Abstract
Background
Chronic obstructive pulmonary disease (COPD) is associated with an increased cardiovascular risk. However, the mechanisms for this association are yet unclear. The aim of this study was to investigate the relationship between brachial intima-media thickness (B-IMT), an independent predictor of cardiovascular risk, systemic inflammation, and asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide synthase, in patients with COPD and respective controls.
Methods
The study sample consisted of 60 patients with stable COPD, free from overt cardiovascular disorders, as well as 20 smoking and 20 nonsmoking controls. Ultrasound assessment of B-IMT, spirometry, venous blood sampling for quantification of inflammatory markers and ADMA levels were carried out, and individual cardiovascular risk was calculated via the Framingham risk score.
Results
Patients with COPD showed significantly higher B-IMT compared to smoking (P=0.007) and nonsmoking controls (P=0.033). COPD patients with elevated B-IMT had a twofold increased calculated 10-year risk for cardiovascular events compared to those below the recommended cutoff (P=0.002). B-IMT was significantly associated with systemic inflammation (interleukin-6 [IL-6]; r=0.365, P=0.006) and ADMA (r=0.331, P=0.013) in COPD. Multivariate linear regression revealed male sex and ADMA as independent predictors of B-IMT in this study sample.
Conclusion
B-IMT is significantly increased in patients with COPD and is associated with systemic inflammation and ADMA levels.
Keywords: cardiovascular risk, chronic obstructive pulmonary disease, comorbidity, subclinical atherosclerosis, biomarker
Introduction
Chronic obstructive pulmonary disease (COPD) is associated with a two- to threefold increased risk of cardiovascular events.1,2 In fact, autopsy studies revealed cardiovascular disease (CVD) as the most frequent, but undetected and potentially modifiable, cause of death in patients with COPD.3 The underlying mechanisms for the relationship between COPD and CVD exceed the effects of traditional risk factors and remain largely unknown.
A long latency period between disease initiation and clinical manifestation allows the identification of subjects at risk at a subclinical, potentially reversible stage of CVD. For this purpose, several markers of subclinical atherosclerosis have been identified as predictors of cardiovascular risk.4–6 Intima-media thickness of the brachial artery (B-IMT)7 is a novel and easily accessible parameter for the quantification of subclinical atherosclerosis. Recent studies validating markers of subclinical atherosclerosis against coronary angiography revealed B-IMT as an independent predictor of cardiovascular events.8 In a 12-year follow-up study, B-IMT clearly outperformed the predictive power of flow-mediated dilation (FMD), the gold standard of noninvasive cardiovascular risk prediction via endothelial function.9 B-IMT has not been studied in COPD so far.
Consequently, this study aimed to assess B-IMT in patients with COPD compared to healthy controls and to investigate potential associations between B-IMT and clinical as well as laboratory markers of COPD (ie, airflow limitation and systemic inflammation). In this context, the present analysis specifically aimed to assess the relationship between asymmetric dimethylarginine (ADMA) and COPD. ADMA is an endogenous inhibitor of nitric oxide synthase, providing a potential link between chronic inflammatory disorders and subclinical atherosclerosis.10,11
It was hypothesized that:
B-IMT is significantly increased in patients with COPD compared to smoking and nonsmoking controls.
B-IMT is significantly correlated with markers of systemic inflammation as well as ADMA levels in patients with COPD.
Materials and methods
Study sample
For this observational study, a sample of patients with COPD (n=60) previously recruited via the outpatient clinic database of the Otto Wagner Hospital, Vienna, was analyzed. Inclusion criteria comprised evidence of airflow limitation on spirometry according to current guidelines,12 a smoking history of at least 20 pack-years and age >40 years. Subjects were excluded if they were on long-term oxygen therapy, had evidence of an acute exacerbation of COPD within the previous 4 months (requiring antibiotics, oral steroids, and/or hospitalization), or suffered from any other relevant lung disease. Other exclusion criteria comprised overt cardiovascular disorders (ie, coronary artery disease, chronic heart failure, cardiomyopathy, arterial hypertension, atrial fibrillation, and peripheral arterial disease), vasoactive medication, malignant diseases, diabetes mellitus, chronic renal failure, or chronic inflammatory conditions other than COPD. The control groups consisted of lifelong nonsmokers (n=20) as well as apparently healthy smokers (n=20). Exclusion criteria were the same as in the study group plus the absence of obstructive lung disease confirmed by clinical evaluation and spirometry. Control subjects were recruited from the general population during a public awareness campaign on spirometry at the Otto Wagner Hospital.
The study was planned and conducted in accordance with the Declaration of Helsinki. The protocol was approved by the local ethics committee (Vienna City Council – EK-number: EK05-150-1205). Prior to enrollment, all the participants gave their written informed consent. Collected data were entirely anonymized. Data handling and reporting followed the STROBE-Statement (Strengthening the Reporting of Observational Studies in Epidemiology).13
Measurements
Ultrasound studies
Ultrasound studies of the brachial artery were conducted as previously reported.8 Subjects were instructed to refrain from food, caffeine, tobacco, and vasoactive drugs for 4–6 h prior to the study visit. Measurements were performed at the antecubital fossa of the right arm after 10 min of rest with subjects placed in a supine position. Investigators blinded to clinical and spirometric results used high-resolution ultrasound (13 MHz) for depiction of the intimal and medial layer of the brachial artery in a longitudinal plane. Using a stereotactic device, the transducer position was fixed and images were stored at an adequate gain for subsequent offline analysis. B-IMT was assessed from the leading edge between lumen and intima to the leading edge between media and adventitia. A cutoff value ≥0.345 mm was regarded as elevated B-IMT14 and was therefore used as a discriminator for cardiovascular risk using the Framingham risk score.15 Lumen diameter was measured as the distance between the leading edge of the near and far intima–lumen interface. Finally, the cross-sectional intima-media area (B-IMA) was calculated following a previously described equation.16
Lung function testing
Lung function testing was performed according to the current guidelines12 for the verification of COPD diagnosis, the classification of the GOLD stage, and the quantification of the distinct parameters of lung function (forced expiratory volume in 1 s [FEV1], % predicted; forced vital capacity [FVC], % predicted; FEV1/FVC ratio).
Physical examination
A physical examination was conducted in every participant for height, weight, heart rate, and arterial blood pressure measurement.
Laboratory analysis
Venous blood samples were collected and analyzed for inflammatory biomarkers (neutrophil count, interleukin [IL]-6) and levels of N-type pro-BNP (pro b-type natriuretic peptide). Circulating ADMA concentrations were measured via reversed-phase high-performance liquid chromatography following a previously described method.17 The 10-year risk for cardiac events was calculated by means of the Framingham risk score15 including the independent cardiovascular risk factors: age, sex, smoking history, arterial blood pressure, cholesterol (total and high-density lipoprotein [HDL]-cholesterol), and diabetes.
Data analysis
Variables of interest are expressed as counts (%) for categorical variables, as mean ± standard deviation or median and interquartile range for interval variables depending on the respective data distribution. Variables with non-normal distribution were logarithm transformed when possible. Between-group comparisons were performed using independent samples t-test or Mann–Whitney U-test depending on the respective data distribution. Univariate correlations were performed by means of Pearson’s correlation coefficient or Kendall’s tau depending on the respective data distribution. A multiple linear regression model was used to identify the strongest predictors of B-IMT. Through forward selection, the sequence of considered covariates was chosen by means of the highest increase in R2. Variables remained in the model if they altered the coefficient by at least 10%. All calculations were two-tailed with a predefined significance level of P<0.05. Analyses were performed using the Statistical Package for Social Sciences (SPSS [version 15 for Windows]; SPSS Inc., Chicago, IL, USA, 2006). Artwork was created using the GraphPad software, version 5 for Windows (GraphPad Inc., San Diego, CA, USA, 2005).
Results
Baseline characteristics
Sixty patients with stable COPD, free from overt cardiovascular disorders, were available for analysis. The sample of COPD patients comprised 32% GOLD stage II, 32% GOLD stage III, and 36% GOLD stage IV. The control groups comprised 20 apparently healthy smoking and nonsmoking subjects each. Clinical characteristics are listed in Table 1. The three groups were matched for age, sex, body mass index, blood pressure, and lipid levels. There were significant differences in heart rate, smoking history, and lung function between patients with COPD and smoking, as well as nonsmoking controls (P<0.05).
Table 1.
Subject characteristics
| Subject characteristics | COPD | Smokers | Never-smokers | P-value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, years | 64±7.8 | 60±9.2 | 64±11.0 | 0.068A, 0.881B, 0.191C |
| Male sex, % | 53 | 40 | 30 | 0.339A, 0.083B, 0.520C |
| Body mass index, kg/m2 | 24±3.6 | 26±3.4 | 25±3.1 | 0.074A, 0.406B, 0.379C |
| Systolic blood pressure, mmHg | 125±13 | 121±12 | 126±9.8 | 0.220A, 0.649B, 0.119C |
| Diastolic blood pressure, mmHg | 76±6.6 | 73±8.5 | 75±5.5 | 0.223A, 0.536B, 0.598C |
| Heart rate, beats per minute | 88±18 | 73±16 | 73±13 | 0.02A, <0.001B, 0.938C |
| Smoking history, pack-years | 65±37 | 39±23 | 0±0 | 0.04A, <0.001B, <0.001C |
| Acute exacerbation rate, n/year | 1.0 (0–1.6) | |||
| Lung function testing | ||||
| FVC, % predicted | 78±17 | 104±13 | 103±16 | <0.001A, <0.001B, 0.880C |
| FEV1, % predicted | 41±18 | 99±12 | 101±16 | <0.001A, <0.001B, 0.655C |
| FEV1/FVC ratio | 43±15 | 82±8.2 | 84±10 | <0.001A, <0.001B, 0.503C |
| Laboratory parameters | ||||
| LDL/HDL ratio | 1.6±0.80 | 1.7±0.72 | 1.4±0.69 | 0.543A, 0.584B, 0.269C |
| HbA1c, % | 5.4±0.35 | 5.3±0.16 | 5.5±0.37 | 0.054A, 0.946B, 0.058C |
| Creatinine, mg/dL | 0.8±0.19 | 0.82±0.20 | 0.81±0.18 | 0.415A, 0.469B, 0.923C |
| Neutrophils, ×103/mL | 4.7±1.6 | 4.3±1.2 | 4.3±1.4 | 0.302A, 0.208B, 0.844C |
| Interleukin-6, pg/mL | 2.7 (1.6–5.3) | 2.2 (1.5–3.4) | 0.97 (0.74–1.8) | 0.051A, <0.001B, 0.123C |
| Cardiovascular parameters | ||||
| B-IMT, mm | 0.37±0.08 | 0.32±0.06 | 0.33±0.06 | 0.007A, 0.033B, 0.559C |
| B-IMA, mm2 | 4.7±1.4 | 3.9±1.2 | 3.9±1.3 | 0.027A, 0.022B, 0.910C |
| Pro-BNP, pg/mL | 82 (47–161) | 74 (45–166) | 76 (36–177) | 0.975A, 0.771B, 0.814C |
| ADMA, μmol/L | 0.47 (0.42–0.54) | 0.55 (0.52–0.62) | 0.54 (0.43–0.59) | 0.131A, 0.817B, 0.135C |
Notes: P-values are not significant unless presented.
COPD versus smokers;
COPD versus nonsmokers;
nonsmokers versus smokers.
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HbA1c, glycated hemoglobin; B-IMT, brachial intima-media thickness; B-IMA, brachial intima-media area; Pro-BNP, pro b-type natriuretic peptide; ADMA, asymmetric dimethylarginine.
Brachial intima-media thickness
Results of B-IMT measurements for the different study groups are depicted in Figure 1. In patients with COPD, significantly higher B-IMT (0.37±0.08 mm vs 0.32±0.06 mm; P=0.007; vs 0.33±0.06 mm; P=0.033) as well as B-IMA (4.7±1.4 mm vs 3.9±1.2 mm; P=0.027; vs 3.9±1.3 mm; P=0.022) was observed compared to smoking and nonsmoking controls. Within the COPD group, male participants showed significantly higher B-IMT levels (0.41±0.06 mm) than females (0.33±0.07 mm) (P<0.001). Prediction of cardiovascular risk through the Framingham risk score revealed a twofold increased 10-year risk for cardiovascular events in COPD patients with elevated B-IMT (12±7.6) compared to those with B-IMT levels below the recommended cutoff (5.9±1.8) (P=0.002) (Figure 2). Univariate correlations between B-IMT and variables of interest are listed in Table 2.
Figure 1.
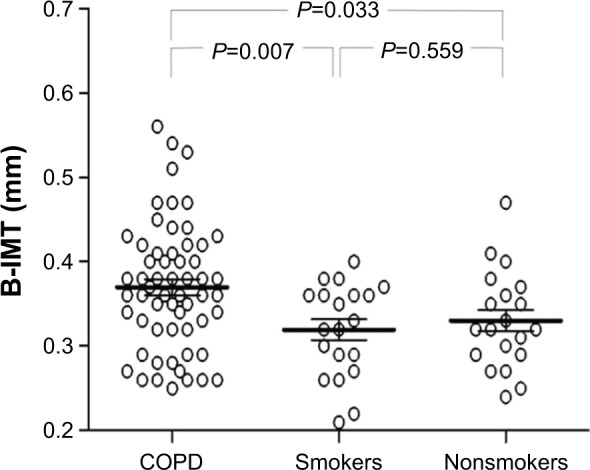
B-IMT in patients with stable COPD (n=60) versus smoking (n=20) and nonsmoking controls (n=20).
Abbreviations: COPD, chronic obstructive pulmonary disease; B-IMT, brachial intima-media thickness.
Figure 2.
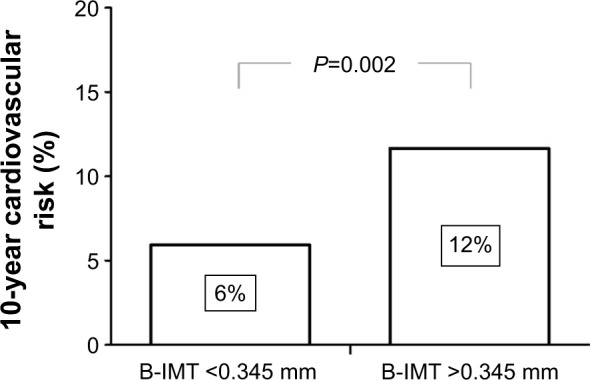
10-year risk for cardiovascular events expressed as Framingham risk score in COPD patients above (n=39) and below (n=21) the suggested reference value for B-IMT (0.345 mm).
Abbreviations: COPD, chronic obstructive pulmonary disease; B-IMT, brachial intima-media thickness.
Table 2.
Univariate associations (Pearson’s correlation coefficient) between brachial intima-media thickness and characteristics of patients with COPD and the complete sample
| Subject characteristics | COPD
|
Smokers
|
Never-smokers
|
|||
|---|---|---|---|---|---|---|
| r-value | P-value | r-value | P-value | r-value | P-value | |
| Age, years | 0.421 | <0.001 | 0.408 | 0.074 | 0.468 | 0.037 |
| Body mass index, m/kg2 | 0.131 | 0.321 | −0.235 | 0.319 | 0.108 | 0.650 |
| Smoking history, pack-years | 0.046 | 0.732 | 0.331 | 0.154 | ||
| Systolic blood pressure, mmHg | 0.314 | 0.016 | −0.101 | 0.672 | 0.224 | 0.357 |
| Acute exacerbation rate, n/year | 0.251 | 0.014 | ||||
| FEV1, % predicted | −0.185 | 0.160 | 0.303 | 0.194 | 0.181 | 0.445 |
| FVC, % predicted | 0.034 | 0.799 | 0.355 | 0.125 | 0.094 | 0.693 |
| FEV1/FVC ratio | −0.286 | 0.028 | 0.115 | 0.631 | −0.280 | 0.231 |
| HbA1c, % | 0.180 | 0.188 | 0.058 | 0.808 | 0.329 | 0.156 |
| HDL/LDL ratio | 0.047 | 0.69 | 0.376 | 0.151 | 0.440 | 0.115 |
| Neutrophil count, ×103/mL | −0.056 | 0.681 | 0.018 | 0.943 | 0.278 | 0.235 |
| Ln interleukin-6, pg/mL | 0.365 | 0.006 | 0.229 | 0.346 | −0.229 | 0.331 |
| Ln pro-BNP, pg/mL | 0.225 | 0.101 | 0.185 | 0.477 | 0.338 | 0.169 |
| Ln ADMA, μmol/L | 0.331 | 0.013 | 0.048 | 0.840 | −0.283 | 0.226 |
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Ln, natural logarithm; Pro-BNP, pro b-type natriuretic peptide; ADMA, asymmetric dimethylarginine.
Systemic Inflammation and ADMA
White blood cell count showed normal levels of neutrophils in the study sample without significant differences between COPD patients, smokers, and nonsmokers. IL-6 plasma levels were higher in patients with COPD compared to controls. There was a significant positive correlation between IL-6 plasma levels and B-IMT in patients with COPD (Figure 3).
Figure 3.
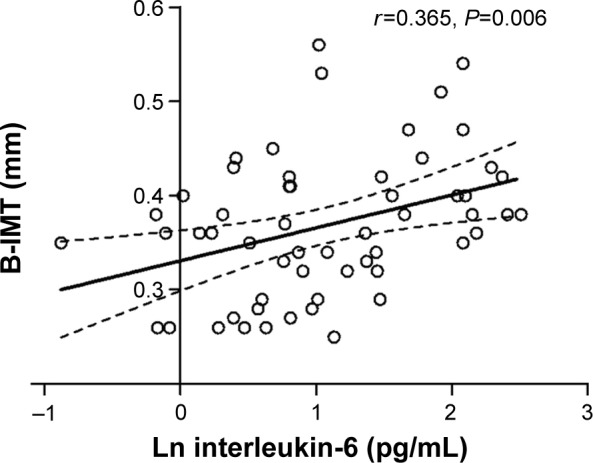
Linear regression analysis between B-IMT and interleukin-6 in patients with COPD (n=60).
Abbreviations: COPD, chronic obstructive pulmonary disease; B-IMT, brachial intima-media thickness; Ln, natural logarithm.
There were no significant differences of circulating ADMA levels between the three study groups. In patients with COPD, B-IMT, however, was positively correlated with ADMA levels (Figure 4), an observation that was not observed in controls. In a multivariable linear regression model (Table 3) with B-IMT as the dependent variable, this study included age, male sex, systolic blood pressure, exacerbation rate, FEV1/FVC ratio, Ln IL-6, Ln ADMA, and Ln pro-BNP as independent variables. Through forward selection, male sex, and ADMA remained independent predictors of B-IMT in the model.
Figure 4.
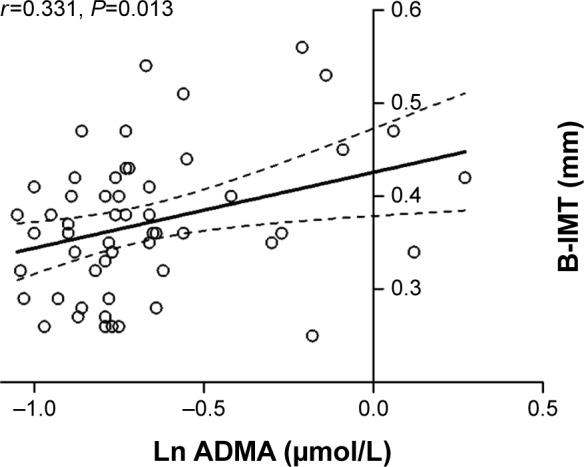
Linear regression analysis between ADMA and B-IMT in patients with COPD (n=60).
Abbreviations: COPD, chronic obstructive pulmonary disease; B-IMT, brachial intima-media thickness; Ln, natural logarithm; ADMA, asymmetric dimethylarginine.
Table 3.
Independent predictors of brachial intima-media thickness in patients with COPD
| Subject characteristics | β | Standardized β | P-value |
|---|---|---|---|
| Male sex | 0.075 | 0.487 | <0.001 |
| Ln ADMA | 0.084 | 0.311 | 0.011 |
Notes: Forward selection multiple linear regression including the following covariates: age, male sex, ln IL-6, ln ADMA, systolic blood pressure, FEV1/FVC ratio, exacerbation rate, and ln pro-BNP. R2=0.402.
Abbreviations: COPD, chronic obstructive pulmonary disease; Ln, natural logarithm; IL-6, interleukin-6; ADMA, asymmetric dimethylarginine; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; Pro-BNP, pro b-type natriuretic peptide.
Discussion
In the present study, significantly higher B-IMT was observed in patients with COPD compared to smoking and nonsmoking controls. Patients with elevated B-IMT had a twofold higher risk for future cardiovascular events calculated by means of the Framingham risk score. B-IMT was correlated with airflow limitation, systemic inflammation, and circulating levels of ADMA. The data support further evidence for an increased cardiovascular risk in patients with stable COPD, independent from traditional cardiovascular risk factors. Although ADMA plasma levels were not different between the groups, the correlation with B-IMT in COPD patients suggests an interaction with circulating ADMA levels as a determinant of subclinical atherosclerosis in COPD. Several markers of subclinical atherosclerosis were identified and previously validated in the general population. This is the first study to investigate B-IMT in patients with COPD free from overt cardiovascular disorders.
These findings support a previous study by Iwamoto et al, who assessed intima-media thickness of the carotid artery as a predictor of CVD risk in smokers with airflow limitation.18 The authors similarly observed a thickening of the arterial wall in COPD compared to healthy smoking and nonsmoking controls. Direct comparison of brachial versus carotid artery intima-media thickness in patients with coronary artery disease showed comparable diagnostic accuracy of both markers to detect diffuse coronary arteriosclerotic lesions.19 Using the brachial artery, however, enables study of both structural and functional properties of systemic vasculature. Using brachial artery measurements of FMD, impaired endothelial function has been previously demonstrated in COPD compared to matched controls.20–22 In a direct comparison, however, B-IMT appears to be a better and independent predictor of coronary artery disease when compared with FMD, in both cross-sectional23 and prospective studies.9
Indeed, stratification of the COPD sample via a previously suggested cutoff14 for B-IMT of 0.345 mm revealed a twofold higher risk of cardiac events in the Framingham risk score for COPD patients with elevated B-IMT compared to those below the recommended cutoff. The available literature suggests several potential mechanisms contributing to elevated subclinical atherosclerosis in COPD. In this study, male sex was identified as an independent predictor for B-IMT. This observation is consistent with findings from the epidemiological Gutenberg heart study.24 In the absence of traditional cardiovascular risk factors, these findings suggest potential vasoprotective effects of estrogen, a hypothesis that is supported by other studies.25–27
Tobacco smoke remains the major risk factor for both COPD and CVD. Patients with COPD, however, are at an increased risk of CVD, independent of smoking.21,28,29 Indeed, analyses of the Framingham study identified airflow obstruction as an independent predictor of cardiac events, with an effect size stronger than traditional cardiovascular risk factors.30,31 In line with these findings, the univariate analysis in this study showed an inverse correlation between B-IMT and FEV1/FVC ratio in patients with COPD.
Both patients with airflow obstruction and those with CVD exhibit systemic inflammation.28,32,33 Significantly higher levels of circulating IL-6 were observed in patients with COPD compared to controls. Importantly, IL-6 levels were strongly associated with B-IMT in univariate analysis. These observations are consistent with previous reports suggesting a link between systemic inflammation and subclinical atherosclerosis.18,34 Systemic inflammation may drive the development of CVD via impairments of the nitric oxide pathway, a hypothesis that is supported by earlier findings of the relationship between circulating inflammatory markers and endothelial function in COPD.21
ADMA is an endogenous inhibitor of nitric oxide synthase, and implicated in the pathogenesis of CVD.35 Indeed, a meta-analysis10 of 22 original articles recently confirmed the relationship between ADMA and B-IMT. Furthermore, circulating ADMA levels predict clinical endpoints, such as myocardial infarction or stroke.36,37 The lung has been identified as one of the most important sources of ADMA formation;38 however, the role of ADMA in COPD remains unknown yet. Recent studies provide conflicting results, demonstrating either higher39 or lower40 circulating ADMA concentrations in patients with COPD compared with controls. Lower concentrations of ADMA were also found in smokers without COPD compared to nonsmokers.41,42 In the present report, there were no statistically significant differences between COPD patients and controls with respect to blood ADMA levels; however, a relationship of the latter with B-IMT exists exclusively in COPD. Thus, these findings may suggest a potential role for ADMA in the development of subclinical atherosclerosis in COPD, rather than a marker of lung function impairment as a consequence of smoking. The relationship between ADMA and impaired B-IMT in COPD in this report is novel and may extend earlier findings, providing a further potential interaction between structural, functional, and molecular properties of the systemic vasculature.
A number of potential limitations in the current study need to be acknowledged including but not limited to the small sample size and a highly selected patient population, free from overt CVDs. However, the present study aimed to investigate pathobiological associations by stringent exclusion of potentially confounding comorbid conditions. Nevertheless, it has to be admitted that the exclusion of coronary artery disease was based on patient surveys and medical history alone, as coronary angiography was not performed in this asymptomatic population. Finally, due to the observational nature of the study further data are needed to characterize the role of ADMA within the observed associations between intima-media thickness and cardiovascular risk in patients with COPD.
Conclusion
In conclusion, the present study reveals B-IMT as a promising and easily accessible tool for stratification of future cardiovascular risk in patients with COPD. Confirming previous observations, a significant association was found between subclinical atherosclerosis and systemic inflammation. Additionally, ADMA was characterized as a potential cofactor in the pathogenesis of COPD related cardiovascular comorbidity. These findings help in understanding the complex mechanisms linking COPD to the associated excess cardiovascular risk.
Acknowledgments
The authors wish to thank the laboratory staff from the Otto Wagner Hospital as well as the laboratory staff from the Department of Clinical Pharmacology (Medical University Vienna) for their help in analyzing the blood samples. This research was supported by the Ludwig Boltzmann Institute for COPD and Respiratory Epidemiology at the Department of Respiratory and Critical Care Medicine, Otto Wagner Hospital, Vienna, Austria. The abstract of this paper was presented at the annual congress of the European Respiratory Society, on September 21, 2010, in Barcelona, as well as at the annual congress of the Austrian Society of Pneumology, on September 9, 2011, in Vienna, as a presentation with interim findings. The presentation’s abstract was published in Wien Klin Wochenschr 2011;123(17–18):A31.
Footnotes
Author contributions
AV as the principal investigator is the guarantor for this study. He had full access to the data and will vouch for the integrity of the data analysis. He is responsible for conception of the study, analysis and interpretation of the data, and revision and approval of the final manuscript. MHU was responsible for data collection, analysis and interpretation of the data, drafting revision, and approval of the final manuscript. PE was responsible for patient recruitment, data collection, and revision and approval of the final manuscript. MW was responsible for laboratory analyses, interpretation and analysis of the data, and revision the final manuscript. GCF was responsible for analysis and interpretation of the data, and revision and approval of the final manuscript. OCB was responsible for analysis and interpretation of the data, revision and approval of the final manuscript, and provided support through the Ludwig Boltzmann Institute.
Disclosure
MHU, PE, OCB, and MW have nothing to disclose. GCF reports personal fees from GlaxoSmithKline, Boehringer Ingelheim, Astra Zeneca, and Menarini, during the conduct of the study. AV reports grants and personal fees from Boehringer Ingelheim, and personal fees from Novartis, Chiesi, GlaxoSmithKline, Pulmonx, PneumRx, and Olympus, outside the submitted work.
The authors report no other conflicts of interest in this work.
References
- 1.Hansell AL, Walk JA, Soriano JB. What do chronic obstructive pulmonary disease patients die from? A multiple cause coding analysis. Eur Respir J. 2003;22(5):809–814. doi: 10.1183/09031936.03.00031403. [DOI] [PubMed] [Google Scholar]
- 2.Mannino DM, Doherty DE, Sonia Buist A. Global Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir Med. 2006;100(1):115–122. doi: 10.1016/j.rmed.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 3.Zvezdin B, Milutinov S, Kojicic M, et al. A postmortem analysis of major causes of early death in patients hospitalized with COPD exacerbation. Chest. 2009;136(2):376–380. doi: 10.1378/chest.08-2918. [DOI] [PubMed] [Google Scholar]
- 4.Held C, Hjemdahl P, Eriksson SV, Bjorkander I, Forslund L, Rehnqvist N. Prognostic implications of intima-media thickness and plaques in the carotid and femoral arteries in patients with stable angina pectoris. Eur Heart J. 2001;22(1):62–72. doi: 10.1053/euhj.1999.2006. [DOI] [PubMed] [Google Scholar]
- 5.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 6.Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J hypertens. 2012;30(3):445–448. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 7.Iwamoto Y, Maruhashi T, Fujii Y, et al. Intima-media thickness of brachial artery, vascular function, and cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2012;32(9):2295–2303. doi: 10.1161/ATVBAHA.112.249680. [DOI] [PubMed] [Google Scholar]
- 8.Weidinger F, Frick M, Alber HF, Ulmer H, Schwarzacher SP, Pachinger O. Association of wall thickness of the brachial artery measured with high-resolution ultrasound with risk factors and coronary artery disease. Am J Cardiol. 2002;89(9):1025–1029. doi: 10.1016/s0002-9149(02)02269-5. [DOI] [PubMed] [Google Scholar]
- 9.Suessenbacher A, Dorler J, Wunder J, et al. Comparison of brachial artery wall thickness versus endothelial function to predict late cardiovascular events in patients undergoing elective coronary angiography. Am J Cardiol. 2013;111(5):671–675. doi: 10.1016/j.amjcard.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Bai Y, Sun L, Du L, et al. Association of circulating levels of asymmetric dimethylarginine (ADMA) with carotid intima-media thickness: evidence from 6168 participants. Ageing Res Rev. 2013;12(2):699–707. doi: 10.1016/j.arr.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Surdacki A, Martens-Lobenhoffer J, Wloch A, et al. Elevated plasma asymmetric dimethyl-L-arginine levels are linked to endothelial progenitor cell depletion and carotid atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2007;56(3):809–819. doi: 10.1002/art.22424. [DOI] [PubMed] [Google Scholar]
- 12.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 14.Hafner F, Kieninger A, Meinitzer A, et al. Endothelial dysfunction and brachial intima-media thickness: long term cardiovascular risk with claudication related to peripheral arterial disease: a prospective analysis. PloS One. 2014;9(4):e93357. doi: 10.1371/journal.pone.0093357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P, CHD Risk, Prediction Group Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 16.Agewall S, Henareh L, Jogestrand T. Intima-media complex of both the brachial artery and the common carotid artery are associated with left ventricular hypertrophy in patients with previous myocardial infarction. J Hypertens. 2005;23(1):119–125. doi: 10.1097/00004872-200501000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Mittermayer F, Namiranian K, Pleiner J, Schaller G, Wolzt M. Acute Escherichia coli endotoxaemia decreases the plasma l-arginine/asymmetrical dimethylarginine ratio in humans. Clin Sci. 2004;106(6):577–581. doi: 10.1042/CS20030363. [DOI] [PubMed] [Google Scholar]
- 18.Iwamoto H, Yokoyama A, Kitahara Y, et al. Airflow limitation in smokers is associated with subclinical atherosclerosis. Am J Respir Crit Care Med. 2009;179(1):35–40. doi: 10.1164/rccm.200804-560OC. [DOI] [PubMed] [Google Scholar]
- 19.Canga A, Kocaman SA, Durakoglugil ME, et al. Increased carotid and brachial intima-media thickness is related to diffuse coronary involvement rather than focal lesions. Angiology. 2013;64(5):356–363. doi: 10.1177/0003319712445373. [DOI] [PubMed] [Google Scholar]
- 20.Marchetti N, Ciccolella DE, Jacobs MR, et al. Hospitalized acute exacerbation of COPD impairs flow and nitroglycerin-mediated peripheral vascular dilation. COPD. 2011;8(2):60–65. doi: 10.3109/15412555.2011.558541. [DOI] [PubMed] [Google Scholar]
- 21.Eickhoff P, Valipour A, Kiss D, et al. Determinants of systemic vascular function in patients with stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178(12):1211–1218. doi: 10.1164/rccm.200709-1412OC. [DOI] [PubMed] [Google Scholar]
- 22.Moro L, Pedone C, Scarlata S, Malafarina V, Fimognari F, Antonelli-Incalzi R. Endothelial dysfunction in chronic obstructive pulmonary disease. Angiology. 2008;59(3):357–364. doi: 10.1177/0003319707306141. [DOI] [PubMed] [Google Scholar]
- 23.Frick M, Schwarzacher SP, Alber HF, et al. Morphologic rather than functional or mechanical sonographic parameters of the brachial artery are related to angiographically evident coronary atherosclerosis. J Am Coll Cardiol. 2002;40(10):1825–1830. doi: 10.1016/s0735-1097(02)02480-4. [DOI] [PubMed] [Google Scholar]
- 24.Sinning C, Wild PS, Echevarria FM, et al. Sex differences in early carotid atherosclerosis (from the community-based Gutenberg-Heart Study) Am J Cardiol. 2011;107(12):1841–1847. doi: 10.1016/j.amjcard.2011.02.318. [DOI] [PubMed] [Google Scholar]
- 25.Sumino H, Murakami M. Investigation of atherosclerosis in postmenopausal women: alteration of atherosclerosis-associated factors and vascular atherosclerosis by oral and transdermal estrogen replacement. Rinsho byori. 2013;61(3):256–262. Japanese. [PubMed] [Google Scholar]
- 26.Radetti G, Mazzanti L, Di Somma C, et al. Evaluation of function and structure of arterial wall in girls and young women with Turner syndrome. J Endocrinol Invest. 2015;38(9):963–970. doi: 10.1007/s40618-015-0268-9. [DOI] [PubMed] [Google Scholar]
- 27.Maturana MA, Franz RF, Metzdorf M, da Silva TR, Spritzer PM. Subclinical cardiovascular disease in postmenopausal women with low/medium cardiovascular risk by the Framingham risk score. Maturitas. 2015;81(2):311–316. doi: 10.1016/j.maturitas.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127(6):1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 29.Barr RG, Mesia-Vela S, Austin JH, et al. Impaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: the Emphysema and Cancer Action Project (EMCAP) Study. Am J Respir Crit Care Med. 2007;176(12):1200–1207. doi: 10.1164/rccm.200707-980OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorlie PD, Kannel WB, O’Connor G. Mortality associated with respiratory function and symptoms in advanced age. The Framingham Study. Am Rev Respir Dis. 1989;140(2):379–384. doi: 10.1164/ajrccm/140.2.379. [DOI] [PubMed] [Google Scholar]
- 31.Hole DJ, Watt GC, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313(7059):711–715. doi: 10.1136/bmj.313.7059.711. discussion 715–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 33.Valipour A, Schreder M, Wolzt M, et al. Circulating vascular endothelial growth factor and systemic inflammatory markers in patients with stable and exacerbated chronic obstructive pulmonary disease. Clin Sci. 2008;115(7):225–232. doi: 10.1042/CS20070382. [DOI] [PubMed] [Google Scholar]
- 34.Sabit R, Bolton CE, Edwards PH, et al. Arterial stiffness and osteoporosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(12):1259–1265. doi: 10.1164/rccm.200701-067OC. [DOI] [PubMed] [Google Scholar]
- 35.Cooke JP. Asymmetrical dimethylarginine: the Uber marker? Circulation. 2004;109(15):1813–1818. doi: 10.1161/01.CIR.0000126823.07732.D5. [DOI] [PubMed] [Google Scholar]
- 36.Leong T, Zylberstein D, Graham I, et al. Asymmetric dimethylarginine independently predicts fatal and nonfatal myocardial infarction and stroke in women: 24-year follow-up of the population study of women in Gothenburg. Arterioscl Thromb Vasc Biol. 2008;28(5):961–967. doi: 10.1161/ATVBAHA.107.156596. [DOI] [PubMed] [Google Scholar]
- 37.Schnabel R, Blankenberg S, Lubos E, et al. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: results from the AtheroGene Study. Circ Res. 2005;97(5):e53–e59. doi: 10.1161/01.RES.0000181286.44222.61. [DOI] [PubMed] [Google Scholar]
- 38.Bulau P, Zakrzewicz D, Kitowska K, et al. Analysis of methylarginine metabolism in the cardiovascular system identifies the lung as a major source of ADMA. Am J Physiol Lung Cell Mol Physiol. 2007;292(1):L18–L24. doi: 10.1152/ajplung.00076.2006. [DOI] [PubMed] [Google Scholar]
- 39.Aydin M, Altintas N, Cem Mutlu L, et al. Asymmetric dimethylarginine contributes to airway nitric oxide deficiency in patients with COPD. Clin Respir J. 2015 Jun 16; doi: 10.1111/crj.12337. Epub. [DOI] [PubMed] [Google Scholar]
- 40.Costanzo L, Pedone C, Battistoni F, Chiurco D, Santangelo S, Antonelli-Incalzi R. Relationship between FEV1 and arterial stiffness in elderly people with chronic obstructive pulmonary disease. Aging Clin Exp Res. 2016 Mar 21; doi: 10.1007/s40520-016-0560-3. Epub. [DOI] [PubMed] [Google Scholar]
- 41.Maas R, Schulze F, Baumert J, et al. Asymmetric dimethylarginine, smoking, and risk of coronary heart disease in apparently healthy men: prospective analysis from the population-based Monitoring of Trends and Determinants in Cardiovascular Disease/Kooperative Gesundheitsforschung in der Region Augsburg study and experimental data. Clin Chem. 2007;53(4):693–701. doi: 10.1373/clinchem.2006.081893. [DOI] [PubMed] [Google Scholar]
- 42.Eid HM, Arnesen H, Hjerkinn EM, Lyberg T, Seljeflot I. Relationship between obesity, smoking, and the endogenous nitric oxide synthase inhibitor, asymmetric dimethylarginine. Metabolism. 2004;53(12):1574–1579. doi: 10.1016/j.metabol.2004.06.026. [DOI] [PubMed] [Google Scholar]


