Abstract
Using experimental and computational methods, we study the role of behavioural variability in activity bursts (or temporal activity patterns) for individual and collective regulation of foraging in A. senilis ants. First, foraging experiments were carried out under special conditions (low densities of ants and food and absence of external cues or stimuli) where individual-based strategies are most prevalent. By using marked individuals and recording all foraging trajectories, we were then able to precisely quantify behavioural variability among individuals. Our main conclusions are that (i) variability of ant trajectories (turning angles, speed, etc.) is low compared with variability of temporal activity profiles, and (ii) this variability seems to be driven by plasticity of individual behaviour through time, rather than the presence of fixed behavioural stereotypes or specialists within the group. The statistical measures obtained from these experimental foraging patterns are then used to build a general agent-based model (ABM) which includes the most relevant properties of ant foraging under natural conditions, including recruitment through pheromone communication. Using the ABM, we are able to provide computational evidence that the characteristics of individual variability observed in our experiments can provide a functional advantage (in terms of foraging success) to the group; thus, we propose the biological basis underpinning our observations. Altogether, our study reveals the potential utility of experiments under simplified (laboratory) conditions for understanding information-gathering in biological systems.
Keywords: search theory, random walk, agent-based models
1. Introduction
Understanding how informational inputs received by living organisms from external stimuli (the landscape, interindividual interactions, etc.) are combined with prior experience or (internal) motivational states to generate dynamic motor responses is one of the main occupations of the cognitive sciences. Within this context, animal foraging is an appealing topic for study. So far, efforts in this direction have considered a wide variety of model organisms, from bacteria to humans [1]. The typical procedure has been to explore how organisms modify their foraging strategies or performance in response to a variable external stimuli (e.g. food density, the presence of obstacles or landmarks, etc.). Such approaches mainly emphasize the stimuli (or the environment in a more general sense) and assign the organism a more reactive role in which it adapts to the external conditions by choosing a more or less standardized response. By contrast, when animals forage under conditions of minimal information and high uncertainty (low or null external stimuli), such standard or automatized responses are no longer applicable or convenient, and instead it is expected that new, active strategies will be promoted. Understanding the mechanisms generating this active component is the main goal of the recently proposed ecological paradigm of foraging under uncertainty [2], which over the past few years has received systematic study [3–6].
Making a clear distinction between informed and uninformed scenarios is, however, often complex and delicate due to the large variety and number of informational sources that organisms are able to sense and process. Even if external stimuli can be largely suppressed in laboratory conditions, it is relevant to assess, for instance, how social species may integrate individual and collective information for foraging under uncertainty. Understanding the details of social foraging is still a challenging problem for ecologists despite the large efforts that have been made. For instance, collective mechanisms for information transfer and regulation have been extensively explored for social insects within the contexts of labour and specialization [7–12], group activation/recruitment [13–17], synchronization between individuals [18], activity bursting [19] or behavioural syndromes at different scales [20,21]. In addition to the classical exploration/exploitation trade-off [2,3,22,23], social foragers are known to face a dilemma between the exploitation of individual information or the use of signals obtained through interindividual communication [23–25]. Information processing involves social recruitment (e.g. through pheromone trails) and individual retrieving, including all of the potential sampling strategies associated with individual information-gathering. These distinctions are important because relying only on the information shared by the group can result in a poor exploration of new sources of information, thus reducing flexibility and adaptability [26,27].
Ants are very appropriate organisms for exploring all these ideas because it has been proven that they are capable of switching between individual-based and collective-based foraging strategies as a function of environmental conditions; when studying this organism, the prevalence of group recruitment and trail formation can be used as criteria for differentiating ‘individual’ versus ‘collective’ effects. In this article, we aim to demonstrate how experimental approaches under simplified (minimal stimuli) laboratory conditions combined with other tools (computational models) can be a powerful set-up for addressing biologically relevant questions in social insect systems. We illustrate this by exploring the question of how behavioural variability between foragers modifies the overall performance of the group. Other authors have already explored the relationship between individual variability and foraging efficiency in ants and other social insects beginning with seminal works in the 1980s which have examined variability in movement patterns [28,29], information spreading [30] and space use [31,32] of individuals, leading up to more recent references (see [7,9,10,33–35] for a short compilation). As a continuation of this line of work, in this study we have addressed variability at the level of activity bursts in order to characterize temporal activity profiles exhibited by the different ants, a topic that to our knowledge has never been considered directly. The specific steps we have followed are:
(i) First, we experimentally studied the foraging properties of Aphaenogaster senilis ants under conditions that are known to promote individual-based foraging strategies [14,36]. This represents a continuation of our previous work in [36] where similar experiments were done without marking the individuals. In this article, we present new results with marked individuals, allowing us to identify individual activity and motion patterns to quantify behavioural variability in great detail. The data obtained reveal that, at least in the case of A. senilis, it is the dynamics of activity bursts, rather than the trajectory details, which capture most of the behavioural variability among foragers.
(ii) We then use the properties of the foraging trajectories obtained experimentally as an input to feed an agent-based model (ABM) which includes three spatially explicit foraging mechanisms: random search, site fidelity (as a paradigm of memory-wise behaviour) and communication through pheromone trails (collective behaviour). The weight of any mechanism in the model can be tuned by varying some probabilities, which are conditioned to the local presence of pheromones and/or to the prior experience of the individual. This general ABM is used to determine how the individual variability in activity bursts observed experimentally affects foraging performance of the group. While some level of variability is generally expected to enhance foraging success, here we go one step further and try to discriminate between two possible sources of this variability: (i) variability originating from the existence of specific individuals behaving systematically in a different manner (we denote this option as the behavioural stereotypes hypothesis), or (ii) variability occurring because individuals dynamically change their behaviour through time (the behavioural plasticity hypothesis). Concordant with the experimental results, our ABM shows that the behavioural plasticity hypothesis leads to higher levels of foraging success than the behavioural stereotypes hypothesis, so it is expected that the former will be favoured evolutionarily.
2. Experimental material and methods
2.1. Experimental setting
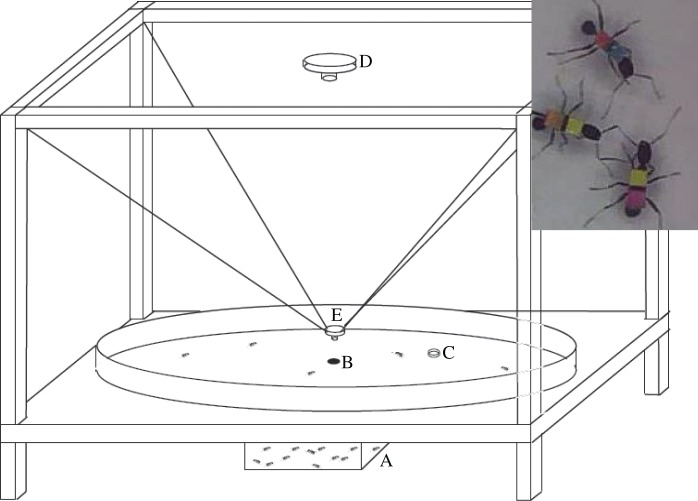
A detailed description of the experimental setting is provided in [36]. We employed a white, wooden, circular arena (radius = 1 m) with a small hole in the centre which is connected to the ant nest below by a plastic tube (internal diameter = 1 cm) (figure 1). The arena is enveloped by a wooden structure covered with a homogeneous dark cloth, making it so that the arena is completely symmetric if seen from its centre and avoiding directional biases to the greatest extent possible. Individual trajectories of foraging ants were tracked using a two-camera system (figure 1). A small wireless camera was put just over the nest entry hole to obtain close-up images of the ants when leaving or returning to the nest, while a wide-angle lens camera mounted on the top of the structure was used to capture the trajectories using a single device.
Figure 1.
Schematic picture of the experimental setting used, where the nest (A) is connected to the center of a circular arena (B) of radius = 1 m which is continuously monitored through two video cameras (D and E) while ants look for food (C). Inset: snapshot of ants used in the experiment, where the two-colour codes used for labelling can be seen. (Online version in colour.)
2.2. Experiments
A group of 70 workers of A. senilis was separated from their colony (previously collected on the Campus of the Universitat Autònoma de Barcelona) and put in a plastic nest together with several eggs and larvae to keep them stimulated for working. The eggs and larvae were not renovated but they were left to grow and become adult during the duration of the experiments; while this introduced some non-stationarity in experimental conditions, we note that the duration of the experiment (one month) was relatively short compared with the time required for the ants to mature to the adult stage, so we expect that this bias had minimum effects on our results.
All of the workers were marked with labels containing a two-colour code (figure 1, inset) glued to their thorax. Though we consulted the methods of previous studies (e.g. [10,37–39]), A.senilis ants turned out to be particularly difficult to mark without affecting their behavioural responses (they exhibited high levels of social grooming and were very sensitive to glues and other chemical products), which prevented us from attempting to reproduce our experiments with additional, or maybe larger, groups of workers. The experiment was conducted during July 2014 in a dark room under conditions of constant temperature and humidity (around 23°C and 50% RH). The experiment consisted of daily trials or replicates. For 50 min each day, ants were allowed to explore the circular arena where they searched for a single small Petri dish containing several food items deposited randomly within the arena. Once the time elapsed, all ants outside the nest were picked up and returned, and the arena was carefully washed with ethanol to remove all traces of chemicals. The trials carried out during the first week were considered as an acclimation period, while the next 24 days were used to collect results. Food items offered to the forager ants consisted of fresh mealworms (one whole mealworm of medium size, ranging between 100 and 150 mg of weight, per day) in order to keep the colony stimulated for foraging at normal levels. By using this controlled set-up—consisting of low group sizes with low resource density in a setting lacking landmarks—we try to reduce social interaction and prevent the formation of pheromone trails.
2.3. Experimental data analysis
All videos recorded were analysed with open source software (Scilab Image and Video Processing Toolbox). Out of the 70 workers initially marked, 48 remained alive and marked at the end of the experimental period (10 died and 12 lost their tags). From these, a total set of 874 excursions and 1038 trajectories were computed and used to calculate population-averaged results. These excursions and trajectories were compared with the individual results obtained from the 14 most active workers, which were responsible for 76% of all excursions. Note that we define an excursion as starting when an ant leaves the nest and enters the arena and finishing when it returns to the nest, while a trajectory (as in [36]) starts when an ant moves at least 5 cm from the nest entry and finishes when it returns to the same 5 cm distance; therefore, a single excursion can involve 0,1,2,… trajectories.
We used the trajectories of the ants to characterize the motion patterns of the individuals. For this, we identified the centre of mass of the ants in each frame, and we compared this centre of mass between consecutive frames (separated by 0.2 s) in order to compute the local displacement and direction of motion, just as described in [36].
The excursions dataset was used to quantify activity levels of individuals throughout time. Individual workers of A. senilis show significant levels of burstiness as they combine periods of high foraging activity (one or several days) with periods of inactivity in which presumably they become engaged in other tasks within the nest. We used standard techniques to quantify burstiness based on the statistics of the activity/inactivity periods. It has been previously shown [40,41] that time burstiness can be appropriately characterized through a (B,M) phase diagram, with B as the burstiness parameter and M the memory coefficient. If τ1, τ2,…,τj represents the series of random times between consecutive excursions of an individual (with mean  and variance
and variance 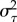 ) then these two parameters are defined as
) then these two parameters are defined as
| 2.1 |
so B is a relative ratio between the fluctuations and the mean value of the series τ1, τ2,…, τj, and Corr(τi, τi+1) represents the correlation coefficient between consecutive interexcursion times.
To carry out the analysis of burstiness, some prior treatment of the data series of excursions was necessary. First, because the trials lasted only 50 min per day we did not consider the pauses or periods of inactivity. Instead, we use a timeline in which consecutive trials are embedded one after the other (each trial is initiated immediately after the previous one). Although this may introduce some biases in our subsequent analyses, we note that some kind of time redefinition is necessary in most analyses of burstiness in animal and human activity [42]. Second, our experiment has the additional complication that foraging activity was not constant during each (daily) trial. In general, activity (the number of excursions) was very low during the first minutes of the trial (because most ants in the nest may not be aware yet that the arena is accessible) and increased gradually until reaching a relatively constant level for the rest of the trial. As a consequence, it was necessary to homogenize foraging intensity, removing any possible trends produced by this inhomogeneity. This was done using the de-seasoning technique described in [43]. This technique basically allows the definition of a new experimental timeline as a function of activity level, such that periods with real-time, systematic observation of low activity are shrunk, and periods of high activity are dilated. To proceed, we denote by n(t) the activity (number of excursions) initiated by ants up to time t. Because the data consists of N = 25 consecutive trials of duration T = 50 min, the relative activity rate for a given point in time of the trial is given by
 |
2.2 |
From this, we can then introduce the de-seasoned (or homogenized) timeline t* as a function of the original t in the form
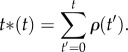 |
2.3 |
So, in practice we use n(t*) instead of n(t) to quantify activity (the reader can go to the original reference [43] for a more detailed discussion and interpretation of the rescaling t*). In any case, though we consider this de-seasoning as a reasonable and necessary step for removing any trends from the data, a posteriori we compared the results with those obtained using the original data series in order to confirm that our general conclusions exposed in the Results section remained the same.
3. Experimental results
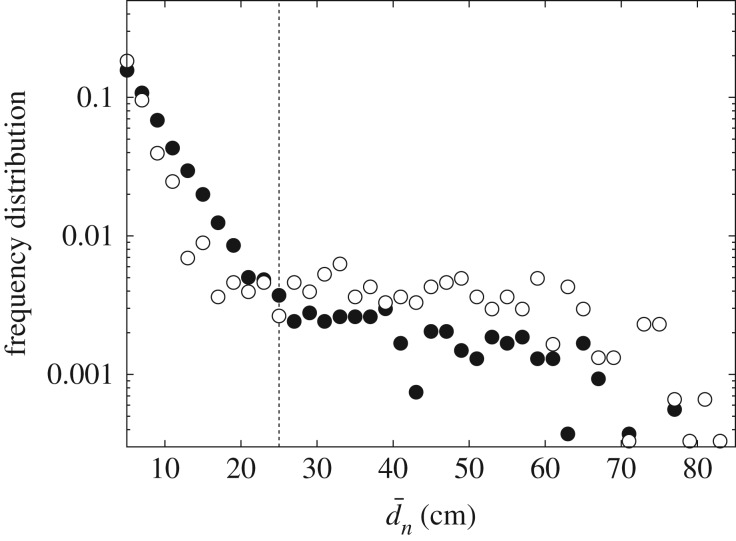
First, we averaged several properties (mean turning angles, mean speed, etc.) over all the trajectories of all individuals. Then, we compared these results (see electronic supplementary material, file S1) with those obtained from previous experiments [36] in which ants were not marked but very similar experimental conditions were used. This demonstrates the reproducibility of our results and verifies that marking the ants did not significantly alter their foraging behaviour. Particularly important is the existence of two well-differentiated types of excursions, which in [36] we termed risk-prone and risk-averse excursions. The former are those trajectories that considerably depart from the nest and last a significant amount of time, while the latter are shorter trajectories that tend to stay relatively close to the nest. The distinction between these is easy to capture visually by plotting the frequency distribution of the mean distance to the nest 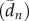 for each excursion (distance is averaged over the entire excursion time); this is shown in figure 2, where a clear bimodal pattern (separated by the vertical dotted line) can be seen.
for each excursion (distance is averaged over the entire excursion time); this is shown in figure 2, where a clear bimodal pattern (separated by the vertical dotted line) can be seen.
Figure 2.
Frequency distribution of the mean distance of each single trajectory to the centre of the arena. Full circles correspond to the experiments from this study, while empty circles correspond to data in [36]. A bimodal decay clearly emerges (separated by a vertical dotted line to facilitate visualization), such that the left and right regions of the plot correspond to risk-averse and risk-prone trajectories, respectively.
These two different patterns clearly show the existence of some behavioural variability whose origin will be explored here with the help of individualized tagging. First, we studied the motion patterns of individuals compared with average (population) behaviour. The distribution of turn angles for ants between consecutive frames is plotted in figure 3a, from which an average turn angle of 0.50 ± 0.39 radians is obtained. We also include the results obtained for the 14 most active individuals. Differences between individuals are rather small, so reorientation patterns show a reduced individual variability (see below and electronic supplementary material, file S2 for further details). Regarding speed distributions (figure 3b), although most individuals show a clear peak in the distribution for values between 20 and 30 mm s−1, here larger variability between individuals is observed. However, we think that speed variability can be hardly interpreted as an adaptive trait for foraging because search efficiency is in general positively correlated with walking speed.
Figure 3.
Comparison between statistics of the trajectories for the most active individuals (thin lines) versus population-averaged behaviour (thick lines). The probability density of instantaneous turn angles (a) and speeds (b) is shown (see electronic supplementary material, file S2 for a further analysis).
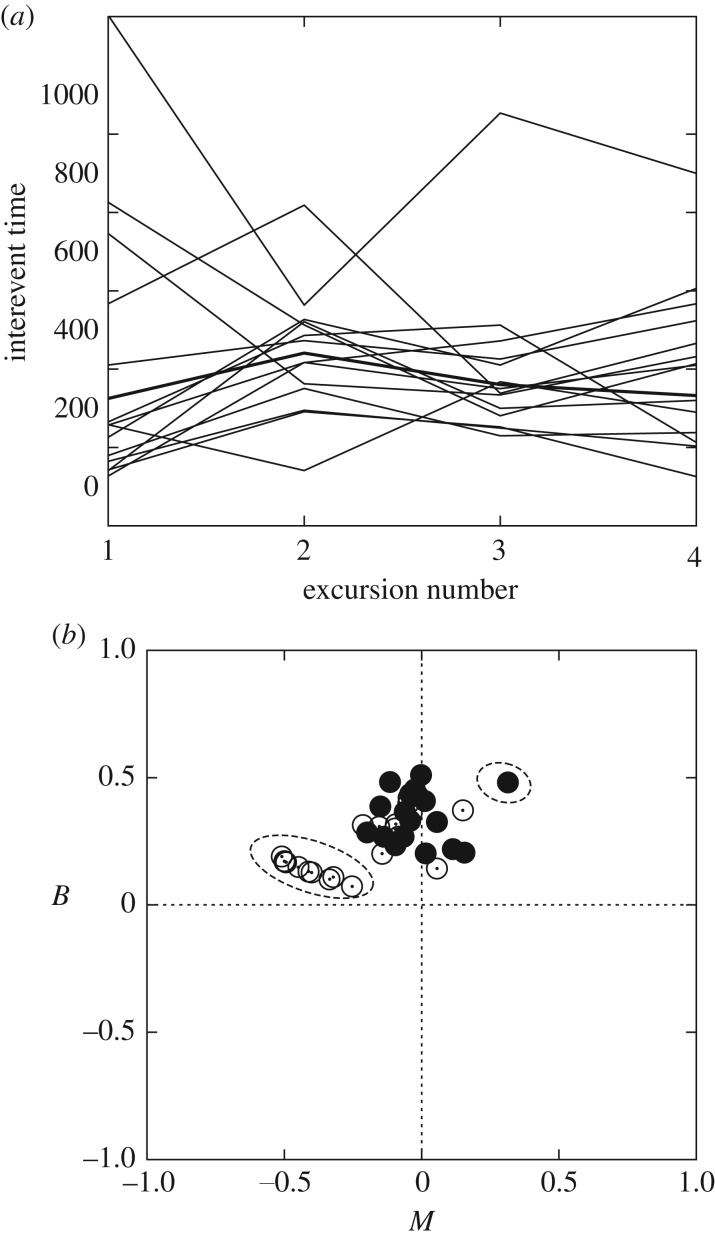
Notably, when foraging activity patterns were studied in detail we obtained much more significant differences between individuals. The mean values of the first, second, third and fourth interexcursion times for each single trial are plotted in figure 4a, showing the comparison between individual and population-averaged values. It is remarkable that variability does not necessarily lead to the existence of some individuals doing longer or shorter excursions than the average systematically; instead, every forager combines both long and short excursions but using different activity cycles. For instance, some individuals use a more cautious strategy based initially on short excursions (possibly used to scan the area surrounding the nest and/or look for information through antennal contact) whose durations gradually increase or saturate. Other individuals, instead, carry out long excursions from the very beginning, which may then be followed by shorter excursions. Also, intermediate or mixed strategies between these two extremes are observed. This variability cannot be explained in terms of the success of previous excursions/individuals because food items present in the arena were scarce and successful excursions represented a small fraction (less than 2%) of the total. Therefore, we interpret that foraging success was not a significant driving force of individual strategies (or at least we were not able to detect such a relation).
Figure 4.
Temporal activity patterns of individuals in the foraging experiments. (a) Comparison between the durations of excursions of the 14 most active individuals (thin lines) versus population-averaged behaviour (thick line). Values plotted correspond to the average duration of the first, second, third and fourth excursions of individuals over the 25 experimental trials performed. (b) (B − M) diagram characterizing the essential properties of burstiness in the activity patterns observed. Full circles correspond to the 14 most active individuals (those represented in figure 2) and empty circles correspond to all others. Dashed ellipses indicate those cases showing significant levels of memory, in contrast with the memoryless patterns exhibited by most individuals.
The (B, M) diagram (figure 4b) reflects that intermediate levels of burstiness, combined with very low levels of memory, are characteristic of foraging activity patterns of most individuals. There were, however, a few individuals who showed significant levels of memory (understood as behavioural time correlation), which were usually negative but with one positive case (marked with ellipses in figure 4b). The specific meaning of those memory effects in these cases is not completely clear from our data, but we suspect that it reflects the strategies of individuals using rather deterministic patterns alternating between short and long excursions. As M measures correlations between consecutive excursion lengths, if the alternation is of type short–long–short then M should be negative, but for patterns including several short or long consecutive excursions a positive M holds.
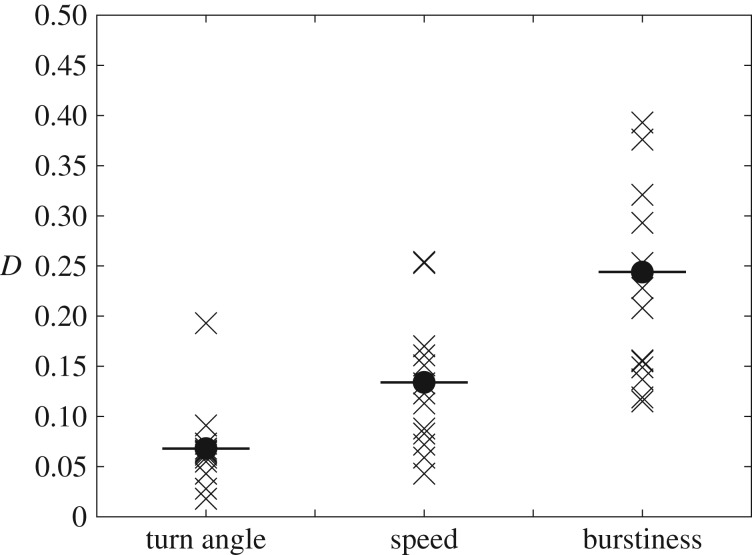
Finally, to summarize our findings about individual variability we computed the statistical distance D between the population-averaged and individual turn angles, speeds and times between excursions using a Kolmogorov–Smirnov test. These results are provided in figure 5, where it is seen that the statistical distance between individual and population-averaged patterns of excursion lengths—as might be intuitively expected from our previous comments and results—is quite large (average of 〈D〉 = 0.244 ± 0.120 for the 14 individuals studied). By contrast, D is rather small for turn angles (〈D〉 = 0.068 ± 0.040), while speed shows intermediate values (〈D〉 = 0.134 ± 0.065). All this indicates that variability between A. senilis foragers is mainly attributable to their activity patterns rather than the characteristics of their individual movement trajectories.
Figure 5.
Statistical distance D between the dataset of turn angles, speed and excursion durations computed for the most active individuals in the colony (crosses). The distance has been computed by carrying out a Kolmogorov–Smirnov test between the data series for these individuals and the complete data series comprising the whole colony. The average of all individuals is also provided for completeness (full circles and horizontal lines).
4. Agent-based model for ant foraging
The next question posed by our study concerns whether the experimentally observed individual variability in temporal patterns of excursions represents a functional advantage in a more biologically relevant scenario including social interaction and communication. If this were true, we can affirm that experiments (like ours) conducted under simplified and controlled conditions actually provide (at least implicitly) relevant information about these processes. With this in mind, we implement an ABM to simulate ant foraging in which the motion rules governing the ants' trajectories are adjusted to fit our experimental results here. For the sake of realism, in the ABM we also explicitly include memory effects and communication between individuals through pheromone deposition.
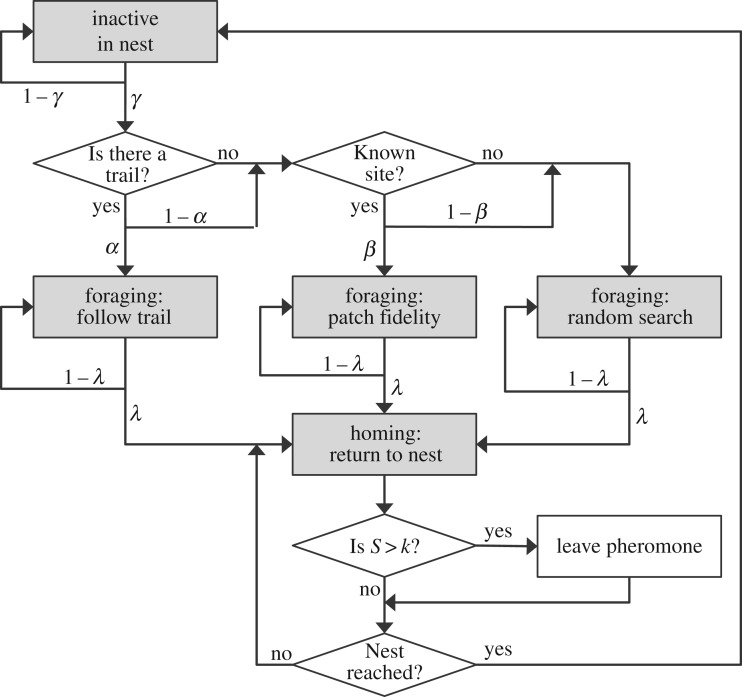
4.1. General rules of the agent-based model algorithm
The main structure of our ABM is largely inspired by recent numerical works on ant foraging [44,45]. In our model, each individual can be in five possible states (grey boxes in figure 6): one for INACTIVE (in-nest) individuals, three different FORAGING states (or strategies), and one for HOMING once the individual decides to return to the nest (with or without food). The spatial domain is continuous, with the nest located at the origin, and it is large enough so that in practice ants will never reach its boundaries. We consider that food is homogeneously distributed with an overall density of 0.015 items/L2 (where L represents the arbitrary unit of length in the ABM). Food is grouped in stacks made of S items (e.g. seeds) as a convenient and simplified way to reproduce the properties of a patchy environment. So, whenever an individual detects one of these stacks, it collects a single item from it causing the stack size to decrease (e.g. from S to S−1) and immediately returns to the nest, thereby assuming a HOMING state. Regarding the detection of food within the framework of the model, we assume that food is found when the ants come within a distance of R = 0.5L, so the parameter R represents their effective detection radius. Finally, communication between individuals is controlled through pheromone deposition once large stacks are found; if the size of the stack detected by the ant is above a certain threshold k, the individual will leave a constant concentration of pheromone on its way back to the nest. The pheromone concentration, however, will also vanish with time at the rate ω, and cannot be detected by the ants once below a certain threshold.
Figure 6.
Scheme of the main algorithm used in the ant foraging ABM, which includes three different foraging mechanisms (random search, patch fidelity and pheromone trail) for assessing both individual and collective information processing.
Preference between foraging states will be determined by the past history of the system and by the parameters α, β, γ and λ, as shown in figure 6 (see also electronic supplementary material, file S3 for further details). First, at each time step the ants are allowed to leave the nest and begin foraging with probability γ. Whenever a pheromone trail is present around the nest, the individual is allowed to follow it with probability α. Otherwise, the individual can assume a site fidelity strategy in which it returns to the last site where it found food; provided such a site exists, the site fidelity strategy will be selected with probability β. If none of these strategies are selected, then by default a random-search strategy is initiated.
To determine the motion rules followed by the ants while in each of the FORAGING and HOMING states we used several fits obtained from the experimental trajectories of our experiments with A. senilis (see electronic supplementary material, file S3 for details).
In the case that food is not reached while in any of the three FORAGING states, the ant proceeds as follows: (i) for follow trail the ant continues searching as long as a pheromone trail is available; otherwise, the ant switches to the HOMING state; (ii) for site fidelity the strategy will terminate once the ant reaches the site where food was found previously, and in the case that food is no longer there, the individual switches to a random-search state; and (iii) an ant engaged in the random-search state will maintain this strategy at each time step with probability 1 − λ or will abandon it with probability λ, in which case it will then switch to the HOMING state.
4.2. Computational results
Next, we compare results obtained from the ABM when stacks of food are given different sizes S = 1, 5, 20, 100 (because food density is kept constant, a larger S implies that stacks are further from each other).
Our main interest is to understand if variability in activity patterns (i.e. excursion durations) provides some functional advantage for foraging. Accordingly, we considered that the parameter λ (measuring the rate at which foragers engaged in random search abandon that state; see figure 6) varied among individuals, and we computed the number of food items collected by the colony after a relatively long time of 104 time steps; this number was used as a measure of foraging success.
For simplicity we divided individuals into two groups: using the behavioral stereotypes hypothesis, we assigned values of λ1 = 0.001 to a fraction p of the individuals (assigning them as risk-prone ants) and λ2 = 0.1 to the others (risk-averse ants). Figure 7a,c shows the corresponding foraging success as a function of p. Figure 7a corresponds to the case β = 0.1 so patch fidelity is almost absent, and this case basically represents competition between the uninformed individual (random search) and collective (follow trail) strategies. In Figure 7c, instead, we use β = 0.5 to evaluate the additional effect of patch fidelity. A remarkable result is obtained in this case, which is the emergence of optimal p values when stack sizes S are large enough to cause the formation of pheromone trails (note that we use k = 5 so pheromone deposition is possible only if S > 5). For small values of S, collective information is not flowing so a larger fraction of risk-prone ants (that is, larger values of p) leads to exploration of a greater area, effectively increasing the number of items found by the group. On the other hand, for large values of S a balance between long-range exploration and the constant presence of workers near the nest (facilitating recruitment when necessary) must be reached, so the optimal value of p found by the model reflects a compromise between individual exploration and collective exploitation. This picture does not change much qualitatively for β = 0.5 (figure 7c); the only difference is that small values of S lead to higher foraging efficiencies because the site fidelity strategy can be used once stacks are found.
Figure 7.
Search efficiency of colonies measured as total number of items collected after 104 time steps (with a time step equal to 0.2 s, the time between frames in the experimental data), averaged over 100 realizations as a function of p and for different values of the stack size S which were 1 (squares), 5 (circles), 20 (triangles) and 100 (inverted triangles). For panels (a,c), ants are considered either as risk-prone or risk-averse and p represents the fraction of risk-prone individuals, while for panels (b) or (d) all individuals can exhibit both personalities, p in this case being the overall fraction of risk-prone excursions. Panels (a,b) correspond to (β = 0.1), while (c,d) correspond to β = 0.5. The values of the other parameters used in the model are L = 1, γ = 0.01, α = 0.5, k = 5 and ω = 0.01.
Our experimental data has revealed that a behavioural plasticity hypothesis (in which the same individual alternates between both long and short excursions) rather than a behavioural stereotypes hypothesis seems to be a much better fit of the real dynamics of foraging in groups of ant workers. In figure 7b,d, we show the results obtained when excursions (not individuals) are assigned different values of λ. Whenever a new excursion starts, we assign it λ1 = 0.001 with probability p, and in all other cases λ = 0.1 (p now represents the fraction of risk-prone excursions). In this case, we observe that (i) search efficiencies are always higher than for behavioural stereotypes of figure 7a,c and (ii) the optimum value of p decreases, especially for small values of S. Altogether, this illustrates the notable benefits that behavioural plasticity may convey for the maximization of foraging success.
5. Discussion
Although the foraging success of ant groups and colonies under natural conditions is strongly conditioned by an effective flow of information and social communication between individuals, here we have tried to assess the potential role that other elements (behavioural variability in activity patterns, in this case) can play within this process. The classical ecological approach to this question would consist of experimentally altering behavioural variability by introducing some external stimuli or varying the environmental conditions of the system and examining the resulting response. Instead, here we have tried to illustrate how a combination of toy experiments under simplistic conditions with additional computational analysis can also yield relevant results and conclusions. Foraging experiments under conditions of low information or uncertain scenarios have been sometimes criticized as simplistic or irrelevant because they obviate essential (typically landscape-driven) components [46]. In contrast with that point of view, we believe that reduced systems such as those proposed under the foraging under uncertainty paradigm [2] provide the proper grounds for understanding particular aspects of foraging (e.g. behavioural stochasticity or noise generation, which are indispensable for living beings as they offer them the necessary flexibility for dealing with new or unexpected situations).
We have also been able to detect several properties governing behavioural variability between A. senilis foragers:
(i) bursting in the individual activity pattern of the ants can change dynamically between individuals and through time. It is remarkable that previous studies in fruit flies [47,48], rats [49], or even in humans [47] reported similar bursting properties. This intriguing result suggests the possible existence of some universal (neuronal) principles driving regulation of motor properties in living beings, though at the moment this is still speculative;
(ii) the variability of individuals' behaviour in foraging trajectories is rather small compared with the variability observed in their activity patterns (interexcursion lengths). This finding can be used to infer that the motion mode of ants is more or less standardized, and possibly driven only through physiological constraints. Likewise, the study of activity patterns might be more than adequate in order to disentangle questions about ant personality and idiosyncrasy; and
(iii) because ants are capable of combining short and long excursions in very different ways, we conclude that a behavioural plasticity hypothesis is much more compatible with our observations than a behavioural stereotypes hypothesis. As shown by the results of the ABM, such behaviour would provide the group a functional advantage for foraging. This conclusion is valid even for groups in which long excursions represent a small fraction (this would correspond to small values of p in figure 7), as could be the case in natural environments with a high risk of predation. Indeed, additional elements could be introduced in our ABM scheme in order to assess this and other questions of interest.
The study of generator mechanisms of behavioural variability in living beings is also a topic of major interest, which is implicitly connected to our work here. It involves several unsolved questions such as: is variability between individuals a manifestation of intrinsic stochasticity in the internal functioning of organisms, or can it rather be attributed to particular traits of the organism (e.g. its past history, physiological properties, etc.)? Can its effects be modulated, and how? Has natural selection shaped generators of variability through history? Further studies as well as carefully designed experiments (possibly benefiting from the joint experimental/computational approach used here) will be needed to address such questions and others.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Eduardo del Rio for help with the experimental work.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
D.C. and F.B. designed the study and wrote the manuscript. D.C. and X.E. conducted the experiments. D.C., V.M. and J.S.A. performed all analysis and simulations. All authors contributed to revisions and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This research has been partially supported by the Spanish government through grants nos. FIS 2012-32334 (V.M. and D.C.) and 2009-04133 and BFU2010-22337 (F.B.), by the Catalan government through grant no. SGR 2013-00924 (V.M., D.C. and F.B.) and by the Brazilian agencies CNPq, CAPES, FUNCAP (J.S.A.). F.B. acknowledges the support of the Human Frontier Science Program (grant no. RGY0084/2011).
References
- 1.Stephens DW, Brown JS, Ydenberg RC (eds). 2007. Foraging: behavior and ecology. Chicago, IL: University of Chicago Press. [Google Scholar]
- 2.Bartumeus F, Campos D, Ryu WS, Lloret-Cabot R, Méndez V, Catalan J. 2016. Foraging success under uncertainty: search tradeoffs and optimal space use. Ecol. Lett. 19, 1299–1313. ( 10.1111/ele.12660) [DOI] [PubMed] [Google Scholar]
- 3.Méndez V, Campos D, Bartumeus F.. 2013. Stochastic foundations in movement ecology: anomalous diffusion, front propagation and random searches. Berlin, Germany: Springer. [Google Scholar]
- 4.Maye A, Hsieh C-H, Sugihara G, Brembs B. 2007. Order in spontaneous behavior. PLoS ONE 2, e443 ( 10.1371/journal.pone.0000443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazazi S, Bartumeus F, Hale JJ, Couzin ID.. 2012. Intermittent motion in desert locusts: behavioural complexity in simple environments. PLoS Comput. Biol. 8, e1002498 ( 10.1371/journal.pcbi.1002498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kölzsch A, Alzate A, Bartumeus F, de Jager M, Weerman EJ, Hengeveld GM, Naguib M, Nolet BA, van de Koppel J. 2015. Experimental evidence for inherent Lévy search behaviour in foraging animals. Proc. R. Soc. B 282, 20150424 ( 10.1098/rspb.2015.0424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravary F, Lecoutey E, Kaminski G, Châline N, Jaisson P. 2007. Individual experience alone can generate lasting division of labor in ants. Curr. Biol. 17, 1308–1312. ( 10.1016/j.cub.2007.06.047) [DOI] [PubMed] [Google Scholar]
- 8.Dornhaus A. 2008. Specialization does not predict individual efficiency in an ant. PLoS Biol. 6, e285 ( 10.1371/journal.pbio.0060285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langridge EA, Sendova-Franks AB, Franks NR. 2008. How experienced individuals contribute to an improvement in collective performance in ants. Behav. Ecol. Sociobiol. 62, 447–456. ( 10.1007/s00265-007-0472-5) [DOI] [Google Scholar]
- 10.Robinson EJH, Richardson TO, Sendova-Franks AB, Feinerman O, Franks NR. 2009. Radio tagging reveals the roles of corpulence, experience and social information in ant decision making. Behav. Ecol. Sociobiol. 63, 627–636. ( 10.1007/s00265-008-0696-z) [DOI] [Google Scholar]
- 11.Beverly BD, McLendon H, Nacu S, Holmes S, Gordon DM. 2009. How site fidelity leads to individual differences in the foraging activity of harvester ants. Behav. Ecol. 20, 633–638. ( 10.1093/beheco/arp041) [DOI] [Google Scholar]
- 12.Holbrook CT, Wright CM, Pruitt JN. 2014. Individual differences in personality and behavioural plasticity facilitate division of labour in social spider colonies. Anim. Behav. 97, 177–183. ( 10.1016/j.anbehav.2014.09.015) [DOI] [Google Scholar]
- 13.Dornhaus A, Chittka I. 2004. Why do honey bees dance? Behav. Ecol. Sociobiol. 55, 395–401. ( 10.1007/s00265-003-0726-9) [DOI] [Google Scholar]
- 14.Cerdá X, Angulo E, Boulay R, Lenoir A. 2009. Individual and collective foraging decisions: a field study of worker recruitment in the gypsy ant Aphaenogaster senilis. Behav. Ecol. Sociobiol. 63, 551–562. ( 10.1007/s00265-008-0690-5) [DOI] [Google Scholar]
- 15.Prabhakar B, Dektar KN, Gordon DM. 2012. The regulation of ant colony foraging activity without spatial information. PLoS Comput. Biol. 8, e1002670 ( 10.1371/journal.pcbi.1002670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinter-Wollman N, Bala A, Merrell A, Queirolo J, Stumpe MC, Holmes S, Gordon DM. 2013. Harvester ants use interactions to regulate forager activation and availability. Anim. Behav. 86, 197–207. ( 10.1016/j.anbehav.2013.05.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schürch R, Grüter C. 2014. Dancing bees improve colony foraging success as long-term benefits outweigh short-term costs. PLoS ONE 9, e104660 ( 10.1371/journal.pone.0104660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelblum A, Pinkoviezky I, Fonio E, Ghosh A, Gov N, Feinerman O.. 2015. Ant groups optimally amplify the effect of transiently informed individuals. Nat. Commun. 6, 7729 ( 10.1038/ncomms8729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole BJ. 1991. Short-term activity cycles in ants: generation of periodicity by worker interaction. Am. Nat. 137, 244–259. ( 10.1086/285156) [DOI] [Google Scholar]
- 20.Chapman BB, Thain H, Coughlin J, Hughes WOH.2011. Behavioural syndromes at multiple scales in Myrmica ants. Anim. Behav. 82, 391–397. (doi:10.1016/j.anbehav.2011.05.019)
- 21.Bengston SE, Dornhaus A. 2014. Be meek or be bold? A colony-level behavioural syndrome in ants. Proc. R. Soc. B 281, 20140518 ( 10.1098/rspb.2014.0518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calhoun AJ,, Chalasani SH, Sharpee TO et al. 2014. Maximally informative foraging by Caenorhabditis elegans. eLife 3, e04220 ( 10.7554/eLife.04220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hills TT, Todd PM, Lazer D, Redish AD, Couzin ID, the Cognitive Search Research Group. 2015. Exploration versus exploitation in space, mind, and society. Trends Cogn. Sci. 19, 46–54. ( 10.1016/j.tics.2014.10.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Czaczkes TJ, Czaczkes B, Iglhaut C, Heinze J. 2015. Composite collective decision-making. Proc. R. Soc. B 282, 20142723 ( 10.1098/rspb.2014.2723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czaczkes TJ, Grüter C, Jones SM, Ratnieks LW. 2011. Synergy between social and private information increases foraging efficiency in ants. Biol. Lett. 7, 521–524. ( 10.1098/rsbl.2011.0067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dechaume-Monchamont F-X, Dornhaus A, Houston AI, McNmara JM, Collins EJ, Frank NR. 2005. The hidden cost of information in collective foraging. Proc. R. Soc. B 272, 1689–1695. ( 10.1098/rspb.2005.3137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grüter C, Leadbeater E. 2014. Insights from insects about adaptive social information use. Trends Ecol. Evol. 29, 177–184. ( 10.1016/j.tree.2014.01.004) [DOI] [PubMed] [Google Scholar]
- 28.Kareiva PM, Shigesada N. 1983. Analyzing insect movement as a correlated random walk. Oecologia 56, 234–238. ( 10.1007/BF00379695) [DOI] [PubMed] [Google Scholar]
- 29.Bovet P, Benhamou S. 1988. Spatial analysis of animals’ movements using a correlated random walk model. J. Theor. Biol. 131, 419–433. ( 10.1016/S0022-5193(88)80038-9) [DOI] [Google Scholar]
- 30.Adler FR, Gordon DM. 1992. Information collection and spread by networks of patrolling ants. Am. Nat. 140, 373–400. ( 10.1086/285418) [DOI] [PubMed] [Google Scholar]
- 31.Thomson JD, Peterson SC, Harder LD. 1987. Response of traplining bumble bees to competition experiments: shifts in feeding location and efficiency. Oecologia 71, 295–300. ( 10.1007/BF00377298) [DOI] [PubMed] [Google Scholar]
- 32.Savolainen R, Vepsäläinen K. 1988. A competition hierarchy among boreal ants: impact on resource partitioning and community structure. Oikos 51, 135–155. ( 10.2307/3565636) [DOI] [Google Scholar]
- 33.Stroeymeyt N, Franks NR, Giurfa M. 2011. Knowledgeable individuals lead collective decisions in ants. J. Exp. Biol. 214, 3046–3054. ( 10.1242/jeb.059188) [DOI] [PubMed] [Google Scholar]
- 34.Mersch DP, Crespi A, Keller L. 2013. Tracking individuals shows spatial fidelity is a key regulator of ant social organization. Science 340, 1090–1093. ( 10.1126/science.1234316) [DOI] [PubMed] [Google Scholar]
- 35.Pamminger T, Foitzik S, Kaufmann KC, Schützler N, Menzel F. 2014. Worker personality and its association with spatially structured division of labor. PLoS ONE 9, e79616 ( 10.1371/journal.pone.0079616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campos D, Bartumeus F, Méndez V, Espadaler X. 2013. Reorientation patterns in central-place foraging: internal clocks and klinokinesis. J. R. Soc. Interface 11, 20130859 ( 10.1098/rsif.2013.0859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker TJ, Wineriter SA. 1981. Marking techniques for recognizing individual insects. Florida Entomol. 64, 18–29. ( 10.2307/3494598) [DOI] [Google Scholar]
- 38.Wojcik DP, Burges RJ, Blanton CM, Focks DA. 2000. An improved and quantified technique for marking individual fire ants (Hymenoptera: Formicidae). Florida Entomol. 83, 74–78. ( 10.2307/3496231) [DOI] [Google Scholar]
- 39.Holbrook CT. 2009. Marking individual ants for behavioral sampling in a laboratory colony. Cold Spring Harb. Protoc. 2009, ppdb.prot5240. ( 10.1101/pdb.prot5240) [DOI] [PubMed] [Google Scholar]
- 40.Karsai M, Kaski K, Barabási A-L, Kertész J. 2012. Universal features of correlated bursty behaviour. Sci. Rep. 2, 397 ( 10.1038/srep00397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goh K-I, Barabási A-L. 2008. Burstiness and memory in complex systems. EPL 81, 48002. [Google Scholar]
- 42.Kim J, Lee D, Kahng B. 2013. Microscopic modelling circadian and bursty pattern of human activities. PLoS ONE 8, e58292 ( 10.1371/journal.pone.0058292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jo H-H, Karsai M, Kertész J, Kaski K. 2012. Circadian pattern and burstiness in mobile phone communication. New J. Phys. 14, 013055 ( 10.1088/1367-2630/14/1/013055) [DOI] [Google Scholar]
- 44.Letendre K, Moses ME. 2013. Synergy in ant foraging strategies: memory and communication alone and in combination. In GECCO'13 . Proc.15th Ann. Conf. on Genetic and Evolutionary Computation, Amsterdam, The Netherlands 6–10 July, pp. 41–48. New York, NY: ACM ( 10.1145/2463372.2463389). [DOI]
- 45.Dornhaus A. 2012. Finding optimal collective strategies using individual-based simulations: colony organization in social insects. Math. Comp. Model. Dyn. 18, 25–37. [Google Scholar]
- 46.Benhamou S. 2014. Of scales and stationarity in animal movements. Ecol. Lett. 17, 261–272. ( 10.1111/ele.12225) [DOI] [PubMed] [Google Scholar]
- 47.Sorribes A, Armendariz BG, Lopez-Pigozzi D, Murga C, Polavieja GG. 2011. The origin of behavioral bursts in decision-making circuitry. PLoS Comput. Biol. 7, e1002075 ( 10.1371/journal.pcbi.1002075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koganezawa M, Hara H, Hayakawa Y, Shimada I.2009. Memory effects on scale-free dynamics in foraging Drosophila. J. Theor. Biol. 260, 353–358. (idoi:10.1016/j.jtbi.2009.06.018) [DOI] [PubMed]
- 49.Jung K, Jang H, Kralik JD, Jeong J. 2014. Bursts and heavy tails in temporal and sequential dynamics of foraging decisions. PLoS Comput. Biol. 10, e1003759 ( 10.1371/journal.pcbi.1003759) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.