Abstract
The evolution of the eye has been a major subject of study dating back centuries. The advent of molecular genetics offered the surprising finding that morphologically distinct eyes rely on conserved regulatory gene networks for their formation. While many of these advances often stemmed from studies of the compound eye of the fruit fly, Drosophila melanogaster, and later translated to discoveries in vertebrate systems, studies on vertebrate lens development far outnumber those in Drosophila. This may be largely historical, since Spemann and Mangold’s paradigm of tissue induction was discovered in the amphibian lens. Recent studies on lens development in Drosophila have begun to define molecular commonalities with the vertebrate lens. Here, we provide an overview of Drosophila lens development, discussing intrinsic and extrinsic factors controlling lens cell specification and differentiation. We then summarize key morphological and molecular events in vertebrate lens development, emphasizing regulatory factors and networks strongly associated with both systems. Finally, we provide a comparative analysis that highlights areas of research that would help further clarify the degree of conservation between the formation of dioptric systems in invertebrates and vertebrates.
Keywords: Semper cell, Corneagenous, Fiber cell, Cone cell, Corneal lens
Drosophila compound eye development: an overview
The adult Drosophila compound eye consists of ~750 individual eye units, called ommatidia. While this organization is morphologically distinct from the single lens camera eye of vertebrates, each ommatidium is composed of the same three basic structures as the vertebrate eye: a neural retina, a pigmented epithelium, and a cornea/lens. Drosophila develop from an egg to an adult in approximately 10 days. During this time, the embryo transitions through three larval stages, followed by ~ 4 days as a pupa before enclosing as an intact adult. Fly eye development begins during embryogenesis, when a small number of cells (~6) are set aside on each side of the animal as an eye field. During the first two and a half larval stages, a subset of these cells will continue to proliferate within a flat epithelial sheet known as the eye-antennal imaginal disc, that in the adult will give rise to two compound eyes, the ocelli (another visual system at the vertex of the head), the antennae, and much of the head cuticle (Held 2002). The compound eye field itself is determined during the second larval stage as a consequence of the overlapping expression of a network of “master eye regulators,” which includes the transcriptional regulators Pax6 (Eyeless and Twin of Eyeless), Sine Oculis (So), Eyes Absent (Eya), and Dachshund (Dac). This network is remarkably conserved across phyla, and its universal role in specifying ocular tissue has been extensively reviewed (see Kumar 2001, 2010; Gehring 2005).
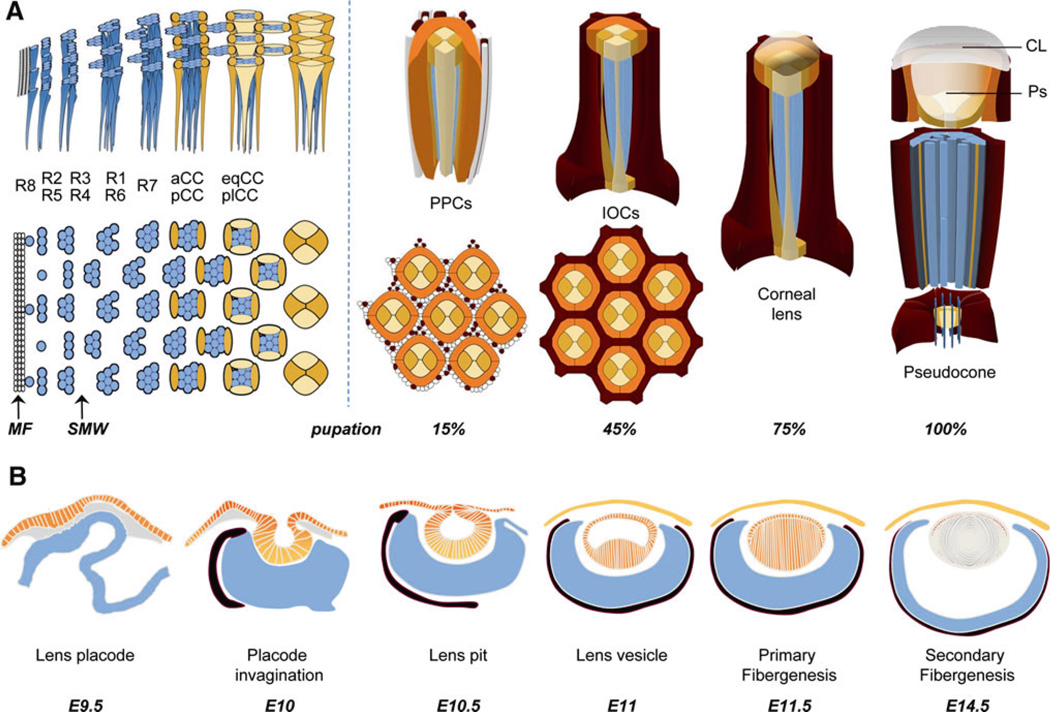
During the third larval stage, signaling from Hedgehog (Hh) and the bone morphogenetic protein (BMP)-related factor, Decapentaplegic (Dpp), initiates at the most posterior region of the eye field, leading to G1 cell cycle arrest and retinal cell fate determination. This signaling occurs within a stripe of cells that are apically constricted, known as the morphogenetic furrow (MF). The MF moves anteriorly across the eye field, leaving behind a new row of regularly spaced ommatidial “founder” cells approximately every 2 h. Each founder cell represents the first of eight retinal neurons present in the adult eye, called the R8 photoreceptor. Soon after its specification, the R8 secretes the EGF ligand, Spitz, and this initiates a sequential and stereotypical recruitment of the R1–R7 photoreceptors (reviewed by Wolff and Ready 1993; Freeman 1997; Frankfort and Mardon 2002; Hsiung and Moses 2002; Roignant and Treisman 2009). The R8 first recruits the R2/ R5 and R3/R4 cells pairwise. The remaining unspecified precursors are then released from G1 and undergo a single additional round of proliferation, termed the “second mitotic wave” (SMW). The SMW is important for creating enough precursors to generate the remaining cell types in the adult eye (Wolff and Ready 1991; de Nooij and Hariharan 1995). After the SMW, the R1/R6 pair and R7 photoreceptor are recruited to complete neuronal specification, followed shortly by pairwise recruitment of the four cone cells (CCs)—first the anterior/posterior (a/p) CCs, then the equatorial/polar (eq/pl) CCs. CCs are the last to be specified prior to pupation, and are the first non-neuronal cells produced in the eye. Shortly after puparium formation (~15% pupation), the CCs recruit the two primary pigment cells (PPCs), and together, these cells contribute to the survival and patterning of about 12 shared interommatidial cells (IOCs), consisting of six secondary pigment cells (SPCs) interlaced with alternating tertiary pigment cells (TPCs) and a mechanosensory bristle (Fig. 1, Cagan and Ready 1989a; Wolff and Ready 1993). Once the correct complement of cells is reached—approximately 35% through pupation—the remaining unspecified cells (2–3 cells/ommatidia) are eliminated by apoptosis, and the specified cells begin terminal differentiation (for further details of these processes, see reviews by Wolff and Ready 1993; Bao 2010; Charlton-Perkins and Cook 2010; Zipursky and Sanes 2010).
Fig. 1.
Schematic representation of invertebrate and vertebrate eye development. a In the Drosophila third larval stage, the morphogenetic furrow (MF) moves anteriorly across the eye imaginal disc, blocking proliferative progenitors in G1. From this progenitor pool, the R8, R2/R5, and R3/R4 cells of the neural retina (light blue) are recruited. This is followed by the second mitotic wave, where the remaining progenitors undergo one additional round of mitosis. From these progenitors, the remaining photoreceptors, R1/R6 and R7 are recruited. After photoreceptor recruitment is complete, the anterior and posterior cone cells (aCC/pCC) (yellow) and equatorial and polar cone cells (eqCC/plCC) (light orange) are recruited pairwise. In early pupation, primary pigment cells (PPCs, dark orange) are added, which together with the CCs, complete the full complement of the lens-secreting cells. Soon afterwards, specification of interommatidial pigment cells (dark red) occurs and any remaining non-specified progenitors are eliminated by apoptosis. By ~45% pupation, all cells in the eye begin to terminally differentiate. In the lens, this includes secretion of the corneal lens (CL), followed by secretion of the pseudocone (Ps). IOCs accumulate pigment, while the light-sensing apical membranes (rhabdomeres) of the photoreceptors (PRs) elongate. CCs span the depth of the retina, with the feet ensheathing the PR axons proximally, and the apical surfaces secreting the corneal lens and pseudocone distally. b The onset of lens development in the vertebrate eye is first observed as a thickening of the surface ectoderm into the lens placode (orange) and requires the underlying neural ectoderm (blue) and surrounding mesenchyme (grey). In mouse, this occurs at embryonic day 9.5 (E9.5). The lens placode then invaginates to form the lens pit which pinches off to form the lens vesicle. Primary fibers first form to create a lifelong central core in the lens. Proliferation of precursors continues in the anterior epithelial layer (AEL) (orange nuclei) and the formation and continued addition of secondary fiber cells occurs at the equator and periphery of the lens. The neural retina (light blue) is surrounded by the pigmented epithelium (black). The vitreous humor (white) occupies the space between the lens and retina
The dioptric system in each adult ommatidium is comprised of a biconvex corneal lens and an underlying vitreal pseudocone (Cagan and Ready 1989a; Wolff and Ready 1993). Developmentally, the corneal lens is the first structure to be formed, beginning at ~50% pupation, and it is secreted from microvilli projections present on the apical surfaces of both the CCs and PPCs (Cagan and Ready 1989a; Frohlich 2001). Corneal lens secretion is, for the most part, complete by ~75% pupation, although radio-labelling of metabolites suggests continuous addition to this structure throughout the fly’s lifetime (Yoon et al. 1997). After corneal lens secretion, the CCs retract their microvilli and begin secreting the pseudocone, a clear, gelatinous substance that likely does not provide focusing power itself, but rather serves as a vitreous layer to distance the corneal lens from the photoreceptors to achieve the correct focal length (Cagan and Ready 1989a; Wolff and Ready 1993; Frohlich 2001; Nilsson and Kelber 2007). Because the hard corneal lens is continuous with the cephalic exoskeleton, as the CCs secrete the pseudocone, their cell bodies are pushed away from the surface and become flattened between the base of the corneal lens and the apical surfaces of the photoreceptors. The PPCs provide the lateral lining of the pseudocone (Cagan and Ready 1989a). The highly organized hexagonal patterning of the IOC apical surfaces contributes to restricting the corneal lenses to individual ommatidia. Therefore, developmental defects in CC, PPCs and IOCs disrupt the regular array of corneal lenses that are visible on the fly eye surface. Mature corneal lenses, observed by transmission electron microscopy, display regularly repeating lamellae of electrondense microfibrils, spanning the width of each ommatidium. At the tapering edges of the lens, an intermediate electrondense substance secreted by SPCs likely produces the lens’ convex shape (Cagan and Ready 1989a; Wolff and Ready 1993).
Despite our general understanding of the anatomy of the dioptric system, essentially nothing is known about its molecular makeup. In most species, lenses primarily consist of highly concentrated, water-soluble, proteins that can crystallize into optically clear structures. These proteins, called Crystallins, frequently function as metabolic enzymes or stress proteins in non-ocular tissues, and are co-opted to the lens based on their crystallization and refractive properties (Piatigorsky 2003). To identify fly Crystallins, Komori et al. (1992) isolated highly enriched proteins present in freeze-fractured fly corneal lens preparations. They further purified and characterized the most abundant of these, naming it Drosocrystallin (Komori et al. 1992). The two additional proteins isolated in this study remain uncharacterized. Immunogold staining localized Drosocrystallin to the electron-dense microfibrils of the adult corneal lens, and developmental studies identified the CCs as its primary source, although it is also expressed by PPCs (Komori et al. 1992; Charlton-Perkins and Cook 2010). Consistent with Crystallins often being involved in non-refractive processes, Drosocrystallin expression has also recently been reported in developing mechanosensory bristles (Dziedzic et al. 2009), although its function here is unknown. α-Crystallins are a subclass of stress-inducible heat shock proteins that are evolutionarily conserved across many species and are present in all vertebrate lenses (Piatigorsky 2003). Drosophila encodes genes for at least four α-Crystallin-related proteins: hsp22, hsp23, hsp26 and hsp27 (Ingolia and Craig 1982). Hsp23 is specifically upregulated in adult CCs under heat-stress conditions (Marin et al. 1996), and is likely to be expressed in CCs and PPCs during late pupation (Pauli et al. 1989), but whether any of the putative α-Crystallin proteins are expressed in the normal adult corneal lens is unknown. Another protein reported as being expressed in the fly corneal lens, called Retinin, is produced in photoreceptors, requires Eya for its expression, and is secreted into the corneal lens (Kim et al. 2008). Currently, no function for retinin has been reported. While few proteins are known to be expressed in the adult corneal lenses, our knowledge of pseudocone components is even more bleak. To date, only two monoclonal antibodies have been shown to recognize this structure, but their target proteins were never defined (Fujita et al. 1982; Edwards and Meyer 1990), and these reagents are no longer available. Thus, identifying the molecular components of the corneal lens and vitreous pseudocone is a necessary step for advancing our understanding of how these ocular structures develop.
Drosophila lens specification
During Drosophila eye development, the successive specification of an individual cell’s fate depends on signals from previously specified cell types (see Nagaraj and Banerjee 2004; Doroquez and Rebay 2006). Thus, photoreceptors (PRs) initiate recruitment of CCs, which in turn signal PPC recruitment, and the CCs and PPCs together provide instructive cues for IOC survival, apoptosis and patterning. Considerable progress has been made toward elucidating how the different neuronal cell populations arise in the Drosophila retina, and how the exquisite patterning of the pigmented epithelium is achieved (Brachmann and Cagan 2003; Nagaraj and Banerjee 2004; Voas and Rebay 2004; Bao 2010). However, significantly less is known regarding the development of the cells that give rise to the lens: CCs and PPCs. Here, we summarize our current understanding of the molecular and developmental events underlying the formation of these cell types (Fig. 2), as well as how these cells help pattern the highly organized crystalline lattice of the secondary and TPCs, which comprise the pigmented epithelia and help shape the fly lens.
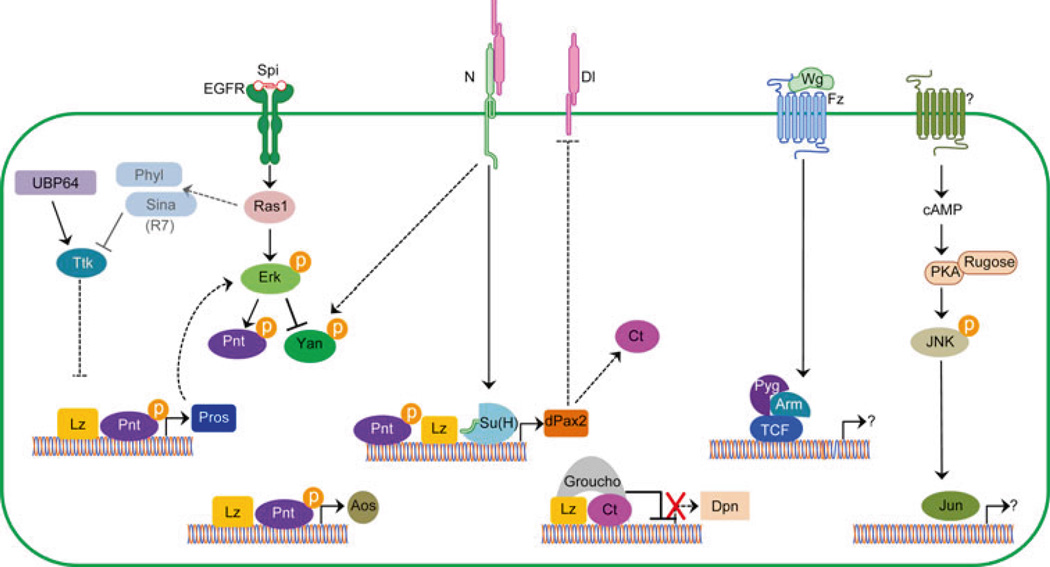
Fig. 2.
Schematic representation of cone cell specification. Summary of intrinsic and extrinsic factors involved in the differentiation of the lens-secreting cells in the fly eye. Solid lines are indicative of direct targets while dashed lines represent genetic interactions. Note that Sina and Phyl are expressed in the R7 to prevent Ttk expression, thus preventing R7 photoreceptors from becoming lens-secreting CCs. See text for further details
Intrinsic factors
A hierarchy of transcription factors orchestrate fly lens development. First, sine oculis (so), glass (gl), and lozenge (lz) specify a multipotential progenitor cell population arising from the SMW, which will ultimately generate the R1, R6 and R7 photoreceptors, as well as CCs, PPCs and IOCs. Second, tramtrack (ttk), prospero (pros), dPax2 and cut restrict these multipotential cells into neuronal versus non-neuronal cell types. pros and dPax2 are also important for generating different subsets of CCs. Third, dPax2 and BarH1 participate in PPC specification and differentiation. Finally, dPax2 and SoxN are likely to participate in terminal differentiation events such as activation of Crystallin expression. Below, we describe each of these events in more detail.
Generation of a multipotential precursor pool: roles for So, Gl, and Lz
sine oculis (so) is necessary for the induction and formation of the entire eye structure (Cheyette et al. 1994; Serikaku and O’Tousa 1994); hence, so mutants completely lack eyes. glass (gl) mutants, on the other hand, form eyes, but these eyes are small and its many fused corneal lens facets make the eyes appear “glassy” by light microscopy (Moses et al. 1989) (e.g. see Fig. 3). Finally, lozenge (lz) was identified based on the lozenge-like shape of its mutant eyes, but these mutants also appear “glossy” or “spectacled”. Both classes of lz mutants lack lenses, while “spectacled” mutants also have pigment cell defects (Patterson and Muller 1930; Clayton 1957).
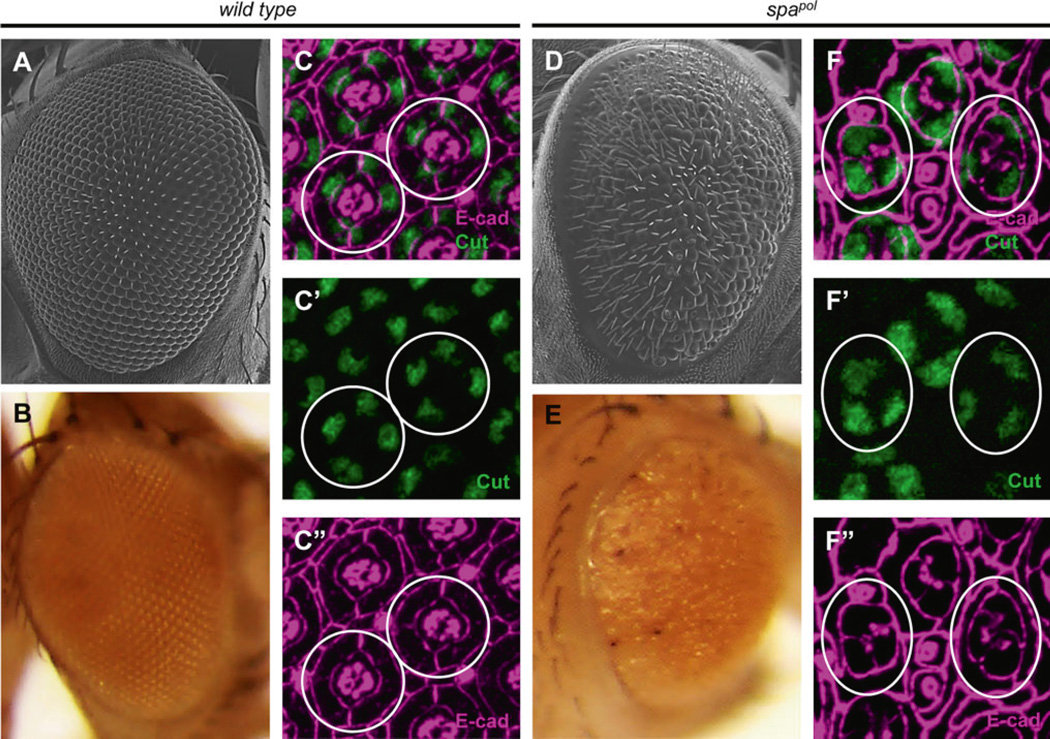
Fig. 3.
Morphology of wild-type and mutant fly lens facets. Scanning electron microscopy (a) and light microscopy (b) of the external surface of a wild-type adult fly eye shows the characteristic repeating hexagonal arrangement of corneal lens facets. Immunostaining of pupal retinas with E-cad (magenta c, c″) shows the highly regular, organized patterning of the primary pigment cells and interommatidial cells (see Fig. 5 for more details), while the transcripton factor Cut (green c′, c″) reveals the quartet of four cone cells present in the center of each ommatidia. Circles highlight individual ommatidia. d–f In spapol mutants, disorganized, fused, and smaller lens facets are apparent as a rough eye by scanning electron microscopy (d) and a “sparkling” appearance by light microscopy (e). Developmentally, this phenotype results from loss of an average of one CC per ommatidia, aberrant PPC differentiation, and premature IOC death (Fu and Noll 1997; Siddall et al. 2003; Charlton-Perkins et al. 2011), apparent by E-cad (magenta f, f″) and Cut (green f, f″) expression
The so retinal determination gene sits at the top of the lens specification cascade. One of its downstream targets is gl, a zinc finger transcription factor-encoding gene activated in all cells after the MF (Yan et al. 2003). Together, So and Gl activate lz, a Runx1 homologue that is necessary for R1/ 6, R7, CC and PPC specification, and prospero, a Prox1 homologue required for R7 terminal differentiation and CC specification (Xu et al. 2000; Cook et al. 2003; Yan et al. 2003; Hayashi et al. 2008; Charlton-Perkins et al. 2011). Like vertebrate Runx orthologs, Lz physically interacts with several other DNA-binding partners to regulate downstream target genes (Canon and Banerjee 2003; Jackson Behan et al. 2005). For example, Lz functions together with the Ras/MAPK-activated ETS-related transcriptional activator PntP2 to induce expression of several genes important for fly lens development, including pros, dPax2, and argos (Fu and Noll 1997; Xu et al. 2000; Wildonger et al. 2005). Lz also cooperatively binds with the homeobox transcription factor, Cut, to repress expression of deadpan (dpn), a proneural bHLH-encoding gene, in CCs (Canon and Banerjee 2003). While less is known about direct targets of Lz in R1 and R6 cells, it genetically suppresses expression of a COUP-TF-related nuclear receptor encoding gene, seven-up, and promotes expression of the homeobox transcription factor-encoding gene BarH1 (Daga et al. 1996). Thus, Lz both activates and represses a number of genes involved in retina and lens formation and further restricts the developmental potential of So- and Gl-expressing cells.
Restricting neuronal from non-neuronal cell fates: roles for Ttk, Cut, Pros, and dPax2
Several factors are involved in further restricting Lz-competent cells into neuronal (photoreceptors) versus non-neuronal (CCs and PPCs) cell types. tramtrack (ttk) encodes two zinc finger transcriptional repressors with important roles in early CC fate determination, and mutants produce mild to severe rough eye phenotypes (Xiong and Montell 1993). The zinc finger-encoding region in ttk is alternatively spliced to generate two isoforms (Ttk69 and Ttk88) with different DNA-binding specificities (Read and Manley 1992). In eye imaginal discs, both ttk isoforms are expressed in CCs, where they prevent these cells from adopting a neuronal cell fate (Xiong and Montell 1993; Lai et al. 1997), much like their roles during peripheral sensory organ precursor development (Guo et al. 1996). Although the R1, R6 and R7 PRs also express ttk, their proteins are degraded in these cells by polyubiquitination via Sina, a RING domain E3 ligase, and Phyllopod, a Ras-dependant adapter protein (Li et al. 1997; Tang et al. 1997). In contrast, Ttk is stabilized in CC precursors through deubiquitylation by the ubiquitin protease Ubp64 (Bajpe et al. 2008). Ttk also promotes cell cycle exit after the SMW through direct transcriptional repression of the cell cycle-promoting Cdc25 phosphatase, String, suggesting that Ttk proteins have dual roles during CC development (Baonza et al. 2002). Besides String, no other directs targets of Ttk have been defined in the eye, although pros and lz, normally expressed higher in R7s than CCs, have been proposed as potential Ttk targets (Xu et al. 2000; Siddall et al. 2009).
prospero (pros) encodes an atypical homeodomain transcription factor that is expressed highly and continuously in R7 PRs, and at lower levels and transiently in CC precursors (MCP and TC personal observation, Kauffmann et al. 1996; Cook et al. 2003). As mentioned earlier, pros is up-regulated by So and Gl, as well as by the Ras-activated ETS transcription factor PntP2 (Xu et al. 2000; Hayashi et al. 2008). R7s and CCs arise from a subset of equipotential cells known as the “R7 equivalence group” (Dickson et al. 1992; Greenwald and Rubin 1992; Tomlinson and Struhl 2001). All five cells are stimulated by EGFR-dependent Ras/MAPK signaling, but only one of these cells, the presumptive R7, receives additional input from the R8 photoreceptor to activate another tyrosine kinase receptor expressed in the R7 equivalence group, called Sevenless. Sevenless, like EGFR, signals through Ras/ MAPK, and the additional Ras/MAPK activation provided by its activation stimulates this cell to differentiate into a neuron. Those cells that do not receive Sevenless signaling have less Ras/MAPK activation and subsequently differentiate into CCs (see Greenwald and Rubin 1992; Tomlinson and Struhl 2001 for review). Thus, the decision to become a neuronal R7 versus a non-neuronal CC is dependent on Ras/MAPK levels. Importantly, pros is not only a downstream target of Ras/MAPK signaling, but also positively feeds back into this pathway to further stimulate Ras/MAPK signaling. By doing so, pros functions as a proneural switch during R7 versus CC fate choices (Charlton-Perkins et al. 2011).
dPax2 was originally identified for its role in lens development by its eye-specific allele, spapol, that appears sparkling or polished by light microscopy (Fig. 3, Rickenbacher 1954). dPax2 encodes a paired domain transcription factor, is expressed in CCs and PPCs, and as discussed more below, is important for various aspects of CC/PPC differentiation. dPax2 was also recently shown to prevent CCs from adopting an R7 photoreceptor fate (Charlton-Perkins et al. 2011). This latter function is mediated by promoting Notch signaling in two ways: first, by up-regulating E(spl) expression, and second, by repressing Delta expression, two events commonly linked to non-neuronal fate specification in the developing nervous system (Beatus and Lendahl 1998; Artavanis-Tsakonas et al. 1999; Doroquez and Rebay 2006).
The transcription factor Cut is currently the only known CC-restricted gene product (Blochlinger et al. 1993), and it is often used as a universal marker for CCs. However, besides its ability to function with Lz to repress dpn in the imaginal disc (Canon and Banerjee 2003), cut gene function during lens formation has not been explored. In addition, despite the widely accepted view that Cut expression is the “gold-standard” for defining specified CCs, it is not expressed in dPax2-mutant CC precursors in the imaginal disc although CC number is only mildly affected in these mutants (Fu and Noll 1997; Charlton-Perkins et al. 2011). This indicates that Cut expression is not required for CC specification. Curiously, however, Cut is expressed later in pupation in dPax2-mutant CCs (Fu and Noll 1997), suggesting that cut undergoes two temporally distinct phases of regulation during CC maturation—early in the imaginal disc, and later during pupation. Consistent with dPax2 contributing to its early expression, Cut displays a similar differential expression pattern with dPax2 in early CC precursors, being more highly expressed in a/p than eq/pl CCs (Charlton-Perkins et al. 2011, see next section). Future studies await to determine what contributes to Cut’s late non-dPax2-dependent expression during pupation, and its potential role(s) in lens development.
Specifying distinct cone cell populations: roles for Pros and dPax2
The four CCs are recruited pairwise: the anterior and posterior CCs (a/p CCs) are recruited immediately after the R7 PR, followed closely by the equatorial and polar CCs (eq/pl CCs) (Wolff and Ready 1993). Although all four CCs are often considered identical, earlier-born CCs are both genetically and morphologically distinct from later-born CCs (Wolff and Ready 1993; Charlton-Perkins et al. 2011). Molecularly, these two CC subtypes can be distinguished by the differential expression of dPax2 and Pros: both factors are expressed in all four CCs, but dPax2 is enriched in a/p CCs, while Pros is enriched in the eq/pl CCs (Charlton-Perkins et al. 2011). Importantly, CC number and lens formation are only mildly affected in loss-of-function mutants for either pros or dPax2, but simultaneously removal of both factors completely abolishes CC specification and lens formation (Charlton-Perkins et al. 2011). Epistasis experiments indicate that pros and dPax2 do not rely on each other for their expression, suggesting that they have partially overlapping functions during lens formation. Currently, the significance of the two separate CC subtypes is unclear, but it is perhaps relevant for establishing different local signaling environments to allow appropriate PPC and IOC specification, patterning and survival (see below).
Primary pigment cell differentiation: roles for dPax2/BarH1
Early in pupation, Pros is no longer detected in CCs, and dPax2/Cut expression increases to uniform levels in all CCs (Kauffmann et al. 1996; Cook et al. 2003) (MCP and TC, unpublished). dPax2 expression is also expanded into the recently recruited PPCs (Fu and Noll 1997). Here, dPax2 does not appear to be important for PPC formation per se, but is critical for activating the expression of two related homeodomain transcription factors, BarH1 and BarH2 (Fu and Noll 1997; Charlton-Perkins et al. 2011). Mutations in BarH1/2 cause a “blueberry” eye phenotype (a small hole in the center of each lens), with lenses often fused (Higashijima et al. 1992). In addition, TEM analysis of BarH1/2 mutants shows that PPCs fail to extend to the surface of the eye, and instead, remain at the level of the CCs, causing the pseudocones from separate ommatidia to fuse (Higashijima et al. 1992). These genetic studies support previous EM-based models that PPCs are important for providing pseudocone structure, while CCs are involved in pseudocone secretion. Interestingly, BarH1 misexpression converts a/p CCs, but not eq/pl CCs, into PPCs (Hayashi et al. 1998). Since a/p CCs express higher levels of dPax2 than eq/pl CCs, these data suggest that BarH1 and high dPax2 together promote PPC differentiation, whereas Pros and dPax2 are important to promote CC differentiation.
Terminal differentiation: roles for dPax2/SoxN
Despite architectural differences between vertebrate and invertebrate lenses, the expression and regulation of lens-specific genes is likely conserved (Fig. 4, Tomarev and Piatigorsky 1996; Kozmik 2005). Particularly compelling evidence for this was provided by experiments demonstrating that the enhancer of the chicken δ-Crystallin gene was specifically activated in CCs when introduced into Drosophila (Blanco et al. 2005). In chick, this enhancer is under direct control of Pax6 and Sox1, but in Drosophila, dPax2 activates this enhancer by binding to the Pax6 sites, while fly Sox Neuro (SoxN) mediates Sox1’s function (Blanco et al. 2005) (Fig. 4a). These findings provided further support for the hypothesis that Pax2 and Pax6 are derived from a common PaxB ancestor (Kozmik et al. 2003) (Fig. 4b), and contributed the first evidence that a Sox factor was expressed in the fly lens. Despite the significance of this study, there is currently no evidence that a Drosophila Crystallin is expressed during the early stages of CC development when the chick Crystallin enhancer was expressed. In addition, although recent studies have implicated dPax2 in activating Drosocrystallin expression, whether this is direct or indirect is unclear (Dziedzic et al. 2009). Finally, it remains uncertain what role, if any, SoxN plays during Drosophila CC or PPC specification, Crystallin expression, or corneal lens secretion. Nevertheless, further research on dPax2 and SoxN during terminal differentiation events in the fly eye should deepen our insight into the evolutionary conservation of lens development across phyla.
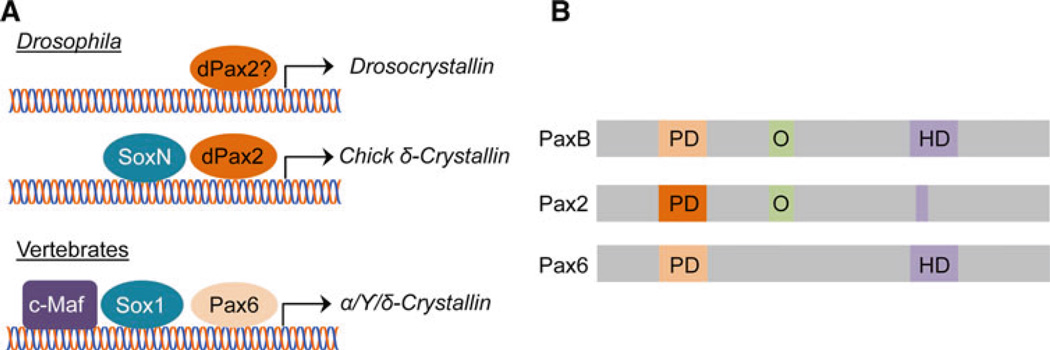
Fig. 4.
Crystallin regulation by PaxB-derived dPax2 and Pax6. a Currently, only one Crystallin-encoding gene, Drosocrystallin, has been studied in Drosophila. Drosocrystallin expression is lost in dPax2 mutants, but whether dPax2 regulates this gene directly or indirectly is not clear (Dziedzic et al. 2009). Interestingly, however, the chicken δ-Crystallin enhancer, normally regulated by vertebrate Pax6 and Sox1, is activated in Drosophila CCs by dPax2 and SoxN (Blanco et al. 2005), revealing that common regulatory mechanisms are likely to be used to control lens-specific gene expression in vertebrates and invertebrates. b dPax2 and Pax6 appear to be derived from a common ancestor, PaxB, which contains a paired domain (PD) and homeodomain (HD) similar to Pax6, and an octapeptide (O) region that is found in Pax2 (Kozmik et al. 2003). While the PD of dPax2 shares little sequence identity with the PD of Pax6, they are capable of binding similar sequences (Blanco et al. 2005)
Extrinsic factors
EGFR and Notch signaling
The EGFR/Ras/MAPK and Notch/Delta signaling pathways are reiteratively and combinatorially used for specifying almost all cell types in the fly eye, including CCs (see reviews by Freeman 1997; Tomlinson and Struhl 2001; Doroquez and Rebay 2006; Vivekanand and Rebay 2006). Indeed, because of the high sensitivity of R7 versus CC fate decisions on levels of Ras-dependent signaling, discussed earlier, this cell fate choice provided an important model system for defining the conserved components of the EGFR/Ras/MAPK signaling pathway (Dickson and Hafen 1994; Wassarman et al. 1995; Freeman 1996; Doroquez and Rebay 2006). Significant cross-talk between EGF and Notch signaling occurs during CC specification, however, making it difficult to separate these pathways in this cellular context. For instance, EGF signaling (via activated MAPK, or pERK) leads to increased Delta expression (Tsuda et al. 2002) and degradation of the intracellular domain of Notch (Nagaraj and Banerjee 2009), together resulting in a cell autonomous reduction in Notch signaling. Conversely, Notch signaling promotes expression of yan, a gene encoding an ETS-related transcriptional repressor that negatively regulates downstream targets of Ras/MAPK signaling (Rohrbaugh et al. 2002). Thus, in both cases, EGF and Notch signaling function antagonistically. In contrast, EGF and Notch signaling function additively to activate dPax2 expression—the MAPK-activated ETS transcriptional activator, PntP2, and the NotchICD/Su(H) activation complex both provide positive input on the CC-specific enhancer of dPax2 (Flores et al. 2000; Swanson et al. 2010).
Consistent with the fact that both EGF and Notch activate dPax2, many studies have demonstrated that EGF and Notch signaling are both critical for CC and PPC development. The source of ligands for the EGFR and Notch receptors, Spitz and Delta, respectively, are initially provided by previously specified photoreceptors. Delta is also transiently expressed in CCs toward the end of larval development, is quickly shut off in early pupation, and is then re-expressed in CCs just prior to PPC specification (Parks et al. 1995). Early Delta expression is likely to be important for CC subtype specification (Charlton-Perkins et al. 2011), but its down-regulation is necessary to prevent ectopic PPC formation (Nagaraj and Banerjee 2007). The later increase of Delta in CCs important for proper PPC recruitment, and results from a cell autonomous increase in EGFR signaling. EGFR signaling in PPCs, however, does not appear to influence their specification: no pERK activation is observed in PPCs, dominant-negative alleles of EGFR show no changes in PPC specification, and argos, a downstream target of EGFR, is not expressed in developing PPCs (Wildonger et al. 2005; Nagaraj and Banerjee 2007). Moreover, dPax2 expression in PPCs is no longer dependent on PntP2 activation, but instead requires high levels of Lz and Notch signaling (Nagaraj and Banerjee 2007). Thus, PPCs are one of the few cell types within the eye that do not directly require EGF signaling for their specification. However, EGFR signaling indirectly affects PPC formation early in the eye imaginal disc by promoting survival of the otherwise unspecified cells (see below) and later, by activating Dl in CCs.
Although both EGF and Notch signaling are required for CC specification, careful examination of the literature shows that not all CCs are equally sensitive to perturbations in these two pathways. For instance, mutations in EGFR, canoe, pnt, yan, pros, Notch, Su(H), dPax2, ebi, strawberry notch (sno) and extramacrochaetae (emc), only lead to a partial loss of CCs (Cagan and Ready 1989b; Lai and Rubin 1992; Brunner et al. 1994; Matsuo et al. 1997; Tsuda et al. 2002; Bhattacharya and Baker 2009). These data suggest that Ras and Notch signaling may differentially affect different subpopulations of CCs. In this respect, it is particularly noteworthy that pros and dPax2 differentially feed back into the Ras and Notch pathways, respectively, and are expressed at different levels in different CCs: pros is more highly expressed in eq/plCCs and up-regulates MAPK, whereas dPax2 is more highly expressed in a/p CCs and represses Notch signaling (Charlton-Perkins et al. 2011).
It is still unclear what the developmental consequence is for such differences in signaling levels and different CCs. One possibility is that it is important for preventing premature apoptosis of unspecified precursors in the imaginal disc. During this time, high EGF signaling blocks pro-apoptotic signals mediated by the Notch pathway (Baker and Yu 2001). As the eye matures into pupation, EGF signaling decreases, allowing Notch activity to eliminate any unspecified cells (Yu et al. 2002; Rusconi et al. 2004). Since dPax2 is important for repressing Delta expression, and pERK indirectly inhibits Notch signaling, it is reasonable to speculate that pros and dPax2 function cooperatively in CCs to block both the Notch receptor and its ligand, thus promoting precursor cell survival. Consistent with this model, loss of dPax2 in the imaginal disc leads to extensive and early cell death, resulting in a significant decrease in precursor cells during pupation (Siddall et al. 2003). Whether pros influences cell death is not known, but its expression is down-regulated in CCs during early pupation when cell pruning begins, placing pros at the right time and place to help prevent premature cell death by inhibiting Notch activity.
Wingless signaling
Wingless (Wg), the first Wnt molecule to be identified, is primarily recognized during eye development for its role in preventing expansion of the eye field into the head cuticle region (Lim and Tomlinson 2006). The Wg receptors Frizzled (Fz)-1 and −2 are also critical regulators of PCP decisions in the R3/R4 photoreceptors, although Wg itself may not be responsible for initiating this process (see review by McNeill 2002). More recently, however, studies have begun to place Wg signaling in processes controlling CC specification and their ability to regulate IOC patterning and death (Cordero et al. 2004; Cordero and Cagan 2010). During CC specification, Wg is secreted by PRs, signals in CC precursors through both Frizzled receptors and the canonical Wg transcriptional activator TCF, and blocks the formation of a subset of CCs (Cordero and Cagan 2010). Pygopus, a downstream effector of Wg that can function as a TCF co-activator (Thompson et al. 2002), is not essential for wg’s role in CC number but is important for CC morphology, suggesting that wg plays multiple and genetically distinct roles during CC development (Cordero and Cagan 2010). Later, during early pupation, Wg is secreted by the CCs and promotes a wave of cell death in the surrounding unspecified cells (Cordero et al. 2004). Consistent with the requirement for Notch in mediating this same process, wg genetically interacts with Notch at this time. Since Notch and wg interact in many developmental contexts, including vertebrate lens development, future studies aimed at better understanding how these pathways intersect during fly eye patterning should be broadly relevant.
BMP signaling
Other signaling pathways that contribute to Drosophila lens development are not well understood. In some cases, as with Dpp signaling, this is likely due to their earlier roles in eye development. As previously mentioned, dpp is necessary for MF progression, so mutations in this pathway abolish eye development (Heberlein et al. 1993). However, relatively recent studies have shown that dpp is also expressed later in PPCs, and activated Dpp targets, such as phosphorylated Mad (Mothers against Dpp) protein, are dynamically expressed in CCs and IOCs (Cordero et al. 2007). While no role for Dpp signaling has been defined for CC development, activation of the Dpp Type I and II receptors (Thick Veins and Punt, respectively) specifically in SPCs promotes proper cell elongation (Cordero et al. 2007). Mutants in the Dpp signaling pathway also lead to excess IOCs, likely through interactions with E-cadherin (Cordero et al. 2007). However, given that activated Mad is expressed and maintained in CCs after their specification, future studies that manipulate late Dpp signaling may be useful for uncovering otherwise unknown aspects of CC terminal differentiation.
JNK and/or PKA signaling
The JNK signaling pathway is a central regulator of cell death in a number of systems, and this appears to be no exception in the fly eye (Kanda and Miura 2004; Xia and Karin 2004; Dhanasekaran and Reddy 2008; Igaki 2009). Indeed, many components of the JNK signaling pathway have been uncovered in modifier screens aimed at identifying suppressors of eye defects caused by protein overexpression (Wang et al. 2005; Franciscovich et al. 2008). Since overexpressing proteins often leads to non-specific cell death, and since CC/PPC cell death is readily detected as “glassy” eyes by light microscopy, the eye has been frequently used for such misexpression studies. It is then perhaps no surprise that mutations in the JNK pathway rescue such defects, presumably by blocking cell death. Whether JNK plays a role during normal lens development, however, remains less clear. Perhaps the strongest evidence for this possibility thus far comes from studies of rugose. rugose mutants were initially identified based on their rough eye appearance, and when the rugose locus was cloned, it was found to encode an A kinase anchor protein, normally involved in cAMP/PKA-dependent signaling (Wech and Nagel 2005). While little attention has been paid to PKA signaling in the fly lens, early analysis of Pkac1 mutants described defects in pigmentation and lens formation (Han et al. 1997). Further studies of rugose mutants revealed defects in CC specification and genetic interactions with JNK signaling as well as dPax2, EGF, Notch (Shamloula et al. 2002). Hypomorphic rugose alleles, which still generate some CCs, showed increased JNK activation and reduced EGF signaling (Wech and Nagel 2005). Moreover, suppressing JNK signaling, removing Notch, blocking apoptosis, or activating EGF signaling completely restored CC formation in rugose mutants (Wech and Nagel 2005). As Notch is critical for triggering apoptosis after IOC recruitment, and EGF signaling antagonizes this function, one could postulate that JNK helps to mediate Notch-dependent cell death, while rugose functions in conjunction with EGF in CCs to prevent their inappropriate apoptosis. In this respect, it is worth noting that PPCs form in rugose mutants, while CCs do not (Wech and Nagel 2005), suggesting that rugose promotes CC survival after PPC specification, but is not involved in CC specification per se. Future experiments testing these hypotheses are certainly necessary, as well as studies that specifically address the contribution of JNK and PKA signaling to lens formation.
Cell adhesion and early CC/PPC morphogenesis events
During the first half of pupation, CCs, PPCs and IOCs undergo dramatic and highly regulated cell sorting and morphogenic event that require cell surface molecules. These include cadherins (E- and N-), integrins, and integrin-related molecules (IRMs)—Hibris (Hbs) and Sticks-N-Stones (Sns) (two Nephrin-related proteins), and Roughest (Rst) and Kirre (Kir) (two Neph-like proteins) (Bao 2010). Here, we highlight the role of these proteins as they relate to CC and PPC patterning, as well as CC/PPC-dependent patterning of the IOCs.
By the end of larval development and early into pupation, the a/p CCs spread over the surface of the PRs and their apical surfaces converge at the center of the ommatidia (Cagan and Ready 1989a). The a/pCC contacts spread basally through integrin-mediated processes and meet at the floor of the retina, physically separating the PRs into two populations—the R1/6/7/8 and R2/3/4/5 (Cagan and Ready 1989a; Longley and Ready 1995). After PPC recruitment, the apical surfaces of the eq/pl CCs begin to migrate over the top of the a/p CCs and meet in the middle, forcing a/p CC separation. Like the a/p CCs earlier, the eq/ pl CC junctions also expand downward through integrin receptor-mediated contacts, further separating the PRs into four groups—R1/8, R 2/3, R4/5, and R6/7 (Wolff and Ready 1993; Longley and Ready 1995; Grillo-Hill and Wolff 2009). Integrin receptors are comprised of a β-subunit complexed with either an α-ps1 or ps2 subunit, and are required for retinal floor integrity (Wilcox et al. 1984; Bogaert et al. 1987; Leptin et al. 1987; Longley and Ready 1995). The β-subunit is expressed at the base of the CCs and the IOCs, while the α-subunits are differentially expressed in the CC plate (Longley and Ready 1995).
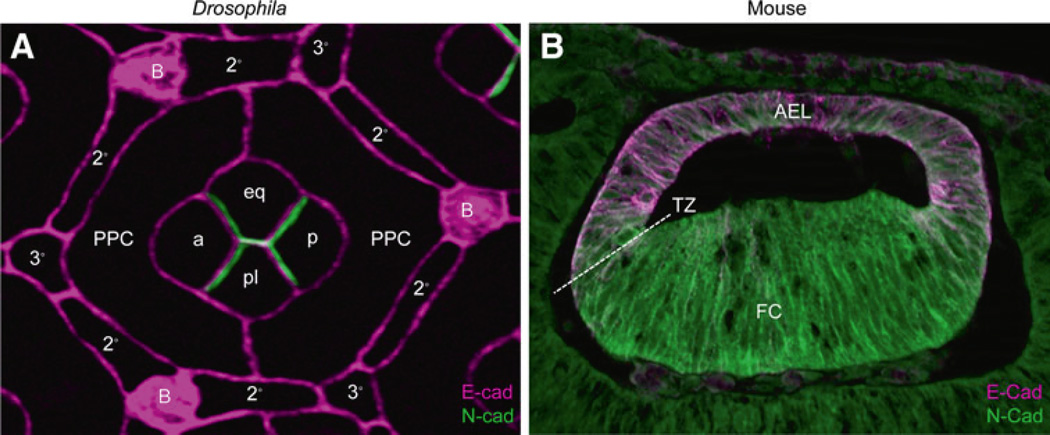
While the basal CC feet produce the retinal floor plate and ensheathe the PR axons exiting the retina, the majority of the CC body remains at the distal region of the retina to produce the corneal lens and pseudocone. Here, the apical surfaces of the pupal CCs form a tight cluster that closely resembles soap bubbles (Hayashi and Carthew 2004). This CC conformation appears to be driven by homophillic N-cadherin interactions (Hayashi and Carthew 2004). N- and E-cadherin are broadly expressed in the developing eye, with E-cadherin present in all cells, and N-cadherin restricted to CCs and the underlying PRs (Fig. 5a, Hayashi and Carthew 2004). Removing N-cadherin affects CC shape, and occasionally disrupts the signature CC quartet, whereas misexpressing N-cadherin in individual PPCs causes inappropriate CC-PPC contacts and significant disruption of an intact CC quartet (Hayashi and Carthew 2004). Therefore, N-cadherin is critical for maintaining CC–CC interactions, and does so at least in part by increasing CC–CC surface areas and minimizing CC–PPC interactions. While N-cadherin is important for deciding CC–CC versus CC-PPC contacts, E- and N-cadherin play redundant roles for zonula adherens function in CCs, as removing both factors leads to complete loss of CC contacts in the eye.
Fig. 5.
Expression of N- and E-Cadherin in the Drosophila and mouse lens. a In the fly lens, by mid-pupation, E-cadherin (magenta) is expressed at the apical junction of all cells while N-cad is exclusively expressed at the junctions between the cone cells. a anterior CC, p posterior CC, eq equatorial CC, pl polar CC, PPC primary pigment cell, 2° secondary pigment cell, 3° tertiary pigment cell, B bristle. b In the mouse lens, shown here at embryonic day 11.5, E-Cadherin (magenta) is lost from posterior lens vesicle cell membranes as these cells initiate lens primary fiber cell (FC) differentiation, while N-Cadherin (green) is expressed by all elongated lens fiber cells. The establishment of the anterior epithelial layer (AEL) boundary at the lens equator or transition zone (TZ) is apparent and maintained into adulthood
In contrast to CC–CC interactions, CC–PPC and PPC–IOC contacts require E-cadherin homophilic interactions, Rst-Hbs heterophilic interactions, and the ZO-1-related apical protein Polychaetoid (Bao and Cagan 2005; Seppa et al. 2008; Grillo-Hill and Wolff 2009). Hbs and Rst are also highly integrated with cell signaling pathways to allow proper patterning and cell number restriction (see Bao 2010 for review). Early in pupation, Rst–Hbs interactions between PPCs are important for zippering these two cells together as they encircle the CCs (Bao and Cagan 2005). Later, in response to Notch signaling, Rst and Hbs become restricted to IOCs and PPCs, respectively, to allow proper patterning (Gorski et al. 2000). This Notch-dependent restriction of Rst is also critical to ensure that the correct number of IOCs form, and in either Notch or rst mutants, precursors normally destined to die are retained in the pigmented epithelial population (Cagan and Ready 1989b; Bao and Cagan 2005). Hbs-Rst heterodimerization is also important for preventing Dpp/Tkv signaling between PPCs and SPCs to ensure proper elongation of the SPC apical surfaces (Cordero et al. 2007). Based on the growing evidence that apical junction markers interact and influence signaling proteins during normal development, as well as in cancer, it is likely that further characterization of the role of cadherins and IRMs in fly eye patterning will be a valuable resource for defining these interactions.
Vertebrate lens development
Multiple works have comprehensively reviewed anterior segment and lens development in vertebrate model organisms (Wride 1996; Fisher and Grainger 2004; Glass and Dahm 2004; Gould et al. 2004; Lovicu and Robinson 2004; Martinez and de Iongh 2010). In addition, the early steps of lens formation, particularly the signals and intrinsic factors that regulate induction, have been discussed numerous times (Fisher and Grainger 2004; Lang 2004; Cvekl and Duncan 2007; Lachke and Maas 2010). Therefore, this section briefly summarizes the fundamental anatomical and cellular changes that underlie lens organogenesis in the vertebrate eye, as a basis for comparison with the fly lens. In particular, we focus on the successive stages of lens formation that lead to the production of temporally distinct waves of differentiated lens fiber cells.
Vertebrate eye development begins with complex morphogenetic movements in both the embryonic surface ectoderm and anterior neural plate (see Fig. 1b). First, ventral diencephalon cells in the brain evaginate to the surface ectoderm, giving rise to the optic vesicle. Lens development starts in the surface ectoderm overlaying the optic vesicle, known as the presumptive lens ectoderm (PLE). This tissue undergoes localized thickening to form the lens placode. Subsequently, cells of the lens placode change shape to produce a lens pit, which fills the space created when the optic vesicle invaginates to form an optic cup. For decades, it has been known that signals from the optic vesicle induce lens formation (Spemann 1938; Mathers et al. 1997), but only very recently was it demonstrated that optic vesicle-to-cup morphogenesis can occur in the absence of the lens placode and surrounding mesenchyme (Eiraku et al. 2011). Once the lens pit closes off fully and detaches from the surface ectoderm, it forms a hollow lens vesicle. The remaining overlying embryonic ectoderm later gives rise to the cornea and other tissues of the anterior eye.
The lens vesicle is comprised of proliferating progenitor cells that enlarge the tissue and differentiate into a single cell type—fiber cells. The first phase of lens cell differentiation, termed primary fiber formation, initiates in the posterior pole of the lens vesicle, as it separates from the surface ectoderm. The farthest posterior cells exit mitosis, lose progenitor cell characteristics and begin to differentiate. Nascent primary fiber cells also activate a cocktail of intrinsic factors that promote terminal differentiation (see below). Primary fibergenesis spreads circumferentially in the lens vesicle, while elongating fiber cells encroach on the vesicle lumen and eventually contact the anterior-most cells (see Fig. 5b). Lens progenitor cells that reside in this anterior region partially differentiate, producing a specialized epithelial cell layer (AEL) that remains proliferative. The apposition of AEL cells and primary fiber cells establishes an anterior–posterior (A–P) boundary. Once this polarity is established, secondary lens fiber differentiation initiates. During secondary fibergenesis, AEL cells migrate circumferentially to the equator, or boundary, of the lens vesicle, exit the cell cycle, elongate and differentiate. Terminal differentiation of fiber cells involves a gradual loss of nuclei and cell organelles (Golgi, mitochondria and the endoplasmic reticulum), which is important to provide optical clarity and achieve the appropriate refractive index necessary for a functional lens (Lovicu and Robinson 2004). The primary fiber cells are the first to undergo this transition and are maintained in the center of the lens, while the secondary fiber cells are stratified around the primary fiber cells and are continuously added to the lens throughout life. Currently, the best-established markers for lens differentiation are the Crystallin proteins. During mammalian lens pit and vesicle morphogenesis, all lens cells express α-Crystallin (Crya), while β-Crystallin (Cryb) and γ-Crystallin (Cryg) are found in differentiating fiber cells. The latter proteins first appear in posterior primary fibers, and later are specifically expressed throughout the fiber cell compartment (McAvoy 1978).
While the genetic network responsible for lens development continues to be elucidated, many questions remain open regarding how these molecular pathways orchestrate vesicle morphogenesis, A-P patterning, epithelial cell proliferation, cell cycle exit, and fiber cell elongation and differentiation. As an example, although retina and lens cells simultaneously initiate differentiation (E11 in mouse), it is unclear whether these are independent or coordinated events. Moreover, it is unclear whether primary and secondary fiber cells are molecularly and/or functionally distinct. The following sections summarize what is known about a subset of intrinsic transcription factors, extracellular signaling pathways and intracellular cell adhesion molecules that are required for proper lens growth and fiber cell differentiation (also see Fig. 6).
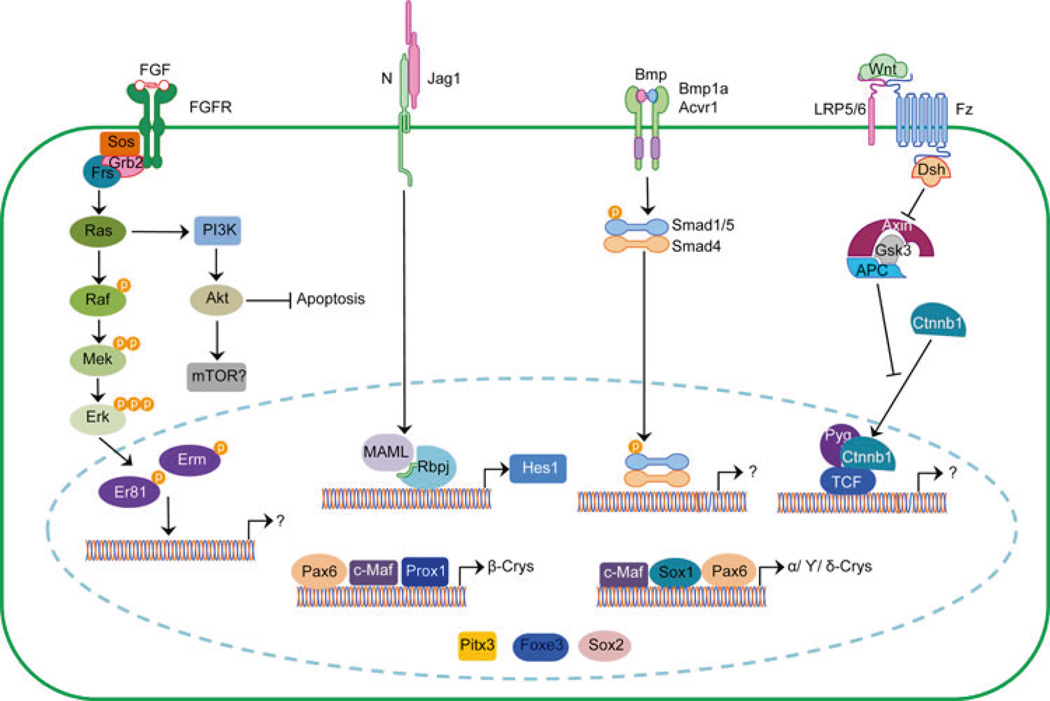
Fig. 6.
Intrinsic and extrinsic factors used during vertebrate lens development. The intrinsic factors Pax6, Sox2, Foxe3, Pitx1, Sox1, c-Maf, and Prox1 are required at different stages of lens development. Some of the extrinsic factors pathways involved in lens development are summarized here. Fgf signaling: there are 22 fibroblast growth factor ligands (Fgf) ligands and four receptors (Fgfr) in vertebrates, with some variability in ligands among species. Fgfs are secreted morphogens that act both short- and long-range to induce cell proliferation, morphogenesis, and cell type differentiation. Ligands bind to a heterodimeric receptor (Fgfr) complex having a C-terminal tyrosine kinase domain that phosphorylates intracellular proteins upon ligand binding. Targets of Fgfr protein kinase activity include Ras and adaptor proteins Frs, Sos and Grb2 (reviewed in Bottcher and Niehrs 2005). Activated Ras initiates a protein phosphorylation cascade— Ras to Raf to Mek to Erk—which terminates with translocation of phospho-Erk into the nucleus to stimulate factors such as Fos and Ets factors. Er81 and Erm are ETS factors expressed in the lens (Pan et al. 2010). Ras also leads to phosphatidylinositol 3 kinase (PI3K) and AKT activation to promote cell survival during lens fiber differentiation (Weber and Menko 2006). Notch signaling: the Notch pathway transduces a direct, cell-to-cell signal initiated by the binding of a membrane-bound ligand (Jagged or Delta-like proteins) on one cell to a Notch receptor on an adjacent cell (reviewed in Fortini 2009; Kopan and Ilagan 2009). Ligand binding triggers a series of proteolytic cleavages to the Notch receptor, ultimately releasing the Notch intracellular domain (NICD), which translocate into the nucleus where it forms a complex with the transcriptional regulators Rbpj/Su(H) and Mastermind-like (MAML) to activate transcription of target genes such as the bHLH repressors Hes1 or Hes5. The ligand Jag1 is critically required during lens fiber cell differentiation (Le et al. 2009). Bmp/Tgfβ signaling: the transforming growth factor β (Tgfβ) gene superfamily includes the Tgfβ, bone morphogenetic protein (Bmp), growth and differentiation factor (Gdf), Activin and Nodal subfamilies. These secreted ligands bind to heterodimeric Type I and Type II receptor complexes (reviewed in Yang 2004; Moustakas and Heldin 2009). There are seven Type I receptor genes and five Type II receptor genes in vertebrates, all containing cytoplasmic serine/ threonine kinase domains. Upon ligand binding, an active Type II receptor phosphorylates the cytoplasmic domain of a Type I receptor, activating its kinase activity, which then phosphorylates intracellular Smad effectors and other proteins. An activated Smad then complexes with a common mediator, Smad4, and translocates into the nucleus to regulate downstream gene transcription. These signals can be modulated by natural agonists, like Noggin, or by negative feedback from inhibitory Smads (reviewed in Moustakas and Heldin 2009). In lens development, Bmpr1a and Acvr1 are important and signal via Smad5. The Wnt/βcatenin Pathway: Wnts (wingless-type MMTV integration site proteins) are secreted signaling molecules that bind to a Frizzled (Fz) receptor and Lrp co-receptor. Once formed, this complex activates Dishevelled (Dvl), recruits Axin to the cell membrane, and this prevents phosphorylation of the intracellular β-catenin (Ctnnb1). This stabilized β-catenin protein translocates to the nucleus where it interacts with Tcf/Lef transcription factors, as well as the Pygopus (Pyg) co-activator to activate target genes. (reviewed in Fuhrmann 2008). There are 19 Wnt ligands and 10 Fz receptors in vertebrates, of which at least six ligands (Wnts 5a, 5b, 7a, 7b, 8a and 8b), five Fz receptors (Fz 1, 2, 3, 4, 6), two co-receptors (Lrp 5 and 6) and multiple pathway agonists (Dkk, Sfrps) are all expressed in the developing mouse lens (Stump et al. 2003)
Intrinsic factors
Transcription factors regulate virtually all aspects of lens development and are often classified as either (a) present in the lens placode, pit, and/or AEL (Pax6, Sox2, Foxe3, Pitx3) or (b) activated during fiber cell differentiation (Prox1, Sox1, c-Maf). As described below, however, these factors are involved in many distinct aspects of lens formation.
Factors present in the lens placode, pit, and/or AEL: Pax6, Sox2, FoxE3, and Pitx3
One of the earliest expressed genes known to regulate lens formation is Pax6, which encodes a protein containing both a paired and a homeodomain DNA-binding motif. Pax6 is present at all stages of lens development, except within differentiated fiber cells. Multiple studies have demonstrated that Pax6 is both necessary and sufficient for lens induction in vertebrates (Chow et al. 1999; Ashery-Padan et al. 2000). However, removal of Pax6 during fiber cell differentiation shows that it is also important for AEL cell cycle exit and fiber cell differentiation (Shaham et al. 2009). Pax6 does not regulate these later processes via Sox1, Prox1 or c-Maf, as these factors are still present in Pax6 conditional knockout mice. Instead, Pax6 is important for activating the Wnt antagonist Sfrp2 in the AEL and suppressing Sox2 expression at the lens equator. Whether these events are responsible for the defects in fiber cell differentiation observed in conditional Pax6 mutants, however, is unclear (Shaham et al. 2009). Pax6 has also been implicated in regulating multiple Crystallin-encoding genes (Cvekl et al. 2004; Kamachi et al. 2001; Kondoh et al. 2004). During placode formation, Pax6 cooperatively binds with Sox2 to activate α-Crystallin, but neither factor is required for its maintenance. Additionally, Pax6 represses β- and γ-Crystallins independently of Sox2 (Kamachi et al. 2001; Kondoh et al. 2004; Shaham et al. 2009). Hence, Pax6 and Sox2, together and separately, regulate multiple downstream target genes at discrete times during lens development.
Foxe3 (in Xenopus, Xlens1) encodes a forkhead transcription factor, requires Pax6 for its expression, and displays the earliest known lens-specific expression pattern (Blixt et al. 2007). Foxe3 initially appears in the PLE, and its expression persists in the lens vesicle and AEL, but it is absent from differentiated fiber cells (Kenyon et al. 1999; Blixt et al. 2000; Brownell et al. 2000). Two distinct mouse Foxe3 mutations highlight its role in lens development (reviewed in Medina-Martinez and Jamrich 2007). The classic mutation, dysgenetic (dyl), reduces the DNA-binding ability of Foxe3, leading to microphthalmic lenses and incomplete separation of the AEL and the overlying cornea (Blixt et al. 2000; Brownell et al. 2000). Complete removal of Foxe3 by targeted gene deletion severely disrupts AEL cell proliferation, and prevents AEL-cornea separation, fiber cell elongation and denucleation. These mutant lenses are also microphthalmic and contain cataracts. The underlying molecular mechanisms of some of these phenotypes have been defined. For examples, loss of Foxe3 deregulates Prox1 expression, which in turn up-regulates the cell cycle exit gene Cdkn1c/p57Kip2, thereby causing progenitor cells to stop dividing. Similarly, Foxe3 can activate platelet-derived growth factor alpha expression (Pdgfα), which is important to induce proliferation (Blixt et al. 2000). Thus, down-regulating Foxe3 blocks multiple lens cell proliferation pathways. The fiber cell deformities in Foxe3 mutants occur because Foxe3 normally promotes DNase2β expression, which is important for degrading nuclear DNA during fiber cell differentiation (Medina-Martinez et al. 2005).
Another critical gene product during vertebrate lens development is Pitx3, which encodes a Bicoid-like homeodomain transcription factor (Semina et al. 1997). aphakia (ak) mutant mice lack Pitx3 mRNA and develop only rudimentary lenses, due to the requirement for Pitx3 to promote progenitor cell proliferation and differentiation and to block apoptosis (Rieger et al. 2001; Medina-Martinez et al. 2009). Lens progenitor cells lacking Pitx3 prematurely exit the mitotic cell cycle, activate the cell cycle inhibitors Cdkn1b/p27Kip1 and Cdkn1c/p57Kip2 and precociously express β and γ-Crystallin (Ho et al. 2009). These defects are partially due to down-regulation of Foxe3 and mis-expression of Prox1 in the lens vesicle (Ho et al. 2009). These data strongly suggest that Pitx3 acts in parallel and upstream of Foxe3 to maintain lens epithelial cells by blocking inappropriate fiber cell differentiation.
Factors activated during fiber cell differentiation: Prox1, Sox1, and L-Mafs
Prox1, Sox1 and L-Maf factors are each activated as primary and secondary fiber cells initiate terminal cell cycle exit, elongate and differentiate. Prox1 encodes a homeobox protein and is the vertebrate ortholog of the Drosophila prospero gene (Tomarev et al. 1998). Sox1, like Sox2, encodes a member of the HMG-box group of transcription factors (Nishiguchi et al. 1998). Large Maf (L-Maf) factors encode a basic leucine zipper DNA-binding domain, plus an N-terminal transactivation domain (Reza and Yasuda 2004). In the chick and frog eye, three large Maf factors, L-Maf, MafB and c-Maf are required for lens formation, but in mammals, only c-Maf and MafB are present in the lens. Loss of Prox1, Sox1 or c-Maf in mice renders progenitor cells unable to complete fiber cell differentiation. Mutations in either Sox1 or Prox1 block fiber cell elongation and γ-Crystallin transcription, with no effect on either α- or β-Crystallin expression (Nishiguchi et al. 1998; Wigle et al. 1999). Removal of c-Maf during mouse lens development also causes incomplete primary fiber elongation, but completely blocks secondary fiber formation, leaving a hollow lens vesicle (Kawauchi et al. 1999; Kim et al. 1999; Ring et al. 2000). In contrast to Sox1 and Prox1 mutant mice, loss of c-Maf causes multiple Crystallin genes to be dramatically down-regulated or prematurely lost. Sox1 and Prox1 expression, however, is unaffected by the loss of c-Maf (Ring et al. 2000), implying that each factor regulates cell cycle exit and/or fiber cell differentiation genes independently. It would be very interesting to simultaneously remove all three transcription factors and test whether primary fibergenesis is abolished, since this process initiates normally in each individual mutant.
Extrinsic factors
The environment surrounding the developing lens contains a multitude of signals for growth and differentiation. Lens cells themselves also synthesize signaling molecules, growth factor receptors and intracellular signaling pathway components. While numerous signaling pathways have been tested for lens induction capabilities, it is equally important to understand how extrinsic signals in the anterior aqueous stimulate the formation of the AEL and maintain its proliferative capability. Likewise, the mechanisms by which secreted factors from the developing optic cup/retina and posterior vitreous control lens fiber cell differentiation remain unresolved.
Fgf signaling
The first evidence that Fgf ligands stimulate lens fiber cell differentiation came from in vitro lens explant studies, which also showed that the posterior vitreous humor is a significant source for these signals (Chamberlain and McAvoy 1987). This assay system was also instrumental for understanding that lens cells respond differently to various concentrations of Fgf ligands (alone or in combination) (McAvoy and Chamberlain 1989). A low amount of Fgf signaling promotes AEL cell proliferation, but high concentrations induce fiber cell elongation and differentiation. These studies culminated in the “Fgf gradient hypothesis”, whereby the lens environment contains a gradient of Fgf signaling, with signals originating from the retina, vitreous, lens and anterior aqueous humor (McAvoy et al. 1999; Lovicu and McAvoy 2005). As the highest levels of signaling come from the vitreous, primary fibergenesis is induced at the posterior of the lens vesicle. During secondary fibergenesis, AEL cells receive the threshold of Fgf signaling needed for differentiation when they reach the equator. This gradient is likely composed of particular combinations of Fgf ligand proteins, and is modifiable by mechanisms such as heparin sulfate binding.
In vivo support for this hypothesis has come from both gain- and loss-of-function studies. Transgenic mice in which various Fgf ligands or dominant-negative Fgfr constructs were specifically misexpressed in the lens resulted in AEL cells prematurely differentiating into fiber cells, or blocking fibergenesis, respectively (Robinson et al. 1995; Lovicu and Overbeek 1998). Ligand overexpression, however, may trigger inappropriate AEL differentiation by blocking proliferation, directly promoting differentiation, or both. Loss-of-function studies have been more challenging, owing to the early lethality of many Fgf pathway mutations and issues regarding ligand and/or receptor redundancy. Recently, three of the four Fgfr genes were conditionally removed in the developing mouse lens (Zhao et al. 2008). Although the lens vesicle formed and progenitor markers like Foxe3, Pax6 and α-Crystalllin were expressed normally, overall vesicle size was reduced. In addition, triple mutant lenses failed to activate c-Maf, Prox1, and β- and γ-Crystallins, and showed persistent E-Cad/Cdh1 expression in the posterior vesicle, resulting in a hollow lens devoid of fiber cells (Zhao et al. 2008). These studies clearly indicate the importance of Fgf signaling for proper lens development.
Notch signaling
At minimum, Notch 1–3, Delta-like factors Dll1 and Dll3, Jag1, Hes1 and Hes5 are expressed during lens formation. Notch1, Notch2, Jag1 and Hes1 are expressed in the lens placode and lens vesicle cells, prior to primary fiber cell formation (Bao and Cepko 1997; Lee et al. 2005). In frogs, optic vesicle cells induce lens formation via Delta–Notch signaling. Cooperative binding of a NotchICD-Rbpj-MAML complex in the lens placode, together with the Otx2 transcription factor, activates Foxe3 transcription (Ogino et al. 2008). Moreover, misexpression of the Xenopus Notch1ICD promotes ectopic lenses and RPE formation, much like ectopic Pax6 expression (Onuma et al. 2002). These early roles for Notch signaling, however, appear specific to the frog eye. During mouse lens fibergenesis, Jag1 protein becomes localized to primary fiber cells and then within lens equator cells during secondary fibergenesis (Le et al. 2009). Tissue-specific deletion of Jag1 or Rbpj demonstrates that this pathway is critical for the expression of cell cycle inhibitors such as Cdkn1c/p57Kip2 and for cell cycle exit (Jia et al. 2007; Rowan et al. 2008; Le et al. 2009). In the absence of Jag1 or Rbpj, the lens equator never fully forms, secondary fibergenesis breaks down and postnatal lens aphakia ensues. Interestingly, Jag1 mRNA and protein are localized on the anterior side of nascent secondary fiber cells, suggesting there may be a feedback signal to the AEL. Indeed, loss of Jag1 causes a dramatic down-regulation of Foxe3 in the AEL, and a loss of epithelial character, via loss of E-Cad expression (Rowan et al. 2008; Le et al. 2009). Understanding which Notch receptor(s) is active during fiber cell differentiation and/or AEL progenitor cell growth would better elucidate the molecular mechanisms for this pathway. Notch signaling acts downstream of Fgf in the lens, evidenced by the finding that Fgfs induce Jag1 expression in vitro (Saravanamuthu et al. 2009).
BMP/TGFβ signaling
Bmp proteins are capable of inducing lens vesicle formation, with Bmps 4 and 7 originating from the optic vesicle (Luo et al. 1995; Karsenty et al. 1996; Furuta and Hogan 1998). Overexpression of these ligands can also induce fiber cell differentiation, as shown by γ-Crystallin expression or other fiber-specific marker activation in lens placode or AEL cell culture (de Iongh et al. 2001, 2005; Belecky-Adams et al. 2002). Blocking Bmp signaling by Noggin overexpression, dominant-negative receptor mis-expression or conditional deletion of Type1 receptor genes, causes microphthalmic lenses, with a diminished AEL, incomplete fiber elongation and, in some cases, fiber cell degeneration (Faber et al. 2002; Beebe et al. 2004; Rajagopal et al. 2008). TGFβ and Activin ligands do not appear to affect the lens, but there is some role for their Type I receptors during fiber cell maintenance, as suggested by misexpression studies (Shull and Doetschman 1994; Vassalli et al. 1994; Matzuk et al. 1995; Proetzel et al. 1995; Sanford et al. 1997). Because particular receptors, like Acvr1, can transduce either a Bmp or Activin signal, the analysis of signal-specific Smads has recently shown that Bmp (Smad 1/5/8), but not Activin/ TGFβ (Smad 2/3), stimulate Acvr1 in the lens during AEL cell cycle exit at the equator. In contrast, Bmp’s separate activation of the Bmpr1a receptor blocks apoptosis in the lens placode and AEL (Rajagopal et al. 2008). Therefore, the multiple functions of this pathway are segregated to distinct receptors, as well as at least one noncanonical mechanism (Rajagopal et al. 2009). Bmp signaling acts downstream of Fgf, but these two pathways also synergize during lens induction and exert feedback regulation during fiber cell differentiation (Faber et al. 2002; Boswell et al. 2008a, b).
The Wnt/β-catenin pathway
The most highly conserved role for Wnt signaling is to induce the development and maintenance of epithelial cell characteristics. Wnt signaling in the surface ectoderm must be down-regulated for lens induction to occur, though it is also required for formation and maintenance of the AEL (Smith et al. 2005; Cain et al. 2008). Loss of the Wnt co-receptor Lrp6 in the prenatal mouse lens causes the AEL cells to lose epithelial cell characteristics and prematurely express β-Crystallin within the anterior compartment (Stump et al. 2003). Similarly, conditional removal of β-catenin keeps AEL cells from coalescing and leads to cell cycle deregulation via precocious Cdkn1c/p57 and c-Maf expression; however, unlike Lrp6 mutants, β-catenin mutant AEL cells do not aberrantly express β-Crystallin, indicating that Lrp6 regulates some functions in a β-catenin-independent manner. Conversely to its loss-of-function phenotype, constitutive activation of β-catenin results in a failure of AEL cells to exit the cell cycle at the equator and the persistence of progenitor cell markers such as Pax6 and E-Cadherin within the posterior compartment (Martinez et al. 2009). There is evidence from lens explant cultures that Wnt signaling, particularly in cooperation with the Fgf pathway, also regulates aspects of fiber cell differentiation. However, the phenotypes of β-catenin mutant lenses suggest that canonical Wnt signaling also acts at the lens equator in cells initiating fibergenesis, but not in older, fully differentiated and elongated lens fibers (Cain et al. 2008). Therefore, loss of Wnt/β-catenin signaling affects AEL and fiber cell development similarly to loss of Bmp or Notch signaling. At present, however, nothing is known about epistatic relationships among these three signaling pathways in the vertebrate lens. There is also Wnt-stimulated, β-catenin-independent, planar cell polarity (PCP) signaling in the mammalian lens. PCP signaling can promote lens induction in the frog eye, and is important for coordinating the arrangement and orientation of fully differentiated lens fibers (Rasmussen et al. 2001; Chen et al. 2008).
Cadherins
There are multiple features that distinguish anterior epithelial cells from posterior lens fiber cells. Differences in Crystallin protein expression have already been discussed, but cells in each compartment also express distinct cell-to-cell communication, cell adhesion and cytoskeletal molecules (see Martinez and de Iongh 2010 for review). The roles of two adhesion molecules, E-Cadherin (Cdh1) and N-Cadherin (Cdh2), are of particular relevance here, given the similarities in expression patterns within invertebrate and vertebrate lenses (see later discussion and Fig. 5b). Cadherins belong to a family of calcium-dependent, cell adhesion molecules that maintain cell–cell contacts at adherens junctions via homophilic interactions (reviewed in Niessen et al. 2011). Cadherins are differentially expressed during development, often in a cell type-restricted manner. E- and N-Cadherin proteins display overlapping expression domains in the vertebrate lens. Both are expressed at relatively high levels in the lens vesicle. When anterior epithelial cells coalesce into the AEL, they maintain expression of both cadherins, but primary fiber cells down-regulate E-Cadherin, while maintaining N-Cadherin expression (Fig. 5b). This is in keeping with the transition of nascent lens fiber cells from epithelial- to mesenchymal-like cellular characteristics. Loss of E-Cadherin expression is a hallmark of lens fiber differentiation, with the interface of E-Cadherin+ /N-Cadherin+ anterior cells versus N-Cadherin+ posterior cells defining the lens equator or transition zone.
Despite robust expression in the lens vesicle, studies show that conditional deletion of either E-Cadherin or N-Cadherin has no effect on vesicle separation from the overlying ectoderm. Single mutant lenses initially form a normal AEL and differentiated fiber cells, but are microphthalmic with late onset AEL deterioration and fiber cell vacuolization (Pontoriero et al. 2009). Double conditional mutants for E- and N-Cadherin had severe microphthalmia, abnormal lens vesicle separation and AEL cell adhesion, all resulting in apoptotic cell death, indicating that these proteins act redundantly (Pontoriero et al. 2009). Thus, in addition to cell adhesion properties, these Cadherin molecules also protect lens cells from prematurely dying. The Wnt and Notch signaling pathways are known to maintain E-Cadherin expression within the anterior epithelial layer, while Fgf signaling emanating from the vitreous downregulates E-Cadherin at the lens equator (Smith et al. 2005; Jia et al. 2007; Cain et al. 2008; Rowan et al. 2008; Zhao et al. 2008).
Comparing vertebrate and Drosophila lens development
The mature single lens in vertebrates and the facet lenses in fruit fly ommatidia show vast morphological differences. Regardless, many common signaling pathways and transcription factors seem to drive related developmental processes in these analogous ocular structures. Below, we discuss genes and pathways that are critical for lens formation in Drosophila and vertebrates, examining both similarities and differences in how these molecular networks orchestrate the development of such a critical ocular structure (see Table 1).
Table 1.
Comparison of factors involved in vertebrate and Drosophila lens development
| Vertebrate | Invertebrate | ||
|---|---|---|---|
| lens placode/ MF | |||
| Pax6 | Pax6 | ||
| Six3 | So/Optix | ||
| Eya1 | Eya | ||
| BMP | Dpp | ||
| AEL/ SMW | |||
| FGF | Proliferation | EGF | Proliferation |
| Foxe3 | Proliferation | Jumuea | Proliferation? |
| Pitx3 | Proliferation | ||
| PDGF | Proliferation | ||
| Transition zone | |||
| FGF | Cell cycle exit | EGF | Cell cycle exit |
| Notch | Cell cycle exit | Notch | Cell cycle exit |
| Six5 | So | pros expression | |
| Eya1 | Eya | pros expression | |
| Ttk | cell cycle exit | ||
| Glass | pros expression | ||
| Lz | pros and dPax2 expression | ||
| Cut | dpn repression | ||
| Lens differentiation/ Specification | |||
| Pax6 | Fiber cell elongation | dPax2 | a/p CCs, PPCs; promotes N signaling |
| Prox1 | Fiber cell elongation | Pros | eq/pl CCs and pErk up regulation |
| FGF | Prox1 and Jag 1 expression | EGF | pros, dPox2 and Delta Expression |
| Notch | Pax6 expression | Notch | dPax2 expression |
| Wnt | Fiber cell differentiation | Wg | CC specification |
| Sox1 | Secondary fibergenesis | SoxN | |
| L-Mafs | Fiber cell differentiation | ||
| BMP | Fiber cell differentiation | Dpp | |
| BarH1 | PPC differentiation | ||
| Terminal Differentiation | |||
| Pax6 | α-, β- & γ-Crystollin regulation | Pax2 | Drosocrystallin expression |
| Sox1 | α- & γ- Crystatlin regulation | SoxN | (chick δ-Crystallin regulation) |
| Prox1 | β-, γ-Crystallin regulation | Pros | |
| L-Mafs | α-, δ- & γ-Crystollin regulation | ||
| Adhesion | |||
| E-Cad | AEL | E-cad | CCs, PPCs, IOCs |
| N-Cad | Differentiating lens fiber cells | N-cad | CC-specific |
Initiation of the lens competence: Pax6 and SIX factors
In virtually every visual system analyzed to date, Pax6 sits atop a network of genes required for ocular development. This network includes member of the So/SIX, Eya, and Dac family of proteins (Quiring et al. 1994; Halder et al. 1995, 1998; Gehring 1996, 2005; Kumar and Moses 1997; Gehring and Ikeo 1999; Kumar 2001, 2010). In vertebrates, Pax6 is primarily known for its role in lens induction. In Drosophila, since the lens arises from the same tissue as the retina, it is unlikely that a direct equivalent to lens induction exists. However, two Pax6-encoding genes, eyeless (ey) and twin of eyeless (toy), are expressed anteriorly to the morphogenetic furrow, and activate so and eya, which are important for activating genes required for lens formation (e.g. lz and pros). Moreover, so and eya activate Dpp/BMP signaling, important for MF progression, and together with EGF/Ras/MAPK signaling, promote downstream cell fate decisions and cell survival that are essential for lens development (Heberlein et al. 1993; Baker and Yu 2001). Comparably, vertebrate Pax6 expression is initiated very early, is required for lens induction and is important for the expression and/or function of Six3 and Eya1 (Xu et al. 1997; Ashery-Padan et al. 2000). Furthermore, Bmp4/Bmp7 and Fgf/Ras/Mapk signaling are required for placode formation, lens competence and the prevention of premature cell death (Faber et al. 2002; Boswell et al. 2008a, b). This suggests that many of the upstream molecular pathways involved in specifying lens competence and maintenance are conserved.
Remarkably little is known regarding the Pax6-Six-Eya network after lens induction, but it is interesting to note that Six3 appears to function upstream, rather than downstream, of Pax6 during lens development (Liu et al. 2006). In this respect, it is unclear whether Six3 is functionally orthologous to so in flies (Donner and Maas 2004; Purcell et al. 2005; Donner et al. 2006). Indeed, Six family members fall into three categories: Six1/2 are most highly related to Drosophila sine oculis, Six3/6 are similar to Drosophila optix, and Six 4/5 are most related to Drosophila dSix4 (Seo et al. 1999). Currently, no loss-of-function studies for optix or dSix4 have been performed in Drosophila, so whether they play a role in lens formation is not known. However, like Six3, optix functions independently of Pax6 in Drosophila to induce ectopic eyes (Seimiya and Gehring 2000). Hence, it is possible that Six factors other than Six3 mediate downstream effects of Pax6 during vertebrate lens development. Six5, for example, is expressed in the AEL and is critical to prevent cataract formation in mice, similar to Pax6 (Klesert et al. 2000; Sarkar et al. 2000), making it an interesting candidate. It should also prove useful to determine the roles of the Eya genes in both vertebrates and invertebrates. Eya1 has been reported to be expressed in the lens placode downstream of Pax6, and a mutation in human EYA1 has been associated with congenital cataracts, but to date no functional studies have been performed for Eya genes during lens development (Azuma et al. 2000). Similarly, in flies, although Eya is expressed in all precursors early in eye specification, after pupation, Eya is restricted to only non-neuronal cell types (CCs, PPCs, and IOCs) (Bonini et al. 1998), suggesting that it performs later functions. However, such functions have not yet been reported. Hence, although the discovery of the Pax–Six–Eya network provided critical support for a monophyletic origin of eyes, many questions remain regarding the function of these factors during later stages of eye formation.
Transition from proliferating precursors to the initiation of specification: Ras/MAPK, Pros/Prox1, and Fox factors
One major distinction between the vertebrate and fly lens is that the vertebrate lens has a proliferative tissue, the AEL, which continually produces lens fiber cells, while cells that give rise to the fly lens undergo their final mitosis in the SMW of the eye imaginal disc. In this respect, it is possible that primary, rather than secondary, fibergenesis is more related to fly lens genesis. Importantly, however, many of the same factors required for the transition between the last mitosis and initiation of lens cell specification are shared in both systems. For example, one of best characterized pathways involved in controlling lens epithelial proliferation in vertebrates is Fgf signaling. Reducing Fgf signaling reduces proliferation, while increasing Fgf signaling causes premature cell cycle exit and promotes differentiation (McAvoy et al. 1999; Lovicu and McAvoy 2005). In flies, no role for Fgf signaling has yet been defined during lens formation. Instead, it is likely that the same Ras/MAPK signaling events initiated by the Fgf receptor in vertebrates is regulated by the EGF receptor in flies. Consistent with this hypothesis, low levels of EGF signaling are required to promote mitosis at the SMW in Drosophila (Baonza et al. 2002), while overactivating EGFR signaling prior to the SMW blocks further mitosis and promotes premature differentiation (Baker and Yu 2001). Therefore, both systems are highly tuned to interpreting different levels of Ras/ MAPK signaling to control proliferation vs differentiation.
In flies, one of the known direct targets of Ras/MAPK signaling in lens-forming cells is pros. Prior to EGFR/Sev activation, pros expression is repressed by the ETS-related transcriptional repressor, Yan (Xu et al. 2000). Upon Ras/ MAPK activation by EGFR/Sev, pros is then up-regulated by the ETS-related transcriptional activator, PntP2 (Xu et al. 2000). pros then feeds back to further stimulate Ras/ MAPK signaling (Charlton-Perkins et al. 2011). Interestingly, during vertebrate lens formation, Prox1 expression also positively correlates with Fgf/Ras signaling. In Fgfr triple knockout mice, Prox1 RNA is severely down-regulated at the transition zone (Zhao et al. 2008), and in transgenic mice overexpressing constitutively active Ras using the Pax6 lens enhancer, Prox1 expression is significantly up-regulated (Burgess et al. 2010). Moreover, two Ras-dependent ETS-related transcription factors are present in the vertebrate lens which may function similarly to Yan and PntP2—Er81 is present in the AEL where Prox1 levels are low, and Erm is present at the transition zone where Prox1 levels increase (Pan et al. 2010). Thus, it would be interesting to test if Er81, like Yan, restricts Prox1 expression, whereas Erm, like PntP2, could promote Prox1 expression.
Another similarity between flies and vertebrate with respect to Ras/MAPK signaling is the activation of Notch ligands—Delta in flies, and Jag1 in vertebrates (Tsuda et al. 2002; Saravanamuthu et al. 2009). In the fly, Ras/MAPK activity leads to a cell autonomous reduction in N function and activation of Delta (Tsuda et al. 2002; Nagaraj and Banerjee 2009). Analogously, in mice, Notch activity is high in the AEL where Fgf signals are low, and as Fgf signals increase at the transition zone, Notch activity is down-regulated and Jag1 expression is up-regulated. Morevoer, Prox1 levels correlate with Fgf signaling and Jag1 levels, much like pros levels correlate with EGF signaling activity and Delta expression. Thus, it is tempting to speculate that Pros/Prox1 commonly link Ras/MAPK signaling with Notch signaling during lens cell differentiation.
Do flies also use similar factors such as Foxe3, Pitx3, and large Maf factors to control lens development? Currently, the answer is that we don’t know. Flies (and ecdysozoans in general) do not encode members of the Fox-E subfamily of proteins, and no functional characterization of the closest Fox family member, the Fox-D factor Fd3, has been performed in Drosophila. Likewise, although flies encode one large Maf factor and one Pitx family member, no roles for these genes during eye development have been described. Regarding Fox family members, though, it is noteworthy that Fox-O and Fox-N-related proteins do participate in Drosophila lens formation. Fox-O is a common downstream target of the insulin receptor/Akt and TOR signaling pathways in many species (Hay 2011; Zhang et al. 2011), and although Fox-O has not been directly tested for its role during fly eye development, InR/ TOR/Akt signaling has been shown to genetically interact with EGF signaling to control the timing of cell differentiation in the fly imaginal disc, including cells expressing pros (Bateman and McNeill 2004). Thus, in this respect, Fox-O in flies may function similarly to FoxE3 in the vertebrate lens by controlling the timing of lens cell differentiation. This argument is also compelling given that insulin and insulin-like growth factors, as well as Akt signaling, genetically interact with Fgf signaling to promote fiber cell differentiation in the vertebrate lens (Iyengar et al. 2006; Weber and Menko 2006). Whether these processes are linked to FoxE3 function, however, has not yet been determined. The other Fox factor that has been analyzed in the fly eye is the Fox-N ortholog jumeau (also known as Domina) (Lee and Frasch 2004). jumeau is expressed near the SMW, and its mutation leads to smaller eyes, fused lenses, and fewer cells (Strodicke et al. 2000), a phenotype similar to that observed when mitosis at the SMW is blocked (de Nooij and Hariharan 1995). Thus, it is possible that the two Drosophila Fox factors together contribute to many of the same processes as FoxE3 in vertebrates: Fox-N to promote cell proliferation and Fox-O to prevent premature differentiation. Alternatively, and not mutually exclusive, is the possibility that Fox-O factors are also important during vertebrate lens development.
Lens cell differentiation: Pax6/dPax2, Sox factors, and Crystallin regulation
In vertebrates, progenitor cells exit the cell cycle in the germinal zone, initiate differentiation at the equator, and begin to elongate. As these cells further mature, they lose organelles to achieve transparency. Fly lenses are acellular, and thus, do not require this later event. Despite the differences at the cellular levels, again, some molecular events during the differentiation of lens fiber cells in vertebrates, and CC/PPC differentiation in flies, rely on common mechanisms. One of the best-established relies on the finding that vertebrate Pax6 and Drosophila dPax2 regulate Crystallin expression through the same DNA-binding sites, as previously discussed (Fig. 4, Kozmik et al. 2003; Blanco et al. 2005). Indeed, in vitro binding assays show that Pax2 and Pax6 bind very similar sequences, and both dPax2 and Pax6 can induce ectopic eyes in flies, providing further support these two factors can regulate at least some common target genes (Epstein et al. 1994; Kozmik et al. 2003; Cvekl et al. 2004). This, together with the fascinating discovery that the PaxB transcription factor in jellyfish shares features with both Pax6 and Pax2 and is expressed in lentoid bodies to regulate crystallin expression (Kozmik et al. 2003), suggest that once the Pax2 and Pax6 genes evolutionarily diverged from PaxB, the Pax2 gene was co-opted into invertebrate lens development, whereas Pax6 was co-opted in the vertebrate lens. Given this, it is not unreasonable to speculate that dPax2 and Pax6 also play other orthologous roles in early lens differentiation events. In Drosophila, dPax2 is activated early in CC specification by EGF and Notch signaling and is required for their maturation, while later in PPCs, dPax2 is specifically activated by Notch where it is also participates in their maturation (Flores et al. 2000; Nagaraj and Banerjee 2007; Charlton-Perkins et al. 2011). Similarly, early Pax6 expression in the lens placode is reduced in Fgfr mutants, while its later expression in the AEL is eliminated in Rbpj mutants (Rowan et al. 2008), suggesting control by Notch signaling. Pax6 is also required for several aspects of fiber cell specification. Thus, from a gene regulation standpoint, one could reason that Pax6 in the lens placode and transition zone and dPax2 in the CCs and PPCs are controlled using similar mechanisms and for mediating similar functions.
Another intriguing correlation stems from recent studies in Drosophila showing the pros and dPax2 cooperate during lens differentiation (Charlton-Perkins et al. 2011). These factors show largely complementary expression patterns during early CC specification, mutants in either factor lead to relatively subtle effects on subsets of CCs, and they combinatorially control all aspects of lens formation. This can be likened to the fact that Pax6 and Prox1 expression are somewhat complementary (Pax6 is high in the AEL and absent in differentiating fiber cells, whereas Prox1 is low in the AEL and is up-regulated at the transition zone and in differentiating fiber cells), that both Pax6 and Prox1 independently regulate cell cycle exit at the transition zone, and that these factors appear to play complementary roles during fiber cell differentiation and Crystallin expression (Wigle et al. 1999; Ashery-Padan et al. 2000; Lengler et al. 2001; Duncan et al. 2002; Cui et al. 2004). Unlike in vertebrate systems, the molecular control of fly Crystallin expression are far less understood. While dPax2 function is necessary for Drosocrystallin expression, it is unclear whether this affect is direct or indirect (Dziedzic et al. 2009). In addition, even though pros does not appear to be required for Drosocrystallin expression (MCP and TC, unpublished), it is not yet possible to test whether it is involved in regulating other Crystallin-encoding genes. Indeed, until other markers of the corneal lens and pseudocone are identified, studies aimed at testing whether dPax2, Pros, SoxN or MAF factors contribute to similar regulatory processes as found in vertebrates will have to wait. Nevertheless, addressing these possibilities will certainly lend important insight into the degree of similarities that exist in the development of cells that give rise to invertebrate and vertebrate lenses.
Adhesion molecules
A compelling similarity between vertebrate and invertebrate lens development is the expression pattern of Cadherin molecules in terminally differentiated cells (Fig. 5). In the Drosophila eye, E-cad is expressed in all cells, but in CCs specifically, N-cad becomes up-regulated, and this expression is maintained through the rest of development (Fig. 5a, MCP, personal observation, Hayashi and Carthew 2004). The CCs rely predominantly N-cad for proper cell contacts and morphogenesis, but E-cad and N-cad function redundantly in CC-CC adhesion (Hayashi and Carthew 2004). Similarly, in vertebrate lens development, E-Cad is predominantly expressed in cells prior to fiber cell differentiation (in the AEL and transition zone), but is dramatically down-regulated as cells undergo terminal differentiation, leaving N-cadherin as the primary cadherin in fully differentiated lens fiber cells. E-Cad and N-Cad also function redundantly during vertebrate lens development (Pontoriero et al. 2009). In vertebrates, the Wnt and Notch signaling pathways are important for maintaining E-Cad expression in the AEL, while Fgf signaling down-regulates E-Cad at the lens equator (Smith et al. 2005; Jia et al. 2007; Cain et al. 2008; Rowan et al. 2008; Zhao et al. 2008). Notably, E-cad expression is maintained at high levels in Prox1 mutant fiber cells (Wigle et al. 1999), suggesting that Prox1 is critical for mediating the Fgf-dependent down-regulation of E-cad. Comparably, in flies, wg and Notch promote functions in cells expressing high levels of E-cad (PPC and IOCs), EGF primarily functions in cells expressing low levels of E-cad and high levels of N-Cad (CCs), and Pros is expressed in low E-Cad/high N-cad-expressing cells. Thus, it may be informative to test the relationships between Pros/Prox1, EGF/ Fgf-dependent signaling and cadherin expression during lens development in both systems. Finally, based on the importance of Neph/Neprin-related molecules for regulating Notch and Bmp/Dpp signaling and morphogenesis in the fly eye, examining the role of these factors in related processes in the vertebrate lens is warranted.
Conclusion
Despite the morphological differences in structure, the correlation of signaling pathways, transcriptional regulation and adhesion molecules involved in vertebrate and invertebrate lens morphogenesis provides robust evidence that their development is highly conserved. The future identification of novel factors with functional roles in either system will likely deepen our understanding of the developmental networks that interact to regulate lens development in general.
Acknowledgments
We thank Tien Le for vertebrate lens immunofluorescent images. We also wish to thank Ruth Ashery-Padan, Mike Robinson, Ross Cagan, Don Ready, and members of the Visual Systems Group at Cincinnati Children’s Hospital for many insightful discussions on this topic. This work was supported by NIH RO1-EY18097 (NLB), and the Research to Prevent Blindness Foundation, the Ziegler Foundation for the Blind and NIH R01-EY017907 (TAC).
Abbreviations
- CC
Cone cell
- PPC
Primary pigment cell
- MF
Morphogenetic furrow
- SMW
Second mitotic wave
- IOC
Interommatidial cell
- SPC
Secondary pigment cell
- TPC
Tertiary pigment cell
- PR
Photoreceptor
- PLE
Presumptive lens ectoderm
- AEL
Anterior epithelial layer
- PCP
Planar cell polarity
Contributor Information
Mark Charlton-Perkins, Divisions of Pediatric Ophthalmology and Developmental Biology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229, USA; Molecular and Developmental Biology Graduate Program, University of Cincinnati, Cincinnati, OH 45229, USA.
Nadean L. Brown, Divisions of Pediatric Ophthalmology and Developmental Biology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229, USA Molecular and Developmental Biology Graduate Program, University of Cincinnati, Cincinnati, OH 45229, USA.
Tiffany A. Cook, Divisions of Pediatric Ophthalmology and Developmental Biology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229, USA Molecular and Developmental Biology Graduate Program, University of Cincinnati, Cincinnati, OH 45229, USA.
References
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma N, Hirakiyama A, Inoue T, Asaka A, Yamada M. Mutations of a human homologue of the Drosophila eyes absent gene (EYA1) detected in patients with congenital cataracts and ocular anterior segment anomalies. Hum Mol Genet. 2000;9:363–366. doi: 10.1093/hmg/9.3.363. [DOI] [PubMed] [Google Scholar]
- Bajpe PK, van der Knaap JA, Demmers JA, Bezstarosti K, Bassett A, van Beusekom HM, Travers AA, Verrijzer CP. Deubiquitylating enzyme UBP64 controls cell fate through stabilization of the transcriptional repressor tramtrack. Mol Cell Biol. 2008;28:1606–1615. doi: 10.1128/MCB.01567-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NE, Yu SY. The EGF receptor defines domains of cell cycle progression and survival to regulate cell number in the developing Drosophila eye. Cell. 2001;104:699–708. doi: 10.1016/s0092-8674(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Bao S. Two themes on the assembly of the Drosophila eye. Curr Top Dev Biol. 2010;93:85–127. doi: 10.1016/B978-0-12-385044-7.00004-7. [DOI] [PubMed] [Google Scholar]
- Bao S, Cagan R. Preferential adhesion mediated by Hibris and Roughest regulates morphogenesis and patterning in the Drosophila eye. Dev Cell. 2005;8:925–935. doi: 10.1016/j.devcel.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Bao ZZ, Cepko CL. The expression and function of Notch pathway genes in the developing rat eye. J Neurosci. 1997;17:1425–1434. doi: 10.1523/JNEUROSCI.17-04-01425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baonza A, Murawsky CM, Travers AA, Freeman M. Pointed and Tramtrack69 establish an EGFR-dependent transcriptional switch to regulate mitosis. Nat Cell Biol. 2002;4:976–980. doi: 10.1038/ncb887. [DOI] [PubMed] [Google Scholar]
- Bateman JM, McNeill H. Temporal control of differentiation by the insulin receptor/tor pathway in Drosophila. Cell. 2004;119:87–96. doi: 10.1016/j.cell.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Beatus P, Lendahl U. Notch and neurogenesis. J Neurosci Res. 1998;54:125–136. doi: 10.1002/(SICI)1097-4547(19981015)54:2<125::AID-JNR1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Beebe D, Garcia C, Wang X, Rajagopal R, Feldmeier M, Kim JY, Chytil A, Moses H, Ashery-Padan R, Rauchman M. Contributions by members of the TGFbeta superfamily to lens development. Int J Dev Biol. 2004;48:845–856. doi: 10.1387/ijdb.041869db. [DOI] [PubMed] [Google Scholar]
- Belecky-Adams TL, Adler R, Beebe DC. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development. 2002;129:3795–3802. doi: 10.1242/dev.129.16.3795. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Baker NE. The HLH protein Extramacrochaetae is required for R7 cell and cone cell fates in the Drosophila eye. Dev Biol. 2009;327:288–300. doi: 10.1016/j.ydbio.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco J, Girard F, Kamachi Y, Kondoh H, Gehring WJ. Functional analysis of the chicken delta1-crystallin enhancer activity in Drosophila reveals remarkable evolutionary conservation between chicken and fly. Development. 2005;132:1895–1905. doi: 10.1242/dev.01738. [DOI] [PubMed] [Google Scholar]
- Blixt A, Mahlapuu M, Aitola M, Pelto-Huikko M, Enerback S, Carlsson P. A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle. Genes Dev. 2000;14:245–254. [PMC free article] [PubMed] [Google Scholar]
- Blixt A, Landgren H, Johansson BR, Carlsson P. Foxe3 is required for morphogenesis and differentiation of the anterior segment of the eye and is sensitive to Pax6 gene dosage. Dev Biol. 2007;302:218–229. doi: 10.1016/j.ydbio.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Blochlinger K, Jan LY, Jan YN. Postembryonic patterns of expression of cut, a locus regulating sensory organ identity in Drosophila. Development. 1993;117:441–450. doi: 10.1242/dev.117.2.441. [DOI] [PubMed] [Google Scholar]
- Bogaert T, Brown N, Wilcox M. The Drosophila PS2 antigen is an invertebrate integrin that, like the fibronectin receptor, becomes localized to muscle attachments. Cell. 1987;51:929–940. doi: 10.1016/0092-8674(87)90580-0. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Leiserson WM, Benzer S. Multiple roles of the eyes absent gene in Drosophila. Dev Biol. 1998;196:42–57. doi: 10.1006/dbio.1997.8845. [DOI] [PubMed] [Google Scholar]
- Boswell BA, Lein PJ, Musil LS. Cross-talk between fibroblast growth factor and bone morphogenetic proteins regulates gap junction-mediated intercellular communication in lens cells. Mol Biol Cell. 2008a;19:2631–2641. doi: 10.1091/mbc.E08-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell BA, Overbeek PA, Musil LS. Essential role of BMPs in FGF-induced secondary lens fiber differentiation. Dev Biol. 2008b;324:202–212. doi: 10.1016/j.ydbio.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottcher RT, Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr Rev. 2005;26:63–77. doi: 10.1210/er.2003-0040. [DOI] [PubMed] [Google Scholar]
- Brachmann CB, Cagan RL. Patterning the fly eye: the role of apoptosis. Trends Genet. 2003;19:91–96. doi: 10.1016/S0168-9525(02)00041-0. [DOI] [PubMed] [Google Scholar]
- Brownell I, Dirksen M, Jamrich M. Forkhead Foxe3 maps to the dysgenetic lens locus and is critical in lens development and differentiation. Genesis. 2000;27:81–93. doi: 10.1002/1526-968x(200006)27:2<81::aid-gene50>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Brunner D, Ducker K, Oellers N, Hafen E, Scholz H, Klambt C. The ETS domain protein pointed-P2 is a target of MAP kinase in the sevenless signal transduction pathway. Nature. 1994;370:386–389. doi: 10.1038/370386a0. [DOI] [PubMed] [Google Scholar]
- Burgess D, Zhang Y, Siefker E, Vaca R, Kuracha MR, Reneker L, Overbeek PA, Govindarajan V. Activated Ras alters lens and corneal development through induction of distinct downstream targets. BMC Dev Biol. 2010;10:13. doi: 10.1186/1471-213X-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagan RL, Ready DF. The emergence of order in the Drosophila pupal retina. Dev Biol. 1989a;136:346–362. doi: 10.1016/0012-1606(89)90261-3. [DOI] [PubMed] [Google Scholar]
- Cagan RL, Ready DF. Notch is required for successive cell decisions in the developing Drosophila retina. Genes Dev. 1989b;3:1099–1112. doi: 10.1101/gad.3.8.1099. [DOI] [PubMed] [Google Scholar]
- Cain S, Martinez G, Kokkinos MI, Turner K, Richardson RJ, Abud HE, Huelsken J, Robinson ML, de Iongh RU. Differential requirement for beta-catenin in epithelial and fiber cells during lens development. Dev Biol. 2008;321:420–433. doi: 10.1016/j.ydbio.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Canon J, Banerjee U. In vivo analysis of a developmental circuit for direct transcriptional activation and repression in the same cell by a Runx protein. Genes Dev. 2003;17:838–843. doi: 10.1101/gad.1064803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain CG, McAvoy JW. Evidence that fibroblast growth factor promotes lens fibre differentiation. Curr Eye Res. 1987;6:1165–1169. doi: 10.3109/02713688709034890. [DOI] [PubMed] [Google Scholar]
- Charlton-Perkins M, Cook TA. Building a fly eye: terminal differentiation events of the retina, corneal lens, and pigmented epithelia. Curr Top Dev Biol. 2010;93:129–173. doi: 10.1016/B978-0-12-385044-7.00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton-Perkins M, Whitaker SL, Fei Y, Xie B, Li-Kroeger D, Gebelein B, Cook T. Prospero and Pax2 combinatorially control neural cell fate decisions by modulating Ras- and Notch-dependent signaling. Neural Dev. 2011;6:20. doi: 10.1186/1749-8104-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Stump RJ, Lovicu FJ, Shimono A, McAvoy JW. Wnt signaling is required for organization of the lens fiber cell cytoskeleton and development of lens three-dimensional architecture. Dev Biol. 2008;324:161–176. doi: 10.1016/j.ydbio.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyette BN, Green PJ, Martin K, Garren H, Hartenstein V, Zipursky SL. The Drosophila sine oculis locus encodes a homeodomain-containing protein required for the development of the entire visual system. Neuron. 1994;12:977–996. doi: 10.1016/0896-6273(94)90308-5. [DOI] [PubMed] [Google Scholar]
- Chow RL, Altmann CR, Lang RA, Hemmati-Brivanlou A. Pax6 induces ectopic eyes in a vertebrate. Development. 1999;126:4213–4222. doi: 10.1242/dev.126.19.4213. [DOI] [PubMed] [Google Scholar]
- Clayton FE. The effect of Lozenge Pseudoalleles on eye pigmentation in Drosophila melanogaster. I. Brown pigment and pigmentation in Lozenge males. Genetics. 1957;42:28–41. doi: 10.1093/genetics/42.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook T, Pichaud F, Sonneville R, Papatsenko D, Desplan C. Distinction between color photoreceptor cell fates is controlled by Prospero in Drosophila. Dev Cell. 2003;4:853–864. doi: 10.1016/s1534-5807(03)00156-4. [DOI] [PubMed] [Google Scholar]
- Cordero JB, Cagan RL. Canonical wingless signaling regulates cone cell specification in the Drosophila retina. Dev Dyn. 2010;239:875–884. doi: 10.1002/dvdy.22235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero J, Jassim O, Bao S, Cagan R. A role for wingless in an early pupal cell death event that contributes to patterning the Drosophila eye. Mech Dev. 2004;121:1523–1530. doi: 10.1016/j.mod.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Cordero JB, Larson DE, Craig CR, Hays R, Cagan R. Dynamic decapentaplegic signaling regulates patterning and adhesion in the Drosophila pupal retina. Development. 2007;134:1861–1871. doi: 10.1242/dev.002972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Tomarev SI, Piatigorsky J, Chepelinsky AB, Duncan MK. Mafs, Prox1, and Pax6 can regulate chicken betaB 1-crystallin gene expression. J Biol Chem. 2004;279:11088–11095. doi: 10.1074/jbc.M312414200. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Duncan MK. Genetic and epigenetic mechanisms of gene regulation during lens development. Prog Retin Eye Res. 2007;26:555–597. doi: 10.1016/j.preteyeres.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl A, Yang Y, Chauhan BK, Cveklova K. Regulation of gene expression by Pax6 in ocular cells: a case of tissue-preferred expression of crystallins in lens. Int J Dev Biol. 2004;48:829–844. doi: 10.1387/ijdb.041866ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daga A, Karlovich CA, Dumstrei K, Banerjee U. Patterning of cells in the Drosophila eye by Lozenge, which shares homologous domains with AML1. Genes Dev. 1996;10:1194–1205. doi: 10.1101/gad.10.10.1194. [DOI] [PubMed] [Google Scholar]
- de Iongh RU, Lovicu FJ, Overbeek PA, Schneider MD, Joya J, Hardeman ED, McAvoy JW. Requirement for TGFbeta receptor signaling during terminal lens fiber differentiation. Development. 2001;128:3995–4010. doi: 10.1242/dev.128.20.3995. [DOI] [PubMed] [Google Scholar]
- de Iongh RU, Wederell E, Lovicu FJ, McAvoy JW. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs. 2005;179:43–55. doi: 10.1159/000084508. [DOI] [PubMed] [Google Scholar]
- de Nooij JC, Hariharan IK. Uncoupling cell fate determination from patterned cell division in the Drosophila eye. Science. 1995;270:983–985. doi: 10.1126/science.270.5238.983. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson B, Hafen E. Genetics of signal transduction in invertebrates. Curr Opin Genet Dev. 1994;4:64–70. doi: 10.1016/0959-437x(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Dickson B, Sprenger F, Hafen E. Prepattern in the developing Drosophila eye revealed by an activated torso—sevenless chimeric receptor. Genes Dev. 1992;6:2327–2339. doi: 10.1101/gad.6.12a.2327. [DOI] [PubMed] [Google Scholar]
- Donner AL, Maas RL. Conservation and non-conservation of genetic pathways in eye specification. Int J Dev Biol. 2004;48:743–753. doi: 10.1387/ijdb.041877ad. [DOI] [PubMed] [Google Scholar]
- Donner AL, Lachke SA, Maas RL. Lens induction in vertebrates: variations on a conserved theme of signaling events. Semin Cell Dev Biol. 2006;17:676–685. doi: 10.1016/j.semcdb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Doroquez DB, Rebay I. Signal integration during development: mechanisms of EGFR and Notch pathway function and crosstalk. Crit Rev Biochem Mol Biol. 2006;41:339–385. doi: 10.1080/10409230600914344. [DOI] [PubMed] [Google Scholar]
- Duncan MK, Cui W, Oh DJ, Tomarev SI. Prox1 is differentially localized during lens development. Mech Dev. 2002;112:195–198. doi: 10.1016/s0925-4773(01)00645-1. [DOI] [PubMed] [Google Scholar]
- Dziedzic K, Heaphy J, Prescott H, Kavaler J. The transcription factor D-Pax2 regulates crystallin production during eye development in Drosophila melanogaster . Dev Dyn. 2009;238:2530–2539. doi: 10.1002/dvdy.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JS, Meyer MR. Conservation of antigen 3G6: a crystalline cone constituent in the compound eye of arthropods. J Neurobiol. 1990;21:441–452. doi: 10.1002/neu.480210306. [DOI] [PubMed] [Google Scholar]
- Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y. Self-organizing opticcup morphogenesis in three-dimensional culture. Nature. 2011;472:51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- Epstein J, Cai J, Glaser T, Jepeal L, Maas R. Identification of a Pax paired domain recognition sequence and evidence for DNA-dependent conformational changes. J Biol Chem. 1994;269:8355–8361. [PubMed] [Google Scholar]
- Faber SC, Robinson ML, Makarenkova HP, Lang RA. Bmp signaling is required for development of primary lens fiber cells. Development. 2002;129:3727–3737. doi: 10.1242/dev.129.15.3727. [DOI] [PubMed] [Google Scholar]
- Fisher M, Grainger RM. Lens induction and determination. In: Lovicu FJ, Robinson ML, editors. Development of the ocular lens. New York: Cambridge University Press; 2004. pp. 21–41. [Google Scholar]
- Flores GV, Duan H, Yan H, Nagaraj R, Fu W, Zou Y, Noll M, Banerjee U. Combinatorial signaling in the specification of unique cell fates. Cell. 2000;103:75–85. doi: 10.1016/s0092-8674(00)00106-9. [DOI] [PubMed] [Google Scholar]
- Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009;16:633–647. doi: 10.1016/j.devcel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Franciscovich AL, Mortimer AD, Freeman AA, Gu J, Sanyal S. Overexpression screen in Drosophila identifies neuronal roles of GSK-3 beta/shaggy as a regulator of AP-1-dependent developmental plasticity. Genetics. 2008;180:2057–2071. doi: 10.1534/genetics.107.085555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankfort BJ, Mardon G. R8 development in the Drosophila eye: a paradigm for neural selection and differentiation. Development. 2002;129:1295–1306. doi: 10.1242/dev.129.6.1295. [DOI] [PubMed] [Google Scholar]
- Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell. 1996;87:651–660. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- Freeman M. Cell determination strategies in the Drosophila eye. Development. 1997;124:261–270. doi: 10.1242/dev.124.2.261. [DOI] [PubMed] [Google Scholar]
- Frohlich A. A scanning electron-microscopic study of apical contacts in the eye during postembryonic development of Drosophila melanogaster . Cell Tissue Res. 2001;303:117–128. doi: 10.1007/s004410000306. [DOI] [PubMed] [Google Scholar]
- Fu W, Noll M. The Pax2 homolog sparkling is required for development of cone and pigment cells in the Drosophila eye. Genes Dev. 1997;11:2066–2078. doi: 10.1101/gad.11.16.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann S. Wnt signaling in eye organogenesis. Organogenesis. 2008;4:60–67. doi: 10.4161/org.4.2.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita SC, Zipursky SL, Benzer S, Ferrus A, Shotwell SL. Monoclonal antibodies against the Drosophila nervous system. Proc Natl Acad Sci USA. 1982;79:7929–7933. doi: 10.1073/pnas.79.24.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Hogan BL. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ. The master control gene for morphogenesis and evolution of the eye. Genes Cells. 1996;1:11–15. doi: 10.1046/j.1365-2443.1996.11011.x. [DOI] [PubMed] [Google Scholar]
- Gehring WJ. New perspectives on eye development and the evolution of eyes and photoreceptors. J Hered. 2005;96:171–184. doi: 10.1093/jhered/esi027. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Ikeo K. Pax 6: mastering eye morphogenesis and eye evolution. Trends Genet. 1999;15:371–377. doi: 10.1016/s0168-9525(99)01776-x. [DOI] [PubMed] [Google Scholar]
- Glass AS, Dahm R. The zebrafish as a model organism for eye development. Ophthal Res. 2004;36:4–24. doi: 10.1159/000076105. [DOI] [PubMed] [Google Scholar]
- Gorski SM, Brachmann CB, Tanenbaum SB, Cagan RL. Delta and notch promote correct localization of irreC-rst. Cell Death Differ. 2000;7:1011–1013. doi: 10.1038/sj.cdd.4400742. [DOI] [PubMed] [Google Scholar]
- Gould DB, Smith RS, John SW. Anterior segment development relevant to glaucoma. Int J Dev Biol. 2004;48:1015–1029. doi: 10.1387/ijdb.041865dg. [DOI] [PubMed] [Google Scholar]
- Greenwald I, Rubin GM. Making a difference: the role of cell-cell interactions in establishing separate identities for equivalent cells. Cell. 1992;68:271–281. doi: 10.1016/0092-8674(92)90470-w. [DOI] [PubMed] [Google Scholar]
- Grillo-Hill BK, Wolff T. Dynamic cell shapes and contacts in the developing Drosophila retina are regulated by the Ig cell adhesion protein hibris. Dev Dyn. 2009;238:2223–2234. doi: 10.1002/dvdy.21981. [DOI] [PubMed] [Google Scholar]
- Guo M, Jan LY, Jan YN. Control of daughter cell fates during asymmetric division: interaction of Numb and Notch. Neuron. 1996;17:27–4. doi: 10.1016/s0896-6273(00)80278-0. [DOI] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila . Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Flister S, Walldorf U, Kloter U, Gehring WJ. Eyeless initiates the expression of both sine oculis and eyes absent during Drosophila compound eye development. Development. 1998;125:2181–2191. doi: 10.1242/dev.125.12.2181. [DOI] [PubMed] [Google Scholar]
- Han JD, Baker NE, Rubin CS. Molecular characterization of a novel A kinase anchor protein from Drosophila melanogaster . J Biol Chem. 1997;272:26611–26619. doi: 10.1074/jbc.272.42.26611. [DOI] [PubMed] [Google Scholar]
- Hay N. Interplay between FOXO, TOR, and Akt. Biochim Biophys Acta-Mol Cell Res. 2011 doi: 10.1016/j.bbamcr.2011.03.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Carthew RW. Surface mechanics mediate pattern formation in the developing retina. Nature. 2004;431:647–652. doi: 10.1038/nature02952. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Kojima T, Saigo K. Specification of primary pigment cell and outer photoreceptor fates by BarH1 homeobox gene in the developing Drosophila eye. Dev Biol. 1998;200:131–145. doi: 10.1006/dbio.1998.8959. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Xu C, Carthew RW. Cell-type-specific transcription of prospero is controlled by combinatorial signaling in the Drosophila eye. Development. 2008;135:2787–2796. doi: 10.1242/dev.006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein U, Wolff T, Rubin GM. The TGF beta homolog dpp and the segment polarity gene hedgehog are required for propagation of a morphogenetic wave in the Drosophila retina. Cell. 1993;75:913–926. doi: 10.1016/0092-8674(93)90535-x. [DOI] [PubMed] [Google Scholar]
- Held LI. Imaginal discs: the genetic and cellular logic of pattern formation. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Higashijima S, Kojima T, Michiue T, Ishimaru S, Emori Y, Saigo K. Dual Bar homeo box genes of Drosophila required in two photoreceptor cells, R1 and R6, and primary pigment cells for normal eye development. Genes Dev. 1992;6:50–60. doi: 10.1101/gad.6.1.50. [DOI] [PubMed] [Google Scholar]
- Ho HY, Chang KH, Nichols J, Li M. Homeodomain protein Pitx3 maintains the mitotic activity of lens epithelial cells. Mech Dev. 2009;126:18–29. doi: 10.1016/j.mod.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Hsiung F, Moses K. Retinal development in Drosophila: specifying the first neuron. Hum Mol Genet. 2002;11:1207–1214. doi: 10.1093/hmg/11.10.1207. [DOI] [PubMed] [Google Scholar]
- Igaki T. Correcting developmental errors by apoptosis: lessons from Drosophila JNK signaling. Apoptosis. 2009;14:1021–1028. doi: 10.1007/s10495-009-0361-7. [DOI] [PubMed] [Google Scholar]
- Ingolia TD, Craig EA. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci USA. 1982;79:2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar L, Patkunanathan B, Lynch OT, McAvoy JW, Rasko JE, Lovicu FJ. Aqueous humour- and growth factor-induced lens cell proliferation is dependent on MAPK/ERK1/2 and Akt/ PI3-K signalling. Exp Eye Res. 2006;83:667–678. doi: 10.1016/j.exer.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Jackson Behan K, Fair J, Singh S, Bogwitz M, Perry T, Grubor V, Cunningham F, Nichols CD, Cheung TL, Batterham P, Pollock JA. Alternative splicing removes an Ets interaction domain from Lozenge during Drosophila eye development. Dev Genes Evol. 2005;215:423–435. doi: 10.1007/s00427-005-0490-0. [DOI] [PubMed] [Google Scholar]
- Jia J, Lin M, Zhang L, York JP, Zhang P. The notch signaling pathway controls the size of the ocular lens by directly suppressing p57Kip2 expression. Mol Cell Biol. 2007;27:7236–7247. doi: 10.1128/MCB.00780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Tanouchi A, Sekido R, Kondoh H. Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes Dev. 2001;15:1272–1286. doi: 10.1101/gad.887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda H, Miura M. Regulatory roles of JNK in programmed cell death. J Biochem. 2004;136:1–6. doi: 10.1093/jb/mvh098. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Luo G, Hofmann C, Bradley A. BMP 7 is required for nephrogenesis, eye development, and skeletal patterning. Ann N Y Acad Sci. 1996;785:98–107. doi: 10.1111/j.1749-6632.1996.tb56247.x. [DOI] [PubMed] [Google Scholar]
- Kauffmann RC, Li S, Gallagher PA, Zhang J, Carthew RW. Ras1 signaling and transcriptional competence in the R7 cell of Drosophila. Genes Dev. 1996;10:2167–2178. doi: 10.1101/gad.10.17.2167. [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Takahashi S, Nakajima O, Ogino H, Morita M, Nishizawa M, Yasuda K, Yamamoto M. Regulation of lens fiber cell differentiation by transcription factor c-Maf. J Biol Chem. 1999;274:19254–19260. doi: 10.1074/jbc.274.27.19254. [DOI] [PubMed] [Google Scholar]
- Kenyon KL, Moody SA, Jamrich M. A novel fork head gene mediates early steps during Xenopus lens formation. Development. 1999;126:5107–5116. doi: 10.1242/dev.126.22.5107. [DOI] [PubMed] [Google Scholar]
- Kim JI, Li T, Ho IC, Grusby MJ, Glimcher LH. Requirement for the c-Maf transcription factor in crystallin gene regulation and lens development. Proc Natl Acad Sci USA. 1999;96:3781–3785. doi: 10.1073/pnas.96.7.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Choi Y, Lee S, Seo Y, Yoon J, Baek K. Characterization of the Drosophila melanogaster retinin gene encoding a cornea-specific protein. Insect Mol Biol. 2008;17:537–543. doi: 10.1111/j.1365-2583.2008.00822.x. [DOI] [PubMed] [Google Scholar]
- Klesert TR, Cho DH, Clark JI, Maylie J, Adelman J, Snider L, Yuen EC, Soriano P, Tapscott SJ. Mice deficient in Six5 develop cataracts: implications for myotonic dystrophy. Nat Genet. 2000;25:105–109. doi: 10.1038/75490. [DOI] [PubMed] [Google Scholar]
- Komori N, Usukura J, Matsumoto H. Drosocrystallin, a major 52 kDa glycoprotein of the Drosophila melanogaster corneal lens. Purification, biochemical characterization, and subcellular localization. J Cell Sci. 1992;102(Pt 2):191–201. doi: 10.1242/jcs.102.2.191. [DOI] [PubMed] [Google Scholar]
- Kondoh H, Uchikawa M, Kamachi Y. Interplay of Pax6 and SOX2 in lens development as a paradigm of genetic switch mechanisms for cell differentiation. Int J Dev Biol. 2004;48:819–827. doi: 10.1387/ijdb.041868hk. [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozmik Z. Pax genes in eye development and evolution. Curr Opin Genet Dev. 2005;15:430–438. doi: 10.1016/j.gde.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Kozmik Z, Daube M, Frei E, Norman B, Kos L, Dishaw LJ, Noll M, Piatigorsky J. Role of Pax genes in eye evolution: a cnidarian PaxB gene uniting Pax2 and Pax6 functions. Dev Cell. 2003;5:773–785. doi: 10.1016/s1534-5807(03)00325-3. [DOI] [PubMed] [Google Scholar]
- Kumar JP. Signalling pathways in Drosophila and vertebrate retinal development. Nat Rev Genet. 2001;2:846–857. doi: 10.1038/35098564. [DOI] [PubMed] [Google Scholar]
- Kumar JP. Retinal determination the beginning of eye development. Curr Top Dev Biol. 2010;93:1–28. doi: 10.1016/B978-0-12-385044-7.00001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J, Moses K. Transcription factors in eye development: a gorgeous mosaic? Genes Dev. 1997;11:2023–2028. doi: 10.1101/gad.11.16.2023. [DOI] [PubMed] [Google Scholar]
- Lachke SA, Maas RL. Building the developmental oculome: systems biology in vertebrate eye development and disease. Wiley Interdiscip Rev Syst Biol Med. 2010;2:305–323. doi: 10.1002/wsbm.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai ZC, Rubin GM. Negative control of photoreceptor development in Drosophila by the product of the yan gene, an ETS domain protein. Cell. 1992;70:609–620. doi: 10.1016/0092-8674(92)90430-k. [DOI] [PubMed] [Google Scholar]
- Lai ZC, Fetchko M, Li Y. Repression of Drosophila photoreceptor cell fate through cooperative action of two transcriptional repressors Yan and Tramtrack. Genetics. 1997;147:1131–1137. doi: 10.1093/genetics/147.3.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RA. Pathways regulating lens induction in the mouse. Int J Dev Biol. 2004;48:783–791. doi: 10.1387/ijdb.041903rl. [DOI] [PubMed] [Google Scholar]
- Le TT, Conley KW, Brown NL. Jagged 1 is necessary for normal mouse lens formation. Dev Biol. 2009;328:118–126. doi: 10.1016/j.ydbio.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Frasch M. Survey of forkhead domain encoding genes in the Drosophila genome: classification and embryonic expression patterns. Dev Dyn. 2004;229:357–366. doi: 10.1002/dvdy.10443. (an official publication of the American Association of Anatomists) [DOI] [PubMed] [Google Scholar]
- Lee HY, Wroblewski E, Philips GT, Stair CN, Conley K, Reedy M, Mastick GS, Brown NL. Multiple requirements for Hes 1 during early eye formation. Dev Biol. 2005;284:464–478. doi: 10.1016/j.ydbio.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengler J, Krausz E, Tomarev S, Prescott A, Quinlan RA, Graw J. Antagonistic action of Six3 and Prox1 at the gamma-crystallin promoter. Nucleic Acids Res. 2001;29:515–526. doi: 10.1093/nar/29.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leptin M, Aebersold R, Wilcox M. Drosophila position-specific antigens resemble the vertebrate fibronectin-receptor family. EMBO J. 1987;6:1037–1043. doi: 10.1002/j.1460-2075.1987.tb04856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Li Y, Carthew RW, Lai ZC. Photoreceptor cell differentiation requires regulated proteolysis of the transcriptional repressor Tramtrack. Cell. 1997;90:469–478. doi: 10.1016/s0092-8674(00)80507-3. [DOI] [PubMed] [Google Scholar]
- Lim HY, Tomlinson A. Organization of the peripheral fly eye: the roles of Snail family transcription factors in peripheral retinal apoptosis. Development. 2006;133:3529–3537. doi: 10.1242/dev.02524. [DOI] [PubMed] [Google Scholar]
- Liu W, Lagutin OV, Mende M, Streit A, Oliver G. Six3 activation of Pax6 expression is essential for mammalian lens induction and specification. EMBO J. 2006;25:5383–5395. doi: 10.1038/sj.emboj.7601398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley RL, Jr, Ready DF. Integrins and the development of three-dimensional structure in the Drosophila compound eye. Dev Biol. 1995;171:415–433. doi: 10.1006/dbio.1995.1292. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, Overbeek PA. Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Development. 1998;125:3365–3377. doi: 10.1242/dev.125.17.3365. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, Robinson ML. Development of the ocular lens. New York: Cambridge University Press; 2004. [Google Scholar]
- Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9:2808–2820. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- Marin R, Demers M, Tanguay RM. Cell-specific heat-shock induction of Hsp23 in the eye of Drosophila melanogaster. Cell Stress Chaperones. 1996;1:40–46. doi: 10.1379/1466-1268(1996)001<0040:cshsio>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez G, de Iongh RU. The lens epithelium in ocular health and disease. Int J Biochem Cell Biol. 2010;42:1945–1963. doi: 10.1016/j.biocel.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Martinez G, Wijesinghe M, Turner K, Abud HE, Taketo MM, Noda T, Robinson ML, de Iongh RU. Conditional mutations of beta-catenin and APC reveal roles for canonical Wnt signaling in lens differentiation. Invest Ophthalmol Vis Sci. 2009;50:4794–4806. doi: 10.1167/iovs.09-3567. [DOI] [PubMed] [Google Scholar]
- Mathers PH, Grinberg A, Mahon KA, Jamrich M. The Rx homeobox gene is essential for vertebrate eye development. Nature. 1997;387:603–607. doi: 10.1038/42475. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Takahashi K, Kondo S, Kaibuchi K, Yamamoto D. Regulation of cone cell formation by Canoe and Ras in the developing Drosophila eye. Development. 1997;124:2671–2680. doi: 10.1242/dev.124.14.2671. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Kumar TR, Vassalli A, Bickenbach JR, Roop DR, Jaenisch R, Bradley A. Functional analysis of activins during mammalian development. Nature. 1995;374:354–356. doi: 10.1038/374354a0. [DOI] [PubMed] [Google Scholar]
- McAvoy JW. Cell division, cell elongation and distribution of alpha-, beta- and gamma-crystallins in the rat lens. J Embryol Exp Morphol. 1978;44:149–165. [PubMed] [Google Scholar]
- McAvoy JW, Chamberlain CG. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development. 1989;107:221–228. doi: 10.1242/dev.107.2.221. [DOI] [PubMed] [Google Scholar]
- McAvoy JW, Chamberlain CG, de Iongh RU, Hales AM, Lovicu FJ. Lens development. Eye (Lond) 1999;13(Pt 3b):425–437. doi: 10.1038/eye.1999.117. [DOI] [PubMed] [Google Scholar]
- McNeill H. Planar polarity: location, location, location. Curr Biol. 2002;12:R449–R451. doi: 10.1016/s0960-9822(02)00942-9. [DOI] [PubMed] [Google Scholar]
- Medina-Martinez O, Jamrich M. Foxe3 view of lens development and disease. Development. 2007;134:1455–1463. doi: 10.1242/dev.000117. [DOI] [PubMed] [Google Scholar]
- Medina-Martinez O, Brownell I, Amaya-Manzanares F, Hu Q, Behringer RR, Jamrich M. Severe defects in proliferation and differentiation of lens cells in Foxe3 null mice. Mol Cell Biol. 2005;25:8854–8863. doi: 10.1128/MCB.25.20.8854-8863.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Martinez O, Shah R, Jamrich M. Pitx3 controls multiple aspects of lens development. Dev Dyn. 2009;238:2193–2201. doi: 10.1002/dvdy.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses K, Ellis MC, Rubin GM. The glass gene encodes a zinc-finger protein required by Drosophila photoreceptor cells. Nature. 1989;340:531–536. doi: 10.1038/340531a0. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136:3699–3714. doi: 10.1242/dev.030338. [DOI] [PubMed] [Google Scholar]
- Nagaraj R, Banerjee U. The little R cell that could. Int J Dev Biol. 2004;48:755–760. doi: 10.1387/ijdb.041881rn. [DOI] [PubMed] [Google Scholar]
- Nagaraj R, Banerjee U. Combinatorial signaling in the specification of primary pigment cells in the Drosophila eye. Development. 2007;134:825–831. doi: 10.1242/dev.02788. [DOI] [PubMed] [Google Scholar]
- Nagaraj R, Banerjee U. Regulation of Notch and Wingless signalling by phyllopod, a transcriptional target of the EGFR pathway. EMBO J. 2009;28:337–346. doi: 10.1038/emboj.2008.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen CM, Leckband D, Yap AS. Tissue organization by cadherin adhesion molecules: dynamic molecular and cellular mechanisms of morphogenetic regulation. Physiol Rev. 2011;91:691–731. doi: 10.1152/physrev.00004.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson DE, Kelber A. A functional analysis of compound eye evolution. Arthropod Struct Dev. 2007;36:373–385. doi: 10.1016/j.asd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Nishiguchi S, Wood H, Kondoh H, Lovell-Badge R, Episkopou V. Sox1 directly regulates the gamma-crystallin genes and is essential for lens development in mice. Genes Dev. 1998;12:776–781. doi: 10.1101/gad.12.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino H, Fisher M, Grainger RM. Convergence of a head-field selector Otx2 and Notch signaling: a mechanism for lens specification. Development. 2008;135:249–258. doi: 10.1242/dev.009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onuma Y, Takahashi S, Asashima M, Kurata S, Gehring WJ. Conservation of Pax 6 function and upstream activation by Notch signaling in eye development of frogs and flies. Proc Natl Acad Sci USA. 2002;99:2020–2025. doi: 10.1073/pnas.022626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Carbe C, Powers A, Feng GS, Zhang X. Sprouty2-modulated Kras signaling rescues Shp2 deficiency during lens and lacrimal gland development. Development. 2010;137:1085–1093. doi: 10.1242/dev.042820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks AL, Turner FR, Muskavitch MA. Relationships between complex Delta expression and the specification of retinal cell fates during Drosophila eye development. Mech Dev. 1995;50:201–216. doi: 10.1016/0925-4773(94)00336-l. [DOI] [PubMed] [Google Scholar]
- Patterson JT, Muller HJ. Are “Progressive” Mutations Produced by X-Rays? Genetics. 1930;15:495–577. doi: 10.1093/genetics/15.6.495f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli D, Arrigo AP, Vazquez J, Tonka CH, Tissieres A. Expression of the small heat shock genes during Drosophila development: comparison of the accumulation of hsp23 and hsp27 mRNAs and polypeptides. Genome. 1989;31:671–676. doi: 10.1139/g89-123. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Crystallin genes: specialization by changes in gene regulation may precede gene duplication. J Struct Funct Genomics. 2003;3:131–137. [PubMed] [Google Scholar]
- Pontoriero GF, Smith AN, Miller LA, Radice GL, West-Mays JA, Lang RA. Co-operative roles for E-cadherin and N-cadherin during lens vesicle separation and lens epithelial cell survival. Dev Biol. 2009;326:403–417. doi: 10.1016/j.ydbio.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, Ding J, Ferguson MW, Doetschman T. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat Genet. 1995;11:409–414. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell P, Oliver G, Mardon G, Donner AL, Maas RL. Pax6-dependence of Six3, Eya1 and Dach1 expression during lens and nasal placode induction. Gene Expr Patterns. 2005;6:110–118. doi: 10.1016/j.modgep.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Quiring R, Walldorf U, Kloter U, Gehring WJ. Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science. 1994;265:785–789. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- Rajagopal R, Dattilo LK, Kaartinen V, Deng CX, Umans L, Zwijsen A, Roberts AB, Bottinger EP, Beebe DC. Functions of the type 1 BMP receptor Acvr1 (Alk2) in lens development: cell proliferation, terminal differentiation, and survival. Invest Ophthalmol Vis Sci. 2008;49:4953–4960. doi: 10.1167/iovs.08-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal R, Huang J, Dattilo LK, Kaartinen V, Mishina Y, Deng CX, Umans L, Zwijsen A, Roberts AB, Beebe DC. The type I BMP receptors, Bmpr1a and Acvr1, activate multiple signaling pathways to regulate lens formation. Dev Biol. 2009;335:305–316. doi: 10.1016/j.ydbio.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen JT, Deardorff MA, Tan C, Rao MS, Klein PS, Vetter ML. Regulation of eye development by frizzled signaling in Xenopus. Proc Natl Acad Sci USA. 2001;98:3861–3866. doi: 10.1073/pnas.071586298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read D, Manley JL. Alternatively spliced transcripts of the Drosophila tramtrack gene encode zinc finger proteins with distinct DNA binding specificities. EMBO J. 1992;11:1035–1044. doi: 10.1002/j.1460-2075.1992.tb05142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reza HM, Yasuda K. Roles of Maf family proteins in lens development. Dev Dyn. 2004;229:440–448. doi: 10.1002/dvdy.10467. [DOI] [PubMed] [Google Scholar]
- Rickenbacher J. Polished (pol), a new eye mutant in the 4th chromosome in Drosophila melanogaster . Z Indukt Abstamm Vererbungsl. 1954;86:61–68. [PubMed] [Google Scholar]
- Rieger DK, Reichenberger E, McLean W, Sidow A, Olsen BR. A double-deletion mutation in the Pitx3 gene causes arrested lens development in aphakia mice. Genomics. 2001;72:61–72. doi: 10.1006/geno.2000.6464. [DOI] [PubMed] [Google Scholar]
- Ring BZ, Cordes SP, Overbeek PA, Barsh GS. Regulation of mouse lens fiber cell development and differentiation by the Maf gene. Development. 2000;127:307–317. doi: 10.1242/dev.127.2.307. [DOI] [PubMed] [Google Scholar]
- Robinson ML, MacMillan-Crow LA, Thompson JA, Overbeek PA. Expression of a truncated FGF receptor results in defective lens development in transgenic mice. Development. 1995;121:3959–3967. doi: 10.1242/dev.121.12.3959. [DOI] [PubMed] [Google Scholar]
- Rohrbaugh M, Ramos E, Nguyen D, Price M, Wen Y, Lai ZC. Notch activation of yan expression is antagonized by RTK/ pointed signaling in the Drosophila eye. Curr Biol. 2002;12:576–581. doi: 10.1016/s0960-9822(02)00743-1. [DOI] [PubMed] [Google Scholar]
- Roignant JY, Treisman JE. Pattern formation in the Drosophila eye disc. Int J Dev Biol. 2009;53:795–804. doi: 10.1387/ijdb.072483jr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan S, Conley KW, Le TT, Donner AL, Maas RL, Brown NL. Notch signaling regulates growth and differentiation in the mammalian lens. Dev Biol. 2008;321:111–122. doi: 10.1016/j.ydbio.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusconi JC, Fink JL, Cagan R. klumpfuss regulates cell death in the Drosophila retina. Mech Dev. 2004;121:537–546. doi: 10.1016/j.mod.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanamuthu SS, Gao CY, Zelenka PS. Notch signaling is required for lateral induction of Jagged1 during FGF-induced lens fiber differentiation. Dev Biol. 2009;332:166–176. doi: 10.1016/j.ydbio.2009.05.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar PS, Appukuttan B, Han J, Ito Y, Ai C, Tsai W, Chai Y, Stout JT, Reddy S. Heterozygous loss of Six5 in mice is sufficient to cause ocular cataracts. Nat Genet. 2000;25:110–114. doi: 10.1038/75500. [DOI] [PubMed] [Google Scholar]
- Seimiya M, Gehring WJ. The Drosophila homeobox gene optix is capable of inducing ectopic eyes by an eyeless-independent mechanism. Development. 2000;127:1879–1886. doi: 10.1242/dev.127.9.1879. [DOI] [PubMed] [Google Scholar]
- Semina EV, Reiter RS, Murray JC. Isolation of a new homeobox gene belonging to the Pitx/Rieg family: expression during lens development and mapping to the aphakia region on mouse chromosome 19. Hum Mol Genet. 1997;6:2109–2116. doi: 10.1093/hmg/6.12.2109. [DOI] [PubMed] [Google Scholar]
- Seo HC, Curtiss J, Mlodzik M, Fjose A. Six class homeobox genes in Drosophila belong to three distinct families and are involved in head development. Mech Dev. 1999;83:127–139. doi: 10.1016/s0925-4773(99)00045-3. [DOI] [PubMed] [Google Scholar]
- Seppa MJ, Johnson RI, Bao S, Cagan RL. Polychaetoid controls patterning by modulating adhesion in the Drosophila pupal retina. Dev Biol. 2008;318:1–16. doi: 10.1016/j.ydbio.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serikaku MA, O’Tousa JE. sine oculis is a homeobox gene required for Drosophila visual system development. Genetics. 1994;138:1137–1150. doi: 10.1093/genetics/138.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham O, Smith AN, Robinson ML, Taketo MM, Lang RA, Ashery-Padan R. Pax6 is essential for lens fiber cell differentiation. Development. 2009;136:2567–2578. doi: 10.1242/dev.032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamloula HK, Mbogho MP, Pimentel AC, Chrzanowska-Lightowlers ZM, Hyatt V, Okano H, Venkatesh TR. rugose (rg), a Drosophila A kinase anchor protein, is required for retinal pattern formation and interacts genetically with multiple signaling pathways. Genetics. 2002;161:693–710. doi: 10.1093/genetics/161.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull MM, Doetschman T. Transforming growth factor-beta 1 in reproduction and development. Mol Reprod Dev. 1994;39:239–246. doi: 10.1002/mrd.1080390218. [DOI] [PubMed] [Google Scholar]
- Siddall NA, Behan KJ, Crew JR, Cheung TL, Fair JA, Batterham P, Pollock JA. Mutations in lozenge and D-Pax2 invoke ectopic patterned cell death in the developing Drosophila eye using distinct mechanisms. Dev Genes Evol. 2003;213:107–119. doi: 10.1007/s00427-003-0295-y. [DOI] [PubMed] [Google Scholar]
- Siddall NA, Hime GR, Pollock JA, Batterham P. Ttk69-dependent repression of lozenge prevents the ectopic development of R7 cells in the Drosophila larval eye disc. BMC Dev Biol. 2009;9:64. doi: 10.1186/1471-213X-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AN, Miller LA, Song N, Taketo MM, Lang RA. The duality of beta-catenin function: a requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev Biol. 2005;285:477–489. doi: 10.1016/j.ydbio.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Spemann H. Embryonic development and induction. New York: Hafner; 1938. [Google Scholar]
- Strodicke M, Karberg S, Korge G. Domina (Dom), a new Drosophila member of the FKH/WH gene family, affects morphogenesis and is a suppressor of position-effect variegation. Mech Dev. 2000;96:67–78. doi: 10.1016/s0925-4773(00)00371-3. [DOI] [PubMed] [Google Scholar]
- Stump RJ, Ang S, Chen Y, von Bahr T, Lovicu FJ, Pinson K, de Iongh RU, Yamaguchi TP, Sassoon DA, McAvoy JW. A role for Wnt/beta-catenin signaling in lens epithelial differentiation. Dev Biol. 2003;259:48–61. doi: 10.1016/s0012-1606(03)00179-9. [DOI] [PubMed] [Google Scholar]
- Swanson CI, Evans NC, Barolo S. Structural rules and complex regulatory circuitry constrain expression of a Notch- and EGFR-regulated eye enhancer. Dev Cell. 2010;18:359–370. doi: 10.1016/j.devcel.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AH, Neufeld TP, Kwan E, Rubin GM. PHYL acts to down-regulate TTK88, a transcriptional repressor of neuronal cell fates, by a SINA-dependent mechanism. Cell. 1997;90:459–467. doi: 10.1016/s0092-8674(00)80506-1. [DOI] [PubMed] [Google Scholar]
- Thompson B, Townsley F, Rosin-Arbesfeld R, Musisi H, Bienz M. A new nuclear component of the Wnt signalling pathway. Nat Cell Biol. 2002;4:367–373. doi: 10.1038/ncb786. [DOI] [PubMed] [Google Scholar]
- Tomarev SI, Piatigorsky J. Lens crystallins of invertebrates— diversity and recruitment from detoxification enzymes and novel proteins. Eur J Biochem. 1996;235:449–465. doi: 10.1111/j.1432-1033.1996.00449.x. [DOI] [PubMed] [Google Scholar]
- Tomarev SI, Zinovieva RD, Chang B, Hawes NL. Characterization of the mouse Prox1 gene. Biochem Biophys Res Commun. 1998;248:684–689. doi: 10.1006/bbrc.1998.8989. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Struhl G. Delta/Notch and Boss/Sevenless signals act combinatorially to specify the Drosophila R7 photoreceptor. Molecular cell. 2001;7:487–495. doi: 10.1016/s1097-2765(01)00196-4. [DOI] [PubMed] [Google Scholar]
- Tsuda L, Nagaraj R, Zipursky SL, Banerjee U. An EGFR/Ebi/ Sno pathway promotes delta expression by inactivating Su(H)/ SMRTER repression during inductive notch signaling. Cell. 2002;110:625–637. doi: 10.1016/s0092-8674(02)00875-9. [DOI] [PubMed] [Google Scholar]
- Vassalli A, Matzuk MM, Gardner HA, Lee KF, Jaenisch R. Activin/inhibin beta B subunit gene disruption leads to defects in eyelid development and female reproduction. Genes Dev. 1994;8:414–427. doi: 10.1101/gad.8.4.414. [DOI] [PubMed] [Google Scholar]
- Vivekanand P, Rebay I. Intersection of signal transduction pathways and development. Annu Rev Genet. 2006;40:139–157. doi: 10.1146/annurev.genet.40.110405.090555. [DOI] [PubMed] [Google Scholar]
- Voas MG, Rebay I. Signal integration during development: insights from the Drosophila eye. Dev Dyn. 2004;229:162–175. doi: 10.1002/dvdy.10449. [DOI] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–125. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Wassarman DA, Therrien M, Rubin GM. The Ras signaling pathway in Drosophila . Curr Opin Genet Dev. 1995;5:44–50. doi: 10.1016/s0959-437x(95)90052-7. [DOI] [PubMed] [Google Scholar]
- Weber GF, Menko AS. Phosphatidylinositol 3-kinase is necessary for lens fiber cell differentiation and survival. Invest Ophthalmol Vis Sci. 2006;47:4490–4499. doi: 10.1167/iovs.06-0401. [DOI] [PubMed] [Google Scholar]
- Wech I, Nagel AC. Mutations in rugose promote cell type-specific apoptosis in the Drosophila eye. Cell Death Differ. 2005;12:145–152. doi: 10.1038/sj.cdd.4401538. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999;21:318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- Wilcox M, Brown N, Piovant M, Smith RJ, White RA. The Drosophila position-specific antigens are a family of cell surface glycoprotein complexes. EMBO J. 1984;3:2307–2313. doi: 10.1002/j.1460-2075.1984.tb02131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildonger J, Sosinsky A, Honig B, Mann RS. Lozenge directly activates argos and klumpfuss to regulate programmed cell death. Genes Dev. 2005;19:1034–1039. doi: 10.1101/gad.1298105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff T, Ready DF. The beginning of pattern formation in the Drosophila compound eye: the morphogenetic furrow and the second mitotic wave. Development. 1991;113:841–850. doi: 10.1242/dev.113.3.841. [DOI] [PubMed] [Google Scholar]
- Wolff T, Ready DF. Pattern formation in the Drosophila retina. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1993. [Google Scholar]
- Wride MA. Cellular and molecular features of lens differentiation: a review of recent advances. Differentiation. 1996;61:77–93. doi: 10.1046/j.1432-0436.1996.6120077.x. [DOI] [PubMed] [Google Scholar]
- Xia Y, Karin M. The control of cell motility and epithelial morphogenesis by Jun kinases. Trends Cell Biol. 2004;14:94–101. doi: 10.1016/j.tcb.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Xiong WC, Montell C. tramtrack is a transcriptional repressor required for cell fate determination in the Drosophila eye. Genes Dev. 1993;7:1085–1096. doi: 10.1101/gad.7.6.1085. [DOI] [PubMed] [Google Scholar]
- Xu PX, Woo I, Her H, Beier DR, Maas RL. Mouse Eya homologues of the Drosophila eyes absent gene require Pax6 for expression in lens and nasal placode. Development. 1997;124:219–231. doi: 10.1242/dev.124.1.219. [DOI] [PubMed] [Google Scholar]
- Xu C, Kauffmann RC, Zhang J, Kladny S, Carthew RW. Overlapping activators and repressors delimit transcriptional response to receptor tyrosine kinase signals in the Drosophila eye. Cell. 2000;103:87–97. doi: 10.1016/s0092-8674(00)00107-0. [DOI] [PubMed] [Google Scholar]
- Yan H, Canon J, Banerjee U. A transcriptional chain linking eye specification to terminal determination of cone cells in the Drosophila eye. Dev Biol. 2003;263:323–329. doi: 10.1016/j.ydbio.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Yang XJ. Roles of cell-extrinsic growth factors in vertebrate eye pattern formation and retinogenesis. Semin Cell Dev Biol. 2004;15:91–103. doi: 10.1016/j.semcdb.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon CS, Hirosawa K, Suzuki E. Corneal lens secretion in newly emerged Drosophila melanogaster examined by electron microscope autoradiography. J Electron Microsc. 1997;46:243–246. doi: 10.1093/oxfordjournals.jmicro.a023515. [DOI] [PubMed] [Google Scholar]
- Yu SY, Yoo SJ, Yang L, Zapata C, Srinivasan A, Hay BA, Baker NE. A pathway of signals regulating effector and initiator caspases in the developing Drosophila eye. Development. 2002;129:3269–3278. doi: 10.1242/dev.129.13.3269. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta-Mol Cell Res. 2011 doi: 10.1016/j.bbamcr.2011.03.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Zhao H, Yang T, Madakashira BP, Thiels CA, Bechtle CA, Garcia CM, Zhang H, Yu K, Ornitz DM, Beebe DC, Robinson ML. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol. 2008;318:276–288. doi: 10.1016/j.ydbio.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky SL, Sanes JR. Chemoaffinity revisited: dscams, protocadherins, and neural circuit assembly. Cell. 2010;143:343–353. doi: 10.1016/j.cell.2010.10.009. [DOI] [PubMed] [Google Scholar]