Abstract
Accumulating evidence from human and rodent studies suggests that females are more sensitive to the motivating and rewarding properties of drugs of abuse. Numerous reports implicate estradiol in enhancing drug-related responses in females, yet the neurobiological mechanisms underlying this effect of estradiol are unknown. Because dendritic spine plasticity in the nucleus accumbens (NAc) is linked to the addictive effects of drugs, we examined the influence of estradiol on dendritic spines in this region. Previously our lab demonstrated that in female medium spiny neurons estradiol activates metabotropic glutamate receptor subtype five (mGluR5), a G-protein coupled receptor already implicated in the etiology of drug addiction. Thus we sought to determine whether mGluR5 is a part of the mechanism by which estradiol affects dendritic spine density in the NAc. To test this hypothesis, ovariectomized female rats were treated with the mGluR5 antagonist, MPEP, or vehicle prior to estradiol (or oil) treatment and 24hrs later dendritic spine density was evaluated by DiI labeling and confocal microscopy. We found that estradiol decreased dendritic spine density in the NAc core, and that pretreatment with MPEP blocked this effect. In contrast, MPEP had no effect on dendritic spine density in the NAc shell or CA1 region of the hippocampus, two regions in which estradiol increased the density of dendritic spines. As dendritic spine plasticity in the NAc core has behavioral consequences for drug addiction, these data provide a clue as to how estradiol acts in females to enhance behavioral responses to drugs of abuse.
Keywords: estrogen, drug addiction, medium spiny neuron, mGluR
Introduction
Compared with men, women show enhanced behavioral responses to drugs of abuse and consequently are thought to be more vulnerable to addiction(Roth et al. 2004). Ovarian hormones, specifically estradiol, may underlie this vulnerability in women(Justice and de Wit 1999; McCance-Katz et al. 2005). Consistent with the human literature, sex differences in responsiveness to drugs of abuse are apparent in animal models of addiction, with female rats exhibiting greater sensitivity to drugs of abuse than do males (Becker and Hu 2008; Anker and Carroll 2011). These sex differences are eliminated upon removal of ovarian hormones and restored upon estradiol administration (Sircar and Kim 1999; Hu and Becker 2003; Festa and Quinones-Jenab 2004; Segarra et al. 2010). Behaviorally, it is clear that estradiol enhances rewarding and motivating properties of drugs of abuse in females, but the neurobiological underpinnings are unknown.
Due to its prominent role in mediating reward and motivation processes, the nucleus accumbens (NAc) is integral in the development and expression of addictive behaviors. Synaptic plasticity in this region following repeated drug exposure is thought to form a neurobiological basis for addiction(Russo et al. 2010). In particular, long-lasting alterations in neuroanatomical circuitry through dynamic dendritic spine plasticity of medium spiny neurons (MSNs) are believed to underlie persistent behavioral changes following repeated drug exposure (Robinson and Kolb 2004). A recent study from our laboratory demonstrated that estradiol treatment produces dendritic spine plasticity in the female NAc(Staffend et al. 2011)reminiscent of that induced by repeated psychostimulant exposure (Dumitriu et al. 2012). Given that dendritic spine plasticity underlies changes in behavior, the similarity inpsychostimlant- and estradiol-induced dendritic spine plasticity may provide insight as to how estradiol potentiates drug responses in females.
Accumulating evidence suggests that activation of group I metabotropic glutamate receptors (mGluRs) may be a putative mechanism by which estradiol regulates dendritic spines. Both estrogen receptors (Milner et al. 2001; McEwen 2001) and group I mGluRs(Mitrano and Smith 2007) are localized near dendritic spines and membrane-localized estrogen receptors directly interact with group I mGluRs(Meitzen and Mermelstein 2011). Interestingly, this action of estradiol to transactivate group I mGluRs and initiate second-messenger signaling is specific to female neurons (Boulware et al 2009; Grove-Strawser et al 2010). More recently estradiol was shown to regulate dendritic spines in the female hypothalamus through a mechanism involving group I mGluRs (Christiansen et al 2011). Whether a similar group I mGluR-dependent mechanism mediates dendritic spine plasticity in NAcremains unknown. Building off previous studies in our lab that implicate mGluR5 in membrane actions of estradiol in female MSNs (Grove-Strawser et al 2010), we took a pharmacological approach to determine if estradiol affects dendritic spine density in the female rat NAc through an mGluR5-dependent mechanism.
Methods
Animals
Ovariectomized female Sprague Dawley rats 12 weeks of age (175-200g) from Harlan labs (Indianapolis, IN) were housed in pairs, handled daily, and allowed to habituate to the laboratory for one week prior to experimentation. Animals were maintained on at 12 hrlight-dark cycle with lights on at 6:00 am. All animal procedures were in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee at the University of Minnesota.
Drugs and hormones
Estradiol (Sigma, St. Louis, MO) was dissolved in cottonseed oil and injected s.c. at 10 μg in 0.1 ml. Cottonseed oil (0.1 ml s.c.) was injected as the hormone vehicle control. For the first experiment, the group I mGluR receptor antagonist JNJ16259685 (JNJ, Tocris Biosciences, Minneapolis, MN) was dissolved in ethanol to make a 100 mM stock solution and stored at −20 °C. On the day of injection the stock solution was diluted in 80% physiological saline: 20% ethanol to a working solution of 1mg/kg JNJ. Whereas at low doses JNJ is a specific antagonist at mGluR1 receptors (Lavreysen et al. 2004), we administered a higher dose designed to antagonize both of the group I mGluRs, mGluR1 and mGluR5. An identical formulation without JNJ was used for the drug vehicle. In the second experiment, the specific mGluR5 receptor antagonist (Gasparini et al. 1999) 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP; Tocris Biosciences, Minneapolis, MN) was dissolved in physiological saline to a working concentration of 1mg/kg on the day of injection. Physiological saline alone was used as the drug vehicle. All drugs and drug vehicles were injected intraperitoneally (i.p.) at a volume of 1 ml/kg. For each experiment animals were divided into four treatment groups.
Drug vehicle + cottonseed oil
Drug vehicle + estradiol
MPEP or JNJ + vehicle
MPEP or JNJ + estradiol
On the day of experimentation, animals received two injections. The first injection was administered interperotineally (i.p.) and contained JNJ (experiment 1), MPEP (experiment 2), or the appropriate drug vehicle. After 30 min, the second injection was administered subcutaneously (s.c.) containing either estradiol benzoate or cottonseed oil. Twenty-four hrs after the estradiol or oil injection, the animals were anesthetized using Beuthanasia-D (0.3 ml i.p./animal, Schering, Union, NJ), injected with 0.25 ml heparin into the left ventricle of the heart and intracardially perfused with 25 mM phosphate buffered saline (PBS, pH = 7.2) for 3 min at 25 ml/min followed by ice-cold 1.5% paraformaldehyde in 25 mM PBS for 20 min. Brains were removed and coronally blocked to allow for penetration of the post-perfusion fixative (1.5% paraformaldehyde for 1 hr) into the tissue blocks containing either the NAc or hippocampus. Brains were sectioned at 300 μm in serial, coronal sections through the NAc and hippocampus using a Vibratome (Lancer Series 1000, St. Louis, MO). Sections were placed in wells containing 25 mM PBS until DiI labeling.
DiI labeling
Prior to DiI delivery, PBS was removed from wells containing the brain sections. Microcarriers containing DiI-coated tungsten particles (2 mg lipophilic carbocyanineDiI (Molecular Probes, Carlsbad, CA) dissolved in 100 μl of dichloromethane mixed with 90 mg of 1.3 μm tungsten particles (Biorad, Hercules, CA)) were made in Tefzel tubing (Biorad) pre-coated with freshly prepared 10-15 mg/ml polyvinylpyrrolidone (PVP, Sigma-Aldrich, St. Louis, MO) and cut into 1.3 mm segments. A Helios Gene Gun (BioRad) with a modified barrel, 40 mm spacer and 70 μm nylon mesh filter was used to deliver the microcarriers to the lightly fixed brain sections using helium gas at a pressure of 100 PSI. Immediately following DiI delivery, brain sections were re-submerged in PBS for 24 hrs in the dark at room temperature for diffusion of DiI. After 24 hrs, the PBS was removed and replaced with 4% paraformaldehyde in PBS for 1 hr at room temperature. Sections were washed (3x10 min) in PBS then mounted on Superfrost slides and coverslipped with Fluorglo mounting media for lipophilic dyes (Spectra Services, Ontario, NY; experiment 1) or 5% n-proply-gallate in glycerin (w/v; experiment 2). The Fluorglo mounting media used in experiment 1 resulted in a greater resolution of individual dendritic spines compared with the glycerol-based medium used in experiment 2. The pattern of results was unaffected by the different mounting media, though there were greater spine densities in experiment 1compared with those measured in experiment 2. We attribute this difference in spine density with the Fluorgloto a greater resolution of spines that did not protrude much past the dendritic shaft. The impact of the mounting medium was particularly evident in the CA1 region of the hippocampus because spines on CA1 dendrites do not extend as far from the dendrite asspines on medium spiny neuron dendrites.
Confocal imaging
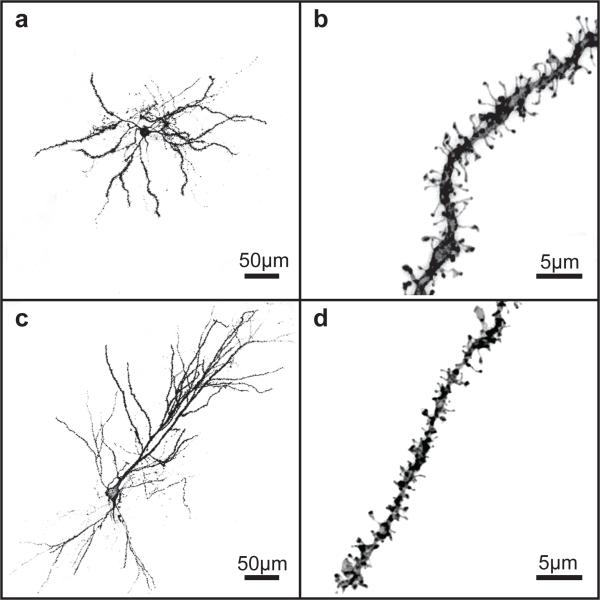
A Leica TCS SPE confocal microscope (Leica, Mannheim, Germany) was used to acquire z-stacks of DiI labeled whole neurons and dendritic segments (Fig. 1). All z-stacks were maintained at an xy pixel distribution of 512 × 512 and scanned at a frequency of 400 Hz. Medium spiny neurons imaged in the nucleus accumbens shell and core were distributed throughout the rostro-caudal axis, with 80% located in the middle third of the accumbens. Additionally, CA1 pyramidal neurons were localized to the rostral extent of the hippocampus leading to the formation of the ventral hippocampus. Whole neurons were imaged with a 20X air objective at 1.0 μm increments in the z-axis and reconstructed using Leica LAS AF software to measure the distance from the soma of target dendritic segments: 70-200 μm from soma in medium spiny neurons from the NAc or 250-400 μm from pyramidal neurons in the CA1 region of the hippocampus. Dendritic segments were imaged with a 63X oil immersion objective with a zoom of 5.61 and z-stacks were acquired in 0.12 μm increments. By adjusting the laser power and photomultiplier, each dendrite was imaged in its full dynamic range to ensure that no part of the dendritic segment was saturated. For each brain region, 2 to 3 neurons and 3 dendritic segments per neuron were imaged to generate a total of 6 to 9 segments for each brain region from every animal. Depending on the experiment and treatment conditions, a final sample size of 3-8 animals was obtained for each brain region per treatment group (see figure legends for group sample sizes).
Fig. 1.
Representative 3D reconstructed images of DiI-labeled neurons and dendritic segments. a Low power DiI-labeled nucleus accumbens medium spiny neuron, scale bar 50 μm. b High power deconvoluted dendritic segment from an accumbal medium spiny neuron, scale bar 5μm. c Low powerDiI-labeled pyramidal neuron in CA1, scale bar 50 μm. d High power deconvoluted dendritic segment from a hippocampal pyramidal neuron, scale bar 5μm
Quantitation
Confocal z-stacks of dendritic segments were first subjected to a 3D-deconvolution process using Autoquant X AutoDeblur Gold CF software (version X2.2, Media Cybernetics, Bethesda, MD). Deconvoluted images were then reconstructed in 3D using the Imaris software (version 7.6, Bitplane Inc., St. Paul, MN), with dendritic shaft and spines traced manually in the xy plane using the Filament tool and the Autodepth function to generate dendritic spine density normalized to 10 μm of dendritic length. A contrast threshold of 0.4-0.7 enabled accurate 3D reconstruction of dendritic shaft and spines. Following 3D reconstruction, dendritic spine head diameters were obtained for each spine on a given segment.
Data analysis
For each animal, 2-3 neurons were analyzed in each brain region, though each animal was used as the unit of statistical analysis. A two-way ANOVA with subsequent Tukey HSD post hoc tests were used to evaluate the effects of drug, hormone and drug-hormone interactions among treatment groups for each brain region using STATISTICA software (StatsoftInc, version 9, Tulsa, OK). For all statistical tests, results were considered to be statistically significant if p< 0.05.
Results
Experiment 1: Estradiol-mediated dendritic spine plasticity depends on group I mGluRs
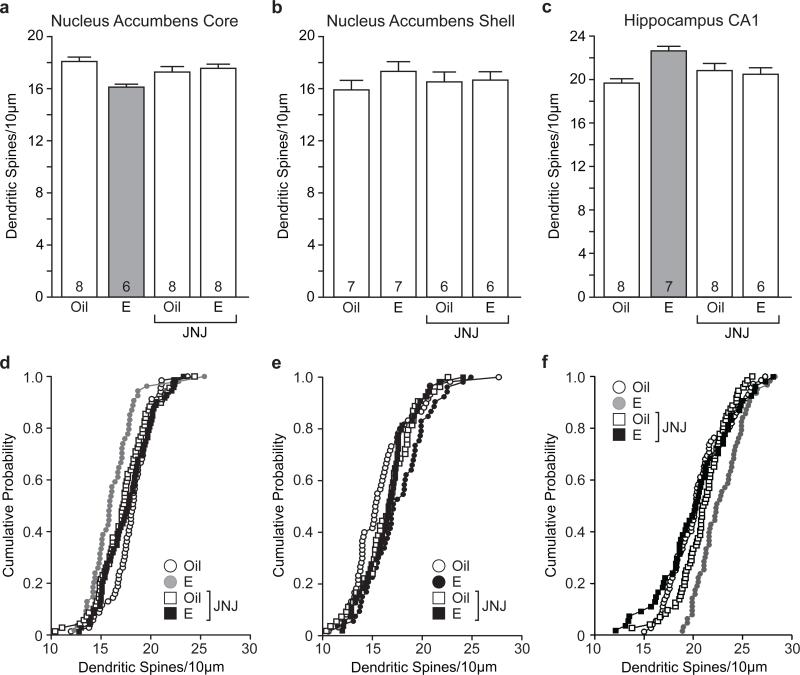
Recent findings from our laboratory demonstrated a novel effect of estradiol on dendritic spine density in that estradiol decreased dendritic spine density in medium spiny neurons (MSN) in the NAc of female Syrian hamsters (Staffend et al 2011). To determine whether this effect is conserved in rats, we treated ovariectomized female rats with estradiol and analyzed dendritic spine density of MSNs. Representative images of a DiI-labeled MSN and dendritic segment used to quantify dendritic spine density are shown in Fig. 1a and Fig. 1b, respectively. Consistent with previous observations, we found that estradiol treatment produced a significant decrease in dendritic spine density in NAccore MSNs (main effect of hormone: F(1,26) = 5.77, p< 0.05). Next, to determine if estradiol decreased dendritic spine density through a mechanism involving group I mGluRs, we antagonized group I mGluRs with JNJ administration prior to estradiol treatment. The effect of group I mGluR antagonism differed across treatment groups (hormone by drug interaction: F(1, 26) = 9.57, p< 0.005). Specifically, females pretreated with JNJ prior to estradiol showed increased dendritic spine density compared with estradiol-treated females that received vehicle pretreatment (Tukey HSD p< 0.01). These data indicate that pretreatment with the group I mGluR antagonist blocked the estradiol-mediated decrease in dendritic spine density in the NAc core (Fig. 2a,d).
Fig. 2.
Estradiol-mediated dendritic spine plasticity in the nucleus accumbens core and hippocampal CA1 depends on group I mGluRs. a Estradiol decreased dendritic spine density of medium spiny neurons in the nucleus accumbens core and this effect was attenuated by pretreatement with JNJ. b Neither estradiol nor JNJ had a significant effect on medium spiny neurons of the nucleus accumbens shell. c Estradiol increased dendritic spine density in hippocampal CA1 pyramidal neurons and pretreatment with JNJ blocked this effect. The number inside of each bar represents the number of animals per treatment condition. Grey bars are significantly different than white bars p< 0.05. d-f Cumulative probability distribution plots of dendritic spine density within treatment groups for nucleus accumbens core (d), nucleus accumbens shell (e), and CA1 region of the hippocampus (f)
In addition to the NAc core we examined estradiol effects on MSN dendritic spine density in the NAc shell. In this region, there was a trend toward increased dendritic spine density of MSNs following estradiol treatment and pretreatment with JNJ appeared to block increased dendritic spine density (Fig. 2b,e). However, these effects were not significant. This is in contrast to what was observed in experiment 2 (see below). The possible variability in observance of an estrogen effect is described in the discussion.
Finally, we chose to analyze dendritic spine density of hippocampal pyramidal neurons in CA1 as a positive control for the effects in the nucleus accumbens because it has been repeatedly demonstrated that estradiol increases dendritic spine density of pyramidal neurons within this region (Woolley 1998; Staffend et al. 2011). Representative images of a DiI-labeled pyramidal neuron in CA1 and dendritic segment used to quantify dendritic spine density are shown in Fig. 1c and Fig. 1d, respectively. In agreement with previous findings, we showed that estradiol increased pyramidal cell dendritic spine density in the CA1 region of the female hippocampus but only in females pretreated with vehicle (hormone by drug interaction (F(1,24) = 13.37, p< 0.01). Estradiol-treated females pretreated with JNJ exhibited lower dendritic spine density compared to estradiol-treated females that received vehicle pretreatment (Tukey HSD p< 0.01). These data show that antagonism of group I mGluRs prior to estradiol treatment attenuated the estradiol-mediated increase in dendritic spine density in hippocampal CA1 pyramidal neurons (Fig. 2c,f). In addition to analyzing dendritic spine densities in the NAc core, shell and CA1 hippocampus, we also examined dendritic spine head diameters for each dendritic segment within treatment groups. We found that there was no effect of estradiol or JNJ on dendritic spine head diameter (data not shown).
Experiment 2: Estradiol-mediated decreases in dendritic spine density in the nucleus accumbenscore depend on mGluR5
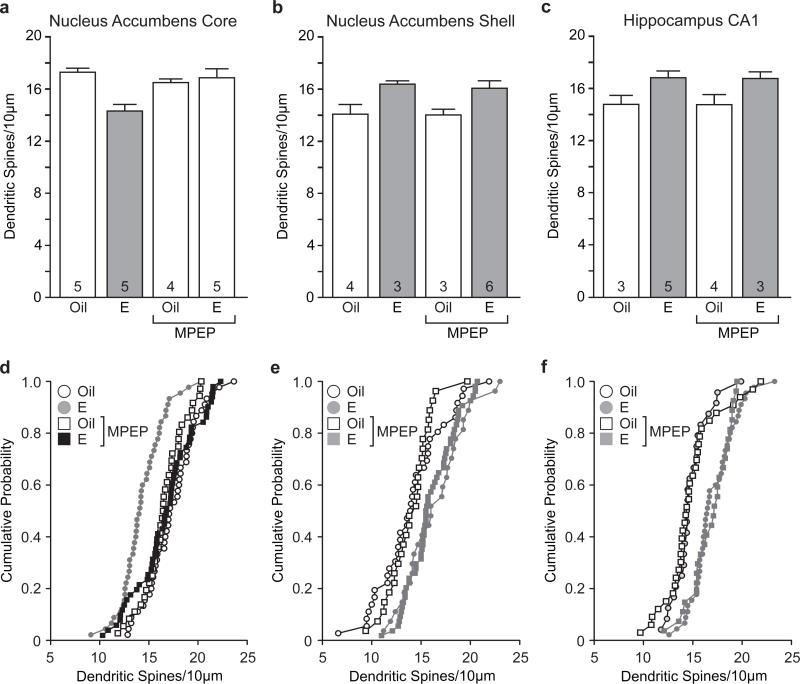
To further decipher the specific group I mGluR underlying estradiol changes in dendritic spines, we treated females with a specific mGluR5 antagonist prior to estradiol treatment. As in experiment 1, estradiol treatment decreased dendritic spine density of MSNs in the NAc core (main effect of hormone: F(1,16) = 7.47, p < 0.05). Hormone treatment conditions were affected by pretreatment with the mGluR5 antagonist (hormone by drug interaction: F(1,16) = 10.17, p<0.05). Estradiol-treated females that received MPEP pretreatment showed increased MSN dendritic spine density in the core compared to those that received vehicle pretreatment (Tukey HSD p< 0.01). These data demonstrate that pretreatment with the selective mGluR5 antagonist MPEP blocked the estradiol-mediated decrease in dendritic spine density (Fig. 3a,d).
Fig. 3.
Estradiol-mediated decreases in dendritic spine density in the nucleus accumbens core depend on mGluR5. a Estradiol decreased dendritic spine density of medium spiny neurons in the nucleus accumbens core and this decrease was blocked by pretreatment with MPEP. b Estradiol increased dendritic spine density in medium spiny neurons of the nucleus accumbens shelland mGluR5 antagonism had no effect on this action of estradiol. c Estradiol increased dendritic spine density of CA1 hippocampal pyramidal neurons and pretreatement with MPEP did not affect this increase. The number inside of each bar represents the number of animals per condition. Grey bars are significantly different than white bars p< 0.05. d-f Cumulative probability distribution plots of dendritic spine density within treatment groups for nucleus accumbens core (d), nucleus accumbens shell (e), and CA1 region of the hippocampus (f)
Whereas in experiment 1 we saw a non-significant trend towards an effect of estradiol on MSN dendritic spine density in the shell of the NAc, in experiment 2 estradiol produced a significant increase in MSN dendritic spine density in this region (main effect of hormone: F(1,12) = 12.36, p< 0.001). Furthermore, antagonism of mGluR5 did not affect the increased spine density observed with estradiol treatment (Fig. 3b,e).
As before, we found that estradiol treatment significantly increased dendritic spine density in CA1 pyramidal neurons (main effect of hormone: F(1,11) = 15.67, p< 0.01) and that pretreatment with the mGluR5 antagonist, MPEP, had no effect on this increase (Fig. 3c,f). Similar to experiment 1, neitherestradiol nor MPEPaffected dendritic spine head diameter (data not shown).
Discussion
Converging evidence suggests that estradiol plays a prominent role in enhancing drug-related behaviors in females and that synaptic plasticity in the NAc is thought to form a neurobiological basis for addiction. The goal of this research was to begin elucidating the mechanism by which estradiol can affect synaptic plasticity to promote rewarding/addictive properties of drugs.
The effects of estradiol on dendritic spine plasticity in the NAc have only recently been investigated(Staffend et al. 2011). In contrast, the effect of psychostimulants on dendritic spines has been the focus of numerous studies(Golden and Russo 2012). The results of these studies suggest that patterns of dendritic spine plasticity depend on a number of factors, including route and context of drug administration, drug administration regime, and length of withdrawal after drug cessation (Golden and Russo 2012). However, what is particularly intriguing is that dendritic spine density in the NAc core is decreased 24 hours after repeated psychostimulant exposure (Dumitrui et al 2012). We observe the same effect in ovariectomized female rats 24 hours following an acute estradiol treatment. The similarity in these findings suggests that estradiol might prime MSNs in the NAc core to respond more strongly to the actions of psychostimulants.
To examine the involvement of group I mGluRs in estradiol-mediated dendritic spine plasticity in the NAc core, we took a pharmacological approach. By blocking both mGluR1 and mGluR5 with a high dose of JNJ we were able to attenuate the reduction in dendritic spine density produced by estradiol. Because we were able to produce the same effect with the specific mGluR5 antagonist MPEP, our assumption is that estradiol activates signaling through mGluR5 to decrease dendritic spine density in the NAc core. This assumption is supported by biochemical evidence that estradiol activates mGluR5 to phosphorylate CREB in medium spiny neurons from female rats both in vitro and in vivo (Grove-Strawser et al. 2010) and that mGluR5 alone can produce changes in dendritic spines (Fujii et al. 2005; Asrar and Jia 2013).
We included the CA1 hippocampus as a positive control because estradiol increases dendritic spine density in this region (Woolley 1998; Staffend et al. 2011). In the same animals that exhibited decreased dendritic spine density in the NAc core, we observed the well-established increase in spine density in hippocampal CA1 pyramidal neurons. JNJ, having mixed effects on mGluR1 and mGluR5 at the dose used, blocked the increase in dendritic spine by estradiol, whereas MPEP did not have any effect in this region. These data suggest that unlike the core of NAc where estradiol acts through mGluR5, in the hippocampus estradiol produces changes in dendritic spine density through mGluR1 signaling. This is the first indication of the mechanism by which estradiol increases dendritic spine density in CA1 and is consistent with how estradiol affects biochemical and electrophysiological properties of pyramidal neurons in this brain region (Boulware et al. 2005; Huang and Woolley 2012). Further support that estradiol may act through mGluR1 to increase dendritic spine density in CA1 comes from the recent result that action of estradiol on mGluR1 underlies increased dendritic spine density in the hypothalamus (Christensen et al. 2011).
Based on our prior study in female hamsters (Staffend et al. 2011), we did not expect to see any changes in dendritic spine density in the NAc shell with estradiol treatment. Indeed, in the first experiment there was no effect of estradiol on MSNs in this region. However, in the second experiment estradiol produced an increase in dendritic spine density that was unaffected by mGluR5 antagonism. We do not know the source of this variability among the experiments in this study and our prior study in hamsters. One possibility is that the time window for observing dendritic spine plasticity following estradiol treatment may differ by brain region. In support of this idea, the time course for the expression of dendritic spine plasticity following repeated psychostimulant exposure appears to be different between the NAc core and shell, with changes in the shell occurring more rapidly (Dumitriu et al. 2012). Thus, it is possible that a similar phenomenon occurs following estradiol treatment and that our timing of sacrifice following estradiol treatment was on the cusp of changes in dendritic spine plasticity in the shell. Clearly, a more in-depth time course analysis may provide insight into this hypothesis. That said, when estradiol effects on dendritic spines in the shell were observed, they were similar to the increases in hippocampal CA1 spine density than the decreased spine density in the NAc core. In this regard the inability of MPEP to antagonize estradiol effects in either the hippocampus or NAc shell may suggest that, like in the hippocampus, estradiol acts through mGluR1 to affect spine density in the NAcshell.
Each subregion of the NAc is thought to serve a different function in terms of mediating drug related behaviors. Specifically, the shell seems to be more involved in shorter-terms aspects of addiction, such as reward, whereas the core seems to play a larger role in behaviors such as the development of patterned motor programs to obtain rewards (Ito et al. 2004; Meredith et al. 2008). Behaviorally, estradiol has been shown to affect both shell and core dependent responses. For example, estradiol potentiates the rewarding effects of psychostimulants, insofar as enhancing positive subjective ratings of drugs in women (Justice and de Wit 1999; McCance-Katz et al. 2005). In addition, estradiol is known to enhance acquisition and escalation of psychostimulant intake in females as well as locomotor sensitization in female rats (Ridenour et al. 2005; Becker and Hu 2008; Carroll and Anker 2010). An obvious conclusion is that the estradiol induced dendritic spine plasticity we observed in the NAc underlies the ability of estradiol to potentiate drug-related responses in females, as decreased dendritic spine density in the NAccore has been associated with locomotor sensitization (Waselus et al. 2013) and our lab has recently demonstrated that mGluR5 is involved in the potentiating effect of estradiol on psychostimulantlocomotor sensitization in female rats (Martinez et al unpublished).
Our research has implications for future therapeutic approaches for addiction, especially in women. Several groups have shown that modulation of group I mGluRs have therapeutic potential for drug related cellular and behavioral plasticity (Olive 2010; Duncan and Lawrence 2012; Olive et al. 2012; Wang et al. 2013; Loweth et al. 2013). If group I mGluRs and estradiol share a common mechanism to enhance drug related cellular and behavioral plasticity in females, group I mGluR antagonism may serve as a viable target for developing treatments for drug addiction in women.
Acknowledgements
This material is based upon work supported by the National Institutes of Health DA035008 and National Science Foundation IOS-114616 and Grant No. 00006595. We would like to thank Dr. Luis Martinez for advice and technical assistance.
Footnotes
Ethical Standards
This manuscript does not contain clinical studies or patient data.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci. 2011;8:73–96. doi: 10.1007/7854_2010_93. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- Asrar S, Jia Z. Molecular mechanisms coordinating functional and morphological plasticity at the synapse: role of GluA2/N-cadherin interaction-mediated actin signaling in mGluR-dependent LTD. Cell Signal. 2013;25:397–402. doi: 10.1016/j.cellsig.2012.11.007. doi: 10.1016/j.cellsig.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, et al. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Christensen A, Dewing P, Micevych P. Membrane-initiated estradiol signaling induces spinogenesis required for female sexual receptivity. J Neurosci. 2011;31:17583–17589. doi: 10.1523/JNEUROSCI.3030-11.2011. doi: 10.1523/JNEUROSCI.3030-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Laplant Q, Grossman YS, et al. Subregional, dendritic compartment, and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus accumbens. J Neurosci. 2012;32:6957–6966. doi: 10.1523/JNEUROSCI.5718-11.2012. doi: 10.1523/JNEUROSCI.5718-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JR, Lawrence AJ. The role of metabotropic glutamate receptors in addiction: evidence from preclinical models. Pharmacol Biochem Behav. 2012;100:811–824. doi: 10.1016/j.pbb.2011.03.015. doi: 10.1016/j.pbb.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Festa ED, Quinones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Horm Behav. 2004;46:509–519. doi: 10.1016/j.yhbeh.2004.04.009. doi: 10.1016/j.yhbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Fujii R, Okabe S, Urushido T, et al. The RNA binding protein TLS is translocated to dendritic spines by mGluR5 activation and regulates spine morphology. Curr Biol CB. 2005;15:587–593. doi: 10.1016/j.cub.2005.01.058. doi: 10.1016/j.cub.2005.01.058. [DOI] [PubMed] [Google Scholar]
- Gasparini F, Lingenhöhl K, Stoehr N, et al. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38:1493–1503. doi: 10.1016/s0028-3908(99)00082-9. doi: 10.1016/S0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- Golden SA, Russo SJ. Mechanisms of psychostimulant-induced structural plasticity. Cold Spring Harb Perspect Med. 2012 doi: 10.1101/cshperspect.a011957. doi: 10.1101/cshperspect.a011957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170:1045–1055. doi: 10.1016/j.neuroscience.2010.08.012. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23:693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74:801–808. doi: 10.1016/j.neuron.2012.03.035. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Lavreysen H, Wouters R, Bischoff F, et al. JNJ16259685, a highly potent, selective and systemically active mGlu1 receptor antagonist. Neuropharmacology. 2004;47:961–972. doi: 10.1016/j.neuropharm.2004.08.007. doi: 10.1016/j.neuropharm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Loweth JA, Tseng KY, Wolf ME. Using metabotropic glutamate receptors to modulate cocaine's synaptic and behavioral effects: mGluR1 finds a niche. Curr Opin Neurobiol. 2013;23:500–506. doi: 10.1016/j.conb.2013.01.009. doi: 10.1016/j.conb.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccance-katz EF, Hart CL, Boyarsky B, et al. Gender Effects Following Repeated Administration of Cocaine and Alcohol in Humans. Subst Use Misuse. 2005;40:511–528. doi: 10.1081/ja-200030693. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Invited Review: Estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Mermelstein PG. Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. J Chem Neuroanat. 2011;42:236–241. doi: 10.1016/j.jchemneu.2011.02.002. doi: 10.1016/j.jchemneu.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct Funct. 2008;213:17–27. doi: 10.1007/s00429-008-0175-3. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, et al. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- Mitrano DA, Smith Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J Comp Neurol. 2007;500:788–806. doi: 10.1002/cne.21214. doi: 10.1002/cne.21214. [DOI] [PubMed] [Google Scholar]
- Olive MF. Cognitive effects of Group I metabotropic glutamate receptor ligands in the context of drug addiction. Eur J Pharmacol. 2010;639:47–58. doi: 10.1016/j.ejphar.2010.01.029. doi: 10.1016/j.ejphar.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Cleva RM, Kalivas PW, Malcolm RJ. Glutamatergic medications for the treatment of drug and behavioral addictions. Pharmacol Biochem Behav. 2012;100:801–810. doi: 10.1016/j.pbb.2011.04.015. doi: 10.1016/j.pbb.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridenour TA, Maldonado-Molina M, Compton WM, et al. Factors associated with the transition from abuse to dependence among substance abusers: Implications for a measure of addictive liability. Drug Alcohol Depend. 2005;80:1–14. doi: 10.1016/j.drugalcdep.2005.02.005. doi: 10.1016/j.drugalcdep.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;1(47 Suppl):33–46. doi: 10.1016/j.neuropharm.2004.06.025. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, et al. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra AC, Agosto-Rivera JL, Febo M, et al. Estradiol: a key biological substrate mediating the response to cocaine in female rats. Horm Behav. 2010;58:33–43. doi: 10.1016/j.yhbeh.2009.12.003. doi: 10.1016/j.yhbeh.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sircar R, Kim D. Female Gonadal Hormones Differentially Modulate Cocaine-Induced Behavioral Sensitization in Fischer, Lewis, and Sprague-Dawley Rats. J Pharmacol Exp Ther. 1999;289:54–65. [PubMed] [Google Scholar]
- Staffend NA, Loftus CM, Meisel RL. Estradiol reduces dendritic spine density in the ventral striatum of female Syrian hamsters. Brain Struct Funct. 2011;215:187–194. doi: 10.1007/s00429-010-0284-7. doi: 10.1007/s00429-010-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Moussawi K, Knackstedt L, et al. Role of mGluR5 neurotransmission in reinstated cocaine-seeking. Addict Biol. 2013;18:40–49. doi: 10.1111/j.1369-1600.2011.00432.x. doi: 10.1111/j.1369-1600.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselus M, Flagel SB, Jedynak JP, et al. Long-term effects of cocaine experience on neuroplasticity in the nucleus accumbens core of addiction-prone rats. Neuroscience. 2013;248C:571–584. doi: 10.1016/j.neuroscience.2013.06.042. doi: 10.1016/j.neuroscience.2013.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm Behav. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]