Abstract
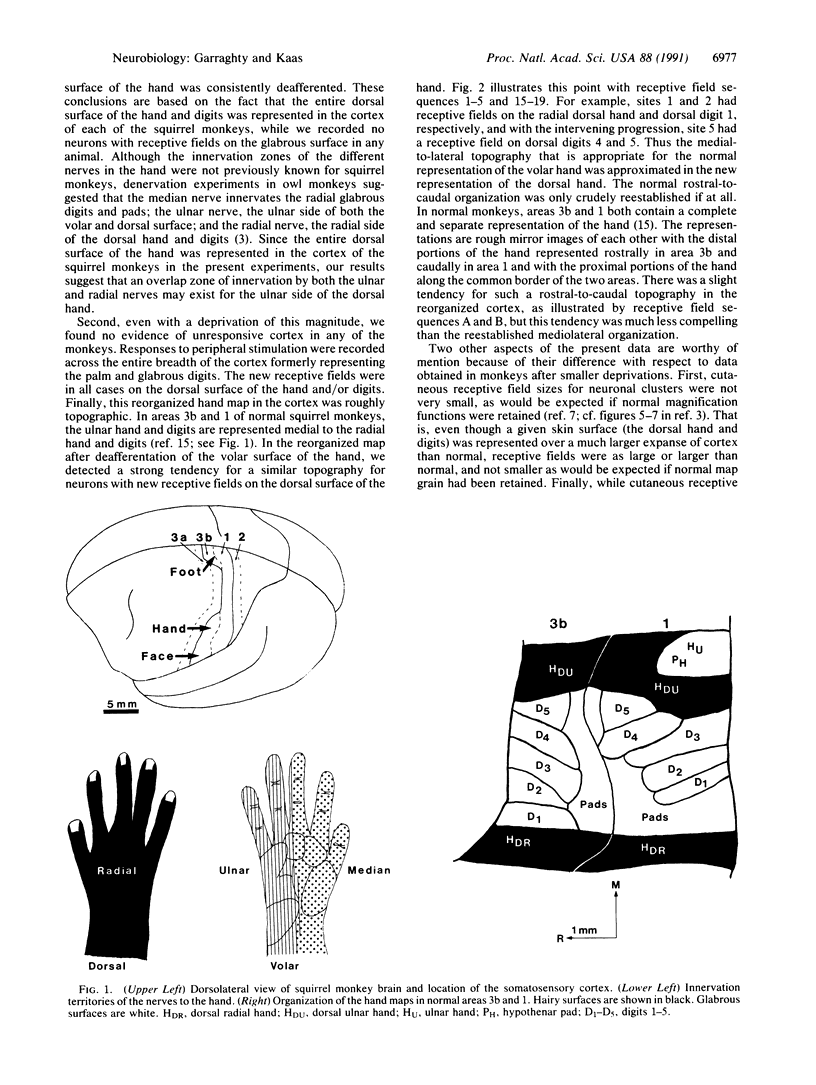
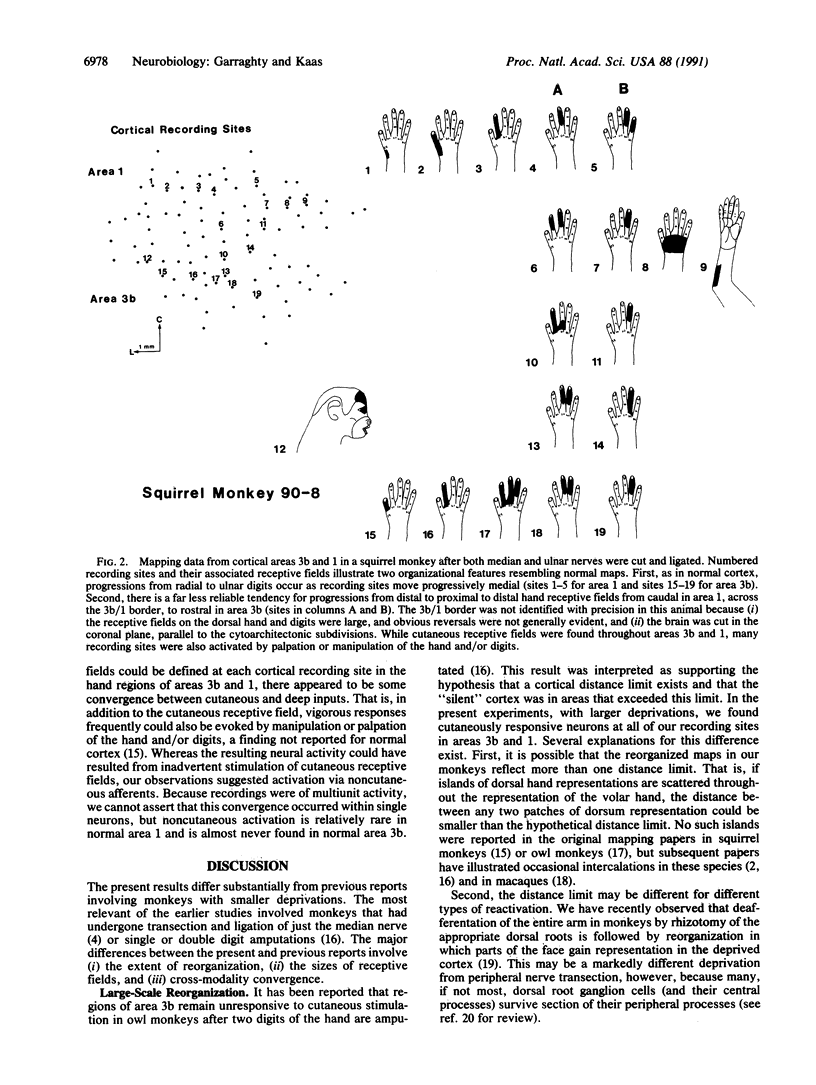
In adult monkeys, peripheral nerve injuries induce dramatic examples of neural plasticity in somatosensory cortex. It has been suggested that a cortical distance limit exists and that the amount of plasticity that is possible after injury is constrained by this limit. We have investigated this possibility by depriving a relatively large expanse of cortex by transecting and ligating both the median and the ulnar nerves to the hand. Electrophysiological recording in cortical areas 3b and 1 in three adult squirrel monkeys no less than 2 months after nerve transection has revealed that cutaneous responsiveness is regained throughout the deprived cortex and that a roughly normal topographic order is reestablished for the reorganized cortex.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alloway K. D., Rosenthal P., Burton H. Quantitative measurements of receptive field changes during antagonism of GABAergic transmission in primary somatosensory cortex of cats. Exp Brain Res. 1989;78(3):514–532. doi: 10.1007/BF00230239. [DOI] [PubMed] [Google Scholar]
- Andersson S. A., Norrsell K., Norrsell U. Spinal pathways projecting to the cerebral first somatosensory area in the monkey. J Physiol. 1972 Sep;225(3):589–597. doi: 10.1113/jphysiol.1972.sp009958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley K. J. Spatial relationships between the terminations of somatic sensory and motor pathways in the rostral brainstem of cats and monkeys. I. Ascending somatic sensory inputs to lateral diencephalon. J Comp Neurol. 1980 Sep 1;193(1):283–317. doi: 10.1002/cne.901930119. [DOI] [PubMed] [Google Scholar]
- Boivie J. Anatomical observations on the dorsal column nuclei, their thalamic projection and the cytoarchitecture of some somatosensory thalamic nuclei in the monkey. J Comp Neurol. 1978 Mar 1;178(1):17–48. doi: 10.1002/cne.901780103. [DOI] [PubMed] [Google Scholar]
- Calford M. B., Tweedale R. Immediate and chronic changes in responses of somatosensory cortex in adult flying-fox after digit amputation. Nature. 1988 Mar 31;332(6163):446–448. doi: 10.1038/332446a0. [DOI] [PubMed] [Google Scholar]
- Cusick C. G., Gould H. J., 3rd Connections between area 3b of the somatosensory cortex and subdivisions of the ventroposterior nuclear complex and the anterior pulvinar nucleus in squirrel monkeys. J Comp Neurol. 1990 Feb 1;292(1):83–102. doi: 10.1002/cne.902920106. [DOI] [PubMed] [Google Scholar]
- Dostrovsky J. O., Millar J., Wall P. D. The immediate shift of afferent drive to dorsal column nucleus cells following deafferentation: a comparison of acute and chronic deafferentation in gracile nucleus and spinal cord. Exp Neurol. 1976 Sep;52(3):480–495. doi: 10.1016/0014-4886(76)90219-3. [DOI] [PubMed] [Google Scholar]
- Dykes R. W., Landry P., Metherate R., Hicks T. P. Functional role of GABA in cat primary somatosensory cortex: shaping receptive fields of cortical neurons. J Neurophysiol. 1984 Dec;52(6):1066–1093. doi: 10.1152/jn.1984.52.6.1066. [DOI] [PubMed] [Google Scholar]
- Flink R., Westman J. Convergence on the same neurons in the feline ventrobasal thalamus of terminals from the dorsal column and the lateral cervical nuclei: an ultrastructural study combining orthograde degeneration and anterograde axonal transport of lectin conjugated horseradish peroxidase. Neurosci Lett. 1985 Nov 11;61(3):243–248. doi: 10.1016/0304-3940(85)90471-9. [DOI] [PubMed] [Google Scholar]
- Garraghty P. E., Florence S. L., Kaas J. H. Ablations of areas 3a and 3b of monkey somatosensory cortex abolish cutaneous responsivity in area 1. Brain Res. 1990 Sep 24;528(1):165–169. doi: 10.1016/0006-8993(90)90213-u. [DOI] [PubMed] [Google Scholar]
- Garraghty P. E., Pons T. P., Sur M., Kaas J. H. The arbors of axons terminating in middle cortical layers of somatosensory area 3b in owl monkeys. Somatosens Mot Res. 1989;6(4):401–411. doi: 10.3109/08990228909144683. [DOI] [PubMed] [Google Scholar]
- Garraghty P. E., Sur M. Morphology of single intracellularly stained axons terminating in area 3b of macaque monkeys. J Comp Neurol. 1990 Apr 22;294(4):583–593. doi: 10.1002/cne.902940406. [DOI] [PubMed] [Google Scholar]
- Hicks T. P., Dykes R. W. Receptive field size for certain neurons in primary somatosensory cortex is determined by GABA-mediated intracortical inhibition. Brain Res. 1983 Sep 5;274(1):160–164. doi: 10.1016/0006-8993(83)90533-4. [DOI] [PubMed] [Google Scholar]
- Kaas J. H., Merzenich M. M., Killackey H. P. The reorganization of somatosensory cortex following peripheral nerve damage in adult and developing mammals. Annu Rev Neurosci. 1983;6:325–356. doi: 10.1146/annurev.ne.06.030183.001545. [DOI] [PubMed] [Google Scholar]
- Kaas J. H., Nelson R. J., Sur M., Dykes R. W., Merzenich M. M. The somatotopic organization of the ventroposterior thalamus of the squirrel monkey, Saimiri sciureus. J Comp Neurol. 1984 Jun 10;226(1):111–140. doi: 10.1002/cne.902260109. [DOI] [PubMed] [Google Scholar]
- Kalil K. Projections of the cerebellar and dorsal column nuclei upon the thalamus of the rhesus monkey. J Comp Neurol. 1981 Jan 1;195(1):25–50. doi: 10.1002/cne.901950105. [DOI] [PubMed] [Google Scholar]
- Kenshalo D. R., Jr, Giesler G. J., Jr, Leonard R. B., Willis W. D. Responses of neurons in primate ventral posterior lateral nucleus to noxious stimuli. J Neurophysiol. 1980 Jun;43(6):1594–1614. doi: 10.1152/jn.1980.43.6.1594. [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Kaas J. H., Sur M., Lin C. S. Double representation of the body surface within cytoarchitectonic areas 3b and 1 in "SI" in the owl monkey (Aotus trivirgatus). J Comp Neurol. 1978 Sep 1;181(1):41–73. doi: 10.1002/cne.901810104. [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Kaas J. H., Wall J. T., Sur M., Nelson R. J., Felleman D. J. Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience. 1983 Nov;10(3):639–665. doi: 10.1016/0306-4522(83)90208-7. [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Kaas J. H., Wall J., Nelson R. J., Sur M., Felleman D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983 Jan;8(1):33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Nelson R. J., Kaas J. H., Stryker M. P., Jenkins W. M., Zook J. M., Cynader M. S., Schoppmann A. Variability in hand surface representations in areas 3b and 1 in adult owl and squirrel monkeys. J Comp Neurol. 1987 Apr 8;258(2):281–296. doi: 10.1002/cne.902580208. [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Nelson R. J., Stryker M. P., Cynader M. S., Schoppmann A., Zook J. M. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984 Apr 20;224(4):591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- Metzler J., Marks P. S. Functional changes in cat somatic sensory-motor cortex during short-term reversible epidural blocks. Brain Res. 1979 Nov 16;177(2):379–383. doi: 10.1016/0006-8993(79)90790-x. [DOI] [PubMed] [Google Scholar]
- Pons T. P., Garraghty P. E., Ommaya A. K., Kaas J. H., Taub E., Mishkin M. Massive cortical reorganization after sensory deafferentation in adult macaques. Science. 1991 Jun 28;252(5014):1857–1860. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- Pons T. P., Wall J. T., Garraghty P. E., Cusick C. G., Kaas J. H. Consistent features of the representation of the hand in area 3b of macaque monkeys. Somatosens Res. 1987;4(4):309–331. doi: 10.3109/07367228709144612. [DOI] [PubMed] [Google Scholar]
- Rasmusson D. D. Reorganization of raccoon somatosensory cortex following removal of the fifth digit. J Comp Neurol. 1982 Mar 10;205(4):313–326. doi: 10.1002/cne.902050402. [DOI] [PubMed] [Google Scholar]
- Rhoades R. W., Belford G. R., Killackey H. P. Receptive-field properties of rat ventral posterior medial neurons before and after selective kainic acid lesions of the trigeminal brain stem complex. J Neurophysiol. 1987 May;57(5):1577–1600. doi: 10.1152/jn.1987.57.5.1577. [DOI] [PubMed] [Google Scholar]
- Shanks M. F., Pearson R. C., Powell T. P. The ipsilateral cortico-cortical connexions between the cytoarchitectonic subdivisions of the primary somatic sensory cortex in the monkey. Brain Res. 1985 Apr;356(1):67–88. doi: 10.1016/0165-0173(85)90019-0. [DOI] [PubMed] [Google Scholar]
- Sur M., Merzenich M. M., Kaas J. H. Magnification, receptive-field area, and "hypercolumn" size in areas 3b and 1 of somatosensory cortex in owl monkeys. J Neurophysiol. 1980 Aug;44(2):295–311. doi: 10.1152/jn.1980.44.2.295. [DOI] [PubMed] [Google Scholar]
- Sur M., Nelson R. J., Kaas J. H. Representations of the body surface in cortical areas 3b and 1 of squirrel monkeys: comparisons with other primates. J Comp Neurol. 1982 Oct 20;211(2):177–192. doi: 10.1002/cne.902110207. [DOI] [PubMed] [Google Scholar]
- Wall J. T., Cusick C. G., Migani-Wall S. A., Wiley R. G. Cortical organization after treatment of a peripheral nerve with ricin: an evaluation of the relationship between sensory neuron death and cortical adjustments after nerve injury. J Comp Neurol. 1988 Nov 22;277(4):578–592. doi: 10.1002/cne.902770410. [DOI] [PubMed] [Google Scholar]



