APC and Axin are key negative regulators of Wnt signaling in development and oncogenesis. They form a multiprotein complex targeting the key Wnt effector β-catenin for destruction. Essential components of APC and Axin required for their cooperative function are identified, and the data are used to design a minimal β-catenin–destruction machine.
Abstract
Negatively regulating key signaling pathways is critical to development and altered in cancer. Wnt signaling is kept off by the destruction complex, which is assembled around the tumor suppressors APC and Axin and targets β-catenin for destruction. Axin and APC are large proteins with many domains and motifs that bind other partners. We hypothesized that if we identified the essential regions required for APC:Axin cooperative function and used these data to design a minimal β-catenin-destruction machine, we would gain new insights into the core mechanisms of destruction complex function. We identified five key domains/motifs in APC or Axin that are essential for their function in reconstituting Wnt regulation. Strikingly, however, certain APC and Axin mutants that are nonfunctional on their own can complement one another in reducing β-catenin, revealing that the APC:Axin complex is a highly robust machine. We used these insights to design a minimal β-catenin-destruction machine, revealing that a minimized chimeric protein covalently linking the five essential regions of APC and Axin reconstitutes destruction complex internal structure, size, and dynamics, restoring efficient β-catenin destruction in colorectal tumor cells. On the basis of our data, we propose a new model of the mechanistic function of the destruction complex as an integrated machine.
INTRODUCTION
The canonical Wnt pathway is one of the most-studied signaling pathways in animal cells. Its key roles during development and in diseases such as cancer underline the importance of fully understanding Wnt signaling regulation (Cadigan and Peifer, 2009; Clevers and Nusse, 2012; Kandoth et al., 2013). Cells evolved mechanisms to keep powerful signaling pathways like this in an off state in the absence of ligand. Two key negative regulators of Wnt signaling, Adenomatous polyposis coli (APC) and Axin, are each critical for embryonic development and also act as important tumor suppressors in the colon and other tissues (web.stanford.edu/group/nusselab/cgi-bin/wnt/). Eighty percent of sporadic colorectal cancers begin with mutations in APC (Kandoth et al., 2013), and mutations in Axin are found in hepatocellular carcinomas, ovarian cancer, and adenocarcinoma (Satoh et al., 2000; Salahshor and Woodgett, 2005).
Canonical Wnt signaling culminates by regulating intracellular levels of the transcriptional coactivator β-catenin (βcat; Clevers and Nusse, 2012). APC and Axin, together with two kinases, GSK3 and CK1, are core components of the βcat destruction complex (Stamos and Weis, 2013), which constitutively phosphorylates βcat. This creates a binding site for the SCFβTrCP E3-ligase, which ubiquitinates βcat and transfers it to the proteasome for degradation. When Wnt signaling is activated through the binding of a Wnt ligand to the Wnt receptor complex, destruction complex function is inhibited and βcat levels increase (MacDonald and He, 2012). βcat enters the nucleus, binds Tcf/Lef transcription factors, and drives expression of Wnt target genes.
Despite the importance of Wnt signaling, although we know that the destruction complex is key in maintaining low levels of βcat, we are just starting to understand the mechanistic function of each core component (Stamos and Weis, 2013). Axin is the scaffold of the destruction complex. It self-polymerizes (Kishida et al., 1999; Schwarz-Romond et al., 2007), forming a hub that recruits APC, βcat, and the two kinases that carry out βcat phosphorylation. APC’s role inside the Axin complex has been less clear. Several hypotheses have been ruled out, including a role in localizing the destruction complex to a particular subcellular location or an essential role in shuttling βcat in and out of the nucleus (Roberts et al., 2012). Current hypotheses of APC’s role in the destruction complex propose that it helps protect βcat from dephosphorylation, stabilizes assembly of the Axin scaffold, and promotes efficient transfer of βcat to the E3-ligase by a GSK3-regulated mechanism (Su et al., 2008; Pronobis et al., 2015).
Although elucidating the individual functions of Axin or APC contributes to our understanding of destruction complex function, we believe that a more complete understanding will be gained by viewing the APC:Axin complex as an entity in which APC and Axin work cooperatively to reduce levels of βcat. Our recent superresolution microscopy provided new insights into the cooperative assembly of these two proteins into a large multiprotein machine (Pronobis et al., 2015). We now carry this further by defining the minimal components required for a functional destruction complex. Both Axin and APC are large, multidomain proteins (Figure 1, A and B; Stamos and Weis, 2013). Each has one or more large globular domains that mediate multiple protein interactions, and each also contains long, intrinsically unstructured regions containing peptide-binding sites for other protein partners. Surprisingly, some features of the two proteins seem overlapping—for example, both contain βcat-binding sites (multiple 15– and 20–amino acid repeats in APC and a single βcat-binding site in Axin), and each has a domain facilitating self-oligomerization (the combined APC Self-Association Domain [ASAD; Kunttas-Tatli et al., 2014] and Armadillo [Arm] repeats in APC and the DIX domain in Axin; Figure 1, A and B).
FIGURE 1:
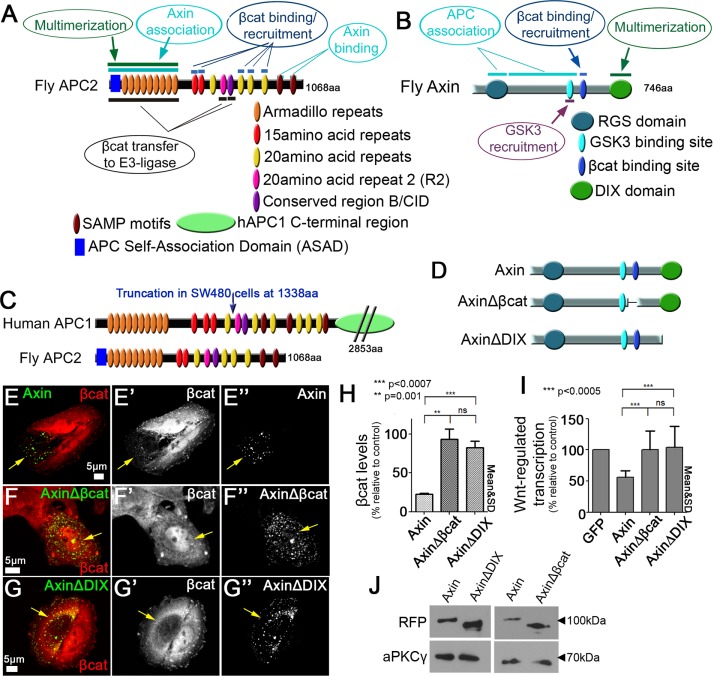
APC and Axin have regions with potentially redundant functions, and Axin has two domains/motifs essential for βcat destruction. (A) Drosophila APC2. Top, regions that may have redundant functions. Bottom, unique functions of APC2. (B) Drosophila Axin. Top, regions that may have redundant functions. Bottom, regions with unique functions. (C) Comparison of human APC1 and fly APC2, indicating truncated protein present in SW480 cells. (D) Schematic representation of Axin mutants. (E) Wild-type Axin can reduce βcat levels. Axin-RFP expressed in SW480 cells and stained for βcat via antibody. Arrows, transfected cells. (F, G) AxinΔβcat-RFP (F) and AxinΔDIX-RFP (G) cannot facilitate βcat destruction. (H) Deleting either the βcat-binding site or DIX domain of Axin impairs its ability to reduce βcat levels. Quantification of βcat relative to untransfected cells in 10 cells each measured in three independent experiments. (I) Wnt-regulated transcription remains high in cells expressing Axin mutants that delete the βcat-binding or DIX domain. Quantification of TOP/FOPflash reporter gene assay of indicated constructs in SW480 cells. Triplicates were measured in three independent experiments. Student’s t test was used. (J) Immunoblot of indicated constructs to compare expression levels. aPKCγ is a loading control.
We hypothesized that if we identified the essential regions required for APC:Axin cooperative function and used these data to design a minimal βcat-destruction machine, we would gain new insights into the core mechanisms of destruction complex function. Furthermore, this effort might provide a paradigm for understanding large multiprotein complexes that assemble by multivalent interactions (Toretsky and Wright, 2014). Our investigation revealed several important new insights. We found that 1) the core mechanism of the destruction machine depends on five domains/motifs found in APC or Axin, 2) APC and Axin mutants that are nonfunctional when expressed alone can complement one another when expressed together, suggesting that some domains/motifs of Axin and APC share partially redundant functions inside the βcat-destruction complex, and 3) a chimera that we designed to covalently link these five regions can reconstitute the wild-type APC:Axin complex in its internal structure, size, and dynamics and restores efficient βcat destruction as effectively as wild-type APC:Axin complexes. On the basis of our data, we propose a new model for the mechanistic function of the destruction complex as an integrated entity.
RESULTS
Establishing a model system in which to define essential features of the minimal destruction complex
In our effort to design the minimal destruction machine and thus probe the mechanism of the APC:Axin complex, we used the human colorectal cancer cell line SW480. These cells have high levels of βcat due to truncation of endogenous human APC1 at 1338aa (Figure 1C; Rubinfeld et al., 1997). In this APC mutant cell line, transfecting exogenous APC can restore βcat destruction. In previous work, we established a system that uses the Drosophila homologues APC2 and Axin, which allowed us to assess the function of full-length proteins, since human APC1 (2853aa) is too large be effectively transfected and expressed in cells (Figure 1C). Fly APC2 has all of the functional regions needed to down-regulate Wnt signaling and reduce βcat levels in human SW480 cells, emphasizing the conservation of the Wnt pathway in all animals (Figure 1C; Roberts et al., 2011). Fly Axin also shares all regions found in human Axin (Figure 1B; Willert et al., 1999). Coexpressing APC2 and Axin in SW480 cells also allowed us to directly visualize the destruction complex, which assembles into large protein puncta that can be readily visualized by light microscopy and function in targeting βcat for destruction (Roberts et al., 2011; Pronobis et al., 2015). It is important to note that we are expressing APC, Axin, and their mutants at levels significantly higher than endogenous (Roberts et al., 2011; Pronobis et al., 2015). Nevertheless, our previous work on APC suggested that in most cases, the functional requirements identified in this assay closely parallel those seen when these proteins are expressed at endogenous levels in a whole animal (Roberts et al., 2011, 2012; Pronobis et al., 2015).
When expressed at endogenous levels, both APC and Axin are essential for βcat destruction, and overexpressing Axin cannot compensate for complete loss of APC (Mendoza-Topaz et al., 2011). SW480 cells provide a useful model for assessing the key functions of the destruction complex, as their strong but not null mutant phenotype in Wnt regulation, due to the presence of truncated human APC1, provides a place to identify the essential core functions of the destruction complex machine. Consistent with this, Axin overexpression can reduce βcat in the APC mutant SW480 cell line, but its ability to down-regulate βcat is not as efficient as that provided by APC2 plus Axin–transfected cells (Pronobis et al., 2015). This parallels the observation that overexpressing Axin in hypomorphic APC2 mutant flies (point mutation N175K in the Arm repeats [rpts]) can restore Wnt regulation, unlike what is observed in APC2-null mutants (Mendoza-Topaz et al., 2011). This is consistent with the idea that APC and Axin must work cooperatively to create the most efficient βcat destruction machine. Thus SW480 cells provide a sensitized system in which to define the most essential domains/motifs of each protein and thus define the core functions of the destruction complex machine.
APC and Axin have 5 regions that are essential for their individual function in βcat degradation
To define the core mechanisms underlying destruction complex function, we first identified the regions in APC2 and Axin that are essential for their individual function in reducing βcat levels when ectopically expressed in SW480 cells. Our previous work and that of others revealed that three regions in APC2 are essential: the combined ASAD and Arm rpts, 20–amino acid repeat 2 (R2), and conserved region B (B is also known as the catenin-inhibitory domain [CID]; Supplemental Figure S1A (Kohler et al., 2009; Roberts et al., 2011, 2012; Kunttas-Tatli et al., 2012, 2014; Pronobis et al., 2015). We previously confirmed that all APC2 mutant proteins were effectively expressed in SW480 cells (Roberts et al., 2011, 2012; Pronobis et al., 2015). Whereas wild-type APC2 decreased βcat effectively, APC2ΔArm (lacking both the ASAD and the Arm rpts), APC2ΔR2, and APC2ΔB mutants could not reduce βcat levels (Supplemental Figure S1, B–E). Quantification of βcat immunofluorescence confirmed that βcat levels were >80% of those in control SW480 cells, and Wnt-regulated transcription, as measured by the TOP/FOPflash transcriptional reporter, was only weakly reduced (to ≥70%; Supplemental Figure S1, F and G; the remaining βcat-binding sites can sequester some βcat; Roberts et al., 2011), thus confirming the requirement of these regions for APC2 function. We next identified regions in Axin that are essential for its ability to reduce βcat levels in SW480 cells (Figure 1D). Overexpressing wild-type Axin reduced βcat in the SW480 cells (Figure 1E; Nakamura et al., 1998). However, deleting either Axin’s βcat-binding site or its DIX domain rendered Axin nonfunctional (Figure 1, D–G; both Axin mutants are expressed at levels similar to that of wild-type Axin; Figure 1J), consistent with studies in Axin-mutant flies (Peterson-Nedry et al., 2008). Quantification confirmed that AxinΔβcat and AxinΔDIX failed to reduce βcat levels or Wnt-regulated transcription; both remained ≥80% of those in untransfected control cells (Figure 1, H and I). Although the lack of function of AxinΔDIX in βcat destruction matched earlier experiments, it is somewhat surprising that AxinΔDIX retains the ability to form puncta. Others also observed the ability of Axin∆DIX to form puncta, albeit less efficiently (Schwarz-Romond et al., 2007), perhaps via interaction with endogenous Axin. Another somewhat surprising result was the failure of Axin∆DIX to provide any reduction of Wnt-regulated transcription, despite its ability to retain at least some βcat in the cytoplasm (Figure 1G’). These two features are worth exploring in more detail in the future. Taken together, these data suggest that APC has three regions and Axin has two regions that are essential for their individual function in promoting βcat destruction in this APC hypomorphic cell line (Figure 2A).
FIGURE 2:
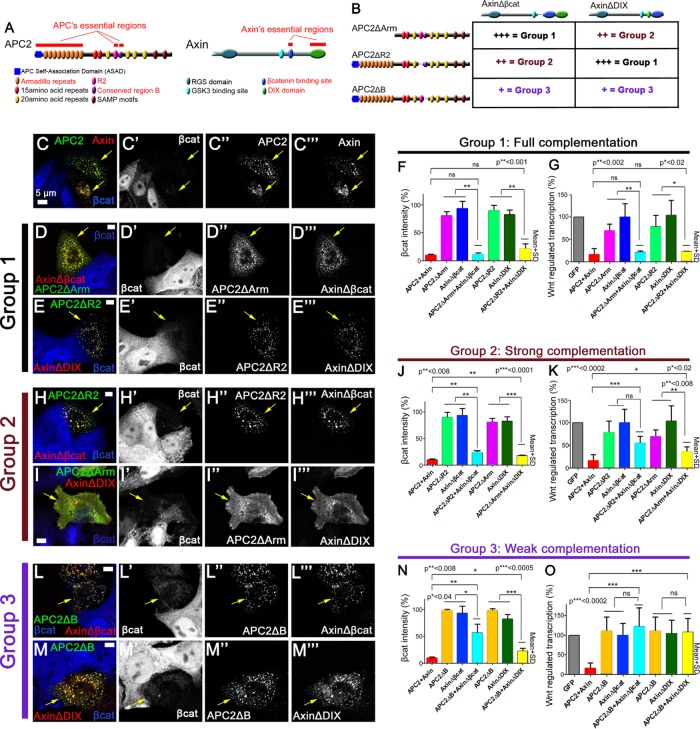
Certain APC and Axin mutants that are nonfunctional on their own can complement one another to facilitate βcat destruction. (A) Schematic representation of APC2 and Axin. Essential regions are highlighted in red. (B) Combinations of APC2 and Axin mutants tested. Groups 1–3 exhibit different levels of complementation. (C–E, H–I, L–M) Immunofluorescence, wild-type or mutant versions of GFP-APC2 and Axin-RFP expressed in SW480 cells and stained for βcat via antibody (blue). Arrows = transfected cells. (C) Wild-type APC2 and Axin form cytoplasmic complexes and effectively reduce βcat. (D, E) The pairs GFP-APC2ΔArm + AxinΔβcat-RFP (D) and GFP-APC2ΔR2 + AxinΔDIX-RFP (E) colocalize in puncta and show full complementation in βcat reduction, and they are thus categorized into group 1. (F) Quantification, βcat fluorescence intensity relative to untransfected control SW480 cells, group 1. Constructs are indicated. Ten cells each in three independent experiments. (G) Quantification, Wnt regulated transcription in SW480 cells of group 1. Group 1 APC and Axin mutants are indistinguishable from wild-type APC2 plus Axin in inhibiting Wnt-regulated transcription. Three triplicates measured in three independent experiments. (H, I) The pairs GFP-APC2ΔR2 + AxinΔβcat-RFP (H) and GFP-APC2ΔArm + AxinΔDIX-RFP (I) show strong complementation in βcat reduction and are thus categorized into group 2. Of interest, whereas GFP-APC2ΔR2 and AxinΔβcat-RFP colocalize in puncta (H), GFP-APC2ΔArm and AxinΔDIX-RFP (I) do not form large cytoplasmic puncta. (J) Quantification of βcat levels, group 2 mutants, as in F. (K) Quantification of Wnt-regulated transcription in group 2, as in G. (L, M) The pairs GFP-APC2ΔB + AxinΔβcat-RFP (L) and GFP-APC2ΔB + AxinΔDIX-RFP (M) show weak complementation in βcat reduction and are categorized into group 3. (N) Quantification of βcat levels of group 3 mutants. (O) Quantification of Wnt-regulated transcription in group 3. Student’s t test was used.
APC and Axin share regions with redundant functions
APC and Axin both possess domains that perform seemingly similar functions, suggesting that these regions may be partially dispensable in a fully formed destruction complex (Figure 1, A and B, top), potentially allowing the retention of partial function, at least when both proteins are overexpressed. These functions include 1) the ability to multimerize, 2) the ability to bind and recruit βcat into the complex, and 3) two mechanisms by which APC and Axin can interact (APC’s SAMP motifs bind Axin’s RGS domain, and APC’s Arm rpts interact with the region near Axin’s GSK3 site [ Nakamura et al., 1998; Kishida et al., 1999; Spink et al., 2000; Liu et al., 2006; Roberts et al., 2012; Pronobis et al., 2015]). However, there are also certain functions that appear unique to APC or to Axin, such as Axin’s ability to bind to GSK3 or APC’s ability to increase transfer of βcat to the E3-ligase (Nakamura et al., 1998; Pronobis et al., 2015).
To examine whether some of these potentially redundant regions are in fact dispensable when APC and Axin work together and define other functional redundancies, we examined whether APC and Axin mutants that lack their essential regions can complement one another in βcat destruction and Wnt signaling inhibition when expressed together. In earlier work, we were surprised to learn that APC’s multiple βcat-binding sites are dispensable for targeting βcat for destruction, although they do modulate signaling by retaining βcat in the cytoplasm (Roberts et al., 2011; Yamulla et al., 2014). Because deleting the βcat-binding site of Axin strongly reduced its activity, we hypothesized that binding of βcat to APC might compensate for loss of βcat binding to Axin. To test this hypothesis, we coexpressed AxinΔβcat with each nonfunctional APC mutant (APC2ΔArm, APC2ΔR2, APC2ΔB), each of which retains βcat-binding sites (Figure 2B). To test for other functional redundancies, we also expressed each APC mutant with AxinΔDIX (Figure 2B).
Strikingly, APC and Axin mutants that lack essential regions and thus could not reduce βcat when expressed separately were often able to facilitate βcat destruction when two mutants were coexpressed (Figure 2, B–O). However, some combinations reduced βcat and Wnt-regulated transcription better than others. On the basis of their rescue ability, we classified these combinations into three groups (Figure 2B). Group 1 (APC2ΔArm + AxinΔβcat and APC2ΔR2 + AxinΔDIX) showed full complementation ability in down-regulating βcat levels and Wnt-regulated transcriptional activity (Figure 2, D–G). The levels of βcat and inhibition of Wnt-regulated transcription were indistinguishable from those for wild-type APC + Axin, suggesting that these APC and Axin mutants fully complement each other (Figure 2, F and G). Group 2 (APC2ΔR2 + AxinΔβcat and APC2ΔArm + AxinΔDIX) exhibited strong complementation ability (Figure 2, H–K). This group had reduced βcat levels and Wnt-regulated transcription, although the reduction was less efficient than that seen in wild-type APC2 + Axin–transfected cells (Figure 2, J and K). Group 3 (APC2ΔB coexpressed with either AxinΔβcat or AxinΔDIX) exhibited weak complementation ability (Figure 2, L–O). Although a modest reduction in βcat levels was detected, Wnt-regulated transcription was as high as when APC2ΔB, AxinΔβcat, or AxinΔDIX was expressed individually (Figure 2, N and O), suggesting that loss of certain regions such as region B interferes with complementation.
These data revealed that deleting Axin’s βcat-binding site was compensated for by each of the APC mutants, although APC2∆B was less effective in this regard. Similarly, removal of the self-polymerization domain in Axin∆DIX could be compensated for by APC∆R2 and more weakly by APC∆B. Surprisingly, Axin∆DIX was also complemented by APC2∆Arm, despite the fact that this pair lacked the predicted self-polymerization domain of both proteins and was unable to form and colocalize in large cytoplasmic complexes (Figure 2I). However, Axin∆DIX and APC2∆Arm can still associate, as detected by coimmunoprecipitation, consistent with their retention of the RGS domain and SAMP motifs (Supplemental Figure S1H). This may suggest that when APC and Axin are expressed at elevated levels, the reduced efficiency of complexes that cannot self-polymerize may be sufficient for βcat destruction. Alternatively, the endogenous truncated human APC, which retains the Arm repeats, may mediate formation of smaller but still functional supramolecular complexes. Together our data suggest that when working together, APC and Axin can each complement functional deficits in the other to efficiently target βcat for destruction.
It was possible that the reduced ability of certain pairs to form efficient destruction complexes might make them more sensitive to expression levels. For our group 2 complementing pairs, we addressed this idea in two ways. First, we assessed whether the level of expression of the constructs, as assessed by green fluorescent protein (GFP) fluorescence, affected their ability to reduce βcat levels by assessing both properties in individual cells. In earlier work, we assessed this for wild-type APC2 and Axin. Of interest, for each of these, increasing levels of expression actually led to reduced rather than increased ability to reduce βcat levels (Pronobis et al., 2015; Supplemental Figure S2A). We interpreted this, as others had suggested (Salic et al., 2000), as reflecting the fact that when one component is overexpressed without the others, it may lead to assembly of partial complexes that are nonfunctional. These earlier data also revealed that APC (plus endogenous Axin) was more effective at reducing βcat levels than was Axin, regardless of their level of expression. We thus carried out a similar analysis of the effectiveness of APC2ΔArm + AxinΔDIX and the APC2ΔR2 + AxinΔβcat. These data did not suggest strong dosage dependence for APC2ΔArm + AxinΔDIX, although there was some suggestion that APC2ΔR2 + AxinΔβcat was less effective at the lowest concentrations (Supplemental Figure S2A). To further assess this, we attempted to reduce the levels of these constructs further by transfecting lower amounts of each plasmid pair into SW480 cells and assessing effects on both reduction of βcat levels and Wnt-regulated transcription (TOPflash reporter). We verified that this led to lower average levels of protein accumulation (Supplemental Figure S2E). We saw no substantial effect of different dilutions on ability to reduce βcat levels—at each concentration, wild-type APC2 + Axin was most effective, followed by APC2ΔArm + AxinΔDIX and then APC2ΔR2 + AxinΔβcat (as assessed by examining average reduction [Supplemental Figure S2F] or graphing construct levels vs. βcat levels [Supplemental Figure S2H]). However, reducing the amount of construct transfected did reduce the ability to silence Wnt-regulated transcription (TOPflash reporter); intriguingly, this effect was also seen with wild-type APC2 + Axin (Supplemental Figure S2G). We suspect that this latter effect reflects reduced ability to retain βcat in the cytoplasm.
Each of APC’s and Axin’s essential regions makes important but partially overlapping contributions to the efficiency of βcat destruction
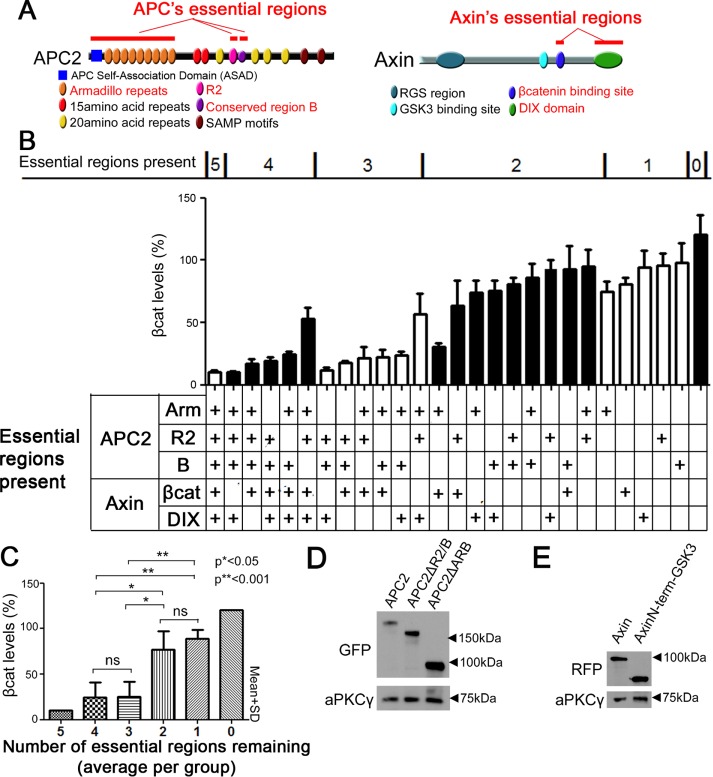
The foregoing data suggest that APC2 and Axin create a robust destruction machine in which some regions are partially redundant, such that coexpression at elevated levels can lead to complementation. To further probe this, we assessed the function of the destruction complex as we systematically decreased the number of essential regions, by coexpressing different APC and Axin mutants lacking particular essential domains (Figure 3, A and B). We confirmed via immunoblot that all APC and Axin mutants that had not been previously tested were expressed (Roberts et al., 2011, 2012; Pronobis et al., 2015; Figure 3, D and E). When we sorted these APC2 + Axin combinations based on the number of essential regions present, we found a generally gradual decrease in destruction complex function and thus increase in βcat levels as we progressed from five essential regions present to zero regions present (Figure 3B). However, within a group defined by number of essential regions present, we saw variations in βcat reduction; in particular, mutant combinations seemed strongly affected by deleting APC2’s region B. When we averaged the βcat levels within a group, we found that the APC:Axin complex was still able to reduce βcat levels fairly effectively when two essential regions were deleted, whereas deleting three regions increased βcat levels significantly (Figure 3C). Thus the destruction complex is a robust machine that can withstand on average the loss of two of the essential regions. Taken together, our data suggest that the five essential regions of APC and Axin make important but partially overlapping contributions to the function of the destruction complex.
FIGURE 3:
The essential regions in APC and Axin each make important but partially overlapping contributions to a fully functional destruction complex. (A) Schematic representation of APC2 and Axin. Essential regions are indicated in red. (B) The essential regions in APC2 and Axin work cooperatively to ensure efficient reduction of βcat levels. Coexpression of indicated APC2 and Axin mutants in SW480 cells, followed by quantification of βcat fluorescence intensity. Top, mutants are sorted into groups that are defined by how many essential regions remain present. Middle, βcat average intensity. Bottom, table describing which essential regions remain present. (C) For βcat reduction, up to two essential regions are largely dispensable. Columns represent the averages of the groups that were defined in B. Student’s t test was used. (D) Immunoblot analysis of expression levels of a subset of the APC2 mutants used; others are given in other figures or in Roberts et al. (2011, 2012) or Pronobis et al. (2015). (E) Expression levels of indicated Axin mutant.
The essential regions of APC and Axin are sufficient to down-regulate Wnt signaling, but mutants carrying only these essential regions are not as effective at reducing βcat as the wild-type proteins
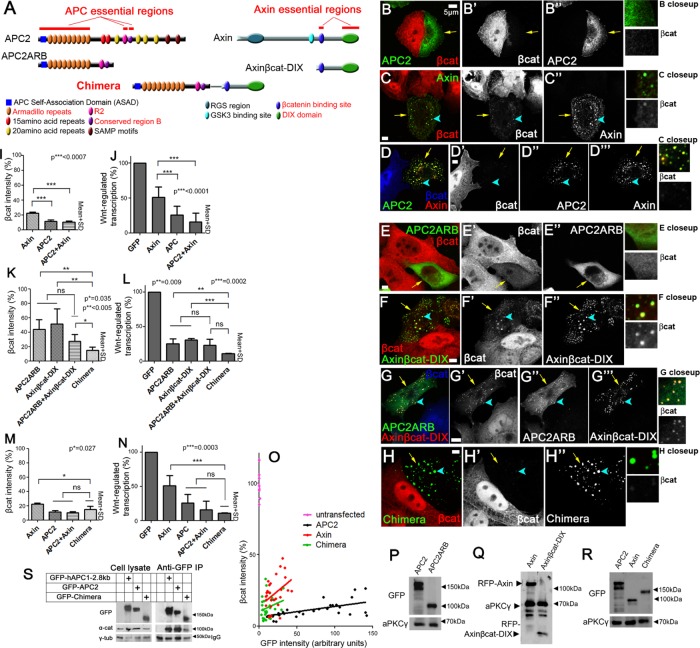
Having defined regions of APC2 and Axin that are necessary for their function, we next tested whether APC’s and Axin’s essential regions are sufficient to facilitate down-regulation of βcat- and Wnt-regulated transcription. To do so, we reduced each protein to its essential regions, generating APC2ARB (Figure 4A, left), which has only the Arm rpts, R2 and region B, and Axinβcat-DIX (Figure 4A, right), which consists of the C-terminal half of Axin, which contains its two essential regions (βcat-binding and DIX). First, we confirmed that expression levels of APC2ARB and Axinβcat-DIX were similar to those for wild-type proteins (Figure 4, P and Q). Then we tested their function in reducing βcat and inhibiting Wnt-regulated transcription. Each retained significant function when misexpressed, reducing βcat levels to ∼40–50% of those in control untransfected cells (Figure 4, E, F, and K). However, neither mutant was as effective as wild-type APC2 or wild-type Axin in reducing overall βcat levels (Figure 4, E and F vs. B and C, and K vs. I). We also saw differences in retention of βcat: APC2ARB lacked βcat-binding sites and did not exhibit retention of βcat in the cytoplasm (Figure 4E’). Axinβcat-DIX was able to assemble into puncta and accumulated somewhat more βcat in the cytoplasmic puncta than did wild-type Axin (Figure 4, C vs. F, arrowheads and close-ups), also suggesting reduced ability to effectively target βcat for destruction. Strikingly, both minimized constructs were indistinguishable from wild-type APC2 or Axin in inhibiting Wnt-regulated transcription (Figure 4, L vs. J). Thus the essential regions of APC and Axin have a moderate ability to reduce βcat levels but can fully down-regulate Wnt-activated transcription.
FIGURE 4:
The APC:Axin Chimera reduces βcat as efficiently as wild-type APC plus Axin. (A) Schematic, APC and Axin constructs plus Chimera. Top, wild-type APC and Axin with essential regions indicated. Middle, mutants incorporating essential regions alone. Bottom, essential regions were combined into a single polypeptide to create the Chimera. (B–H) Immunofluorescence, GFP-tagged or RFP-tagged wild-type or mutant constructs misexpressed in SW480 cells and stained for βcat via antibody. Arrows point to transfected cells. Close-ups of a transfected cell are shown to the right to reveal which constructs retain βcat in puncta. (B) Wild-type GFP-APC2 reduces βcat levels. (C) Wild-type GFP-Axin also reduces βcat levels, but some βcat remains in puncta (arrowheads, close-up). (D) Coexpressing GFP-APC and Axin-RFP effectively reduces βcat levels. (E) GFP-APC2ARB, which consists of APC’s three essential regions, can moderately reduce βcat levels, but βcat remains higher than is seen after wild-type APC2 transfection. (F) Axinβcat-DIX-RFP also moderately reduces βcat levels, and detectable βcat remains in the puncta (arrowhead, close-up). (G) Coexpressing APC2ARB and Axinβcat-DIX does not further decrease βcat levels, and βcat remains in puncta (arrowhead, close-up). (H) Expressing the GFP-Chimera leads to strong reduction of βcat, and no βcat is seen in puncta (arrowhead, close-up). (I, K, M) Quantification, βcat fluorescence intensity in SW480 cells transfected with indicated constructs, normalized to untransfected cells. Ten cells each in three independent experiments were measured. (J, L, N) Quantification of Wnt-regulated transcription in SW480 cells (TOPflash activity, normalized to untransfected cells). Three triplicates were measured in three independent experiments. (I) Axin cannot reduce βcat levels as effectively as APC2 or APC2 + Axin. (J) Axin cannot inhibit Wnt-regulated transcription as effectively as APC2 or APC2 + Axin. (K) Mutants carrying only the essential regions of APC2 or Axin only moderately reduce βcat levels, whereas covalently linking the essential regions of APC and Axin into the Chimera increases βcat reduction. (L) The Chimera strongly inhibits Wnt-regulated transcription. (M) The Chimera reduces βcat levels better than wild-type Axin. (N) Wnt-regulated transcription is as effectively inhibited by the Chimera as it is by wild-type APC2 + Axin or APC2. Student’s t test was used. (O) APC2, Axin, and the Chimera are not dosage dependent in βcat degradation. GFP levels (reflecting expression level of APC or Axin construct) vs. βcat signal in individual cells expressing each construct. βcat signal is normalized to nearby untransfected cells. A set of individual values of untransfected cells shows the degree of variability among cells in the same population. Thirty cells total for each condition. Both the Chimera and APC2 are more effective at reducing βcat than is Axin over a wide range of expression levels. (P–R) Immunoblot, expression levels of indicated constructs. aPKCγ is a loading control. Relative expression levels vary somewhat from experiment to due to transfection efficiency. (S) α-Catenin coimmunoprecipitates with the Chimera. Left, cell lysates from cells expressing the indicated constructs. γ-Tubulin serves as a loading control. Right, anti-GFP immunoprecipitates from cells expressing the indicated constructs. Bottom, effectiveness of antibody pull down.
Covalently linking the essential regions of APC and Axin increases their negative-regulatory activity
Next we tested whether we could decrease βcat levels further by coexpressing APC2ARB and Axinβcat-DIX. Coexpression of both did not lead to a statistically significant further reduction in total βcat levels (assessed by fluorescence quantification; Figure 4, G vs. E, F, and K). When expressed together, APC2ARB and Axinβcat-DIX continued to retain βcat in puncta (Figure 4G, close-up, arrowhead). One reason for reduced function may be the failure of APC2ARB to be as effectively recruited into Axinβcat-DIX puncta (Figure 4, G” vs. D”), likely due to the lack of the SAMP motifs.
In their natural state, APC and Axin act in a complex in which APC binds to Axin’s RGS region via its SAMP motif (Spink et al., 2000), both of which are deleted in our minimized constructs. We thus hypothesized that covalently linking APC2ARB to Axinβcat-DIX might improve rescue ability. We therefore created a Chimera linking the C-terminus of APC2ARB to the N-terminus of Axinβcat-DIX (Figure 4A, bottom). This mimics the interaction of APC’s C-terminal SAMP motifs and Axin’s N-terminal RGS domain (Figure 1, A and B). We confirmed that the level of Chimera protein expression was very similar to the expression of wild-type Axin (Figure 4R).
Strikingly, the APC:Axin Chimera strongly decreased βcat levels (to ∼10%; Figure 4, H and K). It also eliminated βcat in puncta (Figure 4H, close-up, arrowheads), doing so as well as wild-type APC + Axin (Figure 4D, close-up, arrowheads). The Chimera also effectively inhibited Wnt-regulated transcription (Figure 4L). Thus the Chimera down-regulates βcat levels and Wnt-regulated transcription better than APC2ARB and Axinβcat-DIX expressed individually or coexpressed. In fact, the Chimera down-regulated βcat levels and Wnt-regulated transcription as effectively as wild-type APC2 coexpressed with Axin (Figure 4, H vs. D, M, and N). Strikingly, when we compared the Chimera to Axin, we found that it reduced βcat and Wnt-regulated transcription even more effectively than expressing Axin alone (Figure 4, C vs. H, M, and N).
Next we assessed whether the reduction of βcat was dependent on protein levels of the Chimera, APC, or Axin. In previous work, we found that there is an inverse relationship between the level of overexpression of wild-type APC2 or Axin and the down-regulation of βcat levels, suggesting that at very high levels, they may assemble into partial, nonfunctional complexes that sequester βcat (Pronobis et al., 2015). The Chimera exhibited the same inverse relationship between levels of GFP-tagged protein and βcat, revealing that it was more effective than wild-type Axin even at the lowest levels of expression we assessed (Figure 4O). Together these data suggest that covalently linking the five essential regions of APC and Axin together generates a machine that is even more efficient than Axin in down-regulating βcat and Wnt signaling in this APC mutant cell line.
One of the regions contained in the Chimera, R2/B (B is also known as the CID), is a peptide motif that is essential for APC function but did not have a known binding partner. Recent work suggests that this region acts as a binding site for α-catenin (Choi et al., 2013), which has been known to bind APC since the earliest connection of APC to βcat was made (Rubinfeld et al., 1993). We thus asked whether the Chimera, when expressed in SW480 cells, coimmunoprecipitated with α-catenin. We used a human APC1 fragment containing this region (hAPC1-2.8 kB, which begins before the 20 amino acid repeat 1 [20R1] and includes 20R2, B, 20R3, 20R4, and the first SAMP motif) and full-length APC2 as positive controls, using the GFP tag to immunoprecipitate each of them. Strikingly, α-catenin coimmunoprecipitated with all of them but not with control immunoglobulin G (Figure 4S). This is consistent with a possible role for α-catenin in APC and Chimera function.
APC’s and Axin’s essential regions remain indispensable in the Chimera
Because we physically linked APC’s and Axin’s regions together into an artificial chimeric protein, we next assessed whether all five essential regions remain indispensable when linked in the Chimera. We created variants sequentially deleting each of the five regions (Supplemental Figure S3A). We confirmed that all Chimera mutants are expressed at levels similar to that of the Chimera (Supplemental Figure S3, J and K). Next we tested the ability of each Chimera mutant to facilitate βcat destruction and repress Wnt-regulated transcription. Deleting APC2’s Arm rpts, R2, or region B or Axin’s βcat-binding site or DIX domain each substantially reduced the ability of the Chimera to reduce βcat levels and Wnt-regulated transcription (Supplemental Figure S3, B–I). Each of the Chimera mutants had βcat levels >50% of those of untransfected control cells, and Wnt-regulated transcription was >40% of that in control cells (Supplemental Figure S3, H and I). We observed one additional intriguing aspect: whereas most Chimera mutants accumulated βcat in their complexes, ChimeraΔβcat did not (Supplemental Figure S3, F, arrowheads, vs. C–E and G, arrowheads), suggesting that the βcat-binding site of Axin is required to recruit βcat into the Chimera complex. Thus the Chimera needs all five essential regions to fully function in βcat destruction.
The internal complex size and structure of the Chimera are similar to those of APC:Axin complexes
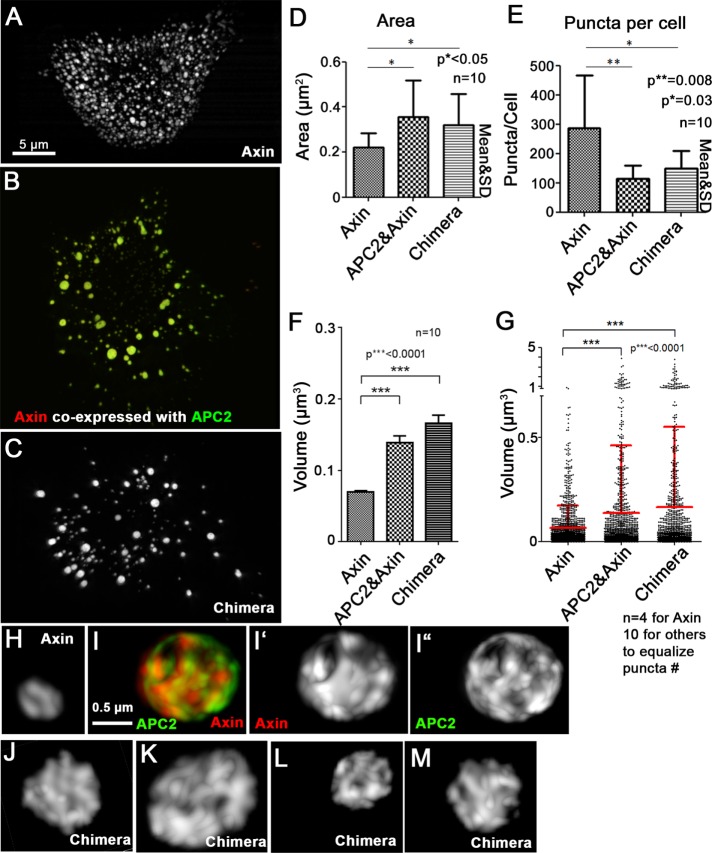
Our earlier work revealed that one role of APC is to promote Axin polymerization, increasing the size, complexity, and effectiveness of destruction complexes (Pronobis et al., 2015). We next assessed the effect on destruction complex size and internal structure of fusing the essential regions of APC and Axin into the Chimera. When overexpressed in SW480 cells, Axin assembles into numerous puncta (Figure 5A). Axin recruits APC2 into these puncta when coexpressed (Figure 5B). We previously found that APC2:Axin coexpression increases puncta size (Pronobis et al., 2015), as measured either by cross-sectional area (Figure 5D) or puncta volume (Figure 5, F and G); this increase in puncta size coincided with a decrease in the number of puncta (Figure 5E). Strikingly, when expressed in SW480 cells, the Chimera also formed larger, less numerous puncta than those assembled by Axin alone and thus were similar to those created by coexpressing Axin and APC2 (Figure 5, C–G).
FIGURE 5:
The Chimera mimics the APC2:Axin complex in internal structure and size. (A–C) SIM images. SW480 cells transfected with Axin-RFP (A), GFP-APC2 plus Axin-RFP (B), or GFP-Chimera (C). (D) The cross-sectional area of the chimeric complexes is similar to those produced by APC2 plus Axin and larger than those produced by Axin alone. (E) The number of puncta in Chimera-transfected cells is similar to that in APC2 + Axin–transfected cells and reduced relative to cells expressing Axin alone. Area and number obtained using LSM 710 images and the ImageJ Particle Analyzer. Ten cells quantified per construct. (F, G) Chimera puncta are similar in volume to APC2:Axin complexes and larger than those produced by Axin alone. Puncta volume assessed from SIM images of indicated constructs using Imaris Software (Bitplane). (F) Average puncta volume of indicated constructs. Ten cells used for each condition. (G) Volume comparison of equal numbers of puncta from Axin-transfected (n = 4 cells quantified), APC2 + Axin–transfected (n = 10 cells), or Chimera-transfected (n = 10 cells) cells. Fewer Axin cells were analyzed to equalize puncta number. Student’s t test was used. (H–M) SIM close-up three-dimensional projections of puncta from cells like those in A–C. (H, I) APC2 coexpression (I) leads to a more internal complex structure of Axin puncta than that of puncta assembled from Axin alone (H). (J–M) The Chimera has a complex internal structure resembling that of APC:Axin complexes. Representative images of GFP-Chimera expressed in two different SW480 cells.
Structured illumination microscopy (SIM) imaging previously revealed that the increase in puncta size reflects the ability of APC to promote Axin polymerization, converting Axin puncta from simple strands and sheets (Figure 5H) into a more complex set of intertwined strands of Axin and APC2 (Figure 5I; Pronobis et al., 2015). SIM imaging of the Chimera revealed that many Chimera puncta were also more complex in structure (Figure 5, J–M, representative large puncta from two different cells), mimicking Axin:APC2 puncta internal structure. Thus the Chimera retains two properties of APC2:Axin-based destruction complexes: more complex internal structure and increased complex size.
The Chimera mimics dynamics of the APC:Axin complex
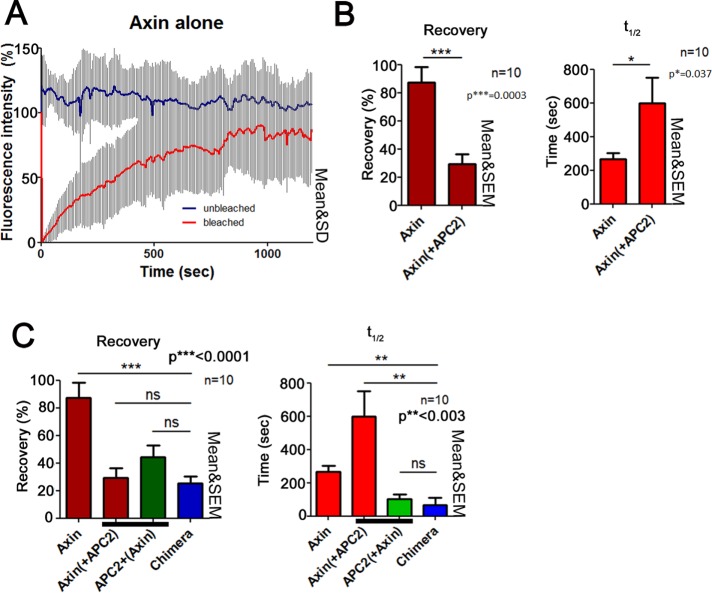
As a final comparison of the Chimera to coexpression of Axin and APC2, we examined the dynamic behavior of the Chimera using fluorescence recovery after photobleaching (FRAP). We previously found that puncta formed by Axin alone are highly dynamic, with a recovery plateau of >80% and a t1/2 = 220 s (Figure 6, A and B; Pronobis et al., 2015). However, in the presence of APC, Axin’s dynamics slows—the recovery plateau decreases to 30%, and the t1/2 extends to almost 600 s, suggesting that APC stabilizes Axin multimerization (Figure 6B; Pronobis et al., 2015). Of interest, although APC’s recovery plateau when in puncta with Axin is similar to that of Axin, APC shows a faster turnover time (t1/2 = 100 s; Figure 6C). This observation is consistent with a model in which Axin acts as a scaffold and therefore its turnover is slower, whereas APC constantly reassembles into the complex, perhaps bringing in new βcat. When we tested dynamics of the Chimera, we found that its recovery plateau is very similar to that of both APC and Axin, suggesting a stable core of Chimera polymer. Strikingly, however, the t1/2 of the Chimera is 100 s and therefore more similar to that of APC (100 s) than that of Axin (600 s; Figure 6C). Thus the artificial Chimera reconstitutes different aspects of the dynamics of the wild-type APC:Axin complex.
FIGURE 6:
Dynamics of the Chimera are similar to that of APC in APC2 + Axin complexes. (A–C) FRAP analysis of complexes formed by Axin-RFP alone, Axin-RFP coexpressed with GFP-APC2, or GFP-Chimera in SW480 cells. Ten complexes from three independent experiments. (A) Axin complexes are dynamic. Example of FRAP traces of complexes formed by Axin-RFP in SW480 cells. Unbleached (blue) and bleached cells (red). (B) Axin’s dynamics slows when APC is coexpressed. GFP-APC2 and Axin-RFP were expressed in SW480 cells. Left, recovery plateau; right, t1/2. (C) APC2 and the Chimera have similar dynamics. The recovery plateau of the Chimera is similar to that of both Axin and APC2. However, the time needed for the Chimera to recover is more similar to that of APC2.
The Chimera remains functional if the endogenous truncated human APC1 is knocked down
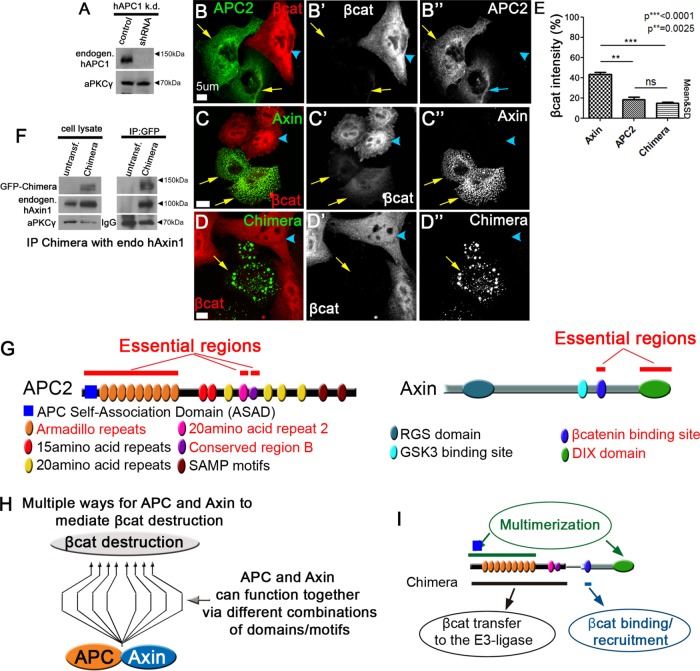
These data demonstrate that the Chimera can restore Wnt regulation to SW480 cells roughly as well as APC2 plus Axin. Like other APC mutant colorectal tumors, SW480 cells express an endogenous truncated human APC1, which end in the R2/B region or earlier. In SW480 cells, the truncation is at amino acid 1338; the truncation thus removes most of the 20–amino acid repeats and all of the SAMP motifs, but the truncated protein retains the coiled-coil self-association domain and Arm repeats, 15 amino acid repeats, and the 20R1 (Figure 1C). This truncated protein is unable to down-regulate βcat, but there remained the possibility that it could contribute to function of the Chimera. In a final set of tests, we explored whether the endogenous truncated human APC1 expressed in this cell line was essential for function of the Chimera. To do so, we generated an SW480 cell line stably transfected with a short hairpin RNA (shRNA) directed against the truncated APC1 (Materials and Methods). We verified knockdown of the truncated APC1 by immunoblotting (Figure 7A). Knockdown cells retained high levels of βcat, similar to those of the parental SW480 cells (Figure 7B; arrowhead; unpublished data).
FIGURE 7:
High levels of the truncated endogenous APC1 are not essential for function of the Chimera and new models of the destruction complex and its key functions. (A) Immunoblot assessing level of knockdown in SW480 cells stably transduced with shRNA targeting the endogenous truncated human APC1. aPKC serves as a loading control; representative of three experiments. (B–D) SW480 cells stably transduced with shRNA targeting the endogenous truncated human APC1 and then transfected with the indicated GFP-tagged constructs. Arrows, transfected cells; arrowheads, untransfected cells. (E) Quantification, βcat fluorescence intensity in cells like those in B–D. Constructs are indicated. Ten cells each in three independent experiments. Student’s t test was used. (F) The Chimera coimmunoprecipitates endogenous human Axin. Left, cell lysates from cells expressing the indicated constructs. aPKC serves as a loading control. Right, anti-GFP immunoprecipitates, bringing down the GFP-tagged-Chimera. Bottom, effectiveness of antibody pull down. (G) Essential regions of APC2 and Axin. (H) APC and Axin can use different combinations of domains/motifs to secure low levels of βcat. The nature of the combination defines the efficiency of the complex. (I) The Chimera helps define all the essential functions of the APC:Axin destruction complex.
We next transfected GFP-tagged APC2, Axin, or the Chimera into these cells and assessed their ability to restore down-regulation of βcat. Both Axin (Figure 7C) and the Chimera (Figure 7D) still formed puncta, as in the parental SW480 cells. Strikingly, APC2, Axin, and the Chimera all retained the ability to down-regulate βcat (Figure 7, B–D; quantified in E), even in these knockdown cells, in which the levels of the truncated APC1 were reduced substantially. Further, in this assay, quantitation of βcat fluorescence revealed that both APC2 and the Chimera each remained more effective in this regard than was Axin alone (Figure 7E), as we observed in parental SW480 cells in which truncated APC1 had not been knocked down (Figure 4I). However, although APC2, Axin, and the Chimera all retained the ability to restore βcat down-regulation, quantification revealed that they were not quite as effective after truncated APC1 knockdown: βcat levels were roughly twice as high in the knockdown cell lines relative to untransfected controls (Figure 7E vs. Figure 4, I and M).Together these data suggest that high levels of truncated endogenous APC1 are not essential for the function of the Chimera. However, they are consistent with the idea that the truncated APC1 may increase the efficiency of βcat destruction. Consistent with this, our earlier work revealed that the endogenous truncated APC1 can coimmunoprecipitate with transfected human Axin (Pronobis et al., 2015). It also is possible that endogenous human Axin is important for the function of the Chimera, as coimmunoprecipitations reveal they can associate (Figure 7F).
DISCUSSION
The βcat-destruction complex is key in regulating Wnt signaling. Our goal was to use a simplified system to design the minimal destruction complex, providing insights into its core mechanisms. APC and Axin proteins are both complex scaffolding proteins (Figure 7G). We began by defining the domains/motifs of APC2 and Axin essential for function in SW480 cells. Three domains/motifs in APC2 (the ASAD + Arm rpts, R2, and region B; Figure 7G) and two domains/motifs in Axin (the βcat-binding region and the DIX domain; Figure 7G) are essential for the function of each in regulating βcat levels when expressed alone in SW480 cells, consistent with prior analysis of Drosophila mutants (McCartney et al., 2006; Peterson-Nedry et al., 2008; Roberts et al., 2011, 2012; Kunttas-Tatli et al., 2012, 2014).
We next carried out complementation assays, revealing the robust nature of the integrated destruction complex. Strikingly, when they were expressed together, otherwise nonfunctional mutants of APC2 and Axin could complement one another. In some cases, this complementation could be traced to overlapping functions. Although APC and Axin are evolutionarily unrelated, a subset of their domains/motifs have shared functions; most strikingly, each has one or more βcat-binding sites, and each has a domain mediating self-association. The clear complementation of the nonfunctional Axin∆βcat mutant by several APC mutants that were not functional on their own, together with earlier analysis of an APC2 mutant lacking all its βcat-binding sites (Roberts et al., 2011; Yamulla et al., 2014), suggests that βcat recruitment to the destruction complex need only be provided by either APC or Axin.
When we expanded this analysis to systematically delete each of the five essential regions in APC and Axin (Figure 7G) and test them in combination for βcat reduction, we found that on average two essential regions were dispensable for function of the destruction complex when overexpressed. These data suggest that the APC:Axin complex may have multiple mechanistic pathways to target βcat for destruction (Figure 7H), using different combinations of domains and motifs, and that these different pathways differ in their efficiencies. This robustness could help absolutely ensure low levels of βcat in the absence of Wnt signals, a key to mediating normal developmental fate decisions and preventing tumor initiation. Further, it may allow for the evolution of mechanisms that fine-tune destruction rather than turning it off entirely, thus fine-tuning Wnt activity. Consistent with this, earlier (Ahmed et al., 2002; Akong et al., 2002) and recent work (Lee et al., 2003; Li et al., 2012b) reinforce the idea that Wnt signals turn down but do not turn off the destruction complex. Thus we propose that assembling a cooperative destruction machine with different domains/motifs in APC and Axin that share partially redundant functions creates a robust βcat-destruction complex that can be easily fine-tuned in its destruction rate.
One curious finding was that although the minimized APC or Axin mutants (the three essential regions of APC or the two essential regions of Axin) were each somewhat less effective at mediating βcat destruction relative to wild-type APC2 or Axin, they repressed Wnt-regulated transcription as well as wild-type APC or Axin, respectively. These data suggest that these two proteins may have yet-unknown mechanisms by which they down-regulate βcat-activated transcription separately from its destruction. Because APC and Axin can each shuttle into the nucleus (Neufeld et al., 2000; Cong and Varmus, 2004), it will be interesting to investigate how they use their essential regions to repress Wnt-regulated transcription. Another place for further analysis is the match between the ability of APC2 and/or Axin mutants to retain βcat in the cytoplasm, which we earlier showed allows APC2 to modulate downstream Wnt signaling and downstream transcriptional activity above and beyond its role in βcat destruction (Roberts et al., 2011). This potential mismatch was raised by the fact that Axin∆DIX still had some ability to retain βcat in the cytoplasm but had little or no effect on Wnt-regulated transcription. Finally, questions remain about the precise domains required for Axin and/or APC2 to form puncta. When the proposed multimerization domains of each protein are removed (Axin∆DIX and APC2∆Arm), neither can restore βcat destruction alone. However, it remains surprising that, as previously observed (Schwarz-Romond et al., 2007), Axin∆DIX can still form puncta. Perhaps it can interact with endogenous human Axin, restoring the ability to polymerize. More puzzling, when coexpressed with APC2∆Arm, Axin∆DIX no longer formed puncta, though this pair of constructs was able to largely restore βcat destruction. Our data reveal that they still can coimmunoprecipitate, perhaps as part of smaller, still functional supramolecular complexes. These data suggest that the relationship between forming puncta and function is not as simple as we suggested in our earlier model.
Our minimization efforts culminated in creation of the Chimera, linking the essential regions of APC and Axin together in one polypeptide (Figure 7I). Strikingly, the Chimera facilitates βcat destruction and inhibition of Wnt-regulated transcription as efficiently as wild-type APC coexpressed with wild-type Axin and better than Axin alone. Further, the Chimera reconstitutes the internal complex structure, size, and dynamics of the APC plus Axin destruction complex. All regions in the Chimera are essential for keeping the Wnt pathway down-regulated, suggesting that it constitutes the minimal destruction machine.
On the basis of this, we propose a model defining the minimal activities that ensure function of the destruction complex in down-regulating Wnt signaling (Figure 7I; the Chimera bypasses one essential feature—domains mediating APC:Axin association—by virtue of covalent linkage). These are as follows: 1) Multimerization via APC’s ASAD + Arm rpts and Axin’s DIX domain generates a cellular compartment into which all other destruction-complex proteins are recruited to allow the most efficient βcat destruction. This type of phase transition model, in which multivalent proteins create a compartment-like structure in the cytoplasm, was proposed to play a major role in signal transduction (Brangwynne et al., 2009; Li et al., 2012a). It will be interesting to test whether the polymerization domains in the Chimera could be replaced by polymerization domains of proteins not involved in Wnt signaling. It is also worth noting that co-overexpressing APC2 and Axin mutants each defective in polymerization (APC2∆Arm + Axin∆DIX) restored some function in βcat destruction; this may suggest that reduced efficiency is less critical when levels of the APC:Axin complex are elevated. 2) Recruitment of βcat into the APC:Axin complex is another key feature. Earlier work suggested that APC’s βcat-binding sites act redundantly with one another and in fact are dispensable for destruction (Roberts et al., 2011; Yamulla et al., 2014). Our new data suggest that Axin’s binding site alone is sufficient to pull βcat into the complex and that Axin’s βcat-binding site is dispensable if βcat-binding sites remain in APC. It would be interesting to pursue these observations in vivo, using, for example, clustered regularly interspaced short palindromic repeats (CRISPR) to precisely replace Axin’s βcat-binding site with APC’s 20R3, which has the highest affinity for βcat (Liu et al., 2006), to determine whether there is anything unique about Axin’s binding site. 3) We recently proposed that the Arm rpts, R2, and region B function in the efficient transfer of βcat to the E3-ligase, another key step in βcat degradation (Pronobis et al., 2015). The Chimera remains dependent on each of these three regions, since deleting any of them results in loss of βcat down-regulation and accumulation of βcat in the destruction complex. It will be interesting to test whether the Arm rpts have dual functions in the complex, working together with the ASAD in multimerization and acting in βcat transfer, or whether only one of these functions is required for the minimal βcat machine. Our previous work suggested that R2 and B act together as a unit (Pronobis et al., 2015), but our data here suggest that region B plays an even more essential role than R2. It will be important to determine whether this suggests differential or additive roles.
Thus the APC:Axin complex is a robust machine that uses the redundancy of domains/motifs to facilitate βcat destruction in multiple ways, and its key functions can be reduced to five essential regions that can reconstitute the destruction complex in the absence of Wnt signaling. However, additional domains/motifs may be critical for the destruction complex to be turned down by Wnt signaling. Consistent with this, a subset of Axin mutants leads to constitutively active destruction in Drosophila axin-mutant flies (Peterson-Nedry et al., 2008). It will be interesting to test whether and how the Chimera responds to Wnt activation. To address this and other questions, it will be of interest to probe function of the Chimera when expressed at levels similar to those of endogenous APC or Axin and, in their absence, by CRISPR-based engineering in either cultured mammalian cells or in vivo in Drosophila. This will provide the ability to assess Chimera function in the complete absence of APC family proteins (e.g., in APC2 APC1 double-mutant flies) or in the absence of endogenous Axin and also will allow the function of the Chimera to be assessed in the context of cells receiving or not receiving Wnt signals. Finally, our experiments raise the question of why nature evolved two proteins when one can suffice. The separate roles of APC proteins in cytoskeletal regulation (Näthke, 2006) may provide one potential answer.
MATERIALS AND METHODS
Constructs
Drosophila APC2 and Axin constructs were cloned using pECFP-N1 (Clontech) as a backbone vector via Gateway (Invitrogen) as described previously (Roberts et al., 2011; Pronobis et al., 2015). N-terminal GFP tags (Roberts et al., 2011) and C-terminal red fluorescent protein tags were used (Pronobis et al., 2015). APC2 mutants were APC2ΔARB (amino acids [aa] 94–536 + 595–621 + 733–1068), APC2ARB (aa 1–494 + 536–595 + 621–733), and APC2ΔR2/B (Δ aa645–715); Axin mutants were AxinΔβcat (Δ aa493–531), AxinΔDIX (∆ aa666–746), Axinβcat-DIX (Δ aa493–746), and AxinN-term-GSK3 (aa 1–494). The Chimera consisted of APC2’s Arm rpts (aa 1–495), R2, and region B (aa 621–733) linked with a 6-aa Gly-Ser-Gly linker to Axin’s C-terminus (aa 493–746). All other APC mutants have been described previously (Roberts et al., 2011, 2012; Pronobis et al., 2015). Plasmids for transfection were prepared as in Pronobis et al. (2016).
Cell culture and immunofluorescence
SW480 cells were cultured in L15 medium (Corning) with 10% heat-inactivated fetal bovine serum plus 1× penicillin/streptomycin (Life Technologies) at 37°C without CO2. Lipofectamine 2000 (Invitrogen) was used for transfections, following the manufacturer’s protocol. For immunostaining, cells were processed after 24 h. Immunostaining was as described in Roberts et al. (2011). Primary antibody was βcat (1:1000; BD Transduction). Secondary antibodies were Alexa 568 and 647 (Invitrogen, 1:1000).
Microscopy
Immunostained samples were imaged on a LSM Pascal microscope (Zeiss) and processed with the LSM image browser (Zeiss). SIM microscopy was carried out on the N-SIM superresolution microscope (Nikon) using 4% formaldehyde-fixed samples mounted in Aquapolymount. Images were processed using Imaris 5.5 (Bitplane), ImageJ, and the LSM Image Browser.
Quantification
Maximum-intensity Z-projections of cell image stacks were generated using ImageJ. βcat fluorescence intensity was measured as described in Pronobis et al. (2015). In short, cells were outlined, mean intensity was measured, background was subtracted, and the βcat average intensity of a transfected cell was normalized to the mean βcat intensity of two or three adjacent untransfected cells. APC:Axin complex area and volume quantification were measured as described (Pronobis et al., 2015) using the ImageJ Particle Analyzer and Imaris 5.5.
FRAP
FRAP was conducted using an Eclipse TE2000-E microscope (Nikon) 24–72 h after transfection. Movies were taken at 1 frame/6 s for 20 min, and bleaching was conducted for 8 s with 100% laser power. Movies were processed using the FRAP analyzer in ImageJ and GraphPad as described previously (Pronobis et al., 2015).
Reporter gene assay for Wnt-regulated transcription
The TOP/FOPflash luciferase and pRL Renilla constructs (transfection control) were gifts from Hans Clevers (Hubrecht Institute, Utrecht, Netherlands). The Dual Glow Luciferase System (Promega, Madison, WI) was used for reporter assays, following the manufacturer’s instructions, as previously described (Roberts et al., 2011). Transcriptional activity was defined as the ratio of TOPflash normalized to Renilla. None of the constructs affected FOPflash values.
Protein work
Coimmunoprecipitations were conducted as described previously (Pronobis et al., 2015). For immunoblots, cells were lysed in 2× SDS buffer and incubated at 96°C for 10 min. Proteins were run on 8 or 7% SDS gels and blotted to nitrocellulose membrane. Primary antibodies were GFP (1:10,000; Abcam), γ-tubulin (1:5000; Sigma-Aldrich), tagRFP (1:5000; Evrogen), and aPKCγ (1:2000; Santa Cruz Biotechnology). Secondary antibodies were horseradish peroxidase anti-mouse and anti-rabbit (1:50,000; Pierce).
Generating a stable SW480 cell line in which the endogenous truncated APC1 is knocked down
Lentiviral vectors expressing shRNAs under U6 promoters (pLVSIN-U6-pur) were generated by substituting the EF1α promoter of pLVSIN-EF1α Pur vector (TaKaRa, Japan) for the U6 promoter from the pSIREN-RetroQ vector (TaKaRa, Japan). The pLVSIN-U6-bsr vector was generated by substituting the puromycin (pur) resistance gene of the pLVSIN-U6-pur vector for the blasticidin (bsr) resistance gene. The target sequence of shRNA against human APC was 5’-GGAGAAATCAACATGGCAACT-3’, and thus the 66-nucleotide oligonucleotide 5’-gggagaagtcagcatggtaactgtgtgctgtccagttgccatgttgatttctccctttttaagctt-3’ was inserted into pLVSIN-U6-bsr vectors to express shRNA in cells. Lentivirus was generated in HEK293T(Lenti-X) cells (TaKaRa, Japan) using the Lentiviral High Titer Packaging Mix (TaKaRa, Japan). SW480 cells were infected for 24 h with virus in the presence of 10 mg/ml Polybrene (Santa Cruz Biotechnology), washed, and allowed to recover for 48 h before selection with 20 μg/ml puromycin (InvivoGen). Knockdown was verified by immunoblotting. The APC KD SW480 cells were frozen after the virus particles were completely removed.
Supplementary Material
Acknowledgments
We thank M. Price for advice on three-dimensional imaging, T. Perdue for help with the Nikon-SIM superresolution microscopy, M. Tegowski for helping to define the essential regions in Axin, and B. Duronio, B. Major, V. Bautch, S. Rogers, K. Slep, K. Schaefer, and D. McKay for helpful advice and comments. This work was supported by National Institutes of Health Grants RO1 GM67236 and R35 GM118096 to M.P. M.I.P. was supported by a Howard Hughes Medical Institute International Student Research Fellowship, N.D. by a William W. and Ida W. Taylor Summer Undergraduate Research Fellowship, and Y.M.-K. by the Japan Society for the Promotion of Science–NEXT program LS128, the Takeda Science Foundation, and an intramural grant from the RIKEN Center for Life Science Technologies.
Abbreviations used:
- APC
adenomatous polyposis coli
- aPKC
atypical protein kinase C
- Arm rpts
armadillo repeats
- ASAD
APC Self-Association Domain
- B
conserved region B
- ßcat
beta-catenin
- CID
catenin inhibitory domain
- CK1
casein kinase 1
- DIX domain
Dishevelled/Axin domain
- GFP
green fluorescent protein
- GSK3
glycogen synthase kinase 3
- LEF
lymphoid enhancer factor
- R2
20–amino acid repeat 2
- RFP
red fluorescent protein
- 20RX
20–amino acid repeat X
- SCF
Skp1-Cullin1-Fbox complex
- shRNA
short hairpin RNA
- TCF
T-cell factor.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E16-07-0557) on November 16, 2016.
REFERENCES
- Ahmed Y, Nouri A, Wieschaus E. Drosophila Apc1 and Apc2 regulate Wingless transduction throughout development. Development. 2002;129:1751–1762. doi: 10.1242/dev.129.7.1751. [DOI] [PubMed] [Google Scholar]
- Akong K, Grevengoed E, Price M, McCartney B, Hayden M, DeNofrio J, Peifer M. Drosophila APC2 and APC1 play overlapping roles in wingless signaling in the embryo and imaginal discs. Dev Biol. 2002;250:91–100. doi: 10.1006/dbio.2002.0776. [DOI] [PubMed] [Google Scholar]
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Julicher F, Hyman AA. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–1732. doi: 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Peifer M. Wnt signaling from development to disease: insights from model systems. Cold Spring Harb Perspect Biol. 2009;1:a002881. doi: 10.1101/cshperspect.a002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Estarás C, Moresco JJ, Yates JR, Jones KA. α-Catenin interacts with APC to regulate β-catenin proteolysis and transcriptional repression of Wnt target genes. Genes Dev. 2013;27:2473–2488. doi: 10.1101/gad.229062.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Cong F, Varmus H. Nuclear-cytoplasmic shuttling of Axin regulates subcellular localization of beta-catenin. Proc Natl Acad Sci USA. 2004;101:2882–2887. doi: 10.1073/pnas.0307344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida S, Yamamoto H, Hino SI, Ikeda S, Kishida M, Kikuchi A. DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol Cell Biol. 1999;19:4414–4422. doi: 10.1128/mcb.19.6.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler EM, Chandra SH, Behrens J, Schneikert J. Beta-catenin degradation mediated by the CID domain of APC provides a model for the selection of APC mutations in colorectal, desmoid and duodenal tumours. Hum Mol Genet. 2009;18:213–226. doi: 10.1093/hmg/ddn338. [DOI] [PubMed] [Google Scholar]
- Kunttas-Tatli E, Roberts DM, McCartney BM. Self-association of the APC tumor suppressor is required for the assembly, stability, and activity of the Wnt signaling destruction complex. Mol Biol Cell. 2014;25:3424–3436. doi: 10.1091/mbc.E14-04-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunttas-Tatli E, Zhou MN, Zimmerman S, Molinar O, Zhouzheng F, Carter K, Kapur M, Cheatle A, Decal R, McCartney BM. Destruction complex function in the Wnt signaling pathway of Drosophila requires multiple interactions between Adenomatous polyposis coli 2 and Armadillo. Genetics. 2012;190:1059–1075. doi: 10.1534/genetics.111.133280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Salic A, Kruger R, Heinrich R, Kirschner MW. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1:E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li PL, Banjade S, Cheng HC, Kim S, Chen B, Guo L, Llaguno M, Hollingsworth JV, King DS, Banani SF, et al. Phase transitions in the assembly of multivalent signalling proteins. Nature. 2012a;483:336–U129. doi: 10.1038/nature10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP, Mohammed S, Heck AJ, Maurice MM, Mahmoudi T, Clevers H. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell. 2012b;149:1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Liu J, Xing Y, Hinds TR, Zheng J, Xu W. The third 20 amino acid repeat is the tightest binding site of APC for beta-catenin. J Mol Biol. 2006;360:133–144. doi: 10.1016/j.jmb.2006.04.064. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, He X. A finger on the pulse of Wnt receptor signaling. Cell Res. 2012;22:1410–1412. doi: 10.1038/cr.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney BM, Price MH, Webb RL, Hayden MA, Holot LM, Zhou M, Bejsovec A, Peifer M. Testing hypotheses for the functions of APC family proteins using null and truncation alleles in Drosophila. Development. 2006;133:2407–2418. doi: 10.1242/dev.02398. [DOI] [PubMed] [Google Scholar]
- Mendoza-Topaz C, Mieszczanek J, Bienz M. The APC tumour suppressor is essential for Axin complex assembly and function, and opposes Axin’s interaction with Dishevelled. Open Biol. 2011;1:110013. doi: 10.1098/rsob.110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Hamada F, Ishidate T, Anai K, Kawahara K, Toyoshima K, Akiyama T. Axin, an inhibitor of the Wnt signalling pathway, interacts with beta- catenin, GSK-3beta and APC and reduces the beta-catenin level. Genes Cells. 1998;3:395–403. doi: 10.1046/j.1365-2443.1998.00198.x. [DOI] [PubMed] [Google Scholar]
- Näthke I. Cytoskeleton out of the cupboard: colon cancer and cytoskeletal changes induced by loss of APC. Nat Rev Cancer. 2006;6:967–974. doi: 10.1038/nrc2010. [DOI] [PubMed] [Google Scholar]
- Neufeld KL, Nix DA, Bogerd H, Kang Y, Beckerle MC, Cullen BR, White RL. Adenomatous polyposis coli protein contains two nuclear export signals and shuttles between the nucleus and cytoplasm. Proc Natl Acad Sci USA. 2000;97:12085–12090. doi: 10.1073/pnas.220401797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson-Nedry W, Erdeniz N, Kremer S, Yu J, Baig-Lewis S, Wehrli M. Unexpectedly robust assembly of the Axin destruction complex regulates Wnt/Wg signaling in Drosophila as revealed by analysis in vivo. Dev Biol. 2008;320:226–241. doi: 10.1016/j.ydbio.2008.05.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronobis MI, Deuitch N, Peifer M. The Miraprep: a protocol that uses a Miniprep kit and provides Maxiprep yields. PLoS One. 2016;11:e0160509. doi: 10.1371/journal.pone.0160509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronobis MI, Rusan NM, Peifer M. A novel GSK3-regulated APC:Axin interaction regulates Wnt signaling by driving a catalytic cycle of efficient betacatenin destruction. Elife. 2015;4:e08022. doi: 10.7554/eLife.08022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DM, Pronobis MI, Poulton JS, Kane EG, Peifer M. Regulation of Wnt signaling by the tumor suppressor APC does not require ability to enter the nucleus nor a particular cytoplasmic localization. Mol Biol Cell. 2012;23:2041–2056. doi: 10.1091/mbc.E11-11-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DM, Pronobis MI, Poulton JS, Waldmann JD, Stephenson EM, Hanna S, Peifer M. Deconstructing the beta-catenin destruction complex: mechanistic roles for the tumor suppressor APC in regulating Wnt signaling. Mol Biol Cell. 2011;22:1845–1863. doi: 10.1091/mbc.E10-11-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Munemitsu S, Polakis P. Loss of ß-catenin regulation by the apc tumor suppressor protein correlates with loss of structure due to common somatic mutations of the gene. Cancer Res. 1997;57:4624–4630. [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Müller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- Salahshor S, Woodgett JR. The links between axin and carcinogenesis. J Clin Pathol. 2005;58:225–236. doi: 10.1136/jcp.2003.009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salic A, Lee E, Mayer L, Kirschner MW. Control of beta-catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Mol Cell. 2000;5:523–532. doi: 10.1016/s1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Fiedler M, Shibata N, Butler PJ, Kikuchi A, Higuchi Y, Bienz M. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Biol. 2007;14:484–492. doi: 10.1038/nsmb1247. [DOI] [PubMed] [Google Scholar]
- Spink KE, Polakis P, Weis WI. Structural basis of the Axin-adenomatous polyposis coli interaction. EMBO J. 2000;19:2270–2279. doi: 10.1093/emboj/19.10.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamos JL, Weis WI. The beta-catenin destruction complex. Cold Spring Harb Perspect Biol. 2013;5:a007898. doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Fu C, Ishikawa S, Stella A, Kojima M, Shitoh K, Schreiber EM, Day BW, Liu B. APC is essential for targeting phosphorylated beta-catenin to the SCFbeta-TrCP ubiquitin ligase. Mol Cell. 2008;32:652–661. doi: 10.1016/j.molcel.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Toretsky JA, Wright PE. Assemblages: functional units formed by cellular phase separation. J Cell Biol. 2014;206:579–588. doi: 10.1083/jcb.201404124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Logan CY, Arora A, Fish M, Nusse R. A Drosophila Axin homolog, Daxin, inhibits Wnt signaling. Development. 1999;126:4165–4173. doi: 10.1242/dev.126.18.4165. [DOI] [PubMed] [Google Scholar]
- Yamulla RJ, Kane EG, Moody AE, Politi KA, Lock NE, Foley AV, Roberts DM. Testing models of the APC tumor suppressor/beta-catenin interaction reshapes our view of the destruction complex in Wnt signaling. Genetics. 2014;197:1285–1302. doi: 10.1534/genetics.114.166496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.