Abstract
Studies of cognitive control in attention-deficit/hyperactivity disorder (ADHD) have emphasized the ability to suppress motor responses (i.e., behavioral inhibition) rather than the ability to actively suppress prepotent mental representations (i.e., cognitive inhibition). Further, working memory deficits are suspected in ADHD, yet their distinction from cognitive inhibition is unclear. Two hundred and eighty-eight adolescent and adult participants, 115 of whom met criteria for ADHD and 173 of whom were for non-ADHD comparison, completed a sentence processing task that required the suppression of an incorrect interpretation and a working memory task. The results failed to support cognitive inhibition problems in ADHD. Moreover, the ability to reanalyze sentences with a temporary misinterpretation was at least partially related to working memory performance. The results challenge a unitary inhibition problem in ADHD and suggest inhibition problems do not extend to cognitive suppression in this age range.
Keywords: ADHD, syntactic ambiguity resolution, cognitive inhibition, working memory, interference control
Attention-deficit/hyperactivity disorder (ADHD) is now recognized as persisting into adolescence and adulthood in a substantial percentage of cases (Barkley, Fischer, Smallish, & Fletcher, 2002), with some 4% of adults affected in the United States (Kessler et al., 2006). However, whereas cognitive control abilities are heavily studied in children with ADHD, the profile of cognitive control problems in adolescents and adults is less well studied. This is particularly important because during the developmental period from adolescence to adulthood, substantial changes occur in the maturation of cognitive control brain circuits and their corresponding abilities (Biederman & Faraone, 2002; Tannock, 1998). Moreover, relations among the three clinical subtypes of ADHD remain poorly characterized, even in children (American Psychiatric Association, 2000). Subtype validity in adolescents and adults is unclear and may benefit from studies of cognitive control abilities (Milich, Balentine, & Lynam, 2001). With regard to cognitive control mechanisms, extensive research in children suggests that at least one mechanism involved in ADHD is a breakdown in strategic behavior, or a deliberate alteration of behavior, particularly suppressing a triggered motor or oculomotor response (Barkley, 1997; Carr, Nigg, & Henderson, 2006; Nigg, 2001; Nigg, 2006 Pennington & Ozonoff, 1996; Schachar, Tannock, Marriott, & Logan, 1995; Willcutt et al., 2001). Yet, several further issues remain when one considers adolescents and adults.
It remains in debate whether inhibitory control is a unitary construct across cognitive and behavioral domains (Logan & Cowan, 1984; MacLeod & Gorfein, 2007). Thus, it is sometimes assumed that ADHD is associated with difficulty in suppressing or inhibiting mental information in addition to motor responses. But, these abilities may be distinct, and cognitive suppression is little studied in ADHD. Harnishfeger and Bjorkland (1993) defined cognitive inhibition as the active suppression of some previously activated cognitive representation, such as the ability to clear incorrect inferences from memory (see also Dempster, 1993). Subsequently, Harnishfeger (1995) argued that behavioral inhibition and cognitive inhibition are distinct. Nigg (2000), in contrast, suggested that interference control as it applies to protecting working memory should be distinguished from cognitive inhibition in analyzing ADHD, similar to Barkley (1997). This distinction seems to have merit because tasks purported to measure working memory tend to show an ADHD weakness (Martinussen, Hayden, Hogg-Johnson, & Tannock, 2005; Willcutt et al., 2001).
However, data in which these distinctions are used to narrow down the cognitive control problems in ADHD are quite limited. First, virtually no working memory studies of ADHD used a competing dual-task paradigm in which items must be held in mind while other cognitive operations are carried out (Engle, Tuholski, Laughlin, & Conway, 1999). Instead, most studies used reverse span tasks that were heavily confounded with short-term storage (Martinussen et al., 2005). Second, studies purporting to measure cognitive inhibition as a separate ability have generally failed to find ADHD effects, albeit in fairly small samples and in younger children who may not have developed sufficient cognitive control to exhibit a clear ADHD weakness, if such a weakness exists. Barkley (1997) tested children on the Matching Familiar Figures task and concluded that cognitive inhibition is not impaired in ADHD. Gaultney, Kipp, Weinstein, and McNeill (1999) tested ADHD children with a directed forgetting paradigm and also found no ADHD-related weakness in cognitive inhibition. Thus, very few studies have attempted to assess cognitive suppression in ADHD, either as the ability to suppress prior information for new information or conceptualized as the ability to protect working memory contents while carrying out other mental operations. More data are needed on these points to evaluate key theories about ADHD (Barkley, 1997; Nigg, 2000, 2001; Martinussen et al., 2005).
As the studies cited above attest, agreement on how to operationally assess cognitive inhibition is lacking. To do so, a task is needed that introduces information that must be suppressed and that then detects suppression failures. Here, we selected a task that has been well validated for this purpose in the language processing literature yet that has never before been applied to ADHD. Several considerations lead to this decision. For one, language abilities are likely related to cognitive control (Barkley, 1997). For another, poor suppression in the language processing could help explain ADHD’s frequent co-occurrence with learning and reading disabilities (August, & Garfinkel, 1990; Beitchman & Young, 1997; Blaskey, 2004; Cohen, Davine, Horodezky, Lipsett, & Isaacson, 1992; Denckla, 1996; Hinshaw, 1992; Tirosh & Cohen, 1998). Third, language processing is important to social adaptation, as children with ADHD have weakness in pragmatic aspects of language output, such as talking excessively, interrupting others, and blurting out answers (Lorch et al., 2000) and, in some cases, have frank language delays (Cantwell, 1996; Tirosh & Cohen, 1998). They also appear to have weakness in story comprehension (Oram, Fine, & Tannock, 1999; Purvis & Tannock, 1997; Rashid, Morris, & Morris, 2001; Shaywitz et al., 1995; Tannock, Purvis & Schachar, 1993; Tannock & Schachar, 1996). Thus, if a cognitive inhibition problem is going to be detectable, the language domain would be a promising place to look for it.
A key complication in evaluating cognitive inhibition is the need to determine whether this problem is distinct from working memory—a more well studied problem in ADHD. Working memory is defined as the capacity to hold and process information over short periods of time (Baddeley & Hitch, 1974; Engle et al., 1999). Data so far suggest that ADHD is associated with weakness in some types of working memory (Martinussen et al., 2005). Yet more data are needed in this domain because, as Martinussen et al. (2005) pointed out, virtually all prior studies used simple reverse span tasks. These tasks may not actually tap working memory because they do not include a storage and processing design of the sort recently advocated in the working memory literature (Engle et al., 1999). Therefore, studies are needed that examine in the same ADHD sample both working memory (i.e., the ability to protect short-term storage while carrying out other mental operations) and cognitive suppression (i.e., the ability to suppress prior content with new information; Gernsbacher, & Faust, 1991; Gernsbacher, Varner, & Faust, 1990). Moreover, working memory has been shown to be important in language comprehension (Daneman & Carpenter, 1980; Just & Carpenter, 1992; King & Just, 1991; Waters & Caplan, 1996), so it is important to determine whether any weaknesses in language processing could be explained by weakness in working memory.
To evaluate working memory, we chose a Stroop-based working memory task that met the dual-task requirements suggested by Engle et al. (1999). Participants saw a series of Stroop trials (2–7) in which they were required to name the color of ink in which a color word was presented, and at the end of a series of words, participants had to recall the named colors in serial order. This task consists of a processing component (i.e., naming the color of ink as the words appears) and a storage component because the already named colors must be held in memory. This particular task specifically assesses the ability to protect working memory from interference, which is the core of the ability according to Engle et al. (1999).
To investigate cognitive inhibition via language processing, we chose a task, which has been well-studied and validated in the language processing literature but has not been previously studied in ADHD, called a garden path task (cf. Blaskey, 2004). Garden path sentences contain a misleading opening (called a temporary ambiguity), in which the reader is led toward one interpretation. Subsequent information in the same sentence signals that the initial interpretation was wrong and has to be revised (Bever, 1970). The faulty initial interpretation has to be suppressed in order to correctly process the sentence. Here is an example: While Anna bathed the baby played in the crib. In that sentence, most readers will momentarily take the baby to be the direct object of bathed, meaning Anna bathed the baby. However, the second verb played signals that the baby is actually the subject of played (meaning that in fact, Anna bathed herself, not the baby). Revision of the incorrect interpretation is accomplished by determining whether bathed can be interpreted reflexively (i.e., that Anna bathed herself). This task is well studied and well validated in typically developing populations as detecting a suppression process because the initial misinterpretation is simply and easily processed, whereas the correct interpretation requires additional processing to overcome the misleading opening (Bever, 1970; Christianson, Hollingworth, Halliwell, & Ferreira, 2001). College-aged students have difficulty suppressing this incorrect interpretation, and, thus, more often than chance, answer yes to a comprehension question, such as Did Anna bathe the baby? If ADHD involves poor cognitive inhibition then an ADHD group should be even more likely to answer yes to this question.
The sentence processing task included two manipulations in order to achieve a range of difficulty with respect to task demands. The first manipulation contrasts a misleading sentence (i.e., a garden path sentence) with a straightforward sentence (i.e., a non–garden path sentence). The two types of sentence pairs are illustrated in Table 1. Sentence 1 in Table 1 shows a misleading structure with a reflexive verb. Sentence 2 in the table shows the exact same information in a straightforward structure. Notice that the only difference is the order of the two clauses. More errors occur when answering a question like Sentence 3 after reading a sentence such as Sentence 1 because that sentence demands suppressing the misleading information in order to get the correct interpretation. Often this suppression fails, which leads to errors on comprehension questions. Sentences 4 and 5 respectively contain an ambiguous and a straightforward sentence; again, both contain an identical number of words and information. However, in the case of Sentences 3 and 4, the type of ambiguity is slightly different because these sentences involve a different type of verb. The ambiguity in Sentence 1 is created by a reflexive verb. Reflexive verbs imply that the action of the verb is done to oneself, such as dressing, shaving, bathing, and so on. The ambiguity in Sentence 4 is created by an optionally transitive verb, which simply means that the verb does not have to take a direct object. The literature on this task distinguishes these two types of garden paths, in that the optionally transitive one, Sentence 4, is more difficult to revise. It takes longer to process, and it is more difficult to suppress the misleading information in Sentence 4 than that in Sentence 1 (Christianson et al., 2001).
Table 1.
Example Language Comprehension Sentences of Varying Type and Difficulty for the Cognitive Inhibition Task
| Type | Example |
|---|---|
| Reflexive verbs | 1. While Anna bathed the baby that was small and cute spit up on the bed. |
| 2. The baby that was small and cute spit up on the bed while Anna bathed. |
|
| Comprehension question | 3. Did Anna bathe the baby?a |
| Optionally transitive verbs | 4. While Susan wrote the letter that was long and eloquent fell off the table. |
| 5. The letter that was long and eloquent fell off the table while Susan wrote. |
|
| Comprehension question | 6. Did Susan write the letter?b,c |
Reflexive verbs are obligatorily transitive, the direct object must refer back to the subject. For example, in Sentence 1 the correct interpretation is that Anna bathed herself. Optionally transitive verbs are optionally transitive, which means that they can take a direct object but are not required to do so.
Errors more likely for Sentence 1, which contains a misleading opening phrase, than Sentence 2 which does not.
Errors are more likely for Sentence 4, which contains a misleading opening phrase, than for Sentence 5, which does not.
Also, errors are more likely for Sentence 4 than for Sentence 1, because the error in Sentence 4 is harder to overcome.
If ADHD is associated with poor cognitive inhibition then the ADHD group should have more difficulty suppressing the misinterpretation in the garden path condition when compared with the control group. Comparison of the two types of verbs provides an additional measure of the ability to obtain the correct interpretation. The aims in the current study were (a) to determine whether ADHD is associated with inability to suppress temporary and incorrect interpretations in language processing (Harnishfeger, 1995), (b) to determine whether ADHD is associated with working memory weakness, and if ADHD is associated with both, (c) to determine whether these associations are distinct or related.
Method
Participants
Overview
Participants were 288 adolescents and adults between the ages of 13 years and 37 years. Table 2 shows the intercorrelations between demographic variables and dependent variables. Demographic data are broken down by diagnostic group in Table 3. Note that the ADHD group comprised 60 participants with ADHD primarily inattentive subtype (ADHD-PI) and 55 participants with ADHD combined subtype (ADHD-C). Cases of ADHD primarily hyperactive–impulsive type were rare and were excluded to avoid having a group too small to analyze.
Table 2.
Intercorrelations Between Demographic Variables and Dependent Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Group | — | .16** | −.28** | .23** | .22** | .20** | .16** | .25** | .04 | .14** | .22** |
| 2. | Age | — | −.07 | .14** | .04 | .84** | .11 | .06 | .05 | .11 | .09 | |
| 3. | Sex | — | .08 | .02 | −.12* | −.06 | .06 | −.05 | −.02 | −.02 | ||
| 4. | FSIQ | — | .51** | .23** | .20** | .28** | .31** | .35** | .19** | |||
| 5. | Reading | — | .15* | .15** | .22** | .33** | .37** | .19** | ||||
| 6. | Education | — | .18** | .12* | .09 | .15* | .13* | |||||
| 7. | M-S OPT | — | .35** | .38** | .32** | .12 | ||||||
| 8. | M-S RV | — | .20** | .20** | .12 | |||||||
| 9. | S-M OPT | — | .75** | .10 | ||||||||
| 10. | S-M RV | — | .19** | |||||||||
| 11. | Incongruent WM trials | — |
Note. N = 288. Groups were coded ADHD = 0 and control = 1. Sexes were coded female = 0 and male = 1. Incongruent WM (working memory) trials are the average proportion of interference trials correct across all six set sizes. FSIQ = free scale intelligence quotient. Reading = word reading on Wechsler Individual Achievement Test—Second Edition; M-S = non-garden path sentence; S-M = garden path sentence; OPT = optionally transitive verb; RV = reflexive verb; ADHD = attention-deficit/hyperactivity disorder.
p < .05.
p < .01.
Table 3.
Sample Characteristics
| Control group |
ADHD group |
|||
|---|---|---|---|---|
| Variable | M | SD | M | SD |
| Age (years) | 19.93 | 5.37 | 18.31 | 4.53 |
| Sex | ||||
| % Male | 43.4 | 71.3 | ||
| % Female | 56.6 | 28.7 | ||
| Estimated full scale IQ | 112.99 | 12.84 | 106.94 | 12.60 |
| Reading standard score | 105.03 | 9.08 | 100.87 | 10.92 |
| Education | 12.91 | 2.61 | 11.60 | 2.00 |
| Ethnicity | ||||
| % White | 78.0 | 80.0 | ||
| % African American | 10.0 | 7.0 | ||
| % Latino | 2.0 | 6.0 | ||
| % Asian/Asian American | 2.0 | 1.0 | ||
| Other/mixed/unreported | 8.0 | 6.0 | ||
| Corners Index T score | 45.18 | 7.81 | 65.45 | 11.49 |
Note. For control group, n = 173; for ADHD group, n = 115. Conners Index T score is self-reported for adults and is the average of mother and teacher ratings for adolescents. The ADHD group consisted of 60 primarily inattentive subtype participants and 55 combined subtype participants.
Recruitment
Prospective participants were recruited from the community via widespread public advertisements (i.e., radio, newspapers, and movie theaters) designed to access as broad and as representative a sample as possible. ADHD participants were recruited by advertisements asking for individuals who had a possible, suspected, or definite history of problems with attention, impulsivity, activity, ADHD, or ADD. The non-ADHD participants were recruited by advertisements for healthy participants for a study of attention. All who called in were then evaluated in a standard, multistage, screening and diagnostic evaluation procedure for identification of cases and control participants that met our study criteria. In this procedure, prospective participants contacted the project office, at which point key rule outs were checked (no sensory-motor handicap, no neurological illness, no nonstimulant psychiatric medications, and native speaker of English). Eligible participants were then scheduled for a diagnostic visit wherein they completed semistructured clinical interviews as described below.
Tasks were administered to in two laboratory sessions. Procedures were deliberately altered slightly for children under the age of 18 years to accommodate their developmental stage and legal status, although the diagnostic logic was the same. In the first session, full scale IQ was assessed with a reliable and valid five-subtest (i.e., Picture Completion, Vocabulary, Similarities, Arithmetic, and Matrix Reasoning) short form of the Wechsler Adult Intelligence Scale—Third Edition (Wechsler, 1997a; for adults age 17 years and above) or the Wechsler Intelligence Scale for Children—Fourth Edition (for adolescents age 16 years and below; Wechsler, 1997b). Reading ability was assessed with the Wechsler Individual Achievement Test—Second Edition Word Reading subtest (Wechsler, 2001). Reading disability was diagnosed if reading achievement scores were at least 15 points below full scale IQ and if reading achievement fell below a standard score of 85. All other tasks assessed neuropsychological executive function, and the tasks were administered in a fixed order at the second session.
In the case of adults over age 18 years, assessment of ADHD requires retrospective assessment of childhood ADHD status to establish childhood onset and inclusion of informant interviews to verify symptoms and impairment (Wender, Wolf, & Wasserstein, 2001). A retrospective Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS; Puig-Antich & Ryan, 1986) was administered by a master’s level clinician with extensive training, following previously published procedures for assessing adults (Biederman, Faraone, Keenan, Knee, & Tsuang, 1990). This procedure assessed the adult’s childhood ADHD, conduct disorder, and oppositional defiant disorder symptoms and impairment. Self-reported recall of these symptoms by adults with ADHD may lead to underreporting (Murphy & Barkley, 1996). Therefore, an informant who had known the participant as a child (usually a parent) also reported on the participant’s childhood behaviors via an ADHD rating scale and a structured clinical interview (a retrospective K-SADS ADHD module adapted to be appropriate for an informant). In the case of adolescents, a K-SADS interview of the parent was conducted to ascertain current and lifetime symptoms of ADHD and all Axis I disorders in the same manner. Teacher ratings were obtained to evaluate cross-situational symptom display.
For adults over the age of 18 years and out of high school, current adult ADHD symptoms were assessed by self-report and by interview with a second informant who knew the participant well (Wender et al., 2001), usually a spouse, a roommate, or a close friend. We again used K-SADS ADHD questions worded appropriately for current adult symptoms, following Biederman et al. (1990). This interview was supplemented with the Barkley and Murphy (1998) Current ADHD Symptoms Rating Scale (as recommended by Weiss, Hechtman, & Weiss, 1999). To allow us to ensure that ADHD participants exceeded normative cutoffs for level of ADHD symptoms, adult participants also completed the Conners, Erhart, and Sparrow (1999) Young Adult ADHD Rating Scale, the Achenbach (1991b) Young Adult Self Report Scale, and the Brown (1996) Adult ADHD Rating Scale. Their peer informants completed the Conners et al. peer rating form, the Barkley and Murphy peer ratings on adult symptoms, and a brief screen of antisocial behavior and drug and alcohol use. The informant also completed a structured interview about the participant’s current ADHD symptoms, using the modified K-SADS for current symptoms. All informant interviews were conducted by clinically trained interviewers (graduate students in clinical psychology or a master’s level clinical social worker) via telephone after appropriate consent procedures. In the case of adolescents, all of this information was based on parent report for the adolescents, except that concurrent informant reports were also obtained from teacher ratings on the Childhood Behavior Checklist (Achenbach, 1991a), on the Conners et al. Rating Scale Revised, and on the ADHD Rating Scale.
Best estimate diagnosis for ADHD
For all participants, a diagnostic team (a licensed clinical psychologist and a board certified psychiatrist) arrived at a best estimate diagnosis (Faraone et al., 2000). The same team evaluated all cases. Each team member independently reviewed all available information from Structured Clinical Interview for Axis I Disorders, K-SADS, and informant rating scales to arrive at a clinical judgment about ADHD presence or absence, ADHD subtype, and comorbid disorders. Because there is no agreement on age-appropriate cutoffs for adolescents and adults, the team conservatively followed Diagnostic and Statistical Manual of Mental Disorders— Fourth Edition DSM-IV; (American Psychiatric Association, 1994) criteria by requiring the six symptoms that DSM-IV specifies. This ensured minimal false positives in the ADHD group. (Note that false positives in the control group were avoided by requiring four or fewer symptoms of ADHD and no past history of ADHD). DSM-IV criteria regarding comorbidity were carefully followed so that although comorbid disorders were diagnosed when present, the participant was excluded from the study if he or she met criteria for ADHD but clinicians judged that symptoms were better explained by a co-occurring mood or other major disorder (American Psychiatric Association, 1994). This provided some control against obtaining a sample with extreme levels of comorbid disorders. The clinical interviewers rated and noted evidence of impairment, and the diagnostic team required such evidence to make the ADHD diagnosis. Interclinician agreement on presence or absence of ADHD (any type) was satisfactory (k = .80), and agreement on ADHD subtype was also adequate, ranging from k = .74 to k = .85. Diagnostician reliability for comorbid disorders was excellent (past major depression, k =.96; any current anxiety disorder, k = 0.98; antisocial personality disorder, k = 0.93; substance or alcohol dependence, k = 0.97).
Exclusionary criteria
Potential participants were excluded from both groups if they had a current, major depressive or manic/hypomanic episode; a current substance dependence preventing sober testing; a history of psychosis; a history of autism; a history of head injury with loss of consciousness greater than 1 min, a sensory-motor handicap or neurological illness; a native language that was not English; or any currently prescribed antipsychotic, antidepressant, or anticonvulsant medications. We used IQ < 75 as a rule out, in keeping with the field’s consensus definition of mental impairment in DSM-IV and to maximize generalizability.
Medication washout
Participants prescribed psychostimulant medications (Adderall, Ritalin, Concerta, and Focalin in this sample) were tested after a minimum of 24 hr (for short acting preparations) to 48 hr washout (for long acting preparations); actual mean washout time was 95.3 hrs for ADHD, 160.3 hr for ADHD-residual, 81.8 hr for ADHD-C, and 91.3 hr for ADHD-PI groups. These washout periods should be sufficient to minimize medication effects.
Materials and Measures
Experiments were programmed with E-Prime (Version 1.1) software. Participants completed both experiments on a Dell Optiplex GX 400 computer with a 19 in. (48.26 cm) monitor. Two tasks were run, one to assess cognitive inhibition and one to assess working memory.
Cognitive inhibition: Sentence processing task
A total of 24 different sentence item pairs were created (12 with optionally transitive verbs and 12 with reflexive verbs). For each sentence, both clause order conditions were created: garden path (ambiguous) and non– garden path (non-ambiguous). Each participant saw only one version of each sentence item but saw an equal number of items in each condition (i.e., six items). The experimental items were presented along with 72 filler sentences. Twenty-four fillers had questions that required a no response, and 48 fillers had a question that required a yes response.
With regard to procedure, participants were given a written description of the experiment, followed by verbal instructions on how to perform the task. The instructions informed participants that they would have to read a sentence and then answer a comprehension question about it. Participants were seated at a computer workstation with a four-button response box. At the beginning of each trial, a message appeared instructing the participant to press any button when ready. After the button press, a fixation cross appeared in the center of the screen for 500 ms. The full sentence replaced the fixation cross, and after the participant finished reading the sentence, he or she pressed any button to view the comprehension question. The sentence and question were separated by a delay of 500 ms, and the question remained on the screen until the participant responded either yes or no. Participants completed 10 practice trials: 1 sentence in each of the 4 experimental conditions and 6 filler sentences. The practice sentences were not included in the experimental session. The participants then saw all 96 sentences in the experimental session. The order of sentence presentation was determined randomly for each participant. Upon completing all of the trials, participants were given a forced-choice memory test in which they had to identify sentences from the experiment, to confirm attention to the task. The entire experiment lasted approximately 45 min.
Working memory: Stroop working memory task
Participants completed a Stroop-based working memory task (McCabe, Robertson, & Smith, 2005). In it, they completed a series of Stroop trials in which they had to name aloud the color of ink in which a color word was presented. After a series of words (two–seven), the participant was asked to recall the color of ink in which the series of words were printed. For example, participants might see a four-item sequence of the word green printed in green type, the word blue printed in red type, the word red printed in green type, and the word yellow printed in blue type, in which case they would have to say out loud “green,” “red,” “green,” and “blue.” At the end of this sequence, participants were required to write G, R, G, and B. The colors used in the experiment were blue, green, red, and yellow. These stimuli were presented in the center of the computer screen for 1 s each, and words were presented in 72-point, lower-case, Times New Roman font. Each color and word was presented randomly, as congruent and incongruent trials, an equal number of times across the entire task. Across the entire experiment, half the words were congruent, and half were incongruent. The same color words never appeared consecutively.
With regard to procedure, participants were informed that they would see a series of color words presented in the center of the computer screen and that their task was to name and remember the color of ink that the words were presented in. After a series of these words, participants saw the word recall in the center of the screen. This was their cue to attempt to write down the first letter of the ink color in the same order in which the colors were presented. This task requires participants to hold information in memory while inhibiting the prepotent response to name the color word (McCabe et al., 2005). Participants were given four practice trials with feedback. The practice was followed by 36 regular session trials, 6 in each of the 6 different set size conditions. The entire experimental session lasted approximately 30 min.
Design and Analysis
For the sentence processing task, the design was 2 × 2 × 2 (Group × Sentence Structure × Verb Type) mixed-model analysis of covariance (ANCOVA), in which sentence structure and verb type were within subject, and group was between subjects. We included three covariates. The first was age. The rationale for including this covariate was to determine whether there is a developmental change with regard to cognitive suppression. The second was gender. This covariate was included because the control group and the ADHD group differed in gender ratio (see Table 3), and gender may be related to language processing (Berry, Shaywitz, & Shaywitz, 1985). Third and finally, we report results after covarying reading ability, as assessed by word reading on the Wechsler Individual Achievement Test—Second Edition (Wechsler, 2001).We viewed this decision as somewhat complex, in that problems with cognitive suppression could conceivably influence reading ability. However, because the task relied on reading, we judged it most conservative to check results with reading ability adjusted.1 The dependent variable was accuracy on comprehension questions. For the working memory task, the design was a 2 × 2 × 6 (Group × Trial Type × Working Memory Span) mixed-model analysis of variance, with group as a between subjects variable and with trial type and set size as within subject variables. The dependent variable was the proportion of correct trials recalled per set size, broken down by congruent and incongruent color words.
Results
Experiment 1: Sentence Processing
Before proceeding with the inferential analyses, we checked the sentence recognition scores, which were obtained in a forced-choice memory test at the end of the sentence processing task, to evaluate whether the task had been engaged by both groups.2 Control participants were 85% accurate (63%–100%), and ADHD participants were 79% accurate (60%–97%). Both were significantly above chance: for control participants, t(172) = 61.31, p < .01, and for ADHD participants, t(114) = 31.16, p < .01, indicating that participants in both groups were fairly accurate in identifying the sentences from the experiment. The control group did have better recognition than did the ADHD group, t(286) = 5.86, p < .01. But, when this was covaried, no change in findings emerged.
All analyses reported below were checked, with the ADHD-PI and the ADHD-C subtypes compared against one another. In no analysis did the two subtypes differ, and there were no interactions of subtype by condition. Therefore, to simplify the presentation, we present results collapsed across the two ADHD subtypes, using a two-group analysis comparing control participants with ADHD participants. Exact age was included as a covariate to assess possible developmental change in effects. That is, if age interacted with any other variable, we would investigate that interaction further. The results of reading times for both the sentences and the questions are presented in the Appendix, for specialist readers interested in that detail.
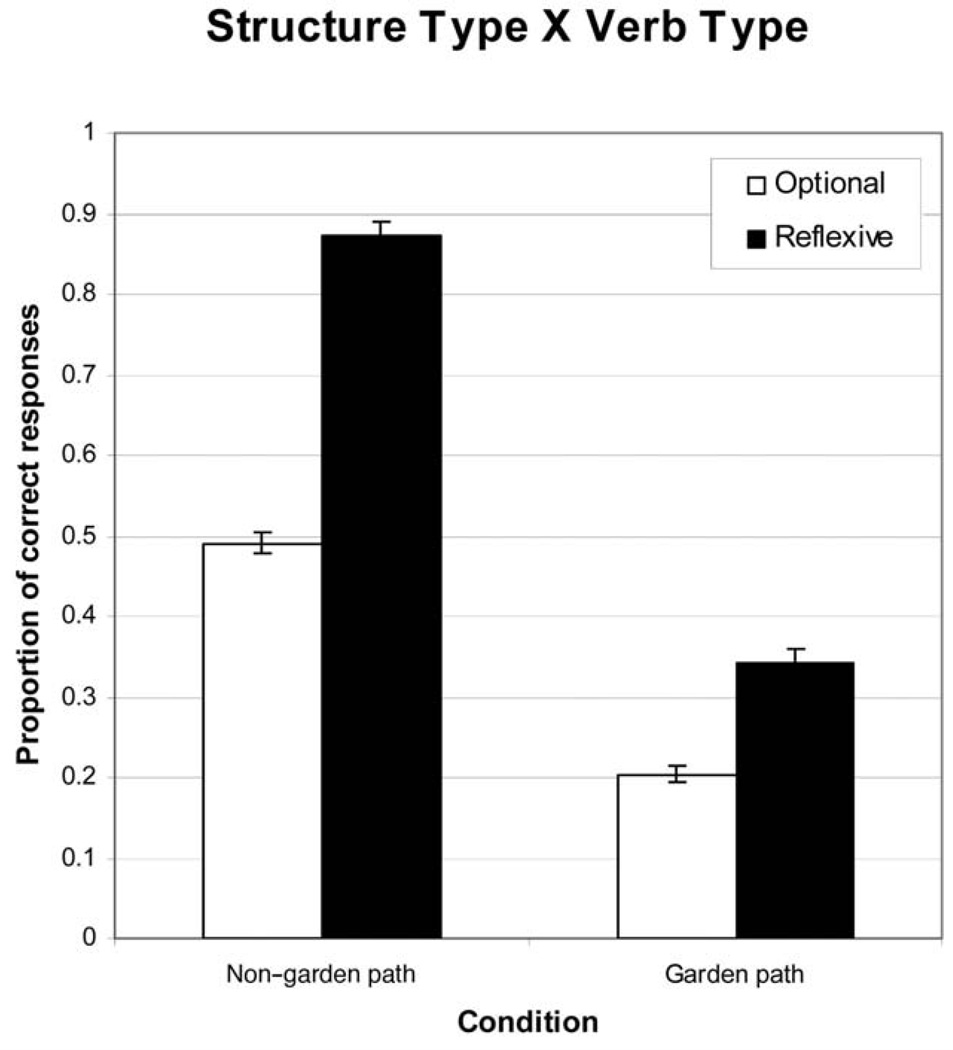
The analytic decomposition of the full ANCOVA model comparing the control group with the ADHD group in the sentence processing task is shown in Table 4. The main effects of sentence structure and group were significant. The main effect of group indicates that the ADHD group was less accurate than was the control group, and the main effect of sentence structure indicates that comprehension performance was better for the non–garden path sentences. The main effect of verb type was not significant when reading ability was controlled. The three-way (Group × Sentence Structure × Verb Type) interaction was not significant; however, two of the two-way interactions were significant. The Sentence Structure × Verb Type interaction was significant, and an examination of the four possible paired-comparisons revealed that all were significantly different from one another. However, the greatest differences were between the two verbs types with the non–garden path sentences (see the left two bars in Figure 1). This disparity in comprehension performance indicates chance performance for non–garden path sentences with the optionally transitive verbs. The reflexive verbs, in contrast, produced over 85% correct.
Table 4.
Analyses of Covariance: Group (ADHD vs. Control) × Sentence Structure × Verb Type
| Interaction | Analysis |
|---|---|
| 3-way | |
| Group × Sentence Structure × Verb Type | F(1, 283) = 0.44, p > .10, η2 = .002 |
| 2-way | |
| Sentence Structure × Verb Type | F(1, 283) = 9.27, p < .05, η2 = .032 |
| Sentence Structure × Group | F(1, 283) = 4.30, p < .05, η2 = .015 |
| Verb Type × Group | F(1, 283) = 2.84, p > .05, η2 = .010 |
| Main effects | |
| Sentence structure | F(1, 283) = 44.68, p < .05, η2 = .136 |
| Verb type | F(1, 283) = 3.00, p > .05, η2 = .010 |
| Group | F(1, 283) = 5.53, p < .05, η2 = .019 |
Note. ADHD = attention-deficity/hyperactivity disorder.
Figure 1.
Results showing the 2 × 2 interaction of sentence structure and verb type. Error bars show the standard error of the mean.
The second factor driving this interaction is the difference between garden path and non–garden path sentences with reflexive verbs (i.e., the solid bars in Figure 1). Here, as expected, the sentence with the temporary ambiguity resulted in significantly more incorrect interpretations than did the sentences that did not contain a misleading opening. These results are consistent with previous studies and indicate that the task was operating as intended with this sample (Christianson et al., 2001). The Group × Verb type interaction was not significant when reading ability was covaried (see Table 4 & Figure 2). As Figure 2 shows, the main story here was that the ADHD group performed more poorly than did the control group, and the difference between groups was greater with the reflexive verbs. However, because the interaction was not robust to reading ability, we do not discuss this result further.
Figure 2.
Results showing the 2 × 2 interaction of verb type and group, comparing the attention-deficit/hyperactivity disorder (ADHD) group with the control group. Error bars show the standard error of the mean.
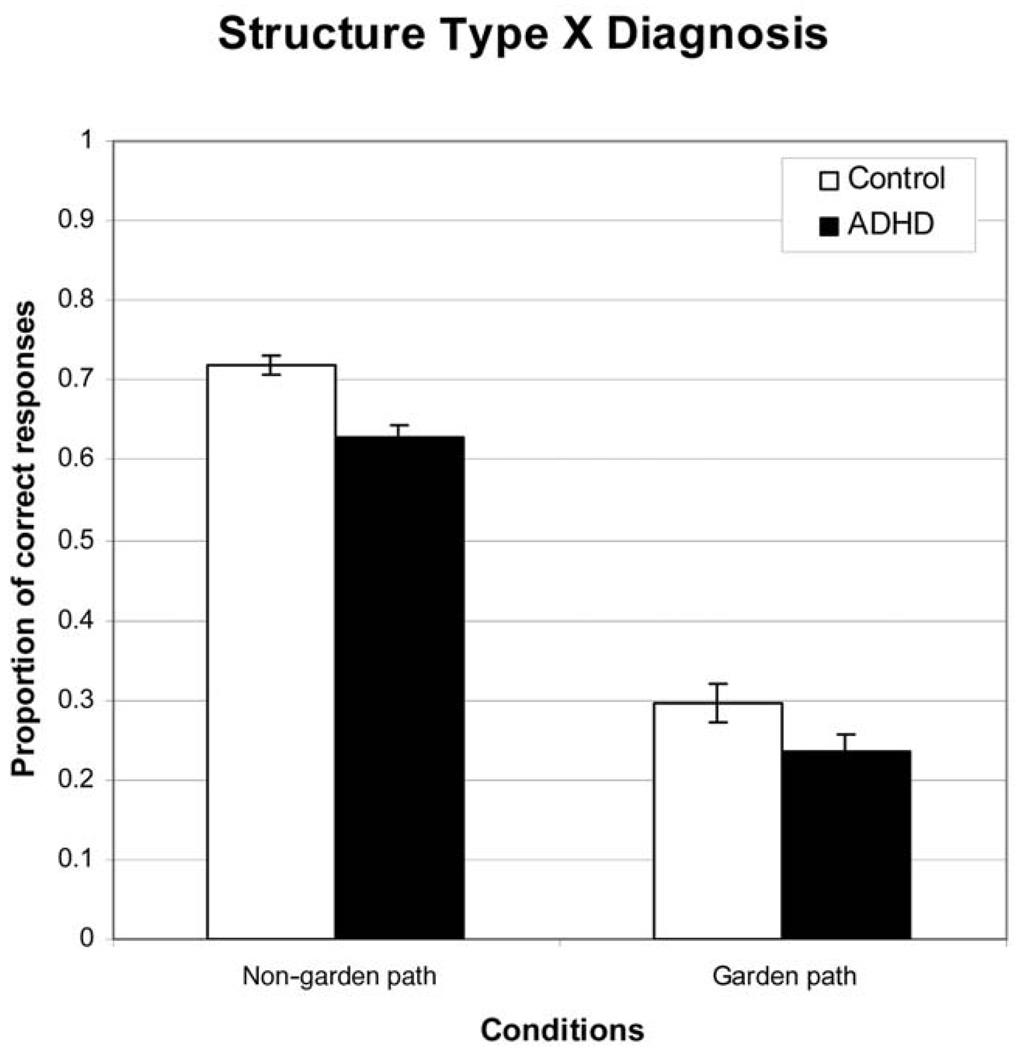
The interaction of most interest for the study hypotheses was the Group × Sentence Structure interaction. It was decomposed via paired comparisons (see Figure 3). The difference between groups for the garden path conditions was not significant when reading ability was covaried, F(1, 283) = .11, p > .10, η2 = .001. Thus, ADHD participants had some difficulty suppressing the misinterpretation as hypothesized, but the difference can be explained by weak reading ability. In the non–garden path conditions, the ADHD group was significantly worse than were control participants, and this effect was robust when reading ability was controlled, F(1, 283) = 11.81, p < .01, η2 = .040. This difference indicates that the ADHD group has an additional comprehension weakness that is not due to poor reading or cognitive inhibition. One possible explanation for this result is that the ADHD participants have reduced working memory capacity, and as a result, they cannot retain information in memory to answer the comprehension question correctly. We turn next to the analysis of the working memory task, but we return to these differences with the non–garden path sentences in the General Discussion.
Figure 3.
Results showing the 2 × 2 interaction of sentence structure and group, comparing the attention-deficit/hyperactivity disorder (ADHD) group with the control group. Error bars show the standard error of the mean.
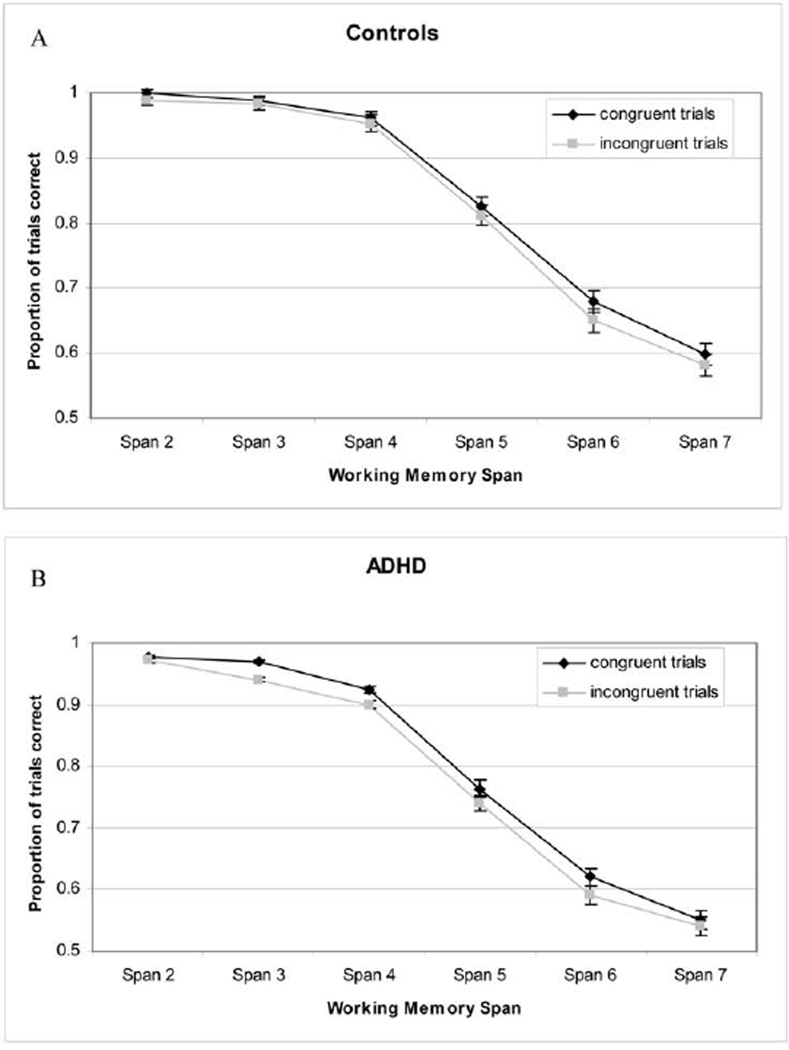
Experiment 2: Working Memory
Following McCabe et al. (2005), we present the results comparing congruent and incongruent trials for each group separately in Figure 4. The results of a 2 × 2 × 6 analysis of variance showed that all three main effects were significant: ADHD participants were less accurate than were control participants, resulting in a main effect of group, F(1, 268) = 14.13, p < .05, η2 = .05; incongruent trials were more difficult than were congruent trials, F(1, 268) = 44.60, p < .01, η2 = .143, for trial type; and longer spans were harder than were shorter spans, F(5, 1340) = 685.40, p < .01, η2 = .719, for memory span.3 The interaction between group and trial type just missed significance, F(1, 286) = 3.27, p < .07, η2 = .012, but the interactions of Group × Span and Span × Trial type were not significant (p > .10). In addition, the three-way interaction was not significant, F(5, 1340) = 1.00, p > .20, η2 = .004. Because floor and ceiling effects could obscure group differences on this task, we ran a follow up analysis of variance in which we analyzed the data using Spans 3–6 (same as McCabe et al.). The results of the follow-up were similar with respect to the main effects (i.e., all were significant). However, the Group × Trial type, F(1, 268) = 4.37, p < .05, η2 = .016 interaction was significant.
Figure 4.
A: Panel shows the results of the working memory Stroop span task for the control group. B: Panel shows the results for attention-deficit/hyperactivity disorder (ADHD) participants. In both panels, error bars show the standard error of the mean.
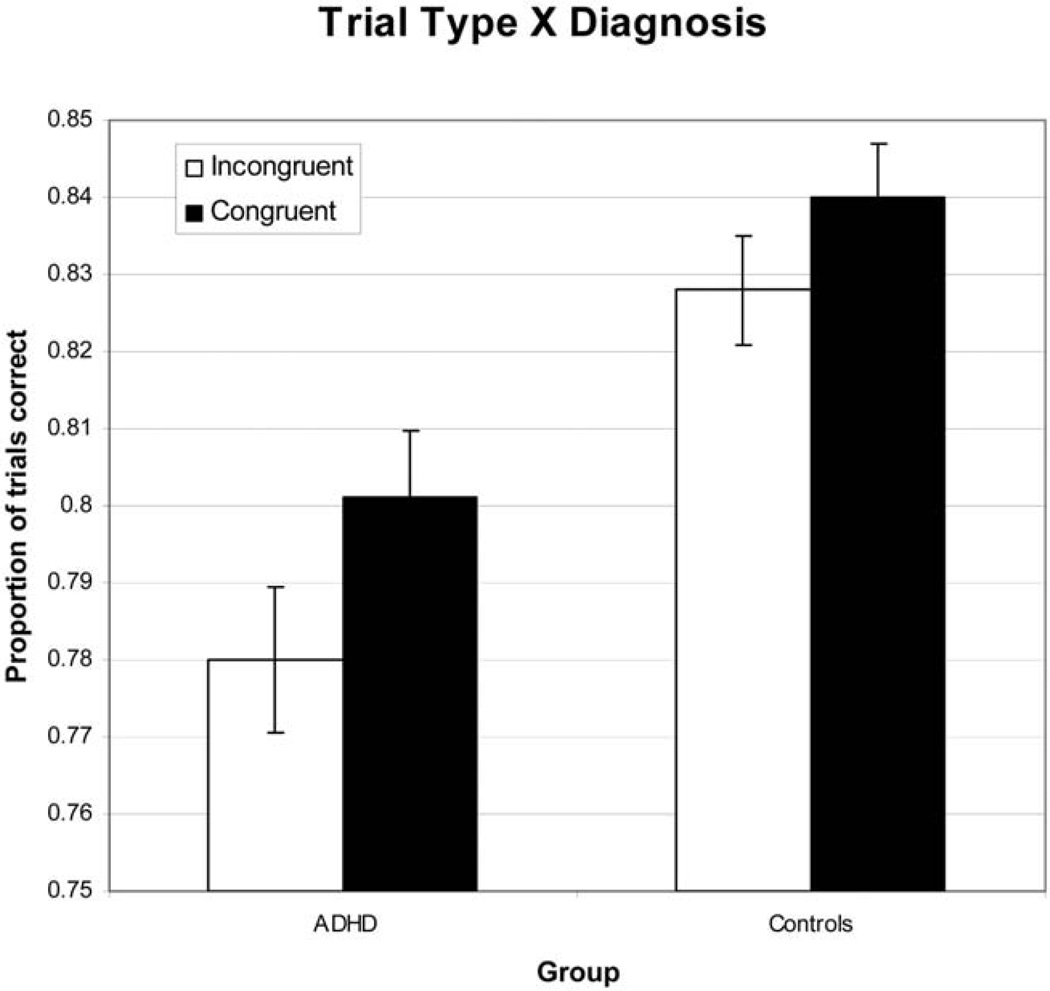
Collapsing across both analyses of the working memory data, we can see that the strongest effect is the Group × Trial type interaction (see Figure 5 for the means for this analysis). The ADHD group showed worse performance for both congruent and incongruent color words when compared with the control group; however, the difference between congruent and incongruent color words was greater for the ADHD group than for the control group. This suggests that the ADHD group had particular difficulty with the incongruent color words. Thus, ADHD participants had more problems protecting the contents of working memory from interference, as shown by their disproportionate decrease in accuracy for incongruent color words. This pattern of results indicates (a) that the ADHD group performed more poorly than did the control group and (b) that incongruent color words resulted in more errors for the ADHD group. Overall, these data suggest problems in working memory in ADHD, such that controlling Stroop interference was more problematic for ADHD participants.
Figure 5.
Results showing the 2 × 2 interaction of trial type and group, comparing the attention-deficit/hyperactivity disorder (ADHD) group with the control group. Error bars show the standard error of the mean.
To evaluate whether working memory was a potential explanation of processing problems on the sentence processing task, partial correlations were computed. The variance in working memory was removed from the correlations between group and accuracy on the sentence processing task. The results of bivariate correlations between group and proportion of correct responses in the four conditions of sentence processing task are shown in the first line of Table 5. The second line of the table has the results of the partial correlations, for which the proportion of incongruent color words correct (i.e., the open bars in Figure 5) were used as an index of the ability to control interference in working memory. The partial correlations show that the relationship between group and sentence processing task drops from significant to nonsignificant for the garden path sentences with reflexive verbs. Thus, the ability to revise the misleading opening with reflexive verbs is at least partially accounted for by working memory. The relationship between the group and the other three conditions was unaffected when the variance due to working memory was removed.
Table 5.
Partial Correlations of Group and Proportion of Correct Responses on the Garden Path Task with Working Memory Partialed
| Optionally transitive |
Reflexive absolute |
|||
|---|---|---|---|---|
| Category | Non-garden path |
Garden path |
Non-garden path |
Garden path |
| Group | .16** | .04 | .25** | .14** |
| Group WM | .13* | .01 | .22** | .09 |
Note. N = 258. WM = working memory.
p < .05.
p < .01.
Summary
The sentence processing task showed no support for a cognitive inhibition deficit in ADHD after controlling for differences in reading ability. Garden path sentences require a reader to actively suppress an incorrect interpretation, but we found no inhibition deficits, as ADHD participants were no worse than were control participants with garden path sentences. However, we did find that ADHD participants performed more poorly with non–garden path sentences. We interpret this result as showing that the ADHD group relies on the plausibility of events in the real world rather than on the actual content of the sentence when evaluating its meaning. For example, with a sentence such as The deer ran through the woods while the man hunted, real-world knowledge makes it highly plausible that the man was hunting deer, but crucially this interpretation is not specified by the sentence. No effects in sentence processing were moderated by age or gender. The working memory task showed an ADHD weakness in working memory related to the ability to protect working memory from interference, as the ADHD group had significantly worse performance for incongruent color words when compared with the control group.
General Discussion
Aside from limitations noted below, the current data suggest two conclusions concerning cognitive control and working memory in ADHD. The first is that suppression of a temporary incorrect interpretation in language is not impaired in ADHD (see also Christianson et al., 2006; for a similar result in an aging study). Thus, we found no evidence to support a distinction between behavioral and cognitive inhibition as hypothesized by Harnishfeger (1995). The second conclusion is that ADHD involves a weakness in controlling interference in working memory. Our working memory task indicated that ADHD participants were worse across all conditions, but crucially, the ADHD group showed larger differences between congruent and incongruent color words when compared with the control group.
This is the first study to assess cognitive inhibition with language processing in adolescents and adults with ADHD, and one of the first to use a dual-task approach to assess working memory. We suspected that language functioning in ADHD may be a locus of cognitive control problems, in view of theoretical assertions concerning internalized speech on self-regulation (Barkley, 1997) and also because of ADHD’s overlap with learning and reading disability (Purvis & Tannock, 1997). Indeed, some effects in the current study were accounted for by reading ability, but others were not.
The primary purpose of the current study was to examine the revision of temporary, incorrect interpretations in language processing. These types of misinterpretations occur often in natural language and are virtually impossible to avoid; confirming this, the ADHD and control groups had significantly more errors in the garden path than in the non–garden path conditions. Thus, the task manipulation was valid. Yet the critical result showed that ADHD participants were no worse at revising misinterpretations than were control participants, when reading ability was controlled. On this basis, we conclude that ADHD does not involve weakness with cognitive suppression. Thus, these results support models of ADHD, which assume deficits in behavioral inhibition (i.e., suppressing a triggered motor responses) rather than weaknesses inhibiting mental representations (i.e., cognitive inhibition) or a global inhibitory control problem. The lack of a convincing ADHD deficit in cognitive suppression is consistent with other reports in the literature, all of which failed to show cognitive inhibition deficits in children with other paradigms (Gaultney et al., 1999; Harnishfeger, & Pope, 1996; Kipp & Pope, 1997; cf. Casey et al., 1997).
In the current study, we used a question-answering paradigm to assess cognitive inhibition, but because this task is offline, the possibility exists that some intervening process, such as memory limitations, could affect the results. We think this possibility is unlikely because a recent study from our lab did show a relationship between online processing, as measured by pupil dilation, and comprehension accuracy (Engelhardt, Patsenko, & Ferreira, 2007). In that study, there was a marginal correlation (r = .434, p = .072) between increases in pupil diameter and accuracy on the comprehension questions. In that study, we tested spoken garden path sentences, and there were no know cases of ADHD in the sample. We take this result as evidence suggesting that performance on the comprehension questions does reflect processing that took place as the sentence was read. At this time, however, we cannot conclusively rule out an intervening process hypothesis, which could limit the ability of the task to detect group differences. In future work, we would like to investigate the online processing of garden path sentences in ADHD, using either reading time measures or pupil response.
The other difference we observed between groups was that ADHD participants were worse than were control participants in the non– garden path conditions, and these effects were not explained by variations in working memory or reading ability. We hypothesized that worse performance with the nonambiguous sentences is likely due to a greater reliance on general-world knowledge or, more specifically, on a plausibility heuristic rather than on the actual content of the sentence. The good-enough approach to language processing assumes that the language comprehension system does not use an algorithmic mechanism when determining sentence meaning (see Ferreira, Bailey, & Ferraro, 2002, for a review of good-enough processing in language comprehension). Such effects have been observed with both garden path and passive sentences. For example, Ferreira (2003) found that participants rated a sentence, such as The dog was bitten by the man, as plausible over 25% of the time. Thus, when people process a passive sentence, it seems that they use real-world knowledge to determine who did what to whom. Ferreira et al. (2002) have interpreted this pattern of results as showing that people use fast and frugal heuristics when evaluating the meaning of a sentence (Gigerenzer, & Goldstein, 1996). Moreover, Christianson et al. (2006) argued that these types of heuristics are more likely in patient groups to compensate for deficits in other domains, such as working memory. One possibility is that the ADHD group relies on this type of strategy when processing the non– garden path sentences. Alternatively, some other mechanism or process could be responsible for these differences, such as state or energetic factors (Seargeant, Oosterlann, & Van Der Meere, 1999).
Our working memory task, which followed recent suggestions by Engle et al. (1999), required the simultaneous storage and processing of information. Moreover, the processing component of this task was based on a well-validated neuropsychological task designed to measure interference control (i.e., the Stroop task; see Kane & Engle, 2003 for a review of the relationship between errors and response latencies on the Stroop task compared with other measures of working memory). The results from our task showed that ADHD participants made more errors when recalling incongruent Stroop trials from working memory. This finding suggests that the ADHD group was more susceptible to interference. However, the data did not show an increase in errors as a function of memory load because group did not interact with memory span size. Thus, these results support conceptions of working memory deficits in ADHD as an inability to effectively allocate controlled attention under conditions of interference rather than as a limitation in the number of items that can be held in memory (Unsworth & Engle, 2005). When we removed the variance due to working memory from the sentence processing results, we found that it only affected the relationship between group and garden path condition with reflexive verbs, suggesting that the ability to control interference in working memory is partially related to overcoming the temporary misinterpretation, at least with this particular class of verbs.
Even though the current study used a very large sample of community recruited participants, it was not without limitation. The first is that the sentences tested in the garden path task were somewhat long and complex. These sentences result in a large number of misinterpretations for typically developing, healthy college students. It is possible that the difficulty level of these stimuli might have masked some differences in our adolescent participants, as they do not likely have as much experience with complex syntactic structures. The second limitation is that we were only able to collect a single measure of working memory. It is often the norm to collect several different measures of working memory (e.g., reading span, operation span, or spatial span), and our study in particular could have benefited from a measure of reading span. This is especially true considering the ongoing theoretical debate about whether there are domain-specific or domain-general pools of working memory resources. The third potential limitation is related to the relationship between working memory deficits and anxiety (Eysenck, 1992; Eysenck, Payne, & Derakshan, 2005). Deficits for those with anxiety disorders are thought to include a decrease in the ability of the central executive to control attention within the two slave systems. In the current sample, participants were not excluded on the basis of current anxiety disorder, thus, it is possible that some of the differences between groups on the working memory task could be due to the inclusion of participants with anxiety problems.
In conclusion, our data suggest that ADHD does not involve deficits in cognitive inhibition but does involve deficits in working memory, not merely in short-term storage (Martinussen et al., 2005). Further, we showed some language comprehension deficits that are independent of reading ability and working memory. That result suggested a greater reliance on good-enough processing or, more specifically, on plausibility of events in the real world. The working memory findings are theoretically important in their own right because most prior studies of working memory in ADHD did not include tasks that required the simultaneous storage and processing of information. Thus, cognitive control problems in ADHD do not extend to the cognitive suppression domain but do involve interference control in working memory.
Acknowledgments
This research was supported by National Institutes of Health, National Institute of Mental Health Grant R01-MH63146 to Joel T. Nigg and Fernanda Ferreira. Paul E. Engelhardt was supported by National Science Foundation–Integrative Graduate Education and Research Traineeship Grant DGE-0114378 and by National Institutes of Health National Institute of Mental Health Grant MH-65310.
We thank the participants and families for their contribution and Colleen Schmitt and Lin Leslie for their assistance with data collection. In addition, we thank Kelly Spegel, Christian Harvey, Elizabeth Davis, and Jessica Leak for help with this study.
Appendix
Results of Reading Times for Sentences and Questions
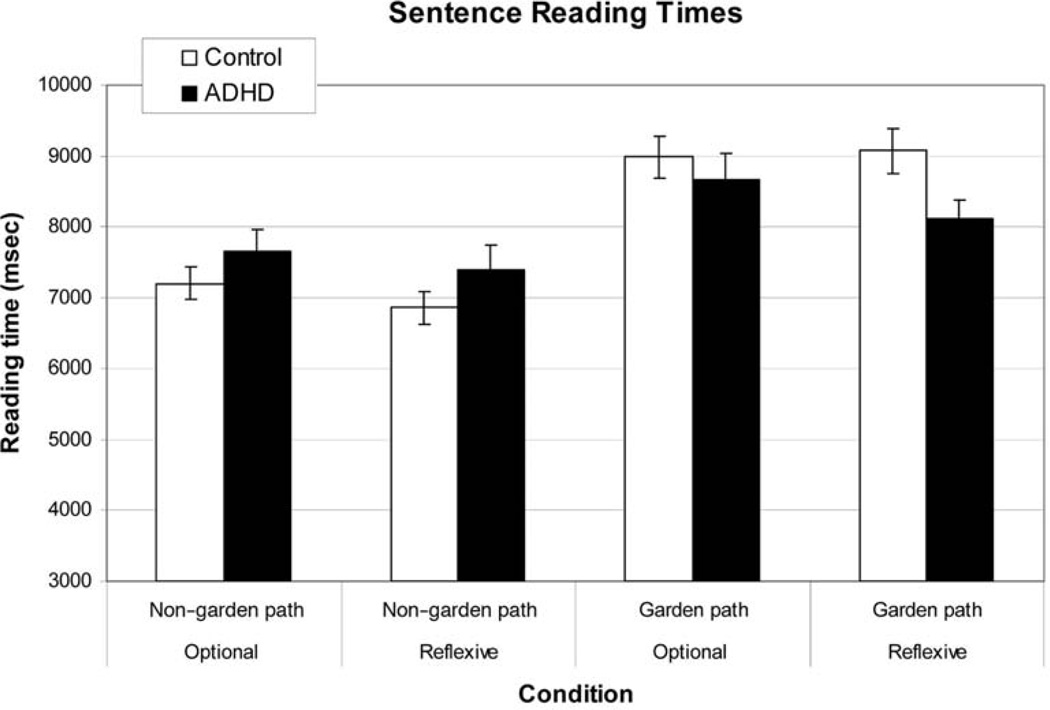
A 2 × 2 × 2 (Group × Sentence Structure × Verb Type) mixed-model ANCOVA was conducted on the sentence reading times. Note that we included the same covariates as in the accuracy data. As discussed in the Method section, participants were instructed to read the sentences for content rather than for speed. The purpose of this analysis was to ensure that there was no speed–accuracy tradeoff between groups. The results showed a main effect of sentence structure, F(1, 258) = 3.82, p = .05 (see Figure A1). In addition, there was a significant interaction between group and sentence structure, F(1, 258) = 6.40, p < .05. Paired comparisons revealed that there was a significant difference between groups with the garden path sentences, F(1, 258) = 5.03, p < .05, and no differences with the non–garden path sentences, F(1, 258) = 0.287, p > .10. Recall that the only group effect on comprehension accuracy was with non–garden path sentences. In these conditions, the ADHD group was not different from control participants and actually showed slightly greater reading times. Therefore, we can conclude that there was no speed–accuracy tradeoff with regard to sentence reading time for the non–garden path sentences. With the garden path sentences, the ADHD group was not worse than the control group on comprehension accuracy; however, this group did read the sentences more quickly, which leaves open the possibility of a speed–accuracy tradeoff. We think this is unlikely because the correlations between accuracy and sentence reading time was so low (r = −.03, for garden path– optional verb; r = .04, for the garden path–reflexive verb).
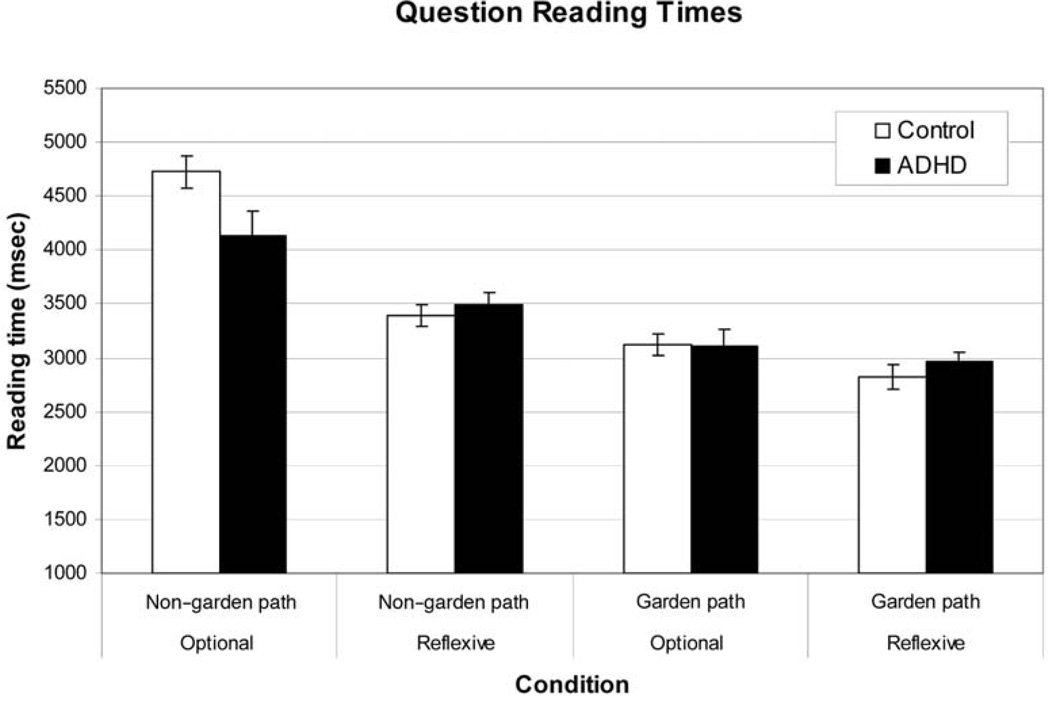
A 2 × 2 × 2 (Group × Sentence Structure × Verb Type) mixed-model ANCOVA was conducted on the question reading times (see Figure A2). Note that we included the same covariates as in the accuracy data. The results showed only a main effect of sentence structure, F(1, 258) = 7.75, p < .01. For this analysis, there was no main effect of group and no interaction involving group; therefore, we can conclude that there was no speed–accuracy tradeoff with regard to question reading time. Note that the one condition that appears to show a group effect (i.e., non–garden path/optional verb) is not significantly correlated with comprehension accuracy (r = .07; Note that data from 29 participants was lost due to technical problems with the experiment program.
Figure A1. Results showing the sentence reading times for each of the four conditions. Error bars show the standard error of the mean. ADHD = attention-deficit/hyperactivity disorder.
Figure A2. Results showing the sentence reading times for each of the four conditions. Error bars show the standard error of the mean. ADHD = attention-deficit/hyperactivity disorder.
Footnotes
By the criteria outlined by Purvis and Tannock (1997), only 2 control participants and 9 ADHD participants met reading disability criteria. We ran the analyses with these subjects in and out of the data set. The results were virtually identical; therefore, we retained them in the analysis for the sake of power.
We used a cutoff of 60% correct on the forced-choice memory task as the criterion for retaining participants in the study. Therefore, the participants reported in the Method section are those who scored 60% or higher on the memory test. In addition, we included this measure as a covariate in the sentence processing ANCOVA; it did not interact with any other variables, nor did it produce a significant main effect.
Note that 30 participants did not comply with the procedure for the working memory task (12 control participants & 18 ADHD participants). We ran the partial correlations using only the subjects who had data for both tasks.
Contributor Information
Paul E. Engelhardt, Department of Psychology, Michigan State University
Joel T. Nigg, Department of Psychology, Michigan State University
Laurie A. Carr, Department of Psychology, Michigan State University
Fernanda Ferreira, Department of Psychology, University of Edinburgh, Edinburgh, United Kingdom.
References
- Achenbach T. Manual for the Childhood Behavior Checklist. Burlington: University of Vermont, Department of Psychiatry; 1991a. [Google Scholar]
- Achenbach T. Manual for the Young Adult Self-Report and Young Adult Behavior Checklist. Burlington: University of Vermont, Department of Psychiatry; 1991b. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: Author; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text rev. Washington, DC: Author; 2000. [Google Scholar]
- August GJ, Garfinkel BD. Comorbidity of ADHD and reading disability among clinic-referred children. Journal of Abnormal Child Psychology. 1990;18:29–45. doi: 10.1007/BF00919454. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch G. Working memory. In: Ross B, editor. The psychology of learning and motivation. Vol. 8. New York: Academic Press; 1974. pp. 47–89. [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;1:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. Journal of Abnormal Psychology. 2002;111:279–289. [PubMed] [Google Scholar]
- Barkley RA, Murphy KR. Attention-deficit/hyperactivity disorder: A clinical workbook. 2nd. New York: Guilford Press; 1998. [Google Scholar]
- Beitchman JH, Young AR. Learning disorders with a special emphasis on reading disorder: A review of the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:1020–1032. doi: 10.1097/00004583-199708000-00009. [DOI] [PubMed] [Google Scholar]
- Berry CA, Shaywitz SE, Shaywitz BA. Girls with attention deficit disorder: A silent minority? A report on behavior and cognitive characteristics. Pediatrics. 1985;76:801–809. [PubMed] [Google Scholar]
- Bever TG. The cognitive basis for linguistic structures. In: Hayes JR, editor. Cognition and the development of language. Englewood Cliffs, NJ: Prentice Hall; 1970. pp. 279–362. [Google Scholar]
- Biederman J, Faraone SV. Current concepts on the neurobiology of attention-deficit/hyperactivity disorder. Journal of Attention Disorders. 2002;6:7–16. doi: 10.1177/070674370200601s03. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Keenan K, Knee D, Tsuang MT. Family genetic and psychosocial risk factors in DSM-III attention deficit disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1990;29:526–533. doi: 10.1097/00004583-199007000-00004. [DOI] [PubMed] [Google Scholar]
- Blaskey LG. Unpublished doctoral dissertation. Michigan State University; 2004. Inhibitory language deficits in attention-deficit/hyperactivity disorder and reading disorder: A candidate shared deficit. [Google Scholar]
- Brown TE. Brown Attention-Deficit Disorder Scales. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Cantwell DP. Attention-deficit disorder: A review of the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:978–987. doi: 10.1097/00004583-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Carr LA, Nigg JT, Henderson JM. Attentional versus motor inhibition in adults with attention-deficit/hyperactivity disorder. Neuropsychology. 2006;20:430–441. doi: 10.1037/0894-4105.20.4.430. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Castellanos X, Giedd J, Marsh W, Hamburger S, Schubert A, et al. Involvement of right front ostriatal circuitry in response inhibition deficits of ADHD. Journal of the American Academy for Child and Adolescent Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Christianson K, Hollingworth A, Halliwell JF, Ferreira F. Thematic roles assigned along the garden path linger. Cognitive Psychology. 2001;42:368–407. doi: 10.1006/cogp.2001.0752. [DOI] [PubMed] [Google Scholar]
- Christianson K, Williams CC, Zacks RT, Ferreira F. Younger and older adults’ ”good enough” interpretations of garden path sentences. Discourse Processes. 2006;42:205–238. doi: 10.1207/s15326950dp4202_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NJ, Davine M, Horodezky N, Lipsett L, Isaacson L. Unsuspected language impairment in psychiatrically disturbed children: Prevalence, language, and behavioral characteristics. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;32:595–603. doi: 10.1097/00004583-199305000-00016. [DOI] [PubMed] [Google Scholar]
- Conners CK, Erhardt D, Sparrow E. Adult ADHD Rating Scales: Technical manual. Toronto, Ontario, Canada: Multi-Health Systems; 1999. [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. Journal of Verbal Learning & Verbal Behavior. 1980;19:450–466. [Google Scholar]
- Dempster FN. Resistance to interference: Developmental changes in a basic processing mechanism. In: Howe ML, Pasnak R, editors. Emerging themes in cognitive development: Volume 1. Foundations. New York: Springer-Verlag; 1993. pp. 3–27. [Google Scholar]
- Denckla MB. Biological correlates of learning and attention: What is relevant to learning disability and attention-deficit/hyperactivity disorder? Developmental and Behavioral Pediatrics. 1996;17:114–119. [PubMed] [Google Scholar]
- Engelhardt PE, Patsenko EG, Ferreira F. Pupillometric indices of visual and prosodic information on spoken language comprehension. In: McNamara DS, Trafton JG, editors. Proceedings of the 29th annual conference of the Cognitive Science Society. Mahwah, NJ: Erlbaum; 2007. pp. 971–976. [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway AR. Working memory, short-term memory, and general fluid intelligence: A latent variable approach. Journal of Experimental Psychology: General. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Eysenck MW. Anxiety: The cognitive perspective. Hove, England: Erlbaum; 1992. [Google Scholar]
- Eysenck MW, Payne S, Derakshan N. Trait anxiety, visuospatial processing, and working memory. Cognition and Emotion. 2005;19:1214–1228. [Google Scholar]
- Faraone SV, Biederman J, Spencer T, Wilens T, Seidman LJ, Mick E, Doyle E. Attention-deficit/hyperactivity disorder in adults: An overview. Biological Psychiatry. 2000;48:9–20. doi: 10.1016/s0006-3223(00)00889-1. [DOI] [PubMed] [Google Scholar]
- Ferreira F. The misinterpretation of noncanonical sentences. Cognitive Psychology. 2003;47:721–749. doi: 10.1016/s0010-0285(03)00005-7. [DOI] [PubMed] [Google Scholar]
- Ferreira F, Bailey KGD, Ferraro V. Good-enough representations in language comprehension. Current Directions in Psychological Science. 2002;11:11–15. [Google Scholar]
- Gaultney JF, Kipp K, Weinstein J, McNeil J. Inhibition and mental effort in attention-deficit/hyperactivity disorder. Journal of Developmental and Physical Disabilities. 1999;11:105–114. [Google Scholar]
- Gernsbacher MA, Faust ME. The mechanism of suppression: A component of general comprehension skill. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1991;17:245–262. doi: 10.1037//0278-7393.17.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernsbacher MA, Varner KR, Faust ME. Investigating differences in general comprehension skill. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1990;16:430–445. doi: 10.1037//0278-7393.16.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigerenzer G, Goldstein DG. Reasoning the fast and frugal way: Models of bounded rationality. Psychological Review. 1996;103:650–669. doi: 10.1037/0033-295x.103.4.650. [DOI] [PubMed] [Google Scholar]
- Harnishfeger KK. The development of cognitive inhibition: Theories, definitions, and research evidence. In: Dempster FN, Brainerd CJ, editors. Interference and inhibition in cognition. New York: Academic Press; 1995. pp. 175–201. [Google Scholar]
- Harnishfeger KK, Bjorkland DF. The ontogeny of inhibition mechanisms: A renewed approach to cognitive development. In: Howe ML, Pasnak R, editors. Emerging themes in cognitive development: Volume 1. Foundations. New York: Springer-Verlag; 1993. pp. 29–49. [Google Scholar]
- Harnishfeger KK, Pope RS. Intending to forget: The development of cognitive inhibition in directed forgetting. Journal of Experimental Psychology. 1996;62:292–315. doi: 10.1006/jecp.1996.0032. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP. Academic underachievement, attention deficits, and aggression: Comorbidity and implications for intervention. Journal of Consulting and Clinical Psychology. 1992;60:893–903. doi: 10.1037//0022-006x.60.6.893. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA. A capacity theory of comprehension: Individual differences in working memory. Psychological Review. 1992;99:122–149. doi: 10.1037/0033-295x.99.1.122. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. Working memory capacity and the control of attention; The contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology: General. 2003;132:47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O. The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. American Journal of Psychiatry. 2006;163:716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J, Just MA. Individual differences in syntactic processing: The role of working memory. Journal of Memory and Language. 1991;30:580–602. [Google Scholar]
- Kipp K, Pope S. The development of cognitive inhibition in streams-of-consciousness and directed speech. Cognitive Development. 1997;12:239–262. [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychological Review. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Lorch EP, Milich R, Sanchez RP, van den Broek P, Baer S, Hooks K, Hartung C, Welsh R. Comprehension of televised stories in boys with attention-deficit/hyperactivity disorder and nonreferred boys. Journal of Abnormal Psychology. 2000;109:321–330. doi: 10.1037/0021-843X.109.2.321. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Gorfein D. Inhibition in cognition. Washington, DC: American Psychological Association; 2007. [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- McCabe DP, Robertson CL, Smith AD. Age differences in Stroop interference in working memory. Journal of Clinical and Experimental Neuropsychology. 2005;27:633–644. doi: 10.1080/13803390490919218. [DOI] [PubMed] [Google Scholar]
- Milich R, Balentine A, Lynam DR. ADHD combined type and inattentive type are distinct and unrelated disorders. Clinical Psychology: Science and Practice. 2001;8:463–488. [Google Scholar]
- Murphy K, Barkley RA. Attention-deficit/hyperactivity disorder adults: Comorbidities and adaptive impairments. Comprehensive Psychiatry. 1996;37:393–401. doi: 10.1016/s0010-440x(96)90022-x. [DOI] [PubMed] [Google Scholar]
- Nigg JT. On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and working inhibition taxonomy. Psychological Bulletin. 2000;126:220–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Is ADHD a disinhibition disorder? Psychological Bulletin. 2001;127:571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- Nigg JT. What causes ADHD? Understanding what goes wrong and why. New York: Guilford Press; 2006. [Google Scholar]
- Oram J, Fine J, Tannock R. Assessing the language of children with attention-deficit/hyperactivity disorder. American Journal of Speech-Language Pathology and Audiology. 1999;8:72–80. [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Puig-Antich J, Ryan N. The Schedule for Affective Disorders and Schizophrenia for School-Age Children (Kiddie-SADS)—1986. Pittsburg, PA: Western Psychiatric Institute and Clinic; 1986. [Google Scholar]
- Purvis K, Tannock R. Language abilities in children with attention-deficit/hyperactivity disorder, reading disabilities, and normal controls. Journal of Abnormal Child Psychology. 1997;25:133–144. doi: 10.1023/a:1025731529006. [DOI] [PubMed] [Google Scholar]
- Rashid FL, Morris MK, Morris R. Naming and verbal memory skills in adults with attention-deficit/hyperactivity disorder and reading disability. Journal of Clinical Psychology. 2001;57:829–838. doi: 10.1002/jclp.1052. [DOI] [PubMed] [Google Scholar]
- Schachar R, Tannock R, Marriott M, Logan GD. Deficient inhibitory control and attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 1995;23:411–437. doi: 10.1007/BF01447206. [DOI] [PubMed] [Google Scholar]
- Seargeant JA, Oosterlann J, Van Der Meere J. Information processing and energetic factor in attention-deficit/hyperactivity disorder. In: Quay HC, Hogan AE, editors. Handbook of disruptive behavior disorders. New York: Kluwer Academic; 1999. pp. 75–104. [Google Scholar]
- Shaywitz BA, Fletcher JM, Holahan JM, Shneider AE, Marchione KE, Stuebing KK. Interrelationships between reading disability and attention-deficit/hyperactivity disorder. Child Neuropsychology. 1995;1:170–186. [Google Scholar]
- Tannock R. Attention-deficit/hyperactivity disorder: Advances in cognitive, neurobiological, and genetic research. Journal of Child Psychology and Psychiatry. 1998;39:65–99. [PubMed] [Google Scholar]
- Tannock R, Purvis K, Schachar R. Narrative abilities in children with attention-deficit/hyperactivity disorder and normal peers. Journal of Abnormal Child Psychology. 1993;21:103–117. doi: 10.1007/BF00910492. [DOI] [PubMed] [Google Scholar]
- Tannock R, Schachar R. Executive dysfunction as an underlying mechanism of behavior and language problems in attention–deficit/hyperactivity disorder. In: Beitchman J, Cohen N, Konstantearas MM, Tannock R, editors. Language, learning, and behavior disorders. Cambridge, England: University Press; 1996. pp. 128–155. [Google Scholar]
- Tirosh E, Cohen A. Language deficit with attention-deficit disorder: A prevalent comorbidity. Journal of Child Neurology. 1998;13:493–497. doi: 10.1177/088307389801301005. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. Working memory capacity and fluid abilities: Examining the correlation between Operation Span and Raven. Intelligence. 2005;33:67–81. [Google Scholar]
- Waters GS, Caplan D. The capacity theory of sentence comprehension: Critique of Just and Carpenter (1992) Psychological Review. 1996;103:761–772. doi: 10.1037/0033-295x.103.4.761. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd. San Antonio, TX: The Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 4th. San Antonio, TX: The Psychological Corporation; 1997b. [Google Scholar]
- Wechsler D. Wechsler Individual Achievement Test. 2nd. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Weiss M, Hechtman LT, Weiss G. ADHD in adulthood: A guide to current theory, diagnosis, and treatment. Baltimore: Johns Hopkins University Press; 1999. [Google Scholar]
- Wender PH, Wolf LE, Wasserstein J. Adults with ADHD: An overview. In: Wasserstein J, Wolf LE, LeFever FF, editors. Annals of the New York Academy of Sciences: Vol 931. Adult attention deficit disorder: Brain mechanisms and life outcomes. New York: New York Academy of Sciences; 2001. pp. 1–16. [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Boada R, Ogline JS, Tunick RA, Chabildas NA, Olson RK. A comparison of the cognitive deficits in reading disability and attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2001;110:157–172. doi: 10.1037//0021-843x.110.1.157. [DOI] [PubMed] [Google Scholar]