Abstract
The human commensal bacterium Staphylococcus aureus can cause a wide range of infections ranging from skin and soft tissue infections to invasive diseases like septicemia, endocarditis, and pneumonia. Muticellular organization almost certainly contributes to S. aureus pathogenesis mechanisms. While there has been considerable focus on biofilm formation and its role in colonizing prosthetic joints and indwelling devices, less attention has been paid to non-surface attached group behavior like aggregation and clumping. S. aureus is unique in its ability to coagulate blood, and it also produces multiple fibrinogen-binding proteins that facilitate clumping. Formation of clumps, which are large, tightly-packed groups of cells held together by fibrin(ogen), has been demonstrated to be important for S. aureus virulence and immune evasion. Clumps of cells are able to avoid detection by the host’s immune system due to a fibrin(ogen) coat that acts as a shield, and the size of the clumps facilitates evasion of phagocytosis. In addition, clumping could be an important early step in establishing infections that involve tight clusters of cells embedded in host matrix proteins, such as soft tissue abscesses and endocarditis. In this review we discuss clumping mechanisms and regulation, as well as what is known about how clumping contributes to immune evasion.
Keywords: Staphylococcus aureus, clumping, agglutination, fibrinogen, coagulation
1. Introduction
The gram-positive bacterium Staphylococcus aureus is a common human commensal, colonizing the nostrils and skin of ~30% of the population (Gorwitz et al., 2008; Miller and Diep, 2008). It is also a formidable opportunistic pathogen, causing superficial skin and soft tissue infections as well as potentially life-threatening invasive diseases such as bacteremia, pneumonia, endocarditis, and osteomyelitis (Lowy, 1998). S. aureus is able to thrive in a wide range of sites within the body, in part due to its impressive array of virulence factors, including adhesins, toxins, and immune evasion proteins (Foster et al., 2014; Thammavongsa et al., 2015). Treatment has become more challenging, as methicillin resistant S. aureus (MRSA) is already widespread in the clinic and in communities, and strains resistant to the last-line antibiotic vancomycin have emerged in recent years (Chambers and Deleo, 2009). A deeper understanding of how S. aureus interacts with the host will facilitate the development of novel therapeutic strategies, particularly as antibiotic resistance becomes more prevalent.
Traditionally, S. aureus and other bacterial pathogens have been grown either as free-floating planktonic cells or as a biofilm. The term biofilm is used loosely in the literature, but usually it describes a multilayered community of cells attached to a surface. These cells are embedded in an extracellular matrix, composed of some combination of secreted polysaccharides, proteins, and DNA, and exhibit increased resistance to antibiotics (Davies, 2003; Lebeaux et al., 2014; Paharik and Horswill, 2016). Some staphylococcal infections clearly involve biofilm formation, such as colonization of indwelling devices including catheters, artificial joints, and pacemakers (Tong et al., 2015). However, not all S. aureus infections involve the biofilm mode of growth, and there is increasing evidence that aggregation or “microcolony” formation is more relevant in many cases (Bjarnsholt et al., 2013). Often these aggregates are embedded in host material, such as extracellular matrix proteins like fibrinogen, fibronectin, and collagen. These microcolonies are not necessarily surface attached, are generally smaller than typical biofilms, and do not form the mushroom-like towers that have been observed with in vitro biofilm growth (Bjarnsholt et al., 2013). Examples of infections that may involve staphylococcal aggregates or microcolonies, rather than typical biofilms, include chronic wound infections (Fazli et al., 2009), osteomyelitis (Horst et al., 2012), soft tissue abscesses (Cheng et al., 2011), and endocarditis (Salgado-Pabon et al., 2013). In these cases interactions with host matrix molecules are particularly important, both in colonization of the site and evasion of the immune response.
In this review we examine the molecular underpinnings of S. aureus aggregation and microcolony formation. Our primary focus is on cell clustering mediated by the abundant host plasma protein fibrinogen/fibrin, the fibrous component of the coagulation cascade responsible for blood clotting. One of the hallmarks of S. aureus is its ability to coagulate blood (Loeb, 1903), allowing for clinical differentiation of S. aureus from coagulase-negative Staphylococci (CoNS) such as S. epidermidis (Lowy, 1998). S. aureus secretes two soluble coagulases that interact with prothrombin to catalyze conversion of fibrinogen to fibrin (McAdow et al., 2012b). S. aureus can also interact directly with fibrinogen to form large clusters of cells, a process that has been termed clumping or agglutination and is mediated by cell surface proteins that bind to fibrinogen. For simplicity we will refer to this process as clumping going forward. We will review the molecular mechanisms and regulation of S. aureus clumping and its importance to human disease. We will also briefly discuss S. aureus aggregation that is mediated by bacterial products rather than human matrix proteins. Aggregation is distinct from clumping, and is dependent on either secreted polysaccharides or surface proteins. We will describe how these aggregates form and to explore their relevance to disease progression.
2. Fibrinogen/fibrin mediated clumping
2.1 S. aureus interactions with the coagulation cascade
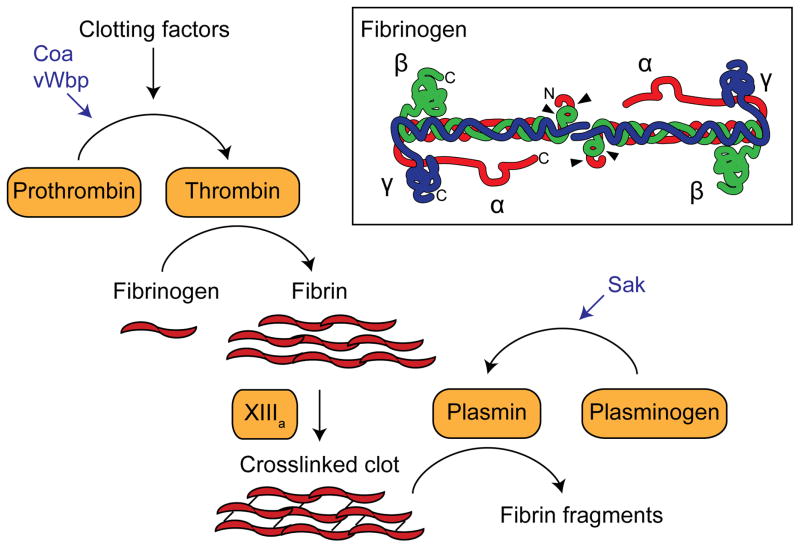
The coagulation cascade consists of a hierarchy of zymogens that is finely tuned to respond to and patch breaches in blood vessels (Adams and Bird, 2009). Vascular damage triggers a local proteolytic cascade, leading to activation of prothrombin to thrombin. Thrombin processes fibrinogen to fibrin, which aggregates and forms a dense fibrous clot that is later strengthened by the crosslinking activity of the transglutaminase factor XIIIa (Fig. 1). To complete the wound healing process, the clot is ultimately broken down in a process called fibrinolysis. This is catalyzed by plasmin, which exists in the bloodstream as a zymogen, plasminogen, until it is activated. The central player in clotting, fibrinogen, is an abundant plasma glycoprotein present at concentrations of 2–4 mg/ml (6–12 μM) in blood (Ariens, 2013). Fibrinogen is a hexameric protein composed of two copies each of Aα-, Bβ-, and γ-chains (Fig. 1). The chains come together to form coiled coils, meeting at the center at their N-termini and forming globular domains at the C-termini. The result is an ~50 nm propeller-shaped dimeric protein with globular ends (Yang et al., 2000b). Upon activation, thrombin cleaves off the A and B peptide extensions of the α- and β-chains of fibrinogen, releasing them as A and B fibrinopeptides and allowing thick fibrin cables to assemble into a clot (Yang et al., 2000a).
Fig. 1.
A simplified schematic of the coagulation cascade that mediates fibrin clot formation. S. aureus coagulases Coa and vWbp, and staphylokinase Sak are shown in blue. Inset shows a representation of fibrinogen adapted from (Hassouna, 2009). Each molecule is composed of two copies each of the Aα-, Bβ-, and γ-chains, with their N-terminal ends interfacing at the center of the dimer. N- and C-terminal ends of the polypeptides are indicated for the left half of the molecule, and arrowheads point to thrombin cleavage sites.
S. aureus has developed multiple strategies to locally interfere with the coagulation cascade. Most S. aureus clinical isolates secrete two coagulases, the classical coagulase (Coa) and von Willibrand Factor-binding protein (vWbp) (Kawabata et al., 1985; Kroh et al., 2009), which are able to activate prothrombin by forming a tight 1:1 complex with the zymogen. This “staphylothrombin” complex is catalytically active in converting fibrinogen to fibrin, circumventing the need for proteolytic processing of prothrombin to thrombin (Friedrich et al., 2003; Kroh et al., 2009). Both coagulases contain conserved D1 and D2 prothrombin binding domains within the N-terminal half of the protein (Friedrich et al., 2003). The C-terminal half of Coa includes 5 to 8 copies of a 27-residue repeat that binds to fibrinogen (Ko et al., 2016; McDevitt et al., 1992; Thomer et al., 2013). The C-terminal half of vWbp differs from Coa, and was originally identified as a binding partner for von Willibrand factor using a phage display library (Bjerketorp et al., 2002). Recent work suggests that vWbp may not associate with von Willibrand factor in plasma, but may instead form a complex with prothrombin, fibrinogen, and factor XIII that is able to non-proteolytically activate the transglutaminase activity of factor XIII (Thomer et al., 2013). The role of staphylococcal coagulases in disease progression was unknown for many years, but recent work indicates that they promote formation of a fibrin shield around the bacterial cells during the development of abscesses (Cheng et al., 2010) (discussed in more detail below).
2.2 Staphylococcal clumping factors
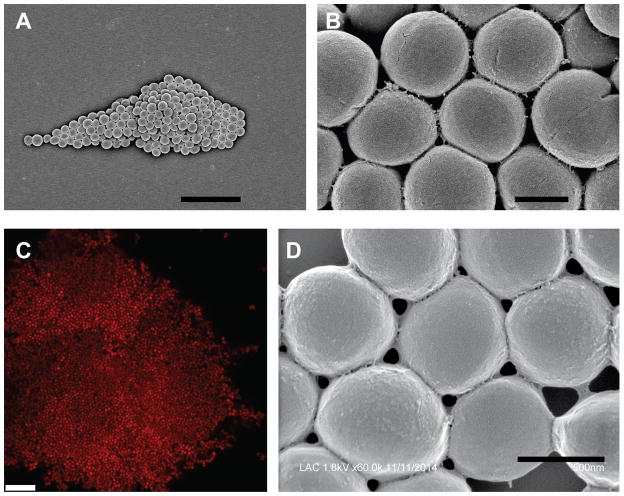
It has been known for over a century that S. aureus forms large clumps of cells in the presence of plasma (Much, 1908). This clumping was later found to be due to interactions with soluble fibrinogen, and specifically the C-terminal 27 residues of the γ-chain of fibrinogen (Fig. 1) (Hawiger et al., 1982; Lipinski et al., 1967). Representative images of a S. aureus clumps formed with purified human fibrinogen are shown in Fig. 2. Clumping is possible because S. aureus interacts with the distal ends of the fibrinogen dimer, allowing fibrinogen to act as a bridge between neighboring cells (Hawiger et al., 1982). Clumping was found to be independent of coagulation (McDevitt et al., 1992), and required a cell surface protein named clumping factor A (ClfA) (McDevitt et al., 1994; McDevitt et al., 1997). ClfA has a canonical LPXTG motif at its C-terminus for covalent anchoring to the cell wall by sortase. It is a member of the microbial surface component recognizing adhesive matrix molecules (MSCRAMM) family, a group of surface proteins recently redefined as having two adjacent IgG fold domains in their N-terminal regions (Foster et al., 2014). ClfA is divided into an N-terminal A domain, composed of three independently folded subdomains (N1, N2, and N3) and a C-terminal R domain, containing a long stretch of serine-aspartate (SD) dipeptide repeats (Fig. 3). The serine residues in the SD repeat region are modified with N-acetylglucosamine disaccharides, apparently to protect this part of the protein from proteolysis (Hazenbos et al., 2013; Thomer et al., 2014). Structural analysis of a fragment containing the N2 and N3 domains, which is the minimal portion required for fibrinogen binding (McDevitt et al., 1997), showed that N2 and N3 adopt separate IgG folds and bind to fibrinogen using a three step “dock, lock and latch” mechanism (Deivanayagam et al., 2002; Ganesh et al., 2008). ClfA has a similar binding affinity for both fibrinogen and fibrin, with a Kd of ~0.5 μM, allowing it to mediate clumping with both forms of the molecule (McAdow et al., 2011; McDevitt et al., 1997).
Fig. 2.
Images of S. aureus clumps. Washed cells of USA300 strain LAC were incubated with 18.5 μg/ml human fibrinogen and imaged using scanning electron microscopy (A, B, D) or confocal laser scanning microscopy (C). In panel C the cells were expressing DsRed from a plasmid. Scale bars represent 5 μm (A), 0.5 μm (B), 10 μM (C), and 0.5 μm (D).
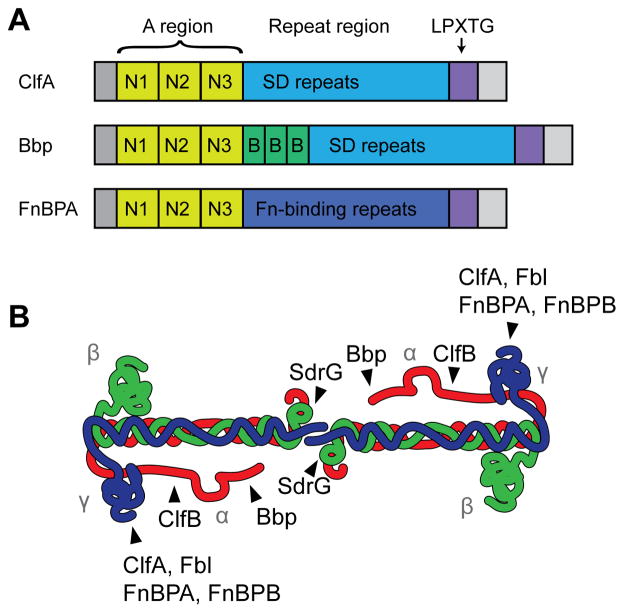
Fig. 3.
MSCRAMM domain organization and interactions with fibrinogen. (A) Representatives of the three known types of fibrinogen-binding MSCRAMMs. The A region, consisting of the N1, N2, and N3 subdomains, is conserved and responsible for fibrinogen binding. The repeat region is variable, with ClfA and Bbp having a series of serine-aspartate dipeptide repeats, while FnBPA contains tandem repeats of a fibronectin-binding domain. Bbp also has three B repeats between the A region and SD repeat region. All of them have a secretion signal at their N-termini and a cell wall anchoring LPXTG sortase signal at their C-termini. S. aureus ClfB and S. lugdunensis Fbl are similar to ClfA, S. aureus SdrE and S. epidermidis SdrG are similar to Bbp (although the number of B repeats is variable), and FnBPA and FnBPB share similar domain architectures. (B) Schematic of fibrinogen, showing where each MSCRAMM binds. Figure is adapted from (Ko and Flick, 2016).
S. aureus has a second clumping factor, ClfB, that is structurally similar to ClfA, although their binding domains are only 26% identical (Ni Eidhin et al., 1998). ClfB also binds to fibrinogen, but unlike ClfA it binds to the α-chain (Walsh et al., 2008). Expression of clfB is under the control of the Agr quorum sensing system and is only expressed in the early exponential phase of growth; consequentially, the contribution of ClfB to clumping is often overshadowed by that of ClfA (Ni Eidhin et al., 1998; Xue et al., 2012). Interestingly, ClfB also binds to cytokeratin 10 and loricrin, facilitating adhesion to nasal epithelial cells (Mulcahy et al., 2012; O’Brien et al., 2002b; Walsh et al., 2004). Structural analysis of a portion of the binding domain of ClfB showed that it binds to fibrinogen and cytokeratin 10 in a similar manner, with the ligands binding to a hydrophobic trough between the N2 and N3 subdomains via the dock, lock and latch mechanism (Xiang et al., 2012). ClfB appears to recognize a glycine and serine rich motif, GSSGXGXXG, that is also present in loricrin, explaining how it is able to bind to several different ligands with similar affinities (Mulcahy et al., 2012; Xiang et al., 2012). Of note, mouse fibrinogen lacks this motif, explaining why ClfB binds to human fibrinogen with higher affinity (Ko and Flick, 2016). Studies in both mice and humans have shown that ClfB is important for nasal colonization (Schaffer et al., 2006; Wertheim et al., 2008), and this appears to be primarily due to its ability to bind loricrin (Mulcahy et al., 2012).
The CoNS species S. lugdunensis and S. schleiferi also produce clumping factors and can give a positive result in latex bead agglutination tests (Freney et al., 1988). For this reason these species have often been misidentified as S. aureus in the clinic, leading to an under appreciation of their contribution to disease (Argemi et al., 2015; Elamin et al., 2015). S. lugdunensis is particularly virulent among CoNS species, and can cause a range of invasive infections, including endocarditis, sepsis, abscesses and wound infections (Frank et al., 2008). The clumping factor Fbl appears to be one of the few virulence factors produced by S. lugdunensis (Frank et al., 2008), suggesting that fibrinogen binding and clumping may be important for establishing these invasive infections. Fbl is highly similar to ClfA and appears to be the main fibrinogen-binding surface protein in S. lugdunensis (Mitchell et al., 2004; Nilsson et al., 2004). Both Fbl and ClfA have similar affinities for fibrinogen and bind to the same region of the fibrinogen γ-chain (Geoghegan et al., 2010b).
The more common CoNS species S. epidermidis also encodes a fibrinogen-binding MSCRAMM called SdrG, also known as Fbe (Hartford et al., 2001; Nilsson et al., 1998). While SdrG is important for binding to immobilized fibrinogen, it does not promote clumping (Nilsson et al., 1998). This can be explained by the fact that SdrG recognizes the N-terminus of the Bβ-chain of fibrinogen (Davis et al., 2001; Pei et al., 1999), which is located at the center of the fibrinogen dimer rather than at the distal ends, preventing bridging between neighboring cells (Fig. 3B). In addition, unlike ClfA and ClfB, SdrG is only able to bind fibrinogen and not fibrin, as the B fibrinopeptide is required for recognition (Davis et al., 2001). Interestingly, the SdrG binding site overlaps with the thrombin cleavage site for removal of the B fibrinopeptide and binding by SdrG inhibits thrombin cleavage (Davis et al., 2001).
2.3 Other fibrinogen-binding surface proteins in S. aureus
S. aureus produces a surprising number of cell wall anchored and secreted fibrinogen-binding proteins in addition to ClfA and ClfB. These proteins do not appear to contribute appreciably to clumping and will be covered only briefly here. Two of these surface proteins, which share structural homology to the clumping factors, are fibronectin binding proteins A and B (FnBPA and FnBPB, Fig. 3). Both were originally characterized as binding to fibronectin (Flock et al., 1987; Froman et al., 1987; Jonsson et al., 1991), an extracellular matrix glycoprotein that is present in blood at ~1/10 the concentration of fibrinogen (Mosher, 2006). The N-terminal A domains of FnBPA and FnBPB are similar to that of ClfA, with three subdomains, N1, N2, and N3, but the C-terminal stalk region consists of 11 tandem repeats rather than an SD repeat region. Fibronectin binding is localized to the tandem repeats in the C-terminal stalk (Flock et al., 1987; Meenan et al., 2007; Signas et al., 1989), and it was later found that the N2–N3 region of FnBPA mediates binding to fibrinogen and elastin (Keane et al., 2007; Roche et al., 2004; Wann et al., 2000). FnBPA and ClfA use a similar binding mechanism to interact with the same region of fibrinogen, and indeed addition of exogenous FnBPA A domain can interfere with clumping (Wann et al., 2000). Yet the fibronectin binding proteins don’t contribute appreciably to clumping, as strains expressing FnBPA and FnBPB but lacking both clfA and clfB are almost completely unable to clump (Wann et al., 2000). However, overexpressing FnBPA from a multicopy plasmid does restore clumping in a clfA clfB double mutant, demonstrating that FnBPA can facilitate clumping when expressed at high levels (Wann et al., 2000). It is unclear why FnBPA and FnBPB don’t promote clumping when expressed at native levels, but it is possible that they are not abundant enough or are overshadowed by larger surface proteins. In addition, recent evidence suggests that binding to fibronectin may sterically exclude fibrinogen binding when both matrix proteins are present (Stemberk et al., 2014), which may explain why FnBPA and FnBPB don’t appear to contribute to clumping in plasma.
Bone sialoprotein binding protein (Bbp, Fig. 3), another member of the MSCRAMM family in S. aureus, has been implicated in fibrinogen binding. Bbp is an allelic variant of SdrE, sharing 76% identity in the A domain responsible for ligand binding and ~95% homology over the rest of the sequence (Tung et al., 2000). The overall domain organization of Bbp/SdrE is similar to that of ClfA, except that there are three B repeats inserted between the A domain and SD repeat region (Josefsson et al., 1998a). These B repeats, which bind Ca2+, each adopt a β-sandwich fold and are thought to act as a spring-like linker connecting the A domain to the SD repeats (Josefsson et al., 1998b; Wang et al., 2013). Although Bbp was originally identified as a bone sialoprotein binding protein (Tung et al., 2000), it was later found to bind fibrinogen with high affinity (Vazquez et al., 2011). A purified fragment of Bbp spanning the N2–N3 region of the A domain binds to residues 561–575 of the Aα-chain of fibrinogen with a Kd of ~0.5 μM (Vazquez et al., 2011). Structural analysis indicates that Bbp binds fibrinogen using a dock, lock and latch mechanism similar to what has been observed with other MSCRAMM proteins (Zhang et al., 2015). The bbp gene appears to be present in only ~20–40% of clinical isolates, with a slightly increased prevalence in osteomyelitis isolates (Peacock et al., 2002; Tristan et al., 2003). The sdrE isoform is more common and is found in ~55–65% of clinical isolates (Li et al., 2013; Peacock et al., 2002; Yu et al., 2012). The function of SdrE is less clear, although preliminary results suggest that it also binds fibrinogen, with a higher affinity for non-human fibrinogen (Ko and Flick, 2016). In addition, SdrE has been reported to bind to complement regulator factor H (Sharp et al., 2012), and it can also promote platelet aggregation (O’Brien et al., 2002a).
2.4 Secreted fibrinogen-binding proteins in S. aureus
S. aureus expresses a group of secreted proteins that interact with host molecules known as SERAMs (secretable expanded repertoire adhesive molecules), many of which can bind fibrinogen (Chavakis et al., 2005). These include Coa (discussed above), Efb, Emp and Eap, which are all regulated by the SaeRS two-component system (Rogasch et al., 2006). In general less is known about the secreted fibrinogen-binding proteins, which do not share the same fibrinogen binding domains and mechanisms as the cell wall anchored proteins. One of the better-understood proteins is Efb (extracellular fibrinogen binding protein), a 16 kDa secreted protein that is involved in immune evasion. The N-terminal half of Efb has two repeats that bind fibrinogen, and the C-terminal half of Efb binds to the complement component C3b (Lee et al., 2004a; Lee et al., 2004b; Palma et al., 1998). The fibrinogen-binding repeats are intrinsically disordered and share homology with the fibrinogen-binding repeats of Coa (Ko et al., 2016). Efb is not involved in cellular adhesion to fibrinogen (Palma et al., 1996), and instead facilitates the formation of a fibrinogen shield at the cell surface (Ko et al., 2013). Cells are coated with C3b during opsonization, which provides a binding surface for secreted Efb. Fibrinogen then interacts with the bound Efb to create a shield around the cell, masking complement factors and protecting the cell from phagocytosis (Ko et al., 2013).
Two other secreted fibrinogen-binding proteins, Emp and Eap, are thought to associate with the cell surface after secretion. Emp (extracellular matrix protein-binding protein, also known as Empbp and Ssp), is a ~38 kDa protein that interacts with fibronectin, fibrinogen, and vitronectin (Hussain et al., 2001). The mechanisms for binding to these ligands are currently unknown. While clumping has not been tested, an emp mutant does have a slight defect in binding to immobilized fibrinogen (Hussain et al., 2001). Emp can be detected surrounding staphylococcal cells in abscess communities, and an emp mutant shows defects in abscess formation, suggesting that its ability to bind extracellular matrix proteins is important for virulence (Attia et al., 2013; Cheng et al., 2009; Guggenberger et al., 2012). Eap (extracellular adherence protein, also called Map) is a 50–70 kDa secreted protein consisting of 4–6 tandem repeats, each of which adopts a β-grasp fold (Geisbrecht et al., 2005). It was originally identified as a cell surface associated protein that could bind to a wide variety of extracellular matrix proteins, including fibrinogen, fibronectin, vitronectin, thrombospondin, and collagen (Jonsson et al., 1995; McGavin et al., 1993). Its lack of specificity was speculated to be due to its high density of lysine residues (it has a pI of 9.9) and also its high hydrophobicity (McGavin et al., 1993). Later work showed that eap wasn’t required for S. aureus binding to any of these matrix proteins, suggesting Eap might have alternative functions (Chavakis et al., 2002; Kreikemeyer et al., 2002). It now appears that Eap may interfere with the immune response by binding to and inhibiting neutrophil serine proteases (Stapels et al., 2014) and the complement component C4b (Woehl et al., 2014), as well as reducing neutrophil recruitment to the site of infection and altering the development of adaptive immunity (Harraghy et al., 2003).
2.5 Clumping in synovial fluid
Historically S. aureus clumping has been studied in the presence of plasma or purified fibrinogen, and under these conditions essentially all of the observed clumping is due to ClfA and ClfB (Ni Eidhin et al., 1998). Recently it was observed that S. aureus forms large clumps in synovial fluid from patients undergoing knee arthroplasty (Dastgheyb et al., 2015a). Synovial fluid clump formation was prevented by pre-incubation with plasmin, suggesting that fibrin plays a crucial role in the clump structure. This was supported by the observation that strains lacking the fibrinogen-binding proteins clfA, clfB, fnbA, or fnbB formed fewer macroscopic clumps than the wild-type (Dastgheyb et al., 2015a). Expression of fnbA and fnbB was elevated in synovial fluid compared to serum, but based on absolute expression levels ClfA was predicted to be the dominant MSCRAMM expressed under these conditions (Dastgheyb et al., 2015b). While these results are consistent with fibrin being the main host matrix molecule in synovial fluid clumps, it was surprising that pre-formed synovial fluid clumps could not be dismantled with plasmin (Dastgheyb et al., 2015a). Work from our lab suggests that the large glycosaminoglycan hyaluronic acid, which is abundant in synovial fluid, also contributes to formation of these large clumps (Ibberson et al., 2016). Future studies may provide more information on the molecular structure of synovial fluid clumps and their importance in prosthetic joint infections.
2.6 Regulation of clumping
Almost all S. aureus clinical isolates express both ClfA and ClfB (Peacock et al., 2002; Tristan et al., 2003), making clumping in the presence of plasma a nearly universal phenotype. This is reflected in the widespread clinical use of rapid agglutination tests with fibrinogen-coated beads. However, there are situations where clumping is inhibited, generally through expression of other surface structures that interfere with fibrinogen binding. For example, a subset of MRSA strains express a large surface protein called Pls that can interfere with clumping and give a negative result in rapid agglutination tests (Kuusela et al., 1994; Vaudaux et al., 1998). Pls is a 230 kDa cell wall attached adhesin encoded within the staphylococcal chromosome cassette mec (SCCmec) type I element conferring methicillin resistance in a subset of MRSA strains (Hilden et al., 1996; Ito et al., 2001). Deletion of pls restores binding to both fibrinogen and fibronectin, suggesting that Pls inhibits interactions with these matrix proteins by steric hindrance (Hussain et al., 2009; Savolainen et al., 2001). Likewise, when capsule polysaccharide is expressed at high levels it can prevent clumping, likely by masking clumping factors (Risley et al., 2007).
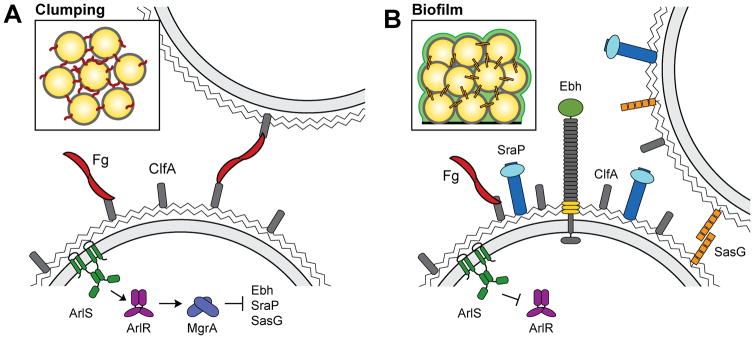
Our group has recently shown that inactivation of the ArlRS two-component regulatory system inhibits clumping (Fig. 4A), suggesting that clumping may be controlled in response to environmental signals (Walker et al., 2013). ArlS is a membrane bound histidine kinase that phosphorylates ArlR, a transcriptional regulator, in response to an unknown signal. The ArlRS system has been previously linked to biofilm formation and virulence, although the mechanism of action was largely unknown (Benton et al., 2004; Liang et al., 2005; Toledo-Arana et al., 2005). It is becoming clear that ArlRS activates expression of the global regulator MgrA, which in turn controls expression of more than 100 genes (Crosby et al., 2016; Luong et al., 2006; Luong and Lee, 2006). Among the genes under the control of this regulatory cascade are those for eight surface proteins, several of which appear to be responsible for blocking clumping. Specifically, MgrA represses expression of the large surface proteins Ebh, SraP, and SasG, and increased production of any one of these proteins can interfere with clumping (Crosby et al., 2016). We hypothesize that these proteins act is a similar fashion as Pls, and that when they are de-repressed they block interactions with fibrinogen through steric hindrance. This is line with previous work showing that overexpressing SasG from a plasmid can block fibrinogen binding (Corrigan et al., 2007).
Fig. 4.
Regulation of clumping and biofilm formation by the ArlRS/MgrA regulatory cascade. (A) In response to an unknown signal, the ArlRS two-component system activates expression of the DNA binding protein MgrA. MgrA affects expression of many genes, and among these it represses production of the large surface proteins Ebh, SraP, and SasG. In the absence of these surface proteins ClfA can interact with fibrinogen, allowing clumping to occur. (B) When the ArlRS/MgrA cascade is interrupted, expression of Ebh, SraP, and SasG is high. These proteins interfere with clumping of planktonic cells, and SasG promotes biofilm formation of surface attached cells. Figure reproduced from (Crosby et al., 2016).
To further underscore that clumping and biofilm formation are distinct processes, strains lacking arlRS or mgrA are defective in clumping but actually produce increased amounts of biofilm (Crosby et al., 2016; Toledo-Arana et al., 2005; Trotonda et al., 2008; Walker et al., 2013). Upregulation of large surface proteins in these mutants appears to interfere with the ability of planktonic cells to approach each other and interact via fibrinogen bridges. Yet increased production of one of these surface proteins, SasG, also seems to facilitate biofilm formation among surface-attached cells (Fig. 4B) (Crosby et al., 2016). SasG is known to promote biofilm formation (Geoghegan et al., 2010a), but it has not previously been appreciated that expression of proteins like SasG can simultaneously block clumping and promote biofilm formation, depending on the growth conditions and human matrix proteins present. Determining the environmental signal sensed by ArlS will shed light on when expression of these surface proteins is normally induced.
3. Role of clumping/agglutination in disease
3.1 Involvement of clumping in disease models
The most convincing evidence that S. aureus clumping is involved in pathogenesis comes from animal infection models. Strains that lack arlRS or mgrA, and thus are unable to clump, consistently show reduced virulence in models of endocarditis, sepsis, arthritis and skin abscesses (Benton et al., 2004; Chen et al., 2006; Crosby et al., 2016; Gupta et al., 2013; Liu et al., 2014; Walker et al., 2013). Also, mutations in genes of individual proteins responsible for clumping and/or coagulation, such as clfA, coa and vWbp, lead to decreased virulence. ClfA is involved in various types of infections including sepsis (Josefsson et al., 2008; McAdow et al., 2011), endocarditis (Moreillon et al., 1995; Que et al., 2001; Stutzmann Meier et al., 2001), kidney abscesses (Cheng et al., 2009), arthritis (Josefsson et al., 2001; Josefsson et al., 2008), and skin infections (Kwiecinski et al., 2014). There is, however, uncertainty regarding how ClfA affects virulence. In addition to clumping, ClfA can also promote bacterial adhesion to host tissues (Heilmann, 2011), activate platelets (O’Brien et al., 2002a) and inactivate complement (Hair et al., 2010). Initial attempts to disentangle these mechanisms relied on the use of strains carrying point mutations in ClfA that prevent fibrinogen binding, while retaining the general structure and expression of the ClfA protein (Josefsson et al., 2008; Scully et al., 2015). Those results pointed to fibrinogen binding as the main mechanism of ClfA action, but subsequent discovery that the same point mutations also interfere with anti-complement activity of ClfA raised questions on the original interpretation (Hair et al., 2010). Another study demonstrating that ClfA affects virulence even in the absence of fibrinogen and clumping again raises questions about the relative roles of different activities of ClfA for disease outcome (Palmqvist et al., 2004a).
The essential role of fibrinogen binding for ClfA activity is apparent in experimental S. aureus vaccine studies. Many groups found that the ability of anti-ClfA antibodies to inhibit fibrinogen binding was essential for protection of animals against infection. Antibodies that bind ClfA without inhibiting its interaction with fibrinogen failed to induce efficient protection (Hall et al., 2003; Scully et al., 2015; Vernachio et al., 2003). As final evidence, mice expressing a mutant form of fibrinogen that cannot bind to ClfA and does not promote clumping have improved survival in sepsis challenge (Flick et al., 2013). Taken together, animal studies on ClfA indicate that, despite having other important functions, ClfA increases virulence of S. aureus mainly through fibrinogen binding and bacterial clumping.
Similar to ClfA, the two staphylococcal coagulases, Coa and vWbp, are important for virulence in diverse infection models. As activities of these two proteins partly overlap, they are functionally redundant, and the most profound effect on virulence can be observed in engineered strains lacking both proteins. In this regard, Coa and vWbp were shown to jointly promote virulence in sepsis, kidney abscesses and skin abscesses (Cheng et al., 2010; McAdow et al., 2011; Vanassche et al., 2011). Lack of either protein was sufficient to abrogate skin infection (Loof et al., 2015; Malachowa et al., 2016), and a coa single mutant was impaired in formation of lung abscesses (Sawai et al., 1997). Surprisingly, no effect was seen of Coa on endocarditis (Baddour et al., 1994; Moreillon et al., 1995; Stutzmann Meier et al., 2001), though it might have been merely an example of vWbp compensating for the lack of Coa. Notably, in mouse abscess models, the coagulases were responsible for formation of protective layers of fibrin around a central core of bacteria. Coa is associated with the S. aureus cell surface and is probably responsible for formation of a fibrin shield directly around the bacterial cells, while vWbp can diffuse away from the surface and induces fibrin formation closer to the abscess periphery (Guggenberger et al., 2012; Thomer et al., 2016). There are, however, differences in the relative contributions of Coa and vWbp to survival in mouse and human blood (Cheng et al., 2010). The role of coagulases in fibrin deposition around the infection site in the skin of rabbits, whose coagulation/fibrinolysis system is more similar to that of humans, is also not as clear as in mouse models (Malachowa et al., 2016). Therefore, more studies are needed to determine the degree to which the role of staphylococcal coagulases in animal models is applicable to human infections.
3.2 Clumping and immune evasion
Professional phagocytes constitute the most important host defense against S. aureus infection. Neutrophils are the predominant leukocytes in the blood and they are amongst the first phagocytes arriving at the infection site, while macrophages appear with a significant delay (Bremell et al., 1992; Kwiecinski et al., 2014). This sequence of events, combined with observed aggravated infection severity in mice lacking neutrophils (Molne et al., 2000; Verdrengh and Tarkowski, 1997), and in humans suffering from neutrophil-related disorders (Spaan et al., 2013), led some to speculate that neutrophils are the only cell type involved in the efficient response against staphylococci. However, macrophages are also important for controlling staphylococcal growth in a mouse model of systemic infection (Verdrengh and Tarkowski, 2000), and ingestion of bacteria by macrophages and other antigen-presenting cells is essential for development of subsequent adaptive immunity. Therefore phagocytosis of staphylococci by all kinds of professional phagocytes is a crucial part of host defenses. S. aureus has evolved effective immune evasion strategies for these phagocytes, and the process of clumping is probably one of those strategies. One of the earliest papers describing clumping in vivo noticed that staphylococci in this state were protected from immune attacks. More specifically, neutrophils were approaching the surface of clumps formed in the peritoneal cavity, but failed to penetrate inside or to ingest any of the bacteria (Kapral, 1966). There are several potential mechanisms explaining the apparent resistance of clumps to immune attacks.
Phagocytes are capable of ingesting particles of a wide range of sizes, but there is an upper size limit dictated by the available cell membrane needed to fully engulf the prey (Cannon and Swanson, 1992; Simon and Schmid-Schonbein, 1988). Therefore phagocytes can ingest only targets with a total volume smaller or slightly larger than the volume of the phagocyte itself (Champion and Mitragotri, 2006; Herant et al., 2005). For neutrophils this means that clumps of several hundred staphylococci might already exceed the upper size limit for phagocytosis. Interestingly, the presence of phagocytic targets too large for ingestion appears to decrease global phagocytosis in the infected area, providing bystander protection to neighboring smaller targets (Okagaki and Nielsen, 2012). Thus the presence of even a couple of large staphylococcal clumps could improve the survival of neighboring S. aureus cells. The shape of the phagocytic target also plays a dominant role in susceptibility. The local curvature at the point of initial contact with the phagocyte’s membrane determines whether ingestion will occur (Champion and Mitragotri, 2006). Individual round cells, symmetrical and with a low aspect ratio, provide the optimal angle of contact with the phagocyte membrane in all orientations. Unlike individual cocci, bacterial clumps are more likely to develop asymmetric shapes, with non-optimal aspect ratios, requiring re-positioning of the phagocyte for successful ingestion and delaying the phagocytic process.
A similar impact of size on phagocytosis has been observed for other microorganisms. Many fungal pathogens form large cells that evade phagocyte ingestion due to their size, e.g. the giant cells of Cryptococcus or spherules of Coccidioides (Hung et al., 2007; Okagaki et al., 2010; Zaragoza et al., 2010). Mycobacterial formation of cords (large, twisted, rope-like cell aggregates) protects the pathogen from phagocytosis (Bernut et al., 2014). Phagocytes readily approach the surface of multicellular, flee-floating aggregates of Pseudomonas aeruginosa but are not able to ingest or penetrate them (Alhede et al., 2011). The effect of size and shape has been most extensively studied in Escherichia coli and Legionella pneumophila, which can form large filaments during infection. These filaments display both increased size and aspect ratio, and consequentially their phagocytosis by neutrophils and macrophages is decreased (Horvath et al., 2011; Moller et al., 2012). Even when phagocytosis occurs, it is delayed, leading to disturbed phagosome maturation and decreased killing of ingested filaments (Prashar et al., 2013). One can easily imagine a similar process happening during the slow ingestion of large S. aureus clumps, potentially contributing to survival of staphylococci after phagocytosis. In situations where microbes resist phagocytosis, and in cases when successful phagosome formation is not possible, neutrophils trap pathogens in neutrophil extracellular traps (NETs) (Branzk et al., 2014). NETs are composed of expelled neutrophil chromosomal DNA and decorated with bactericidal proteins, and they are expected to entrap the invading pathogen, limiting its spread and killing the immobilized bacteria (Spaan et al., 2013). However, S. aureus secretes a potent nuclease that can cleave the NETs (Berends et al., 2010) and thus circumvent this host defense mechanism.
There are some mechanisms by which the increased size of clumps can lead to improved phagocytosis and bacterial killing. First, the larger size of bacterial aggregates increases deposition of complement on the bacterial surface and allows for a better receptor-ligand interaction and recognition of pathogen by phagocytes, thus promoting opsonophagocytosis (Dalia and Weiser, 2011). As S. aureus possess several mechanisms that prevent complement deposition and activation on its surface (reviewed in (Zipfel and Skerka, 2014)), it is likely that this pathogen is not affected by the potentially increased complement deposition on larger clumps. Second, agglutination of bacteria by host antibodies is essential for efficient pathogen clearance in many diseases (Weiser, 2013; Yang et al., 2016), and when S. aureus is agglutinated by specific antibodies or pulmonary surfactant proteins it has been shown to increase phagocytosis (Hartshorn et al., 1998; Varrone et al., 2014). However, these host-induced agglutinates are relatively small (up to couple dozen cells (Varrone et al., 2014)) and differ in composition from the clumps induced by fibrinogen binding. Therefore, these immune-induced agglutinates most likely have different properties than the clumps discussed in this review, including differences in their ability to promote phagocytic uptake.
Additionally, S. aureus clumps might modulate phagocytosis due to fibrinogen present between the individual cocci and on the surface, and through the protective layers of fibrin around the infecting bacteria. Interactions of the immune system with fibrin and fibrinogen are complex, and in some instances the presence of fibrinogen in infected sites clearly promotes containment and clearance of invading staphylococci (Ko and Flick, 2016). However, in most settings S. aureus takes advantage of host fibrinogen and hijacks the human coagulation system for its own survival. The anti-phagocytic effect of fibrinogen bound on the surface of S. aureus has been well established (Higgins et al., 2006; Ko and Flick, 2016). There are probably several distinct mechanisms responsible for this outcome, such as shielding opsonins from recognition by immune cells using a fibrinogen layer (Ko and Flick, 2016), or the recruitment of factor H, which binds directly to fibrinogen and inhibits complement activation (Horstmann et al., 1992). While in certain experimental settings the effect of fibrinogen binding and bacterial clumping on phagocytosis seems to be minimal (Palmqvist et al., 2004b), the protective potential of fibrinogen on staphylococcal surfaces has been confirmed in most studies (Ko and Flick, 2016). Therefore fibrinogen likely has a dual function in clumps: both as a mediator of clump formation and as an important constituent that shields the bacterium from host defenses. In addition to the protective effect of fibrinogen itself, additional fortification can be conferred by fibrin, the product of fibrinogen polymerization. Coagulases secreted by S. aureus can induce this polymerization and cover bacteria with a protective fibrin coat. Fibrin-encased bacteria, in a biofilm state or free-floating, do not activate immune cells and thus escape phagocytosis (Kwiecinski et al., 2016; Vanassche et al., 2011). When an in vitro abscess model was investigated, two types of fibrin barriers (one closely associated with the bacterial surface, probably mainly due to Coa activity, the other a more diffuse meshwork of fibers due to vWbp) acted as a physical obstacle for incoming neutrophils (Guggenberger et al., 2012). In animal infection models, formation of this fibrin coat was essential for full virulence (Cheng et al., 2010; Loof et al., 2015). The ability to form fibrinogen-rich clumps is most likely a prerequisite for the subsequent coagulation and fibrin-shielding, which further underlines the importance of clumping for staphylococcal virulence and immune evasion.
3.3 Clumping and antibiotic resistance
One of the major challenges associated with treatment of biofilm infections is their resistance to antimicrobials (Paharik and Horswill, 2016), and it is possible that S. aureus clumping may also increase resistance. Fibrin-rich staphylococcal clumps forming in synovial fluid showed decreased susceptibility to vancomycin and cefazolin, which disappeared once the clumps were dispersed (Dastgheyb et al., 2015a). The exact mechanism of this resistance is not known, although the presence of fibrin in biofilm was previously linked with increased antibiotic resistance (Kwiecinski et al., 2016; Vanassche et al., 2013). There seems to be a common theme of staphylococci associated with fibrin becoming resistant to various antimicrobials. This also includes host-produced compounds, as antibacterial phospholipase A2 had decreased killing activity towards S. aureus inside clumps (Dominiecki and Weiss, 1999).
4. Anti-clumping mechanisms
4.1 S. aureus escape from clumps
Formation of clumps gives staphylococci a distinctive advantage for survival in the host. However, as illustrated by the case of fibrin-dependent killing of S. aureus in the peritoneal cavity (Prasad et al., 2015), in some instances it is beneficial for the pathogen to escape from clumps, and thus avoid entrapment by the immune system and freely disseminate to other infection sites. Therefore S. aureus may have evolved mechanisms for leaving clumps and fibrin aggregates. As already discussed above, expression of capsule or, in some MRSA strains, the surface protein Pls can prevent clumping and retain staphylococci in a single-cell state. Alternatively, the ArlRS-MgrA regulatory axis can switch the entire pattern of surface protein expression from “clumping” to “free-living/biofilm” type (described in detail above). Additionally, staphylococci can quickly degrade existing clumps by secretion of staphylokinase (Sak), and this mechanism will be reviewed below.
Sak is expressed by the majority of human S. aureus isolates, and its gene is encoded on a prophage (usually the beta-converting phage) (Bokarewa et al., 2006). It is a potent activator of human plasminogen – a zymogen circulating in blood – into a broad-spectrum, fibrinolytic protease, plasmin (Fig. 1). Notably, staphylococci bind plasminogen on their cell surface. And while the activity of plasmin in the bloodstream in the absence of fibrin is normally controlled by the inhibitor α-2-antiplasmin, this inhibition does not occur with plasmin bound to the staphylococcal surface (Molkanen et al., 2002). Therefore, by secreting Sak, S. aureus hijacks the host fibrinolytic system and generates its own, surface-associated, strong proteolytic activity. The presence of Sak can lead to both cleavage of fibrinogen, dispersing the clumps, and unraveling the protective fibrin sheath surrounding clumps and abscesses. Therefore secretion of Sak could lead to dissemination of individual cocci away from the original clumps and clots. This mechanism is somewhat understudied, in part because Sak is highly selective for human plasminogen and has very little activity towards plasminogen in mouse models (Kwiecinski et al., 2016; Okada et al., 2000). Considering mouse models are the dominant means of assessing S. aureus pathogenesis, the lack of Sak activity likely leads to a “hyper-coagulative” phenotype in different types of model infections, potentially biasing the interpretation. Nevertheless, the use of humanized mouse models and in vitro human abscess-like systems allows for validation of the role of Sak, and in these models Sak activity led to dissemination of individual bacteria from in vitro fibrin-coated abscesses (Guggenberger et al., 2012), wider spread of infection inside skin tissues, spreading from initial injection sites (Peetermans et al., 2014) and detachment of bacteria from fibrin-rich biofilms (Kwiecinski et al., 2016). While the effect of Sak on clumps was not examined, externally added plasmin disperses staphylococci agglutinated in synovial fluid (Dastgheyb et al., 2015a), suggesting S. aureus-secreted Sak should achieve the same outcome.
4.2 Targeting clumping as therapy
The importance of clumping for S. aureus virulence makes it a promising target for therapeutic development. Different attempts at inhibiting staphylococcal clumping can be roughly divided into those using small molecule inhibitors and those relying on immunotherapies. The most obvious target is regulation of clumping, and several potential drugs blocking MgrA activity have been identified. One MgrA inhibitor, 5,5-methylenedisalicylic acid, has been shown to prevent formation of organ abscesses during S. aureus sepsis (Sun et al., 2011). Some of the α-methylene-γ-butyrolactones inhibit both MgrA and other staphylococcal transcriptional regulators like SarA and SarR, thus exhibiting potentially powerful, combined antivirulent action (Kunzmann et al., 2014). Extracts of Sargassum pallidium seaweed and Cinnamomum cassia tree were also shown to interfere with MgrA, yet the exact active compounds were not identified(Wang et al., 2015).
Most of the “traditional” anticoagulants cannot prevent S. aureus-induced coagulation, but this can be achieved with novel drugs of the univalent direct thrombin inhibitor family (Vanassche et al., 2010), such as dabigatran and argatroban. Their use in animal models confirmed their efficacy and potential to decrease disease severity, similar to the effect of coa and vWbp mutants (Peetermans et al., 2015). Using an in vitro model, argatroban prevented formation of fibrin barriers around abscesses (Guggenberger et al., 2012), and in mouse models dabigatran decreased the severity of skin infections (Vanassche et al., 2011) and prevented mortality in sepsis (McAdow et al., 2011). However, as these inhibitors are not selective for staphylococcal coagulases and also prevent the patient’s own coagulation, it is unclear if they would be safe to use as a treatment approach in all situations.
In addition to small molecule inhibitors, immune-based therapies against coagulases have received significant attention. Both vaccination and passive transfer of antibodies against Coa and vWbp protected from lethal sepsis, development of kidney abscesses and appearance of thromboembolic lesions in tissues (Cheng et al., 2010; McAdow et al., 2011). As there are many serotypes of coagulases, vaccination against a particular one will provide poor protection against infection with a S. aureus strain secreting a different serotype. One attempt to overcome this barrier is to vaccinate with hybrid protein, containing relevant domains from the most common serotypes (McAdow et al., 2012a). Another approach is to vaccinate against the non-variable region of Coa, which is the fibrinogen-binding domain (Thomer et al., 2016). The latter approach has an added advantage of creating antibodies that would prevent binding of Coa to fibrinogen/fibrin and subsequent formation of a fibrin shield around the staphylococcal surface (Thomer et al., 2016). Despite promising performance in infection models, coagulase-targeting vaccines appear to be inefficient in neutropenic (immunosuppressed) animals, while some other anti-S. aureus vaccines retain their potency (Rauch et al., 2014). It remains unclear if this is due to lack of coagulation and clumping in the absence of inflammation, or if it is because an incomplete immune system cannot deal with individual, un-clumped staphylococci.
Similarly to coagulases, it is possible to vaccinate against the ClfA surface protein. Numerous reports indicate high efficacy of such vaccines (or ready antibody preparations) in prevention and treatment of a whole range of infections, including mastitis (Gong et al., 2010; Tuchscherr et al., 2008), arthritis (Josefsson et al., 2001), necrotic wound infection (Schennings et al., 2012), endocarditis (Domanski et al., 2005), and sepsis (Josefsson et al., 2008; McAdow et al., 2011; Scully et al., 2015). Many of the studies revealed that the essential requirement for antibodies to be efficient is their ability to inhibit binding of fibrinogen to ClfA (Scully et al., 2015; Vernachio et al., 2003). Efficacy of anti-ClfA antibodies was highest when they were combined with inhibitors of coagulases, ensuring complete blockade of clumping and coagulation (McAdow et al., 2011). While some recent experiments failed to demonstrate as impressive a protective effect of ClfA vaccination as the earlier studies (Li et al., 2016), ClfA nevertheless remains one of the most promising targets for vaccination in order to prevent clumping and decrease virulence.
5. Polysaccharide based aggregation
5.1 Staphylococcal aggregation
Many strains of S. aureus and S. epidermidis are able to form multicellular clusters in the absence of host factors, which is commonly referred to in the literature as aggregation. Usually aggregation is due to production of the extracellular polysaccharide poly-N-acetyl-glucosamine (PNAG), also known as polysaccharide extracellular adhesin (PIA). PIA was initially identified as a secreted factor important for intercellular adhesion, a necessary step for the accumulation phase of biofilm formation, subsequent to the initial attachment to a surface (Heilmann et al., 1996a; Heilmann et al., 1996b; Mack et al., 1994). This intercellular adhesion can also occur in planktonic cultures, resulting in cellular aggregates that can be >0.5 mm in diameter (Haaber et al., 2012). In support of this, heterologous expression of the S. epidermidis PIA producing genes in S. carnosus resulted in pronounced aggregation in liquid culture (Heilmann et al., 1996b). PIA is composed of linear polymers of β-1,6-linked N-acetylglucosamine (GlcNAc) residues, though ~15% of these GlcNAc residues are later deacetylated, resulting in a net positive charge (Vuong et al., 2004a). While most bacterial extracellular polysaccharides have no net charge or are anionic (Limoli et al., 2015), the positive charge of PIA appears to be essential for its association with the negatively charged cell surface (Vuong et al., 2004a). Once synthesized, PIA forms a rope-like network that encases and holds together neighboring cells (Fluckiger et al., 2005; Haaber et al., 2012; Vuong et al., 2004b). In addition to polysaccharide-based aggregation, overexpression of certain cell wall anchored proteins can also lead to intercellular adhesion and aggregation. For example, overexpression of the IgG-binding Protein A can promote both aggregation and biofilm formation in S. aureus (Merino et al., 2009). Likewise, overproduction of SasG (Geoghegan et al., 2010a; Kuroda et al., 2008) or surface protein C (SasC) (Schroeder et al., 2009), a large surface protein of unknown function, results in bacterial aggregation. However, surface protein-mediated cellular aggregation seems to be the exception, with most aggregation resulting from polysaccharide production.
The PIA synthesis machinery is encoded by the four-gene icaADBC operon, with a transcriptional regulator, icaR, located upstream and divergent from icaA (Cramton et al., 1999; Heilmann et al., 1996b). IcaA and IcaD are integral membrane proteins that polymerize UDP-GlcNAc to ~20-mer chains, which appear to be further elongated and perhaps transported across the cell membrane by IcaC (Gerke et al., 1998). IcaB is a secreted deacetylase that subsequently deacetylates a fraction of the GlcNAc residues (Vuong et al., 2004a). Regulation of icaADBC expression is complex and varies not only between Staphylococcal species, but also among individual strains (Cue et al., 2012). This is reflected in the observation that the degree of aggregation varies from strain to strain, with one study finding that ~30% of S. aureus strains tested aggregated to some degree in rich media (Haaber et al., 2016). Conditions that induce icaADBC expression include high temperature, anaerobiosis, high NaCl concentrations, high glucose levels, ethanol, and subinhibitory concentrations of some antibiotics (Conlon et al., 2002; Cramton et al., 2001; Knobloch et al., 2001; Rachid et al., 2000). Intriguingly, there is evidence that PIA production is increased in vivo compared to typical laboratory culture conditions (Fluckiger et al., 2005; McKenney et al., 1999).
5.2 Role of aggregates in infection
The significance of polysaccharide-based aggregation in staphylococcal infections is largely unexplored. This is due in part to inconsistencies in terminology, where aggregates and biofilms are sometimes referred to interchangeably. In an infection setting, it may be more accurate to consider polysaccharide-based aggregates and biofilms as different phases of the same process, with aggregates seeding biofilms, and biofilms dispersing into free-floating aggregates (Kragh et al., 2016; Melaugh et al., 2016). Several studies have focused on the contribution of PIA to biofilm formation, where there is a great deal of heterogeneity between strains and infection sites. There are examples where the icaADBC locus is required for biofilm formation in vivo (Lin et al., 2015; Vuong et al., 2004a), as well as cases where the biofilm matrix appears to be predominantly composed of protein and DNA, and PIA production is dispensable for virulence (Beenken et al., 2004; Schaeffer et al., 2015; Vergara-Irigaray et al., 2009). One of the main advantages of biofilm formation is enhanced tolerance of some antibiotics, with S. aureus exhibiting 100–1000 fold higher MICs (Ceri et al., 1999). Likewise, S. aureus aggregates are better able to tolerate antibiotic treatment than planktonic cells, suggesting that aggregation does provide a degree of protection (Haaber et al., 2012). It is possible that aggregation also confers enhanced protection from phagocytosis, similar to what has been postulated for clumping. Production of PIA appears to be dispensable for renal abscess formation (Cheng et al., 2009), suggesting that fibrinogen-based clumping is more important in this type of infection.
6. Conclusions
S. aureus has evolved to interact with multiple components of the coagulation cascade, allowing it to take advantage of a system that is normally part of the host defense against bacterial pathogens. For example, fibrin clots form at breaches in the skin in part to trap invading bacteria and prevent their dissemination throughout the body (Ko and Flick, 2016). S. aureus, on the other hand, secretes its own coagulases, which allow it to use the host’s resources to build a fibrin shield around abscess communities, protecting the bacteria from infiltrating neutrophils (Cheng et al., 2011). In addition to catalyzing the deposition of a fibrin wall, S. aureus also possesses multiple cell wall anchored and secreted fibrinogen-binding proteins. Among the MSCRAMMs, ClfA appears to play the largest role in fibrinogen binding. It facilitates adhesion to fibrin coated surfaces and platelets, and also promotes clumping between staphylococcal cells. From a clinical standpoint, clumping is a defining characteristic of S. aureus, although a few of the more virulent coagulase negative staphylococci, like S. lugdunensis, can also clump. Formation of large clumps of cells in the presence of fibrinogen is likely a defensive strategy against phagocytosis, although it may also confer increased resistance to antibiotics. Intriguingly, there is evidence that clumping also promotes activation of the quorum sensing system, hastening production of virulence factors (Rothfork et al., 2003).
We propose that fibrin(ogen)-mediated clumping and agglutination are distinct from biofilm formation, and should be considered as different processes. At the molecular level, clumping relies on interaction with a host plasma protein, rather than bacterial production of extracellular matrix material such as polysaccharide or eDNA. While biofilm formation requires particular environmental conditions and a concerted effort on the part of the bacterial cells, the ability to clump is a “default state” for S. aureus, since clumping factors are expressed by nearly all strains at all phases of growth. Yet it is possible to inhibit clumping, typically through expression of other large surface proteins that interfere with fibrinogen binding. Interestingly, the ArlRS/MgrA regulatory cascade affects clumping and biofilm formation in opposite directions (Fig. 4). Mutants lacking these regulators fail to clump, but are hyper-biofilm formers (Crosby et al., 2016). From an infection standpoint, it seems likely that biofilm formation and clumping have distinct niches, although there is likely overlap. Biofilms are most commonly associated with infections of artificial joints and indwelling devices. These artificial surfaces are quickly coated with host proteins (Francois et al., 2000; Vaudaux et al., 1993), and binding to these matrix proteins is crucial for both biofilm formation and clumping. Once established, surface-attached biofilms often occur as large lawns of cells, many of which are metabolically inactive. Clumping, on the other hand, may be more relevant for establishment of abscesses, heart valve vegetations in infective endocarditis, osteomyelitis, and chronic wound infections. These infections often involve compact groups of bacterial cells in a milieu of host matrix proteins, with the bacteria harnessing these host proteins to protect themselves from phagocytosis.
Although we have known for over a century that S. aureus can coagulate blood and form large clumps in the presence of fibrinogen, it has been less clear how these traits promote virulence. The fact that expression of coagulases and clumping factors is nearly universal among clinical isolates suggests that they are important for pathogenesis. There is growing evidence that coagulation and clumping promote abscess formation (Cheng et al., 2010; McAdow et al., 2011), and that clumping plays a role in endocarditis (Moreillon et al., 1995; Walker et al., 2013). Future work may shed light on the role of clumping and agglutination in other infection types, such as bacteremia, chronic wound infections, osteomyelitis, and septic arthritis. It is possible that S. aureus takes advantage of additional host matrix material to form protective structures in other body sites, such as in bone tissue and synovial fluid.
Acknowledgments
We thank Tom Moninger at the University of Iowa Central Microscopy Research Facility for assistance with SEM. H. Crosby was supported by an NIH Training Grant T32 AI007511 and an American Heart Association postdoctoral fellowship. J. Kwiecinski was supported by a Swedish Society for Medical Research postdoctoral fellowship. Research in the lab of A. R. Horswill was supported by project 3 of NIH grant AI083211.
References
- Adams RL, Bird RJ. Review article: Coagulation cascade and therapeutics update: relevance to nephrology. Part 1: Overview of coagulation, thrombophilias and history of anticoagulants. Nephrology. 2009;14:462–470. doi: 10.1111/j.1440-1797.2009.01128.x. [DOI] [PubMed] [Google Scholar]
- Alhede M, Kragh KN, Qvortrup K, Allesen-Holm M, van Gennip M, Christensen LD, Jensen PO, Nielsen AK, Parsek M, Wozniak D, Molin S, Tolker-Nielsen T, Hoiby N, Givskov M, Bjarnsholt T. Phenotypes of non-attached Pseudomonas aeruginosa aggregates resemble surface attached biofilm. PloS one. 2011;6:e27943. doi: 10.1371/journal.pone.0027943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argemi X, Riegel P, Lavigne T, Lefebvre N, Grandpre N, Hansmann Y, Jaulhac B, Prevost G, Schramm F. Implementation of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry in Routine Clinical Laboratories Improves Identification of Coagulase-Negative Staphylococci and Reveals the Pathogenic Role of Staphylococcus lugdunensis. Journal of clinical microbiology. 2015;53:2030–2036. doi: 10.1128/JCM.00177-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariens RA. Fibrin(ogen) and thrombotic disease. Journal of thrombosis and haemostasis: JTH. 2013;11(Suppl 1):294–305. doi: 10.1111/jth.12229. [DOI] [PubMed] [Google Scholar]
- Attia AS, Cassat JE, Aranmolate SO, Zimmerman LJ, Boyd KL, Skaar EP. Analysis of the Staphylococcus aureus abscess proteome identifies antimicrobial host proteins and bacterial stress responses at the host-pathogen interface. Pathogens and disease. 2013 doi: 10.1111/2049-632X.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddour LM, Tayidi MM, Walker E, McDevitt D, Foster TJ. Virulence of coagulase-deficient mutants of Staphylococcus aureus in experimental endocarditis. Journal of medical microbiology. 1994;41:259–263. doi: 10.1099/00222615-41-4-259. [DOI] [PubMed] [Google Scholar]
- Beenken KE, Dunman PM, McAleese F, Macapagal D, Murphy E, Projan SJ, Blevins JS, Smeltzer MS. Global gene expression in Staphylococcus aureus biofilms. Journal of bacteriology. 2004;186:4665–4684. doi: 10.1128/JB.186.14.4665-4684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton BM, Zhang JP, Bond S, Pope C, Christian T, Lee L, Winterberg KM, Schmid MB, Buysse JM. Large-scale identification of genes required for full virulence of Staphylococcus aureus. Journal of bacteriology. 2004;186:8478–8489. doi: 10.1128/JB.186.24.8478-8489.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berends ET, Horswill AR, Haste NM, Monestier M, Nizet V, von Kockritz-Blickwede M. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. Journal of innate immunity. 2010;2:576–586. doi: 10.1159/000319909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernut A, Herrmann JL, Kissa K, Dubremetz JF, Gaillard JL, Lutfalla G, Kremer L. Mycobacterium abscessus cording prevents phagocytosis and promotes abscess formation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E943–952. doi: 10.1073/pnas.1321390111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnsholt T, Alhede M, Alhede M, Eickhardt-Sorensen SR, Moser C, Kuhl M, Jensen PO, Hoiby N. The in vivo biofilm. Trends in microbiology. 2013;21:466–474. doi: 10.1016/j.tim.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Bjerketorp J, Nilsson M, Ljungh A, Flock JI, Jacobsson K, Frykberg L. A novel von Willebrand factor binding protein expressed by Staphylococcus aureus. Microbiology. 2002;148:2037–2044. doi: 10.1099/00221287-148-7-2037. [DOI] [PubMed] [Google Scholar]
- Bokarewa MI, Jin T, Tarkowski A. Staphylococcus aureus: Staphylokinase. The international journal of biochemistry & cell biology. 2006;38:504–509. doi: 10.1016/j.biocel.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, Papayannopoulos V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nature immunology. 2014;15:1017–1025. doi: 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremell T, Abdelnour A, Tarkowski A. Histopathological and serological progression of experimental Staphylococcus aureus arthritis. Infection and immunity. 1992;60:2976–2985. doi: 10.1128/iai.60.7.2976-2985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon GJ, Swanson JA. The macrophage capacity for phagocytosis. Journal of cell science. 1992;101(Pt 4):907–913. doi: 10.1242/jcs.101.4.907. [DOI] [PubMed] [Google Scholar]
- Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. Journal of clinical microbiology. 1999;37:1771–1776. doi: 10.1128/jcm.37.6.1771-1776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nature reviews Microbiology. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavakis T, Hussain M, Kanse SM, Peters G, Bretzel RG, Flock JI, Herrmann M, Preissner KT. Staphylococcus aureus extracellular adherence protein serves as anti-inflammatory factor by inhibiting the recruitment of host leukocytes. Nature medicine. 2002;8:687–693. doi: 10.1038/nm728. [DOI] [PubMed] [Google Scholar]
- Chavakis T, Wiechmann K, Preissner KT, Herrmann M. Staphylococcus aureus interactions with the endothelium: the role of bacterial “secretable expanded repertoire adhesive molecules” (SERAM) in disturbing host defense systems. Thrombosis and haemostasis. 2005;94:278–285. doi: 10.1160/TH05-05-0306. [DOI] [PubMed] [Google Scholar]
- Chen PR, Bae T, Williams WA, Duguid EM, Rice PA, Schneewind O, He C. An oxidation-sensing mechanism is used by the global regulator MgrA in Staphylococcus aureus. Nature chemical biology. 2006;2:591–595. doi: 10.1038/nchembio820. [DOI] [PubMed] [Google Scholar]
- Cheng AG, DeDent AC, Schneewind O, Missiakas D. A play in four acts: Staphylococcus aureus abscess formation. Trends in microbiology. 2011;19:225–232. doi: 10.1016/j.tim.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2009;23:3393–3404. doi: 10.1096/fj.09-135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AG, McAdow M, Kim HK, Bae T, Missiakas DM, Schneewind O. Contribution of coagulases towards Staphylococcus aureus disease and protective immunity. PLoS pathogens. 2010;6:e1001036. doi: 10.1371/journal.ppat.1001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon KM, Humphreys H, O’Gara JP. icaR encodes a transcriptional repressor involved in environmental regulation of ica operon expression and biofilm formation in Staphylococcus epidermidis. Journal of bacteriology. 2002;184:4400–4408. doi: 10.1128/JB.184.16.4400-4408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan RM, Rigby D, Handley P, Foster TJ. The role of Staphylococcus aureus surface protein SasG in adherence and biofilm formation. Microbiology. 2007;153:2435–2446. doi: 10.1099/mic.0.2007/006676-0. [DOI] [PubMed] [Google Scholar]
- Cramton SE, Gerke C, Schnell NF, Nichols WW, Gotz F. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infection and immunity. 1999;67:5427–5433. doi: 10.1128/iai.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramton SE, Ulrich M, Gotz F, Doring G. Anaerobic conditions induce expression of polysaccharide intercellular adhesin in Staphylococcus aureus and Staphylococcus epidermidis. Infection and immunity. 2001;69:4079–4085. doi: 10.1128/IAI.69.6.4079-4085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby HA, Schlievert PM, Merriman JA, King JM, Salgado-Pabon W, Horswill AR. The Staphylococcus aureus Global Regulator MgrA Modulates Clumping and Virulence by Controlling Surface Protein Expression. PLoS pathogens. 2016;12:e1005604. doi: 10.1371/journal.ppat.1005604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cue D, Lei MG, Lee CY. Genetic regulation of the intercellular adhesion locus in staphylococci. Frontiers in cellular and infection microbiology. 2012;2:38. doi: 10.3389/fcimb.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalia AB, Weiser JN. Minimization of bacterial size allows for complement evasion and is overcome by the agglutinating effect of antibody. Cell host & microbe. 2011;10:486–496. doi: 10.1016/j.chom.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastgheyb S, Parvizi J, Shapiro IM, Hickok NJ, Otto M. Effect of biofilms on recalcitrance of staphylococcal joint infection to antibiotic treatment. The Journal of infectious diseases. 2015a;211:641–650. doi: 10.1093/infdis/jiu514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastgheyb SS, Villaruz AE, Le KY, Tan VY, Duong AC, Chatterjee SS, Cheung GY, Joo HS, Hickok NJ, Otto M. Role of Phenol-Soluble Modulins in Formation of Staphylococcus aureus Biofilms in Synovial Fluid. Infection and immunity. 2015b;83:2966–2975. doi: 10.1128/IAI.00394-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. Understanding biofilm resistance to antibacterial agents. Nature reviews Drug discovery. 2003;2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- Davis SL, Gurusiddappa S, McCrea KW, Perkins S, Hook M. SdrG, a fibrinogen-binding bacterial adhesin of the microbial surface components recognizing adhesive matrix molecules subfamily from Staphylococcus epidermidis, targets the thrombin cleavage site in the Bbeta chain. The Journal of biological chemistry. 2001;276:27799–27805. doi: 10.1074/jbc.M103873200. [DOI] [PubMed] [Google Scholar]
- Deivanayagam CC, Wann ER, Chen W, Carson M, Rajashankar KR, Hook M, Narayana SV. A novel variant of the immunoglobulin fold in surface adhesins of Staphylococcus aureus: crystal structure of the fibrinogen-binding MSCRAMM, clumping factor A. The EMBO journal. 2002;21:6660–6672. doi: 10.1093/emboj/cdf619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanski PJ, Patel PR, Bayer AS, Zhang L, Hall AE, Syribeys PJ, Gorovits EL, Bryant D, Vernachio JH, Hutchins JT, Patti JM. Characterization of a humanized monoclonal antibody recognizing clumping factor A expressed by Staphylococcus aureus. Infection and immunity. 2005;73:5229–5232. doi: 10.1128/IAI.73.8.5229-5232.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominiecki ME, Weiss J. Antibacterial action of extracellular mammalian group IIA phospholipase A2 against grossly clumped Staphylococcus aureus. Infection and immunity. 1999;67:2299–2305. doi: 10.1128/iai.67.5.2299-2305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elamin WF, Ball D, Millar M. Unbiased species-level identification of clinical isolates of coagulase-negative Staphylococci: does it change the perspective on Staphylococcus lugdunensis? Journal of clinical microbiology. 2015;53:292–294. doi: 10.1128/JCM.02932-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazli M, Bjarnsholt T, Kirketerp-Moller K, Jorgensen B, Andersen AS, Krogfelt KA, Givskov M, Tolker-Nielsen T. Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. Journal of clinical microbiology. 2009;47:4084–4089. doi: 10.1128/JCM.01395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick MJ, Du X, Prasad JM, Raghu H, Palumbo JS, Smeds E, Hook M, Degen JL. Genetic elimination of the binding motif on fibrinogen for the S. aureus virulence factor ClfA improves host survival in septicemia. Blood. 2013;121:1783–1794. doi: 10.1182/blood-2012-09-453894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock JI, Froman G, Jonsson K, Guss B, Signas C, Nilsson B, Raucci G, Hook M, Wadstrom T, Lindberg M. Cloning and expression of the gene for a fibronectin-binding protein from Staphylococcus aureus. The EMBO journal. 1987;6:2351–2357. doi: 10.1002/j.1460-2075.1987.tb02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluckiger U, Ulrich M, Steinhuber A, Doring G, Mack D, Landmann R, Goerke C, Wolz C. Biofilm formation, icaADBC transcription, and polysaccharide intercellular adhesin synthesis by staphylococci in a device-related infection model. Infection and immunity. 2005;73:1811–1819. doi: 10.1128/IAI.73.3.1811-1819.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TJ, Geoghegan JA, Ganesh VK, Hook M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nature reviews Microbiology. 2014;12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois P, Schrenzel J, Stoerman-Chopard C, Favre H, Herrmann M, Foster TJ, Lew DP, Vaudaux P. Identification of plasma proteins adsorbed on hemodialysis tubing that promote Staphylococcus aureus adhesion. The Journal of laboratory and clinical medicine. 2000;135:32–42. doi: 10.1016/s0022-2143(00)70018-7. [DOI] [PubMed] [Google Scholar]
- Frank KL, Del Pozo JL, Patel R. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clinical microbiology reviews. 2008;21:111–133. doi: 10.1128/CMR.00036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freney J, Brun Y, Bes M, Meugnier H, Grimont F, Grimont PAD, Nervi C, Fleurette J. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38:168–172. [Google Scholar]
- Friedrich R, Panizzi P, Fuentes-Prior P, Richter K, Verhamme I, Anderson PJ, Kawabata S, Huber R, Bode W, Bock PE. Staphylocoagulase is a prototype for the mechanism of cofactor-induced zymogen activation. Nature. 2003;425:535–539. doi: 10.1038/nature01962. [DOI] [PubMed] [Google Scholar]
- Froman G, Switalski LM, Speziale P, Hook M. Isolation and characterization of a fibronectin receptor from Staphylococcus aureus. The Journal of biological chemistry. 1987;262:6564–6571. [PubMed] [Google Scholar]
- Ganesh VK, Rivera JJ, Smeds E, Ko YP, Bowden MG, Wann ER, Gurusiddappa S, Fitzgerald JR, Hook M. A structural model of the Staphylococcus aureus ClfA-fibrinogen interaction opens new avenues for the design of anti-staphylococcal therapeutics. PLoS pathogens. 2008;4:e1000226. doi: 10.1371/journal.ppat.1000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbrecht BV, Hamaoka BY, Perman B, Zemla A, Leahy DJ. The crystal structures of EAP domains from Staphylococcus aureus reveal an unexpected homology to bacterial superantigens. The Journal of biological chemistry. 2005;280:17243–17250. doi: 10.1074/jbc.M412311200. [DOI] [PubMed] [Google Scholar]
- Geoghegan JA, Corrigan RM, Gruszka DT, Speziale P, O’Gara JP, Potts JR, Foster TJ. Role of surface protein SasG in biofilm formation by Staphylococcus aureus. Journal of bacteriology. 2010a;192:5663–5673. doi: 10.1128/JB.00628-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoghegan JA, Ganesh VK, Smeds E, Liang X, Hook M, Foster TJ. Molecular characterization of the interaction of staphylococcal microbial surface components recognizing adhesive matrix molecules (MSCRAMM) ClfA and Fbl with fibrinogen. The Journal of biological chemistry. 2010b;285:6208–6216. doi: 10.1074/jbc.M109.062208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke C, Kraft A, Sussmuth R, Schweitzer O, Gotz F. Characterization of the N-acetylglucosaminyltransferase activity involved in the biosynthesis of the Staphylococcus epidermidis polysaccharide intercellular adhesin. The Journal of biological chemistry. 1998;273:18586–18593. doi: 10.1074/jbc.273.29.18586. [DOI] [PubMed] [Google Scholar]
- Gong R, Hu C, Xu H, Guo A, Chen H, Zhang G, Shi L. Evaluation of clumping factor A binding region A in a subunit vaccine against Staphylococcus aureus-induced mastitis in mice. Clinical and vaccine immunology: CVI. 2010;17:1746–1752. doi: 10.1128/CVI.00162-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorwitz RJ, Kruszon-Moran D, McAllister SK, McQuillan G, McDougal LK, Fosheim GE, Jensen BJ, Killgore G, Tenover FC, Kuehnert MJ. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. The Journal of infectious diseases. 2008;197:1226–1234. doi: 10.1086/533494. [DOI] [PubMed] [Google Scholar]
- Guggenberger C, Wolz C, Morrissey JA, Heesemann J. Two distinct coagulase-dependent barriers protect Staphylococcus aureus from neutrophils in a three dimensional in vitro infection model. PLoS pathogens. 2012;8:e1002434. doi: 10.1371/journal.ppat.1002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Alba J, Xiong YQ, Bayer AS, Lee CY. MgrA activates expression of capsule genes, but not the alpha-toxin gene in experimental Staphylococcus aureus endocarditis. The Journal of infectious diseases. 2013;208:1841–1848. doi: 10.1093/infdis/jit367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaber J, Cohn MT, Frees D, Andersen TJ, Ingmer H. Planktonic aggregates of Staphylococcus aureus protect against common antibiotics. PloS one. 2012;7:e41075. doi: 10.1371/journal.pone.0041075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaber J, Cohn MT, Petersen A, Ingmer H. Simple method for correct enumeration of Staphylococcus aureus. Journal of microbiological methods. 2016;125:58–63. doi: 10.1016/j.mimet.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Hair PS, Echague CG, Sholl AM, Watkins JA, Geoghegan JA, Foster TJ, Cunnion KM. Clumping factor A interaction with complement factor I increases C3b cleavage on the bacterial surface of Staphylococcus aureus and decreases complement-mediated phagocytosis. Infection and immunity. 2010;78:1717–1727. doi: 10.1128/IAI.01065-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AE, Domanski PJ, Patel PR, Vernachio JH, Syribeys PJ, Gorovits EL, Johnson MA, Ross JM, Hutchins JT, Patti JM. Characterization of a protective monoclonal antibody recognizing Staphylococcus aureus MSCRAMM protein clumping factor A. Infection and immunity. 2003;71:6864–6870. doi: 10.1128/IAI.71.12.6864-6870.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harraghy N, Hussain M, Haggar A, Chavakis T, Sinha B, Herrmann M, Flock JI. The adhesive and immunomodulating properties of the multifunctional Staphylococcus aureus protein Eap. Microbiology. 2003;149:2701–2707. doi: 10.1099/mic.0.26465-0. [DOI] [PubMed] [Google Scholar]
- Hartford O, O’Brien L, Schofield K, Wells J, Foster TJ. The Fbe (SdrG) protein of Staphylococcus epidermidis HB promotes bacterial adherence to fibrinogen. Microbiology. 2001;147:2545–2552. doi: 10.1099/00221287-147-9-2545. [DOI] [PubMed] [Google Scholar]
- Hartshorn KL, Crouch E, White MR, Colamussi ML, Kakkanatt A, Tauber B, Shepherd V, Sastry KN. Pulmonary surfactant proteins A and D enhance neutrophil uptake of bacteria. The American journal of physiology. 1998;274:L958–969. doi: 10.1152/ajplung.1998.274.6.L958. [DOI] [PubMed] [Google Scholar]
- Hassouna HI. Thrombophilia and hypercoagulability. Medical principles and practice: international journal of the Kuwait University, Health Science Centre. 2009;18:429–440. doi: 10.1159/000235891. [DOI] [PubMed] [Google Scholar]
- Hawiger J, Timmons S, Strong DD, Cottrell BA, Riley M, Doolittle RF. Identification of a region of human fibrinogen interacting with staphylococcal clumping factor. Biochemistry. 1982;21:1407–1413. doi: 10.1021/bi00535a047. [DOI] [PubMed] [Google Scholar]
- Hazenbos WL, Kajihara KK, Vandlen R, Morisaki JH, Lehar SM, Kwakkenbos MJ, Beaumont T, Bakker AQ, Phung Q, Swem LR, Ramakrishnan S, Kim J, Xu M, Shah IM, Diep BA, Sai T, Sebrell A, Khalfin Y, Oh A, Koth C, Lin SJ, Lee BC, Strandh M, Koefoed K, Andersen PS, Spits H, Brown EJ, Tan MW, Mariathasan S. Novel staphylococcal glycosyltransferases SdgA and SdgB mediate immunogenicity and protection of virulence-associated cell wall proteins. PLoS pathogens. 2013;9:e1003653. doi: 10.1371/journal.ppat.1003653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann C. Adhesion mechanisms of staphylococci. Advances in experimental medicine and biology. 2011;715:105–123. doi: 10.1007/978-94-007-0940-9_7. [DOI] [PubMed] [Google Scholar]
- Heilmann C, Gerke C, Perdreau-Remington F, Gotz F. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infection and immunity. 1996a;64:277–282. doi: 10.1128/iai.64.1.277-282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Gotz F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Molecular microbiology. 1996b;20:1083–1091. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- Herant M, Heinrich V, Dembo M. Mechanics of neutrophil phagocytosis: behavior of the cortical tension. Journal of cell science. 2005;118:1789–1797. doi: 10.1242/jcs.02275. [DOI] [PubMed] [Google Scholar]
- Higgins J, Loughman A, van Kessel KP, van Strijp JA, Foster TJ. Clumping factor A of Staphylococcus aureus inhibits phagocytosis by human polymorphonuclear leucocytes. FEMS microbiology letters. 2006;258:290–296. doi: 10.1111/j.1574-6968.2006.00229.x. [DOI] [PubMed] [Google Scholar]
- Hilden P, Savolainen K, Tyynela J, Vuento M, Kuusela P. Purification and characterisation of a plasmin-sensitive surface protein of Staphylococcus aureus. European journal of biochemistry/FEBS. 1996;236:904–910. doi: 10.1111/j.1432-1033.1996.00904.x. [DOI] [PubMed] [Google Scholar]
- Horst SA, Hoerr V, Beineke A, Kreis C, Tuchscherr L, Kalinka J, Lehne S, Schleicher I, Kohler G, Fuchs T, Raschke MJ, Rohde M, Peters G, Faber C, Loffler B, Medina E. A novel mouse model of Staphylococcus aureus chronic osteomyelitis that closely mimics the human infection: an integrated view of disease pathogenesis. The American journal of pathology. 2012;181:1206–1214. doi: 10.1016/j.ajpath.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Horstmann RD, Sievertsen HJ, Leippe M, Fischetti VA. Role of fibrinogen in complement inhibition by streptococcal M protein. Infection and immunity. 1992;60:5036–5041. doi: 10.1128/iai.60.12.5036-5041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath DJ, Jr, Li B, Casper T, Partida-Sanchez S, Hunstad DA, Hultgren SJ, Justice SS. Morphological plasticity promotes resistance to phagocyte killing of uropathogenic Escherichia coli. Microbes and infection/Institut Pasteur. 2011;13:426–437. doi: 10.1016/j.micinf.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CY, Xue J, Cole GT. Virulence mechanisms of coccidioides. Annals of the New York Academy of Sciences. 2007;1111:225–235. doi: 10.1196/annals.1406.020. [DOI] [PubMed] [Google Scholar]
- Hussain M, Becker K, von Eiff C, Schrenzel J, Peters G, Herrmann M. Identification and characterization of a novel 38.5-kilodalton cell surface protein of Staphylococcus aureus with extended-spectrum binding activity for extracellular matrix and plasma proteins. Journal of bacteriology. 2001;183:6778–6786. doi: 10.1128/JB.183.23.6778-6786.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M, Schafer D, Juuti KM, Peters G, Haslinger-Loffler B, Kuusela PI, Sinha B. Expression of Pls (plasmin sensitive) in Staphylococcus aureus negative for pls reduces adherence and cellular invasion and acts by steric hindrance. The Journal of infectious diseases. 2009;200:107–117. doi: 10.1086/599359. [DOI] [PubMed] [Google Scholar]
- Ibberson CB, Parlet CP, Kwiecinski J, Crosby HA, Meyerholz DK, Horswill AR. Hyaluronan Modulation Impacts Staphylococcus aureus Biofilm Infection. Infection and immunity. 2016;84:1917–1929. doi: 10.1128/IAI.01418-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Katayama Y, Asada K, Mori N, Tsutsumimoto K, Tiensasitorn C, Hiramatsu K. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrobial agents and chemotherapy. 2001;45:1323–1336. doi: 10.1128/AAC.45.5.1323-1336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson K, McDevitt D, McGavin MH, Patti JM, Hook M. Staphylococcus aureus expresses a major histocompatibility complex class II analog. The Journal of biological chemistry. 1995;270:21457–21460. doi: 10.1074/jbc.270.37.21457. [DOI] [PubMed] [Google Scholar]
- Jonsson K, Signas C, Muller HP, Lindberg M. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. European journal of biochemistry/FEBS. 1991;202:1041–1048. doi: 10.1111/j.1432-1033.1991.tb16468.x. [DOI] [PubMed] [Google Scholar]
- Josefsson E, Hartford O, O’Brien L, Patti JM, Foster T. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. The Journal of infectious diseases. 2001;184:1572–1580. doi: 10.1086/324430. [DOI] [PubMed] [Google Scholar]
- Josefsson E, Higgins J, Foster TJ, Tarkowski A. Fibrinogen binding sites P336 and Y338 of clumping factor A are crucial for Staphylococcus aureus virulence. PloS one. 2008;3:e2206. doi: 10.1371/journal.pone.0002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson E, McCrea KW, Ni Eidhin D, O’Connell D, Cox J, Hook M, Foster TJ. Three new members of the serine-aspartate repeat protein multigene family of Staphylococcus aureus. Microbiology. 1998a;144(Pt 12):3387–3395. doi: 10.1099/00221287-144-12-3387. [DOI] [PubMed] [Google Scholar]
- Josefsson E, O’Connell D, Foster TJ, Durussel I, Cox JA. The binding of calcium to the B-repeat segment of SdrD, a cell surface protein of Staphylococcus aureus. The Journal of biological chemistry. 1998b;273:31145–31152. doi: 10.1074/jbc.273.47.31145. [DOI] [PubMed] [Google Scholar]
- Kapral FA. Clumping of Staphylococcus aureus in the peritoneal cavity of mice. Journal of bacteriology. 1966;92:1188–1195. doi: 10.1128/jb.92.4.1188-1195.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata S, Morita T, Iwanaga S, Igarashi H. Enzymatic properties of staphylothrombin, an active molecular complex formed between staphylocoagulase and human prothrombin. Journal of biochemistry. 1985;98:1603–1614. doi: 10.1093/oxfordjournals.jbchem.a135430. [DOI] [PubMed] [Google Scholar]
- Keane FM, Loughman A, Valtulina V, Brennan M, Speziale P, Foster TJ. Fibrinogen and elastin bind to the same region within the A domain of fibronectin binding protein A, an MSCRAMM of Staphylococcus aureus. Molecular microbiology. 2007;63:711–723. doi: 10.1111/j.1365-2958.2006.05552.x. [DOI] [PubMed] [Google Scholar]
- Knobloch JK, Bartscht K, Sabottke A, Rohde H, Feucht HH, Mack D. Biofilm formation by Staphylococcus epidermidis depends on functional RsbU, an activator of the sigB operon: differential activation mechanisms due to ethanol and salt stress. Journal of bacteriology. 2001;183:2624–2633. doi: 10.1128/JB.183.8.2624-2633.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko YP, Flick MJ. Fibrinogen Is at the Interface of Host Defense and Pathogen Virulence in Staphylococcus aureus Infection. Seminars in thrombosis and hemostasis. 2016;42:408–421. doi: 10.1055/s-0036-1579635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko YP, Kang M, Ganesh VK, Ravirajan D, Li B, Hook M. Coagulase and Efb of Staphylococcus aureus Have a Common Fibrinogen Binding Motif. mBio. 2016;7:e01885–01815. doi: 10.1128/mBio.01885-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko YP, Kuipers A, Freitag CM, Jongerius I, Medina E, van Rooijen WJ, Spaan AN, van Kessel KP, Hook M, Rooijakkers SH. Phagocytosis escape by a Staphylococcus aureus protein that connects complement and coagulation proteins at the bacterial surface. PLoS pathogens. 2013;9:e1003816. doi: 10.1371/journal.ppat.1003816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragh KN, Hutchison JB, Melaugh G, Rodesney C, Roberts AE, Irie Y, Jensen PO, Diggle SP, Allen RJ, Gordon V, Bjarnsholt T. Role of Multicellular Aggregates in Biofilm Formation. mBio. 2016:7. doi: 10.1128/mBio.00237-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreikemeyer B, McDevitt D, Podbielski A. The role of the map protein in Staphylococcus aureus matrix protein and eukaryotic cell adherence. International journal of medical microbiology: IJMM. 2002;292:283–295. doi: 10.1078/1438-4221-00212. [DOI] [PubMed] [Google Scholar]
- Kroh HK, Panizzi P, Bock PE. Von Willebrand factor-binding protein is a hysteretic conformational activator of prothrombin. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7786–7791. doi: 10.1073/pnas.0811750106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzmann MH, Bach NC, Bauer B, Sieber SA. [small alpha]-Methylene-[gamma]-butyrolactones attenuate Staphylococcus aureus virulence by inhibition of transcriptional regulation. Chemical Science. 2014;5:1158–1167. [Google Scholar]
- Kuroda M, Ito R, Tanaka Y, Yao M, Matoba K, Saito S, Tanaka I, Ohta T. Staphylococcus aureus surface protein SasG contributes to intercellular autoaggregation of Staphylococcus aureus. Biochemical and biophysical research communications. 2008;377:1102–1106. doi: 10.1016/j.bbrc.2008.10.134. [DOI] [PubMed] [Google Scholar]
- Kuusela P, Hilden P, Savolainen K, Vuento M, Lyytikainen O, Vuopio-Varkila J. Rapid detection of methicillin-resistant Staphylococcus aureus strains not identified by slide agglutination tests. Journal of clinical microbiology. 1994;32:143–147. doi: 10.1128/jcm.32.1.143-147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiecinski J, Jin T, Josefsson E. Surface proteins of Staphylococcus aureus play an important role in experimental skin infection. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 2014;122:1240–1250. doi: 10.1111/apm.12295. [DOI] [PubMed] [Google Scholar]
- Kwiecinski J, Peetermans M, Liesenborghs L, Na M, Bjornsdottir H, Zhu X, Jacobsson G, Johansson BR, Geoghegan JA, Foster TJ, Josefsson E, Bylund J, Verhamme P, Jin T. Staphylokinase Control of Staphylococcus aureus Biofilm Formation and Detachment Through Host Plasminogen Activation. The Journal of infectious diseases. 2016;213:139–148. doi: 10.1093/infdis/jiv360. [DOI] [PubMed] [Google Scholar]
- Lebeaux D, Ghigo JM, Beloin C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiology and molecular biology reviews: MMBR. 2014;78:510–543. doi: 10.1128/MMBR.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LY, Hook M, Haviland D, Wetsel RA, Yonter EO, Syribeys P, Vernachio J, Brown EL. Inhibition of complement activation by a secreted Staphylococcus aureus protein. The Journal of infectious diseases. 2004a;190:571–579. doi: 10.1086/422259. [DOI] [PubMed] [Google Scholar]
- Lee LY, Liang X, Hook M, Brown EL. Identification and characterization of the C3 binding domain of the Staphylococcus aureus extracellular fibrinogen-binding protein (Efb) The Journal of biological chemistry. 2004b;279:50710–50716. doi: 10.1074/jbc.M408570200. [DOI] [PubMed] [Google Scholar]
- Li T, Yu X, Xie J, Xu Y, Shang Y, Liu Y, Huang X, Qin Z, Parsons C, Hu L, Salgado C, Wang L, Yu F. Carriage of virulence factors and molecular characteristics of Staphylococcus aureus isolates associated with bloodstream, and skin and soft tissue infections in children. Epidemiology and infection. 2013;141:2158–2162. doi: 10.1017/S0950268812002919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang X, Thompson CD, Park S, Park WB, Lee JC. Preclinical Efficacy of Clumping Factor A in Prevention of Staphylococcus aureus Infection. mBio. 2016;7:e02232–02215. doi: 10.1128/mBio.02232-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zheng L, Landwehr C, Lunsford D, Holmes D, Ji Y. Global regulation of gene expression by ArlRS, a two-component signal transduction regulatory system of Staphylococcus aureus. Journal of bacteriology. 2005;187:5486–5492. doi: 10.1128/JB.187.15.5486-5492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limoli DH, Jones CJ, Wozniak DJ. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiology spectrum. 2015:3. doi: 10.1128/microbiolspec.MB-0011-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MH, Shu JC, Lin LP, Chong KY, Cheng YW, Du JF, Liu ST. Elucidating the crucial role of poly N-acetylglucosamine from Staphylococcus aureus in cellular adhesion and pathogenesis. PloS one. 2015;10:e0124216. doi: 10.1371/journal.pone.0124216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski B, Hawiger J, Jeljaszewicz J. Staphylococcal clumping with soluble fibrin mmonomer complexes. The Journal of experimental medicine. 1967;126:979–988. doi: 10.1084/jem.126.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CL, Chen ZJ, Wang F, Hou HY, Wang Y, Zhu XH, Jian C, Tian L, Yan SZ, Xu LQ, Sun ZY. The impact of mgrA on progression of Staphylococcus aureus sepsis. Microbial pathogenesis. 2014;71–72:56–61. doi: 10.1016/j.micpath.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Loeb L. The Influence of certain Bacteria on the Coagulation of the Blood. The Journal of medical research. 1903;10:407–419. [PMC free article] [PubMed] [Google Scholar]
- Loof TG, Goldmann O, Naudin C, Morgelin M, Neumann Y, Pils MC, Foster SJ, Medina E, Herwald H. Staphylococcus aureus-induced clotting of plasma is an immune evasion mechanism for persistence within the fibrin network. Microbiology. 2015;161:621–627. doi: 10.1099/mic.0.000019. [DOI] [PubMed] [Google Scholar]
- Lowy FD. Staphylococcus aureus infections. The New England journal of medicine. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- Luong TT, Dunman PM, Murphy E, Projan SJ, Lee CY. Transcription Profiling of the mgrA Regulon in Staphylococcus aureus. Journal of bacteriology. 2006;188:1899–1910. doi: 10.1128/JB.188.5.1899-1910.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luong TT, Lee CY. The arl locus positively regulates Staphylococcus aureus type 5 capsule via an mgrA-dependent pathway. Microbiology. 2006;152:3123–3131. doi: 10.1099/mic.0.29177-0. [DOI] [PubMed] [Google Scholar]
- Mack D, Nedelmann M, Krokotsch A, Schwarzkopf A, Heesemann J, Laufs R. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infection and immunity. 1994;62:3244–3253. doi: 10.1128/iai.62.8.3244-3253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malachowa N, Kobayashi SD, Porter AR, Braughton KR, Scott DP, Gardner DJ, Missiakas DM, Schneewind O, DeLeo FR. Contribution of Staphylococcus aureus Coagulases and Clumping Factor A to Abscess Formation in a Rabbit Model of Skin and Soft Tissue Infection. PloS one. 2016;11:e0158293. doi: 10.1371/journal.pone.0158293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdow M, DeDent AC, Emolo C, Cheng AG, Kreiswirth BN, Missiakas DM, Schneewind O. Coagulases as determinants of protective immune responses against Staphylococcus aureus. Infection and immunity. 2012a;80:3389–3398. doi: 10.1128/IAI.00562-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdow M, Kim HK, Dedent AC, Hendrickx AP, Schneewind O, Missiakas DM. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS pathogens. 2011;7:e1002307. doi: 10.1371/journal.ppat.1002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdow M, Missiakas DM, Schneewind O. Staphylococcus aureus secretes coagulase and von Willebrand factor binding protein to modify the coagulation cascade and establish host infections. Journal of innate immunity. 2012b;4:141–148. doi: 10.1159/000333447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt D, Francois P, Vaudaux P, Foster TJ. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Molecular microbiology. 1994;11:237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- McDevitt D, Nanavaty T, House-Pompeo K, Bell E, Turner N, McIntire L, Foster T, Hook M. Characterization of the interaction between the Staphylococcus aureus clumping factor (ClfA) and fibrinogen. European journal of biochemistry/FEBS. 1997;247:416–424. doi: 10.1111/j.1432-1033.1997.00416.x. [DOI] [PubMed] [Google Scholar]
- McDevitt D, Vaudaux P, Foster TJ. Genetic evidence that bound coagulase of Staphylococcus aureus is not clumping factor. Infection and immunity. 1992;60:1514–1523. doi: 10.1128/iai.60.4.1514-1523.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavin MH, Krajewska-Pietrasik D, Ryden C, Hook M. Identification of a Staphylococcus aureus extracellular matrix-binding protein with broad specificity. Infection and immunity. 1993;61:2479–2485. doi: 10.1128/iai.61.6.2479-2485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney D, Pouliot KL, Wang Y, Murthy V, Ulrich M, Doring G, Lee JC, Goldmann DA, Pier GB. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science. 1999;284:1523–1527. doi: 10.1126/science.284.5419.1523. [DOI] [PubMed] [Google Scholar]
- Meenan NA, Visai L, Valtulina V, Schwarz-Linek U, Norris NC, Gurusiddappa S, Hook M, Speziale P, Potts JR. The tandem beta-zipper model defines high affinity fibronectin-binding repeats within Staphylococcus aureus FnBPA. The Journal of biological chemistry. 2007;282:25893–25902. doi: 10.1074/jbc.M703063200. [DOI] [PubMed] [Google Scholar]
- Melaugh G, Hutchison J, Kragh KN, Irie Y, Roberts A, Bjarnsholt T, Diggle SP, Gordon VD, Allen RJ. Shaping the Growth Behaviour of Biofilms Initiated from Bacterial Aggregates. PloS one. 2016;11:e0149683. doi: 10.1371/journal.pone.0149683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino N, Toledo-Arana A, Vergara-Irigaray M, Valle J, Solano C, Calvo E, Lopez JA, Foster TJ, Penades JR, Lasa I. Protein A-mediated multicellular behavior in Staphylococcus aureus. Journal of bacteriology. 2009;191:832–843. doi: 10.1128/JB.01222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LG, Diep BA. Clinical practice: colonization, fomites, and virulence: rethinking the pathogenesis of community-associated methicillin-resistant Staphylococcus aureus infection. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2008;46:752–760. doi: 10.1086/526773. [DOI] [PubMed] [Google Scholar]
- Mitchell J, Tristan A, Foster TJ. Characterization of the fibrinogen-binding surface protein Fbl of Staphylococcus lugdunensis. Microbiology. 2004;150:3831–3841. doi: 10.1099/mic.0.27337-0. [DOI] [PubMed] [Google Scholar]
- Molkanen T, Tyynela J, Helin J, Kalkkinen N, Kuusela P. Enhanced activation of bound plasminogen on Staphylococcus aureus by staphylokinase. FEBS letters. 2002;517:72–78. doi: 10.1016/s0014-5793(02)02580-2. [DOI] [PubMed] [Google Scholar]
- Moller J, Luehmann T, Hall H, Vogel V. The race to the pole: how high-aspect ratio shape and heterogeneous environments limit phagocytosis of filamentous Escherichia coli bacteria by macrophages. Nano letters. 2012;12:2901–2905. doi: 10.1021/nl3004896. [DOI] [PubMed] [Google Scholar]
- Molne L, Verdrengh M, Tarkowski A. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infection and immunity. 2000;68:6162–6167. doi: 10.1128/iai.68.11.6162-6167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreillon P, Entenza JM, Francioli P, McDevitt D, Foster TJ, Francois P, Vaudaux P. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infection and immunity. 1995;63:4738–4743. doi: 10.1128/iai.63.12.4738-4743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher DF. Plasma fibronectin concentration: a risk factor for arterial thrombosis? Arteriosclerosis, thrombosis, and vascular biology. 2006;26:1193–1195. doi: 10.1161/01.ATV.0000223342.15969.7a. [DOI] [PubMed] [Google Scholar]
- Much H. A preliminary stage of the fibrin enzymes in cultures of staphyloccus aureus. Biochem Z. 1908;14:143–155. [Google Scholar]
- Mulcahy ME, Geoghegan JA, Monk IR, O’Keeffe KM, Walsh EJ, Foster TJ, McLoughlin RM. Nasal colonisation by Staphylococcus aureus depends upon clumping factor B binding to the squamous epithelial cell envelope protein loricrin. PLoS pathogens. 2012;8:e1003092. doi: 10.1371/journal.ppat.1003092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Eidhin D, Perkins S, Francois P, Vaudaux P, Hook M, Foster TJ. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Molecular microbiology. 1998;30:245–257. doi: 10.1046/j.1365-2958.1998.01050.x. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Bjerketorp J, Guss B, Frykberg L. A fibrinogen-binding protein of Staphylococcus lugdunensis. FEMS microbiology letters. 2004;241:87–93. doi: 10.1016/j.femsle.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Frykberg L, Flock JI, Pei L, Lindberg M, Guss B. A fibrinogen-binding protein of Staphylococcus epidermidis. Infection and immunity. 1998;66:2666–2673. doi: 10.1128/iai.66.6.2666-2673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien L, Kerrigan SW, Kaw G, Hogan M, Penades J, Litt D, Fitzgerald DJ, Foster TJ, Cox D. Multiple mechanisms for the activation of human platelet aggregation by Staphylococcus aureus: roles for the clumping factors ClfA and ClfB, the serine-aspartate repeat protein SdrE and protein A. Molecular microbiology. 2002a;44:1033–1044. doi: 10.1046/j.1365-2958.2002.02935.x. [DOI] [PubMed] [Google Scholar]
- O’Brien LM, Walsh EJ, Massey RC, Peacock SJ, Foster TJ. Staphylococcus aureus clumping factor B (ClfB) promotes adherence to human type I cytokeratin 10: implications for nasal colonization. Cellular microbiology. 2002b;4:759–770. doi: 10.1046/j.1462-5822.2002.00231.x. [DOI] [PubMed] [Google Scholar]
- Okada K, Ueshima S, Tanaka M, Fukao H, Matsuo O. Analysis of plasminogen activation by the plasmin-staphylokinase complex in plasma of alpha2-antiplasmin-deficient mice. Blood coagulation & fibrinolysis: an international journal in haemostasis and thrombosis. 2000;11:645–655. doi: 10.1097/00001721-200010000-00009. [DOI] [PubMed] [Google Scholar]
- Okagaki LH, Nielsen K. Titan cells confer protection from phagocytosis in Cryptococcus neoformans infections. Eukaryotic cell. 2012;11:820–826. doi: 10.1128/EC.00121-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okagaki LH, Strain AK, Nielsen JN, Charlier C, Baltes NJ, Chretien F, Heitman J, Dromer F, Nielsen K. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS pathogens. 2010;6:e1000953. doi: 10.1371/journal.ppat.1000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paharik AE, Horswill AR. The Staphylococcal Biofilm: Adhesins, Regulation, and Host Response. Microbiology spectrum. 2016:4. doi: 10.1128/microbiolspec.VMBF-0022-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma M, Nozohoor S, Schennings T, Heimdahl A, Flock JI. Lack of the extracellular 19-kilodalton fibrinogen-binding protein from Staphylococcus aureus decreases virulence in experimental wound infection. Infection and immunity. 1996;64:5284–5289. doi: 10.1128/iai.64.12.5284-5289.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma M, Wade D, Flock M, Flock JI. Multiple binding sites in the interaction between an extracellular fibrinogen-binding protein from Staphylococcus aureus and fibrinogen. The Journal of biological chemistry. 1998;273:13177–13181. doi: 10.1074/jbc.273.21.13177. [DOI] [PubMed] [Google Scholar]
- Palmqvist N, Josefsson E, Tarkowski A. Clumping factor A-mediated virulence during Staphylococcus aureus infection is retained despite fibrinogen depletion. Microbes and infection/Institut Pasteur. 2004a;6:196–201. doi: 10.1016/j.micinf.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Palmqvist N, Patti JM, Tarkowski A, Josefsson E. Expression of staphylococcal clumping factor A impedes macrophage phagocytosis. Microbes and infection/Institut Pasteur. 2004b;6:188–195. doi: 10.1016/j.micinf.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Peacock SJ, Moore CE, Justice A, Kantzanou M, Story L, Mackie K, O’Neill G, Day NP. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infection and immunity. 2002;70:4987–4996. doi: 10.1128/IAI.70.9.4987-4996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peetermans M, Vanassche T, Liesenborghs L, Claes J, Vande Velde G, Kwiecinksi J, Jin T, De Geest B, Hoylaerts MF, Lijnen RH, Verhamme P. Plasminogen activation by staphylokinase enhances local spreading of S. aureus in skin infections. BMC microbiology. 2014;14:310. doi: 10.1186/s12866-014-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peetermans M, Verhamme P, Vanassche T. Coagulase Activity by Staphylococcus aureus: A Potential Target for Therapy? Seminars in thrombosis and hemostasis. 2015;41:433–444. doi: 10.1055/s-0035-1549849. [DOI] [PubMed] [Google Scholar]
- Pei L, Palma M, Nilsson M, Guss B, Flock JI. Functional studies of a fibrinogen binding protein from Staphylococcus epidermidis. Infection and immunity. 1999;67:4525–4530. doi: 10.1128/iai.67.9.4525-4530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad JM, Gorkun OV, Raghu H, Thornton S, Mullins ES, Palumbo JS, Ko YP, Hook M, David T, Coughlin SR, Degen JL, Flick MJ. Mice expressing a mutant form of fibrinogen that cannot support fibrin formation exhibit compromised antimicrobial host defense. Blood. 2015;126:2047–2058. doi: 10.1182/blood-2015-04-639849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prashar A, Bhatia S, Gigliozzi D, Martin T, Duncan C, Guyard C, Terebiznik MR. Filamentous morphology of bacteria delays the timing of phagosome morphogenesis in macrophages. The Journal of cell biology. 2013;203:1081–1097. doi: 10.1083/jcb.201304095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que YA, Francois P, Haefliger JA, Entenza JM, Vaudaux P, Moreillon P. Reassessing the role of Staphylococcus aureus clumping factor and fibronectin-binding protein by expression in Lactococcus lactis. Infection and immunity. 2001;69:6296–6302. doi: 10.1128/IAI.69.10.6296-6302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachid S, Ohlsen K, Witte W, Hacker J, Ziebuhr W. Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilm-forming Staphylococcus epidermidis. Antimicrobial agents and chemotherapy. 2000;44:3357–3363. doi: 10.1128/aac.44.12.3357-3363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch S, Gough P, Kim HK, Schneewind O, Missiakas D. Vaccine protection of leukopenic mice against Staphylococcus aureus bloodstream infection. Infection and immunity. 2014;82:4889–4898. doi: 10.1128/IAI.02328-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risley AL, Loughman A, Cywes-Bentley C, Foster TJ, Lee JC. Capsular polysaccharide masks clumping factor A-mediated adherence of Staphylococcus aureus to fibrinogen and platelets. The Journal of infectious diseases. 2007;196:919–927. doi: 10.1086/520932. [DOI] [PubMed] [Google Scholar]
- Roche FM, Downer R, Keane F, Speziale P, Park PW, Foster TJ. The N-terminal A domain of fibronectin-binding proteins A and B promotes adhesion of Staphylococcus aureus to elastin. The Journal of biological chemistry. 2004;279:38433–38440. doi: 10.1074/jbc.M402122200. [DOI] [PubMed] [Google Scholar]
- Rogasch K, Ruhmling V, Pane-Farre J, Hoper D, Weinberg C, Fuchs S, Schmudde M, Broker BM, Wolz C, Hecker M, Engelmann S. Influence of the two-component system SaeRS on global gene expression in two different Staphylococcus aureus strains. Journal of bacteriology. 2006;188:7742–7758. doi: 10.1128/JB.00555-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfork JM, Dessus-Babus S, Van Wamel WJ, Cheung AL, Gresham HD. Fibrinogen depletion attenuates Staphyloccocus aureus infection by preventing density-dependent virulence gene up-regulation. Journal of immunology. 2003;171:5389–5395. doi: 10.4049/jimmunol.171.10.5389. [DOI] [PubMed] [Google Scholar]
- Salgado-Pabon W, Breshears L, Spaulding AR, Merriman JA, Stach CS, Horswill AR, Peterson ML, Schlievert PM. Superantigens are critical for Staphylococcus aureus Infective endocarditis, sepsis, and acute kidney injury. mBio. 2013:4. doi: 10.1128/mBio.00494-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen K, Paulin L, Westerlund-Wikstrom B, Foster TJ, Korhonen TK, Kuusela P. Expression of pls, a gene closely associated with the mecA gene of methicillin-resistant Staphylococcus aureus, prevents bacterial adhesion in vitro. Infection and immunity. 2001;69:3013–3020. doi: 10.1128/IAI.69.5.3013-3020.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T, Tomono K, Yanagihara K, Yamamoto Y, Kaku M, Hirakata Y, Koga H, Tashiro T, Kohno S. Role of coagulase in a murine model of hematogenous pulmonary infection induced by intravenous injection of Staphylococcus aureus enmeshed in agar beads. Infection and immunity. 1997;65:466–471. doi: 10.1128/iai.65.2.466-471.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer CR, Woods KM, Longo GM, Kiedrowski MR, Paharik AE, Buttner H, Christner M, Boissy RJ, Horswill AR, Rohde H, Fey PD. Accumulation-associated protein enhances Staphylococcus epidermidis biofilm formation under dynamic conditions and is required for infection in a rat catheter model. Infection and immunity. 2015;83:214–226. doi: 10.1128/IAI.02177-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer AC, Solinga RM, Cocchiaro J, Portoles M, Kiser KB, Risley A, Randall SM, Valtulina V, Speziale P, Walsh E, Foster T, Lee JC. Immunization with Staphylococcus aureus clumping factor B, a major determinant in nasal carriage, reduces nasal colonization in a murine model. Infection and immunity. 2006;74:2145–2153. doi: 10.1128/IAI.74.4.2145-2153.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schennings T, Farnebo F, Szekely L, Flock JI. Protective immunization against Staphylococcus aureus infection in a novel experimental wound model in mice. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 2012;120:786–793. doi: 10.1111/j.1600-0463.2012.02907.x. [DOI] [PubMed] [Google Scholar]
- Schroeder K, Jularic M, Horsburgh SM, Hirschhausen N, Neumann C, Bertling A, Schulte A, Foster S, Kehrel BE, Peters G, Heilmann C. Molecular characterization of a novel Staphylococcus aureus surface protein (SasC) involved in cell aggregation and biofilm accumulation. PloS one. 2009;4:e7567. doi: 10.1371/journal.pone.0007567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully IL, Timofeyeva Y, Keeney D, Matsuka YV, Severina E, McNeil LK, Nanra J, Hu G, Liberator PA, Jansen KU, Anderson AS. Demonstration of the preclinical correlate of protection for Staphylococcus aureus clumping factor A in a murine model of infection. Vaccine. 2015;33:5452–5457. doi: 10.1016/j.vaccine.2015.08.029. [DOI] [PubMed] [Google Scholar]
- Sharp JA, Echague CG, Hair PS, Ward MD, Nyalwidhe JO, Geoghegan JA, Foster TJ, Cunnion KM. Staphylococcus aureus surface protein SdrE binds complement regulator factor H as an immune evasion tactic. PloS one. 2012;7:e38407. doi: 10.1371/journal.pone.0038407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signas C, Raucci G, Jonsson K, Lindgren PE, Anantharamaiah GM, Hook M, Lindberg M. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:699–703. doi: 10.1073/pnas.86.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SI, Schmid-Schonbein GW. Biophysical aspects of microsphere engulfment by human neutrophils. Biophysical journal. 1988;53:163–173. doi: 10.1016/S0006-3495(88)83078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan AN, Surewaard BG, Nijland R, van Strijp JA. Neutrophils versus Staphylococcus aureus: a biological tug of war. Annual review of microbiology. 2013;67:629–650. doi: 10.1146/annurev-micro-092412-155746. [DOI] [PubMed] [Google Scholar]
- Stapels DA, Ramyar KX, Bischoff M, von Kockritz-Blickwede M, Milder FJ, Ruyken M, Eisenbeis J, McWhorter WJ, Herrmann M, van Kessel KP, Geisbrecht BV, Rooijakkers SH. Staphylococcus aureus secretes a unique class of neutrophil serine protease inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:13187–13192. doi: 10.1073/pnas.1407616111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemberk V, Jones RP, Moroz O, Atkin KE, Edwards AM, Turkenburg JP, Leech AP, Massey RC, Potts JR. Evidence for steric regulation of fibrinogen binding to Staphylococcus aureus fibronectin-binding protein A (FnBPA) The Journal of biological chemistry. 2014;289:12842–12851. doi: 10.1074/jbc.M113.543546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann Meier P, Entenza JM, Vaudaux P, Francioli P, Glauser MP, Moreillon P. Study of Staphylococcus aureus pathogenic genes by transfer and expression in the less virulent organism Streptococcus gordonii. Infection and immunity. 2001;69:657–664. doi: 10.1128/IAI.69.2.657-664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Zhou L, Zhao BC, Deng X, Cho H, Yi C, Jian X, Song CX, Luan CH, Bae T, Li Z, He C. Targeting MgrA-mediated virulence regulation in Staphylococcus aureus. Chemistry & biology. 2011;18:1032–1041. doi: 10.1016/j.chembiol.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thammavongsa V, Kim HK, Missiakas D, Schneewind O. Staphylococcal manipulation of host immune responses. Nature reviews Microbiology. 2015;13:529–543. doi: 10.1038/nrmicro3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomer L, Becker S, Emolo C, Quach A, Kim HK, Rauch S, Anderson M, Leblanc JF, Schneewind O, Faull KF, Missiakas D. N-acetylglucosaminylation of serine-aspartate repeat proteins promotes Staphylococcus aureus bloodstream infection. The Journal of biological chemistry. 2014;289:3478–3486. doi: 10.1074/jbc.M113.532655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomer L, Emolo C, Thammavongsa V, Kim HK, McAdow ME, Yu W, Kieffer M, Schneewind O, Missiakas D. Antibodies against a secreted product of Staphylococcus aureus trigger phagocytic killing. The Journal of experimental medicine. 2016;213:293–301. doi: 10.1084/jem.20150074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomer L, Schneewind O, Missiakas D. Multiple ligands of von Willebrand factor-binding protein (vWbp) promote Staphylococcus aureus clot formation in human plasma. The Journal of biological chemistry. 2013;288:28283–28292. doi: 10.1074/jbc.M113.493122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Arana A, Merino N, Vergara-Irigaray M, Debarbouille M, Penades JR, Lasa I. Staphylococcus aureus develops an alternative, ica-independent biofilm in the absence of the arlRS two-component system. Journal of bacteriology. 2005;187:5318–5329. doi: 10.1128/JB.187.15.5318-5329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG., Jr Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clinical microbiology reviews. 2015;28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristan A, Ying L, Bes M, Etienne J, Vandenesch F, Lina G. Use of multiplex PCR to identify Staphylococcus aureus adhesins involved in human hematogenous infections. Journal of clinical microbiology. 2003;41:4465–4467. doi: 10.1128/JCM.41.9.4465-4467.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotonda MP, Tamber S, Memmi G, Cheung AL. MgrA represses biofilm formation in Staphylococcus aureus. Infection and immunity. 2008;76:5645–5654. doi: 10.1128/IAI.00735-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchscherr LP, Buzzola FR, Alvarez LP, Lee JC, Sordelli DO. Antibodies to capsular polysaccharide and clumping factor A prevent mastitis and the emergence of unencapsulated and small-colony variants of Staphylococcus aureus in mice. Infection and immunity. 2008;76:5738–5744. doi: 10.1128/IAI.00874-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung H, Guss B, Hellman U, Persson L, Rubin K, Ryden C. A bone sialoprotein-binding protein from Staphylococcus aureus: a member of the staphylococcal Sdr family. The Biochemical journal. 2000;345(Pt 3):611–619. [PMC free article] [PubMed] [Google Scholar]
- Vanassche T, Peetermans M, Van Aelst LN, Peetermans WE, Verhaegen J, Missiakas DM, Schneewind O, Hoylaerts MF, Verhamme P. The role of staphylothrombin-mediated fibrin deposition in catheter-related Staphylococcus aureus infections. The Journal of infectious diseases. 2013;208:92–100. doi: 10.1093/infdis/jit130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanassche T, Verhaegen J, Peetermans WE, Hoylaerts MF, Verhamme P. Dabigatran inhibits Staphylococcus aureus coagulase activity. Journal of clinical microbiology. 2010;48:4248–4250. doi: 10.1128/JCM.00896-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanassche T, Verhaegen J, Peetermans WE, JVANR, Cheng A, Schneewind O, Hoylaerts MF, Verhamme P. Inhibition of staphylothrombin by dabigatran reduces Staphylococcus aureus virulence. Journal of thrombosis and haemostasis: JTH. 2011;9:2436–2446. doi: 10.1111/j.1538-7836.2011.04529.x. [DOI] [PubMed] [Google Scholar]
- Varrone JJ, de Mesy Bentley KL, Bello-Irizarry SN, Nishitani K, Mack S, Hunter JG, Kates SL, Daiss JL, Schwarz EM. Passive immunization with anti-glucosaminidase monoclonal antibodies protects mice from implant-associated osteomyelitis by mediating opsonophagocytosis of Staphylococcus aureus megaclusters. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2014;32:1389–1396. doi: 10.1002/jor.22672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudaux P, Pittet D, Haeberli A, Lerch PG, Morgenthaler JJ, Proctor RA, Waldvogel FA, Lew DP. Fibronectin is more active than fibrin or fibrinogen in promoting Staphylococcus aureus adherence to inserted intravascular catheters. The Journal of infectious diseases. 1993;167:633–641. doi: 10.1093/infdis/167.3.633. [DOI] [PubMed] [Google Scholar]
- Vaudaux PE, Monzillo V, Francois P, Lew DP, Foster TJ, Berger-Bachi B. Introduction of the mec element (methicillin resistance) into Staphylococcus aureus alters in vitro functional activities of fibrinogen and fibronectin adhesins. Antimicrobial agents and chemotherapy. 1998;42:564–570. doi: 10.1128/aac.42.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez V, Liang X, Horndahl JK, Ganesh VK, Smeds E, Foster TJ, Hook M. Fibrinogen is a ligand for the Staphylococcus aureus microbial surface components recognizing adhesive matrix molecules (MSCRAMM) bone sialoprotein-binding protein (Bbp) The Journal of biological chemistry. 2011;286:29797–29805. doi: 10.1074/jbc.M110.214981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdrengh M, Tarkowski A. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infection and immunity. 1997;65:2517–2521. doi: 10.1128/iai.65.7.2517-2521.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdrengh M, Tarkowski A. Role of macrophages in Staphylococcus aureus-induced arthritis and sepsis. Arthritis and rheumatism. 2000;43:2276–2282. doi: 10.1002/1529-0131(200010)43:10<2276::AID-ANR15>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Vergara-Irigaray M, Valle J, Merino N, Latasa C, Garcia B, Ruiz de Los Mozos I, Solano C, Toledo-Arana A, Penades JR, Lasa I. Relevant role of fibronectin-binding proteins in Staphylococcus aureus biofilm-associated foreign-body infections. Infection and immunity. 2009;77:3978–3991. doi: 10.1128/IAI.00616-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernachio J, Bayer AS, Le T, Chai YL, Prater B, Schneider A, Ames B, Syribeys P, Robbins J, Patti JM. Anti-clumping factor A immunoglobulin reduces the duration of methicillin-resistant Staphylococcus aureus bacteremia in an experimental model of infective endocarditis. Antimicrobial agents and chemotherapy. 2003;47:3400–3406. doi: 10.1128/AAC.47.11.3400-3406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong C, Kocianova S, Voyich JM, Yao Y, Fischer ER, DeLeo FR, Otto M. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. The Journal of biological chemistry. 2004a;279:54881–54886. doi: 10.1074/jbc.M411374200. [DOI] [PubMed] [Google Scholar]
- Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, Otto M. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cellular microbiology. 2004b;6:269–275. doi: 10.1046/j.1462-5822.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- Walker JN, Crosby HA, Spaulding AR, Salgado-Pabon W, Malone CL, Rosenthal CB, Schlievert PM, Boyd JM, Horswill AR. The Staphylococcus aureus ArlRS two-component system is a novel regulator of agglutination and pathogenesis. PLoS pathogens. 2013;9:e1003819. doi: 10.1371/journal.ppat.1003819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh EJ, Miajlovic H, Gorkun OV, Foster TJ. Identification of the Staphylococcus aureus MSCRAMM clumping factor B (ClfB) binding site in the alphaC-domain of human fibrinogen. Microbiology. 2008;154:550–558. doi: 10.1099/mic.0.2007/010868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh EJ, O’Brien LM, Liang X, Hook M, Foster TJ. Clumping factor B, a fibrinogen-binding MSCRAMM (microbial surface components recognizing adhesive matrix molecules) adhesin of Staphylococcus aureus, also binds to the tail region of type I cytokeratin 10. The Journal of biological chemistry. 2004;279:50691–50699. doi: 10.1074/jbc.M408713200. [DOI] [PubMed] [Google Scholar]
- Wang X, Ge J, Liu B, Hu Y, Yang M. Structures of SdrD from Staphylococcus aureus reveal the molecular mechanism of how the cell surface receptors recognize their ligands. Protein & cell. 2013;4:277–285. doi: 10.1007/s13238-013-3009-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang H, Zhang Q, Liang Y, Ma L, Tan H, Lao Y, Xu H, Li Z. Genetically encoded fluorescence screening probe for MgrA, a global regulator in Staphylococcus aureus. RSC Advances. 2015;5:87216–87220. [Google Scholar]
- Wann ER, Gurusiddappa S, Hook M. The fibronectin-binding MSCRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. The Journal of biological chemistry. 2000;275:13863–13871. doi: 10.1074/jbc.275.18.13863. [DOI] [PubMed] [Google Scholar]
- Weiser JN. The battle with the host over microbial size. Current opinion in microbiology. 2013;16:59–62. doi: 10.1016/j.mib.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheim HF, Walsh E, Choudhurry R, Melles DC, Boelens HA, Miajlovic H, Verbrugh HA, Foster T, van Belkum A. Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLoS medicine. 2008;5:e17. doi: 10.1371/journal.pmed.0050017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woehl JL, Stapels DA, Garcia BL, Ramyar KX, Keightley A, Ruyken M, Syriga M, Sfyroera G, Weber AB, Zolkiewski M, Ricklin D, Lambris JD, Rooijakkers SH, Geisbrecht BV. The extracellular adherence protein from Staphylococcus aureus inhibits the classical and lectin pathways of complement by blocking formation of the C3 proconvertase. Journal of immunology. 2014;193:6161–6171. doi: 10.4049/jimmunol.1401600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang H, Feng Y, Wang J, Liu B, Chen Y, Liu L, Deng X, Yang M. Crystal structures reveal the multi-ligand binding mechanism of Staphylococcus aureus ClfB. PLoS pathogens. 2012;8:e1002751. doi: 10.1371/journal.ppat.1002751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue T, You Y, Shang F, Sun B. Rot and Agr system modulate fibrinogen-binding ability mainly by regulating clfB expression in Staphylococcus aureus NCTC8325. Medical microbiology and immunology. 2012;201:81–92. doi: 10.1007/s00430-011-0208-z. [DOI] [PubMed] [Google Scholar]
- Yang DC, Blair KM, Salama NR. Staying in Shape: the Impact of Cell Shape on Bacterial Survival in Diverse Environments. Microbiology and molecular biology reviews: MMBR. 2016;80:187–203. doi: 10.1128/MMBR.00031-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Mochalkin I, Doolittle RF. A model of fibrin formation based on crystal structures of fibrinogen and fibrin fragments complexed with synthetic peptides. Proceedings of the National Academy of Sciences of the United States of America. 2000a;97:14156–14161. doi: 10.1073/pnas.97.26.14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Mochalkin I, Veerapandian L, Riley M, Doolittle RF. Crystal structure of native chicken fibrinogen at 5.5-A resolution. Proceedings of the National Academy of Sciences of the United States of America. 2000b;97:3907–3912. doi: 10.1073/pnas.080065697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Li T, Huang X, Xie J, Xu Y, Tu J, Qin Z, Parsons C, Wang J, Hu L, Wang L. Virulence gene profiling and molecular characterization of hospital-acquired Staphylococcus aureus isolates associated with bloodstream infection. Diagnostic microbiology and infectious disease. 2012;74:363–368. doi: 10.1016/j.diagmicrobio.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Zaragoza O, Garcia-Rodas R, Nosanchuk JD, Cuenca-Estrella M, Rodriguez-Tudela JL, Casadevall A. Fungal cell gigantism during mammalian infection. PLoS pathogens. 2010;6:e1000945. doi: 10.1371/journal.ppat.1000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wu M, Zhuo W, Gu J, Zhang S, Ge J, Yang M. Crystal structures of Bbp from Staphylococcus aureus reveal the ligand binding mechanism with Fibrinogen alpha. Protein & cell. 2015;6:757–766. doi: 10.1007/s13238-015-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel PF, Skerka C. Staphylococcus aureus: the multi headed hydra resists and controls human complement response in multiple ways. International journal of medical microbiology: IJMM. 2014;304:188–194. doi: 10.1016/j.ijmm.2013.11.004. [DOI] [PubMed] [Google Scholar]