Abstract
The gut microbiota of infants is shaped by both the mode of delivery and the type of feeding. The gut of vaginally and cesarean-delivered infants is colonized at different rates and with different bacterial species, leading to differences in the gut microbial composition, which may persist up to 6 months. In a multicenter, randomized, controlled, double-blind trial conducted in South Africa, we tested the effect of a formula supplemented with a prebiotic (a mixture of bovine milk-derived oligosaccharides [BMOS] generated from whey permeate and containing galactooligosaccharides and milk oligosaccharides such as 3′- and 6′-sialyllactose) and the probiotic Bifidobacterium animalis subsp. lactis (B. lactis) strain CNCM I-3446 on the bifidobacteria levels in the gut of infants born vaginally or via cesarean section in early life. Additionally, the safety of the new formulation was evaluated. A total of 430 healthy, full-term infants born to HIV-positive mothers who had elected to feed their child beginning from birth (≤3 days old) exclusively with formula were randomized into this multicenter trial of four parallel groups. A total of 421 infants who had any study formula intake were included in the full analysis set (FAS). The first two groups consisted of cesarean-delivered infants assigned to the Test formula (n = 92) (a starter infant formula [IF] containing BMOS at a total oligosaccharide concentration of 5.8 ± 1.0 g/100 g of powder formula [8 g/L in the reconstituted formula] + B. lactis [1 × 107 colony-forming units {cfu}/g]) or a Control IF (n = 101); the second two groups consisted of vaginally delivered infants randomized to the same Test (n = 115) or Control (n = 113) formulas from the time of enrollment to 6 months. The primary efficacy outcome was fecal bifidobacteria count at 10 days, and the primary safety outcome was daily weight gain (g/d) between 10 days and 4 months. At 10 days, fecal bifidobacteria counts were significantly higher in the Test formula than in the Control formula group among infants with cesarean birth (median [range] log: 9.41 [6.30–10.94] cfu/g versus 6.30 [6.30–10.51] cfu/g; P = 0.002) but not among those with vaginal birth (median [range] log: 10.06 [5.93–10.77] cfu/g versus 9.85 [6.15–10.79] cfu/g; P = 0.126). The lower bound of the two-sided 95% confidence interval of the difference in the mean daily weight gain between the Test and Control formula groups was more than –3 g/d in both the vaginally and cesarean-delivered infants, indicating that growth in the Test formula-fed infants was not inferior to that of Control formula-fed infants. At 10 days and 4 weeks, the fecal pH of infants fed the Test formula was significantly lower than in those fed the Control formula, irrespective of mode of delivery: for vaginal delivery: 4.93 versus 5.59; P < 0.001 (10 days) and 5.01 versus 5.71; P < 0.001 (4 weeks); for cesarean delivery: 5.14 versus 5.65, P = 0.009 (10 days) and 5.06 versus 5.75, P < 0.001 (4 weeks). At 3 months, this acidification effect only persisted among cesarean-born infants. IF supplemented with the prebiotic BMOS and probiotic B. lactis induced a strong bifidogenic effect in both delivering modes, but more explicitly correcting the low bifidobacteria level found in cesarean-born infants from birth. The supplemented IF lowered the fecal pH and improved the fecal microbiota in both normal and cesarean-delivered infants. The use of bifidobacteria as a probiotic even in infants who are immunologically at risk is safe and well tolerated.
Keywords: Infant formula, oligosaccharides, gut microbiota, cesarean, bifidogenic, B. lactis
Introduction
Infants are born with a sterile gut but quickly acquire their initial colonizing microbes from their environment.1,2 In infants delivered vaginally, the initial inoculum comes mainly from the birth canal.2–4 By contrast, infants who are born by cesarean acquire their colonizing strains primarily from their mother’s skin and the environment.4 Dominguez-Bello et al found that infants born vaginally had a microbiota dominated by Lactobacillus, Prevotella, or Sneathia spp., whereas those delivered by cesarean had predominantly Staphylococcus, Corynebacterium, and Propionibacterium spp., which are typical skin colonizers.4 Other investigators have reported that vaginally delivered infants are colonized by bifidobacteria and lactobacilli within 2–3 days of birth, whereas in cesarean-delivered infants, colonization with these species is delayed until about 10 days.2,3,5 Differences in gut microbial composition between vaginally and cesarean-delivered infants can persist up to 6 months.3 However, by the first year of life, the characteristic infant microbiota in both groups of infants gives way to a more adult-like gut microbiota.2,6
These initial differences in early colonization have been implicated in the health differences observed between vaginally and cesarean-delivered infants. Cesarean birth has been associated with increased incidence of allergic sensitization or allergy in the first years of life7–9 and with increased risk for gastrointestinal infections.10,11 After birth, development of infants’ gut microbiota is largely influenced by the type of feeding, with breast-fed infants exhibiting bifidobacteria- and lactobacilli-predominant microbiota. Formula-fed infants, on the other hand, have a more diversified microbiota that includes Clostridium perfringens, streptococci, and staphylococci.2,12 Bifidobacteria and lactobacilli have been associated with health benefits, such as reduced infection rates and a reduced propensity for allergies.13–16
Human milk contains bioactive components that are thought to contribute to its beneficial health effects. Among these are the relatively large quantities of diverse oligosaccharides found in human milk.17 These oligosaccharides include nondigestible oligosaccharides, which influence the gut microbiota composition by stimulating bifidobacteria growth.17–20 The nondigestible oligosaccharides also help protect against pathogens by inhibiting pathogen binding to host cells and by creating an acidic environment (through their fermentation in the colon) that inhibits pathogen growth.17 These properties may partly explain the reduced infection rate observed in breast-fed infants. These nondigestible oligosaccharides are also present in cow milk, albeit at much lower concentrations than in human milk.21
We have previously reported on the development and safety of infant formulas (IFs) supplemented with a prebiotic, which is a mixture of bovine milk-derived oligosaccharides (BMOS) generated from whey permeate (containing galactooligosaccharides and milk oligosaccharides such as 3′- and 6′-sialyllactose).22 In the current study, the primary aim was to evaluate the effect of a Test formula supplemented with both BMOS prebiotic and the probiotic Bifidobacterium animalis ssp lactis (B. lactis)25 on the early onset of bifidobacteria colonization in the gut of newborn infants delivered via vaginal or cesarean mode and to evaluate the safety of this new formula.
Study Population and Methods
Study design
This was a randomized, controlled, double-blind, multicenter trial conducted between October 2008 and October 2013 at three sites in South Africa affiliated to the University of Witwatersrand: Charlotte Maxeke Johannesburg Academic Hospital, Rahima Moosa Mother and Child Hospital, and Chris Hani Baragwanath Academic Hospital. Infants were enrolled at birth and followed up until they were 1 year old.
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. It conformed to the International Conference on Harmonization Guidelines for Good Clinical Practice and adhered to the applicable regulatory and legal requirements. The study protocol and informed consent form were reviewed and approved by the Human Research Ethics Committee of the University of the Witwatersrand for each site.
The study has been registered under ClinicalTrials.gov registry number NCT01880970.
All aspects of participation in the study were explained to the parents or legally acceptable representatives, and they voluntarily signed the informed consent forms; none of the infants received the study formulas before parents/legal representatives gave their signed consent.
Study population
Healthy, full-term, newborn infants were recruited from human immunodeficiency virus (HIV)-positive mothers who had elected to feed their child exclusively with formula beginning from birth. Inclusion criteria were being a full-term baby (between 37 weeks and 42 weeks gestation), <3 days old, weighing between 2500 g and 4500 g, and being a singleton birth. Infants were excluded if they had a congenital illness or malformation that might affect normal growth, had a significant perinatal disease, had received antibiotics during the first 3 days of life, had caregivers who could have difficulties complying with the study protocol, or if they were currently participating in another clinical trial.
Study formulas and blinding
Both Test and Control formulas contained sufficient amounts of proteins (1.8 g/100 kcal, whey/casein ratio of 70:30), carbohydrates, fats, vitamins, and minerals to support the normal growth of healthy infants from birth to 6 months of age. The Test formula was additionally supplemented with a mixture of BMOS generated from whey permeate (containing galactooligosaccharides and milk oligosaccharides such as 3′- and 6′-sialyllactose) at a total oligosaccharide concentration of 5.8 ± 1.0 g/100 g of powder formula (8 g/L in the reconstituted formula) and a probiotic (B. lactis strain CNCM-I-3446 with 1 × 107 cfu/g of powder formula).
All study subjects received a follow-up formula from 6 months to 12 months of age without pre- and probiotics. Introduction of complementary food progressively started between 4 months and 6 months of age. Formulas were manufactured by the sponsor (Nestlé Product Technology Center, Konolfingen, Switzerland), who also coded the formula cans with a single-letter code. Parents (caregivers), investigators, study support staff, and the clinical project managers were blinded to the identity of the products.
Outcome measures
The primary efficacy outcome was fecal bifidobacteria count at 10 days, and the primary safety outcome was daily weight gain (g/d) between 10 days and 4 months. Secondary outcomes were fecal bifidobacterial and total bacterial counts at 3 days, 4 weeks, and 3 months and total bacterial counts at 10 days of age; detectable fecal B. lactis, Lactobacillus, Bacteroides, Clostridium, Staphylococcus, and enterobacteria (including Escherichia coli and Klebsiella) from 3 days to 3 months of age; anthropometric measurements from 3 days to 12 months of age; fecal pH between 3 days and 3 months of age; lean mass, fat mass, and bone mineral content (BMC) at 4 months and 12 months of age; digestive tolerance from 10 days to 4 months of age; immune parameters (fecal secretory immunoglobulin A [sIgA] from 3 days to 3 months and blood anti-hepatitis B vaccine IgG levels at 6 weeks, 4 months, and 12 months of age); HIV infection status at 6 weeks, 4 months, and 6 months of age; and the frequency of morbidity episodes, including infections, during the study.
Study conduct
Upon enrollment (≤3 days of age), eligible infants were randomized to the Test or Control formula groups. Infants were exclusively fed their assigned formulas ad libitum from enrollment until 6 months of age. Infants who were not enrolled directly after birth received the regular non-acidified hospital formula, which did not contain any pre- or probiotics (for a maximum of 3 days).
At the time the study was started (2008), most women who were HIV negative commenced breast-feeding. The national policy at that time, in accordance with World Health Organization (WHO) recommendations, was that HIV- positive mothers be counseled during pregnancy or immediately after birth regarding the risks and benefits of breast-feeding versus formula feeding. Pregnant mothers could be started on antiretroviral therapy if they had a low cluster of differentiation 4 (CD4) count. National policy was that those who chose not to breast-feed be provided with free formula for 6 months after birth. Only mothers who had already chosen not to breastfeed were approached regarding enrollment into this study. The national policy changed in August 2011 in line with the new WHO recommendations, and pregnant mothers testing positive for HIV were started on full antiretroviral therapy during pregnancy, which continued after birth. Breast-feeding was now advocated as national policy for all babies born to HIV-positive mothers. This policy change happened toward the end of recruitment (362 of the final 430 subjects had been enrolled) for this study, but some HIV-positive mother still chose to formula feed. Thus, the study was at all times conducted in accordance with WHO and South African national guidelines with respect to feeding of babies born to HIV-positive mothers.
Between 4 months and 6 months, infants progressively received weaning foods alongside their assigned study formulas, and starting at 6 months, all infants received the same follow-on formula without pre- or probiotics. All mothers and infants received antiretroviral medication to prevent mother-to-child HIV transmission (beginning at the time of labor for the mother).
Baseline anthropometric measurements and demographic data were recorded at enrollment, before any study formula intake. Thereafter, visits to the study center took place at 10 ± 2 days, 4 weeks ± 3 days, 6 weeks ± 3 days, 3 months ± 3 days, 4 months ± 3 days, 6 months ± 3 days, 9 months ± 14 days, and 12 months ± 14 days. Mothers/caregivers were given 3-day diaries in which they recorded the formula intake and digestive tolerance (stool characteristics, occurrences of flatulence, spitting up and vomiting, and infants’ behavior [crying, fussing, or colic]) for the 3 days preceding each visit up to 4 months. Mothers/caregivers also recorded any incidence or episode of illness in infants between visits.
At each visit, investigators took anthropometric measurements and assessed morbidity. Infants were weighed nude to the nearest 10 g on the same well-calibrated electronic scales. Recumbent length was measured to the nearest 1 mm with the body fully extended and feet flexed. Head circumference was measured approximately 2.5 cm above the eyebrows using a standard measuring tape.
Body composition was measured at 4 months ± 14 days and 12 months ± 14 days using a dual energy x-ray absorptiometry (DEXA) (Hologic) scanner.
Fecal samples were collected at 3 days, 10 days, 4 weeks, and 3 months. Fecal B. lactis CNCM I-3446, Staphylococcus, enterobacteria, Escherichia coli, and Klebsiella counts were determined using plating methods and polymerase chain reaction (PCR) (Advanced Analytical Technologies). Total bacterial counts, bifidobacteria, lactobacilli, Bacteroides, and Clostridium (Clostridium and Eubacterium) were determined using Fluorescent in situ hybridization (FISH) (Biovisible).
Fecal sIgA was quantified using a commercial kit (Bethyl), and the sIgA value of each sample was normalized for total proteins as described previously.23 Fecal pH was measured at each fecal sample collection using a Consort pH meter (model 561).
At 6 weeks, 4 months, and 12 months, blood (2–2.5 mL) was drawn 1.5 hours after the infant’s previous meal to evaluate IgG titers against the hepatitis B vaccine and to run the HIV PCR test. Regarding the latter, for ambiguous results, additional tests were performed at 6 months, and the viral load at this time point was considered definitive.
Digestive tolerance was assessed by the investigators based on the 3-day diaries in which infants’ stool characteristics, occurrences of flatulence, spitting up and vomiting, and infants’ behavior (crying, fussing, or colic) had been recorded by the caregivers. Stool characteristics consisted of its frequency in 24 hours and its consistency. The frequency of flatulence was recorded as never, sometimes, or often, and the frequency of spitting up ranged from never to very much. Behavior patterns were assessed based on crying, fussing (being unsettled or irritable), or being colic. Colic was recorded as being present or absent and identified using Wessel’s criteria.
Adverse events (AEs)
At each visit, investigators specifically recorded any occurrences of respiratory tract infections (including bronchiolitis and otitis media), gastrointestinal conditions, and whether or not infants had been hospitalized between visits or had received medication.
In addition, episodes of diarrhea, constipation, cough, fever, respiratory symptoms, and skin rashes occurring between visits were documented. Diarrhea was defined as ≥3 loose or watery stools in 24 hours. An episode of diarrhea was considered to have ended once there were two consecutive nonwatery stools or no stools for 24 hours. Atopic eczema was graded on a scale of 0 to 3: 0, absent; 1, mild; 2, moderate; and 3, severe.
AEs were coded using the WHO adverse drug reaction terminology (WHO-ART). Investigators assessed AEs for seriousness and relation to study formulas.
Randomization and allocation concealment
Infants were randomized using the delivery mode and sex as the stratification factors. The randomization was performed using the in-house TrialSys software.
Statistical methods
Sample size calculation was based on showing superiority of mean bifidobacteria counts in the Test formula group relative to the Control formula group. A difference of 0.7 log cfu/g was considered clinically relevant.24 Using an estimated standard deviation (SD) of 1.23 log cfu/g, a significance level of 2.5% (due to two independent analyses in the two delivery groups) and a power of 80%, 240 infants (120 per formula group) were required. Accounting for a 30% dropout rate and 10% exclusion rate due to HIV infection, a total of 400 infants had to be recruited into the study. This number of infants was also sufficient to demonstrate noninferiority in daily weight gain using a 3 g/d margin and an estimated SD of 6.1 g/d.
All analyses were performed separately in infants delivered vaginally and by cesarean section. For primary analyses, P-values < 0.025 were considered significant due to multiple testing (in the two delivery groups).
Primary analyses were performed in both the full analysis set (FAS) and the per protocol (PP) population. The FAS was defined as all infants who were randomized into one of the study formula groups and who had any intake of the study formula. The PP population excluded infants who had any major protocol deviations, which were defined prior to unblinding the study. Major protocol deviations for the efficacy analysis were the following: absence of fecal samples at 10 days, receiving antibiotics before collection of the 10-day fecal sample, date of last study formula intake being before the 10-day fecal sample, any interruption of the study formula intake before the 10-day fecal sample, introduction of complementary food before 10 days, and testing positive for HIV. Major protocol deviations for the safety analysis were the following: ≥7 days interruption of study formula intake before the age of 4 months, last visit occurring before 10 days, date of last study formula intake at ≤115 days of age, testing positive for HIV, and a serious AE (SAE) occurring within 1 week before any of the visits during the first 4 months.
Bifidobacteria counts on Day 10 were nonnormally distributed even after log transformation. Thus, treatment effect was estimated using the Wilcoxon nonparametric analysis. The Test formula was compared with the Control formula in the vaginally and cesarean-delivered infants separately, and the Test formula was considered to be superior to the Control formula at a P-value of 0.025. Bifidobacteria counts that were below the detection limit were imputed to the (value of the) detection limit.
Primary safety outcome was compared using analysis of variance within a mixed-model setting, which provided a useful strategy for analyzing longitudinal data with dropouts. The treatment difference and the two-sided 95% confidence intervals (CIs) were estimated using a mixed model for repeated measures using all available body weight measurements obtained between 10 days and 4 months in order to analyze parabolic growth accounting for age, sex, and treatment. Mean daily weight gain in the Test formula group was considered non-inferior to the Control formula group, if the lower margin of the two-sided 95% CI of the difference in weight gain lied to the right of –3 g/d. P-value < 0.025 was considered significant. Growth curves for each treatment group and the delivery mode were compared with WHO reference data (http://www.who.int/childgrowth/software/en/).
Secondary analyses were performed in the FAS, and no adjustments for multiple testing were made. Bifidobacteria and total fecal bacteria counts were compared between groups using the same nonparametric model described for the primary outcome.
Any detection of the various microbial species above the detection limit was considered positive and was modeled for both vaginal and cesarean delivery using logistic regression at each visit. For the 3-day visit in infants born by vaginal delivery, the Fisher’s exact test was used because logistic regression could not be applied for the 0% detection values obtained in the Control formula group.
Fecal pH was analyzed using a mixed model as described for body weight analysis. Fecal sIgA was analyzed at each visit using nonparametric models.
Body weight was compared between formula groups using a model for repeated measures adjusted for baseline value and sex. The treatment effect was compared at each visit for each delivery group, and P-values and two-sided 95% CIs are presented. Body weights measured outside the windows for the various visits (refer the section on “Study conduct”) were excluded from this analysis. Other anthropometric data were analyzed as described for body weight. In addition, weight-for-length, length-for-age, body mass index (BMI)-for-age, and head circumference-for-age Z-scores were estimated for each subject and visit based on the WHO infant growth standards.
Body composition parameters measured by DEXA (fat mass, lean mass, Bone Mineral Content (BMC) mass, and corresponding percentages) were analyzed as described for body weight, except that adjustment for baseline was not performed because DEXA measurements were not performed at baseline.
Daily stool count was computed by summing all stools reported from 10 days until 6 months and dividing it by the number of days where tolerance record was completed. The percentage of days with a specific stool consistency was calculated by dividing the number of days during which stools of a given consistency were reported by the number of days during which any stool consistency was reported (thus, multiple stool consistencies may be present on any given day).
The percentage of days in which the various gastrointestinal symptoms (flatulence, spitting up, vomiting, crying, fussing, and colic) occurred was calculated for the 10-day to the 4-month visits. All fecal characteristics and gastrointestinal symptoms were compared between groups using a two-sided superiority testing for treatment differences using Poisson regression.
The percentages of infants with a positive IgG response to the hepatitis B vaccine or to the HIV test were analyzed as categorical data, and comparisons between groups were made using the exact chi-square test. Similarly, the percentages of infants who had ≥1 SAEs and nonserious AEs were compared between groups using the exact chi-square test.
All statistical analyses were conducted using the Statistical Analysis System (SAS, version 9.1) software.
Results
Study population
Four hundred and thirty infants were randomized into the study. Nine (2.1%) infants were lost to follow-up after randomization but before starting the study formulas. Of the remaining 421 who started taking the study formulas, 228 were born vaginally and 193 by cesarean section. In total, eight infants were found to be HIV infected, seven at the 4-week visit (v2) and one became positive at 6 months (v5). The proportion of HIV-infected infants was not significantly different between the two formula groups regardless of the mode of delivery.
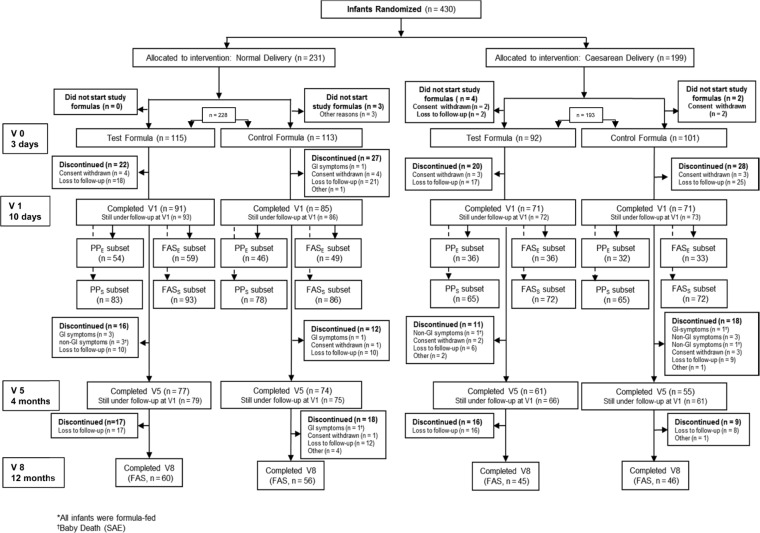
Among those, three infants died and one discontinued the study. The remaining four stayed in the study but their data were not included in the data analysis, as mentioned in the study protocol. The flow of infants in each formula group during the study is shown in Figure 1.
Figure 1.
CONSORT diagram for the four randomized groups by delivery mode and formula type.
Notes: Visits were numerated as follows: V0, enrollment date: 0–3 days of life; V1, 10 ± 2 days; V2, 4 weeks ± 3 days; V3, 6 weeks ± 3 days; V4, 3 months ± 3 days; V5, 4 months ± 3 days; V6, 6 months ± 3 days; V7, 9 months ± 14 days; and V8, 12 months ± 14 days.
Abbreviations: FAS, full analysis data set (FASE for Efficacy, FASS for Safety); PP, per protocol data set (PPE for efficacy, and PPS for safety).
For the primary efficacy outcome (fecal bifidobacteria counts at 10 days), 177 infants were included in the FAS for efficacy (FASE): 108 were born vaginally (59 received the Test formula and 49 received the Control formula) and 69 were born by cesarean section (36 received the Test formula and 33 received the Control formula; Fig. 1). The PP population for efficacy (PPE) included 168 infants: 100 had vaginal birth (54 received the Test formula and 46 received the Control formula) and 68 had cesarean birth (36 received the Test formula and 32 received the Control formula; Fig. 1).
The primary safety outcome (mean daily weight gain) at 4 months of age included data from 323 infants in the FAS population (FASS). Of these, 179 had vaginal birth (93 received the Test formula and 86 received the Control formula) and 144 had cesarean birth (72 received the Test formula and 72 the Control formula; Fig. 1). The PP population for safety (PPS) included 290 infants: 161 had vaginal birth (83 received the Test formula and 78 received the Control formula) and 129 had cesarean birth (65 received the Test formula and 64 received the Control formula; Fig. 1).
Infants delivered by cesarean section were slightly older and more likely to be male than those born vaginally (Table 1). Within each delivery group, demographic data and baseline characteristics were balanced between treatment groups (Table 1).
Table 1.
Demographic data and baseline characteristics, full analysis set.
| VAGINAL DELIVERY | CESAREAN DELIVERY | |||
|---|---|---|---|---|
| TEST FORMULA (n = 115) | CONTROL FORMULA (n = 113) | TEST FORMULA (n = 92) | CONTROL FORMULA (n = 101) | |
| Characteristics mean (SD) or n (%) | ||||
| Age at randomization (days) | 0.9 (0.7) | 0.8 (0.5) | 1.5 (0.8) | 1.5 (0.7) |
| Male, n | 55 (47.8) | 51 (45.1) | 54 (58.7) | 59 (58.4) |
| Gestational age (weeks) | 39.1 (1.5) | 38.9 (1.3) | 39.0 (1.3) | 39.1 (1.5) |
| Body weight at birth (kg) | 3.10 (0.31) | 3.08 (0.32) | 3.18 (0.38) | 3.15 (0.37) |
| Body length at birth (cm) | 50.5 (2.7) | 50.1 (2.1) | 50.6 (2.5) | 50.4 (2.6) |
| BMI at birth (kg/m2) | 12.2 (1.4) | 12.3 (1.2) | 12.4 (1.5) | 12.5 (1.6) |
| Head circumference at birth (cm) | 34.4 (1.3) | 34.4 (1.4) | 35.4 (1.2) | 35.1 (1.5) |
| HIV treatment during pregnancy, n | 100 (87) | 94 (83.2) | 80 (87) | 84 (83.2) |
Abbreviations: SD, standard deviation; BMI, body mass index; HIV, human immunodeficiency virus.
Fecal microbial counts and pH
Approximately 30%–50% of infants had fecal data available for primary outcome analysis (Table 2). Bifidobacteria counts at 10 days (the primary efficacy outcome) were significantly higher in the Test formula group, compared with the Control formula group, among infants with cesarean birth but not among those with vaginal birth (Table 2). PP analysis was consistent with these results, with significant difference between formula groups among infants delivered by cesarean section (median [range] log bifidobacteria counts: 9.41 [6.30–10.94] cfu/g and 6.30 [6.30–10.51] cfu/g in the Test and Control formula groups, respectively; P = 0.002). Among vaginally born infants, bifidobacteria counts in the PP population were not significantly different between formula groups (median [range] log bifidobacteria counts: 10.04 [5.93–10.77] cfu/g and 9.86 [6.15–10.79] cfu/g in the Test and Control formula groups, respectively; P = 0.197).
Table 2.
Fecal bifidobacteria counts (log cfu/g) at 10 days of age, full analysis set.
| VAGINAL DELIVERY | CESAREAN DELIVERY | |||
|---|---|---|---|---|
| TEST FORMULA (n = 115) | CONTROL FORMULA (n = 113) | TEST FORMULA (n = 92) | CONTROL FORMULA (n = 101) | |
| Number of infants (%) available for analysis | 59 (51.3) | 49 (43.4) | 36 (39.1) | 33 (32.7) |
| Mean (SD) | 9.60 (1.09) | 9.28 (1.37) | 8.99 (1.44) | 7.71 (1.68) |
| Median (min – max) | 10.06 (5.93–10.77) | 9.85 (6.15–10.79) | 9.41 (6.30–10.94) | 6.30 (6.30–10.51) |
| Treatment effect P value* | 0.126 | 0.002 | ||
Notes:
Nonparametric Wilcoxon test for Test infant formula versus Control infant formula.
Abbreviations: cfu, colony-forming units; SD, standard deviation; min, minimum; max, maximum.
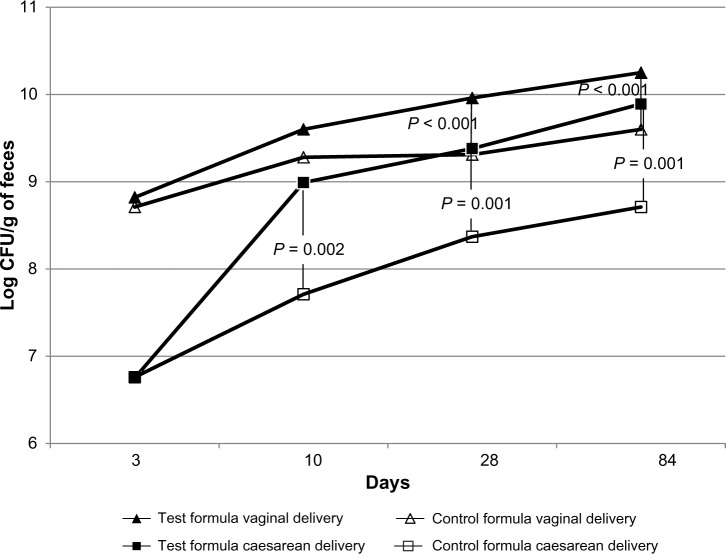
In the FAS population, bifidobacteria counts at 28 days and 3 months (84 days) were also significantly higher in the Test formula group, compared to the Control formula group, in cesarean-born infants (squares in Fig. 2). Then, at 28 days, the median (range) log bifidobacteria counts were 10.15 (6.30–10.96) cfu/g and 9.00 (6.30–10.77) cfu/g in the Test and Control formula groups, respectively (P = 0.001). At 84 days, the median (range) log bifidobacteria counts were 10.40 (6.50–10.79) cfu/g and 9.67 (6.30–10.50) cfu/g in the Test and Control formula groups, respectively (P < 0.001).
Figure 2.
Fecal bifidobacteria counts at 3 days, 10 days, 28 days, and 84 days by delivery mode and formula type.
Among the vaginal-born infants (triangles in Fig. 2), the Test formula group also had significantly increased bifidobacteria counts at 28 days and 84 days compared to the Control formula group. At 28 days, median (range) log bifidobacteria counts were 10.25 (6.75–10.98) cfu/g and 9.66 (6.30–10.31) cfu/g in the Test and Control formula groups, respectively (P < 0.001). At 84 days, the median (range) log bifidobacteria counts were 10.45 (8.22–10.96) cfu/g and 9.95 (6.30–10.17) cfu/g in the Test and Control formula groups, respectively (P < 0.001).
Total bacterial counts at 3 days and 10 days were not significantly different between the Test and Control formula groups among infants with either delivery mode (data not shown). However, at 4 weeks, total bacterial counts were higher in the Test formula groups in both delivery groups, and at 3 months, they were higher in the Test formula group only among vaginally delivered infants (data not shown).
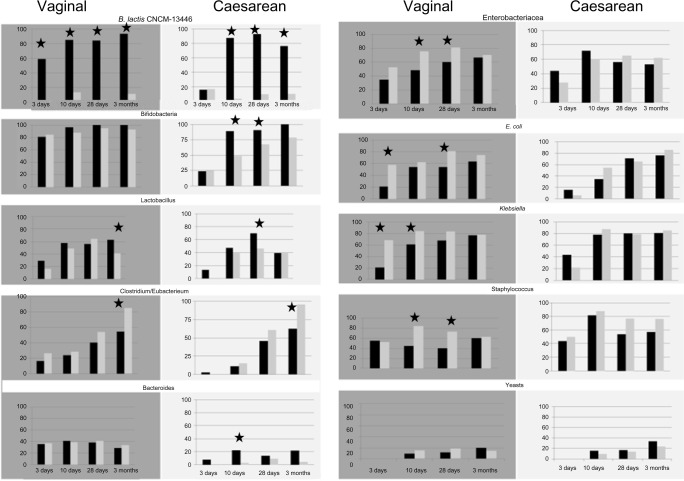
A higher proportion of infants in the Test formula groups had detectable bifidobacteria species compared with Control formula groups up to 4 weeks (Fig. 3). Lactobacillus species were detectable in a higher proportion of infants at 4 weeks and 3 months in respectively cesarean and vaginally delivered infants in the Test formula groups compared to those in the Control formula groups. At 10 days, 4 weeks, and 3 months, B. lactis was detected in a significantly higher proportion of infants in the Test formula groups compared with those in the Control formula groups (Fig. 3). Escherichia coli and Staphylococcus, enterobacteria, and Klebsiella sp. were detected in a significantly higher proportion of infants in the Control formula group compared with the Test formula group (Fig. 3).
Figure 3.
Fecal detection rates of B. lactis CNCM I-3446, bifidobacteria, Lactobacillus, Clostridium, Bacteroides, Enterobacteriaceae, Escherichia coli, Klebsiella sp., staphylococcus, and yeasts in infants from the Test formula groups (black bars) and the Control formula groups (gray bars), respectively, born through vaginal delivery (gray panels) and from the cesarean delivery (white panels).
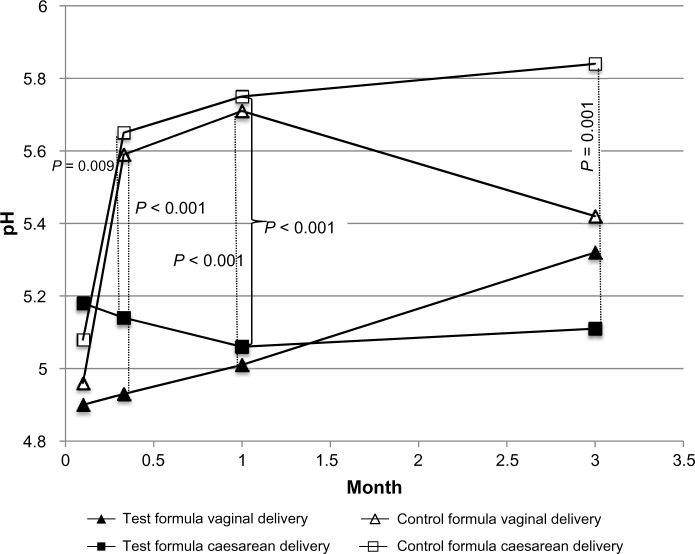
Fecal pH at 3 days was not significantly different between formula groups in either vaginally or cesarean-delivered infants (Fig. 4). At 10 days and 4 weeks, it was lower in the Test formula groups compared with the Control formula groups among both the vaginally and cesarean-delivered infants, but at 3 months, it was lower only in the cesarean-delivered group (Fig. 4).
Figure 4.
Mean fecal pH during the first 3 months by delivery mode and formula type.
Growth and body composition measurements
Mean daily weight gains (the primary safety outcome) were similar between the formula groups among infants with both delivery modes (Table 3). The lower bound of the two-sided 95% CIs of the difference in the mean daily weight gain between the Test and Control formula groups was more than −3 g/d in both the vaginally and cesarean-delivered infants in both the FAS and PP populations (Table 3). This indicates that weight gain in the Test formula group was statistically not inferior to that in the Control formula group based on the preset noninferiority margin.
Table 3.
Comparison of daily weight gain between 10 days and 4 months of age.
| FULL ANALYSIS SET | VAGINAL DELIVERY | CESAREAN DELIVERY | ||
|---|---|---|---|---|
| TEST FORMULA (n = 93) | CONTROL FORMULA (n = 86) | TEST FORMULA (n = 72) | CONTROL FORMULA (n = 72) | |
| Mean (SE) daily weight gain (g/day) | 28.1 (0.8) | 28.2 (0.8) | 29.6 (0.9) | 29.6 (0.9) |
| Treatment effect (1-sided 97.5% CI) | −0.129 (−2.267 to infinity) | −0.049 (−2.532 to infinity) | ||
| Non-inferiority P-value* | 0.004 | 0.010 | ||
| PER PROTOCOL | (n = 83) | (n = 78) | (n = 65) | (n = 64) |
| Mean (SE) daily weight gain (g/day) | 29.2 (0.7) | 28.6 (0.8) | 30.0 (0.9) | 30.2 (0.9) |
| Treatment effect (1-sided 97.5% CI), | 0.595 (−1.429 to infinity) | −0.209 (−2.654 to infinity) | ||
| Non-inferiority P-value* | P < 0.001 | P = 0.013 | ||
Abbreviations: ANOVA, analysis of variance; CI, confidence interval; SE, standard error.
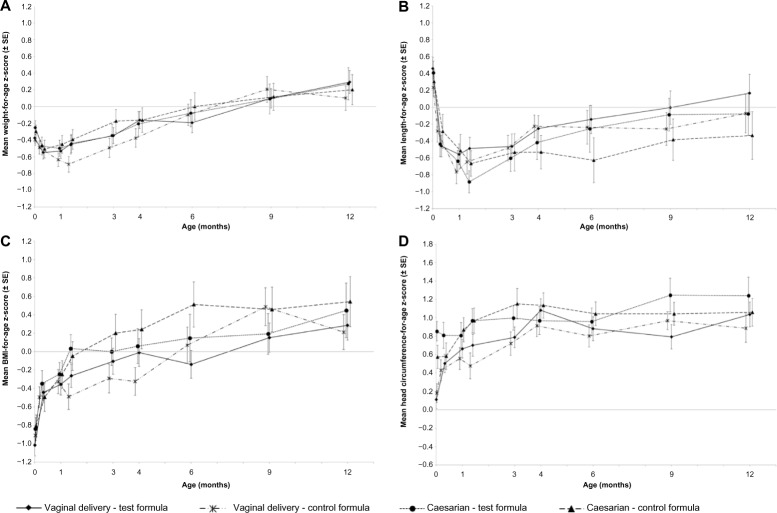
Mean weight-for-age, length-for-age, and head circumference- for-age Z-scores (relative to WHO standards) were not significantly different between the formula groups among infants in either delivery group (Fig. 5).
Figure 5.
(A) Weight-for-age, (B) Length-for-age, (C) BMI-for-age, and (D) head circumference-for-age z-scores relative to WHO standards from birth to 12 months of age.
The fat mass (g) and percentage mean fat mass (measured by DEXA in a subset of infants) at 4 months and 12 months were not significantly different between the formula groups (data not shown). However, among infants born by normal vaginal delivery, those consuming the Test formula had a significantly greater percentage BMC at 4 months (Test formula: 1.78% versus Control formula: 1.69%; P = 0.005), and a significantly greater adjusted mean lean mass (grams) at 12 months (Test: 7531 versus Control: 6881 g; P = 0.002) compared to those consuming the Control formula.
Cesarean-delivered infants showed no difference in these measurements between the formula groups at any time (data not shown).
Immune measurements
The type of formula intake did not have any effect on the immune measures assessed: fecal sIgA concentrations were not significantly different between the infants in the two formula groups at any time point. Moreover, 65% of infants in both Test formula groups and 55% (cesarean-delivered) and 62% (vaginal- delivered) of infants in the Control formula groups had positive anti-hepatitis B IgG antibody response. These response rates were not significantly different (data not shown).
Digestive tolerance
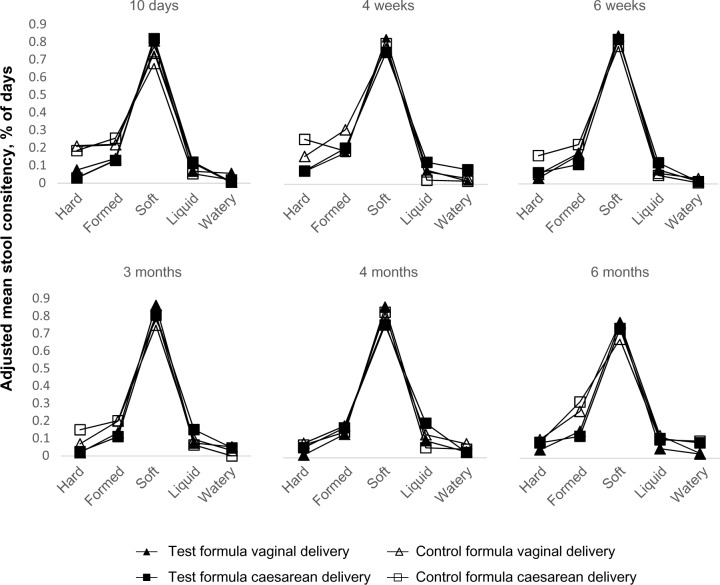
Among the days tested (3 days’ diary before the visits), infants in all groups had an approximate frequency of four daily stools, and this was not significantly different between vaginally and cesarean-delivered infants. The proportion of days in which hard stool was reported was lower in the Test formula group than in the Control formula group among both vaginally (mean % ± SD, 4.1% ± 8.5 versus 10.8% ± 17.2; P = 0.002) and cesarean-delivered infants (6.3% ± 15.4 versus 16.9% ± 22.8; P = 0.001) up to 6 months of age.
The proportion of days with formed stools was also greater in the Control formula group than in the Test formula group among infants delivered by cesarean section (22.5% versus 15.3%; P = 0.045) and among those delivered vaginally (23.5% versus 14.0%; P = 0.055). Liquid stools were more frequent in the Test formula group compared with the Control formula group only among cesarean-delivered infants (13.3% versus 5.7%; P < 0.001) (Fig. 6).
Figure 6.
Adjusted mean stool consistency (percentage of days, calculated as the number of days during which stools of a given consistency were reported divided by the number of days during which any stool consistency was reported) at 10 days; 4 weeks and 6 weeks; 3 months, 4 months, and 6 months, by delivery mode and formula type.
The frequencies of flatulence, spitting up, vomiting, crying, fussing, or colic were not significantly different between the formula groups among infants with either delivery mode (data not shown).
Adverse events
Among vaginally delivered infants, 72.2% of those receiving the Test formula and 63.7% of those receiving the Control formula (P = 0.202) had at least one AE (serious and nonserious) during the 12-month study period. Among those delivered by cesarean section, these figures were 65.2% and 61.4%, respectively (chi-square test, P = 0.182). During the same period, SAEs occurred in 15% of Test formula and 13.3% of the Control formula groups among vaginally delivered infants and in 9.8% of the Test formula group and 11.9% in the Control formula group among cesarean-delivered infants. Overall, and regardless of delivery mode, the number of SAEs was equivalent in the Control and the Test formula groups: 31 versus 32. The number of SAEs in the HIV-positive infants subgroup was two in the Control formula group and six in the Test formula group. Among the seven baby deaths, three were in the Control formula group and four belonged to the Test formula group. Within this subgroup of seven infants, three were HIV+, and among those, two belonged to the Test formula group and one belonged to the Control formula group. Per assessment of the investigators, no SAEs were attributable to the study intervention.
For the AEs, ie, respiratory infections/symptoms, diarrhea, gastrointestinal conditions, and skin disorders, the frequency of occurrence was not significantly different between the formula groups among either vaginally or cesarean-delivered infants (data not shown).
Discussion
In the current study, we have shown that supplementing IFs with BMOS and B. lactis significantly increased the intestinal bifidobacteria counts in infants delivered by cesarean section at 10 days, 28 days, and 84 days after birth compared to the cesarean Control formula group. Additionally, a higher proportion of infants in the Test formula group (90.7%) had detectable Bifidobacterium sp. compared with the Control formula group (67.4%) at 4 weeks. By 3 months, this difference was no longer statistically significant.
Similarly, in the vaginally delivered infants, the bifidobacteria counts became significantly higher at 4 weeks and 3 months in the Test formula group compared to the Control formula group. The lower bifidobacteria counts at 3 days of life and lower proportion of infants with Bifidobacterium sp. among the cesarean-born infants can be restored by supplementation of IF with BMOS and B. lactis when provided from early life onward within a week, as shown by the data presented.
These results are consistent with previous studies showing delays in bifidobacteria colonization in cesarean-delivered infants compared with those vaginally delivered.3 Because vaginally delivered newborns have higher numbers of bifidobacteria compared with those delivered by cesarean section, an effect on bifidobacteria counts by supplementing IF with prebiotics (BMOS) and probiotics (B. lactis) is more likely to be detected in infants who do not already have a sizable bifidobacteria population, which may explain why a significant effect was observed in cesarean-delivered infants early in life.
Having said that, the effect of IF supplementation on the vaginal-born infants, as tested in this study, was measurable at 4 weeks and 3 months of life in terms of bifidobacteria counts and at 3 months by the presence of Lactobacillus sp. Additionally, Simeoni et al.25 showed that the prebiotic (BMOS) stimulated a marked shift to a bifidobacterium-dominated fecal microbiota via increases in endogenous bifidobacteria in healthy term infants from birth.
This study further supports the observations by showing the early effect on bifidobacterial counts in cesarean-born infants (<10 days) after feeding the supplemented IF. As would be expected, B. lactis counts were significantly higher in Test formula-fed infants in both the vaginally and cesarean-delivered infants. This difference was already apparent at 3 days among vaginally delivered infants and at 10 days in cesarean-delivered infants, and it remained significantly higher in both groups until 3 months, when infants were still exclusively fed the study formulas.
Interestingly, fecal pH remained low until 1 month in infants receiving the Test formula independent of delivery mode, while fecal pH increased in Control formula-fed infants at 10 days and 1 month. In cesarean-section-born infants, this difference between Test and Control formula groups continued up to 3 months.
The intestinal microbiota of infants receiving the supplemented IF (Test) likely produced more acid metabolites than the microbiota of the Control group. For the cesarean-born infants receiving the Test formula, the acidification of the gut milieu was even stronger, as reflected by the persistence of a low pH of the stool up to 3 months of age. It is possible that other bacterial species benefit from the acidic metabolism and help to maintain the lower pH. Breast-fed infants also have a low stool pH.25 In both vaginal-and cesarean-born infants, the lower pH is thought to be linked to the composition of the gut microbiota. A dominant lactic acid bacteria flora from early life onward is known to affect the stool pH.
The consumption of the Test formula supplemented with BMOS over the first 6 months of life resulted in an overall softening of the stool consistency in both vaginal- and cesarean-born infants. This was already observed in a previous study by Simeoni et al.25, in which infants fed the same supplemented formula expressed stool consistency closer to the one observed in breast-fed infants.
Finally, no differences in morbidity incidence rates were found between the supplemented formula compared to the Control formula in cesarean-born infants, indicating that the use of bifidobacteria as a probiotic even in infants who are immunologically at risk is safe and well tolerated. The study was not designed to measure differences in morbidity, and infants were not followed up for more than 1 year. Additional studies will be needed to further investigate the long-term health effects in cesarean-born infants.
The current study showed that the IF formula supplemented with BMOS in combination with B. lactis was safe, as shown by both weight gain and anthropometric measurements relative to WHO standards. Infants grew normally in both formula groups and showed no differences in anthropometric measurements between groups. A 2009 meta-analysis of five studies of IFs supplemented with B. lactis concluded that infants of HIV-negative mothers fed these formulas had similar weight gain compared with those fed control, non-supplemented formulas.26 Among these five studies, two studies tested acidified formulas in HIV-negative infants of HIV-positive mothers and showed an effect of B. lactis supplementation on weight gain per day.26 The reason why this study did not show a difference in weight gain is not clear. However, because antiretroviral therapy was commenced during pregnancy in many mothers in this study, which was not the case in the earlier studies, the general health of the infants in this study at birth was probably better than those in the earlier studies and was thus more comparable to the studies on infants born to HIV-negative mothers.
In conclusion, the current study shows that, consistent with previous studies, BMOS as well as B. lactis are safe to add to infant starter formulas. The BMOS prebiotic in combination with B. lactis probiotic stimulated the growth of bifidobacteria in infants born by cesarean delivery at early life (within the first 10 days) when the gut colonization with bifidobacteria is delayed compared to vaginally born infants, suggesting a possible beneficial effect for these infants.
Acknowledgments
The authors wish to thank Samir Dahbane for data management and are grateful to Annemarie Beekman for clinical study management. The authors also thank Drs Firdose Nakwa, Reenu Thomas, Alison van Kwawegen, Lea Chirwa, Tanusha Ramdin, Elizabeth Ho, Delania Lawrence and Gary Reubenson for assisting with recruitment, data collection and follow up.
Footnotes
ACADEMIC EDITOR: Praveen Kumar, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 814 words, excluding any confidential comments to the academic editor.
FUNDING: Nestec Ltd funded the study.
COMPETING INTERESTS: NdG, SEA, SP and PS are employees of Nestec Ltd. SV and PC disclose grants for contract research received from Nestec Ltd for the work presented here. KB discloses grants and non-financial support from Nestec Ltd for the work presented here and for previous contract research.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: PS, SP, SEA. Analysed the data: PC, KB, SV, NdG, SEA, SP, PS. Wrote the first draft of the manuscript: SP, NdG. Contributed to the writing of the manuscript: PC, KB, SV, NdG, SEA, SP, PS. Agree with manuscript results and conclusions: PC, KB, SV, NdG, SEA, SP, PS. Jointly developed the structure and arguments for the paper: PC, KB, SV, SP, NdG, PS. Made critical revisions and approved final version: PC, KB, SV, NdG, SEA, SP, PS. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Lawley TD, Walker AW. Intestinal colonization resistance. Immunology. 2013;138(1):1–11. doi: 10.1111/j.1365-2567.2012.03616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118(2):511–21. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 3.Gronlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after caesarean delivery. J Pediatr Gastroenterol Nutr. 1999;28(1):19–25. doi: 10.1097/00005176-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morelli L. Postnatal development of intestinal microflora as influenced by infant nutrition. J Nutr. 2008;138(9):1791S–5S. doi: 10.1093/jn/138.9.1791S. [DOI] [PubMed] [Google Scholar]
- 6.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5(7):e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang JH, Hsu CY, Lo JC, Chen CP, Huang FY, Yu S. Comparative analysis of neonatal morbidity for vaginal and caesarean section deliveries using hospital charge. Acta Paediatr. 2006;95(12):1561–6. doi: 10.1080/08035250600711066. [DOI] [PubMed] [Google Scholar]
- 8.Eggesbo M, Botten G, Stigum H, Nafstad P, Magnus P. Is delivery by caesarean section a risk factor for food allergy? J Allergy Clin Immunol. 2003;112(2):420–6. doi: 10.1067/mai.2003.1610. [DOI] [PubMed] [Google Scholar]
- 9.Negele K, Heinrich J, Borte M, et al. Mode of delivery and development of atopic disease during the first 2 years of life. Pediatr Allergy Immunol. 2004;15(1):48–54. doi: 10.1046/j.0905-6157.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 10.Hakansson S, Kallen K. Caesarean section increases the risk of hospital care in childhood for asthma and gastroenteritis. Clin Exp Allergy. 2003;33(6):757–64. doi: 10.1046/j.1365-2222.2003.01667.x. [DOI] [PubMed] [Google Scholar]
- 11.Laubereau B, Filipiak-Pittroff B, von Berg A, et al. Caesarean section and gastrointestinal symptoms, atopic dermatitis, and sensitisation during the first year of life. Arch Dis Child. 2004;89(11):993–7. doi: 10.1136/adc.2003.043265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balmer SE, Wharton BA. Diet and faecal flora in the newborn: breast milk and infant formula. Arch Dis Child. 1989;64(12):1672–7. doi: 10.1136/adc.64.12.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalliomaki M, Isolauri E. Role of intestinal flora in the development of allergy. Curr Opin Allergy Clin Immunol. 2003;3(1):15–20. doi: 10.1097/00130832-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Mountzouris KC, McCartney AL, Gibson GR. Intestinal microflora of human infants and current trends for its nutritional modulation. Br J Nutr. 2002;87(5):405–20. doi: 10.1079/BJNBJN2002563. [DOI] [PubMed] [Google Scholar]
- 15.Newburg DS. Oligosaccharides in human milk and bacterial colonization. J Pediatr Gastroenterol Nutr. 2000;30(suppl 2):S8–17. [PubMed] [Google Scholar]
- 16.Salminen SJ, Gueimonde M, Isolauri E. Probiotics that modify disease risk. J Nutr. 2005;135(5):1294–8. doi: 10.1093/jn/135.5.1294. [DOI] [PubMed] [Google Scholar]
- 17.Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr. 2005;25:37–58. doi: 10.1146/annurev.nutr.25.050304.092553. [DOI] [PubMed] [Google Scholar]
- 18.Coppa GV, Pierani P, Zampini L, Carloni I, Carlucci A, Gabrielli O. Oligosaccharides in human milk during different phases of lactation. Acta Paediatr Suppl. 1999;88(430):89–94. doi: 10.1111/j.1651-2227.1999.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 19.Gyorgy P, Jeanloz RW, von Nicolai H, Zilliken F. Undialyzable growth factors for Lactobacillus bifidus var. pennsylvanicus. Protective effect of sialic acid bound to glycoproteins and oligosaccharides against bacterial degradation. Eur J Biochem. 1974;43(1):29–33. doi: 10.1111/j.1432-1033.1974.tb03380.x. [DOI] [PubMed] [Google Scholar]
- 20.Sela DA, Chapman J, Adeuya A, et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci U S A. 2008;105(48):18964–9. doi: 10.1073/pnas.0809584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barile D, Tao N, Lebrilla CB, Coisson JD, Arlorio M, German JB. Permeate from cheese whey ultrafiltration is a source of milk oligosaccharides. Int Dairy J. 2009;19(9):524–30. doi: 10.1016/j.idairyj.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meli F, Puccio G, Cajozzo C, et al. Growth and safety evaluation of infant formulae containing oligosaccharides derived from bovine milk: a randomized, double-blind, noninferiority trial. BMC Pediatr. 2014;14(1):306. doi: 10.1186/s12887-014-0306-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benyacoub J, Perez PF, Rochat F, et al. Enterococcus faecium SF68 enhances the immune response to Giardia intestinalis in mice. J Nutr. 2005;135(5):1171–6. doi: 10.1093/jn/135.5.1171. [DOI] [PubMed] [Google Scholar]
- 24.Chow SC, Wang S, Shao J. Sample Size Calculations in Clinical Research. Second ed. Chapman & Hall/CRC Biostatistics Series; Taylor and Francis Group; Boca Raton: 2007. [Google Scholar]
- 25.Simeoni U, Berger B, Junick J, et al. Modulation of the infant gut microbiota by a starter infant formula containing a synbiotic of bovine milk-derived oligosaccharides and Bifidobacterium animalis subsp. lactis CNCM I-3446. Environ Microbiol. 2016;18(7):2185–95. doi: 10.1111/1462-2920.13144. [DOI] [PubMed] [Google Scholar]
- 26.Steenhout PG, Rochat F, Hager C. The effect of Bifidobacterium lactis on the growth of infants: a pooled analysis of randomized controlled studies. Ann Nutr Metab. 2009;55(4):334–40. doi: 10.1159/000248992. [DOI] [PubMed] [Google Scholar]