Abstract
Aim: Cholesterol levels vary throughout childhood and adolescence. The aim of the present study was to evaluate and identify age- and gender-specific reference values for serum lipid concentrations including non-high-density lipoprotein cholesterol (non-HDL-C) and the triglyceride to HDL-C ratio (TG/HDL-C ratio) in apparently healthy Korean children and adolescents.
Methods: A total of 6197 participants aged 10 to 19 years old from the 2007–2013 Korean National Health and Nutrition Examination Survey were analyzed. Serum lipid concentrations were evaluated according to age and gender.
Results: The overall mean concentration of non-HDL-C was 105.5 ± 25.6 mg/dL, with a significant gender difference: 103.3 ± 26.1 mg/dL in boys and 107.9 ± 24.7 mg/dL in girls (p = 0.028). The median values of non-HDL-C concentrations in boys and girls, respectively, were 111 and 112 mg/dL in the 10-year-old age group, 95 and 103 mg/dL in the 15-year-old age group, and 109 and 103 mg/dL in the 19-year-old age group. The overall mean TG/HDL-C ratio was 1.74 ± 1.22, and there were no significant gender differences: 1.77 ± 1.25 in boys and 1.72 ± 1.22 in girls (p = 0.183). The median values of the TG/HDL-C ratio in boys and girls were 1.16 and 1.00 in the 10-year-olds, 1.54 and 0.95 in the 15-year-olds, and 1.74 and 0.84 in the 19-year-olds, respectively.
Conclusions: Age- and gender-specific reference values for non-HDL-C and for the TG/HDL-C ratio in children and adolescents could provide valuable information for individualized interpretations of lipid profiles and interventions as well as for strategies to prevent and manage childhood and adolescent dyslipidemia.
Keywords: Triglyceride to high-density lipoprotein cholesterol ratio, Non-high-density lipoprotein cholesterol, Dyslipidemia, Children, Adolescents
See editorial vol. 23: 1311–1312
Introduction
The morbidity and mortality because of atherosclerotic cardiovascular disease (CVD) has become the leading cause of death in Korea, accounting for approximately 25% of all deaths1); furthermore, the age of CVD onset now tends to be younger than in previous decades2). Atherosclerosis can begin in childhood, and its development has been related to cardiovascular risk factors, including dyslipidemia3). Early recognition of the risk factors and age-specific interventions are both important in preventing CVD in adulthood. However, the cholesterol levels of children and adolescents have attracted little interest.
Recently, non-conventional lipid profiles have been suggested to be predictors of cardiovascular events. These predictors include the concentrations of non-high-density lipoprotein cholesterol (non-HDL-C) and the triglyceride (TG) to HDL-C ratio (TG/HDL-C ratio). Non-HDL-C, defined as the total cholesterol minus the high-density lipoprotein cholesterol (HDL-C), includes all atherogenic cholesterols, such as low-density lipoprotein (LDL), lipoprotein (a), intermediate-density lipoprotein (IDL), and very-low-density lipoprotein (VLDL) remnants4). In the National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP III), non-HDL-C concentrations have been restricted to being a secondary goal in patients with high triglyceride concentrations of ≥200 mg/dL, whereas low-density lipoprotein cholesterol (LDL-C) concentrations are used as a primary therapeutic target5). However, recent studies have demonstrated that non-HDL-C can be used as an accurate predictor of cardiovascular disease6). The TG/HDL-C ratio has been suggested as a measure to identify overweight individuals with insulin resistance7, 8). In recent studies, the TG/HDL-C ratio was found to be associated with cardiovascular mortality9). It is well established that cholesterol levels vary by nation and ethnicity10). Therefore, caution should be used in the application of reference values from other nations and ethnicities in the Korean population. However, there are scarce reports on the distribution of non-HDL-C and of the TG/HDL-C ratio in Korean children and adolescents.
In the present study, we aimed to evaluate and identify age- and gender-specific reference values for serum lipids, including total cholesterol (TC), HDL-C, TG, LDL-C, non-HDL-C and the TG/HDL-C ratio, in a apparently healthy population of Korean children and adolescents.
Methods
Subjects
Data from the Korean National Health and Nutrition Examination Survey (KNHANES) from 2007–2013 were used. The KNHANES, a cross-sectional, nationally representative and population-based surveillance system, has been conducted annually since 1998 to evaluate the health and nutritional status of the civilian, non-institutionalized population of the Republic of Korea11). The survey is conducted by the Division of Chronic Disease Surveillance at the Korean Centers for Disease Control and Prevention. This survey, a nationally representative study, uses a stratified, multistage probability sampling design to select household units. The participants complete questionnaires that include a health interview survey, a health behavior survey, and a nutrition survey. All participants also undergo a health examination survey that includes fasting glucose, fasting insulin and fasting lipid profiles.
A total of 6,213 subjects had measures for TC, HDL-C, and TG and were 10 to 19 years old in the 2007–2013 KNHANES. Participants who had type 1 or 2 diabetes mellitus (DM) or thyroid disease were excluded from this study. Additionally, we excluded subjects receiving current medication for hyperlipidemia. Current medication for hyperlipidemia was defined as the administration of a drug for more than 20 days each month. Because LDL-C was determined using Friedewald's equation (LDL-C= total cholesterol − HDL-C − TG/5), which cannot be used when the triglyceride concentration is ≥400 mg/dL12), the participants with serum TG concentrations ≥400 mg/dL were also excluded. A final sample of 6,197 participants (3,260 males, 2,937 females) was available for analysis.
Anthropometric and Laboratory Measurements
The anthropometric data collected included height, weight, body mass index (BMI), waist circumference and blood pressure. BMI was determined by the weight in kilograms divided by the square of the height in meters (kg/m2). Blood samples were obtained from all subjects after ≥8 h of fasting. The collected specimens were centrifuged, frozen at −70°C and transported to a central laboratory (Seoul Medical Science Institute, Seoul, Korea before 2008 and NeoDIN Medical Institute, Seoul, Korea since 2008), where they were measured within 24 h. In 2007, the serum TC, HDL-C, and TG concentrations were measured enzymatically using ADIVIA1650 (Siemens/USA) with commercial reagents (Siemens/USA). After 2008, serum lipid concentrations, including total cholesterol (Pureauto S CHO-N; Daiichi, Tokyo, Japan), HDL-C (Cholestest N HDL; Daiichi), and triglycerides (Pureauto S TG-N; Daiichi), were analyzed by an enzymatic method using an automated analyzer (Hitachi Automatic Analyzer 7600, Hitachi). LDL-C concentrations were determined using Friedewald's formula. Non-HDL-C was calculated as: TC–HDL-C. Because the domestic laboratory which used to determine HDL-C changed in 2008, and the respective institutes used different analyzing methods and devices, some differences existed in the HDL-C results. Commutable frozen serum samples were prepared and sent to the Lipid Reference Laboratory at the Centers for Disease Control and Prevention in the USA. These data were compared with those from the two domestic laboratory institutes, and a conversion equation to adjust for the differences was formulated to obtain the true HDL-C value. The total coefficient of variation (CVs) for total cholesterol was 1.0%–2.8%. The total CV for HDL-C was 0.9%–3.2%, and the total CV for triglycerides was 0.9%–3.1%.
Definition
The presence of type 1 or 2 DM and thyroid disease and the current use of medications for hyperlipidemia were assessed using the responses to the questionnaire that the respective subjects had provided during the health interview. Dyslipidemia was defined by the following measures: 1) hyper-TC concentration (TC ≥200 mg/dL), hypo-HDL-C concentration (HDL-C < 40 mg/dL), hyper-TG concentration (TG ≥130 mg/dL), hyper-LDL-C (LDL-C ≥130 mg/dL), and hyper-non-HDL-C (non-HDL-C ≥145 mg/dL)5, 13).
Statistics
The data were analyzed using SPSS for Windows version 21 (IBM SPSS Inc., Chicago, IL, USA). The serum concentrations of TC, HDL-C, TG, LDL-C, and non-HDL-C and the TG/HDL-C ratio were analyzed according to age and gender for each age group and are presented as the mean ± standard deviation (SD) and percentiles (5th, 10th, 25th, 50th, 75th, 90th, 95th). P values less than 0.05 were considered statistically significant. Data are reported as the mean ± SD for the continuous variables.
Results
Clinical Characteristics of the Study Population
The clinical characteristics of the subjects are presented in Table 1. The boys had a significantly greater mean height (163.79 cm vs. 156.98 cm in girls, p < 0.001), weight (56.92 kg vs. 50.02 kg, p < 0.001) waist circumference (71.32 cm vs. 66.96 cm, p < 0.001), BMI (20.89 kg/m2 vs. 20.13 kg/m2, p < 0.001), systolic blood pressure (108.29 mmHg vs. 103.45 mmHg, p < 0.001) and diastolic pressure (66.84 mmHg vs. 65.61 mmHg, p < 0.001) than girls. The mean age was higher in the girls than in the boys (14.20 years old vs. 14.02 years old, respectively, p = 0.011).
Table 1. Clinical characteristics of the study population (n = 6197).
| Total | Boys | Girls | P | |
|---|---|---|---|---|
| Number | 6207 | 3260 | 2937 | |
| Age (years) | 14.1 ± 2.8 | 14.0 ± 2.7 | 14.2 ± 2.8 | 0.011 |
| Height (cm) | 160.6 ± 11.4 | 163.8 ± 12.7 | 157.0 ± 8.4 | <0.001 |
| Weight (kg) | 53.7 ± 13.9 | 56.9 ± 15.5 | 50.0 ± 10.8 | <0.001 |
| Waist circumference (cm) | 69.3 ± 9.7 | 71.3 ± 10.5 | 67.0 ± 8.2 | <0.001 |
| Body mass index (kg/m2) | 20.5 | 20.9 ± 3.8 | 20.1 ± 3.3 | <0.001 |
| Systolic blood pressure (mmHg) | 106.0 ± 10.5 | 108.3 ± 10.9 | 103.5 ± 9.4 | <0.001 |
| Diastolic blood pressure (mmHg) | 66.3 ± 9.3 | 66.9 ± 9.9 | 65.6 ± 8.5 | <0.001 |
Age-specific Reference Values for Serum Lipid Concentrations According to Age and Gender
Table 2 shows the mean, standard deviation, median, and percentile values for the serum lipid levels according to age and gender.
Table 2. Means, standard deviations, medians and percentiles for serum lipid profiles, non-HDL-C and TG/HDL-C ratios by age and gender among Korean children and adolescents (n = 6197).
| Age | n | Mean | SD | Percentile |
Age | n | Mean | SD | Percentile |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 25 | 50 | 75 | 90 | 95 | 5 | 10 | 25 | 50 | 75 | 90 | 95 | |||||||||
| Boys | Girls | |||||||||||||||||||||
| Total cholesterol (mg/dL) | Total cholesterol (mg/dL) | |||||||||||||||||||||
| 10 | 354 | 167.9 | 24.4 | 134 | 139 | 151 | 165 | 181 | 202 | 213 | 10 | 315 | 167.6 | 26.0 | 129 | 135 | 148 | 166 | 184 | 200 | 219 | |
| 11 | 376 | 163.7 | 25.8 | 125 | 133 | 145 | 163 | 183 | 197 | 208 | 11 | 332 | 161.9 | 23.7 | 126 | 131 | 145 | 161 | 178 | 192 | 202 | |
| 12 | 401 | 157.7 | 29.1 | 116 | 126 | 139 | 154 | 172 | 193 | 207 | 12 | 311 | 161.1 | 24.8 | 124 | 132 | 146 | 160 | 177 | 196 | 209 | |
| 13 | 375 | 151.6 | 25.0 | 113 | 123 | 135 | 149 | 166 | 183 | 197 | 13 | 327 | 161.1 | 25.3 | 124 | 131 | 146 | 160 | 173 | 196 | 208 | |
| 14 | 388 | 148.2 | 25.3 | 108 | 117 | 131 | 147 | 163 | 178 | 190 | 14 | 343 | 161.9 | 25.2 | 122 | 128 | 144 | 163 | 177 | 195 | 207 | |
| 15 | 332 | 147.6 | 26.6 | 106 | 115 | 129 | 145 | 166 | 180 | 194 | 15 | 279 | 159.1 | 11.2 | 123 | 128 | 142 | 157 | 173 | 194 | 207 | |
| 16 | 290 | 150.6 | 28.1 | 109 | 118 | 133 | 146 | 165 | 186 | 197 | 16 | 278 | 161.0 | 26.0 | 122 | 129 | 143 | 158 | 177 | 196 | 207 | |
| 17 | 293 | 148.7 | 24.7 | 113 | 121 | 129 | 146 | 166 | 182 | 192 | 17 | 290 | 161.9 | 26.7 | 126 | 132 | 142 | 158 | 178 | 199 | 212 | |
| 18 | 260 | 153.7 | 27.3 | 115 | 123 | 135 | 151 | 169 | 190 | 202 | 18 | 228 | 164.6 | 27.0 | 124 | 134 | 146 | 162 | 178 | 202 | 217 | |
| 19 | 191 | 159.8 | 24.7 | 118 | 130 | 143 | 159 | 176 | 190 | 199 | 19 | 234 | 165.1 | 26.4 | 125 | 133 | 147 | 164 | 181 | 197 | 206 | |
| Total | 3260 | 155.0 | 27.0 | 115 | 123 | 137 | 153 | 171 | 190 | 202 | Total | 2937 | 162.6 | 25.7 | 124 | 132 | 145 | 161 | 178 | 196 | 209 | |
| High-density lipoprotein cholesterol (mg/dL) | High-density lipoprotein cholesterol (mg/dL) | |||||||||||||||||||||
| 10 | 354 | 56.7 | 11.8 | 39 | 43 | 48 | 55 | 64 | 73 | 79 | 10 | 315 | 54.8 | 11.3 | 39 | 41 | 47 | 53 | 62 | 70 | 75 | |
| 11 | 376 | 55.1 | 11.5 | 38 | 41 | 47 | 55 | 62 | 70 | 75 | 11 | 332 | 53.5 | 11.1 | 36 | 41 | 46 | 52 | 61 | 68 | 74 | |
| 12 | 401 | 53.0 | 11.2 | 36 | 39 | 45 | 52 | 60 | 69 | 73 | 12 | 311 | 54.5 | 10.0 | 37 | 41 | 48 | 54 | 61 | 67 | 71 | |
| 13 | 375 | 51.6 | 11.0 | 35 | 39 | 44 | 50 | 59 | 66 | 70 | 13 | 327 | 53.5 | 10.4 | 36 | 41 | 47 | 53 | 60 | 66 | 71 | |
| 14 | 388 | 50.3 | 10.2 | 34 | 37 | 42 | 50 | 57 | 63 | 68 | 14 | 343 | 53.4 | 10.4 | 38 | 40 | 46 | 53 | 60 | 67 | 71 | |
| 15 | 332 | 48.7 | 9.6 | 35 | 37 | 42 | 48 | 55 | 61 | 65 | 15 | 279 | 54.3 | 11.2 | 36 | 40 | 47 | 54 | 62 | 69 | 72 | |
| 16 | 290 | 49.1 | 9.6 | 35 | 37 | 43 | 49 | 54 | 61 | 67 | 16 | 278 | 54.3 | 11.4 | 37 | 40 | 46 | 53 | 62 | 68 | 76 | |
| 17 | 293 | 48.9 | 9.2 | 34 | 37 | 42 | 48 | 55 | 61 | 63 | 17 | 290 | 55.4 | 10.4 | 39 | 42 | 48 | 55 | 62 | 68 | 72 | |
| 18 | 260 | 50.8 | 10.0 | 36 | 38 | 44 | 51 | 57 | 64 | 69 | 18 | 228 | 55.8 | 12.4 | 36 | 40 | 48 | 55 | 65 | 72 | 76 | |
| 19 | 191 | 50.3 | 9.4 | 36 | 39 | 43 | 49 | 56 | 61 | 68 | 19 | 234 | 58.4 | 10.5 | 42 | 45 | 51 | 58 | 65 | 71 | 78 | |
| Total | 3260 | 51.7 | 10.8 | 36 | 39 | 44 | 51 | 58 | 66 | 71 | Total | 2937 | 54.6 | 11.0 | 38 | 41 | 47 | 54 | 62 | 68 | 73 | |
| Triglyceride (mg/dL) | Triglyceride (mg/dL) | |||||||||||||||||||||
| 10 | 354 | 76.7 | 42.6 | 31 | 37 | 47 | 65 | 97 | 125 | 168 | 10 | 315 | 89.4 | 43.9 | 35 | 44 | 59 | 80 | 112 | 146 | 164 | |
| 11 | 376 | 81.5 | 51.1 | 30 | 36 | 48 | 67 | 96 | 148 | 195 | 11 | 332 | 95.1 | 50.2 | 38 | 44 | 59 | 83 | 119 | 160 | 192 | |
| 12 | 401 | 81.6 | 48.3 | 33 | 37 | 50 | 70 | 95 | 140 | 184 | 12 | 311 | 93.3 | 45.4 | 42 | 47 | 65 | 84 | 110 | 147 | 180 | |
| 13 | 375 | 86.3 | 51.0 | 33 | 38 | 52 | 73 | 105 | 151 | 197 | 13 | 327 | 87.4 | 44.5 | 38 | 45 | 58 | 78 | 103 | 132 | 183 | |
| 14 | 388 | 79.3 | 43.4 | 32 | 38 | 48 | 67 | 97 | 142 | 165 | 14 | 343 | 89.0 | 46.1 | 38 | 46 | 57 | 77 | 108 | 149 | 189 | |
| 15 | 332 | 83.6 | 44.9 | 36 | 41 | 55 | 73 | 99 | 142 | 176 | 15 | 279 | 84.5 | 47.7 | 36 | 44 | 54 | 74 | 103 | 140 | 166 | |
| 16 | 290 | 86.7 | 46.1 | 36 | 41 | 56 | 78 | 106 | 146 | 178 | 16 | 278 | 79.8 | 40.4 | 36 | 41 | 54 | 71 | 94 | 130 | 159 | |
| 17 | 293 | 87.5 | 47.9 | 38 | 42 | 55 | 75 | 103 | 153 | 189 | 17 | 290 | 78.0 | 38.4 | 33 | 40 | 51 | 69 | 95 | 126 | 158 | |
| 18 | 260 | 86.5 | 47.0 | 38 | 43 | 54 | 74 | 105 | 143 | 168 | 18 | 228 | 79.2 | 36.0 | 39 | 43 | 55 | 71 | 94 | 127 | 158 | |
| 19 | 191 | 99.0 | 51.6 | 42 | 51 | 65 | 87 | 113 | 169 | 207 | 19 | 234 | 80.1 | 46.3 | 33 | 39 | 51 | 69 | 92 | 130 | 176 | |
| Total | 3260 | 84.1 | 48.3 | 34 | 39 | 52 | 72 | 101 | 144 | 180 | Total | 2937 | 86.3 | 45.0 | 37 | 44 | 56 | 76 | 104 | 140 | 171 | |
| Boys | Girls | |||||||||||||||||||||
| Low-density lipoprotein cholesterol (mg/dL) | Low-density lipoprotein cholesterol (mg/dL) | |||||||||||||||||||||
| 10 | 354 | 95.9 | 21.8 | 64 | 71 | 81 | 95 | 109 | 126 | 135 | 10 | 315 | 94.9 | 22.3 | 59 | 67 | 78 | 94 | 111 | 123 | 134 | |
| 11 | 376 | 92.2 | 22.3 | 58 | 65 | 75 | 91 | 107 | 122 | 130 | 11 | 332 | 89.5 | 21.0 | 54 | 64 | 77 | 89 | 101 | 117 | 127 | |
| 12 | 401 | 88.5 | 25.2 | 55 | 61 | 73 | 85 | 100 | 119 | 131 | 12 | 311 | 89.0 | 21.9 | 55 | 63 | 74 | 88 | 101 | 118 | 130 | |
| 13 | 375 | 82.7 | 20.9 | 52 | 57 | 70 | 80 | 94 | 111 | 120 | 13 | 327 | 90.1 | 22.8 | 59 | 65 | 77 | 86 | 102 | 117 | 133 | |
| 14 | 388 | 82.1 | 22.8 | 48 | 56 | 66 | 80 | 96 | 109 | 119 | 14 | 343 | 90.6 | 22.0 | 58 | 64 | 76 | 89 | 103 | 119 | 130 | |
| 15 | 332 | 82.1 | 22.2 | 49 | 55 | 66 | 80 | 96 | 112 | 119 | 15 | 279 | 87.9 | 22.2 | 56 | 64 | 72 | 86 | 100 | 118 | 128 | |
| 16 | 290 | 84.1 | 25.1 | 47 | 57 | 68 | 82 | 96 | 113 | 129 | 16 | 278 | 90.7 | 22.8 | 58 | 62 | 74 | 90 | 105 | 122 | 132 | |
| 17 | 293 | 82.3 | 22.8 | 48 | 55 | 67 | 80 | 98 | 112 | 121 | 17 | 290 | 90.9 | 23.3 | 59 | 65 | 74 | 88 | 102 | 125 | 139 | |
| 18 | 260 | 85.6 | 23.3 | 50 | 59 | 69 | 84 | 99 | 116 | 127 | 18 | 228 | 93.0 | 23.1 | 62 | 66 | 77 | 90 | 107 | 122 | 138 | |
| 19 | 191 | 89.6 | 21.6 | 54 | 63 | 76 | 89 | 103 | 116 | 125 | 19 | 234 | 90.6 | 22.8 | 58 | 64 | 76 | 88 | 105 | 117 | 127 | |
| Total | 3260 | 86.5 | 23.4 | 52 | 59 | 71 | 84 | 100 | 116 | 126 | Total | 2937 | 90.7 | 22.4 | 58 | 64 | 75 | 89 | 104 | 120 | 131 | |
| Non-high-density lipoprotein cholesterol (mg/dL) | Non-high-density lipoprotein cholesterol (mg/dL) | |||||||||||||||||||||
| 10 | 354 | 111.3 | 23.4 | 78 | 84 | 95 | 111 | 124 | 144 | 156 | 10 | 315 | 112.8 | 23.7 | 76 | 85 | 94 | 112 | 129 | 143 | 153 | |
| 11 | 376 | 108.5 | 24.8 | 73 | 77 | 90 | 106 | 125 | 143 | 151 | 11 | 332 | 108.5 | 23.7 | 71 | 79 | 94 | 107 | 121 | 140 | 149 | |
| 12 | 401 | 104.8 | 27.7 | 67 | 76 | 88 | 99 | 119 | 139 | 156 | 12 | 311 | 107.7 | 23.7 | 70 | 78 | 91 | 106 | 121 | 141 | 156 | |
| 13 | 375 | 100.0 | 24.7 | 64 | 71 | 84 | 97 | 113 | 131 | 143 | 13 | 327 | 107.6 | 24.5 | 72 | 81 | 92 | 104 | 122 | 138 | 154 | |
| 14 | 388 | 98.0 | 25.4 | 61 | 69 | 81 | 96 | 113 | 128 | 140 | 14 | 343 | 108.4 | 23.3 | 74 | 80 | 91 | 107 | 123 | 139 | 154 | |
| 15 | 332 | 98.8 | 25.1 | 62 | 68 | 80 | 96 | 116 | 129 | 143 | 15 | 279 | 104.8 | 25.6 | 68 | 77 | 87 | 101 | 118 | 139 | 152 | |
| 16 | 290 | 101.5 | 28.1 | 63 | 69 | 82 | 98 | 114 | 135 | 154 | 16 | 278 | 106.7 | 25.8 | 69 | 77 | 89 | 104 | 121 | 140 | 155 | |
| 17 | 293 | 99.8 | 25.7 | 63 | 68 | 82 | 99 | 116 | 134 | 146 | 17 | 290 | 106.5 | 25.7 | 71 | 78 | 88 | 102 | 120 | 143 | 158 | |
| 18 | 260 | 102.9 | 27.1 | 65 | 72 | 83 | 101 | 119 | 136 | 150 | 18 | 228 | 108.8 | 25.8 | 73 | 79 | 91 | 105 | 124 | 143 | 154 | |
| 19 | 191 | 109.4 | 25.4 | 70 | 78 | 93 | 109 | 126 | 145 | 151 | 19 | 234 | 106.6 | 26.1 | 71 | 77 | 89 | 103 | 122 | 137 | 155 | |
| Total | 3260 | 103.3 | 26.1 | 66 | 73 | 85 | 101 | 119 | 136 | 150 | Total | 2937 | 107.9 | 24.7 | 72 | 79 | 91 | 105 | 122 | 140 | 153 | |
| Triglyceride to high-density lipoprotein cholesterol ratio | Triglyceride to high-density lipoprotein cholesterol ratio | |||||||||||||||||||||
| 10 | 354 | 1.48 | 1.05 | 0.45 | 0.55 | 0.79 | 1.16 | 1.83 | 2.75 | 3.74 | 10 | 315 | 1.78 | 1.21 | 0.59 | 0.73 | 1.00 | 1.45 | 2.22 | 3.09 | 4.06 | |
| 11 | 376 | 1.63 | 1.29 | 0.51 | 0.60 | 0.83 | 1.21 | 1.88 | 3.35 | 4.20 | 11 | 332 | 1.96 | 1.38 | 0.55 | 0.71 | 1.06 | 1.63 | 2.49 | 3.55 | 4.63 | |
| 12 | 401 | 1.69 | 1.23 | 0.52 | 0.62 | 0.89 | 1.29 | 2.06 | 3.20 | 4.45 | 12 | 311 | 1.85 | 1.19 | 0.66 | 0.80 | 1.13 | 1.59 | 2.24 | 3.06 | 4.00 | |
| 13 | 375 | 1.83 | 1.36 | 0.53 | 0.65 | 0.95 | 1.44 | 2.29 | 3.37 | 4.55 | 13 | 327 | 1.76 | 1.16 | 0.66 | 0.76 | 1.00 | 1.47 | 2.10 | 2.91 | 4.18 | |
| 14 | 388 | 1.70 | 1.15 | 0.59 | 0.69 | 0.93 | 1.35 | 2.06 | 3.17 | 4.11 | 14 | 343 | 1.81 | 1.24 | 0.65 | 0.78 | 1.00 | 1.46 | 2.20 | 3.16 | 4.16 | |
| 15 | 332 | 1.83 | 1.21 | 0.68 | 0.80 | 1.01 | 1.54 | 2.24 | 3.16 | 4.19 | 15 | 279 | 1.72 | 1.45 | 0.63 | 0.69 | 0.95 | 1.34 | 1.98 | 2.91 | 3.92 | |
| 16 | 290 | 1.86 | 1.13 | 0.61 | 0.77 | 1.05 | 1.60 | 2.39 | 3.27 | 3.81 | 16 | 278 | 1.61 | 1.12 | 0.57 | 0.68 | 0.92 | 1.31 | 1.87 | 2.79 | 4.11 | |
| 17 | 293 | 1.92 | 1.30 | 0.68 | 0.79 | 1.07 | 1.55 | 2.29 | 3.71 | 4.40 | 17 | 290 | 1.53 | 1.03 | 0.58 | 0.65 | 0.84 | 1.25 | 1.86 | 2.71 | 3.66 | |
| 18 | 260 | 1.83 | 1.19 | 0.64 | 0.78 | 0.98 | 1.51 | 2.27 | 3.16 | 4.18 | 18 | 228 | 1.56 | 0.98 | 0.56 | 0.67 | 0.91 | 1.26 | 1.86 | 2.73 | 3.79 | |
| 19 | 191 | 2.10 | 1.32 | 0.75 | 0.90 | 1.19 | 1.74 | 2.49 | 4.02 | 4.97 | 19 | 234 | 1.47 | 1.10 | 0.53 | 0.63 | 0.84 | 1.17 | 1.63 | 2.54 | 3.83 | |
| Total | 3260 | 1.77 | 1.25 | 0.57 | 0.68 | 0.94 | 1.44 | 2.17 | 3.25 | 4.20 | Total | 2937 | 1.72 | 1.22 | 0.60 | 0.71 | 0.97 | 1.40 | 2.04 | 3.04 | 4.04 | |
SD; standard deviation
TC Concentrations
The overall mean concentration of TC was 158.6 ± 26.7 mg/dL, with a significant gender difference: 155.0 ± 27.0 mg/dL in boys and 162.6 ± 25.7 mg/dL in girls (p < 0.001).
The median TC level was approximately 160 mg/dL in 10- to 11-year-old boys and decreased to approximately 150 mg/dL in 12- to 18-year-old boys. The 95th percentile value was approximately 210 mg/dL in 10- to 12-year-old boys and approximately 200 mg/dL in 13- to 19-year-old boys. In girls, the median value of TC concentration was approximately 160 mg/dL in the 11- to 19-year-old age groups. The 95th percentile concentration was approximately 210 mg/dL in 12- to 19-year-old girls. The prevalence of a TC concentration >200 mg/dL was 5.6% in boys and 8.3% in girls.
HDL-C Concentrations
The overall mean concentration of HDL-C was 53.1 ± 11.0 mg/dL with a significant gender difference: 51.7 ± 10.8 mg/dL in boys and 54.6 ± 10.6 mg/dL in girls (p < 0.001).
In boys, the median concentration of HDL-C was approximately 55 mg/dL in the 10- to 11-year-old groups and decreased to approximately 50 mg/dL in the 12- to 19-year-old groups. The 5th percentile value of HDL-C was approximately 40 mg/dl in 10- to 19-year-old boys. In girls, the median concentration of HDL-C and the 5th percentile level of HDL-C was similar, approximately 55 mg/dL and 40 mg/dL, respectively, in the 10- to 19-year-old age groups. The prevalence of a HDL-C concentration <40 mg/dL was 12.0% in boys and 7.5% in girls.
TG Concentrations
The overall mean concentration of TG was 85.0 ± 46.2 mg/dL, with no significant gender difference: 84.1 ± 48.3 mg/dL in boys and 86.3 ± 45.0 mg/dL in girls (p = 0.057).
In boys, the median value of TG concentration increased gradually from 65 mg/dL in the 10-year-old age group to approximately 75 mg/dL in the 18-yearold age group. Overall, the 95th percentile concentration was approximately 185 mg/dL in the 10- to 19-year-old age groups. In girls, the median TG concentration decreased gradually from 80 mg/dL in the 10-year-old age group to approximately 70 mg/dL in the 19-year-old age group. Overall, the 95th percentile TG was approximately 170 mg/dL in girls of all ages. The prevalence of a TG concentration ≥130 mg/dL was 13.3% in boys and 12.9% in girls.
LDL-C Concentrations
The overall mean concentration of LDL-C was 88.5 ± 23.0 mg/dL, with a significant gender difference: 86.5 ± 23.4 mg/dL in boys and 90.7 ± 22.4 mg/dL in girls (p < 0.001).
In boys, the median concentration of LDL-C was approximately 90 mg/dL in the 10- to 11-yearold age groups and approximately 85 mg/dL in the 12- to 19-year-old age groups. The 95th percentile LDL-C concentration was approximately 130 mg/dL in 10- to 12-year-olds and decreased to approximately 125 mg/dL in 13- to 19-year-olds. In girls, the median value of LDL-C concentration was relatively consistent at approximately 90 mg/dl. The 95th percentile LDL-C concentration was approximately 130 mg/dL in 10- to 19-year-old girls. The prevalence of a LDL-C concentration ≥130 mg/dL was 4.2% in boys and 5.5% in girls.
Non-HDL-C Concentration
The overall mean concentration of non-HDL-C was 105.5 ± 25.6 mg/dL, and there was a significant gender difference: 103.3 ± 26.1 mg/dL in boys and 107.9 ± 24.7 mg/dL in girls (p = 0.028).
In boys, the median value of non-HDL-C concentrations was approximately 110 mg/dL in the 10- to 11-year-old age groups and approximately 100 mg/dL in the 12- to 17-year-old age groups. Finally, the median value of non-HDL-C concentrations was approximately 110 mg/dL in the 18- to 19-year-olds. The 95th percentile non-HDL-C concentration was approximately 150 mg/dL in the 10- to 19-year-old age groups (Fig. 1-A). The curve of the non-HDL-C was U-shaped in boys. In girls, the median non-HDL-C concentration was approximately 110 mg/dL in the 10- to 11-year-old age groups and approximately 105 mg/dL in the 12- to 19-year-old age groups. The 95th percentile concentration of non-HDL-C was relatively consistent, at an approximate level of 150 mg/dL (Fig. 1-B). The prevalence of a non-HDL-C concentration ≥145 mg/dL was 6.5% in boys and 7.8% in girls.
Fig. 1.

Reference percentile curves for non-HDL-C according to age in Korean boys (A) and girls (B) (n = 6,197)
TG/HDL-C Ratio
The overall mean TG/HDL-C ratio was 1.74 ± 1.22, and there was no significant gender difference: 1.77 ± 1.25 in boys and 1.72 ± 1.22 in girls (p = 0.183).
In boys, the median TG/HDL-C ratio increased gradually from 1.16 in the 10-year-olds to 1.75 in the 19-year-olds. Although the 95th percentile values of the TG/HDL-C ratio varied, they showed an increasing tendency overall (Fig. 2-A). In girls, the median TG/HDL-C ratio decreased gradually from 1.46 in the 10-year-old age group to 1.17 in the 19-year-old age group. The 95th percentile TG/HDL-C ratio decreased gradually from 4.61 in 10-year-old girls to 3.83 in 19-year-old girls (Fig. 2-B).
Fig. 2.
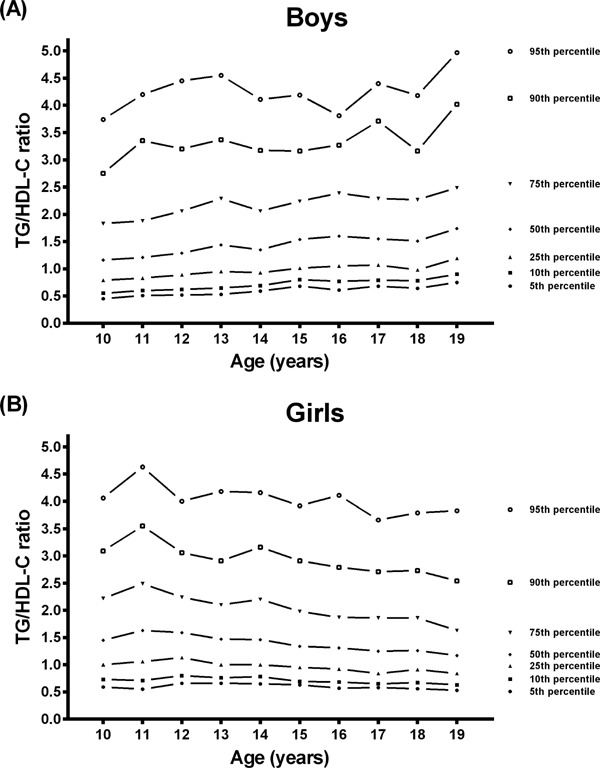
Reference percentile curves for the TG/HDL-C ratio according to age in Korean boys (A) and girls (B) (n = 6,197)
Discussion
In the present study, we identified the reference values of fasting serum lipid profiles including non-HDL-C and TG/HDL-C ratios in Korean children and adolescents from a nationally representative survey. We evaluated the distributions of non-HDL-C and of TG/HDL-C ratios in Korean children and adolescents aged 10 to 19 years. The overall means of the non-HDL-C and of the TG/HDL-C ratio in boys were 103.3 ± 26.1 mg/dL and 1.77 ± 1.25, respectively, and 107.9 ± 24.7 mg/dL and 1.22 ± 0.60 in girls, respectively.
In pediatric fields, measuring non-HDL-C has a distinct advantage because it does not require fasting. The Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents recommends the use of non-fasting non-HDL-C concentrations as a screening method for dyslipidemia. Several lines of evidence have supported non-HDL-C as a good predictor of CVD risk. In the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study, adolescents and adults aged 15 to 34 years with high non-HDL-C concentrations were at a significantly high risk for histological progression from fatty streaks to advanced atherosclerotic lesions14). In the Bogalusa Heart Study, non-HDL-C levels were as good as or better than other lipoprotein measurements, including LDL-C level, for identifying subclinical atherosclerosis among young subjects aged 24 to 48 years15). A study of US children and adolescents aged 12 to 19 years demonstrated that non-HDL-C was significantly associated with metabolic syndrome, which is significantly associated with CVD in adulthood16). In a US study of non-HDL-C, youth with non-HDL-C concentrations ≥120 mg/dL and ≥145 mg/dL were at borderline high and high risk of metabolic syndrome, respectively. Therefore, in the Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents, a non-HDL-C concentration <120 mg/dL, between 120 and 144 mg/dL and ≥145 mg/dL are defined as acceptable, borderline high and high, respectively13). Recently, a study of Japanese children and adolescents aged 9 to 16 years presented reference values for non-fasting non-HDL-C17). In that population, a non-HDL-C concentration ≥122 mg/dL in boys and ≥125 mg/dL in girls were considered borderline high risk (highest 75th percentile), whereas a non-HDL-C concentration of ≥152 mg/dL in boys and ≥159 mg/dL in girls indicated high risk (highest 95th percentile). Another Japanese study reported that obese boys with MetS have elevated non-HDL-C levels and may have a higher risk for the development of atherosclerosis18). In their study, the mean non-HDL-C level in obese boys was 139.0 mg/dl and that in boys with abdominal obesity, pre-MetS (abdominal obesity plus one component of MetS), and MetS were 112.9, 135.4, and 149.0 mg/dl, respectively. However, there have been no available data for non-HDL-C in Korea. In the present study, a non-HDL-C of ≥111 mg/dL in boys and ≥112 mg/dL in girls was the highest 75th percentile, and a non-HDL-C ≥156 mg/dL in boys and ≥158 mg/dL in girls was the highest 95th percentile. The prevalence of a non-HDL-C concentration ≥145 mg/dL was 6.5% in boys and 7.8% in girls. Our results will help clinicians interpret lipid profiles in Korea. Further studies are needed to identify the optimal cutoff points for non-HDL-C concentrations in Korean children and adolescents.
The TG/HDL-C ratio, a recently introduced lipid profile measure, may be a better predictor of small, dense low-density lipoprotein (LDL)19), an atherogenic lipoprotein particle that strongly predicts coronary heart disease and has been related to an increased risk of CVD20). An elevated TG/HDL-C ratio has been associated with coronary artery disease in adults who underwent cardiac catheterization21). TG/HDL-C was found to be an independent predictor of carotid intima-media thickness progression, which is an independent risk factor for CVD in adults22). In a study of US adolescents and adults aged 10 to 26 years, the TG/HDL-C ratio was found to be an independent predictor of arterial stiffness in adolescents and young adults23). In addition, a large Italian study showed that the TG/HDL-C ratio can be a simple and effective tool to identify children and adolescents with atherogenic dyslipidemia and cardio-metabolic risk24). In that Italian study, individuals with a TG/HDL-C ratio ≥2.2 were at high risk of preclinical signs of organ damage. Another study in the US demonstrated that white children and adolescents with a TG/HDL-C ratio ≥2.27 were at a high risk of insulin resistance25). Insulin resistance is a main mechanism involved in metabolic syndrome, which is a significant risk factor for CVD. Thus individuals with a TG/HDL-C ratio ≥2.27 can be considered at risk for CVD. However, in a study by Ostfeld et al.21), adults with a TG/HDL-C ratio ≥3.5 were significantly associated with CAD. Furthermore, in a study on US adolescents and young adults by Urbina et al.23), individuals with a TG/HDL-C ratio ≥2.7 were at high risk (the highest tertile). There is thus controversy over the cutoff point for cardiovascular risk. There have been few studies on the distribution of the TG/HDL-C ratio in healthy children and adolescents. In this study, the overall mean TG/HDL-C ratio was 1.77 in boys and 1.72 in girls. The highest 75th percentile was 2.39 in boys and 2.49 in girls, and the highest 95th percentile was 4.55 in boys and 4.63 in girls. The highest 75th percentile of the TG/HDL-C ratio in both genders was within the values of 2.2 to 2.7, which were the values associated with a high risk of CVD in children adolescents in this study.
In our study, the trends in TG/HDL-C ratio regarding age differed between boys and girls. The TG/HDL-C ratio tended to increase with age in boys, whereas the trend in TG/HDL-C ratio was decreasing with age in girls. Serum lipids are affected by age, gender, ethnicity, nutritional state, pubertal stage, physical activity, and hormonal status including menstrual cycle for women26–29). Although the study populations in a study by Skinner et al.26) did not fast, the curves of TG regarding age had a slightly increasing trend after the age of 10 in boys but a decreasing trend in girls. On the other hand, the HDL-C curve for age showed a decreasing trend in boys, whereas the HDL-C curve for age showed an increasing trend in girls. A Japanese study found a decreasing trend in HDL-C with age in boys and an increasing trend in HDL-C with age in girls17). Moreover, a previous Korean study showed that the HDL-C concentrations had an increasing trend with age in girls but a decreasing trend in boys30). The TG levels regarding age had an increasing trend in boys and a decreasing trend in girls30). Those results showed a similar pattern in TG and HDL-C concentrations as the present study. Although the direct TG/HDL-C ratio was not estimated in that study, we can postulate that the TG/HDL-C ratio for age would have a similar pattern as the one in our study throughout childhood and adolescence.
The results of this study confirmed previous findings that the distribution of serum lipids vary with age and gender26, 31, 32). This can be related to physiologic changes that indicate hormonal influences and lead to lipid variations. However, current guidelines for lipids and treatment based on fixed cutoff values do not reflect a normal distribution of age and gender. Evaluating lipid profiles against age- and gender-specific curves could be helpful in managing lipid problems in children and adolescents. It is expected that these curves could play a role in preventing over- and under-treatment. From the individual's perspective, proper management of lipids within age- and gender-specific reference values can help delay future cardiovascular events33). From a national perspective, these curves could play a significant role in planning national strategies for the prevention and control of cholesterol in children and adolescents.
There were several limitations to this study. The results were based on cross-sectional data; therefore, we cannot track whether each child followed the pattern of the curves. We were unable to evaluate the associations between the distribution of serum lipids and pubertal stage. There were no available data from previous studies to compare with the present study, and we cannot evaluate the trends in serum lipid concentrations. Further studies are needed to evaluate trends in the distribution of serum lipids with an increased sample size.
Conclusion
This study on the distribution of serum lipid concentrations in Korea demonstrated age- and gender-specific reference values of serum lipid profiles including non-HDL-C and TG/HDL-C ratios in children and adolescents based on a nationally representative survey. These results provide not only more information for individualized interpretation of lipid profiles and interventions but also valuable information for planning strategies to prevent and manage childhood and adolescent dyslipidemia.
Acknowledgement
None.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1). Sekikawa A, Kuller LH, Ueshima H, Park JE, Suh I, Jee SH, Lee HK, Pan WH: Coronary heart disease mortality trends in men in the post World War II birth cohorts aged 35–44 in Japan, South Korea and Taiwan compared with the United States. Int J Epidemiol, 1999; 28: 1044-1049 [DOI] [PubMed] [Google Scholar]
- 2). Chang HS, Kim HJ, Nam CM, Lim SJ, Jang YH, Kim Sera, Kang HY: The Socioeconomic Burden of Coronary Heart Disease in Korea. J Prev Med Public Health, 2012; 45: 291-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA: Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med, 1998; 338: 1650-1656 [DOI] [PubMed] [Google Scholar]
- 4). Cui Y, Blumenthal RS, Flaws JA, Whiteman MK, Langenberg P, Bachorik PS, Bush TL: Non-high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch Intern Med, 2001; 161: 1413-1419 [DOI] [PubMed] [Google Scholar]
- 5). Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults: Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Circulation, 2002; 106: 3143-3421 [PubMed] [Google Scholar]
- 6). Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB: Non-high-density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation, 2005; 112: 3375-3383 [DOI] [PubMed] [Google Scholar]
- 7). McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G: Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med, 2003; 139: 802-809 [DOI] [PubMed] [Google Scholar]
- 8). Wang Q, Yin J, Xu L, Cheng H, Zhao X, Xiang H, Lam HS, Mi J, Li M: Prevalence of metabolic syndrome in a cohort of Chinese schoolchildren: comparison of two definitions and assessment of adipokines as components by factor analysis. BMC Public Health, 2013; 13: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Hadaegh F, Khalili D, Ghasemi A, Tohidi M, Sheikholeslami F, Azizi F: Triglyceride/HDL-cholesterol ratio is an independent predictor for coronary heart disease in a population of Iranian men. Nutr Metab Cardiovasc Dis, 2009; 19: 401-408 [DOI] [PubMed] [Google Scholar]
- 10). Kant AK, Graubard BI: Race-ethnic, family income, and education differentials in nutritional and lipid biomarkers in US children and adolescents: NHANES 2003-2006. Am J Clin Nutr, 2012; 96: 601-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Yoon YS, Oh SW, Baik HW, Park HS, Kim WY. Alcohol consumption and the metabolic syndrome in Korean adults: the 1998 Korean National Health and Nutrition Examination Survey. Am J Clin Nutr, 2004; 80: 217-224 [DOI] [PubMed] [Google Scholar]
- 12). Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem, 1972; 18: 499-502 [PubMed] [Google Scholar]
- 13). Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute: Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary report. Pediatrics, 2011; 128: s213-s256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Homma S, Troxclair DA, Zieske AW, Malcom GT, Strong JP: Pathobiological Determinants of Atherosclerosis in Youth Research Group: Histological changes and risk fac tor associations in type 2 atherosclerotic lesions (fatty streaks) in young adults. Atherosclerosis, 2011; 219: 184-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Frontini MG, Srinivasan SR, Xu JH, Tang R, Bond MG, Berenson G: Utility of non-high-density lipoprotein cholesterol versus other lipoprotein measures in detecting subclinical atherosclerosis in young adults (The Bogalusa Heart Study). Am J Cardiol, 2007; 100: 64-68 [DOI] [PubMed] [Google Scholar]
- 16). Li C, Ford ES, McBride PE, Kwiterovich PO, McCrindle BW, Gidding SS: Non-high-density lipoprotein cholesterol concentration is associated with the metabolic syndrome among US youth aged 12-19 years. J Pediatr, 2011; 158: 201-207 [DOI] [PubMed] [Google Scholar]
- 17). Abe Y, Okada T, Sugiura R, Yamauchi K, Murata M: Reference Ranges for the Non-High-Density Lipoprotein Cholesterol Levels in Japanese Children and Adolescents. J Atheroscler Thromb, 2015; 22: 669-675 [DOI] [PubMed] [Google Scholar]
- 18). Saito E, Okada T, Abe Y, Kazama M, Yonezawa R, Kuromori Y, Iwata F, Hara M: Non-high-density Lipoprotein Cholesterol Levels in Japanese Obese Boys with Metabolic Syndrome. J Atheroscler Thromb, 2016; 23: 105-111 [DOI] [PubMed] [Google Scholar]
- 19). Tsimihodimos V, Gazi I, Kostara C, Tselepis AD, Elisaf M: Plasma lipoproteins and triacylglycerol are predictors of small, dense LDL particles. Lipids, 2007; 42: 403-409 [DOI] [PubMed] [Google Scholar]
- 20). Onat A, Can G, Kaya H, Hergenç G: “Atherogenic index of plasma” (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. J Clin Lipidol, 2010; 4: 89-98 [DOI] [PubMed] [Google Scholar]
- 21). Ostfeld R, Mookherjee D, Spinelli M, Holtzman D, Shoyeb A, Schaefer M, Doddamani S, Spevack D, Du Y: A triglyceride/high-density lipoprotein ratio > or = 3.5 is associated with an increased burden of coronary artery disease on cardiac catheterization. J Cardiometab Syndr, 2006; 1: 13-15 [DOI] [PubMed] [Google Scholar]
- 22). Maki KC, Davidson MH, Dicklin MR, Bell M, Witchger M, Feinstein SB: Predictors of anterior and posterior wall carotid intima media thickness progression in men and women at moderate risk of coronary heart disease. J Clin Lipidol, 2011; 5: 141-151 [DOI] [PubMed] [Google Scholar]
- 23). Urbina EM, Khoury PR, McCoy CE, Dolan LM, Daniels SR, Kimball TR: Triglyceride to HDL-C ratio and increased arterial stiffness in children, adolescents, and young adults. Pediatrics, 2013; 131: e1082-e1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Di Bonito P, Valerio G, Grugni G, Licenziati MR, Maffeis C, Manco M, Miraglia del Giudice E, Pacifico L, Pellegrin MC, Tomat M, Baroni MG, CARdiometabolic risk factors in overweight and obese children in ITALY (CARITALY) Study Group : Comparison of non-HDL-cholesterol versus triglycerides-to-HDL-cholesterol ratio in relation to cardiometabolic risk factors and preclinical organ damage in overweight/obese children: the CARITALY study. Nutr Metab Cardiovasc Dis, 2015; 25: 489-494 [DOI] [PubMed] [Google Scholar]
- 25). Giannini C, Santoro N, Caprio S, Kim G, Lartaud D, Shaw M, Pierpont B, Weiss R: The triglyceride-to-HDL cholesterol ratio: association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes care, 2011; 34: 1869-1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Skinner AC, Steiner MJ, Chung AE, Perrin EM: Cholesterol curves to identify population norms by age and sex in healthy weight children. Clin Pediatr, 2012; 51: 233-237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Plasma lipid distributions in selected North American populations: the Lipid Research Clinics Program Prevalence Study. The Lipid Research Clinics Program Epidemiology Committee. Circulation, 1979; 60: 427-439 [DOI] [PubMed] [Google Scholar]
- 28). Mumford SL, Schisterman EF, Siega-Riz AM, Browne RW, Gaskins AJ, Trevisan M, Steiner AZ, Daniels JL, Zhang C, Perkins NJ, Wactawski-Wende J: A longitudinal study of serum lipoproteins in relation to endogenous reproductive hormones during the menstrual cycle: findings from the BioCycle study. J Clin Endocrinol Metab, 2010; 95: e80-e85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Bradlee ML, Singer MR, Daniels SR, Moore LL: Eating patterns and lipid levels in older adolescent girls. Nutr Metab Cardiovasc Dis, 2013; 23: 196-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Yang S, Hwang JS, Park HK, Lee HS, Kim HS, Kim EY, Lim JS: Serum lipid concentrations, prevalence of dyslipidemia, and percentage eligible for pharmacological treatment of Korean children and adolescents; data from the Korea National Health and Nutrition Examination Survey IV (2007-2009). PLoS One, 2012; 7: e49253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Hickman TB1, Briefel RR, Carroll MD, Rifkind BM, Cleeman JI, Maurer KR, Johnson CL: Distributions and trends of serum lipid levels among United States children and adolescents ages 4-19 years: data from the Third National Health and Nutrition Examination Survey. Prev Med, 1998; 27: 879-890 [DOI] [PubMed] [Google Scholar]
- 32). Cook S, Auinger P, Huang TT: Growth curves for cardiometabolic risk factors in children and adolescents. J Pediatr, 2009; 155: e15-e26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Rodenburg J, Vissers MN, Wiegman A, van Trotsenburg AS, van der Graaf A, de Groot E, Wijburg FA, Kastelein JJ, Hutten BA: Statin treatment in children with familial hypercholesterolemia: the younger, the better. Circulation, 2007; 116: 664-668 [DOI] [PubMed] [Google Scholar]


