate across the embryo proper to the genital ridges and pass through the aorta-gonado-mesonephros region (AGM), the first definitive HSCs emerge in the aortic endothelium 87. This potential link between PGC migration—the emergence first of hemangioblasts and later on definitive HSCs—suggests that the developmental origin of VSELs is from migrating PGCs 2, 47, 88, 89.
In support of this concept, both PGCs and VSELs are reportedly able to give rise to HSCs and EPCs 55, 56, 90–92. Moreover, PGC-derived precursors of gametes, VSELs, and HSCs express several functional sex hormone receptors and the erythropoietin (EpO) receptor. Moreover, VSELs highly express certain hemangioblast markers (e.g., Flk1) and, as reported in an elegant study, they can be specified into endothelial cells in vitro and in vivo 47, 59, 88, 89, 93. This concept of the origin of definitive HSCs from migrating PGCs has been recently demonstrated in elegant work by the de Felici group 94 and discussed in an inspiring review by Virant-Klun 95. Nevertheless, as always with new scientific ideas, more experimental evidence is needed to support this novel and intriguing concept.
Evidence for the presence of very small SCs in adult tissues—a roadmap for discovery of very small embryonic-like SCs
SCs are characterized by a high nuclear/cytoplasmic ratio, and one may make the assumption that those that are more quiescent and dormant (e.g., VSELs) compared with those that are actively proliferating (e.g., HSCs or intestinal epithelium SCs) are much smaller and contain only sparse, round mitochondria as signs of low metabolic activity 1, 96, 97. In support of this claim, several types of small SCs were initially described in hematopoietic tissues and later confirmed to be present in other organs as well. The exact size of the small SCs may depend on the method of their measurement (microscopic template grids or size beads) and may also be affected by procedures such as fixation or mounting on slides. Generally, we consider small cells to be up to 5 μm in diameter for mice and slightly larger in humans. We will briefly discuss these cells, keeping in mind the tempting speculations that these SCs could be precursors for MAPCs, MIAMIs, MASCs, MSCs, or Muse SCs. It is likely that these particular SCs, which seem to be somewhat larger, have from the beginning been contaminated by populations of small SCs attached to them or internalized by a process of emperipolesis 98.
Below, we will briefly summarize examples of small SCs that have been isolated from murine, rat, and human tissues. These cells were identified primarily as candidates for quiescent HSCs. However, with time it became obvious that some of them are also endowed with broader differentiation potential across germ layers.
Small SCs with primary hematopoietic potential
Lin−/ALDHhigh long-term repopulating HSCs
The population of small, long-term repopulating HSCs, which were isolated by employing elutriation followed by FACS and selection for high activity of aldehyde dehydrogenase (ALDHhigh) and not hematopoietic lineage markers (which these cells lack), was described by Jones and Sharkis 25, 99. Although the exact size of these cells was not published in the original publication, a recent paper from this group described these SCs as smaller than 5 μm 61. These interesting cells, however, were not analyzed for the expression of pluripotent or multipotent SC markers. However, recent evidence suggests their broader non-hematopoietic differentiation potential (see ELH SCs below) 41, 62.
BM mononuclear cell-derived progenitor-like cells
We identified these small cells (~4–5 μm in diameter) in BM by employing the electron microscopy methods of Matsuoka et al. This population of small cells exhibited certain morphological features characteristic of hematopoietic progenitors 100. Similarly, small cells (~5 μm) were reported by Berardi as the most primitive HSCs in human BM 101.
Lin−/Rhdull/Hodull long-term repopulating HSCs
Radley et al. described a population of small (~4–5 μm), primitive HSCs isolated from murine BM that were capable of long-term hematopoietic reconstitution. These cells were characterized by a lack of hematopoietic lineage markers and an ability to exclude both Rhodamine 123 (Rhdull) and Hoechst 33342 (Hodull) dyes 102.
Small cells with multi-tissue differentiation potential
Elutriation-derived (fraction 25 [Fr25]/Lin−) stem cells (ELH SCs)
As mentioned above, these interesting SCs were initially described as Lin−/ALDHhigh long-term repopulating HSCs 25, 41, 99, 103. More careful analysis of these cells and modification of their isolation strategy by elutriation (E, Fr25), combined with their lineage negativity (L) and transplantation into lethally irradiated mice and recovery by FACS after short-term homing (H), demonstrated that they contribute to multiple epithelial tissues. Recent experiments from the Krause group 62 demonstrated that they are distinct from classical HSCs and may repopulate lung alveolar type II pneumocytes, producing surfactant after transplantation into surfactant-deficient mice. These particular SCs have been recently proposed to overlap with VSELs 61. Similar small Lin− SCs isolated by elutriation (Fr25) from murine BM were shown to contribute to regeneration of retinal epithelium and to differentiate into insulin-producing cells in mice 104, 105.
CD45−Sca-1+c-kit− cells with pluripotent characteristics
These cells have been isolated by employing FACS-based phenotypic analysis of single-cell suspensions from murine brain, blood, and intestinal epithelium by Srour and Yoder and proposed to represent universal PSCs residing in multiple murine tissues 20.
Spore-like SCs
The presence of small SCs (~5 μm) in adult tissues known as “spore-like” SCs was demonstrated by Vacanti et al. Unfortunately, the isolation strategy and the exact markers for these cells were not described in the original publication 19.
VSEL-like SCs in murine and human gonads
Very small SCs with pluripotent characteristics were described in the human and murine ovarian surface epithelium and identified in murine and human testes independently by the Virant-Klun and Bhartiya groups 18, 65–67, 106. These small cells express embryonic markers such as SSEA, Oct-4, Sall4, Nanog, and Sox-2 and are able to form embryoid body-like structures in vitro. It was proposed that these cells are the precursors of gametes.
VSEL-like SCs in rat BM
SSEA-1+Oct-4+ VSEL-like SCs were also purified from rat BM by the Yuzhen Tan group and successfully employed to regenerate damaged myocardium in an experimental model of acute myocardial infarction 107.
Small UCB-derived SCs
McGuckin et al. demonstrated the presence of very small SCs in UCB and estimated the size of these cells as 2–3 μm 17. As reported in the original publication, these Oct-4+, Sox2+ cells exhibit pluripotent characteristics and possess neural differentiation potential.
Small SCs remaining in the BM filtrate
Finally, while isolating MSCs on a double layer culture plate containing 3-μm pores to filter out the relatively large MSCs, Huang et al., isolated a population of very small SCs residing in human BM 108 that were able to migrate through the 3-μm pores.
All of these small SCs listed above, which were isolated from adult tissues, most likely represent overlapping cell populations. Although many of them were not well characterized at the molecular level, we envision that they are closely related to VSELs in the developmental hierarchy. This concept will be presented in more detail below.
The discovery of very small embryonic-like stem cells (VSELs) and their challenge to understanding the adult stem cell compartment
Evidence has accumulated for a very likely scenario in which some primitive epiblast/PGC-derived SCs “escape” specification into TCSCs and thus retain their pluripotent character and survive as VSELs into adulthood, forming a reserve pool of precursors for TCSCs. Thus, VSELs could play an important role in tissue rejuvenation and regeneration.
VSELs were initially purified by employing FACS-based multiparameter sorting of murine BM and several adult murine organs (e.g., brain, liver, skeletal muscle, heart, gonads, and kidney) 45, 49, 50. Murine BM-derived VSELs: i) are very rare (~0.01% of nucleated BM cells); ii) are small in size (~3–5 μm); iii) express several PSC markers, including Oct4, Nanog, Rex-1, and SSEA-1; i.v.) contain sparse, round mitochondria; and v) have large nuclei filled with unorganized chromatin (euchromatin). Importantly, to exclude expression of Oct-4 pseudogenes in these cells, we confirmed the true expression of Oct4 by demonstrating transcriptionally active hypomethylated DNA associated with acetylated histone chromatin in the Oct4 promoter 46, 47. The Oct-4 amplicon was also sequenced for accuracy.
Moreover, a corresponding population of small (~4–7 μm) CD133+Lin−CD45− SCs that display embryonic-like cell morphology have been purified from UCB and mPB 51, 57, 109, 110. Human VSELs, like murine VSELs, have large nuclei that contain unorganized euchromatin and a relatively small rim of cytoplasm with sparse, round mitochondria. These cells also express Oct4 and Nanog in their nuclei and display the SSEA-4 antigen on their surface 109.
Evidence indicates that VSELs are a population of migratory cells, and their number increases both in mice and in humans in PB during stress situations related to tissue or organ injuries (e.g., heart infarct, stroke, skin burns, or acute colitis) 64, 111–114. These cells, which are mobilized into PB where they then circulate, may play a physiologically important surveillance role in repairing minor tissue damage. The elevated number of VSELs observed in UCB may be explained as a physiological mechanism in which these cells are mobilized in newborns, which, due to hypoxia and delivery stress, experience numerous minor tissue injuries. Thus, the mobilization of VSELs into UCB is an inborn protective mechanism, which can be considered as the original physiological stem cell therapy, that everybody experiences in life after delivery. The number of VSELs circulating in PB also increases after administration of certain drugs that are employed on a routine basis in the clinic to mobilize HSCs into PB (e.g., G-CSF or AMD3100) 51, 115. Thus, VSELs could be harvested for potential clinical applications, like HSCs from mPB, by employing similar protocols for leucophoresis. The problem of low recovery of these cells from BM, UCB, and mPB is ameliorated by a recently established ex vivo expansion protocol for these cells (see below).
As recently reported, the number of VSELs in murine BM increases in vivo in response to regular physical activity and prolonged caloric restriction 116, 117. On the other hand, highly quiescent VSELs in murine BM may enter the cell cycle, as confirmed by bromodeoxyuridine (BrdU) accumulation in these cells after administration of sex hormones (SexHs, such as follicle stimulating factor [FSH] or luteinizing hormone [LH]) or erythropoietin (EpO) 88, 89. The responsiveness of VSELs to sex hormones supports a developmental relationship of these cells to PGC progeny and has been recently employed in our ex vivo-expansion protocol for these cells (see below).
Molecular characteristics of VSELs—evidence for their pluripotency or multipotency
According to their definition, PSCs should, at the molecular level, i) express acknowledged markers of pluripotency, ii) have bivalent domains at promoters for homeodomain-containing transcription factors, and iii) have two active X chromosomes in female PSCs. VSELs have been carefully characterized according to these criteria by employing several complementary techniques, including gene expression studies at the mRNA level, miRNA analysis, the creation of cDNA libraries from highly purified cells, DNA methylation studies, the analysis of histone methylation and acetylation, and direct immuno-staining 47, 48, 118. All of these studies revealed that VSELs express several markers characteristic of PSCs. However, at the same time our single-cell-sorted library results indicate that, despite similar morphology and expression of similar surface markers, these cells residing in adult BM are somewhat heterogeneous in the expression of certain lineage-specific genes 48. In support of their pluripotency, VSELs express several genes characteristic of PSCs (e.g., Oct-4, SSEA, Nanog, Sox-2, Klf4, and Rex-1). Moreover, adult murine BM-derived VSELs express genes characteristic of EpiSCs (Gbx2, Fgf5, and Nodal) and of blastocyst ICM-derived ESCs (Rex1, also known as Zfp42) 47. However, it was found that Gbx2, Fgf5, and Nodal transcripts are expressed at higher levels than Rex1 in VSELs, in contrast to the expression pattern in the established murine ESC cell line ESC-D3 47. This finding strongly suggests that VSELs are more differentiated than ICM-derived ESCs and share several markers with the more differentiated EpiSCs 46, 47.
Molecular analysis also revealed that VSELs are developmentally related to epiblast-derived PGCs, as they express transcripts for Stella, Prdm14, Fragilis, Blimp1, Nanos3, and Dnd1 47. The protein expression of PGC markers such as Stella, Blimp1, and Mvh in purified VSELs was subsequently confirmed by immunostaining. More importantly, chromatin immunoprecipitation (ChIP) results revealed that the Stella promoter in VSELs displays transcriptionally active histone modifications (acetylated histone 3 [H3Ac] and trimethylated lysine 4 of histone 3 [H3K4me3]) and was less enriched for transcriptionally repressive histone markers (dimethylated lysine 9 of histone 3 [H3K9me2] and trimethylated lysine 27 of histone 3 [H3K27me3]) 47. At the same time, VSELs also highly express transcripts for Dppa2, Dppa4, and Mvh, which are characteristic of late migratory PGCs; however, they do not express the Sycp3, Dazl, and LINE1 genes, which are markers of post-migratory PGCs 47. Finally, the partial DNA demethylation of repetitive DNA sequences (LINE1 and IAP) and promoters of Mvh and Sycp3 further supports a close relationship between VSELs and late-migratory PGCs 47. As mentioned above, a potential relationship between VSELs and PGCs is also somewhat supported by the expression of several pituitary and gonadal sex hormone receptors by these cells 88 as well as the presence of the receptor for erythropoietin 89.
In further support of murine VSEL pluripotency, these small cells also express bivalent domains at promoters for homeodomain-containing transcription factors (TFs), such as Sox21, Nkx2.2, Dlx1, Lbx1h, Hlxb9, Pax5, and HoxA3, which, as mentioned above, is one of the characteristics of PSCs 48. Bivalent domains represent the state of chromatin structure in which transcriptionally opposite histone codes physically coexist in the same promoter of homeodomain-containing TFs. While in undifferentiated PSCs bivalent domains prevent premature differentiation, during differentiation the transient repressive epigenetic marks in promoters of homeodomain-containing TFs become monovalent in order to activate or repress expression of the appropriate TFs. The presence of transcriptionally active histone codes, such as H3K4me3, physically coexisting with repressive histone codes, like H3K27me3, within bivalent domains was confirmed by employing the carrier-ChIP assay 48.
The phenomenon of X chromosome inactivation in female PSCs (e.g., ESCs isolated from the female blastocyst ICM) is the epigenetic process for transcriptional silencing of one of the two X chromosomes in female cells in order to compensate for gene dosage 119. It is well known that female-derived PSCs reactivate the X chromosome that is inactivated after fertilization, and, as a result, female PSCs display two equivalently activated X chromosomes 120. Reactivation of the silenced X chromosome in female PSCs is one of the important features of pluripotency, and our results also indicate that VSELs purified from female mice partially activate an X chromosome, which indicates that murine VSELs, like ESCs, undergo the process of X chromosome reactivation.
In vivo differentiation of VSELs reveals their pluripotent or multipotent character
It has been demonstrated in several elegant studies that purified VSELs differentiate into cells from different germ layers. Some of these exciting reports will be briefly discussed below. These reports demonstrate the pluripotent or multipotent character of these cells; however, in order to achieve robust differentiation and tissue contribution in vivo, a greater number of injected cells would be needed. Thus, our recent exciting results showing that VSELs can be expanded ex vivo will provide more of these cells for in vivo testing.
VSELs—at the top of the mesenchymal lineage hierarchy
In a very elegant study, Taichman et al. reported that VSELs isolated from GFP+ mice formed bone-like structures when implanted into SCID mice 58. To further confirm that this effect depends on VSELs that exhibit true MSC activity (bone formation), stromal cells were harvested from Col2.3ΔTK mice and implanted into SCID mice to generate thymidine kinase-sensitive ossicles. At 1.5 months after implantation, these ossicles were injected with 2000 GFP+ VSELs. At harvest, colocalization of GFP-expressing cells with antibodies to the osteoblast-specific marker Runx-2, the endothelial marker CD31, and the adipocyte marker PPARγ was observed. Based upon the ability of uncultured VSELs to (i) differentiate in vivo into multiple mesenchymal lineages and (ii) generate osseous tissues at low density, Taichman et al. proposed that VSELs fulfill many of the required characteristics of precursors for MSCs 58. Recently, a similar bone-forming potential of human VSELs has been demonstrated in vivo in an immunodeficient mouse model 57.
VSELs as a source of endothelial progenitors
In another elegant paper, Smadja et al. demonstrated that human VSELs are mobilized into PB in patients with critical limb ischemia, and in in vitro and in vivo assays human VSELs were able to differentiate into endothelial cells 59. Accordingly, VSEL-derived cells in vitro, like endothelial progenitor cells, released low levels of VEGF-A and a similar repertoire of inflammatory cytokines. More importantly, in an in vivo immunodeficient mouse model human VSELs triggered post-ischaemic revascularization, and in neo-vessels from plug sections human CD31+ cells were detected. The authors concluded that VSELs are a potential new source of cells that could be specified into endothelial lineages for therapeutic applications in humans 59. In support of these exciting results, we observed a high expression level of the Flk2 transcript in highly purified VSELs. This demonstrated potential of VSELs to differentiate into the endothelial lineage suggests their possible overlap with hemangioblast-like cells identified in adult BM 93.
VSELs as potential precursors of cardiomyocytes
VSELs are also promising cells for cardiac regeneration. As reported by Zuba-Surma et al. 63, 64, BM-derived VSELs freshly isolated from GFP+ mice and injected into the hearts of mice that had undergone ischemia/reperfusion injury improved global and regional left ventricular (LV) systolic function as assessed by echocardiography and attenuated myocyte hypertrophy in surviving tissue (histology and echocardiography) compared with vehicle-treated controls. What is most important, newly formed GFP+ cardiomyocytes and capillaries in infarcted myocardium were observed in these animals 63, 64. Nevertheless, because not many newly formed cardiomyocytes were observed, the major effect of injected VSELs was most likely due to paracrine actions of the transplanted VSELs.
In another interesting study, VSELs were successfully isolated from rat BM by the Yuzhen Tan group, which demonstrated that VSEL-derived embryoid-like bodies in soft agarose models supplemented with leukemia inhibitory factor and basic fibroblast growth factor can differentiate into cells from the three germ layers, giving rise to cardiomyocytes and endothelial cells 107. In further support of this finding, transplantation of these cells derived from male rats reduced the scar area and significantly improved cardiac function in a female rat myocardium infarction model. Moreover, analyzing cells for the donor-derived Y chromosome, the authors convincingly demonstrated the presence of male VSEL-derived cardiomyocytes and endothelial cells. The authors concluded that cells from VSEL-derived embryoid-like bodies may contribute to cardiomyogenesis and angiogenesis in vivo 107.
VSELs and their contribution to epithelial cells
As mentioned above, ELH SCs isolated from murine BM differentiated into several epithelial lineages after injection into mice 62. Since these cells share several characteristics with VSELs, Krause et al. tested whether VSELs purified from BM overlap with ELH SCs and compared the level of BM-derived epithelial cells after transplantation of i) VSELs, ii) hematopoietic stem/progenitor cells (HSPCs), and iii) other nonhematopoietic cells. It turned out that VSELs clearly had the highest rate of epithelial cell formation in the lung. Furthermore, in these elegant studies employing VSELs from donor mice expressing H2B–GFP under a type 2 pneumocyte-specific promoter, Krause et al. demonstrated that this engraftment occurs by differentiation of VSELs into type 2 pneumocytes, excluded the phenomenon of fusion, and concluded that ELH SCs and Oct4+ VSELs in the adult BM exhibit broad differentiation potential 61.
Hepatocyte differentiation of VSELs
In another recent study, Zou et al. reported that VSELs differentiate into hepatic colonies in the presence of hepatocyte growth factor and, if transplanted into mice with CCl4-induced liver injury, they significantly reduced serum ALT and AST levels 60. It was therefore concluded that VSELs play a role in the repair of liver injury.
VSELs and their role in gametogenesis
Finally, the potential role of VSELs in postnatal gametogenesis has been addressed by independent groups led by Viran-Klunt 18, 106 and Bhartiya 65–67. These investigators identified a population of very small cells in human and animal gonads that mimic VSELs. As they proposed, these cells could be employed as an alternative source of oocytes and sperm in patients with damaged gonads after high-dose chemotherapy. In support of this possibility, VSELs are localized in the ovary surface epithelium and in the basement membrane of seminiferous tubules in testis. In appropriate in vitro and in vivo experiments, these cells gave rise to oocyte-like structures 121. Moreover, they were also able to establish spermatogenesis in testis after a high dose of chemotherapy, followed by injection of MSCs or Sertoli cells 67. These exciting results open up new possibilities for reproductive medicine 18, 106.
In vivo pluripotency criteria, or why do VSELs not contribute to blastocyst development and not grow teratomas? Understanding the consequences of epigenetic modification of parentally imprinted genes
Accumulating evidence has unequivocally demonstrated that murine VSELs exhibit several features of PSCs. However, they do not fulfill two “gold standard” in vivo criteria of pluripotency that are seen in the cases of ESCs and inducible PSCs (iPSCs); namely, they i) do not complete blastocyst development and ii) do not form teratomas after transplantation into immunodeficient mice. These in vivo pluripotency criteria were proposed based on research with ESCs and iPSCs. However, they do not apply to PGCs, which, despite being SCs endowed with developmental totipotency, do not comply with this definition. To explain this difference in PGCs, it has been reported that they modify the methylation of certain crucial parentally imprinted genes 122, and this prevents them from proliferation, complementation of the blastocyst, and teratoma formation and, at the DNA level, explains their quiescent state. Taking into consideration the similarities in gene expression between PGCs and VSELs 47, we have proposed and subsequently demonstrated 123 that a similar phenomenon may occur in VSELs, and this could explain their quiescent state in postnatal tissues. However, there are some differences in these epigenetic changes between PGCs and VSELs, because VSELs, like PGCs, erase imprinting at certain crucial paternally imprinted genes, but PGCs, in contrast to VSELs, erase the imprinting of several maternally imprinted genes as well.
Overall, parental genomic imprinting is an epigenetic program that ensures the parent-of-origin-specific monoallelic transcription of parentally imprinted genes, which play a crucial role in embryogenesis, fetal growth, the totipotential state of the zygote, and the pluripotency of early-development stem cells 124. The expression of parentally imprinted genes from paternal or maternal chromosomes is regulated by DNA methylation at differentially methylated regions (DMRs), which are CpG-rich cis-elements within the gene locus 124. Evidence indicates that VSELs freshly isolated from murine BM erase the paternally methylated imprints (e.g., at Igf2–H19 and Rasgrf1 loci); however, at the same time they hypermethylate the maternally methylated imprints (e.g., at the locus encoding the Igf2 receptor [Igf2R] and at the Kcnq1-p57KIP2 and Peg1 loci).
As a result of these epigenetic changes in the methylation state of DMRs in paternally imprinted genes, VSELs highly express growth-repressive genes (H19, p57KIP2, and Igf2R) and at the same time downregulate growth-promoting genes (Igf2 and Rasgrf1). These epigenetic changes cause a growth-repressive state in VSELs and a quiescent state, mainly by attenuating proteins that are involved in insulin-like growth factor 1 and 2 (IGF-1 and IGF-2) and insulin signaling 123, 125, 126, including those encoded by the Igf2–H19 and Rasgrf1 loci. These erased loci are methylated during development in the gonads in PGC-derived gametes after the first meiotic division by de novo DNA methyltransferases (DNMTs). This step is required for normal development of the embryo after fertilization 127.
Based on these findings, it became obvious to us that, by methylation of erased DMRs at paternally imprinted loci by DNMTs, we would be able to reverse in vitro VSEL cultures more efficiently from the quiescent state 123, 128.
Epigenetic regulators stimulate remethylation of erased loci in paternally imprinted genes and facilitate ex vivo VSEL expansion
As mentioned above, we assumed that the critical step in reversing the quiescent state of VSELs would be remethylation of the erased loci in parentally imprinted genes. We found that treatment of VSELs by the histone deacetylase (HDAC) inhibitor valporic acid (VPA) remethylated the DMRs of parentally imprinted genes that were erased in VSELs. As a result, we were able to force the proliferation of VSELs and expand them ex vivo by ~103 fold in chemically defined media in cultures supplemented with VPA and a cocktail of two pituitary sex hormones, FSH and LH together with bone morphogen protein-4 (BMP-4), IGF-2 and kit ligand (KL). As mentioned above, FSH and LH had been effective in stimulation of murine VSELs in vivo 88. VSELs in these culture conditions began to proliferate, and after 1–2 months of expansion we can distinguish in our in vitro cultures many small cells (Figure 2). In our expansion many of small cells still express Oct-4 and some large cells express certain markers of the germ lineage (e.g., Blimp-1 and Stella) at the protein level. These observations can be explained by the fact that the FSH and LH present in expansion cultures maintained the germline potential of VSELs.
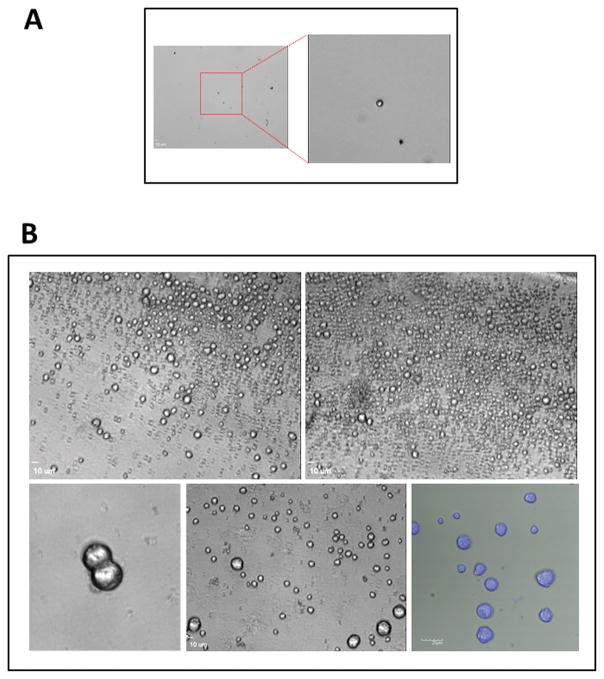
Figure 2. Example of expansion of human umbilical cord blood derived VSELs.
Panel A – Freshly sorted VSELs (5×102) were plated in 0.2 ml of DMEM + 10% FBS, supplemented with VPA and a cocktail of two pituitary sex hormones, FSH and LH together with BMP-4, IGF-2 and KL. Right inset shows enlarged image of freshly sorted VSEL. Cells were cultured for 2 months and half of culture medium has been changed every 7 days. Panel B – Upper panel - VSELs in these culture conditions began to proliferate, and after 2 months of expansion we can distinguish many small cells as well as some larger cells. Maximal expansion is achieved after 2–3 months of culture. Lower panel - cells aspirated from the cultures. Left and middle panel light microscope image. Right panel – Hoe3342 intravital staining of cells aspirated from the expansion.
This novel expansion system is somewhat supported by recent data on the expansion of HSCs from CD133+ or CD34+ cells purified from UCB using immunomagnetic beads 129, 130. These cells were effectively expanded in the presence of the HDAC inhibitors VPA 129, 131 or nicotinamide 132, 133. Since immunomagnetic beads isolate both large and small cells, we propose that efficient expansion of long-term repopulating HSCs (LT-HSCs) from paramagnetically purified CD133+ or CD34+ cells was the result of expansion of small CD133+ or CD34+ VSELs that “contaminated” the immunomagnetically purified cells.
Specifically, in the first paper in which Dr. Hoffman’s group employed VPA for the expansion of CD133+ cells, the authors observed an increase in the number of Oct-4+ cells, and this remarkable effect on the expansion of the most primitive LT-HSCs was reversed after Oct-4 had been downregulated by shRNA during expansion 129. Therefore, since Oct-4+ VSELs can be specified into LT-HSCs 55, 56, we envision that this effect was most likely due to the increased hematopoietic expansion of small CD133+ VSELs.
However, we have to keep in mind that, by employing HDAC inhibitors, not only are paternally methylated imprints released from an inhibitory complex with HDAC 134–136 but the addition of these inhibitors increases the acetylation of histones in several genes. However, consistent with the crucial involvement of HDAC inhibition on the normal expression of paternally imprinted genes, we observed a similar effect when one of the HDACs was downregulated in VSELs by employing an shRNA strategy (manuscript in preparation).
We are aware that this is a first step, and our expansion system is still open for optimization using different lineage-specific growth factors as well as other DNA modifiers—particularly HDAC inhibitors that are more potent and specific than VPA. However, what is very important, by developing this ex vivo-expansion protocol, we have demonstrated that VSELs can be “woken up” from their dormant/quiescent state and expanded ex vivo in cell cultures that are free of third-party feeder layer cells or vectors for overexpressing genes involved in cell pluripotency, as proposed by Yamanaka et al. 137–139.
Finally, our work on VSELs and imprinted genes has for the first time connected the role of caloric restriction, the beneficial effect of regular exercise, insulin and IGF-1/2 signaling, and metformin to the number of VSELs playing a role in tissue and organ rejuvenation. In particular, Sirt1 is an important HDAC that regulates all of these biological processes, and its high expression in VSELs keeps them in a quiescent state. Overall, our results suggest that imprinted genes are involved in longevity as guardians of the insulin/insulin-like growth factor-signaling quiescent state of VSELs. In support of this notion, we demonstrated that BM-residing VSELs can be prematurely depleted by enhanced insulin, IGF-1, or IGF-2 signaling, as seen for example after prolonged administration of IGF-1 or, in the case of transgenic mice that overexpress growth hormone (GH), a stimulator of the IGF-1 level in blood 140, 141.
It is worthwhile mentioning that, as reported by other groups, downregulation of Sirt1 in BM cells leads to premature depletion of HSCs 142, 143. Taking into consideration the possibility that VSELs play the role of precursors for LT-HSCs, these observations explain why HDAC inhibition in VSELs promotes their controlled specification into LT-HSCs. This phenomenon may play a role in VSEL specification into other types of TCSCs and explains at the molecular level the novel role of HDAC in extending longevity. In fact, Laron dwarf and Ames dwarf mice, which have an elevated number of VSELs in tissues, are long-living animals.
Conclusions
Regenerative medicine is still looking for a reliable source of PSCs that could be safely employed in the clinic. One of potential promising candidates are iPSCs, however, with advent of high-throughput technologies including next-generation sequencing evidence accumulated showing genomic instability of these cells.144 In addition, the presence of genetic variations in iPSCs has raised serious safety concerns, hampering the advancement of iPSC-based novel therapies and first clinical trials in humans have been stopped. That is why it is important to emphasize that the only stem cells so far that have been successfully employed in the clinic are those isolated from postnatal tissues. Moreover, several recent publications support the presence of pluripotent or multipotent VSELs in adult tissues. These small cells seem to be at the top of the stem cell hierarchy in adult tissues and most likely play a role in tissue and organ rejuvenation as a source of adult TCSCs. Their premature depletion in adult tissues is prevented by decreasing insulin, IGF-1, and IGF-2 signaling, caloric restriction, metformin supplementation, and regular physical activity. HDACs play a crucial role in preventing remethylation of erased paternally imprinted loci, and their inhibition reverses the quiescent state of these cells in adult tissues. By inhibiting HDAC activity with VPA, we were able to successfully expand human and murine VSELs ex vivo. This latest development opens up new and exciting possibilities for application of these intriguing cells in regenerative medicine and sheds new light on the mechanisms affecting aging.
Acknowledgments
Sources of Funding
This work was supported by NIH grants R01 DK074720, R01HL112788, the Stella and Henry Endowment and the Harmonia NCN grant UMO-2014/14/M/NZ3/00475 to MZR and by Basic Science Research Program through the National Research Foundation of Korea (NRF) (2015R1A2A1A15054754) to SDM.
Non-standard Abbreviations and Acronyms
- ELH SCs
elutriation-derived stem cells
- EPiSCs
epiblast stem cells
- ICM
inner cell mass
- MAPCs
multipotent adult progenitor cells
- MASCs
multipotent adult stem cells
- MIAMI
marrow-isolated adult multilineage inducible cells
- Muse SCs
multilineage-differentiating, stress-enduring stem cells
- PGCs
primordial germ cells
- SexHs
sex hormones
- TCSCs
tissue-committed stem cells
- UCB
umbilical cord blood
- VPA
valporic acid
- VSELs
very small embryonic-like stem cells
Footnotes
Conflicts of interest – None to report
Disclosures
University of Louisville owns IP on VSELs technology. Authors do not have any financial interest to disclose.
References
- 1.Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, Ratajczak MZ. A population of very small embryonic-like (vsel) cxcr4(+)ssea-1(+)oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 2.Ratajczak MZ, Machalinski B, Wojakowski W, Ratajczak J, Kucia M. A hypothesis for an embryonic origin of pluripotent oct-4(+) stem cells in adult bone marrow and other tissues. Leukemia. 2007;21:860–867. doi: 10.1038/sj.leu.2404630. [DOI] [PubMed] [Google Scholar]
- 3.Beltrami AP, Cesselli D, Bergamin N, Marcon P, Rigo S, Puppato E, D’Aurizio F, Verardo R, Piazza S, Pignatelli A, Poz A, Baccarani U, Damiani D, Fanin R, Mariuzzi L, Finato N, Masolini P, Burelli S, Belluzzi O, Schneider C, Beltrami CA. Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow) Blood. 2007;110:3438–3446. doi: 10.1182/blood-2006-11-055566. [DOI] [PubMed] [Google Scholar]
- 4.D’Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (miami) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. Journal of Cell Science. 2004;117:2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 5.Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N, Liedtke S, Sorg RV, Fischer J, Rosenbaum C, Greschat S, Knipper A, Bender J, Degistirici O, Gao J, Caplan AI, Colletti EJ, Almeida-Porada G, Muller HW, Zanjani E, Wernet P. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. The Journal of Experimental Medicine. 2004;200:123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 7.Ling TY, Kuo MD, Li CL, Yu AL, Huang YH, Wu TJ, Lin YC, Chen SH, Yu J. Identification of pulmonary oct-4+ stem/progenitor cells and demonstration of their susceptibility to sars coronavirus (sars-cov) infection in vitro. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9530–9535. doi: 10.1073/pnas.0510232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 9.Anjos-Afonso F, Bonnet D. Nonhematopoietic/endothelial ssea-1+ cells define the most primitive progenitors in the adult murine bone marrow mesenchymal compartment. Blood. 2007;109:1298–1306. doi: 10.1182/blood-2006-06-030551. [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Fang D, Kumar SM, Li L, Nguyen TK, Acs G, Herlyn M, Xu X. Isolation of a novel population of multipotent adult stem cells from human hair follicles. The American Journal of Pathology. 2006;168:1879–1888. doi: 10.2353/ajpath.2006.051170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 13.Wakao S, Kitada M, Kuroda Y, Shigemoto T, Matsuse D, Akashi H, Tanimura Y, Tsuchiyama K, Kikuchi T, Goda M, Nakahata T, Fujiyoshi Y, Dezawa M. Multilineage-differentiating stress-enduring (muse) cells are a primary source of induced pluripotent stem cells in human fibroblasts. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9875–9880. doi: 10.1073/pnas.1100816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 15.Kuroda Y, Wakao S, Kitada M, Murakami T, Nojima M, Dezawa M. Isolation, culture and evaluation of multilineage-differentiating stress-enduring (muse) cells. Nature Protocols. 2013;8:1391–1415. doi: 10.1038/nprot.2013.076. [DOI] [PubMed] [Google Scholar]
- 16.McGuckin CP, Forraz N, Baradez MO, Navran S, Zhao J, Urban R, Tilton R, Denner L. Production of stem cells with embryonic characteristics from human umbilical cord blood. Cell Proliferation. 2005;38:245–255. doi: 10.1111/j.1365-2184.2005.00346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGuckin C, Jurga M, Ali H, Strbad M, Forraz N. Culture of embryonic-like stem cells from human umbilical cord blood and onward differentiation to neural cells in vitro. Nature Protocols. 2008;3:1046–1055. doi: 10.1038/nprot.2008.69. [DOI] [PubMed] [Google Scholar]
- 18.Virant-Klun I, Zech N, Rozman P, Vogler A, Cvjeticanin B, Klemenc P, Malicev E, Meden-Vrtovec H. Putative stem cells with an embryonic character isolated from the ovarian surface epithelium of women with no naturally present follicles and oocytes. Differentiation. 2008;76:843–856. doi: 10.1111/j.1432-0436.2008.00268.x. [DOI] [PubMed] [Google Scholar]
- 19.Vacanti MP, Roy A, Cortiella J, Bonassar L, Vacanti CA. Identification and initial characterization of spore-like cells in adult mammals. Journal of Cellular Biochemistry. 2001;80:455–460. [PubMed] [Google Scholar]
- 20.Howell JC, Lee WH, Morrison P, Zhong J, Yoder MC, Srour EF. Pluripotent stem cells identified in multiple murine tissues. Annals of the New York Academy of Sciences. 2003;996:158–173. doi: 10.1111/j.1749-6632.2003.tb03244.x. [DOI] [PubMed] [Google Scholar]
- 21.Leemhuis T, Yoder MC, Grigsby S, Aguero B, Eder P, Srour EF. Isolation of primitive human bone marrow hematopoietic progenitor cells using hoechst 33342 and rhodamine 123. Experimental Hematology. 1996;24:1215–1224. [PubMed] [Google Scholar]
- 22.Dolle L, Boulter L, Leclercq IA, van Grunsven LA. Next generation of aldh substrates and their potential to study maturational lineage biology in stem and progenitor cells. American Journal of Physiology. 2015;308:G573–578. doi: 10.1152/ajpgi.00420.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reitsma MJ, Lee BR, Uchida N. Method for purification of human hematopoietic stem cells by flow cytometry. Methods in Molecular Medicine. 2002;63:59–77. doi: 10.1385/1-59259-140-X:059. [DOI] [PubMed] [Google Scholar]
- 24.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. The Journal of Experimental Medicine. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones RJ, Collector MI, Barber JP, Vala MS, Fackler MJ, May WS, Griffin CA, Hawkins AL, Zehnbauer BA, Hilton J, Colvin OM, Sharkis SJ. Characterization of mouse lymphohematopoietic stem cells lacking spleen colony-forming activity. Blood. 1996;88:487–491. [PubMed] [Google Scholar]
- 26.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: Cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 27.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 28.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nature Medicine. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 29.Di Campli C, Piscaglia AC, Pierelli L, Rutella S, Bonanno G, Alison MR, Mariotti A, Vecchio FM, Nestola M, Monego G, Michetti F, Mancuso S, Pola P, Leone G, Gasbarrini G, Gasbarrini A. A human umbilical cord stem cell rescue therapy in a murine model of toxic liver injury. Digestive and Liver Disease. 2004;36:603–613. doi: 10.1016/j.dld.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Castro RF, Jackson KA, Goodell MA, Robertson CS, Liu H, Shine HD. Failure of bone marrow cells to transdifferentiate into neural cells in vivo. Science. 2002;297:1299. doi: 10.1126/science.297.5585.1299. [DOI] [PubMed] [Google Scholar]
- 31.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 32.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 33.Ratajczak MZ, Kucia M, Jadczyk T, Greco NJ, Wojakowski W, Tendera M, Ratajczak J. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: Can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia. 2012;26:1166–1173. doi: 10.1038/leu.2011.389. [DOI] [PubMed] [Google Scholar]
- 34.Camussi G, Deregibus MC, Tetta C. Paracrine/endocrine mechanism of stem cells on kidney repair: Role of microvesicle-mediated transfer of genetic information. Current Opinion in Nephrology and Hypertension. 2010;19:7–12. doi: 10.1097/MNH.0b013e328332fb6f. [DOI] [PubMed] [Google Scholar]
- 35.Ratajczak MZ, Kucia M, Reca R, Majka M, Janowska-Wieczorek A, Ratajczak J. Stem cell plasticity revisited: Cxcr4-positive cells expressing mrna for early muscle, liver and neural cells ‘hide out’ in the bone marrow. Leukemia. 2004;18:29–40. doi: 10.1038/sj.leu.2403184. [DOI] [PubMed] [Google Scholar]
- 36.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (mscs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 37.Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Experimental Hematology. 2002;30:896–904. doi: 10.1016/s0301-472x(02)00869-x. [DOI] [PubMed] [Google Scholar]
- 39.Orlic D, Anderson S, Bodine DM. Biological properties of subpopulations of pluripotent hematopoietic stem cells enriched by elutriation and flow cytometry. Blood Cells. 1994;20:107–117. discussion 118–120. [PubMed] [Google Scholar]
- 40.Jones RJ, Wagner JE, Celano P, Zicha MS, Sharkis SJ. Separation of pluripotent haematopoietic stem cells from spleen colony-forming cells. Nature. 1990;347:188–189. doi: 10.1038/347188a0. [DOI] [PubMed] [Google Scholar]
- 41.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 42.Wakao S, Akashi H, Kushida Y, Dezawa M. Muse cells, newly found non-tumorigenic pluripotent stem cells, reside in human mesenchymal tissues. Pathology International. 2014;64:1–9. doi: 10.1111/pin.12129. [DOI] [PubMed] [Google Scholar]
- 43.Kucia M, Wysoczynski M, Ratajczak J, Ratajczak MZ. Identification of very small embryonic like (vsel) stem cells in bone marrow. Cell and Tissue Research. 2008;331:125–134. doi: 10.1007/s00441-007-0485-4. [DOI] [PubMed] [Google Scholar]
- 44.Zuba-Surma EK, Klich I, Greco N, Laughlin MJ, Ratajczak J, Ratajczak MZ. Optimization of isolation and further characterization of umbilical-cord-blood-derived very small embryonic/ epiblast-like stem cells (vsels) European Journal of Haematology. 2010;84:34–46. doi: 10.1111/j.1600-0609.2009.01352.x. [DOI] [PubMed] [Google Scholar]
- 45.Zuba-Surma EK, Kucia M, Wu W, Klich I, Lillard JW, Jr, Ratajczak J, Ratajczak MZ. Very small embryonic-like stem cells are present in adult murine organs: Imagestream-based morphological analysis and distribution studies. Cytometry. Part A. 2008;73A:1116–1127. doi: 10.1002/cyto.a.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin DM, Liu R, Klich I, Ratajczak J, Kucia M, Ratajczak MZ. Molecular characterization of isolated from murine adult tissues very small embryonic/epiblast like stem cells (vsels) Molecules and Cells. 2010;29:533–538. doi: 10.1007/s10059-010-0081-4. [DOI] [PubMed] [Google Scholar]
- 47.Shin DM, Liu R, Klich I, Wu W, Ratajczak J, Kucia M, Ratajczak MZ. Molecular signature of adult bone marrow-purified very small embryonic-like stem cells supports their developmental epiblast/germ line origin. Leukemia. 2010;24:1450–1461. doi: 10.1038/leu.2010.121. [DOI] [PubMed] [Google Scholar]
- 48.Shin DM, Liu R, Wu W, Waigel SJ, Zacharias W, Ratajczak MZ, Kucia M. Global gene expression analysis of very small embryonic-like stem cells reveals that the ezh2-dependent bivalent domain mechanism contributes to their pluripotent state. Stem Cells and Development. 2012;21:1639–1652. doi: 10.1089/scd.2011.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuba-Surma EK, Kucia M, Abdel-Latif A, Dawn B, Hall B, Singh R, Lillard JW, Jr, Ratajczak MZ. Morphological characterization of very small embryonic-like stem cells (vsels) by imagestream system analysis. Journal of Cellular and Molecular Medicine. 2008;12:292–303. doi: 10.1111/j.1582-4934.2007.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuba-Surma EK, Ratajczak MZ. Overview of very small embryonic-like stem cells (vsels) and methodology of their identification and isolation by flow cytometric methods. Current protocols in cytometry. 2010;Chapter 9(Unit 9):29. doi: 10.1002/0471142956.cy0929s51. [DOI] [PubMed] [Google Scholar]
- 51.Sovalat H, Scrofani M, Eidenschenk A, Pasquet S, Rimelen V, Henon P. Identification and isolation from either adult human bone marrow or g-csf-mobilized peripheral blood of cd34(+)/cd133(+)/cxcr4(+)/ lin(−)cd45(−) cells, featuring morphological, molecular, and phenotypic characteristics of very small embryonic-like (vsel) stem cells. Experimental Hematology. 2011;39:495–505. doi: 10.1016/j.exphem.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Halasa M, Baskiewicz-Masiuk M, Dabkowska E, Machalinski B. An efficient two-step method to purify very small embryonic-like (vsel) stem cells from umbilical cord blood (ucb) Folia Histochemica et Cytobiologica. 2008;46:239–243. doi: 10.2478/v10042-008-0036-1. [DOI] [PubMed] [Google Scholar]
- 53.Nakatsuka R, Iwaki R, Matsuoka Y, Sumide K, Kawamura H, Fujioka T, Sasaki Y, Uemura Y, Asano H, Kwon AH, Sonoda Y. Identification and characterization of lineage(−)cd45(−)sca-1(+) vsel phenotypic cells residing in adult mouse bone tissue. Stem Cells and Development. 2016;25:27–42. doi: 10.1089/scd.2015.0168. [DOI] [PubMed] [Google Scholar]
- 54.Chang YJ, Tien KE, Wen CH, Hsieh TB, Hwang SM. Recovery of cd45(−)/lin(−)/ssea-4(+) very small embryonic-like stem cells by cord blood bank standard operating procedures. Cytotherapy. 2014;16:560–565. doi: 10.1016/j.jcyt.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 55.Ratajczak J, Wysoczynski M, Zuba-Surma E, Wan W, Kucia M, Yoder MC, Ratajczak MZ. Adult murine bone marrow-derived very small embryonic-like stem cells differentiate into the hematopoietic lineage after coculture over op9 stromal cells. Experimental Hematology. 2011;39:225–237. doi: 10.1016/j.exphem.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ratajczak J, Zuba-Surma E, Klich I, Liu R, Wysoczynski M, Greco N, Kucia M, Laughlin MJ, Ratajczak MZ. Hematopoietic differentiation of umbilical cord blood-derived very small embryonic/epiblast-like stem cells. Leukemia. 2011;25:1278–1285. doi: 10.1038/leu.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Havens AM, Shiozawa Y, Jung Y, Sun H, Wang J, McGee S, Mishra A, Taichman LS, Danciu T, Jiang Y, Yavanian G, Leary E, Krebsbach PH, Rodgerson D, Taichman RS. Human very small embryonic-like cells generate skeletal structures, in vivo. Stem Cells and Development. 2013;22:622–630. doi: 10.1089/scd.2012.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Havens AM, Sun H, Shiozawa Y, Jung Y, Wang J, Mishra A, Jiang Y, O’Neill DW, Krebsbach PH, Rodgerson DO, Taichman RS. Human and murine very small embryonic-like cells represent multipotent tissue progenitors, in vitro and in vivo. Stem Cells and Development. 2014;23:689–701. doi: 10.1089/scd.2013.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guerin CL, Loyer X, Vilar J, Cras A, Mirault T, Gaussem P, Silvestre JS, Smadja DM. Bone-marrow-derived very small embryonic-like stem cells in patients with critical leg ischaemia: Evidence of vasculogenic potential. Thrombosis and Haemostasis. 2015;113:1084–1094. doi: 10.1160/TH14-09-0748. [DOI] [PubMed] [Google Scholar]
- 60.Chen ZH, Lv X, Dai H, Liu C, Lou D, Chen R, Zou GM. Hepatic regenerative potential of mouse bone marrow very small embryonic-like stem cells. Journal of Cellular Physiology. 2015;230:1852–1861. doi: 10.1002/jcp.24913. [DOI] [PubMed] [Google Scholar]
- 61.Kassmer SH, Jin H, Zhang PX, Bruscia EM, Heydari K, Lee JH, Kim CF, Krouse D. Very small embryonic-like stem cells from the murine bone marrow differentiate into epithelial cells of the lung. Stem Cells. 2013;31:2759–2766. doi: 10.1002/stem.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kassmer SH, Bruscia EM, Zhang PX, Krause DS. Nonhematopoietic cells are the primary source of bone marrow-derived lung epithelial cells. Stem Cells. 2012;30:491–499. doi: 10.1002/stem.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dawn B, Tiwari S, Kucia MJ, Zuba-Surma EK, Guo Y, Sanganalmath SK, Abdel-Latif A, Hunt G, Vincent RJ, Taher H, Reed NJ, Ratajczak MZ, Bolli R. Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells. 2008;26:1646–1655. doi: 10.1634/stemcells.2007-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zuba-Surma EK, Guo Y, Taher H, Sanganalmath SK, Hunt G, Vincent RJ, Kucia M, Abdel-Latif A, Tang XL, Ratajczak MZ, Dawn B, Bolli R. Transplantation of expanded bone marrow-derived very small embryonic-like stem cells (vsel-scs) improves left ventricular function and remodelling after myocardial infarction. Journal of Cellular and Molecular Medicine. 2011;15:1319–1328. doi: 10.1111/j.1582-4934.2010.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parte S, Bhartiya D, Telang J, Daithankar V, Salvi V, Zaveri K, Hinduja I. Detection, characterization, and spontaneous differentiation in vitro of very small embryonic-like putative stem cells in adult mammalian ovary. Stem Cells and Development. 2011;20:1451–1464. doi: 10.1089/scd.2010.0461. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Bhartiya D, Kasiviswananthan S, Shaikh A. Cellular origin of testis-derived pluripotent stem cells: A case for very small embryonic-like stem cells. Stem Cells and Development. 2012;21:670–674. doi: 10.1089/scd.2011.0554. [DOI] [PubMed] [Google Scholar]
- 67.Anand S, Bhartiya D, Sriraman K, Patel H, Manjramkar D, Bakshi G, Dhamankar V, Kurkure P. Quiescent very small embryonic-like stem cells resist oncotherapy and can restore spermatogenesis in germ cell depleted mammalian testis. Stem Cells and Development. 2013 doi: 10.1089/scd.2013.0059. [DOI] [PubMed] [Google Scholar]
- 68.Ratajczak MZ, Zuba-Surma E, Kucia M, Poniewierska A, Suszynska M, Ratajczak J. Pluripotent and multipotent stem cells in adult tissues. Advances in Medical Sciences. 2012;57:1–17. doi: 10.2478/v10039-012-0020-z. [DOI] [PubMed] [Google Scholar]
- 69.Dyce PW, Liu J, Tayade C, Kidder GM, Betts DH, Li J. In vitro and in vivo germ line potential of stem cells derived from newborn mouse skin. PloS One. 2011;6:e20339. doi: 10.1371/journal.pone.0020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song SH, Kumar BM, Kang EJ, Lee YM, Kim TH, Ock SA, Lee SL, Jeon BG, Rho GJ. Characterization of porcine multipotent stem/stromal cells derived from skin, adipose, and ovarian tissues and their differentiation in vitro into putative oocyte-like cells. Stem Cells and Development. 2011;20:1359–1370. doi: 10.1089/scd.2010.0203. [DOI] [PubMed] [Google Scholar]
- 71.Shirazi R, Zarnani AH, Soleimani M, Abdolvahabi MA, Nayernia K, Ragerdi Kashani I. Bmp4 can generate primordial germ cells from bone-marrow-derived pluripotent stem cells. Cell Biology International. 2012;36:1185–1193. doi: 10.1042/CBI20110651. [DOI] [PubMed] [Google Scholar]
- 72.Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, Tschudy KS, Tilly JC, Cortes ML, Forkert R, Spitzer T, Iacomini J, Scadden DT, Tilly JL. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–315. doi: 10.1016/j.cell.2005.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Selesniemi K, Lee HJ, Niikura T, Tilly JL. Young adult donor bone marrow infusions into female mice postpone age-related reproductive failure and improve offspring survival. Aging. 2009;1:49–57. doi: 10.18632/aging.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nayernia K, Lee JH, Drusenheimer N, Nolte J, Wulf G, Dressel R, Gromoll J, Engel W. Derivation of male germ cells from bone marrow stem cells. Laboratory Investigation. 2006;86:654–663. doi: 10.1038/labinvest.3700429. [DOI] [PubMed] [Google Scholar]
- 75.Heo YT, Lee SH, Yang JH, Kim T, Lee HT. Bone marrow cell-mediated production of transgenic chickens. Laboratory Investigation. 2011;91:1229–1240. doi: 10.1038/labinvest.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nature Medicine. 2014;20:847–856. doi: 10.1038/nm.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shaker A, Rubin DC. Intestinal stem cells and epithelial-mesenchymal interactions in the crypt and stem cell niche. Translational research : the journal of laboratory and clinical medicine. 2010;156:180–187. doi: 10.1016/j.trsl.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yovchev MI, Dabeva MD, Oertel M. Isolation, characterization, and transplantation of adult liver progenitor cells. Methods Mol Biol. 2013;976:37–51. doi: 10.1007/978-1-62703-317-6_4. [DOI] [PubMed] [Google Scholar]
- 79.Vogler TO, Gadek KE, Cadwallader AB, Elston TL, Olwin BB. Isolation, culture, functional assays, and immunofluorescence of myofiber-associated satellite cells. Methods Mol Biol. 2016;1460:141–162. doi: 10.1007/978-1-4939-3810-0_11. [DOI] [PubMed] [Google Scholar]
- 80.Oh IH, Kwon KR. Concise review: Multiple niches for hematopoietic stem cell regulations. Stem Cells. 2010;28:1243–1249. doi: 10.1002/stem.453. [DOI] [PubMed] [Google Scholar]
- 81.Koninckx R, Daniels A, Windmolders S, Mees U, Macianskiene R, Mubagwa K, Steels P, Jamaer L, Dubois J, Robic B, Hendrikx M, Rummens JL, Hensen K. The cardiac atrial appendage stem cell: A new and promising candidate for myocardial repair. CardiovascularResearch. 2013;97:413–423. doi: 10.1093/cvr/cvs427. [DOI] [PubMed] [Google Scholar]
- 82.McLaren A. Primordial germ cells in the mouse. Developmental biology. 2003;262:1–15. doi: 10.1016/s0012-1606(03)00214-8. [DOI] [PubMed] [Google Scholar]
- 83.McLaren A. Development of primordial germ cells in the mouse. Andrologia. 1992;24:243–247. doi: 10.1111/j.1439-0272.1992.tb02647.x. [DOI] [PubMed] [Google Scholar]
- 84.Molyneaux K, Wylie C. Primordial germ cell migration. The International Journal of Developmental Biology. 2004;48:537–544. doi: 10.1387/ijdb.041833km. [DOI] [PubMed] [Google Scholar]
- 85.Hayashi K, de Sousa Lopes SM, Surani MA. Germ cell specification in mice. Science. 2007;316:394–396. doi: 10.1126/science.1137545. [DOI] [PubMed] [Google Scholar]
- 86.Mikkola HK, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006;133:3733–3744. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- 87.De Miguel MP, Arnalich Montiel F, Lopez Iglesias P, Blazquez Martinez A, Nistal M. Epiblast-derived stem cells in embryonic and adult tissues. The International Journal of Developmental Biology. 2009;53:1529–1540. doi: 10.1387/ijdb.072413md. [DOI] [PubMed] [Google Scholar]
- 88.Mierzejewska K, Borkowska S, Suszynska E, Suszynska M, Poniewierska-Baran A, Maj M, Pedziwiatr D, Adamiak M, Abdel-Latif A, Kakar SS, Ratajczak J, Kucia M, Ratajczak MZ. Hematopoietic stem/progenitor cells express several functional sex hormone receptors-novel evidence for a potential developmental link between hematopoiesis and primordial germ cells. Stem Cells and Development. 2015;24:927–937. doi: 10.1089/scd.2014.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Suszynska M, Poniewierska-Baran A, Gunjal P, Ratajczak J, Marycz K, Kakar SS, Kucia M, Ratajczak MZ. Expression of the erythropoietin receptor by germline-derived cells - further support for a potential developmental link between the germline and hematopoiesis. Journal of Ovarian Research. 2014;7:66. doi: 10.1186/1757-2215-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kritzenberger M, Wrobel KH. Histochemical in situ identification of bovine embryonic blood cells reveals differences to the adult haematopoietic system and suggests a close relationship between haematopoietic stem cells and primordial germ cells. Histochemistry and Cell Biology. 2004;121:273–289. doi: 10.1007/s00418-004-0629-5. [DOI] [PubMed] [Google Scholar]
- 91.Ohtaka T, Matsui Y, Obinata M. Hematopoietic development of primordial germ cell-derived mouse embryonic germ cells in culture. Biochemical and Biophysical Research Communications. 1999;260:475–482. doi: 10.1006/bbrc.1999.0691. [DOI] [PubMed] [Google Scholar]
- 92.Rich IN. Primordial germ cells are capable of producing cells of the hematopoietic system in vitro. Blood. 1995;86:463–472. [PubMed] [Google Scholar]
- 93.Ratajczak MZ. A novel view of the adult bone marrow stem cell hierarchy and stem cell trafficking. Leukemia. 2015;29:776–782. doi: 10.1038/leu.2014.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scaldaferri ML, Klinger FG, Farini D, Di Carlo A, Carsetti R, Giorda E, De Felici M. Hematopoietic activity in putative mouse primordial germ cell populations. Mechanisms of Development. 2015;136:53–63. doi: 10.1016/j.mod.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 95.Virant-Klun I. Very small embryonic-like stem cells: A potential developmental link between germinal lineage and hematopoiesis in humans. Stem Cells and Development. 2016;25:101–113. doi: 10.1089/scd.2015.0275. [DOI] [PubMed] [Google Scholar]
- 96.Folmes CD, Dzeja PP, Nelson TJ, Terzic A. Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell. 2012;11:596–606. doi: 10.1016/j.stem.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nesti C, Pasquali L, Vaglini F, Siciliano G, Murri L. The role of mitochondria in stem cell biology. Bioscience Reports. 2007;27:165–171. doi: 10.1007/s10540-007-9044-1. [DOI] [PubMed] [Google Scholar]
- 98.Kucia M, Reca R, Jala VR, Dawn B, Ratajczak J, Ratajczak MZ. Bone marrow as a home of heterogenous populations of nonhematopoietic stem cells. Leukemia. 2005;19:1118–1127. doi: 10.1038/sj.leu.2403796. [DOI] [PubMed] [Google Scholar]
- 99.Sharkis SJ, Collector MI, Barber JP, Vala MS, Jones RJ. Phenotypic and functional characterization of the hematopoietic stem cell. Stem Cells. 1997;15(Suppl 1):41–44. doi: 10.1002/stem.5530150807. discussion 44–45. [DOI] [PubMed] [Google Scholar]
- 100.Matsuoka T, Tavassoli M. Electron microscopic identification of hemopoietic progenitor cells by exploiting their sugar-recognizing receptors using a newly developed minibead technique. Experimental Hematology. 1989;17:326–329. [PubMed] [Google Scholar]
- 101.Berardi AC, Wang A, Levine JD, Lopez P, Scadden DT. Functional isolation and characterization of human hematopoietic stem cells. Science. 1995;267:104–108. doi: 10.1126/science.7528940. [DOI] [PubMed] [Google Scholar]
- 102.Radley JM, Ellis S, Palatsides M, Williams B, Bertoncello I. Ultrastructure of primitive hematopoietic stem cells isolated using probes of functional status. Experimental Hematology. 1999;27:365–369. doi: 10.1016/s0301-472x(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 103.Lanzkron SM, Collector MI, Sharkis SJ. Hematopoietic stem cell tracking in vivo: A comparison of short-term and long-term repopulating cells. Blood. 1999;93:1916–1921. [PubMed] [Google Scholar]
- 104.Goldenberg-Cohen N, Avraham-Lubin BC, Sadikov T, Goldstein RS, Askenasy N. Primitive stem cells derived from bone marrow express glial and neuronal markers and support revascularization in injured retina exposed to ischemic and mechanical damage. Stem Cells and Development. 2012;21:1488–1500. doi: 10.1089/scd.2011.0366. [DOI] [PubMed] [Google Scholar]
- 105.Iskovich S, Goldenberg-Cohen N, Stein J, Yaniv I, Fabian I, Askenasy N. Elutriated stem cells derived from the adult bone marrow differentiate into insulin-producing cells in vivo and reverse chemical diabetes. Stem Cells and Development. 2012;21:86–96. doi: 10.1089/scd.2011.0057. [DOI] [PubMed] [Google Scholar]
- 106.Virant-Klun I, Rozman P, Cvjeticanin B, Vrtacnik-Bokal E, Novakovic S, Rulicke T, Dovc P, Meden-Vrtovec H. Parthenogenetic embryo-like structures in the human ovarian surface epithelium cell culture in postmenopausal women with no naturally present follicles and oocytes. Stem Cells and Development. 2009;18:137–149. doi: 10.1089/scd.2007.0238. [DOI] [PubMed] [Google Scholar]
- 107.Wu JH, Wang HJ, Tan YZ, Li ZH. Characterization of rat very small embryonic-like stem cells and cardiac repair after cell transplantation for myocardial infarction. Stem Cells and Development. 2012;21:1367–1379. doi: 10.1089/scd.2011.0280. [DOI] [PubMed] [Google Scholar]
- 108.Hung SC, Chen NJ, Hsieh SL, Li H, Ma HL, Lo WH. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002;20:249–258. doi: 10.1634/stemcells.20-3-249. [DOI] [PubMed] [Google Scholar]
- 109.Kucia M, Halasa M, Wysoczynski M, Baskiewicz-Masiuk M, Moldenhawer S, Zuba-Surma E, Czajka R, Wojakowski W, Machalinski B, Ratajczak MZ. Morphological and molecular characterization of novel population of cxcr4+ ssea-4+ oct-4+ very small embryonic-like cells purified from human cord blood: Preliminary report. Leukemia. 2007;21:297–303. doi: 10.1038/sj.leu.2404470. [DOI] [PubMed] [Google Scholar]
- 110.Shin DM, Suszynska M, Mierzejewska K, Ratajczak J, Ratajczak MZ. Very small embryonic-like stem-cell optimization of isolation protocols: An update of molecular signatures and a review of current in vivo applications. Experimental & Molecular Medicine. 2013;45:e56. doi: 10.1038/emm.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ratajczak MZ, Liu R, Marlicz W, Blogowski W, Starzynska T, Wojakowski W, Zuba-Surma E. Identification of very small embryonic/epiblast-like stem cells (vsels) circulating in peripheral blood during organ/tissue injuries. Methods in Cell Biology. 2011;103:31–54. doi: 10.1016/B978-0-12-385493-3.00003-6. [DOI] [PubMed] [Google Scholar]
- 112.Wojakowski W, Tendera M, Kucia M, Zuba-Surma E, Paczkowska E, Ciosek J, Halasa M, Krol M, Kazmierski M, Buszman P, Ochala A, Ratajczak J, Machalinski B, Ratajczak MZ. Mobilization of bone marrow-derived oct-4+ ssea-4+ very small embryonic-like stem cells in patients with acute myocardial infarction. Journal of the American College of Cardiology. 2009;53:1–9. doi: 10.1016/j.jacc.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marlicz W, Zuba-Surma E, Kucia M, Blogowski W, Starzynska T, Ratajczak MZ. Various types of stem cells, including a population of very small embryonic-like stem cells, are mobilized into peripheral blood in patients with crohn’s disease. Inflammatory Bowel Diseases. 2012;18:1711–1722. doi: 10.1002/ibd.22875. [DOI] [PubMed] [Google Scholar]
- 114.Drukala J, Paczkowska E, Kucia M, Mlynska E, Krajewski A, Machalinski B, Madeja Z, Ratajczak MZ. Stem cells, including a population of very small embryonic-like stem cells, are mobilized into peripheral blood in patients after skin burn injury. Stem Cell Reviews. 2012;8:184–194. doi: 10.1007/s12015-011-9272-4. [DOI] [PubMed] [Google Scholar]
- 115.Kucia MJ, Wysoczynski M, Wu W, Zuba-Surma EK, Ratajczak J, Ratajczak MZ. Evidence that very small embryonic-like stem cells are mobilized into peripheral blood. Stem Cells. 2008;26:2083–2092. doi: 10.1634/stemcells.2007-0922. [DOI] [PubMed] [Google Scholar]
- 116.Grymula K, Piotrowska K, Sluczanowska-Glabowska S, Mierzejewska K, Tarnowski M, Tkacz M, Poniewierska-Baran A, Pedziwiatr D, Suszynska E, Laszczynska M, Ratajczak MZ. Positive effects of prolonged caloric restriction on the population of very small embryonic-like stem cells - hematopoietic and ovarian implications. Journal of Ovarian Research. 2014;7:68. doi: 10.1186/1757-2215-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marycz K, Mierzejewska K, Smieszek A, Suszynska E, Malicka I, Kucia M, Ratajczak MZ. Endurance exercise mobilizes developmentally early stem cells into peripheral blood and increases their number in bone marrow: Implications for tissue regeneration. Stem Cells International. 2016;2016:5756901. doi: 10.1155/2016/5756901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mierzejewska K, Heo J, Kang JW, Kang H, Ratajczak J, Ratajczak MZ, Kucia M, Shin DM. Genome-wide analysis of murine bone marrowderived very small embryonic-like stem cells reveals that mitogenic growth factor signaling pathways play a crucial role in the quiescence and ageing of these cells. International Journal of Molecular Medicine. 2013;32:281–290. doi: 10.3892/ijmm.2013.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Surani MA, Hayashi K, Hajkova P. Genetic and epigenetic regulators of pluripotency. Cell. 2007;128:747–762. doi: 10.1016/j.cell.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 120.Maatouk DM, Kellam LD, Mann MR, Lei H, Li E, Bartolomei MS, Resnick JL. DNA methylation is a primary mechanism for silencing postmigratory primordial germ cell genes in both germ cell and somatic cell lineages. Development. 2006;133:3411–3418. doi: 10.1242/dev.02500. [DOI] [PubMed] [Google Scholar]
- 121.Sriraman K, Bhartiya D, Anand S, Bhutda S. Mouse ovarian very small embryonic-like stem cells resist chemotherapy and retain ability to initiate oocyte-specific differentiation. Reprod Sci. 2015;22:884–903. doi: 10.1177/1933719115576727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Surani MA, Durcova-Hills G, Hajkova P, Hayashi K, Tee WW. Germ line, stem cells, and epigenetic reprogramming. Cold Spring Harbor Symposia on Quantitative Biology. 2008;73:9–15. doi: 10.1101/sqb.2008.73.015. [DOI] [PubMed] [Google Scholar]
- 123.Shin DM, Zuba-Surma EK, Wu W, Ratajczak J, Wysoczynski M, Ratajczak MZ, Kucia M. Novel epigenetic mechanisms that control pluripotency and quiescence of adult bone marrow-derived oct4(+) very small embryonic-like stem cells. Leukemia. 2009;23:2042–2051. doi: 10.1038/leu.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bartolomei MS, Ferguson-Smith AC. Mammalian genomic imprinting. Cold Spring Harbor Perspectives in Biology. 2011:3. doi: 10.1101/cshperspect.a002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ratajczak MZ, Kucia M, Liu R, Shin DM, Bryndza E, Masternak MM, Tarnowski M, Ratajczak J, Bartke A. Rasgrf1: Genomic imprinting, vsels, and aging. Aging. 2011;3:692–697. doi: 10.18632/aging.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ratajczak MZ, Shin DM, Schneider G, Ratajczak J, Kucia M. Parental imprinting regulates insulin-like growth factor signaling: A rosetta stone for understanding the biology of pluripotent stem cells, aging and cancerogenesis. Leukemia. 2013;27:773–779. doi: 10.1038/leu.2012.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hajkova P, Erhardt S, Lane N, Haaf T, El-Maarri O, Reik W, Walter J, Surani MA. Epigenetic reprogramming in mouse primordial germ cells. Mechanisms of Development. 2002;117:15–23. doi: 10.1016/s0925-4773(02)00181-8. [DOI] [PubMed] [Google Scholar]
- 128.Maj M, Schneider G, Ratajczak J, Suszynska M, Kucia M, Ratajczak MZ. The cell cycle- and insulin-signaling-inhibiting mirna expression pattern of very small embryonic-like stem cells contributes to their quiescent state. Exp Biol Med (Maywood) 2015;240:1107–1111. doi: 10.1177/1535370215584940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chaurasia P, Gajzer DC, Schaniel C, D’Souza S, Hoffman R. Epigenetic reprogramming induces the expansion of cord blood stem cells. The Journal of Clinical Investigation. 2014;124:2378–2395. doi: 10.1172/JCI70313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Broxmeyer HE. Inhibiting hdac for human hematopoietic stem cell expansion. The Journal of Clinical Investigation. 2014;124:2365–2368. doi: 10.1172/JCI75803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chaurasia P, Berenzon D, Hoffman R. Chromatin-modifying agents promote the ex vivo production of functional human erythroid progenitor cells. Blood. 2011;117:4632–4641. doi: 10.1182/blood-2010-10-314567. [DOI] [PubMed] [Google Scholar]
- 132.Peled T, Shoham H, Aschengrau D, Yackoubov D, Frei G, Rosenheimer GN, Lerrer B, Cohen HY, Nagler A, Fibach E, Peled A. Nicotinamide, a sirt1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Experimental Hematology. 2012;40:342–355. e341. doi: 10.1016/j.exphem.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 133.Peled T, Adi S, Peleg I, Rosenheimer NG, Daniely Y, Nagler A, Fibach E, Peled A. Nicotinamide modulates ex-vivo expansion of cord blood derived cd34+cells cultured with cytokines and promotes their homing and engraftment in scid mice. Blood. 2006;108:218a–218a. [Google Scholar]
- 134.New M, Olzscha H, La Thangue NB. Hdac inhibitor-based therapies: Can we interpret the code? Molecular Oncology. 2012;6:637–656. doi: 10.1016/j.molonc.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tang J, Yan H, Zhuang S. Histone deacetylases as targets for treatment of multiple diseases. Clin Sci (Lond) 2013;124:651–662. doi: 10.1042/CS20120504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chueh AC, Tse JW, Togel L, Mariadason JM. Mechanisms of histone deacetylase inhibitor-regulated gene expression in cancer cells. Antioxidants & Redox Signaling. 2015;23:66–84. doi: 10.1089/ars.2014.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 138.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 139.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 140.Kucia M, Masternak M, Liu R, Shin DM, Ratajczak J, Mierzejewska K, Spong A, Kopchick JJ, Bartke A, Ratajczak MZ. The negative effect of prolonged somatotrophic/insulin signaling on an adult bone marrow-residing population of pluripotent very small embryonic-like stem cells (vsels) Age (Dordr) 2013;35:315–330. doi: 10.1007/s11357-011-9364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kucia M, Shin DM, Liu R, Ratajczak J, Bryndza E, Masternak MM, Bartke A, Ratajczak MZ. Reduced number of vsels in the bone marrow of growth hormone transgenic mice indicates that chronically elevated igf1 level accelerates age-dependent exhaustion of pluripotent stem cell pool: A novel view on aging. Leukemia. 2011;25:1370–1374. doi: 10.1038/leu.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ou X, Chae HD, Wang RH, Shelley WC, Cooper S, Taylor T, Kim YJ, Deng CX, Yoder MC, Broxmeyer HE. Sirt1 deficiency compromises mouse embryonic stem cell hematopoietic differentiation, and embryonic and adult hematopoiesis in the mouse. Blood. 2011;117:440–450. doi: 10.1182/blood-2010-03-273011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Singh SK, Williams CA, Klarmann K, Burkett SS, Keller JR, Oberdoerffer P. Sirt1 ablation promotes stress-induced loss of epigenetic and genomic hematopoietic stem and progenitor cell maintenance. The Journal of Experimental Medicine. 2013;210:987–1001. doi: 10.1084/jem.20121608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yoshihara M, Hayashizaki Y, Murakawa Y. Genomic Instability of iPSCs: Challenges Towards Their Clinical Applications. Stem Cell Rev. 2016 Sep 5; doi: 10.1007/s12015-016-9680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]