Abstract
Mustard exposures result in epithelial–stromal separations in the cornea and epidermal–dermal separations in the skin. Large blisters often manifest in skin, while the cornea develops microblisters, and, when enough form, the epithelium sloughs. If the exposure is severe, healing can be imperfect and can result in long-term adverse consequences. For the cornea, this could manifest as recurrent corneal erosions. Since the corneal epithelial–stromal separations are in the region identified by electron microscopy as the lamina lucida, the same region as affected by the blistering disease junctional epidermolysis bullosa (JEB), we postulated that the molecules that are defective in JEB would be the same ones cleaved by mustard compounds. These molecules are α6β4 integrin and collagen XVII, which can be cleaved by MMP-9 and ADAM17, respectively. Therefore, our lab has tested MMP-9 and ADAM17 inhibitors as potential therapies to attenuate corneal mustard injury. Our results demonstrated that inhibiting MMP-9 and ADAM17 resulted in less epithelial–stromal separation in the corneas at 24 hours postexposure, as compared to using only medium as a therapy.
Keywords: cornea, mustard injury, mustard therapies, basement membrane zone, α6β4 integrin, collagen XVII
Introduction
Sulfur mustard (SM) is a blistering agent used in World War I (WWI) and in the Iran–Iraq war of the 1980s. When Germany began using mustard gas in WWI to attack their enemies, no one was fully prepared for the eye, lung, and skin damage that exposures caused. Although there are no U.S. Food and Drug Administration (FDA)–approved drugs for SM-induced wounds, some treatments have been suggested. A review of the effects of SM injury and potential treatments for the three affected organs (lung, skin, and eye) can be found in a report by Graham and Schoneboom.1 Background information concentrating on the ocular effects of SM can be found in Chapter 39 of the 2009 Handbook of Toxicology of Chemical Warfare Agents.2 SM is still a concern as a terrorist agent, especially since its use by ISIS was verified in Syria on August 21, 2015.3 Furthermore, in March 2016, the Boston Globe reported that an Islamic detainee gave American Special Operations Forces information about how ISIS has developed a powdered form of the agent to put into artillery shells.4 It is suspected this was the form added to the shells used in the August 2015 attack. The use of the vesicating agents in the last 100 years has resulted in many investigations aimed at identifying appropriate model systems to discern the molecular mechanisms of toxicity. Since there are no FDA-approved therapies for mustard injury, the goal is to use the information learned about mustard's mechanisms of action in eye, skin, and lung injury as clues to predict therapies that might improve outcomes and to test these therapies for efficacy.
SM causes short-term effects in the eye, including pain, tearing, photophobia, blepharospasm, and compromised vision.5 In skin, the short-term phenotype is often large blisters, whereas mustard injury to the cornea results in microblistering. When enough microblisters accrue, the hemidesmosomal attachments round up and stretch until they break, and the corneal epithelium separates from the stroma (Figs. 1 and 2). SM exposure can also result in long-term adverse consequences. Years after ocular mustard exposure, eyes can suffer from ulceration, perforation, opacification, neovascularization, and scarring. Devastating consequences can also include the development of dry eye disease6 and limbal stem cell deficiency.7
Figure 1.
Comparison of unexposed corneas with those with injury caused by NM and SM. Both vesicants induce separation at the epithelial–stromal border, starting with microblisters (D and G). When these coalesce, they progress to larger areas of epithelial–stromal separation (E and H). Eventually the epithelium loses the last tendrils holding on to the stroma, and the epithelial cell layer is released (F and I). Reprinted from Ref. 24.
Figure 2.
Electron micrographs of the epithelial–stromal border in an unexposed organ-cultured rabbit eye (A) and a freshly dissected rabbit eye (B). Note that the hemidesmosomes are flattened at the basal epithelial cell surface (A and B). In corneal organ cultures exposed to NM (C and D), and in rabbit eyes exposed in vivo to SM (E and F), the hemidesmosomes are not flat and sometimes appear quite plump. The lamina luicida after exposure to either vesicant is expanded between the hemidesmosomes, and in some micrographs thin tendrils can be seen reaching from the hemidesmosomes toward the stromal surface (C, D, E, and F). Reprinted from Ref. 24.
After WWI, ocular SM injury remained a topic of interest to scientists and physicians, and this interest increased as Germany began to show aggressive tendencies in the 1930s. Among the first physicians to publish on the damage caused to the eyes by SM was George Derby. In 1919, he described the lesion and its course of healing. Of note was his assertion (with which other ophthalmologists agreed) that eyes exposed to SM should never be bandaged, as this usually made the injury more severe.8
Several years later, Ida Mann began studying ocular mustard injury. A graduate of the Royal Free London School of Medicine for Women, she had chosen ophthalmic research as her specialty and worked at St. Mary's Hospital, and later at the Royal London Ophthalmic Hospital in Moorsfield. Around 1937, there was a general concern in England that SM would be used again if the country were involved in another war with Germany, and Mann knew it was estimated that ~90% of ocular casualties in WWI were from mustard gas. At the time, specific scientific information on mustard injury was scarce. Only clinical studies and postmortem pathology and histology assessments had been documented from human and animal exposures, so Mann began doing her own research on SM-induced ocular injury. Government officials heard of her research efforts, approached her, and convinced her to act as the head of a research team for the Chemical Defence Research Department. The aim was to study the effects of toxic chemicals on the eye and to discover therapies.9 Of all the treatments Mann tested on mustard-exposed animal and human eyes, she found only one that worked, and it worked best on long-term ocular problems, not short-term ones: the recurrence of erosions was found to be reduced when previously exposed patients wore glass contact lenses at least 5 h per day.10
Around the same time, concerns also existed in the United States about mustard being used by Germany in another war. In 1941, the National Research Council Committee on the Treatment of Gas Casualties requested that the Wilmer Institute scientists investigate the mechanisms of ocular mustard toxicity, since SM might be employed again. This led to an Office of Scientific Research and Development contract with Johns Hopkins University. Jonas Friedenwald was the Director of the Ophthalmic Pathology Laboratory at this time, and he directed the majority of the research activities. The resulting data were published in volume 82 of the Bulletin of the Johns Hopkins Hospital, and included papers by Friedenwald, Roy O. Scholz, Heinz Herrmann, W.R. Hughes, Jr., A. Edward Maumenee, and others.11 Friedenwald was an author on seven articles that dealt with the reaction mustard had on the cornea, such as how it affected mitotic activity, nuclear fragmentation, and wound healing, plus a report on a mechanical device for the extraction of tough tissue, such as the cornea. Maumenee and Scholz researched the histopathology of ocular SM and nitrogen mustard (NM) injury. Herrmann, who later became known for his work on protein synthesis during corneal development in the chick embryo, worked with Fay H. Hickman to understand how to combat the loosening of the bovine corneal epithelium from the stroma after mustard exposure. The pair also developed a mechanical scraping device, where the amount of weight on the blade that was needed to remove the epithelium indicated the strength of the epithelial–stromal integrity. In addition, they also studied utilization of ribose, consumption of pyruvate, and other metabolic processes in the cornea after exposure.11
The next major use of SM was in the Iran–Iraq war, which began in 1980 and went on until 1988. Iranian ophthalmologists, pulmonologists, and dermatologists reported extensively on the many injuries incurred by exposed military and civilian populations and on the immediate and delayed toxic effects. They also reported on the diagnosis and medical management of mustard injury (for a review on this, see Ref. 12).
In the 1990s, cell biologists were beginning to identify molecules that might be responsible for connecting the epidermal layer of the skin to the dermis13 and the corneal epithelium to its stroma.14 Components of the hemidesmosomes, the electron-dense structures at the basal surface of the basal epithelial cells (Fig. 2) were clearly important players in this strong attachment. Like the skin, the cornea uses the hemidesomosomal component α6β4 integrin to adhere the epithelial cells to the stroma,15–17 thereby facilitating cell layer integrity. In support of α6β4 being crucial to epidermal–dermal or epithelial–stromal integrity was the fact that certain genetic mutations in the β4 subunit caused blistering disease in patients.18 Defects in the blistering disease bullous pemphigoid greatly contributed to the understanding of the hemidesmosomal components, as it was found that bullous pemphigoid patients made antibodies that localized to hemidesmosome molecules.16 In addition, the bullous pemphigoid antigen was no longer observed if hemidesmosomes were disrupted by scraping the epithelium from the corneal stroma. The antigen was once again detectible after the wound was allowed to heal.15 In 1991, the other hemidesmosomal component, the 180-kDa bullous pemphigoid antigen 2, was cloned and sequenced and was found to be a transmembranous collagen. Receiving the next number in the series of molecules in the collagen family, its official name became collagen XVII.19–21
With the identification of intact α6β4 integrin and collagen XVII as critical hemidesmosomal factors for epithelial–stromal integrity, it was clear that these two molecules must be adversely affected by mustard. A possible explanation was that they were cleaved by enzymes that were activated by vesicants. We had previously shown that MMP-9 was highly activated after mustard exposure of the skin,22 so it was likely that inhibiting the enzyme could help retain epithelial–stromal attachment. As in the skin, MMP-9 is activated in the cornea after mustard exposure, as shown by the immunofluorescence image in Figure 3. In 2011, it was reported that MMP-9 cleaves the β4 ectodomain, leading to recurrent corneal erosions in mice.23 This observation also supported the assertion that mustard activation of MMP-9 adversely affects the hemidesmosomes. Our laboratory showed the efficacy of the MMP-9 inhibitor doxycycline in preserving epithelial–stromal separation,24 as Kadar et al. did previously25 and Horwitz et al.26 did subsequently. Our hypothesis also included the fact that ADAM17 cleaves collagen XVII27 and would likely play a role in disturbing the epithelial–stromal integrity. Therefore, inhibitors of ADAM17 should also reduce mustard injury of the cornea by preserving the epithelial–stromal junction. This was also found to be true in NM-exposed corneal organ cultures treated with ADAM17-inhibiting hydroxamates.28
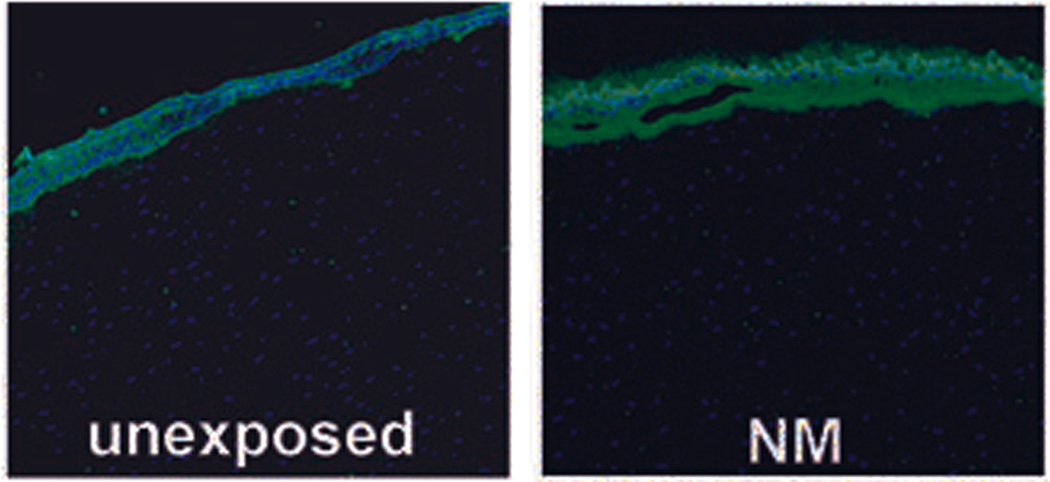
Figure 3.
MMP-9 immunofluorescence (IF): unexposed corneal organ cultures have very low levels of MMP-9 expression as assessed by IF (left panel). At 22 h after a 2-h exposure to NM, MMP-9 is very abundant at the epithelial–stromal border. Reprinted from Ref. 24.
The corneal organ culture system
Mice, rabbits, and pigs are among the animals that have been used for mustard studies. Rabbits were our choice of ocular models. Since we wished to make this a major project in the laboratory, we put off expensive in vivo studies, instead beginning by using rabbit corneal organ cultures to do initial identifications of mustard's mechanisms of mustard action or to screen therapies. The eyes used for organ culture were purchased from PelFreez, a company that sells rabbits for food. NM has been used as the vesicant in the organ culture studies for therapy evaluations, and the best candidate drugs move forward to in vivo exposures at a company like Battelle or MRI, who set up contracts for studies with in vivo SM exposures of rabbit eyes.24
With the evidence that epithelial–stromal separation was very likely a consequence of mustard activating MMP-9 and ADAM17, we tested for activity of these enzymes in corneal organ cultures exposed to NM.24,28 Corneas were dissected from the eyes retaining ~2 mm of scleral rim, then laid epithelial-side down in the curved wells of spot plates. Agar was added to their concave endothelial side, and, once it hardened, the corneas were flipped into culture dishes with the corneal epithelial side up. Medium was added only up to the scleral–corneal junction in order to retain epithelial differentiation. The NM exposure was for 2 h. The organ-cultured corneas could be analyzed immediately or could remain in culture for up to a week to experimentally test therapies. The limitations of the organ culture approach were that (1) the corneal nerves would be cut, and therefore we would not be able to discern how the nerves affected the healing; and (2) we would not be able to assess neovascularization, a very adverse consequence of mustard exposure, until live animal studies were performed. Still, the clear advantage of the organ culture system is that live animals are not used, and therefore many veterinary considerations are avoided. Also, not having to start with dissecting eyes from animals allowed faster setup of the cultures and faster preparation of tissue for histology, immunofluorescence, and protein and DNA analyses. In addition, with the relatively low cost of purchasing eyes (as compared to the cost of rabbits and their care in an in vivo study), we were able to assess a larger number of therapies. From such tests, only the best of the candidate drugs move forward to in vivo ocular studies. A final advantage of organ culture was that the use of animals for research is reduced by this approach, since the rabbit eyes are dissected from animals sacrificed to be sold as a food source.
Information learned from the corneal organ cultures: assessments of MMP-9
Knowing that inhibiting MMP-9, which cleaves α6β4 integrin, would likely reduce the degree of epithelial–stromal separation, corneal organ cultures were set up to evaluate therapies that target the enzyme. The first round of experiments tested whether evaluation of MMP-9 by immunofluorescence analysis was possible, and, as shown in Figure 3, the MMP-9 fluorescent signal was much higher in the NM-exposed corneas than in the unexposed corneas. Since many tetracycline family members have been shown to inhibit MMPs,17–20 we used organ cultures to do 24-h analyses of corneas after a 2-h NM exposure. For example, the effectiveness of sancycline, doxycycline, t-butyl sancycline, and the deaminated tetracycline dedimethyl-aminotetracycline (DDMT) were tested.24 When the corneas were removed from organ culture, the major form of analysis was by histology, similar to that shown in Figure 1. Since the organ cultures indicated that doxycycline was the most efficacious of the tetracyclines tested, we proceeded to in vivo ocular SM-exposure studies using doxycycline in a topical liquid formulation as well as in a hydrogel delivery system. Rabbit eyes were exposed in vivo to SM, and the eyes were followed for 28 days and evaluated at 1, 3, 7, and 28 days postexposure24 (Fig. 4).The liquid formulation was applied to the central cornea as drops and the hydrogel formulation, designed to release the drug over the course of 22 h, was delivered to the bottom eyelid pocket. The SM-exposed and control rabbit eyes were also assessed by visual examination, and corneal thicknesses were measured. The normal rabbit corneal thickness is ~350 µm, but severe mustard injury was able to increase this to close to 600 µm. The doxycycline treatments reduced swelling, and hence some of the cloudiness by 7 days after SM exposure, while corneas receiving no treatment were thicker and still cloudy. By 28 days postexposure, some corneas receiving no treatment were still cloudy, while doxycycline-treated eyes were clear. In addition, the corneal thickness of the doxycycline-treated eyes approached normal levels and showed less neovascularization. The results obtained were in agreement with those of others performing similar exposures and assessments,25,26 and the experiment offered an alternative delivery method for the doxycycline (i.e., hydrogels polymerized from two liquid components, placed in the bottom eyelid pocket). This had the advantage of releasing the drug over the course of 22 h, offsetting the removal of the drug from the cornea by blinking,
Figure 4.
An unexposed rabbit eye (top); the central three vertical images are in vivo SM-exposed rabbit eyes at 1 day, 3 days, and 7 days postexposure that received no treatment, and the bottom three horizontal images are SM-exposed eyes at 28 days postexposure. Note that the SM-exposed cornea receiving no therapy (left) is still cloudy, while the middle and right photos are eyes treated with doxycycline and are fairly well healed. There is some evidence of neovascularization, but less in the SM-exposed eye receiving no therapy. Reprinted from Ref. 24.
We next addressed whether preventing cleavage of the other known hemidesmosomal component, collagen XVII, would help retain the integrity of epithelial–stromal attachment. One of the enzymes known to cleave collagen XVII, ADAM17 (also known as tumor necrosis factor α–converting enzyme), was specifically assessed.28 As shown in Figure 5A, NM exposure produced an ADAM17 immunofluorescent signal which was not seen in the unexposed control. When the Innozyme TACE activity assay kit (Calbiochem) was used to determine how quickly ADAM17 was expressed after NM exposure (since the enzyme is known to be expressed early in wound healing), organ-cultured corneas showed that ADAM17 was activated very shortly after exposure to NM. We tested 0-min exposures (NM was applied and immediately washed off), 5-min exposures before washing NM off, and 10-min exposures followed by washing. As shown in Figure 5B (right panel_, the 0-min exposure had essentially the same ADAM17 activity as the unexposed control for the 24-h culture (Fig. 5B, left panel), but, with 5-min exposures, ADAM17 activity was detected. When analyzed after a 10-min NM exposure, ADAM17 activity was about 25% higher than in the unexposed samples, suggesting that activation had begun (Fig. 5B, right panel). At 22 h after a 2-h NM exposure (Fig. 5B, left panel), the level of ADAM17 activity assessed by the Innozyme kit showed that activity was ~25 times greater than that in unexposed corneas.
Figure 5.
(A) A mouse monoclonal antibody against ADAM17 detected no rabbit ADAM17 in unexposed corneas by immunofluorescence (left panel), but did detect it in NM-exposed corneas (right panel). Both had been cultured for 24 h. (B) The Innozyme TACE activity assay kit showed unexposed corneas kept in culture for 24 h show very little ADAM17 activity (~40 ng/mL) (left panel), while corneas exposed to NM for 2 h then kept in culture for 22 h showed ~ 24 times more ADAM17 activity than the unexposed corneas kept in culture for the same amount of time (right panel). (C) The Innozyme TACE activity kit determined how fast ADAM17 was turned on by NM. When applied and immediately washed off (0 min), or allowed to remain on the cornea for 5 or 10 min, ADAM17 assays indicated the enzyme was immediately activated. Thus, ADAM17 activation is an early response to NM exposure (left panel) Epithelial–stromal separation averaged 85% in the sets of corneas receiving no therapy, but with three applications of the hydroxamate compound NDH4417, separation was only ~17%. (right panel). The statistical analysis included one-way analysis of variance followed by Duncan's multiple comparison tests, then Duncan’s multiple comparison test or the unpaired Student’s t-test. All data published in Ref. 28. *A P value < 0.05 was considered significant.
Hydroxamates are known to inhibit ADAM17; therefore, four hydroxamates were synthesized for evaluation as potential therapies for ocular mustard exposure.28 Retro olvanil-8 (i.e., NDH4417) was found to reduce the ADAM17 levels to more than half that in exposed corneas receiving no therapy (Fig. 5C, right panel). The treatment also resulted in much less epithelial stromal separation (Fig. 5C, left panel). The data suggest that a hydroxamate version of retro olvanil-8 should proceed to in vivo ocular exposures, as was done with doxycycline.
Discussion
Using corneal organ cultures exposed to NM has helped to identify potential candidate therapies for ocular SM exposure. As they are much less expensive than live animal studies, the organ cultures enabled us to keep the number of live rabbits used in our experiments to a minimum until the final phases that needed to be corroborated by such studies. MMP-9 and ADAM17 inhibitors used as treatments at 2 h postexposure and repeated three times over the subsequent 22 h have been shown to improve the histologic outcome of NM-exposed corneas, as found by quantitatively measuring a lesser degree of epithelial–stromal separation. The edema that is a likely contributor to the blistering in in vivo corneas cannot be reliably assessed by the organ cultures, but we did determine in our in vivo rabbit eye exposure studies that therapy with the MMP inhibitor doxycycline correlated with reduced edema.24 Future studies will address other therapies to retain the integrity of the α6β4 integrin and collagen XVII in the adhesion complex, since holding the corneal epithelium to the stroma is essential for vision. We will also test combination therapies to improve outcome, and the best therapies will proceed to in vivo animal testing.
Acknowledgments
This work is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (U54AR055073) and the National Institute of Environmental Health Sciences (P30ES005022).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Graham JS, Schoneboom BA. Historical perspective on effects and treatment of sulfur mustard injuries. Chemico-Biological Interactions. 2013;206:512–522. doi: 10.1016/j.cbi.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Gordon MK, Enzenauer RW, Babin MC. Ocular Toxicity of Sulfur Mustard. In: Gupta RC, editor. Handbook of Toxicology of Chemical Warfare Agents. Chapter 39. 2009. pp. 575–594. [Google Scholar]
- 3.The Guardian. UN watchdog confirms mustard gas attack in Syria Organisation for the Prohibition of Chemical Weapons says child was probably filled by mustard gas in August during fighting between jihadi and rebels. [Accessed April 10, 2016];2015 Nov 6; http://www.theguardian.com/world/2015/nov/06/un-watchdog-confirms-mustard-gas-attack-in-syria. [Google Scholar]
- 4.Boston Globe. Reported by Helene Cooper and Eric Schmitt. ISIS chemical weapons specialist details militants’ plans, US says. [Accessed August 16, 2016];2016 Mar 9; https://www.bostonglobe.com/news/world/2016/03/09/captures-head-isis-chemical-weapons-unit-iraq-says/QDTVuduI6S0GKempf85hvN/story.html. [Google Scholar]
- 5.Solberg Y, Alcalay M, Belkin M. Ocular injury by mustard gas. Surv. Ophthalmol. 1997;41:461–466. doi: 10.1016/s0039-6257(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 6.Rowell MK, Kehe F, Balszuweit H, Thiermann H. The chronic effects of sulfur mustard exposure. Toxicology. 2009;263:9–11. doi: 10.1016/j.tox.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Javadi MA, Jafarinasab MR, Feizi S, et al. Management of mustard gas-induced limbal stem cell deficiency and keratitis. Ophthalmol. 2011;118:1272–1281. doi: 10.1016/j.ophtha.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Derby GS. Ocular Manifestations following exposure to various types of poisonous gases. Trans. Am. Ophthalmol. Soc. 1919;17:90–105. [PMC free article] [PubMed] [Google Scholar]
- 9.Frace DP. Ida Mann--her wartime career, 1939–1949: Clinical and Experimental Ophthalmology. Aust N Z J Ophthalmol. 1989;17:95–101. doi: 10.1111/j.1442-9071.1989.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 10.Mann I. A study of eighty four cases of delayed mustard gas keratitis fitted with contact lenses. Br. J. Ophthalmol. 1944;28:441–444. doi: 10.1136/bjo.28.9.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedenwald JS, Herrmann H, Hickman FH, et al. Volume devoted to Mustard studies, Bulletin of the Johns Hopkins Hospital. Vol. 82. Baltimore: Johns Hopkins Press; 1948. pp. 1–337. [Google Scholar]
- 12.Balali-Mood M, Hefazi M. The pharmacology, toxicology and medical treatment of sulphur mustard poisoning. Fundam. Clin. Pharmacol. 2005;19:297–315. doi: 10.1111/j.1472-8206.2005.00325.x. 2005. [DOI] [PubMed] [Google Scholar]
- 13.Klein CE, Steinmayer T, Mattes JM, et al. Integrins of normal human epidermis: differential expression, synthesis and molecular structure. Br J. Dermatol. 1990;123:171–178. doi: 10.1111/j.1365-2133.1990.tb01844.x. [DOI] [PubMed] [Google Scholar]
- 14.Stepp MA, Spurr-Michaud S, Tisdale A, et al. Alpha 6 beta 4 integrin heterodimer is a component of hemidesmosomes. Proc Natl Acad Sci U S A. 1990;87:8970–8974. doi: 10.1073/pnas.87.22.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujikawa LS, Foster CS, Gipson IK, K I, Colvin RBJ. Basement membrane components in healing rabbit corneal epithelial wounds: immune-fluorescence and ultrastructural studies. Cell Biol. 1984;98:128–138. doi: 10.1083/jcb.98.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anhalt GJ, Jampel HD, Patel HP, et al. Bullous pemphigoid auto-antibodies are markers of corneal epithelial hemidesmosomes. Invest Ophthalmol Vis Sci. 1987;28:903–907. [PubMed] [Google Scholar]
- 17.Pulkkinen L, Rouan F, Bruckner-Tuderman L, et al. Novel ITGB4 mutations in lethal and nonlethal variants of epidermolysis bullosa with pyloric atresia: missense versus nonsense. Am J Hum Genet. 1998;63:1376–1387. doi: 10.1086/302116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vidal F, Aberdam D, Miquel C, et al. Integrin beta 4 mutations associated with junctional epidermolysis bullosa with pyloric atresia. Nat Genet. 1995;10:229–234. doi: 10.1038/ng0695-229. [DOI] [PubMed] [Google Scholar]
- 19.Li KH, Sawamura D, Giudice GJ, et al. Genomic organization of collagenous domains and chromosomal assignment of human 180-kDa bullous pemphigoid antigen-2, a novel collagen of stratified squamous epithelium. J. Biol. Chem. 1991;266:24064–24069. [PubMed] [Google Scholar]
- 20.Giudice GJ, Emery DJ, Diaz LA, A L. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol. 1992;99:243–250. doi: 10.1111/1523-1747.ep12616580. [DOI] [PubMed] [Google Scholar]
- 21.Li K, Tamai K, Tan EM, Uitto J. Cloning of type XVII collagen. Complementary and genomic DNA sequences of mouse 180-kilodalton bullous pemphigoid antigen (BPAG2) predict an interrupted collagenous domain, a transmembrane segment, and unusual features in the 5'-end of the gene and the 3'-untranslated region of the mRNA. J Biol Chem. 1993;268:8825–8834. [PubMed] [Google Scholar]
- 22.Shakarjian MP, Bhatt P, Gordon MK, et al. Preferential expression of matrix metalloproteinase-9 in mouse skin after sulfur mustard exposure. J Appl Toxicol. 2006;26:239–246. doi: 10.1002/jat.1134. [DOI] [PubMed] [Google Scholar]
- 23.Pal-Ghosh S, Blanco T, Tadvalkar G, et al. MMP9 cleavage of the β4 integrin ectodomain leads to recurrent epithelial erosions in mice. J Cell Sci. 2011;124:2666–2675. doi: 10.1242/jcs.085480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon MK, Desantis A, Deshmukh M, et al. Doxycycline hydrogels as a potential therapy for ocular vesicant injury. J Ocul Pharmacol Ther. 2010;26:407–419. doi: 10.1089/jop.2010.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kadar T, Dachir S, Cohen SL, et al. Ocular injuries following sulfur mustard exposure--pathological mechanism and potential therapy. Toxicology. 2009;263:59–69. doi: 10.1016/j.tox.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Horwitz V, Dachir S, Cohen M, et al. The beneficial effects of doxycycline, an inhibitor of matrix metalloproteinases, on sulfur mustard-induced ocular pathologies depend on the injury stage. Curr Eye Res. 2014;39:803–812. doi: 10.3109/02713683.2013.874443. [DOI] [PubMed] [Google Scholar]
- 27.Zimina EP, Bruckner-Tuderman L, Franzke CW. Shedding of collagen XVII ectodomain depends on plasma membrane microenvironment. J Biol Chem. 2005;280:34019–34024. doi: 10.1074/jbc.M503751200. [DOI] [PubMed] [Google Scholar]
- 28.DeSantis-Rodrigues A, Chang YC, Hahn RA, et al. ADAM17 Inhibitors Attenuate Corneal Epithelial Detachment Induced by Mustard Exposure. Invest Ophthalmol Vis Sci. 2016;57:1687–1698. doi: 10.1167/iovs.15-17269. [DOI] [PMC free article] [PubMed] [Google Scholar]