Abstract
The endothelial cells that line blood and lymphatic vessels undergo complex, collective migration and rearrangement processes during embryonic development, and are known to be exquisitely responsive to fluid flow. At present, the molecular mechanisms by which endothelial cells sense fluid flow remain incompletely understood. Here, we report that both the G-protein-coupled receptor sphingosine 1-phosphate receptor 1 (S1PR1) and its ligand sphingosine 1-phosphate (S1P) are required for collective upstream migration of human lymphatic microvascular endothelial cells in an in vitro setting. These findings are consistent with a model in which signalling via S1P and S1PR1 are integral components in the response of lymphatic endothelial cells to the stimulus provided by fluid flow.
Keywords: lymphatic endothelial cell, migration, S1PR1, fluid shear stress
1. Introduction
The lymphatic system drains excess interstitial fluid and returns it to the circulating blood. Improper lymph drainage due to either vessel malformation or valve malfunction can lead to tissue swelling, termed lymphedema, which in addition to disfigurement and debilitation can contribute to the pathophysiology of atherosclerosis, hypertension and the formation of blood clots (thrombosis) [1–5]. Failure to establish proper cardiac lymph drainage is also thought to be a primary factor in organ rejection for heart transplants [6], further illustrating the importance of this sometimes overlooked component of the circulatory system.
Fluid flow is an important cue in the development and stabilization of the lymphatic system [5,7–15]. Lymphatic endothelial cells (LECs), which line lymphatic vessels, experience a wide range in wall shear stress (WSS), ranging from 0 to 12 dynes cm−2 in rat mesenteric prenodal lymphatics [13], and up to 40 dynes cm−2 in models of lymphedema [16]. Interestingly, the physical cue provided by WSS has been proposed to play an essential role in the development of lymphatic valves, which preferentially form at constrictions and vessel junctions, where both geometries feature spatial gradients in WSS [5,11].
Previous studies have examined how endothelial cells (ECs) from the blood vasculature sense and respond to fluid flow [17–19]. PECAM-1, VE-Cadherin, VEGFR-2 and VEGFR-3 have previously been identified as components of a molecular flow sensor that converts the physical stimulus provided by WSS into downstream signalling via VEGFR and PI3K [10,17,20,21]. G-protein-coupled receptors (GPCRs) have also been implicated in flow sensing. Notably, measurements using a Förster resonance energy transfer-based sensor suggest that bradykinin B2 undergoes changes in conformation and activity in response to fluid shear stress, consistent with a direct role in flow sensing [22]. Recent evidence likewise implicates sphingosine 1-phosphate receptor 1 (S1PR1), another GPCR, in the response of endothelial cells to fluid flow [23]. S1PR1 knockout mice die in utero between E13.5 and E14.5 due to defects in vascular stabilization [23–27]. How S1PR1 contributes to vessel stabilization is incompletely understood. However, previous work shows that the presence of S1PR1 was required for human umbilical vein ECs (HUVECs) to initiate downstream signalling via ERK1/2 specifically in response to WSS [23].
More broadly, S1PR1 and its ligand S1P are known to play central roles in individual and collective cell migration. The concentration of S1P is negligible in interstitial fluid, but is ca. 100 nM in blood and lymphatic fluid [28]. Because of this, the local concentration of S1P provides an important cue in the context of immunological function, lymphangiogenesis and blood vessel stabilization. Loss of S1PR1 in RBL-2H3 cells, derived from mast cells (a type of white blood cell), results in decreased chemotactic motility [29,30], and S1PR1 activation via S1P is required for lymphocyte egress from the thymus and secondary lymphoid organs in mice [28,31,32]. S1P promotes LEC sprouting in vitro in a manner that requires S1PR1 [33]. In zebrafish embryos, S1P and its receptors are required for collective migration of prechordal plate progenitor cells [34]. This observation suggests that signalling via S1P may play broader roles in embryonic development than is currently appreciated.
Previously, we developed an impinging flow chamber (IFC) to impart controlled spatial gradients in WSS to a monolayer of adherent cells in a six-well plate fashion [35–37]. Using this device, we found that human lymphatic microvascular endothelial cells (HLMVECs), uniquely among the cells assayed, migrated upstream, against the flow direction [35]. Here, we show that this upstream migratory phenotype requires both S1PR1 and its ligand S1P. As discussed above, the requirement for S1P is significant in that S1P provides a chemical cue unique to the lymphatic and blood circulatory systems. Our data are consistent with a model in which S1P and flow act synergistically during the development and remodelling of the lymphatic system.
2. Material and methods
2.1. Cell culture
Primary HLMVECs (CC-2810) were purchased from Lonza Corporation (Walkersville, MD, USA) and cultured in EGM-2 basal medium (Lonza CC-3156) with supplements and growth factors (Lonza CC-4147) containing 5% FBS (fetal bovine serum), hEGF, VEGF, hFGF-B, R3-IGF-1, hydrocortisone and ascorbic acid. In total, 50 units ml−1 of penicillin and 50 µg ml−1 streptomycin (Life Technologies, Carlsbad, CA, USA) were added to the medium. Unless stated otherwise, cells used for experiments were between passages 6 and 10. Three to five days before the experiment, depending on the desired initial confluency, cells were plated onto a 6-well cell culture dish with a #1.5 glass coverslip bottom (Cellvis, Sunnyvale, CA, USA). These dishes were pre-coated with 0.2% gelatin (Sigma-Aldrich, Saint Louis, MO, USA) for 1 h. Cells were plated at 0.5–1.5 × 105 cells per dish and incubated at 37°C and 5% CO2. We performed parallel plate experiments using commercially available parallel plate flow chambers μ-Slide VI0.4 (80601, Ibidi, Madison, WI, USA). The channels were pre-coated with 0.2% gelatin for 1 h. Cells were plated at 7.5 × 104 cells per well on each chamber and incubated at 37°C and 5% CO2.
Prior to imaging, the EGM-2 medium was exchanged to Leibovitz's L-15 medium (Life Technologies) to allow for imaging independent of CO2. The L-15 medium included 5% FBS, the endothelial growth factor kit from Lonza (CC-4147), 50 units ml−1 of penicillin and 50 µg ml−1 streptomycin (Life Technologies). EC experiments were performed with cells at surface coverage (fraction of the coverslip covered by cells) of greater than 95% and confluency (fraction of maximum cell density) of 80% to ensure sufficient contact with neighbouring cells.
2.2. Fluid dynamics
Details of the fluid dynamics of our IFC have been previously reported [36]. Our IFC contains six replicate pairs of submerged jet orifices that apply impinging flow of cell culture media to a monolayer of adherent cells on standard glass-bottom 6-well dishes (figure 1a). The adherent cells experience an axisymmetric spatial gradient in WSS that can be tuned by changing the volumetric flow rate of media dispensed by a 9-roller dampened peristaltic pump (Idex, Oak Harbor, WA, USA). One orifice is located at the centre of each well and dispenses media while the second orifice aspirates media and is located far away from the inlet such that the exiting of the fluid does not alter the impinging flow region of interest (figure 1a).
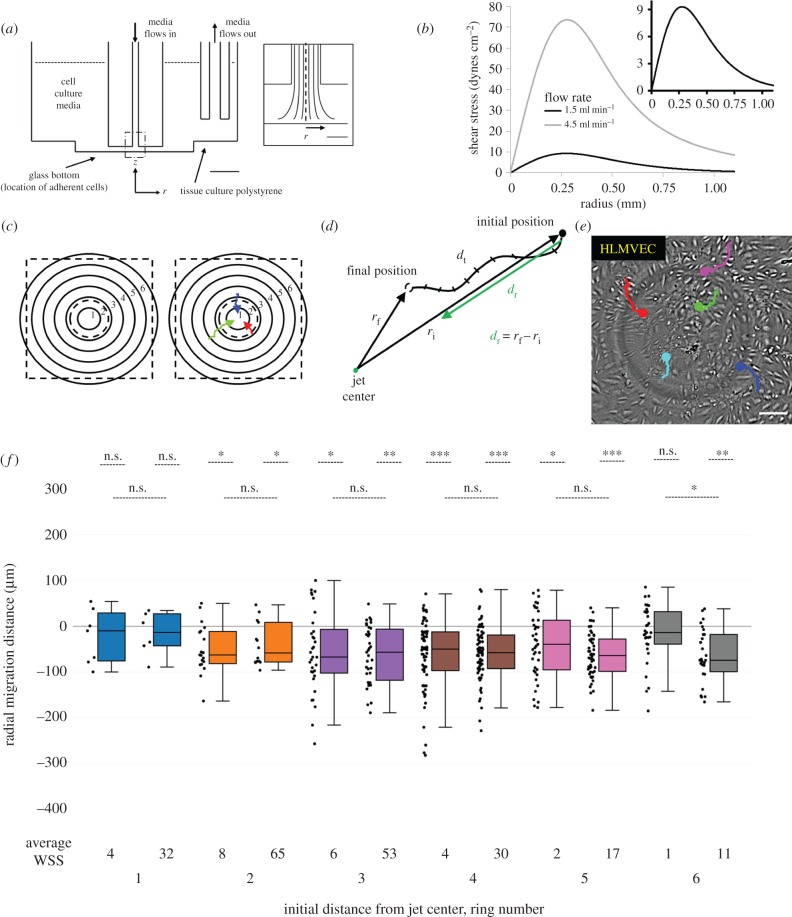
Figure 1.
HLMVECs migrate against the flow direction in an impinging flow device. (a) A cross section showing to scale the location of the two jet orifices, which are submerged in media inside each well. The orifice that applies impinging flow is 1 mm away from the glass bottom of each well, while the orifice that removes media from the well is 4 mm from the polystyrene bottom of the well. Scale bar, 5 mm. Inset: The dashed region of impinging flow in (a). The velocity flow profile and thus WSS profile are axisymmetric about r = 0. Scale bar, 500 µm. This figure is adapted from a prior publication [36]. (b) WSS (i.e. shear experienced at the cell surface) is plotted as a function of radial distance from the jet centre for two representative vascular shear stress profiles: 0–9 dynes cm−2 at a volumetric flow rate of 1.5 ml min−1 (black) and 0–72 dynes cm−2 at a volumetric flow rate of 4.5 ml min−1 (grey). Hereafter, WSS conditions are referred to by the maximum WSS (either 9 or 72 dynes cm−2). (c) Cells are binned by their initial starting position at time t = 0 into one of six concentric 185 µm rings around the jet centre. Ring 1 is the closest to the jet centre and Ring 6 is the farthest. The dashed black square indicates the field of view of captured images during experiments and the inner dashed black circle indicates the approximate location of the maximum WSS, in between Rings 1 and 2. (d) The radial cell migration distance (dr) (green line) is defined by calculating the difference between the initial and final radial distances from the jet centre. The total distance an EC travels (dt) during the duration of an experiment is determined by summing the distance the cell moved frame-by-frame. (e) Live-cell image of HLMVECs near the jet centre, after exposure to a maximum WSS of 9 dynes cm−2 at t = 20 h. The migration trajectories of five cells are shown as coloured lines, starting at t = 0 with the dot indicating its final location at t = 20 h. The light grey circle results from the shadow of the jet orifice. Scale bar, 100 µm. (f) Box-and-whisker plots showing the radial migration distance of tracked cells binned by initial starting position in response to 20 h of impinging flow at a maximum WSS of 9 dynes cm−2 (left plots per ring number) or a maximum WSS of 72 dynes cm−2 (right plots per ring number). The average WSS at the given ring number is presented underneath each box-and-whisker plot. A positive migration distance indicates migration away from the jet centre, while a negative migration distance indicates migration towards the jet centre. N = 200 cells per condition, taken from two independent experiments.
Before exposing the cells to impinging flow, the IFC was prepared by adding 10 ml of L-15 medium to each of the six chambers in the absence of cells followed by the removal of bubbles. Medium was circulated through the device tubing. Once the tubing lines were filled, the device was stopped and the remaining air was removed through a syringe. The flow rate in all experiments was 1.5 ml min−1, corresponding to a peak WSS of 9 dynes cm−2. All flow experiments were performed for 20 h. Cleaning of the device after each experiment was performed analogously to preparation and is detailed in our prior publication [36]. Parallel plate experiments were performed using the same peristaltic pump detailed above. Shear stress calculations for the channels used were provided online by Ibidi. All parallel plate experiments were performed with a uniform WSS of 12 dynes cm−2 corresponding to a flow rate of 7.3 ml min−1.
Cell migration was captured through a Flea 3 camera (Point Grey, British Columbia, Canada) using a Nikon TE inverted microscope (Nikon Corporation, Tokyo, Japan) with a Nikon 4× objective and a 1.5× tube lens. A custom designed temperature control chamber was used to keep the ambient temperature at 37°C.
2.3. Inhibitor and siRNA experiments
Each well of the 6-well IFC is connected to a separate reservoir of medium, allowing for six simultaneous, independent experiments. Generally, four experimental conditions and duplicate control samples were cultured independently and subjected to identical flow conditions.
Stock concentrations of inhibitors were diluted in L-15 medium and added to the cells 1 h prior to the start of flow experiments. The inhibitor was also included in the recirculated medium to maintain its concentration throughout the IFC. W146, a S1PR1 antagonist (Ki = 77 nM), was purchased from Cayman Chemical (Item No. 10009109, Ann Arbor, MI, USA).
Small interfering RNA (siRNA) knockdown experiments were performed using the Lipofectamine® RNAiMAX Transfection Reagent (13778030, Thermo-Fisher Scientific, Waltham, MA, USA) to deliver siGENOME SMARTpool S1PR1 siRNA (GE Dharmacon, Lafayette, CO, USA). Scrambled siRNA (Negative Control of siRNA Duplex, 027210, QIAgen, Hilden, Germany) was used for both quantitative real-time PCR and flow experiments as a negative control to verify that siRNA delivery did not adversely affect the HLMVECs. Delivery of siRNA was performed based on a standardized protocol detailed by Life Technologies: 1 day prior to the experiment, siRNA and Lipofectamine dilutions in Opti-MEM® (Thermo-Fisher Scientific) were prepared separately, mixed and incubated at room temperature for 20 min. The resulting solution was added dropwise to HLMVECs (approx. 80% confluency) plated on the glass-bottom 6-well plate in EGM-2 medium and incubated at 37°C for 24 h. After incubation, the EGM-2 medium with Lipofectamine/siRNA treated medium was exchanged with L-15 and the flow experiment was performed.
2.4. Quantitative real-time PCR
RNA was isolated and purified using the GENEjet RNA Purification Kit (#K0731, Thermo-Fisher Scientific). RNA was converted to cDNA using the Applied Biosystems High Capacity Reverse Transcription Kit (4368814, Thermo-Fisher Scientific). qPCR was performed through use of the Applied Biosystems StepOne Plus Real Time PCR System (Thermo-Fisher Scientific) with Power SYBR® Green PCR Master Mix (4367659, Thermo-Fisher Scientific). To determine the relative mRNA expression levels, two normalization factors were used: the gene ActB and the negative control samples that had been treated with scrambled siRNA. The ActB gene served as a housekeeping gene and the scrambled siRNA samples served as a reference baseline by which all relative S1PR1 mRNA expression levels were compared. S1PR1 qPCR primers used were: Forward, 5′-TGCGGGAAGGGAGTATGTTT-3′ and Reverse, 5′-CGATGGCGAGGAGACTGAAC-3′. ActB primers used were: Forward 5′-TTCTACAATGAGCTGCGTGTG-3′ and Reverse, 5′-ATCACAATGCCAGTGGTACG-3′. All trials were run in triplicate.
2.5. S1P addback experiments
On the day of the experiment, HLMVECs were switched from the EGM-2 medium to non-supplemented Opti-MEM [26]. Cells were starved in Opti-MEM for 2 h. Charcoal-stripped L-15 medium (CS-L15) which contained no lipid/lipophilic molecules was prepared using charcoal-stripped FBS (12676011, Life Technologies) instead of standard FBS and with the addition of the antibiotics and endothelial growth factors (see Cell culture). Sphingosine 1-phosphate (Cayman Chemical, Item No. 62570), dissolved in 0.5% bovine serum albumin (Sigma-Aldrich) in sterile phosphate-buffered saline (Thermo-Fisher Scientific), buffered to pH 7.4 by titration with NaOH, was added in defined amounts to CS-L15. The resulting CS-L15 with or without S1P was added to the serum-starved HLMVECs, and incubated at 37°C for an hour prior to the start of a flow experiment.
2.6. Analysis of cell migration
Bright-field movies were processed with Fiji software and analysed using custom Matlab routines. All image files were bandpass filtered in Fiji using high- and low-frequency cutoffs at 2 and 20 pixels (approx. 2.5 and 25 µm). Cell migration was tracked for 20 h using the Manual Tracking plugin in Fiji for 100 cells per well. Cellular x- and y-positions were determined every 20 min and input into a custom Matlab script. Displacement in the radial direction (figure 1d) relative to the position of the jet centre, along with the total distance a cell travels during the duration of an experiment were calculated for each tracked cell. The persistence of migration was calculated as a ratio between these two values (dr/dt) ranging between −1 and 1, where the limiting cases indicate migration along a radius towards or away from the jet centre, respectively, with a value of 0 indicating random migration with no net displacement. Tracked cells were binned by their initial starting position in the experiment (figure 1c). The migration and radial persistence of HLMVECs were determined for duplicate datasets (200 cells in total per trial) and graphed on box-and-whisker plots using Plotly (plot.ly). Tests for pairwise equal median statistical significance were performed using the Wilcoxon rank sum test, while tests for zero median statistical significance of a dataset were determined using the Wilcoxon signed-rank test. For all box-and-whisker plots, the lower significance bars indicate the pairwise equal median comparison for a given ring. Not significant (n.s.) indicates that the medians of the pair of distributions are statistically indistinguishable. The upper significance bars indicate results from the zero median test, where n.s. indicates that the distribution has a median statistically indistinguishable from zero. Statistical significance is denoted by asterisks above the significance bar. To account for the multiple comparison tests being performed, a Bonferroni correction was applied to conservatively set the p-value for significance to less than or equal to 0.0167 (for three independent comparisons at 5% significance) for each pair of conditions per ring. A single asterisk indicates p < 0.0167, whereas two or three asterisks indicate p < 10−3 or p < 10−7, respectively. All experiments are shown in duplicate, where two independent flow experiments were performed from HLMVECs cultured in different batches.
3. Results
3.1. An impinging flow chamber applies spatial gradients in wall shear stress to cultured endothelial cells
The 6-well IFC apparatus fits on standard 6-well cell culture plates, applies an axisymmetric WSS gradient about the flow-stagnation point (figure 1a), and is compatible with both brightfield and fluorescence live-cell imaging [36]. Advantages of the IFC are that it applies a range of WSSs in one experiment, and moreover replicates the spatially varying WSS found at vessel bends, constrictions and bifurcations. The magnitude of the maximum WSS and WSS gradient can be tuned across a wide range of values by changing the flow rate. In previous work, we assayed a number of adherent cell types [36] and found that, unusually, HLMVECs migrate upstream, against the direction of flow. This upstream migration required cell–cell contact, as cells without nearby neighbours were unable to migrate against the flow direction, indicating that the migratory response was a collective rather than individual response to WSS [35].
Here, we subjected HLMVECs to impinging flow for 20 h, under conditions that generated WSSs of 0–10 dynes cm−2, a range found in the lymphatic system [12]. Consistent with previous results, HLMVECs migrated upstream, against the flow direction (figure 1e,f). Inward cell migration was generally similar for cells subjected to WSS ranges of either 0–10 or 0–72 dynes cm−2, with the exception that cells at the periphery of the field of view (Ring 6) migrated inward at the high, but not low, WSS condition (figure 1f). These observations indicate that (i) inward migration towards the jet centre resulted from an active response to WSS and (ii) even the relatively modest WSSs found in the lymphatic vasculature were sufficient to elicit this response.
3.2. S1PR1 is required for human lymphatic microvascular endothelial cell inward migration in response to wall shear stress
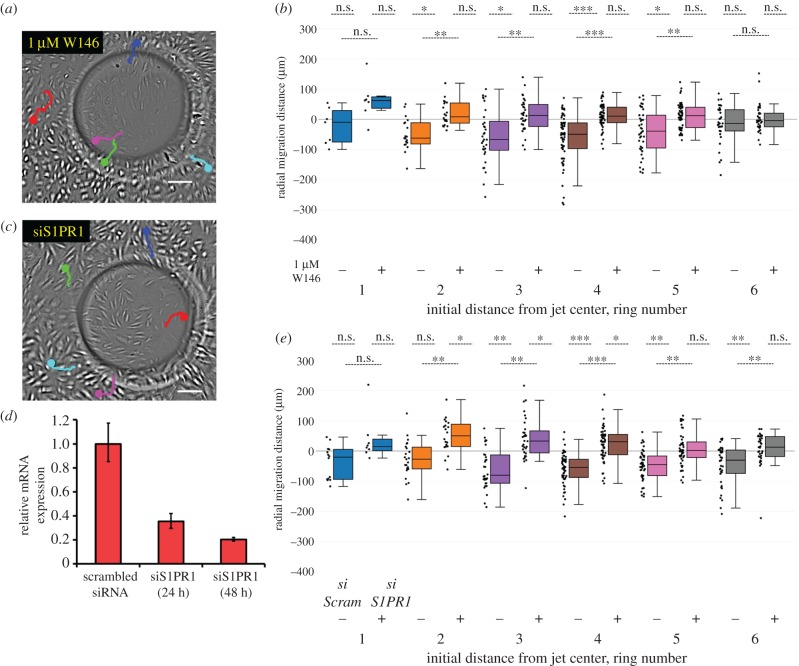
We previously showed that the six-well impinging flow device is well suited to perform medium-throughput screens. Using this device, we previously reported that HLMVEC migration in response to impinging flow required activation of src tyrosine kinase, focal adhesion kinase and phosphoinositide 3-kinase (PI3K) [36]. Here, we report that the G-protein-coupled receptor S1PR1 is also required for the HLMVEC migration against the flow direction (figure 2). Addition of 1 µM W146, a specific and potent S1PR1 inhibitor [24], abolished net inward migration in response to impinging flow (figure 2a,b). S1PR1 inhibition with W146 (1 µM) likewise blocked net inward migration at flow conditions which resulted in peak WSS of 72 dynes cm−2, demonstrating the robustness of the result (electronic supplementary material, figure S6a). To confirm the role of S1PR1 in flow-induced migration, we performed a transient siRNA knockdown of S1PR1 (figure 2d) and saw an analogous prevention of directional migration (figure 2c,e). This result was robust across a wide range of WSSs (electronic supplementary material, figure S7a).
Figure 2.
S1PR1 is required for HLMVEC migration in response to impinging flow. (a) HLMVECs treated with 1 µM W146 do not migrate against the flow direction in response to impinging flow. Live cell image at t = 20 h near the jet centre. The migration trajectories of five cells are shown as coloured lines, starting at t = 0 with the dot indicating its final location at t = 20 h. Scale bar, 100 µm. (b) Box-and-whisker plots showing the radial migration distance of tracked cells that were untreated (−) or treated with 1 µM W146 (+), binned by initial starting position in response to 20 h of impinging flow at a maximum WSS of 9 dynes cm−2. Here, a positive migration distance indicates migration away from the jet centre, while negative migration distance indicates migration towards the jet centre. N = 200 cells per condition, taken from two independent experiments. (c) HLMVECs treated with siRNA for S1PR1 do not migrate against the flow direction in response to impinging flow. Live cell image at t = 20 h around the jet centre. The migration trajectories of five cells are shown as coloured lines, starting at t = 0 with the dot indicating its final location at t = 20 h. Scale bar, 100 µm. (d) Relative S1PR1 mRNA expression (referenced to scrambled siRNA control) for HLMVECs after 24 and 48 h. (e) Box-and-whisker plots showing the radial migration distance of tracked cells both treated with scrambled siRNA (−) and treated with siRNA targeting S1PR1 (+), binned by initial starting position in response to 20 h of impinging flow at a maximum WSS of 9 dynes cm−2. N = 200 cells per condition, taken from two independent experiments.
To determine whether S1PR1 was required for the HLMVEC migratory response across other WSS profiles, we treated cells with 1 µM W146 and compared their migration to untreated cells exposed to spatially uniform WSS at 12 dynes cm−2. HLMVECs exposed to spatially uniform WSS also migrated upstream, against the flow direction (electronic supplementary material, figure S2a). In an earlier work, we reported that HLMVECs migrated with the flow direction when exposed to spatially uniform WSS of 12 dynes cm−2 [35]. In subsequent experiments, we found that the upstream migratory response for HLMVECs is most robust for cells between passages 7 and 10, as used in this study (data not shown). Importantly, HMLVECs treated with W146 and exposed to spatially uniform 12 dynes cm−2 WSS migrated with, as opposed to against, the flow direction (electronic supplementary material, figure S2b). In sum, both spatially uniform and spatially varying WSS could trigger directed, upstream migration by HLMVECs, and this response was mediated by S1PR1.
3.3. S1PR1 is required for persistent, directional migration in response to flow
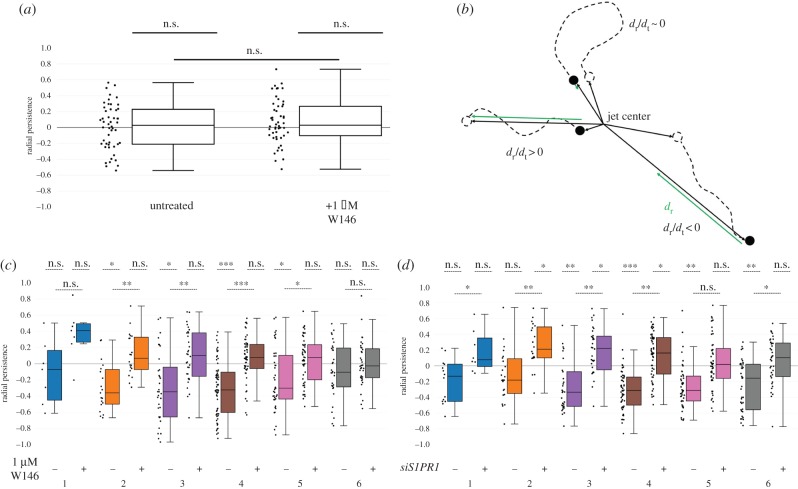
We next sought to determine whether S1PR1 was required for a flow-mediated response to WSS, or was instead required simply for HLMVEC motility. We treated HLMVECs with 1 µM W146 in the absence of flow, and found that cells were motile regardless of W146 treatment (figure 3a). To further quantify the directional nature of HLMVEC migration, we developed a metric termed radial persistence, where a radial persistence of +1 and −1 indicates migration along a radial line away (positive) or towards (negative) the jet centre (figure 3b). Control, untreated HLMVECs exhibited moderately negative radial persistence values for rings 2–5, indicating a persistent inward migration. By contrast, HLMVECs treated with 1 µM W146 on average displayed little to no radial persistence (figure 3c). Similarly, HLMVECs treated with siRNA targeting S1PR1 either displayed no radial persistence at rings close to and far from the jet centre or migrated with the direction of flow (figure 3d). These findings remained unchanged at higher flow rates yielding a maximum WSS of 72 dynes cm−2 (electronic supplementary material, figures S6b and S7b). Taken together, these results indicate that S1PR1 is required for maintaining directionally persistent, inward migration in response to the spatial cue provided by WSS.
Figure 3.
S1PR1 is required for persistent HLMVEC of migration in response to fluid flow. (a) Box-and-whisker plots showing the radial persistence of tracked cells that were untreated (−) or treated with 1 µM W146 (+) in the absence of fluid flow for 20 hours. Average cell displacements were 200±60 and 300±100 μm (mean±standard deviation) for untreated and W146-treated cells, respectively. N = 50 cells. (b) Schematic detailing the calculation of radial persistence for migrating HLMVECs. Analogous to figure 1d, the radial migration distance (dr, green) is calculated with respect to the jet centre, while the total distance travelled dt is indicated by a dashed line. The radial persistence dr/dt ranges from −1 to 1. (c) Box-and-whisker plots showing the radial persistence of tracked cells that were untreated (−) or treated with 1 µM W146 (+), binned by initial position in response to 20 h of impinging flow at a maximum WSS of 9 dynes cm−2. N = 200 cells per condition, taken from two independent experiments. (d) Box-and-whisker plots showing the radial persistence of tracked cells that were treated with scrambled siRNA (−) or treated with siRNA targeting S1PR1 (+), binned by initial starting position in response to 20 h of impinging flow at a maximum WSS of 9 dynes cm−2. N = 200 cells per condition, taken from two independent experiments.
3.4. S1P is required for human lymphatic microvascular endothelial cell migration in response to wall shear stress
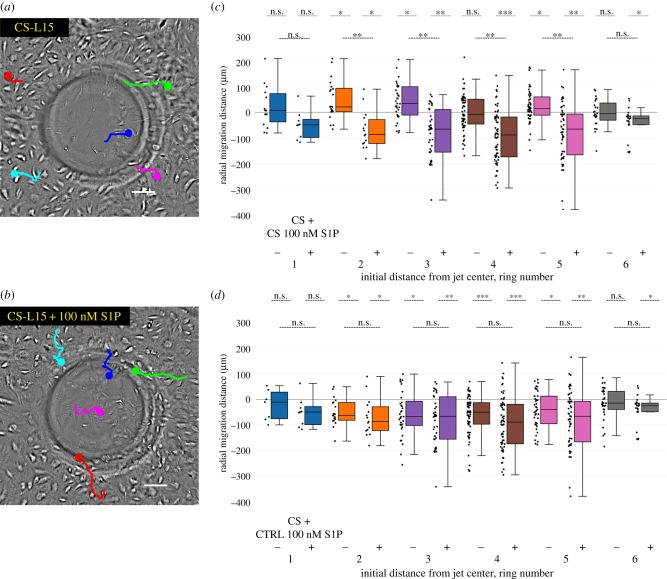
Our next goal was to investigate the mechanism by which S1PR1 may be activated in response to fluid flow. It has been previously shown that S1P is required for lymphangiogenesis and EC motility [33]. We therefore hypothesized that S1P might itself play a necessary role in the inward migration triggered by impinging flow. After starving HLMVECs in serum-free Opti-MEM for 2 h, cells were switched into media prepared with charcoal-stripped FBS (CS-L15), a process that removes lipophilic molecules, including S1P. Under these conditions, HLMVECs did not migrate against the flow direction over the course of 20 h, with many of the cells showing no directional preference (figure 4a,c; electronic supplementary material, figure S4). We next performed the same experiment, but supplemented media prepared with charcoal-stripped FBS and 100 nM of S1P, a physiologically relevant concentration [24,26,38] (figure 4b,c). Under this condition, HLMVECs migrated against the flow direction with near identical radial migration distances and persistence to those of control, untreated, HLMVECs (figure 4d and electronic supplementary material, figure S5) indicating a rescue of their migratory phenotype. To assess whether using CS-L15 prevented EC motility, an identical culture of HLMVECs was imaged in the absence of flow. We observed that there was no statistical difference in the migration of cells in CS-L15 when compared with cells cultured in CS-L15 with 100 nM added S1P in the absence of flow (electronic supplementary material, figure S3), implying a specific role for S1P in mediating the HLMVEC migratory response to flow. Lastly, we examined the importance of S1P in regulating HLMVEC migration if cells were exposed to a higher maximum WSS of 72 dynes cm−2. Under these conditions, HLMVECs were unable to migrate against the flow direction when placed in CS-L15 but similarly regained their inward migratory phenotype when S1P was added back to the CS-L15 (electronic supplementary material, figures S8 and S9).
Figure 4.
S1P is required for HLMVEC migration in response to fluid flow. (a) HLMVECs placed in CS-L15 do not migrate against the flow direction in response to impinging flow. Live cell image at t = 20 h around the jet centre. The migration trajectories of five cells are shown as coloured lines, starting at t = 0 with the dot indicating its final location at t = 20 h. Scale bar, 100 µm. (b) Addition of S1P to CS-L15 rescues the inward migration of HLMVECs. Live cell image at t = 20 h around the jet centre where the maximum WSS is 9 dynes cm−2. The migration trajectories of five cells are shown as coloured lines, starting at t = 0 with the dot indicating its final location at t = 20 h. Scale bar, 100 µm. (c) Box-and-whisker plots showing the radial migration distance of tracked cells in CS-L15 (−) and CS-L15 + 100 nM S1P (+), binned by initial starting position, in response to 20 h of impinging flow at a maximum WSS of 9 dynes cm−2. A positive migration distance indicates migration away from the jet centre. N = 200 cells per condition, taken from two independent experiments. (d) Box-and-whisker plots showing the radial migration distance of tracked cells treated with CS-L15 + 100 nM S1P (+) versus untreated cells (−), binned by initial starting position in response to 20 h of impinging flow at a maximum WSS of 9 dynes cm−2. N = 200 cells per condition, taken from two independent experiments.
4. Discussion
In previous work, we observed that HLMVECs migrated upstream in response to impinging flow. Furthermore, upstream migration was a collective phenomenon, as sparsely seeded HLMVECs are unable to migrate upstream, indicating the need for cell–cell contacts [35]. Here, we extend these observations by showing that upstream migration also occurs at low, physiological WSS levels. In addition, we identify the signalling lipid S1P and its receptor S1PR1 as critical for this response.
Vascular development, and plausibly lymphatic development, relies on long-range, coordinated cell migration in order to pattern developing vessels [39]. The physical mechanisms by which coordinated cell migration arises are incompletely understood [40]. In some such studies, the collective dynamics of confluent (continuous and contacting) sheets of cells exhibit glassy dynamics, meaning that their migration displays properties commonly associated with non-equilibrium matter (foams, polymers, etc.) such as elastic, solid-like behaviour on short timescales and fluid-like cell rearrangements at longer timescales [41–48]. Consistent with this description, confluent epithelial and endothelial sheets show spontaneous stress fluctuations that can propagate many cell lengths across cell monolayers [44,46]. Long-range, coordinated cell movements were shown to be of particular importance in the asthmatic airway epithelium, where large energy barriers to cell reorientation were shown to lock the epithelium in a jammed state that was unable to adapt to global changes in stress [48]. Whether analogous mechanisms underlie the transition from lymphatic (and blood) vessel growth to stabilization remains an important topic for future investigations.
Fluid flow is thought to play a central role in patterning the developing vasculature. In one prominent example, blood flow is required to induce vessel remodelling in the mouse yolk sac, where it triggers upstream migration of ECs from capillaries into growing arterial segments in a process that is coupled to vessel fusion and maturation [39,49,50]. Whether flow may trigger LECs to migrate upstream in an in vivo context has not been examined to our knowledge. At least in principle, upstream migration would provide an appealing mechanism to drive LEC recruitment and hence vessel expansion specifically in vessels experiencing high levels of fluid influx, and hence WSS. We note that in the adult lymphatic vasculature, wound healing also requires a similar process of lymphatic vessel outgrowth followed by stabilization [51]. We speculate that the upstream migratory phenotype displayed by HLMVECs may thus reflect a conserved mechanism for regulating vessel growth and remodelling in a variety of developmental and physiological circumstances.
Our results are consistent with previous work showing that arterial, venous and lymphatic ECs possess a set point for WSS that corresponds to their flow environment in vivo [10]. In particular, VEGFR3 was shown to be required for the heightened sensitivity of LECs to WSS, and siRNA knockdown of VEGFR3 shifted the maximal alignment to flow back to the range shown by blood ECs [10]. Further highlighting their unique sensitivity to WSS, HLMVECs cultured in microchannels and imaged using a ratiometric calcium dye displayed changes in intracellular calcium that scaled with WSS, with a maximum response at 10 dynes cm−2 [12]. Finally, using an ex vivo perfusion system, the contractility of isolated rat thoracic ducts was examined for pressure-driven flow. The mean WSS threshold required to elicit a contractile response was significantly higher at 0.97 dynes cm−2 (5 cmH2O) than 0.64 dynes cm−2 (3 cmH2O) for a modestly higher pressure drop, indicating that the shear-mediated contractile response in the collecting lymphatics is sensitive to changes in WSS as small as 0.3 dynes cm−2 [7].
The mechanism by which fluid flow activates S1PR1 in our study is unclear. One possibility is that the physical stimulus provided by flow activates S1PR1 directly. Consistent with this possibility, a previous study reported that S1PR1 inhibition or knockdown in HUVECs significantly decreased cell alignment in response to spatially uniform fluid flow [23]. Furthermore, the response of these cells to fluid flow could be reconstituted with R120A S1PR1, which cannot be activated by S1P, suggesting that S1PR1 might be activated by fluid shear stress independent of its ligand [23]. However, in our system, S1P appears to be required for the response of HLMVECs to WSS (figure 4). As a provisional interpretation of these results, we suggest that the direct activation of S1PR1 by flow may be context and/or cell-type dependent.
Given that S1P is required for the HLMVEC response to WSS, one plausible explanation for our observations is that the ECs generate a gradient in S1P in response to flow, which in turn triggers directional cell migration via chemotaxis. An S1P concentration gradient could in principle be created through secretion via S1P transporters such as Spinster2 [52], by HLMVEC-mediated depletion of S1P from the media, or both. S1P is a known chemoattractant for a variety of cell types [28,31,32,53,54]. Furthermore, activation of S1PR1 by S1P promotes lymphangiogenesis via Gαi-dependent signalling [33]. However, the full recirculation of cell culture media in the IFC takes 5 min, whereas the HLMVECs migrate on the timescale of multiple hours. It is thus unclear if a chemotactic S1P gradient can be established in the conditions studied here. A possible alternative model is that S1P and S1PR1 act as a permissive signal upstream or in parallel to the signalling pathways that detect the direction of fluid flow. In this model, S1PR1 may not be mechanically activated by flow, but is required to activate downstream signalling involved in flow-mediated collective migration. Given the known requirement of S1PR1 activity in proper VE-Cadherin localization in the developing microvasculature [24], it is possible that signalling via S1PR1 influences the assembly of the flow sensing complex consisting of PECAM, VE-Cadherin and VEGFR2/3 [10,17,21,55], and hence indirectly regulates flow sensing via this pathway.
How activation of S1PR1 drives internal signalling that results in directional cell migration in our study is unclear. Previous work suggests that PI3K is activated downstream of S1P, S1PR1 and Gαi in ECs [56]. Consistent with this understanding, we found that chemical inhibition of PI3K prevented HLMVEC migration against the flow direction in response to impinging flow [36]. In addition, the requirement of S1P could imply an involvement of additional S1P binding receptors such as S1PR2, which has been previously shown to play a role in lymphatic vessel contraction [57]. Additional work, lying outside the scope of this study, will be required to establish the possible role of PI3K and other signalling molecules in mediating the response of LECs to WSS. More broadly, additional experiments examining the roles of S1P and S1PR1 in blood and lymphatic vessel remodelling in vivo present an appealing target for future investigations.
In summary, our results support an important role for S1P and S1PR1 in regulating important aspects of EC migration. In particular, our work suggests that the LEC response to flow requires the simultaneous presence of S1P, a chemical cue that indicates the presence of a functional vessel. We therefore speculate that S1P may act as a permissive cue upstream of the LEC response to flow in a variety of contexts, for example, during lymphatic valve formation and vessel remodelling during development [8,11]. To the best of our knowledge, this model is consistent with extant developmental data, and provides a means by which LECs may combine biochemical and physical information to yield the complex vessel architectures necessary for lymphatic function.
Supplementary Material
Authors' contributions
Research was conducted by V.N.S., E.M. and E.Y.H. Data analysis was performed by V.N.S and E.M. Manuscript and figure preparation was performed by all of the authors Research was supervised and supported by G.G.F. and A.R.D.
Competing interests
There are no actual or potential perceived conflicts of interest among the authors of this paper.
Funding
This work was supported in part by an American Heart Association Predoctoral Fellowship 16PRE27500024 (V.N.S.), National Institutes of Health R01HL128779 (A.R.D. and G.G.F.) and a National Institutes of Health New Innovator Award 1DP2OD007078-01 (A.R.D.).
References
- 1.Rockson SG. 2001. Lymphedema. Am. J. Med. 110, 288–295. ( 10.1016/S0002-9343(00)00727-0) [DOI] [PubMed] [Google Scholar]
- 2.Rockson SG. 2008. Diagnosis and management of lymphatic vascular disease. J. Am. Coll. Cardiol. 52, 799–806. ( 10.1016/j.jacc.2008.06.005) [DOI] [PubMed] [Google Scholar]
- 3.Nakano T, Nakashima Y, Yonemitsu Y, Sumiyoshi S, Chen Y-X, Akishima Y, Ishii T, Iida M, Sueishi K. 2005. Angiogenesis and lymphangiogenesis and expression of lymphangiogenic factors in the atherosclerotic intima of human coronary arteries. Hum. Pathol. 36, 330–340. ( 10.1016/j.humpath.2005.01.001) [DOI] [PubMed] [Google Scholar]
- 4.Alitalo K. 2011. The lymphatic vasculature in disease. Nat. Med. 17, 1371–1380. ( 10.1038/nm.2545) [DOI] [PubMed] [Google Scholar]
- 5.Planas-Paz L, Lammert E. 2013. Mechanical forces in lymphatic vascular development and disease. Cell. Mol. Life Sci. 70, 4341–4354. ( 10.1007/s00018-013-1358-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui Y, Urschel JD, Petrelli NJ. 2001. The effect of cardiopulmonary lymphatic obstruction on heart and lung function. Thorac. Cardiovasc. Surg. 49, 35–40. ( 10.1055/s-2001-9917) [DOI] [PubMed] [Google Scholar]
- 7.Kornuta JA, Nepiyushchikh Z, Gasheva OY, Mukherjee A, Zawieja DC, Dixon JB. 2015. Effects of dynamic shear and transmural pressure on wall shear stress sensitivity in collecting lymphatic vessels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R1122–R1134. ( 10.1152/ajpregu.00342.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabine A, et al. 2015. FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature. J. Clin. Invest. 125, 3861–3877. ( 10.1172/JCI80454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweet DT, Jiménez JM, Chang J, Hess PR, Mericko-Ishizuka P, Fu J, Xia L, Davies PF, Kahn ML. 2015. Lymph flow regulates collecting lymphatic vessel maturation in vivo. J. Clin. Invest. 125, 2995–3007. ( 10.1172/JCI79386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baeyens N, et al. 2015. Vascular remodeling is governed by a VEGFR3-dependent fluid shear stress set point. Elife 4, 3982 ( 10.7554/eLife.04645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabine A, et al. 2012. Mechanotransduction, PROX1, and FOXC2 cooperate to control Connexin37 and calcineurin during lymphatic-valve formation. Dev. Cell 22, 430–445. ( 10.1016/j.devcel.2011.12.020) [DOI] [PubMed] [Google Scholar]
- 12.Jafarnejad M, Cromer WE, Kaunas RR, Zhang SL, Zawieja DC, Moore JE. 2015. Measurement of shear stress-mediated intracellular calcium dynamics in human dermal lymphatic endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 308, H697–H706. ( 10.1152/ajpheart.00744.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE, Zawieja DC. 2006. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 13, 597–610. ( 10.1080/10739680600893909) [DOI] [PubMed] [Google Scholar]
- 14.Cha B, et al. 2016. Mechanotransduction activates canonical Wnt/β-catenin signaling to promote lymphatic vascular patterning and the development of lymphatic and lymphovenous valves. Genes Dev. 30, 1454–1469. ( 10.1101/gad.282400.116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fatima A, et al. 2016. Foxc1 and Foxc2 deletion causes abnormal lymphangiogenesis and correlates with ERK hyperactivation. J. Clin. Invest. 126, 2437–2451. ( 10.1172/JCI80465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahbar E, Akl T, Coté GL, Moore JE, Zawieja DC. 2014. Lymph transport in rat mesenteric lymphatics experiencing edemagenic stress. Microcirculation 21, 359–367. ( 10.1111/micc.12112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tzima E, et al. 2005. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437, 426–431. ( 10.1038/nature03952) [DOI] [PubMed] [Google Scholar]
- 18.DePaola N, Gimbrone MA, Davies PF, Dewey CF. 1992. Vascular endothelium responds to fluid shear stress gradients. Arterioscler. Thromb. Vasc. Biol. 12, 1254–1257. ( 10.1161/01.ATV.12.11.1254) [Erratum in Arterioscler. Thromb. 1993 13, 465.] [DOI] [PubMed] [Google Scholar]
- 19.Sun X, Fu Y, Gu M, Zhang L, Li D, Li H, Chien S, Shyy JY-J, Zhu Y. 2016. Activation of integrin α5 mediated by flow requires its translocation to membrane lipid rafts in vascular endothelial cells. Proc. Natl Acad. Sci. USA 113, 769–774. ( 10.1073/pnas.1524523113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. 2013. Fluid shear stress on endothelial cells modulates mechanical tension across VE-Cadherin and PECAM-1. Curr. Biol. 23, 1024–1030. ( 10.1016/j.cub.2013.04.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coon BG, Baeyens N, Han J, Budatha M, Ross TD, Fang JS, Yun S, Thomas J-L, Schwartz MA. 2015. Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex. J. Cell Biol. 208, 975–986. ( 10.1083/jcb.201408103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chachisvilis M, Zhang Y, Frangos JA. 2006. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc. Natl. Acad. Sci. USA 103, 15 463–15 468. ( 10.1073/pnas.0607224103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung B, et al. 2012. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev. Cell 23, 600–610. ( 10.1016/j.devcel.2012.07.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaengel K, et al. 2012. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev. Cell 23, 587–599. ( 10.1016/j.devcel.2012.08.005) [DOI] [PubMed] [Google Scholar]
- 25.Shoham AB, Malkinson G, Krief S, Shwartz Y, Ely Y, Ferrara N, Yaniv K, Zelzer E. 2012. S1P1 inhibits sprouting angiogenesis during vascular development. Development 139, 3859–3869. ( 10.1242/dev.078550) [DOI] [PubMed] [Google Scholar]
- 26.Chae S-S, Paik J-H, Furneaux H, Hla T, Saba J, Hla T. 2004. Requirement for sphingosine 1–phosphate receptor-1 in tumor angiogenesis demonstrated by in vivo RNA interference. J. Clin. Invest. 114, 1082–1089. ( 10.1172/JCI200422716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunkel GT, Maceyka M, Milstien S, Spiegel S. 2013. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat. Rev. Drug Discov. 12, 688–702. ( 10.1038/nrd4099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnon TI, Xu Y, Lo C, Pham T, An J, Coughlin S, Dorn GW, Cyster JG. 2011. GRK2-dependent S1PR1 desensitization is required for lymphocytes to overcome their attraction to blood. Science 333, 1898–1903. ( 10.1126/science.1208248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, Milstien S, Spiegel S. 2004. Transactivation of sphingosine-1–phosphate receptors by FcεRI triggering is required for normal mast cell degranulation and chemotaxis. J. Exp. Med. 199, 959–970. ( 10.1084/jem.20030680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halova I, Draberova L, Draber P. 2012. Mast cell chemotaxis—chemoattractants and signaling pathways. Front. Immunol. 3, 119 ( 10.3389/fimmu.2012.00119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cyster JG. 2005. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 23, 127–159. ( 10.1146/annurev.immunol.23.021704.115628) [DOI] [PubMed] [Google Scholar]
- 32.Friedl P, Weigelin B. 2008. Interstitial leukocyte migration and immune function. Nat. Immunol. 9, 960–969. ( 10.1038/ni.f.212) [DOI] [PubMed] [Google Scholar]
- 33.Yoon CM, Hong BS, Moon HG, Lim S, Suh P, Kim Y, Chae C-B, Gho YS. 2008. Sphingosine-1-phosphate promotes lymphangiogenesis by stimulating S1P1/Gi/PLC/Ca2+ signaling pathways. Blood 112, 1129–1138. ( 10.1182/blood-2007-11-125203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kai M, Heisenberg C-P, Tada M. 2008. Sphingosine-1-phosphate receptors regulate individual cell behaviours underlying the directed migration of prechordal plate progenitor cells during zebrafish gastrulation. Development 135, 3043–3051. ( 10.1242/dev.020396) [DOI] [PubMed] [Google Scholar]
- 35.Ostrowski MA, et al. 2014. Microvascular endothelial cells migrate upstream and align against the shear stress field created by impinging flow. Biophys. J. 106, 366–374. ( 10.1016/j.bpj.2013.11.4502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostrowski MA, Huang EY, Surya VN, Poplawski C, Barakat JM, Lin GL, Fuller GG, Dunn AR. 2016. Multiplexed fluid flow device to study cellular response to tunable shear stress gradients. Ann. Biomed. Eng. 44, 2261–2272. ( 10.1007/s10439-015-1500-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakayama KH, et al. 2016. Nanoscale patterning of extracellular matrix alters endothelial function under shear stress. Nano Lett. 16, 410–419. ( 10.1021/acs.nanolett.5b04028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hla T, et al. 1999. Sphingosine-1-phosphate: extracellular mediator or intracellular second messenger? Biochem. Pharmacol. 58, 201–207. ( 10.1016/S0006-2952(99)00086-6) [DOI] [PubMed] [Google Scholar]
- 39.Udan RS, Vadakkan TJ, Dickinson ME. 2013. Dynamic responses of endothelial cells to changes in blood flow during vascular remodeling of the mouse yolk sac. Development 140, 4041–4050. ( 10.1242/dev.096255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedl P, Gilmour D. 2009. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 10, 445–457. ( 10.1038/nrm2720) [DOI] [PubMed] [Google Scholar]
- 41.Angelini TE, Hannezo E, Trepat X, Marquez M, Fredberg JJ, Weitz DA. 2011. Glass-like dynamics of collective cell migration. Proc. Natl Acad. Sci. USA 108, 4714–4719. ( 10.1073/pnas.1010059108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bi D, Lopez JH, Schwarz JM, Manning ML, Farhadifar R, Röper J-C. 2014. Energy barriers and cell migration in densely packed tissues. Soft Matter 10, 1885–1890. ( 10.1039/c3sm52893f) [DOI] [PubMed] [Google Scholar]
- 43.Schötz E-M, Lanio M, Talbot JA, Manning ML. 2013. Glassy dynamics in three-dimensional embryonic tissues. J. R Soc. Interface 10, 20130726 ( 10.1098/rsif.2013.0726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trepat X, Fredberg JJ. 2011. Plithotaxis and emergent dynamics in collective cellular migration. Trends Cell Biol. 21, 638–646. ( 10.1016/j.tcb.2011.06.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angelini TE, Hannezo E, Trepat X, Fredberg JJ, Weitz DA. 2010. Cell migration driven by cooperative substrate deformation patterns. Phys. Rev. Lett. 104, 168104 ( 10.1103/PhysRevLett.104.168104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trepat X, et al. 2009. Physical forces during collective cell migration. Nat. Phys. 5, 426–430. ( 10.1038/nphys1269) [DOI] [Google Scholar]
- 47.Sadati M, Taheri Qazvini N, Krishnan R, Park CY, Fredberg JJ. 2013. Collective migration and cell jamming. Differentiation 86, 121–125. ( 10.1016/j.diff.2013.02.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park J-A, et al. 2015. Unjamming and cell shape in the asthmatic airway epithelium. Nat. Mater. 14, 1040–1048. ( 10.1038/nmat4357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lucitti JL, Jones EAV, Huang C, Chen J, Fraser SE, Dickinson ME. 2007. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 134, 3317–3326. ( 10.1242/dev.02883) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Culver JC, Dickinson ME. 2010. The effects of hemodynamic force on embryonic development. Microcirculation 17, 164–178. ( 10.1111/j.1549-8719.2010.00025.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paavonen K, Puolakkainen P, Jussila L, Jahkola T, Alitalo K. 2000. Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing. Am. J. Pathol. 156, 1499–1504. ( 10.1016/S0002-9440(10)65021-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donoviel MS, Hait NC, Ramachandran S, Maceyka M, Takabe K, Milstien S, Oravecz T, Spiegel S. 2015. Spinster 2, a sphingosine-1-phosphate transporter, plays a critical role in inflammatory and autoimmune diseases. FASEB J. 29, 5018–5028. ( 10.1096/fj.15-274936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pappu R, et al. 2007. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 316, 295–298. ( 10.1126/science.1139221) [DOI] [PubMed] [Google Scholar]
- 54.Tanski W, Roztocil E, Davies MG. 2002. Sphingosine-1-phosphate induces Gαi-coupled, PI3 K/ras-dependent smooth muscle cell migration. J. Surg. Res. 108, 98–106. ( 10.1006/jsre.2002.6529) [DOI] [PubMed] [Google Scholar]
- 55.Conway DE, Schwartz MA. 2015. Mechanotransduction of shear stress occurs through changes in VE-cadherin and PECAM-1 tension: implications for cell migration. Cell Adh. Migr. 9, 335–339. ( 10.4161/19336918.2014.968498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spiegel S, Milstien S. 2003. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 4, 397–407. ( 10.1038/nrm1103) [DOI] [PubMed] [Google Scholar]
- 57.Kimizuka K, Kawai Y, Maejima D, Ajima K, Kaidoh M, Ohhashi T. 2013. Sphingosine 1-phosphate (S1P) induces S1P2 receptor-dependent tonic contraction in murine iliac lymph vessels. Microcirculation 20, 1–16. ( 10.1111/micc.12001) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.