Abstract
We review behavioural change models (BCMs) for infectious disease transmission in humans. Following the Cochrane collaboration guidelines and the PRISMA statement, our systematic search and selection yielded 178 papers covering the period 2010–2015. We observe an increasing trend in published BCMs, frequently coupled to (re)emergence events, and propose a categorization by distinguishing how information translates into preventive actions. Behaviour is usually captured by introducing information as a dynamic parameter (76/178) or by introducing an economic objective function, either with (26/178) or without (37/178) imitation. Approaches using information thresholds (29/178) and exogenous behaviour formation (16/178) are also popular. We further classify according to disease, prevention measure, transmission model (with 81/178 population, 6/178 metapopulation and 91/178 individual-level models) and the way prevention impacts transmission. We highlight the minority (15%) of studies that use any real-life data for parametrization or validation and note that BCMs increasingly use social media data and generally incorporate multiple sources of information (16/178), multiple types of information (17/178) or both (9/178). We conclude that individual-level models are increasingly used and useful to model behaviour changes. Despite recent advancements, we remain concerned that most models are purely theoretical and lack representative data and a validation process.
Keywords: behaviour, model, infectious disease, vaccination, game theory, individual-based
1. Introduction
Infectious diseases can have a large impact on society as they can negatively affect, among others, morbidity, mortality, unemployment and inequality. As a result, prevention and control of infectious diseases are important for public health and welfare.
The main objective of infectious disease transmission models is to inform and guide policy-makers to prepare for and respond to (re)emerging infectious diseases, particularly when sufficient information from controlled experiments is lacking. However, the impact of infectious disease transmission and policy interventions are subject to hosts' behaviour. Therefore, there is an interest to incorporate behaviour change in response to disease-related information into models for infectious disease transmission.
Numerous historical infectious disease experiences confirm the existence of a so-called behavioural immune system [1] in humans. For example, during the 2003, severe acute respiratory syndrome (SARS) outbreak people took precautionary actions such as wearing face masks, hand-washing, avoiding public transport, restaurants, shops and other crowded places in Hong Kong [2,3] and Beijing [4]. In addition, the 2009 A/H1N1 influenza pandemic has triggered a significant proportion of the population to adapt their behaviour and take preventive measures such as social distancing [5,6].
We refer to models incorporating behavioural immunity as ‘behavioural change models’ (BCMs), which typically complement models for disease transmission in an attempt to mimic real life dynamics. In essence, a BCM is a model in which individuals are responsive to external information about the disease and as a result take one or more preventive measures to reduce the chance of contracting the disease. The external information individuals respond to can be global (equally available and relevant to all individuals) or local (individual availability and relevance determined by physical or social proximity to the information source). Furthermore, this information can be specified in terms of actual risks (‘prevalence-based’) or of perceptions of these risks (‘belief-based’), as well as a mixture of all the above [7]. Vaccination is a common prevention measure with varying uptake, given historical fluctuations in the trade-off between the perceived risks of vaccine-related side effects (VRSEs) and of vaccine-preventable disease. Other common prevention measures include social distancing and condom use.
A widely used theoretical foundation for the formation and dynamic nature of individuals' behaviour comes from game theory. Game theory has a rich history in social sciences with the Prisoner's Dilemma being a frequently used illustration (see [8] for a comprehensive introduction). Game theory assumes individuals take rational decisions based on a trade-off that embodies the anticipated rational decisions of all other individuals in society. Even though these assumptions are often not observed in real life [9], a multitude of BCMs in the setting of infectious disease transmission still use a game-theoretical foundation that caused the development of, for instance, ‘vaccination games’ [10] and ‘epidemic games with social distancing’ [11].
Another foundation for behaviour change is found in the fields of network science and individual-based modelling (IBM), where there are opportunities to develop more realistic models by introducing (more) heterogeneity. The challenge here is to find a balance between model complexity and computational boundaries. Some examples of behavioural change research for which network science has been used include models using adaptive contact networks [12], vaccinating behaviour in social contact networks [13] and social distancing in sexual contact networks [14].
Although there is increased recognition for the need to incorporate behavioural changes in infectious disease transmission models, a consensus on the proper methodology to do so is lacking. It appears much research is not supported by empirical information but departs from a theoretical foundation with arbitrarily chosen parameter values and no validation process. As a result, there is large heterogeneity in the triggers for behavioural change and the impact on disease transmission, as well as the conclusions of such studies. There is a need for empirical data from, for instance, surveys or discrete choice experiments to support the validity of these models and to guide further research [7,15].
The main goal of this paper is to systematically review and document how and to which extent behavioural immunity has been explored in infectious disease transmission models over the past 5 years. In brief, we aim to investigate to which extent: (i) technological advancements and increased data availability have enriched BCMs, (ii) the literature has coped with the fact that behavioural immunity is often contingent on the disease and not coupled to disease dynamics, (iii) modelling efforts are validated with quantifiable observations and parametrized, (iv) the current models have assessed the importance of social networks in individual decisions, (v) the process of transferring information to behaviour is managed and (vi) irrational behaviour is demonstrated.
In the following sections, we systematically identify and analyse BCMs applied to infectious disease transmission, starting from where a previous review in 2010 left off [7]. These models are categorized in order to distinguish their assumptions, methods, disease and transmission-specific applications and implications. Furthermore, a critical point of view is taken when evaluating these models in terms of their real-life applicability. Current pitfalls and opportunities are identified to support the development of more advanced BCMs in the near future.
2. Methods
The strategy and reporting in this review are based on Cochrane guidelines for systematic reviews of intervention [16] and the PRISMA statement [17]. The eligibility criteria and the search query were determined by consensus between all authors, covering expertise in infectious disease modelling and economics.
2.1. Search
We searched PubMed and Web of Science (WoS) for records published between January 2010 and December 2015. After discussing and defining the inclusion and exclusion criteria, we obtained our final search query which we used in PubMed on 12 January 2016 and in WoS on 13 January 2016: ‘(behavio* OR decision*) AND (change* OR influence* OR dynamic* OR adapta* OR adapt OR adaptive OR strategic*) AND (infect* OR epidemic OR epidemics OR epidemiology OR epidemiological OR epidemiologic OR pandem* OR outbreak*) AND (disease* OR vaccin*) AND (model OR models OR modelling OR modeling OR simulat* OR transmission*)’.
2.2. Selection
In a first step, F.V. screened the results of the search query based on title and abstract in accordance with the following pre-specified eligibility criteria:
Infectious diseases. Only records that concern infectious diseases are included in the selection. Infectious diseases are defined using the WHO definition: infectious diseases are caused by pathogenic microorganisms, such as bacteria, viruses, parasites or fungi; the diseases can be spread, directly or indirectly, from one person to another [18].
Model. Records should consist of a mathematical model for behavioural change, for infectious disease transmission or a coupled model combining these two.
Individual behaviour. Behaviour is considered the consequence of personal and voluntary choices made by an individual, i.e. we exclude studies tackling forced interventions such as school closure or mandatory vaccination, but include government interventions creating awareness, education in prevention, etc.
External trigger(s). At least one trigger for modelled individuals to change their behaviour is external and has to be related to infectious disease. We exclude models with exclusively intrinsic triggers from the selection (e.g. an individual's own human immunodeficiency virus (HIV) status).
Preventive measure. A preventive measure is central to the analysis (e.g. vaccination or social distancing). The behaviour of the individual is defined by the decision (not) to take preventive measures.
Humans. We are interested in diseases in humans and behaviour of humans regarding these diseases, and therefore exclude research on plants, animals, the behaviour of the model itself or the behaviour of governments or institutions.
Original research. We exclude review articles, letters, editorials and comments.
English language. Excluding articles written in other languages.
In a second step, the remaining articles' full texts were screened to confirm eligibility, independently by F.V. and L.W. Whenever there was doubt about eligibility, agreement was sought through discussion.
2.3. Data extraction
Using a common data extraction protocol for each eligible article, F.V. and L.W. independently retrieved from the full text: (i) infectious disease; (ii) disease category (sexually transmitted infection (STI), influenza-like illness (ILI), childhood disease, vector-borne disease (VBD) or other); (iii) prevention measure (vaccination, social distancing etc.); (iv) source of information (global, local or multiple); (v) type of information (prevalence-based, belief-based or multiple); (vi) effect on the model (disease state, model parameters, contact structure or multiple); (vii) disease transmission model description; (viii) BCM description; (ix) whether there was interaction between the behaviour and disease transmission model; (x) whether the analysis incorporated real-life data; and (xi) movement of individuals in the model. When applicable, other interesting information was extracted using free form fields. Again, discrepancies in interpretation were resolved through discussion.
3. Results
3.1. Search results
Our search query resulted in 7193 records from Web of Science and PubMed (figure 1). We identified and removed 1434 duplicates, resulting in 5759 unique records that were screened based on title, abstract, keywords and full-text if necessary. Exclusions were mostly related to (i) topic, including the study of non-infectious diseases or infections in animals, plants and crops; (ii) discipline, including microbiological and clinical trial studies, and to a lesser extent to (iii) language and article type. Eventually, 178 articles were included for full-text analysis.
Figure 1.
PRISMA flow diagram. (Online version in colour.)
The number of articles matching our eligibility criteria increased from 18 in 2010 to 38 in 2015, but there was a single year downward deviation from the trend in 2014 (with 22 eligible studies; figure 2). Compared with Funk et al. [7], we observe a marked increase in BCM publications. Over the 9 year period between 2002 and 2010, Funk et al.'s search yielded 27 eligible articles (i.e. about 15% of our yield over 6 years), but their search and selection procedure lacks transparency to compare these results in depth.
Figure 2.
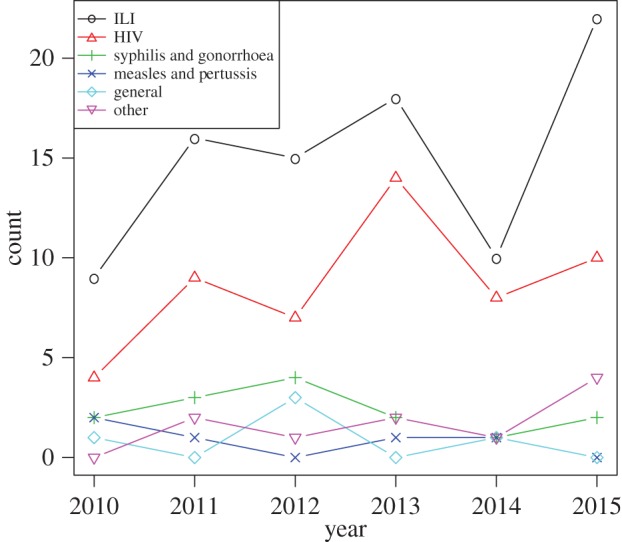
Number of studies over time.
Models applied to influenza or ILI stand out, together with ‘general’ models. In the latter category, a hypothetical infectious disease is modelled, without specification of which disease (but often including optimistic statements about the generalizability of the application).
3.2. Model structure categories
In table 1, we categorized the studies according to disease, prevention measure (topic) and whether the model is implemented at the population level or at the individual-level (i.e. using an IBM or contact network) to simulate infectious disease transmission. Metapopulation models for disease transmission were also identified and are labelled in bold. Furthermore, the columns indicate at which level the impact of prevention measures is modelled, distinguishing whether behavioural change is implemented through a switch in infectious disease state (e.g. vaccination immunizes previously susceptible persons, and this can be modelled by moving them from the susceptible to the immune state), a change in model parameters (e.g. hygiene measures may be assumed to reduce the effectiveness of transmission) or in social contact structure (e.g. social distancing may be mimicked by a link-breaking or rewiring process between susceptible and infectious individuals in contact networks). Studies can appear in multiple categories, as some have multiple prevention strategies or multiple effects on the disease transmission model. For the transmission model category, we interpreted to which extent heterogeneity is introduced in the model. All references are categorized and represented in a spreadsheet that can be found as electronic supplementary material. The model type is often disease-dependent. For instance, all retrieved models for measles and/or pertussis are population models with vaccination as a preventive measure that affects the disease state in the transmission model. Moreover, the models are often prevention-dependent. We observe that most of the models that use vaccination as a prevention strategy will impact the model through a switch in disease state. For instance, in many compartmental susceptible–infectious–recovered (SIR) disease models, vaccinated individuals move to the R compartment. General models with social distancing as a prevention strategy usually impact the model in terms of a modified contact structure, contingent on the disease transmission model. Whereas for influenza applications, this only applies for one out of seven references.
Table 1.
Model structure categories. References in bold represent metapopulation models. References with author names in italics represent references that use empirical data for parametrization and/or validation. PrEP, pre-exposure prohylaxis. References in category ‘Other’ specify a disease other than the above. Hygiene measures include face-mask use, increased hand washing, etc. For more information, see online appendix.
| population level |
network/IBM |
|||||
|---|---|---|---|---|---|---|
| disease | prevention measure | infectious disease state | model parameters | infectious disease state | model parameters | contact structure |
| influenza/ILI | vaccination | Bhattacharyya & Bauch [19], Liu et al. [20], Breban [21], Laguzet & Turinici [22], Shim et al. [23], Cohen et al. [24], Xia & Liu [25] | Vardavas et al. [26] | Liu et al. [20], Cardillo et al. [27], Wu & Zhang [28], Zhang [29], Han & Sun [30], Fukuda et al. [31], Cornforth et al. [32], Fu et al. [33], Loganathan et al. [34], Wells & Bauch [35], Zhang et al. [36], Wells et al. [37], Xia & Liu [38], Fukuda et al. [39], Liao & You [40], Andrews & Bauch [41] | Marathe et al. [42], Mei et al. [43], Zhang et al. [44], Karimi et al. [45] | — |
| Social Distancing | Poletti et al. [46], Mummert & Weiss [47], Zhong et al. [48] | Larson & Nigmatulina [49], Wang [50], Greer [51], Morin et al. [52], Liu et al. [53], Poletti et al. [54], He et al. [55], Springborn et al. [56] | — | Mei et al. [43], Karimi et al. [45], Barrett et al. [57,58], Wang et al. [59], Bayham et al. [60] | Chen et al. [61] | |
| PrEP antivirals | — | — | Liao & You [40] | Liao & You [40], Marathe et al. [42], Mei et al. [43], Barrett et al. [58], Chen et al. [61], Mao & Bian [62], Mao & Yang [63], Andrews & Bauch [41] | — | |
| hygiene measures | — | Larson & Nigmatulina [49], Parikh et al. [64], Poletti et al. [54] | — | Mei et al. [43], Wang et al. [59], Parikh et al. [64] | — | |
| other and general | — | Xiao et al. [65], Pawelek et al. [66] | Liao et al. [67], Collinson et al. [68] | Zhang et al. [69], Fierro & Liccardo [70] | — | |
| HIV | condom use | — | Nyabadza et al. [71], Johnson et al. [72] | — | Vieira et al. [73] | — |
| reduce sexual risk | — | — | Alimadad et al. [74] | Tully et al. [75,76] | — | |
| social distancing | — | Nyabadza et al. [71,77], Reniers & Armbruster [78], Reluga & Li [79], Viljoen et al. [80] | — | Tully et al. [75] | — | |
| other and general | Kassa & Ouhinou [81,82] | — | — | Marshall et al. [83] | — | |
| syphilis and gonorrhoea | condom use | — | Morin et al. [84] | — | Gray et al. [85] | — |
| vaccination | — | Milner & Zhao [86] | — | — | — | |
| reduce sexual risk | — | Milner & Zhao [86] | — | — | — | |
| social distancing | — | Aadland et al. [87] | — | Gray et al. [85] | Althouse & Hébert-Dufresne [88] | |
| measles and pertussis | vaccination | Bhattacharyya & Bauch [89], Shim et al. [90], Bauch & Bhattacharyya [91], d'Onofrio et al. [92], Oraby et al. [93] | — | — | — | — |
| other | social distancing | Perra et al. [94], Sharma & Misra [95], Greenhalgh et al. [96] | Cooper et al. [97], Del Valle et al. [98], Wang et al. [99] | — | Fast et al. [100] | Robinson et al. [14] |
| vaccination | Sharma & Misra [95], Sykes & Rychtar [101] | — | — | — | — | |
| other and general | Misra et al. [102] | Cooper et al. [97] | Williams et al. [103] | — | — | |
| general models | social distancing | Funk et al. [104], Misra et al. [105], Samanta et al. [106], Zuo & Liu [107], Misra et al. [108], Wang et al. [109] | Reluga [110], Brauer [111], Fenichel et al. [112], Buonomo et al. [113], Xiao et al. [114], Liu [115], Wang et al. [116], Li et al. [117], Morin et al. [118], Li et al. [119], Liu et al. [120], Lubuma & Terefe [121] | Sahneh & Scoglio [122] | Wu et al. [123], Wang et al. [124], Cao [125], Zhang et al. [126], Schumm et al. [127], Meloni et al. [128], Sun et al. [129], Bichara et al. [130], Liu & Zheng [131] | Van Segbroeck et al. [12], Shaw & Schwartz [132], Jolad et al. [133], Maharaj & Kleczkowski [134], Rogers et al. [135], Valdez et al. [136], Zhang et al. [137], Juher et al. [138], Liu et al. [139], Noble et al. [140], Valdez et al. [141], Tunc & Shaw [142], Dong et al. [143], Szabo-Solticzky et al. [144], Nicolaides et al. [145], Maharaj et al. [146] |
| vaccination | Bauch et al. [147], d'Onofrio & Manfredi [148], Buonomo et al. [149], d'Onofrio et al. [150], Reluga & Galvani [151], Wu et al. [152], Buonomo et al. [153], Xu & Cressman [154], Bhattacharyya et al. [155], Oraby & Bauch [156], Voinson et al. [157] | Barbagallo & Cojocaru [10] | Mbah et al. [13], Dong et al. [143], Schimit & Monteiro [158], Wells et al. [159], Morsky & Bauch [160], Ruan et al. [161], Campbell & Salathe [162], Wu et al. [163], Cai et al. [164], Wu et al. [165], Han et al. [166], Helbing et al. [167], Liang & Juang [168] | Zhang et al. [169] | — | |
| hygiene measures | Funk et al. [104] | — | — | — | — | |
| other and general | Joshi et al. [170] | Reluga [11], Liu & Stechlinski [171], Samanta & Chattopadhyay [172], Sega et al. [173] | Hatzopoulos et al. [174], Sahneh & Scoglio [175], Sahneh et al. [176], Miller [177], Yuan et al. [178], Goyal & Vigier [179], Guo et al. [180], Juher et al. [181], Liu et al. [182], Chen et al. [183] | Goyal & Vigier [179], Kitchovitch and Lio [184,185], Ni et al. [186], Rutherford et al. [187], Shang [188,189] | — | |
3.3. Prevention measures
Most of the eligible articles use models with vaccination or social distancing as a prevention measure, though other strategies have been considered. The choice of prevention measure naturally depends on the disease under study. For instance, the discovery and implementation of antivirals as a prophylactic for influenza and HIV has resulted in the publication of models with pre-exposure antiviral use as individual behaviour. A minority of models does not specify the preventive action taken by individuals. When an effect on the contact rate was mentioned, we assumed that the preventive action was social distancing. It appears some authors use the term ‘social distancing’ as a synonym for all non-pharmaceutical interventions (NPIs) [11]. In this review, social distancing is interpreted as reducing physical (or sexual) contacts between individuals and their environment.
3.4. Diseases
In table 1, we classified the records based on four specific disease categories, one category for general models (not specifying a disease) and one category for other diseases. Most models retrieved were on influenza or influenza-like illness (ILI) and HIV. Other frequent diseases studied with BCMs are ‘measles & pertussis’ and ‘syphilis & gonorrhoea’. Historically, perceptions of high risks, associated with measles and pertussis vaccination, have adversely affected the uptake of these vaccines. As a result, these are topical applications for transmission models incorporating behavioural changes, as discussed in [19]. The literature on measles is becoming more diverse as VRSE perceptions evolve; Bhattacharyya & Bauch [89] describe a model in which parents delay vaccinating their children as a result of an exogenous vaccine scare, whereas the same authors use social networks of imitation behaviour for VRSE perception spread in response to a vaccine scare [91], and d'Onofrio et al. [92] introduce public interventions in their model to increase vaccine uptake. Diseases in the ‘other’ category are: SARS, smallpox-like disease, malaria, hepatitis B, Ebola, pneumococcus, pneumonic plague, toxoplasmosis and cholera. General models do not explicitly specify a disease, often assuming general applicability. As noted earlier, models tend to be disease-specific. In the case of influenza or influenza-like illness, some models look at seasonal changes in behaviour with backward looking individuals evaluating the success of their (vaccination or social distancing) strategy during previous season(s) [20,27–31,69]. HIV BCMs are often coupled with a public health information/education campaign aimed at evaluating public health measures to control epidemic spread or to study the cost-effectiveness of these control measures [71,72,77,81,83]. An example of a more advanced, game-theoretic model is the model by Tully et al. [75]. They use an agent-based model (ABM) for the spread of risk perception, sexual behaviour and HIV transmission in the context of individual sexual encounters evaluating the behaviour of (potential) partners.
3.5. Emergence-driven research
Between 2010 and 2015, much research has been emergence-driven. That is, the research field responds by focusing on diseases that are of major interest because of a change in the threat they present to public health. The influenza A/H1N1 pandemic of 2009 has largely influenced the development of BCMs for influenza. For example, Poletti et al. [54] use the influenza A/H1N1 pandemic of 2009/2010 to parametrize an influenza transmission model with behavioural changes focusing on the spread of risk perceptions in the population. In addition, a model on Ebola virus disease (EVD) was published in 2015 in response to the epidemic outbreak in Liberia [100]. The authors use WHO and CDC data to parametrize the model suggested in an attempt to mimic disease transmission and to identify behavioural changes as drivers of the disease dynamics. Note that, in the current review, we relate ‘emergence’ not only to disease emergence, but also the emergence of a vaccine scare (such as observed with measles–mumps–rubella (MMR) vaccination and pertussis whole-cell vaccination [91]) or the emergence of new therapies for endemic diseases (such as the development of a multi-season influenza vaccine [26]).
3.6. Disease transmission models
We identify three major categories of models: population-level models, metapopulation and individual-level models. Population-level models traditionally formulate compartments according to health state (e.g. susceptible, infectious and recovered) and simulate transitions between the compartments over time using population averages. These models are often based on the mass-action principle to designate the transmission probability. Each individual has an equal probability of contracting disease given the disease state levels in the population. Metapopulation models split the population into different subpopulations with their own (spatial) general characteristics and disease-related parameters. The individual-level category consists of network models and IBMs. Network models represent disease transmission on a network where nodes (individuals) are connected to each other using links. This allows to model individuals with different degrees, representing how many links a node has (i.e. number of neighbours/direct contacts). IBMs or ABMs typically incorporate more heterogeneity and stochasticity on individuals' characteristics such as spatial location, age, gender, sexual orientation, etc. The model selection depends on disease characteristics, data availability, modelling purpose (i.e. what outcome figures are you interested in?), computational resources, etc.
Individual-level models are gaining interest in the BCM literature since they can introduce heterogeneity in behaviours, tackle clustering of vaccine sentiments and look at stochastic and local outbreaks of infectious diseases with a high vaccination coverage (e.g. measles). Moreover, given an underlying contact structure, individual-level models are well suited to model social distancing behaviour in terms of reduced contacts as a prevention strategy. Remarkably, for measles and pertussis we found deterministic models only, despite the widely acknowledged stochastic nature of outbreaks in highly vaccinated populations. Note that, in table 1, we also made a distinction between individual-level and population-level models in the category ‘disease transmission model’. Metapopulation models are displayed in bold.
3.7. Information gathering
In order for individuals to change their behaviour in relation to prevention measures, they require disease-related information. As defined in the eligibility criteria, we only included papers in which this information is external to the individual. Examples of disease-related, external information include: news broadcasts on a disease outbreak or rumours among friends and family about VRSEs or vaccine-preventable disease. Funk et al. [7] proposed a classification based on type and source of information, distinguishing global and local information as source and prevalence-based and belief-based information as type of information. Global information is defined as information available to all individuals in the population, for example, TV stations and public health campaigns. Local information is information individuals gather from their direct contacts or neighbourhood. Examples are rumours from neighbours or infective individuals in their close contacts. Prevalence-based information is defined as ‘directly relating to disease prevalence’, whereas belief-based information is ‘not directly relating to disease prevalence’. Belief-based information can therefore have its own dynamics, to some extent independent of the disease dynamics. For example, rumours can inflate the perception of disease prevalence, even if the true prevalence is low. In table 2, we classify the studies we identified in a matrix, using the same definitions.
Table 2.
Information gathering.
| type of information |
|||
|---|---|---|---|
| source of information | belief-based | prevalence-based | multiple |
| local | Barbagallo & Cojocaru [10], Mbah et al. [13], Liu et al. [20], Cardillo et al. [27], Wu & Zhang [28], Zhang et al. [69], Zhang [29], Han & Sun [30], Fukuda et al. [31], Xia & Liu [25,38], Wang et al. [59], Alimadad et al. [74], Tully et al. [76], Funk et al. [104] | Van Segbroeck et al. [12], Fu et al. [33], Fukuda et al. [39], Reniers & Armbruster [78], Althouse & Hébert-Dufresne [88], Sahneh & Scoglio [122,175], Cao [125], Zhang et al. [126], Schumm et al. [127], Shaw & Schwartz [132], Rogers et al. [135], Valdez et al. [136], Juher et al. [138], Noble et al. [140], Valdez et al. [141], Tunc & Shaw [142], Dong et al. [143], Szabo-Solticzky et al. [144], Maharaj et al. [146], Wells et al. [159], Morsky & Bauch [160], Ruan et al. [161], Cai et al. [164], Wu et al. [165], Han et al. [166], Helbing et al. [167], Sahneh et al. [176], Juher et al. [181], Liu et al. [182] | Zhang et al. [36], Wells et al. [37], Andrews & Bauch [41], Mao & Bian [62], Mao & Yang [63], Wu et al. [163], Miller [177], Guo et al. [180], Shang [189] |
| global | Bhattacharyya & Bauch [19,89], Bauch & Bhattacharyya [91], Johnson et al. [72], Marshall et al. [83], Laguzet & Turinici [22], Shim et al. [23], Cornforth et al. [32], Karimi et al. [45], Poletti et al. [46], He et al. [55], Parikh et al. [64], Vieira et al. [73], Milner & Zhao [86], Shim et al. [90], Oraby et al. [93], Cooper et al. [97], Fast et al. [100], Sykes & Rychtar [101], Misra et al. [102], Li et al. [117], Voinson et al. [157], Zhang et al. [169], Joshi et al. [170], Durham & Casman [3], Mannberg [190] | Reluga [11], Nyabadza et al. [71,77], Kassa & Ouhinou [81], Zhang et al. [44], Mummert & Weiss [47], Zhong et al. [48], Wang [50], Greer [51], Morin et al. [52], Liu et al. [53], Poletti et al. [54], Barrett et al. [57], Chen et al. [61], Xiao et al. [65], Pawelek et al. [66], Collinson et al. [68], Reluga & Li [79], Viljoen et al. [80], Morin et al. [84], Aadland et al. [87], Sharma & Misra [95], Greenhalgh et al. [96], Del Valle et al. [98], Wang et al. [99], Misra et al. [105], Samanta et al. [106], Zuo & Liu [107], Misra et al. [108], Wang et al. [109], Reluga [110], Fenichel et al. [112], Buonomo et al. [113], Xiao et al. [114], Liu [115], Wang et al. [116], Morin et al. [118], Li et al. [119], Liu et al. [120], Lubuma & Terefe [121], Wang et al. [124], Meloni et al. [128], Sun et al. [129], Bichara et al. [130], Liu & Zheng [131], Jolad et al. [133], Zhang et al. [137], Liu et al. [139], d'Onofrio & Manfredi [148], Buonomo et al. [149], d'Onofrio et al. [150], Reluga & Galvani [151], Wu et al. [152], Xu & Cressman [154], Liu & Stechlinski [171], Samanta & Chattopadhyay [172], Yuan et al. [178], Goyal & Vigier [179], Chen et al. [183] | Breban [21], Bayham et al. [60], Liao et al. [67], Bauch et al. [147], Buonomo et al. [153], Bhattacharyya et al. [155], Oraby & Bauch [156], Sega et al. [173] |
| multiple | d'Onofrio et al. [92], Cohen et al. [24], Loganathan et al. [34], Nicolaides et al. [145], Campbell & Salathe [162], Ni et al. [186] | Vardavas et al. [26], Marathe et al. [42], Larson & Nigmatulina [49], Barrett et al. [58], Kassa & Ouhinou [82], Maharaj & Kleczkowski [134], Schimit & Monteiro [158], Kitchovitch & Lio [184,185], Shang [188] | Tully et al. [75], Wells et al. [37], Liao & You [40], Mei et al. [43], Fierro & Liccardo [70], Perra et al. [94], Wu et al. [123], Liang & Juang [168], Hatzopoulos et al. [174] |
We observe that most BCMs are using information that is globally available and prevalence-based. These models are frequently game-theoretic (or pay-off maximizing) behavioural change frameworks coupled with disease transmission models at the population level. Studies that met our eligibility criteria, but are unclear about the information individuals use [14,56,85,103,111,187] were excluded from figure 2. Given the increasing individual heterogeneity in disease transmission models, it is becoming more interesting to incorporate local information in BCMs. In network models and IBMs, one could for instance model the local spread of information through direct contacts with crucial implications in terms of clustering of both disease prevalence and opinions [186].
In addition, we observe that more articles are using multiple information types and/or sources, making individual behaviour more realistic. For instance, Barrett et al. [58] constructed a model where ‘individual behaviour is triggered by the prevalence level of the virus in the overall society (global prevalence) as well as within one's own demographic class (local prevalence)’. Highly relevant are articles introducing both multiple sources and multiple types of information such as the bij model, Liang & Juang [168], which introduces different forms of information in the individual's risk perception of an epidemic, embodying all four information categories.
3.8. How is the transfer from information to behaviour managed?
Based on full-text analysis, we extracted how individuals were modelled to translate the information they receive into behavioural change. Traditionally, behaviour formation models were composed of a game-theoretic framework in which individuals have perfect information on disease-related data and prevention effectiveness. Individuals are then assumed to use this information in a utility-maximizing game by comparing the expected costs of infection with the expected costs of the prevention measure. However, more advanced and different BCMs have been developed since. We identified five distinct categories for characterizing the decision-making process of individuals, listed in §§3.8.1–3.8.5. (see also electronic supplementary material, appendix). Some referenced papers contain multiple BCMs.
3.8.1. Exogenous behaviour formation (16/178)
We retrieved 16 papers [14,45,51,56,64,72,73,83–85,97,98,103,111,170,187] describing BCMs in which there is no two-way interaction with a disease transmission model. Morin et al. [84] provide an example of such a model by assessing the impact of policies encouraging condom use, on gonorrhoea transmission dynamics; i.e. behaviour (condom use) is parametrized based on different empirical studies and model projections are made to estimate consequential disease transmission and model equilibria. Similarly, Brauer [111] assessed disease model implications of a constant fractional reduction in the number of contacts. A third example is the model by Joshi et al. [170] where a time-dependent education function moves susceptible individuals into lower susceptibility classes with lower transmission rates, independent of disease dynamics. These models are relatively rare and most often focus on policy implementations and short-term effects of behaviour on disease transmission.
3.8.2. Information threshold (29/178)
We retrieved 29 BCMs in which behaviour change is modelled conditional on exceeding a predefined information threshold [12,42,57,58,61–63,70,78,81,88,114,127,132,133,135,136,138–144,162,163,180–182]. The information the individual assesses can be obtained in a direct way (e.g. through prevalence in neighbours) or in an indirect way (e.g. through rumours or opinions). These models do not elaborate on how behaviour is rationally determined or influenced by relevant factors. Instead, behaviour formation is a result of a predefined threshold function. Examples include switching to social distancing when the number of infectives exceeds a threshold [114], social distancing by rewiring once a non-infected node connects to an infected node [132], and—as in Wu et al. [163]—to have an individual's vaccination decision exercised through a risk function exceeding a threshold, which in turn depends on the number of infected neighbours. Mao & Yang [63] used an individual risk function incorporating the proportion of ‘adopters’ among contacts, the perceived pressure of ‘adoption’ and the proportion of infective neighbours. Again, once the risk function exceeds a threshold, individuals adopt preventive behaviour, which in this case consisted of taking prophylactic antivirals.
3.8.3. Information as dynamic parameter (76/178)
The largest category embodies 76 references managing information as a dynamic parameter [3,25,34,40,43,47–50,53,55,60,61,65–68,70,71,74,77,80,82,86,92,94–96,99,100,102,104–109,113,115–117,119–126,128–131,133,134,137,148,149,153,157,161,165,166,168,171–178,184–186,188,189]. In this category, instead of a threshold, the information is a continuous input in the decision-making process of individuals. At the population level, we can characterize these BCMs as information driving the flow in and out the prevention taking compartment. Two subcategories can be distinguished: models with a direct relation between infectious disease parameters and behaviour formation (i.e. behaviour changes vis-à-vis disease dynamics), and models with an indirect relation, through an information spread medium. For the former subcategory, the behaviour or decision-making process is predefined as a functional relation depending on disease transmission parameters. The functional form does not need to be linear. Some examples are vaccination coverage as a positive decreasing function of perceived risk of VRSE [148], the percentage of the susceptible population engaging in avoidance actions increases as the disease becomes more prevalent [48] and a model where the effective contact rate reduces with the number of infectives [119].
The latter subcategory requires a third-party spreading the information for individuals to receive. For instance through mass media, neighbours, formation of opinions in the population, etc. A multitude of these models introduce an ‘aware’ compartment in the model where aware and unaware individuals are assigned distinct disease transmission parameters such that aware individuals have lower susceptibility of acquiring infection. See for example Funk et al. [104], in which a rate introduces people in an ‘aware’ class after which the awareness spreads through the population, coupling disease transmission with a BCM. Interestingly, some models introduce information spread models with characteristics from disease transmission models where individuals are, for example, susceptible to or infected with disease-related information. Misra et al. [105] use a model with media coverage creating awareness in the population, also introducing an ‘aware’ compartment in a population model. Social impact is introduced in a model by Ni et al. [186], where they use a variety of complex networks for the spread of opinions driving the individual probability of prevention behaviour. The use of a network is convenient to model these dynamics as they allow clustering of, for instance, vaccine-related sentiments in the population. Most often these models assign additional characteristics to nodes (which represent individuals), apart from disease state. An example could be that a node is assigned a disease state and an opinion which is either provaccination or contravaccination. When simulating the disease and behaviour dynamics in this network, when nodes interact, transmission of both disease and opinions can occur. Such that if a provaccine node is surrounded by many vaccine sceptics, it might change its opinion towards the opinions of its links (i.e. neighbours) and as a result this will influence the individual's probability of taking vaccination as a prevention measure.
3.8.4. An economic objective function (37/178)
This ‘economic’ class of BCMs is also quite common with 37 articles being retrieved [10,11,13,19,21–24,26,32,35,41,52,59,75,76,79,87,90,101,110,112,118,128,145–147,151,155,157,158,160,167,169,179,183,190]. This approach assumes individuals take their prevention decision based on an objective function, which they attempt to optimize (i.e. by maximizing benefits and/or minimizing costs). Game theory grounded models form an integral part of this category. By way of example, one can assume that individuals have knowledge about both the disease and their options for prevention and make rational decisions based on this knowledge. People accordingly possess a (perceived) cost of infection (ci) and a (perceived) cost of the prevention measure (cp), which can, for instance, be assumed to be 100% effective. Another important input in people's decision-making, their probability of infection (λ) can be assumed to be dependent on disease prevalence, which evolves over time. For instance, one can define this using an SIR model under the mass action principle as the force of infection, i.e. λ = βI, where β is the per-contact transmission rate, and I is the fraction of infectives in the population. This way the behavioural change framework can be coupled to the disease dynamics. The individual makes the following trade-off, with P, the choice of taking the prevention measure
| 3.1 |
In a study by Bhattacharyya & Bauch [19], individuals take their vaccination decision based on the perceived vaccination cost in the context of the 2009 A/H1N1 influenza pandemic. Their BCM model exhibits a ‘wait and see’ Nash equilibrium where individuals incorporate the concept of herd immunity in their prevention behaviour, resulting in free-riding represented by a ‘delayer’ strategy. The model developed by Morin et al. [52] embodies individuals' behaviour by the maximization of expected utility determined by adapting the contact level (i.e. social distancing). Aadland et al. [87] introduce a BCM maximizing an individual's expected lifetime utility by choosing the number of sexual partners, hereby explaining the re-emergence of syphilis.
3.8.5. An economic objective function with social learning/imitation (26/178)
We retrieved 26 papers [13,20,27–31,33,37–39,44,46,54,69,89,91,93,137,143,150,152,154,156,159,164] describing a BCM with an objective function with imitation. It is recognized that some social or peer influence should be incorporated in the decision-making process of the individuals (see also models with information as a dynamic parameter). As a response to this concern, the (rational) ‘game-theoretic’ model has been adapted to include social influence or imitation behaviour. In these models, it is assumed that people compare their own prevention-related behaviour with that of other individuals in society. Through comparison, individuals learn whether their own behaviour is optimal and, to which extent they should adapt it. Typically, a sampling rate is assumed for individuals sampling other individuals from the population. After sampling an individual from the population, the trade-off is compared and people switch strategies with a probability as a function of the pay-off difference. Often, a Fermi-like function is used, guiding the adoption to the better strategy depending on the magnitude of the pay-off difference. Other switching functions/strategies are used, but naturally, the larger the beneficial pay-off difference, the higher the probability of switching your behaviour. An example of a Fermi function, taken from [31] is given in this section. If we represent the pay-off of the strategies of individual i (with strategy si) and individual j (with strategy sj) as ɛi and ɛj respectively, and the pay-off difference is defined by Δɛij = ɛi − ɛj. Then, the probability of individual i switching to the strategy of individual j is
| 3.2 |
where κ denotes the selection pressure representing the sensitivity of individuals to switch strategies in response to a pay-off difference [31]. Parameter κ can be interpreted as expressing ‘stickiness’ in behaviour. Figure 3 indicates that individuals are very responsive even to small differences in the pay-off when κ is low, and that for large values of κ (e.g. 0,9) their behaviour becomes ‘sticky’. Sticky, in the sense that they need to observe a very large pay-off difference before they opt to change. For intermediate values of κ, people have sticky behaviour but when the potential benefit in the pay-off is large enough, people switch to the strategy of individual j. If the behaviour is not assumed to be very sticky, then it could be that individual i still adopts the strategy of individual j even if the pay-off of strategy j is worse. The underlying assumption is here that for some individuals peer influence and social conforming behaviour is—to a certain extent—more important than pay-off maximization. Note that in the majority of these models, assumptions rather than real-life observations guide the choice and distribution of the ‘stickiness’ parameter κ.
Figure 3.
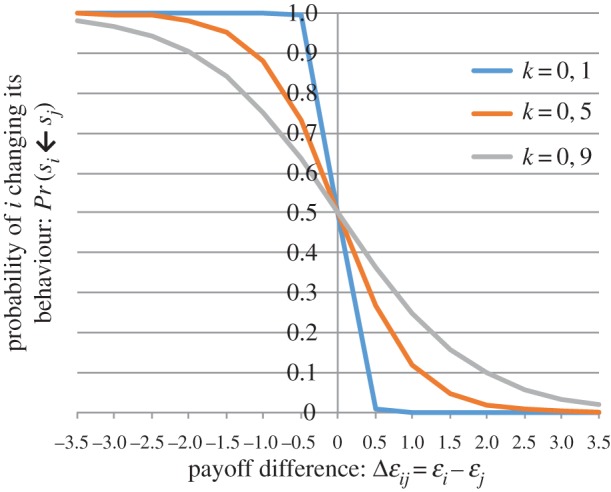
Fermi function for different values of κ.
3.9. Model parametrization and validation
One may question how well BCMs approach reality, as there is a paucity of empirical data on behavioural responses to disease-related information informing these models. We examined whether and how data were used to parametrize BCMs, and to which extent these data support the underlying theoretical model. Moreover, we critically assessed model parametrization, distinguishing data-driven from assumption-driven parametrization, for the disease model, the BCM and the complete integrated model. A first, striking observation is that most models are solely theoretical because they are constructed independently from empirical observations. Often a stability analysis is performed, and equilibria are obtained in order to grasp the dynamics of the model in the absence of parameter values. Others perform numerical simulations with either assumptions on parameters or referring to other studies supporting their choice of parameters. Less than 20% of the studies has (partially) fitted or validated their model to behavioural and/or disease transmission data. Retrospective studies on disease emergence are particularly useful when real-time data on behavioural change and disease transmission during an outbreak are available over a sufficiently long time. Social media data and other electronic sources of information are also increasingly used, thus creating opportunities for ‘big data’ collection on disease transmission, behaviour formation and spatial location [25,60,66]. Next, we briefly describe studies constructing their models using observational data, i.e. studies not exclusively making assumptions or taking parameters from literature.
To underpin BCMs, participatory experiments have been performed to capture social distancing. Maharaj et al. [146] and Chen et al. [183] collected data through a game in which participants trade-off social contacts versus their risk of infection. Such data can be used to parametrize game-theoretic models of social distancing and adaptive networks with link deletion. In addition, survey data have been used to assess behavioural change. Zhong et al. [48] used survey (Public Risk Communication Survey, 2009) data to parametrize their BCM. Robinson et al. [14] surveyed sexual attitudes and lifestyle to build a sexual contact network. The IBM in Gray et al. [85] for syphilis transmission was also informed with survey data on sexual behaviour. Additionally, disease transmission parameters were calibrated from syphilis diagnosis among gay men in Victoria, Australia. A survey on altruism and self-interest was conducted by Shim et al. [23] to calibrate the behavioural change parameters regarding influenza vaccination. In Schumm et al. [127], the BCM is represented by a dynamic social contact network with social distancing, constructed from a survey and census data. Cohen et al. [24] surveyed a convenience sample of students about their risk perceptions for influenza A/H1N1 to estimate the utility values of different behaviours. The study by Fierro & Liccardo [70], used data on awareness and concern about the risk of contagion to populate their model on A/H1N1 influenza transmission with behavioural parameters. Moreover, they also validated their output through comparisons with Italian influenza surveillance data from 2009. The health belief model (HBM) [191] is frequently used to retrieve prevention behaviour and parametrize BCMs. The parameters in the HBM in Durham & Casman [3] were calibrated, using survey data on perceived severity and susceptibility during the 2003 SARS outbreak in Hong Kong. Karimi et al. also use the HBM for their ABM on influenza in 2015 [45]. For validation, the authors compare their model output with similar influenza ABMs in the literature. Another model tackling the influenza A/H1N1 pandemic in 2009 is the model by Bayham et al. [60], who used data from the American time-use survey and the National Health and Activity Patterns Survey (NHAPS). Moreover, Google Trends data are represented as a proxy for subjective risk perception and weather data are used to control for the effects of extreme weather phenomena. Xia et al. [25] constructed a social network using data of an online Facebook-like community to construct a BCM for disease and vaccine awareness on the 2009 influenza A/H1N1 pandemic in Hong Kong. The same pandemic has inspired Springborn et al. to use home television viewing as a proxy for social distancing [56]. Pawelek et al. [66] used Twitter data of self-reporting for awareness spread and ILI surveillance data (UK Health Protection Agency) of the 2009 A/H1N1 influenza pandemic for disease transmission. In addition, Collinson et al. [68] constructed a model on influenza A/H1N1, incorporating mass media report data from the Global Public Health Intelligence Network.
Incidence and outbreak data have been useful to inform the disease dynamics parallel with BCMs. For the 2009 influenza pandemic, Zhong et al. [48] parametrized their transmission model with outbreak data from Arizona and Xiao et al. [65] estimated parameters using outbreak data (laboratory-confirmed cases) from Shaanxi province in China. Schumm et al. [127] focused on observational census and survey data from rural areas. Andrews & Bauch [41] calibrated both disease and behaviour parameters to vaccine coverage and disease incidence data. Althouse & Hébert-Dufresne [88] used surveillance-based incidence rates for syphilis and gonorrhoea from 1941 to 2002. Gray et al. [85] calibrated disease transmission parameters from data on syphilis diagnosis among men who have sex with men in Victoria, Australia. An HIV transmission model including adaptive condom use and sexual partnerships in South Africa is fitted to HIV prevalence data in Nyabadza et al. [71]. The publication makes projections for disease dynamics when scaling up condom use and reducing the number of sexual partners stepwise with 10%. Behavioural change parameters are not calibrated in this publication. The HIV model of Viljoen et al. [80] is fitted to prevalence data in South Africa and Botswana to look at the effect of awareness on disease spread.
BCMs on vaccination dynamics have also been supported by real-life observations. Bauch & Bhattacharyya [91] informed model parameters with historical vaccine coverage and disease incidence data from two vaccine scares (MMR and whole-cell pertussis). The behavioural change framework introduced in the model has a game-theoretic foundation with inclusion of imitation. Likewise, a model for the dynamics of vaccine uptake with a public intervention was proposed by d'Onofrio et al. [92]. Pertussis vaccination uptake and disease dynamics data for the UK are used to fit the model by Oraby et al. [93], which focuses on the inclusion of injunctive social norms in the context of vaccinations for paediatric infectious diseases. The model is validated comparing the model prediction with observed vaccination uptake data during both the UK vaccine-scare period and high coverage period.
Model fitting has been performed through maximum-likelihood and least-squares methods [3,55]. Poletti et al. [54] use ILI incidence data in Italy to calibrate the disease dynamics in their game-theoretic model using least-squares. In addition, data on antiviral drug purchase were used to calibrate the model. In [100], a model of social mobilization is fitted to weekly case counts from CDC and WHO for EVD in Lofa County, Liberia. He et al. [55] investigated three possible explanations for multiple waves of the 1918 influenza pandemic, with one consisting of human behaviour responses. Three proposed models are fitted to historical mortality data using maximum-likelihood in order to determine the extent they can justify the observed disease dynamics. Johnson et al. [72] used prevalence data, antenatal clinic surveys and household surveys for parametrization in order to determine the effects of increased condom use and antivirals on disease dynamics. They calibrated both disease and behaviour parameters to age-specific data using a Bayesian approach for two distinct models.
4. Discussion
4.1. What are current behavioural change models capturing?
It is intuitively logical to include human behaviour in mathematical models for the spread of infectious diseases. After all, disease dynamics are, in essence, dependent on human behaviour dynamics: people interact and take preventive measures on a regularly basis. Because there is much heterogeneity in the ways in which behaviour is included and parametrized in BCMs, it seems the real question is: ‘How should behaviour be taken into account?’ We found that model output may depend on the model specification, to the extent that the selection and development of a model leads in a predictive way towards a predefined conclusion. That is, it seems many of these models serve to justify a theory. For instance, in many pure game-theoretic models, free-rider behaviour emerges resulting in suboptimal vaccination coverage levels, whereas in models including imitation behaviour, the results are often ambiguous. Validation of models with real-life observations is desperately needed to specify an appropriate model, conditional on disease characteristics. Note that model selection implicitly determines the characterization of individuals in the population; models with an economic objective function often assume rational decision-makers, whereas models with imitation or information spread introduce some ‘irrational’ behaviour such as peer influence and social responsibility.
Primary sources such as surveys are needed to empirically underpin the foundations of the models used. The study of Skea et al. [192] on MMR vaccination decisions uses an online chat forum to assess vaccination sentiments and the importance of social responsibility in the parental decision process. The authors find that: ‘participants expressed a desire to both (i) protect their own child and (ii) help protect others by contributing to herd immunity’ [192]. This finding suggests that people are not purely self-interested and herd immunity is not taken as a means to opt for free-riding, on the contrary, establishing herd immunity is seen as an additional incentive, protecting others. A similar conclusion can be drawn from Vietri et al. [9], who tested whether college students consider either free-riding or altruistic motives to decide on (not) receiving vaccinations. They find that individuals both incorporate their own risk of infection and altruistic motives in their decision of whether or not to vaccinate. Determann et al. [193] suggest that these behaviours—and as a result the decision-making process—are country-dependent. They find that focus group participants tend to ‘base their vaccination decision on the trade-off between perceived benefits and barriers of the vaccine…’. Although, in their vaccination strategy, Swedish participants also incorporate: following the rules, doing the right thing, solidarity with other citizens and social influences. The latter drivers are less important in Dutch and Polish participants. This implies that studies may have to be diversified by country-specific characteristics to tackle the inhabitant's behaviour. Dorell et al. [194] conclude that one of the most important factors for vaccination is the healthcare provider's recommendation, which is a determinant that is not included in any of the approaches in the models we found in this extensive review.
In general, there is a need for empirical research to underpin the development of valid models approximating real-life behaviour and disease transmission. Some attempts for recent BCMs illustrate the difficulty of finding suitable observational data. For instance, Springborn et al. [56] used television viewing habits (average viewing time) as a proxy for social distancing, although this proxy is far removed from a direct estimation of social distancing in an outbreak situation. More promising sources of information include: survey data using, for instance, the HBM framework (also see [191,195,196]) or time-use surveys [3,14,23,24,45,48,60,72,85,127,183] or digital sources such as social media [25,60,66,146,197]. Real-life data collection during the influenza A/H1N1 pandemic in 2009 has been a milestone for the parametrization of BCMs with increased collection of both behaviour and disease-related information. For instance, Van Kerckhove et al. [198] studied social contact patterns of symptomatic ILI cases during the pandemic. We encourage the collection of such real-time data in future outbreaks to guide policy-makers in the establishment of an optimal response strategy. For some models, data are just not available, and one needs to resort to assumptions to model behavioural change. Note also that excluding behavioural change from infectious disease models equates to assuming behaviour is unaffected by risk perceptions and disease incidence, and vice versa. Ignoring behavioural responses in the face of substantial changes in risk perceptions is probably worse than making assumptions within a theoretical model in the first place. This review has also met with important limitations in clarity of assumptions and methods in many publications, notwithstanding transparency is an essential part of publishing credible and replicable research.
4.2. Disease-dependent model specification
We observed that the specification of BCMs largely depends on the disease being investigated and the prevention measures considered. Clearly, the transmission characteristics (e.g. air and saliva borne versus STIs), the potential prevention measures (e.g. social distancing versus condom use) and the epidemic stage (e.g. emergence versus endemic equilibrium versus elimination) are interdependent, and determine both the utility and specification of a BCM. For instance, many influenza models use vaccination as a prevention measure with individuals evaluating their previous influenza vaccination decisions to determine the current season's strategy. It would seem unrealistic to require more data to parameterize both behavioural change and disease transmission models with the aim to develop more general models that suit any infectious disease, albeit that behavioural change in response to one disease's risk perceptions could change the risk perceptions of another. At the current stage of BCM development and parametrization, generalized BCMs accommodating multiple pathogens and different transmission routes seem unrealistic. However, it would be easier to combine multiple diseases with the same transmission and prevention properties. For instance, BCMs assessing the combined effects of vaccination scares on MMR and diphtheria, tetanus, pertussis (DTP) disease seem intuitively possible and relevant, though technically challenging and high on data demands.
Developing BCMs with multiple prevention measures is also challenging. Again, we take influenza as an example where we discovered a multitude of prevention measures in our selection (also see table 1): vaccination, social distancing, pre-exposure prophylaxis by antivirals, hygiene measures and others. Interdependencies between these prevention strategies may occur. For instance, a person vaccinated for seasonal influenza may put less effort into hygienic measures such as hand-washing. However, individuals taking hygiene measures may also be more inclined to engage in social distancing if these individuals are more risk-averse. Researchers need to take into account that focusing health policy on one prevention measure may induce ‘crowding out’ of other prevention measures because of such interdependencies. Hence, it is useful to assess the total effect of combined prevention efforts when evaluating policies to reduce the incidence of a disease. Models introducing behavioural change with interdependencies between different prevention measures are influenced by both intrinsic and extrinsic factors.
The popularity of emergence-driven research has many drivers: often new research funding and data collection opportunities arise as an emergence unfolds for the development and parametrization of new models to inform health policy.
4.3. Social networks and individual-based modelling
We observed a rise in the number of studies using (complex) social networks and IBMs to represent disease spread and individual behavioural changes. Social network models impose a structure in the population enabling the identification of model subjects at the individual-level. The implementation of these networks creates a coherent environment to model: social distancing as a prevention measure, the spread and clustering of disease- and prevention-related information and disease dynamics itself. In addition, neighbours can be identified to implement game-theoretic models with imitation dynamics, potentially resulting in clustering of prevention measures. It is clear that the development of these networks has increased the feasibility of modelling local or combined local–global information sources in a BCM. Nevertheless, the selection of an individual-level model is often a trade-off between the desirability for heterogeneity and IBM-specific hurdles such as the computational burden, greater risk of coding errors and potential loss of transparency and reproducibility. Here too, data availability is key to develop relevant models. For example, one could use the POLYMOD study on mixing patterns to construct a synthetic population or a network [199]. Still, more research is needed to enrich the validity of synthetic populations as a representation of real-life dynamics. We refer to a review by Wang et al. [200] focusing on coupling disease dynamics with behaviour in complex networks. A more general work covering BCMs is the book by Manfredi & D'Onofrio [201].
Some models use a single social network for both the disease transmission process and the formation of behaviour. Nonetheless, depending on the background, separate networks may be needed to model the spread of risks and the spread of information influencing behaviour. Take for instance anti-vaccine sentiments. These are often spread through blogs, Facebook groups and other social media [197]. Unlike these sentiments, infections are not spread through the Internet, and as a result require an additional network of physical contacts (see also Grim et al. [202], who make the case for modelling multiple networks). Additionally, the timescale of disease transmission can differ substantially from that of information spread leading to behaviour change. The models by Fukuda et al. [31], Helbing et al. [167] and Maharaj & Kleczkowski [134] are useful examples to guide further development of BCMs with separate parallel and sometimes interacting networks.
4.4. Internet and social media
Information gathering by individuals has evolved over the past decades with the introduction of the Internet, mobile phones and associated social media applications. It is well documented that web-based information can provide a distorted picture about disease risks and adverse events from vaccinations [203–205]. For instance, the search term ‘MMR vaccine’ in Google is automatically complemented by the suggestions ‘autism’ or ‘side effects’. We know individuals retrieve information using these sources for disease-related or prevention-related information and as a result, individuals are exposed to a wide variety of biased information. We recommend policy-makers to implement measures to help individuals to distinguish between evidence-based and unsubstantiated information. A quality label for health-related websites and public health information campaigns are two examples of such measures. Surveys can help understanding how individuals form their perceptions and where they obtain their information.
Another challenge we are faced with, given the popularity of social media, is whether we can still make a distinction between global and local information and how to use these sources of information to construct BCMs. We motivate by example: are Tweets local or global information? In essence, this information can be accessed by anyone, so that they are global. However, at the same time, Tweets are primarily shared among contacts that ‘follow’ each other, which defines local information. In addition, Facebook contacts are not necessarily close in a geographical sense, such that ‘local’ relates more to the possibility of clustering, moving beyond geography. This evolution reinforces the need for having distinct networks in the same model. While social media require reconsidering how information spread is modelled, they also present an opportunity to gather data on behaviour and behavioural changes. A number of studies we identified already integrated social media data [25,60,66,197]. We expect future modelling studies to increasingly use social media as a data source to parametrize BCMs.
4.5. Irrational behaviour and altruism
BCMs have evolved from the perspective of a fully rational ‘Homo economicus’ to a more reasonable, empathic ‘Homo sapiens’. This evolution is conform the findings of surveys examining individuals' drivers to take vaccination [9,192,193] and common sense in general. The study of Shim et al. [23] even considers altruism explicitly as a driver of individuals to take vaccination. In the most recent literature, only few papers are still using a pure, self-centred game-theoretic model. Instead, in the majority of the papers, some form of irrational behaviour has been introduced by the inclusion of social influences or imitation. It is striking, however, that most of the imitation BCMs did not empirically justify their choice of stickiness parameter.
4.6. Level of detail of behaviour
Many BCMs today capture, to some extent, heterogeneity in behaviour; individual-level networks can, for instance, introduce heterogeneity in the number of neighbours that can influence a person to adopt preventive measures. Some population models split the population into compartments representing different levels of risk attitude [89]. Some IBMs introduce personal experiences with disease or prevention measures in behaviour change models [33].
Moreover, heterogeneity in behaviour can be split into two categories: heterogeneity in information an individual receives (e.g. the social contact network of the individual) and heterogeneity in the response to this information (e.g. assigning individual values of stickiness of response in models with imitation). The majority of the publications include individual heterogeneity as the information they are exposed to, whereas only few include the latter category.
The desirability of heterogeneity in behaviour depends on the circumstances and characteristics observed. We illustrate by example: for measles in a highly vaccinated population, it has been observed that unvaccinated individuals and anti-vaccine sentiments are clustered and, as a result, heterogeneity in behaviour should be introduced in behaviour models. For example, one can introduce a distinction between vaccine sceptics and vaccine believers [90].
Again, the availability of real-life observations determines to a large extent the feasibility of introducing heterogeneity in BCMs. Why develop a complex model with large heterogeneity if the parameters cannot be informed by real-life observations? A trade-off needs to be made in terms of computational efficiency, data availability and desirability of heterogeneity given the context of the disease [15].
4.7. Limitations and strengths
Our search was limited to the past 6 years. However, a previous review ended where we start, and since this field is transitioning fast with rapidly increasing computational and research capacity, we believe the most recent years are the most informative. This is also testified by the evolution of our search yield over the 6 year period we covered. Our strength lies in the transparent and systematic way we have searched and analysed the literature according to the standards of systematic review. Nevertheless, as with any systematic review, our search string strikes a balance between completeness and feasibility. Given the current lack of a consistently used common term for the models we review, it is inevitable that we missed some admissible research. Indeed, it came to our attention that, for instance, [206–208] terms were not retrieved by our search, although they would satisfy our eligibility criteria. This emphasizes the need for a specific terminology. We therefore propose the use of the term ‘behavioural change model’ in title, abstract or keywords to facilitate more accurate identification of relevant studies by researchers in different fields.
5. Conclusion
We have systematically reviewed the literature on BCMs published from 2010 until 2015. We analysed and classified 178 references after full-text processing. We proposed a classification of the BCMs based on the decision-making process of the individual. We can summarize our findings in line with the six aims we listed in the introduction. Regarding the technological advancements and increased data availability (i), we find that social media and big data are useful to parametrize BCMs and present an as yet insufficiently explored source of information. Social media can, however, introduce a bias in individuals' prevention- or disease-related perceptions. In addition to the health recommendations they make, policy-makers can optimize their influence by enabling the collection and accessibility of government-owned data (such as surveillance) and by establishing a quality label for disease-related websites. Further, we can confirm that behavioural immunity is often contingent on the disease (ii): BCMs are disease and situation-dependent, which we strongly support. Regarding model validation and parametrization with quantifiable observations (iii), we can state that additional data sources are needed to specify relevant BCMs. Although the 2009 influenza pandemic presented an opportunity for parametrization and validation of both disease transmission and BCMs for flu-like illnesses, there is still much room for improvement in other disease areas. Current models have, without a doubt, assessed the importance of social networks in individual decisions (iv). Individual-level models such as IBMs are extremely useful to tackle behaviour changes and to mimic disease transmission better. More specifically, (v) the diversity observed in BCMs has increased the feasibility of introducing social influences and irrational behaviour (vi). In terms of policy recommendations, it is highly important to think about the total effect of an intervention, with possible implications on all prevention strategies.
The expansion of BCMs has been remarkably valuable. We encourage researchers to incorporate behaviour changes in future disease transmission models and to be transparent about the assumptions they make if data sources for parametrization or validation are sparse.
Supplementary Material
Authors' contributions
P.B. initiated the study. F.W., L.W. and P.B. conceived and designed the inclusion criteria. F.V. and L.W. performed the screening. All authors reviewed the manuscript.
Competing interests
We declare we have no competing interests.
Funding statement
L.W. and F.V. are supported by the Research Foundation Flanders (FWO, G043815N). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Schaller M. 2011. The behavioural immune system and the psychology of human sociality. Phil. Trans. R. Soc. B 366, 3418–3426. ( 10.1098/rstb.2011.0029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau JTF, Yang X, Tsui HY, Pang E. 2004. SARS related preventive and risk behaviours practised by Hong Kong-mainland China cross border travellers during the outbreak of the SARS epidemic in Hong Kong. J. Epidemiol. Community Health 58, 988–996. ( 10.1136/jech.2003.017483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durham DP, Casman EA. 2011. Incorporating individual health-protective decisions into disease transmission models: a mathematical framework. J. R. Soc. Interface 9, 562–570. ( 10.1098/rsif.2011.0325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beutels P, Jia N, Zhou Q-Y, Smith R, Cao W-C, De Vlas SJ. 2009. The economic impact of SARS in Beijing, China. Trop. Med. Int. Health 14, 85–91. ( 10.1111/j.1365-3156.2008.02210.x) [DOI] [PubMed] [Google Scholar]
- 5.Rubin GJ, Amlot R, Page L, Wessely S. 2009. Public perceptions, anxiety, and behaviour change in relation to the swine flu outbreak: cross sectional telephone survey. BMJ 339, b2651 ( 10.1136/bmj.b2651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones JH, Salathe M. 2009. Early assessment of anxiety and behavioral response to novel swine-origin influenza A (H1N1). PLoS ONE 4, e8032 ( 10.1371/journal.pone.0008032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funk S, Salathe M, Jansen VAA. 2010. Modelling the influence of human behaviour on the spread of infectious diseases: a review. J. R. Soc. Interface 7, 1247–1256. ( 10.1098/rsif.2010.0142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbons R. 1992. A primer in game theory. London, UK: Harvester Wheatsheaf. [Google Scholar]
- 9.Vietri JT, Li M, Galvani AP, Chapman GB. 2012. Vaccinating to help ourselves and others. Med. Decis. Making 32, 447–458. ( 10.1177/0272989X11427762) [DOI] [PubMed] [Google Scholar]
- 10.Barbagallo A, Cojocaru MG. 2010. Dynamic vaccination games and variational inequalities on timedependent sets. J. Biol. Dyn. 4, 539–558. ( 10.1080/17513750903398216) [DOI] [PubMed] [Google Scholar]
- 11.Reluga TC. 2013. Equilibria of an epidemic game with piecewise linear social distancing cost. Bull. Math. Biol. 75, 1961–1984. ( 10.1007/s11538-013-9879-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Segbroeck S, Santos FC, Pacheco JM. 2010. Adaptive contact networks change effective disease infectiousness and dynamics. PLoS Comput. Biol. 6, e1000895 ( 10.1371/journal.pcbi.1000895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mbah MLN, Liu JZ, Bauch CT, Tekel YI, Medlock J, Meyers LA, Galvani AP. 2012. The impact of imitation on vaccination behavior in social contact networks. PLoS Comput. Biol. 8, e10024691 (doi:0.1371/journal.pcbi.1002469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson K, Cohen T, Colijn C. 2012. The dynamics of sexual contact networks: effects on disease spread and control. Theor. Popul. Biol. 81, 89–96. ( 10.1016/j.tpb.2011.12.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funk S, Bansal S, Bauch CT, Eames KTD, Edmunds WJ, Galvani AP, Klepac P. 2015. Nine challenges in incorporating the dynamics of behaviour in infectious diseases models. Epidemics 10, 21–25. ( 10.1016/j.epidem.2014.09.005) [DOI] [PubMed] [Google Scholar]
- 16.Higgins pJPT et al. 2008. Cochrane handbook for systematic reviews of interventions, vol. 5 Chichester, UK: Wiley-Blackwell. [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 151, 264–269. ( 10.7326/0003-4819-151-4-200908180-00135) [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. 2016. World Health Organization. Infectious diseases. See http://www.who.int/topics/infectious_diseases/en/.
- 19.Bhattacharyya S, Bauch CT. 2011. ‘Wait and see’ vaccinating behaviour during a pandemic: a game theoretic analysis. Vaccine 29, 5519–5525. ( 10.1016/j.vaccine.2011.05.028) [DOI] [PubMed] [Google Scholar]
- 20.Liu XT, Wu ZX, Zhang LZ. 2012. Impact of committed individuals on vaccination behavior. Phys. Rev. E 86, 051132 ( 10.1103/PhysRevE.86.051132) [DOI] [PubMed] [Google Scholar]
- 21.Breban R. 2011. Health newscasts for increasing influenza vaccination coverage: an inductive reasoning game approach. PLoS ONE 6, e28300 ( 10.1371/journal.pone.0028300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laguzet L, Turinici G. 2015. Individual vaccination as Nash equilibrium in a SIR model with application to the 2009–2010 influenza A (H1N1) epidemic in France. Bull. Math. Biol. 77, 1955–1984. ( 10.1007/s11538-015-0111-7) [DOI] [PubMed] [Google Scholar]
- 23.Shim E, Chapman GB, Townsend JP, Galvani AP. 2012. The influence of altruism on influenza vaccination decisions. J. R. Soc. Interface 9, 2234–2243. ( 10.1098/rsif.2012.0115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen MJ, Brezis M, Block C, Diederich A, Chinitz D. 2013. Vaccination, herd behavior, and herd immunity. Med. Decis. Making 33, 1026–1038. ( 10.1177/0272989x13487946) [DOI] [PubMed] [Google Scholar]
- 25.Xia S, Liu JM. 2014. A belief-based model for characterizing the spread of awareness and its impacts on individuals’ vaccination decisions. J. R. Soc. Interface 11, 20140013 ( 10.1098/rsif.2014.0013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vardavas R, Breban R, Blower S. 2010. A universal long-term flu vaccine may not prevent severe epidemics. BMC Res. Notes 3, 92 ( 10.1186/1756-0500-3-92) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardillo A, Reyes-Suarez C, Naranjo F, Gomez-Gardenes J. 2013. Evolutionary vaccination dilemma in complex networks. Phys. Rev. E, Stat. Nonlin. Soft Matter Phys. 88, 032803 ( 10.1103/PhysRevE.88.032803) [DOI] [PubMed] [Google Scholar]
- 28.Wu ZX, Zhang HF. 2013. Peer pressure is a double-edged sword in vaccination dynamics. EPL 104, 10002 ( 10.1209/0295-5075/104/10002) [DOI] [Google Scholar]
- 29.Zhang Y. 2013. The impact of other-regarding tendencies on the spatial vaccination game. Chaos Solitons Fractals 56, 209–215. ( 10.1016/j.chaos.2013.08.014) [DOI] [Google Scholar]
- 30.Han D, Sun M. 2014. Can memory and conformism resolve the vaccination dilemma? Physica A-Stat. Mech. Appl. 415, 95–104. ( 10.1016/j.physa.2014.07.073) [DOI] [Google Scholar]
- 31.Fukuda E, Tanimoto J, Akimoto M. 2015. Influence of breaking the symmetry between disease transmission and information propagation networks on stepwise decisions concerning vaccination. Chaos Solitons Fractals 80, 47–55. ( 10.1016/j.chaos.2015.04.018) [DOI] [Google Scholar]
- 32.Cornforth DM, Reluga TC, Shim E, Bauch CT, Galvani AP, Meyers LA. 2011. Erratic flu vaccination emerges from short-sighted behavior in contact networks. PLoS Comput. Biol. 7, e1001062 ( 10.1371/journal.pcbi.1001062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu F, Rosenbloom DI, Wang L, Nowak MA. 2011. Imitation dynamics of vaccination behaviour on social networks. Proc. R. Soc. B 278, 42–49. ( 10.1098/rspb.2010.1107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loganathan P, Sundaramoorthy S, Lakshminarayanan S. 2011. Modeling information feedback during H1N1 outbreak using stochastic agent-based models. Asia-Pacific J. Chem. Eng. 6, 391–397. ( 10.1002/apj.571) [DOI] [Google Scholar]
- 35.Wells CR, Bauch CT. 2012. The impact of personal experiences with infection and vaccination on behaviour-incidence dynamics of seasonal influenza. Epidemics 4, 139–151. ( 10.1016/j.epidem.2012.06.002) [DOI] [PubMed] [Google Scholar]
- 36.Zhang HF, Fu F, Zhang WY, Wang BH. 2012. Rational behavior is a ’double-edged sword’ when considering voluntary vaccination. Physica A-Stat. Mech. Appl. 391, 4807–4815. ( 10.1016/j.physa.2012.05.009) [DOI] [Google Scholar]
- 37.Wells CR, Klein EY, Bauch CT. 2013. Policy resistance undermines superspreader vaccination strategies for influenza. PLoS Comput. Biol. 9, e1002945 ( 10.1371/journal.pcbi.1002945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia S, Liu JM. 2013. A computational approach to characterizing the impact of social influence on individuals’ vaccination decision making. PLoS ONE 8, e60373 ( 10.1371/journal.pone.0060373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukuda E, Kokubo S, Tanimoto J, Wang Z, Hagishima A, Ikegaya N. 2014. Risk assessment for infectious disease and its impact on voluntary vaccination behavior in social networks. Chaos Solitons Fractals 68, 1–9. ( 10.1016/j.chaos.2014.07.004) [DOI] [Google Scholar]
- 40.Liao CM, You SH. 2014. Assessing risk perception and behavioral responses to influenza epidemics: linking information theory to probabilistic risk modeling. Stoch. Environ. Res. Risk Assess. 28, 189–200. ( 10.1007/s00477-013-0739-5) [DOI] [Google Scholar]
- 41.Andrews MA, Bauch CT. 2015. Disease interventions can interfere with one another through disease–behaviour interactions. PLoS Comput. Biol., 11, e1004291 ( 10.1371/journal.pcbi.1004291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marathe A, Lewis B, Barrett C, Chen J, Marathe M, Eubank S, Ma Y. 2011. Comparing effectiveness of top-down and bottom-up strategies in containing influenza. PLoS ONE 6, e25149 ( 10.1371/journal.pone.0025149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mei S, Zhu YF, Qiu XG, Zhou X, Zu ZH, Boukhanovsky AV, Sloot PMA. 2014. Individual decision making can drive epidemics: a fuzzy cognitive map study. IEEE Trans. Fuzzy Syst. 22, 264–273. ( 10.1109/Tfuzz.2013.2251638) [DOI] [Google Scholar]
- 44.Zhang HF, Wu ZX, Tang M, Lai YC. 2014. Effects of behavioral response and vaccination policy on epidemic spreading - an approach based on evolutionary-game dynamics. Sci. Rep. 4, ARTN5666 ( 10.1038/srep05666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karimi E, Schmitt K, Akgunduz A. 2015. Effect of individual protective behaviors on influenza transmission: an agent-based model. Health Care Manag. Sci. 18, 318–333. ( 10.1007/s10729-014-9310-2) [DOI] [PubMed] [Google Scholar]
- 46.Poletti P, Ajelli M, Merler S. 2012. Risk perception and effectiveness of uncoordinated behavioral responses in an emerging epidemic. Math. Biosci. 238, 80–89. ( 10.1016/j.mbs.2012.04.003) [DOI] [PubMed] [Google Scholar]
- 47.Mummert A, Weiss H. 2013. Get the news out loudly and quickly: the influence of the media on limiting emerging infectious disease outbreaks. PLoS ONE 8, e71692 ( 10.1371/journal.pone.0071692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhong W, Kim Y, Jehn M. 2013. Modeling dynamics of an influenza pandemic with heterogeneous coping behaviors: case study of a 2009 H1N1 outbreak in Arizona. Comput. Math. Organ. Theory 19, 622–645. ( 10.1007/s10588-012-9146-6) [DOI] [Google Scholar]
- 49.Larson RC, Nigmatulina KR. 2010. Engineering responses to pandemics. Stud. Health Technol. Inform. 153, 311–339. [PubMed] [Google Scholar]
- 50.Wang W. 2012. Modeling adaptive behavior in influenza transmission. Math. Model. Nat. Phenomena 7, 253–262. ( 10.1051/mmnp/20127315) [DOI] [Google Scholar]
- 51.Greer AL. 2013. Can informal social distancing interventions minimize demand for antiviral treatment during a severe pandemic? BMC Public Health 13, 2 ( 10.1186/1471-2458-13-669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morin BR, Fenichel EP, Castillo-Chavez C. 2013. Sir dynamics with economically driven contact rates. Nat. Resour. Model. 26, 505–525. ( 10.1111/nrm.12011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu SH, Pang LY, Ruan SG, Zhang XN. 2015. Global dynamics of avian influenza epidemic models with psychological effect. Comput. Math. Methods Med. 2015, 1–12. ( 10.1155/2015/913726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poletti P, Ajelli M, Merler S. 2011. The effect of risk perception on the 2009 H1N1 pandemic influenza dynamics . PLoS ONE 6, e16460 ( 10.1371/journal.pone.0016460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He D, Dushoff J, Day T, Ma J, Earn DJ. 2013. Inferring the causes of the three waves of the 1918 influenza pandemic in England and Wales. Proc. R. Soc. B 280, 20131345 ( 10.1098/rspb.2013.1345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Springborn M, Chowell G, MacLachlan M, Fenichel EP. 2015. Accounting for behavioral responses during a flu epidemic using home television viewing. BMC Infect. Dis. 15, 21 ( 10.1186/s12879-014-0691-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barrett C, Bisset K, Leidig J, Marathe A, Marathe M. 2010. An integrated modeling environment to study the coevolution of networks, individual behavior, and epidemics. AI Mag. 31, 75–87. [Google Scholar]
- 58.Barrett C, Bisset K, Leidig J, Marathe A, Marathe M. 2011. Economic and social impact of influenza mitigation strategies by demographic class. Epidemics 3, 19–31. ( 10.1016/j.epidem.2010.11.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang ZG, Zhang HF, Wang Z. 2014. Multiple effects of self-protection on the spreading of epidemics. Chaos Solitons Fractals 61, 1–7. ( 10.1016/j.chaos.2014.01.004) [DOI] [Google Scholar]
- 60.Bayham J, Kuminoff NV, Gunn Q, Fenichel EP. 2015. Measured voluntary avoidance behaviour during the 2009 A/H1N1 epidemic. Proc. R. Soc. B 282, 20150814 ( 10.1098/rspb.2015.0814) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen JZ, Marathe A, Marathe M. 2010. Coevolution of epidemics, social networks, and individual behavior: a case study. Adv. Soc. Comput. Proc. 6007, 218–227. ( 10.1007/978-3-642-12079-4_28) [DOI] [Google Scholar]
- 62.Mao L, Bian L. 2011. Agent-based simulation for a dual-diffusion process of influenza and human preventive behavior. Int. J. Geogr. Inf. Sci. 25, 1371–1388. ( 10.1080/13658816.2011.556121) [DOI] [Google Scholar]
- 63.Mao L, Yang Y. 2012. Coupling infectious diseases, human preventive behavior, and networks – a conceptual framework for epidemic modeling. Soc. Sci. Med. 74, 167–175. ( 10.1016/j.socscimed.2011.10.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parikh N, Youssef M, Swarup S, Eubank S. 2013. Modeling the effect of transient populations on epidemics in Washington DC. Sci. Rep. 3, 3152 ( 10.1038/srep03152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiao Y, Tang S, Wu J. 2015. Media impact switching surface during an infectious disease outbreak. Sci. Rep. 5, 7838 ( 10.1038/srep07838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pawelek KA, Oeldorf-Hirsch A, Rong LB. 2014. Modeling the impact of Twitter on influenza epidemics. Math. Biosci. Eng. 11, 1337–1356. ( 10.3934/mbe.2014.11.1337) [DOI] [PubMed] [Google Scholar]
- 67.Liao CM, You SH, Cheng YH. 2015. Network information analysis reveals risk perception transmission in a behaviour-influenza dynamics system. Epidemiol. Infect. 143, 23–36. ( 10.1017/S0950268814000430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Collinson S, Khan K, Heffernan MJ. 2015. The effects of media reports on disease spread and important public health measurements. PLoS ONE 10, e0141423 ( 10.1371/journal.pone.0141423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang HF, Yang ZM, Wu ZX, Wang BH, Zhou T. 2013. Braess's paradox in epidemic game: better condition results in less payoff. Sci. Rep. 3, ARTN3292. ( 10.1038/srep03292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fierro A, Liccardo A. 2013. Lattice model for influenza spreading with spontaneous behavioral changes. PLoS ONE 8, e83641 ( 10.1371/journal.pone.0083641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nyabadza F, Mukandavire Z, Hove-Musekwa SD. 2011. Modelling the HIV/AIDS epidemic trends in South Africa: insights from a simple mathematical model. Nonlinear Anal.-Real World Appl. 12, 2091–2104. ( 10.1016/j.nonrwa.2010.12.024) [DOI] [Google Scholar]
- 72.Johnson LF, Hallett TB, Rehle TM, Dorrington RE. 2012. The effect of changes in condom usage and antiretroviral treatment coverage on human immunodeficiency virus incidence in South Africa: a model-based analysis. J. R. Soc. Interface 9, 1544–1554. ( 10.1098/rsif.2011.0826) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vieira IT, Cheng RCH, Harper PR, de Senna V. 2010. Small world network models of the dynamics of HIV infection. Ann. Operat. Res. 178, 173–200. ( 10.1007/s10479-009-0571-y) [DOI] [Google Scholar]
- 74.Alimadad A, Dabbaghian V, Singhk SK, Tsang HH. 2011. Modeling HIV spread through sexual contact using a cellular automaton. In 2011 IEEE Congress on Evolutionary Computation (Cec), pp. 2345–2350.
- 75.Tully S, Cojocaru M, Bauch CT. 2013. Coevolution of risk perception, sexual behaviour, and HIV transmission in an agent-based model. J. Theor. Biol. 337, 125–132. ( 10.1016/j.jtbi.2013.08.014) [DOI] [PubMed] [Google Scholar]
- 76.Tully S, Cojocaru M, Bauch CT. 2015. Sexual behavior, risk perception, and HIV transmission can respond to HIV antiviral drugs and vaccines through multiple pathways. Sci. Rep. 5, 15411 ( 10.1038/srep15411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nyabadza F, Chiyaka C, Mukandavire Z, Hove-Musekwa SD. 2010. Analysis of an HIV/aids model with public-health information campaigns and individual withdrawal. J. Biol. Syst. 18, 357–375. ( 10.1142/S0218339010003329) [DOI] [Google Scholar]
- 78.Reniers G, Armbruster B. 2012. HIV status awareness, partnership dissolution and HIV Transmission in generalized epidemics. PLoS ONE 7, e50669 ( 10.1371/journal.pone.0050669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reluga TC, Li J. 2013. Games of age-dependent prevention of chronic infections by social distancing. J. Math. Biol. 66, 1527–1553. ( 10.1007/s00285-012-0543-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Viljoen T, Spoelstra J, Hemerik L, Molenaar J. 2014. Modelling the Impact of HIV on the populations of South Africa and Botswana. Acta Biotheor. 62, 91–108. ( 10.1007/s10441-014-9210-3) [DOI] [PubMed] [Google Scholar]
- 81.Kassa SM, Ouhinou A. 2011. Epidemiological models with prevalence dependent endogenous self-protection measure. Math. Biosci. 229, 41–49. ( 10.1016/j.mbs.2010.10.007) [DOI] [PubMed] [Google Scholar]
- 82.Kassa SM, Ouhinou A. 2015. The impact of self-protective measures in the optimal interventions for controlling infectious diseases of human population. J. Math. Biol. 70, 213–236. ( 10.1007/s00285-014-0761-3) [DOI] [PubMed] [Google Scholar]
- 83.Marshall BD, Paczkowski MM, Seemann L, Tempalski B, Pouget ER, Galea S, Friedman SR. 2012. A complex systems approach to evaluate HIV prevention in metropolitan areas: preliminary implications for combination intervention strategies. PLoS ONE 7, e44833 ( 10.1371/journal.pone.0044833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morin BR, Medina-Rios L, Camacho ET, Castillo-Chavez C. 2010. Static behavioral effects on gonorrhea transmission dynamics in a MSM population. J. Theor. Biol. 267, 35–40. ( 10.1016/j.jtbi.2010.07.027) [DOI] [PubMed] [Google Scholar]
- 85.Gray RT, Hoare A, McCann PD, Bradley J, Down I, Donovan B, Prestage G, Wilson DP. 2011. Will changes in gay men's sexual behavior reduce syphilis rates? Sex. Transm. Dis. 38, 1151–1158. ( 10.1097/OLQ.0b013e318238b85d) [DOI] [PubMed] [Google Scholar]
- 86.Milner FA, Zhao R. 2010. A new mathematical model of syphilis. Math. Model. Nat. Phenomena 5, 96–108. ( 10.1051/mmnp/20105605) [DOI] [Google Scholar]
- 87.Aadland D, Finnoff DC, Huang KXD. 2013. Syphilis cycles. BE J. Econ. Anal. Policy 13, 297–348. ( 10.1515/bejeap-2012-0060) [DOI] [Google Scholar]
- 88.Althouse BM, Hébert-Dufresne L. 2014. Epidemic cycles driven by host behaviour. J. R. Soc. Interface 11, 20140575 ( 10.1098/rsif.2014.0575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bhattacharyya S, Bauch CT. 2010. A game dynamic model for delayer strategies in vaccinating behaviour for pediatric infectious diseases. J. Theor. Biol. 267, 276–282. ( 10.1016/j.jtbi.2010.09.005) [DOI] [PubMed] [Google Scholar]
- 90.Shim E, Grefenstette JJ, Albert SM, Cakouros BE, Burke DS. 2012. A game dynamic model for vaccine skeptics and vaccine believers: measles as an example. J. Theor. Biol. 295, 194–203. ( 10.1016/j.jtbi.2011.11.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bauch CT, Bhattacharyya S. 2012. Evolutionary game theory and social learning can determine how vaccine scares unfold. PLoS Comput. Biol. 8, e1002452 ( 10.1371/journal.pcbi.1002452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.d'Onofrio A, Manfredi P, Poletti P. 2012. The interplay of public intervention and private choices in determining the outcome of vaccination programmes. PLoS ONE 7, e45653 ( 10.1371/journal.pone.0045653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oraby T, Thampi V, Bauch CT. 2014. The influence of social norms on the dynamics of vaccinating behaviour for paediatric infectious diseases. Proc. R. Soc. B 281, 20133172 ( 10.1098/rspb.2013.3172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perra N, Balcan D, Goncalves B, Vespignani A. 2011. Towards a characterization of behavior-disease models. PLoS ONE 6, e23084 ( 10.1371/journal.pone.0023084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sharma A, Misra AK. 2014. Modeling the impact of awareness created by media campaigns on vaccination coverage in a variable population. J. Biol. Syst. 22, 249–270. ( 10.1142/S0218339014400051) [DOI] [Google Scholar]
- 96.Greenhalgh D, Rana S, Samanta S, Sardar T, Bhattacharya S, Chattopadhyay J. 2015. Awareness programs control infectious disease - Multiple delay induced mathematical model. Appl. Math. Comput. 251, 539–563. ( 10.1016/j.amc.2014.11.091) [DOI] [Google Scholar]
- 97.Cooper K, et al. 2012. An economic model of school-based behavioral interventions to prevent sexually transmitted infections. Int. J. Technol. Assess. Health Care 28, 407–414. ( 10.1017/S0266462312000475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Del Valle SY, Hyman JM, Chitnis N. 2013. Mathematical models of contact patterns between age groups for predicting the spread of infectious diseases. Math. Biosci. Eng. 10, 1475–1497. ( 10.3934/mbe.2013.10.1475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang X, Gao D, Wang J. 2015. Influence of human behavior on cholera dynamics. Math. Biosci. 267, 41–52. ( 10.1016/j.mbs.2015.06.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fast SM, Mekaru S, Brownstein JS, Postlethwaite TA, Markuzon N. 2015. The role of social mobilization in controlling ebola virus in Lofa County, Liberia. PLoS Curr. 2015, 7. ( 10.1371/currents.outbreaks.c3576278c66b22ab54a25e122fcdbec1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sykes D, Rychtar J. 2015. A game-theoretic approach to valuating toxoplasmosis vaccination strategies. Theor. Popul. Biol. 105, 33–38. ( 10.1016/j.tpb.2015.08.003) [DOI] [PubMed] [Google Scholar]
- 102.Misra AK, Sharma A, Li J. 2013. A mathematical model for control of vector borne diseases through media campaigns. Discrete Contin. Dyn. Syst. B 18, 1909–1927. ( 10.3934/dcdsb.2013.18.1909) [DOI] [Google Scholar]
- 103.Williams AD, Hall IM, Rubin GJ, Amlot R, Leach S. 2011. An individual-based simulation of pneumonic plague transmission following an outbreak and the significance of intervention compliance. Epidemics 3, 95–102. ( 10.1016/j.epidem.2011.03.001) [DOI] [PubMed] [Google Scholar]
- 104.Funk S, Gilad E, Jansen VA. 2010. Endemic disease, awareness, and local behavioural response. J. Theor. Biol. 264, 501–509. ( 10.1016/j.jtbi.2010.02.032) [DOI] [PubMed] [Google Scholar]
- 105.Misra AK, Sharma A, Singh V. 2011. Effect of awareness programs in controlling the prevalence of an epidemic with time delay. J. Biol. Syst. 19, 389–402. ( 10.1142/S0218339011004020) [DOI] [Google Scholar]
- 106.Samanta S, Rana S, Sharma A, Misra AK, Chattopadhyay J. 2013. Effect of awareness programs by media on the epidemic outbreaks: a mathematical model. Appl. Math. Comput. 219, 6965–6977. ( 10.1016/j.amc.2013.01.009) [DOI] [Google Scholar]
- 107.Zuo LX, Liu MX. 2014. Effect of awareness programs on the epidemic outbreaks with time delay. Abstr. Appl. Anal. 2014, 940841 ( 10.1155/2014/940841) [DOI] [Google Scholar]
- 108.Misra AK, Sharma A, Shukla JB. 2015. Stability analysis and optimal control of an epidemic model with awareness programs by media. Biosystems 138, 53–62. ( 10.1016/j.biosystems.2015.11.002) [DOI] [PubMed] [Google Scholar]
- 109.Wang Q, Zhao LJ, Huang RB, Yang YP, Wu JH. 2015. Interaction of media and disease dynamics and its impact on emerging infection management. Discrete Contin. Dyn. Syst. B 20, 215–230. ( 10.3934/dcdsb.2015.20.215) [DOI] [Google Scholar]
- 110.Reluga TC. 2010. Game theory of social distancing in response to an epidemic. PLoS Comput. Biol. 6, e1000793 ( 10.1371/journal.pcbi.1000793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brauer F. 2011. A simple model for behaviour change in epidemics. BMC Public Health 11, 443 ( 10.1186/1471-2458-11-S1-S3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fenichel EP, et al. 2011. Adaptive human behavior in epidemiological models. Proc. Natl Acad. Sci. USA 108, 6306–6311. ( 10.1073/pnas.1011250108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Buonomo B, d'Onofrio A, Lacitignola D. 2012. Globally stable endemicity for infectious diseases with information-related changes in contact patterns. Appl. Math. Lett. 25, 1056–1060. ( 10.1016/j.aml.2012.03.016) [DOI] [Google Scholar]
- 114.Xiao YN, Xu XX, Tang SY. 2012. Sliding mode control of outbreaks of emerging infectious diseases. Bull. Math. Biol. 74, 2403–2422. ( 10.1007/s11538-012-9758-5) [DOI] [PubMed] [Google Scholar]
- 115.Liu WB. 2013. A SIRS epidemic model incorporating media coverage with random perturbation. Abstr. Appl. Anal. 2013, 1–9. ( 10.1155/2013/792308) [DOI] [Google Scholar]
- 116.Wang LY, Huang HL, Xu AC, Wang WM. 2013. Stochastic extinction in an SIRS epidemic model incorporating media coverage. Abstr. Appl. Anal. 2013, 1–8. ( 10.1155/2013/891765) [DOI] [Google Scholar]
- 117.Li YF, Xie DL, Cui JA. 2014. The effect of impulsive vaccination on delayed SEIRS epidemic model incorporating saturation recovery. Discrete Dyn. Nat. Soc. 104, 1–7. ( 10.1155/2014/426456) [DOI] [Google Scholar]
- 118.Morin BR, Perrings C, Levin S, Kinzig A. 2014. Disease risk mitigation: the equivalence of two selective mixing strategies on aggregate contact patterns and resulting epidemic spread. J. Theor. Biol. 363, 262–270. ( 10.1016/j.jtbi.2014.07.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li J, Yulin ZL, Zhu HP. 2015. Bifurcation of an SIS model with nonlinear contact rate. J. Math. Anal. Appl. 432, 1119–1138. ( 10.1016/j.jmaa.2015.07.001) [DOI] [Google Scholar]
- 120.Liu MX, Liz E, Rost G. 2015. Endemic bubbles generated by delayed behavioral response: global stability and bifurcation switches in an SIS model. SIAM J. Appl. Math. 75, 75–91. ( 10.1137/140972652) [DOI] [Google Scholar]
- 121.Lubuma JMS, Terefe YA. 2015. A nonstandard Volterra difference equation for the SIS epidemiological model. Rev. R. Acad. Cienc. Exactas Fis. Nat., A 109, 597–602. ( 10.1007/s13398-014-0203-5) [DOI] [Google Scholar]
- 122.Sahneh FD, Scoglio C. 2011. Epidemic spread in human networks. In 2011 50th IEEE Conf. on Decision and Control and European Control Conference (Cdc-Ecc), pp. 3008–3013. Piscataway, NJ: IEEE.
- 123.Wu QC, Fu XC, Small M, Xu XJ. 2012. The impact of awareness on epidemic spreading in networks. Chaos 22, 013101 ( 10.1063/1.3673573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang Y, Cao JD, Jin Z, Zhang HF, Sun GQ. 2013. Impact of media coverage on epidemic spreading in complex networks. Physica A-Stat. Mech. Appl. 392, 5824–5835. ( 10.1016/j.physa.2013.07.067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cao L. 2014. Infection dynamics in structured populations with disease awareness based on neighborhood contact history. Eur. Phys. J. B 87, ARTN225. ( 10.1140/epjb/e2014-50422-8) [DOI] [Google Scholar]
- 126.Zhang HF, Xie JR, Tang M, Lai YC. 2014. Suppression of epidemic spreading in complex networks by local information based behavioral responses. Chaos 24, 043106 ( 10.1063/1.4896333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schumm P, Schumm W, Scoglio C. 2013. Impact of preventive behavioral responses to epidemics in rural regions. 2013 Int. Conf. Comput. Sci. 18, 631–640. ( 10.1016/j.procs.2013.05.227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Meloni S, Perra N, Arenas A, Gomez S, Moreno Y, Vespignani A. 2011. Modeling human mobility responses to the large-scale spreading of infectious diseases. Sci. Rep. 1, ARTN62. ( 10.1038/srep00062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sun CJ, Yang W, Arino J, Khan K. 2011. Effect of media-induced social distancing on disease transmission in a two patch setting. Math. Biosci. 230, 87–95. ( 10.1016/j.mbs.2011.01.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bichara D, Kang Y, Castillo-Chavez C, Horan R, Perrings C. 2015. SIS and SIR epidemic models under virtual dispersal. Bull. Math. Biol. 77, 2004–2034. ( 10.1007/s11538-015-0113-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu WB, Zheng QB. 2015. A stochastic SIS epidemic model incorporating media coverage in a two patch setting. Appl. Math. Comput. 262, 160–168. ( 10.1016/j.amc.2015.04.025) [DOI] [Google Scholar]
- 132.Shaw LB, Schwartz IB. 2010. Enhanced vaccine control of epidemics in adaptive networks. Phys. Rev. E 81, ARTN046120. ( 10.1103/PhysRevE.81.046120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jolad S, Liu WJ, Schmittmann B, Zia RKP. 2012. Epidemic spreading on preferred degree adaptive networks. PLoS ONE 7, e48686 ( 10.1371/journal.pone.0048686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Maharaj S, Kleczkowski A. 2012. Controlling epidemic spread by social distancing: do it well or not at all. BMC Public Health 12, 499 ( 10.1186/1471-2458-12-679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rogers T, Clifford-Brown W, Mills C, Galla T. 2012. Stochastic oscillations of adaptive networks: application to epidemic modelling. J. Stat. Mech.-Theory Exp. 2012, P08018 ( 10.1088/1742-5468/2012/08/P08018) [DOI] [Google Scholar]
- 136.Valdez LD, Macri PA, Braunstein LA. 2012. Intermittent social distancing strategy for epidemic control. Phys. Rev. E, Stat. Nonlin. Soft Matter Phys. 85, 036108 ( 10.1103/PhysRevE.85.036108) [DOI] [PubMed] [Google Scholar]
- 137.Zhang HF, Small M, Fu XC, Sun GQ, Wang BH. 2012. Modeling the influence of information on the coevolution of contact networks and the dynamics of infectious diseases. Physica D 241, 1512–1517. ( 10.1016/j.physd.2012.05.011) [DOI] [Google Scholar]
- 138.Juher D, Ripoll J, Saldana J. 2013. Outbreak analysis of an SIS epidemic model with rewiring. J. Math. Biol. 67, 411–432. ( 10.1007/s00285-012-0555-4) [DOI] [PubMed] [Google Scholar]
- 139.Liu MX, Rost G, Vas G. 2013. SIS model on homogeneous networks with threshold type delayed contact reduction. Comput. Math. Appl. 66, 1534–1546. ( 10.1016/j.camwa.2013.02.009) [DOI] [Google Scholar]
- 140.Noble C, Bagrow JP, Brockmann D. 2013. The role of caretakers in disease dynamics. J. Stat. Phys. 152, 787–798. ( 10.1007/s10955-013-0787-8) [DOI] [Google Scholar]
- 141.Valdez LD, Macri PA, Braunstein LA. 2013. Temporal percolation of a susceptible adaptive network. Physica A 392, 4172–4180. ( 10.1016/j.physa.2013.05.003) [DOI] [Google Scholar]
- 142.Tunc I, Shaw LB. 2014. Effects of community structure on epidemic spread in an adaptive network. Phys. Rev. E 90, ARTN022801. ( 10.1103/PhysRevE.90.022801) [DOI] [PubMed] [Google Scholar]
- 143.Dong C, Yin Q, Liu W, Yan Z, Shi T. 2015. Can rewiring strategy control the epidemic spreading? Physica A 438, 169–177. ( 10.1016/j.physa.2015.06.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Szabo-Solticzky A, Berthouze L, Kiss IZ, Simon PL. 2015. Oscillating epidemics in a dynamic network model: stochastic and mean-field analysis. J. Math. Biol. 72, 1153–1176. ( 10.1007/s00285-015-0902-3) [DOI] [PubMed] [Google Scholar]
- 145.Nicolaides C, Cueto-Felgueroso L, Juanes R. 2013. The price of anarchy in mobility-driven contagion dynamics. J. R. Soc. Interface 10, 20130495 ( 10.1098/rsif.2013.0495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Maharaj S, McCaldin T, Kleczkowski A. 2011. A participatory simulation model for studying attitudes to infection risk. In Proc the 2011 Summer Computer Simulation Conf., The Hague, The Netherlands, 27–30 June, pp. 8–13.
- 147.Bauch CT, Bhattacharyya S, Ball RF. 2010. Rapid emergence of free-riding behavior in new pediatric immunization programs. PLoS ONE 5, e12594 ( 10.1371/journal.pone.0012594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.d'Onofrio A, Manfredi P. 2010. Vaccine demand driven by vaccine side effects: dynamic implications for SIR diseases. J. Theor. Biol. 264, 237–252. ( 10.1016/j.jtbi.2010.02.007) [DOI] [PubMed] [Google Scholar]
- 149.Buonomo B, d'Onofrio A, Lacitignola D. 2010. Rational exemption to vaccination for non-fatal sis diseases: globally stable and oscillatory endemicity. Math. Biosci. Eng. 7, 561–578. ( 10.3934/mbe.2010.7.561) [DOI] [PubMed] [Google Scholar]
- 150.d'Onofrio A, Manfredi P, Poletti P. 2011. The impact of vaccine side effects on the natural history of immunization programmes: an imitation-game approach. J. Theor. Biol. 273, 63–71. ( 10.1016/j.jtbi.2010.12.029) [DOI] [PubMed] [Google Scholar]
- 151.Reluga TC, Galvani AP. 2011. A general approach for population games with application to vaccination. Math. Biosci. 230, 67–78. ( 10.1016/j.mbs.2011.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu B, Fu F, Wang L. 2011. Imperfect vaccine aggravates the long-standing dilemma of voluntary vaccination. PLoS ONE 6, e20577 ( 10.1371/journal.pone.0020577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Buonomo B, d'Onofrio A, Lacitignola D. 2013. Modeling of pseudo-rational exemption to vaccination for SEIR diseases. J. Math Anal. Appl. 404, 385–398. ( 10.1016/j.jmaa.2013.02.063) [DOI] [Google Scholar]
- 154.Xu F, Cressman R. 2014. Disease control through voluntary vaccination decisions based on the smoothed best response. Comput. Math. Methods Med. 2014, 1–14. ( 10.1155/2014/825734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bhattacharyya S, Bauch CT, Breban R. 2015. Role of word-of-mouth for programs of voluntary vaccination: a game-theoretic approach. Math. Biosci. 269, 130–134. ( 10.1016/j.mbs.2015.08.023) [DOI] [PubMed] [Google Scholar]
- 156.Oraby T, Bauch CT. 2015. Bounded rationality alters the dynamics of paediatric immunization acceptance. Sci. Rep. 5, 10724 ( 10.1038/srep10724) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Voinson M, Billiard S, Alvergne A. 2015. Beyond rational decision-making: modelling the influence of cognitive biases on the dynamics of vaccination coverage. PLoS ONE 10, e0142990 ( 10.1371/journal.pone.0142990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schimit PHT, Monteiro LHA. 2011. A vaccination game based on public health actions and personal decisions. Ecol. Model. 222, 1651–1655. ( 10.1016/j.ecolmodel.2011.02.019) [DOI] [Google Scholar]
- 159.Wells CR, Tchuenche JM, Meyers LA, Galvani AP, Bauch CT. 2011. Impact of imitation processes on the effectiveness of ring vaccination. Bull. Math. Biol. 73, 2748–2772. ( 10.1007/s11538-011-9646-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Morsky B, Bauch CT. 2012. Outcome inelasticity and outcome variability in behaviour-incidence models: an example from an SEIR infection on a dynamic network. Comput. Math. Methods Med. 2012, 1–11. ( 10.1155/2012/652562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ruan ZY, Tang M, Liu ZH. 2012. Epidemic spreading with information-driven vaccination. Phys. Rev. E 86, ARTN036117. ( 10.1103/PhysRevE.86.036117) [DOI] [PubMed] [Google Scholar]
- 162.Campbell E, Salathe M. 2013. Complex social contagion makes networks more vulnerable to disease outbreaks. Sci. Rep. 3, ARTN1905. ( 10.1038/srep01905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wu QC, Liu HX, Small M. 2013. Dynamical diversity induced by individual responsive immunization. Physica A 392, 2792–2802. ( 10.1016/j.physa.2013.02.014) [DOI] [Google Scholar]
- 164.Cai CR, Wu ZX, Guan JY. 2014. Effect of vaccination strategies on the dynamic behavior of epidemic spreading and vaccine coverage. Chaos Solitons Fractals 62–63, 36–43. ( 10.1016/j.chaos.2014.04.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wu QC, Zhang HF, Zeng GH. 2014. Responsive immunization and intervention for infectious diseases in social networks. Chaos 24, 023108 ( 10.1063/1.4872177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Han D, Sun M, Li DD. 2015. Epidemic process on activity-driven modular networks. Physica A 432, 354–362. ( 10.1016/j.physa.2015.03.062) [DOI] [Google Scholar]
- 167.Helbing D, et al. 2015. Saving human lives: what complexity science and information systems can contribute. J. Stat. Phys. 158, 735–781. ( 10.1007/s10955-014-1024-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liang YH, Juang J. 2015. The impact of vaccine failure rate on epidemic dynamics in responsive networks. Chaos 25, 043116 ( 10.1063/1.4919245) [DOI] [PubMed] [Google Scholar]
- 169.Zhang HF, Zhang J, Li P, Small M, Wang BH. 2011. Risk estimation of infectious diseases determines the effectiveness of the control strategy. Physica D 240, 943–948. ( 10.1016/j.physd.2011.02.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Joshi HR, Lenhart S, Hota S, Agusto F. 2015. Optimal control of an SIR model with changing behavior through an education campaign. Electron. J. Diff. Equ. 2015, 1–4. [Google Scholar]
- 171.Liu XZ, Stechlinski P. 2012. Infectious disease models with time-varying parameters and general nonlinear incidence rate. Appl. Math. Model 36, 1974–1994. ( 10.1016/j.apm.2011.08.019) [DOI] [Google Scholar]
- 172.Samanta S, Chattopadhyay J. 2014. Effect of awareness program in disease outbreak - a slow-fast dynamics. Appl. Math. Comput. 237, 98–109. ( 10.1016/j.amc.2014.03.109) [DOI] [Google Scholar]
- 173.Sega L, Maxin D, Eaton L, Latham A, Moose A, Stenslie S. 2015. The effect of risk-taking behaviour in epidemic models. J. Biol. Dyn. 9, 229–246. ( 10.1080/17513758.2015.1065351) [DOI] [PubMed] [Google Scholar]
- 174.Hatzopoulos V, Taylor M, Simon PL, Kiss IZ. 2011. Multiple sources and routes of information transmission: Implications for epidemic dynamics. Math. Biosci. 231, 197–209. ( 10.1016/j.mbs.2011.03.006) [DOI] [PubMed] [Google Scholar]
- 175.Sahneh FD, Scoglio CM. 2012. Optimal information dissemination in epidemic networks. In 2012 IEEE 51st Annual Conf. on Decision and Control (Cdc), Grand Wailea, HI, 10–13 December, pp. 1657–1662.
- 176.Sahneh FD, Chowdhury FN, Scoglio CM. 2012. On the existence of a threshold for preventive behavioral responses to suppress epidemic spreading. Sci. Rep. 2, ARTN632. ( 10.1038/srep00632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Miller JC. 2013. Cocirculation of infectious diseases on networks. Phys. Rev. E 87, ARTN060801. ( 10.1103/PhysRevE.87.060801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yuan XP, Xue YK, Liu MX. 2013. Analysis of an epidemic model with awareness programs by media on complex networks. Chaos Solitons Fractals 48, 1–11. ( 10.1016/j.chaos.2012.12.001) [DOI] [Google Scholar]
- 179.Goyal S, Vigier A. 2015. Interaction, protection and epidemics. J. Public Econ. 125, 64–69. ( 10.1016/j.jpubeco.2015.02.010) [DOI] [Google Scholar]
- 180.Guo QT, Jiang X, Lei YJ, Li M, Ma YF, Zheng ZM. 2015. Two-stage effects of awareness cascade on epidemic spreading in multiplex networks. Phys. Rev. E 91, pARTN012822. ( 10.1103/PhysRevE.91.012822) [DOI] [PubMed] [Google Scholar]
- 181.Juher D, Kiss IZ, Saldana J. 2015. Analysis of an epidemic model with awareness decay on regular random networks. J. Theor. Biol. 365, 457–468. ( 10.1016/j.jtbi.2014.10.013) [DOI] [PubMed] [Google Scholar]
- 182.Liu C, Xie J-R, Chen H-S, Zhang H-F, Tang M. 2015. Interplay between the local information based behavioral responses and the epidemic spreading in complex networks. Chaos 25, 103111 ( 10.1063/1.4931032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chen F, Griffith A, Cottrell A, Wong YL. 2013. Behavioral responses to epidemics in an online experiment: using virtual diseases to study human behavior. PLoS ONE 8, e52814 ( 10.1371/journal.pone.0052814) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kitchovitch S, Lio P. 2010. Risk perception and disease spread on social networks. ICCS 2010 – Int. Conf. on Comput. Sci. Proc. 1, 2339–2348. ( 10.1016/j.procs.2010.04.264) [DOI] [Google Scholar]
- 185.Kitchovitch S, Lio P. 2011. Community structure in social networks: applications for epidemiological modelling. PLoS ONE 6, e22220 ( 10.1371/journal.pone.0022220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ni SJ, Weng WG, Zhang H. 2011. Modeling the effects of social impact on epidemic spreading in complex networks. Physica A-Stat. Mech. Appl. 390, 4528–4534. ( 10.1016/j.physa.2011.07.042) [DOI] [Google Scholar]
- 187.Rutherford G, Friesen MR, McLeod RD. 2012. An agent based model for simulating the spread of sexually transmitted infections. Online J. Public Health Inf. 4 ( 10.5210/ojphi.v4i3.4292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shang YL. 2013. Modeling epidemic spread with awareness and heterogeneous transmission rates in networks. J. Biol. Phys. 39, 489–500. ( 10.1007/s10867-013-9318-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shang YL. 2015. Degree distribution dynamics for disease spreading with individual awareness. J. Syst. Sci. Complex. 28, 96–104. ( 10.1007/s11424-014-2186-x) [DOI] [Google Scholar]
- 190.Mannberg A. 2012. Risk and rationalization-the role of affect and cognitive dissonance for sexual risk taking. Eur. Econ. Rev. 56, 1325–1337. ( 10.1016/j.euroecorev.2012.06.005) [DOI] [Google Scholar]
- 191.Hochbaum G, Rosenstock I, Kegels S. 1952. Health belief model. Washington, DC: United States Public Health Service. [Google Scholar]
- 192.Skea ZC, Entwistle VA, Watt I, Russell E. 2008. ‘Avoiding harm to others’ considerations in relation to parental measles, mumps and rubella (MMR) vaccination discussions–an analysis of an online chat forum. Soc. Sci. Med. 67, 1382–1390. ( 10.1016/j.socscimed.2008.07.006) [DOI] [PubMed] [Google Scholar]
- 193.Determann D, de Bekker-Grob EW, French J, Voeten HA, Richardus JH, Das E, Korfage IJ. 2016. Future pandemics and vaccination: public opinion and attitudes across three European countries. Vaccine 34, 803–808. ( 10.1016/j.vaccine.2015.12.035) [DOI] [PubMed] [Google Scholar]
- 194.Dorell C, Yankey D, Kennedy A, Stokley S. 2013. Factors that influence parental vaccination decisions for adolescents, 13 to 17 years old national immunization survey–teen, 2010. Clin. Pediatr. 52, 162–170. ( 10.1177/0009922812468208) [DOI] [PubMed] [Google Scholar]
- 195.Green EC, Murphy E. 2014. Health belief model. In The Wiley Blackwell encyclopedia of health, illness, behavior, and society. Chichester, UK: John Wiley. [Google Scholar]
- 196.Rosenstock IM, Strecher VJ, Becker MH. 1988. Social learning theory and the health belief model. Health Educ. Behav. 15, 175–183. ( 10.1177/109019818801500203) [DOI] [PubMed] [Google Scholar]
- 197.Salathe M, Khandelwal S. 2011. Assessing vaccination sentiments with online social media: implications for infectious disease dynamics and control. PLoS Comput. Biol. 7, e1002199 ( 10.1371/journal.pcbi.1002199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Van Kerckhove K, Hens N, Edmunds WJ, Eames KTD. 2013. The impact of illness on social networks: implications for transmission and control of influenza. Am. J. Epidemiol. 178, 1655–1662. ( 10.1093/aje/kwt196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mossong J, et al. 2008. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 5, e74 ( 10.1371/journal.pmed.0050074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang Z, Andrews MA, Wu Z-X, Wang L, Bauch CT. 2015. Coupled disease–behavior dynamics on complex networks: a review. Phys. Rev. 15, 1–29. ( 10.1016/j.plrev.2015.07.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Manfredi P, D'Onofrio A. 2013. Modeling the interplay between human behavior and the spread of infectious diseases. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 202.Grim P, Reade C, Singer DJ, Fisher S, Majewicz S. 2010. What you believe travels differently: information and infection dynamics across sub-networks. Connect (Tor) 30, 50–63. [PMC free article] [PubMed] [Google Scholar]
- 203.Betsch C, et al. 2012. Opportunities and challenges of Web 2.0 for vaccination decisions. Vaccine 30, 3727–3733. ( 10.1016/j.vaccine.2012.02.025) [DOI] [PubMed] [Google Scholar]
- 204.Davies P, Chapman S, Leask J. 2002. Antivaccination activists on the world wide web. Arch. Dis. Child. 87, 22–25. ( 10.1136/adc.87.1.22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Larson HJ, et al. 2013. Measuring vaccine confidence: analysis of data obtained by a media surveillance system used to analyse public concerns about vaccines. Lancet Infect. Dis. 13, 606–613. ( 10.1016/S1473-3099(13)70108-7) [DOI] [PubMed] [Google Scholar]
- 206.Massaro E, Bagnoli F. 2014. Epidemic spreading and risk perception in multiplex networks: a self-organized percolation method. Phys. Rev. E 90, 052817 ( 10.1103/PhysRevE.90.052817) [DOI] [PubMed] [Google Scholar]
- 207.Zhou J, Xiao G, Cheong SA, Fu X, Wong L, Ma S, Cheng TH. 2012. Epidemic reemergence in adaptive complex networks. Phys. Rev. E 85, 036107 ( 10.1103/PhysRevE.85.036107) [DOI] [PubMed] [Google Scholar]
- 208.Mao L. 2014. Modeling triple-diffusions of infectious diseases, information, and preventive behaviors through a metropolitan social networkan agent-based simulation. Appl. Geogr. 50, 31–39. ( 10.1016/j.apgeog.2014.02.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.