Abstract
Microtubules (MTs) are cytoskeletal polymers composed of repeating subunits of tubulin that are ubiquitously expressed in eukaryotic cells. They undergo a stochastic process of polymerization and depolymerization from their plus ends termed dynamic instability. MT dynamics is an ongoing process in all cell types and has been the target for the development of several useful anticancer drugs, which compromise rapidly dividing cells. Recent studies also suggest that MT dynamics may be particularly important in neurons, which develop a highly polarized morphology, consisting of a single axon and multiple dendrites that persist throughout adulthood. MTs are especially dynamic in dendrites and have recently been shown to polymerize directly into dendritic spines, the postsynaptic compartment of excitatory neurons in the CNS. These transient polymerization events into dendritic spines have been demonstrated to play important roles in synaptic plasticity in cultured neurons. Recent studies also suggest that MT dynamics in the adult brain function in the essential process of learning and memory and may be compromised in degenerative diseases, such as Alzheimer’s disease. This raises the possibility of targeting MT dynamics in the design of new therapeutic agents.
DYNAMIC MICROTUBULES IN NEURONAL DENDRITES
It is difficult to comprehend how dynamic cells are at the molecular level. The cytoplasm of a living cell consists of a tightly packed assemblage of proteins, lipids, organelles, and nucleic acids in a constant dance. Certain proteins within cells—most notably tubulin, actin, intermediate filaments, and septins—have the ability to form polymeric structures that give rise to what is termed the cytoskeleton. Although the name suggests a rather stable network of filaments, the cytoskeleton is anything but (Uchida and Shumyatsky, 2015). The cytoskeleton is in constant motion through subunit exchange, assembling and disassembling via intrinsic mechanisms inherent in each type of polymer and regulated by myriad polymer-associated proteins. Many years of research on the cytoskeleton have led to the appreciation of its dynamics. Here I focus on one cytoskeletal polymer, the microtubule (MT), and discuss how MT dynamics might play an important role in brain function. I then discuss how MT dynamics in dendrites might be part of the mechanism that influences memory and the implications of this for neurodegenerative disease.
MTs are polar polymers comprising α/β-tubulin dimers that assemble end to end at centrosomes with the aid of the γ-tubulin complex (Oakley et al., 2015) to form tubules usually consisting of 13 protofilaments. The plus ends of MTs stochastically switch from growing to shrinking and back to growing via a process termed dynamic instability, and the minus ends are generally associated with the centrosome (Mitchison and Kirschner, 1984). Dynamic instability is believed to allow MTs to interrogate all regions of a cell in a very efficient manner (Mitchison and Kirschner, 1984). Although MT dynamic instability is stochastic, it is regulated by many MT-associated proteins (MAPs). Different MAPs can either stabilize or destabilize MTs and help convert growing MTs into shrinking MTs (catastrophe) or visa versa (rescue; Akhmanova and Steinmetz, 2015; Bowne-Anderson et al., 2015). Of importance, MTs also serve as the primary substrate for trafficking material throughout the cell. This transport is accomplished through activation of motor proteins. Generally speaking, kinesin motor proteins transport cargo toward the plus ends of MTs, whereas cytoplasmic dynein moves cargo toward the minus ends of MTs (Verhey et al., 2011; Cianfrocco et al., 2015).
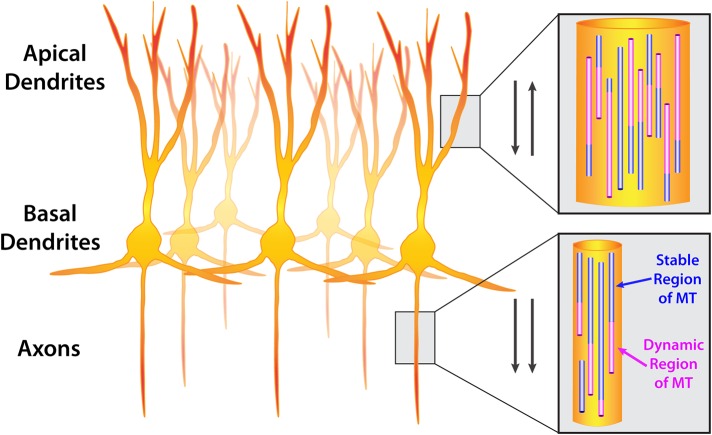
The highly elongated and polarized shape of neurons has resulted in a distinctive MT array. Instead of being attached to the centrosome, MTs are severed from centrosomes (Yu et al., 1993; Roll-Mecak and McNally, 2010) and exist in axons and dendrites as autonomous structures with a dynamic plus end and a stable minus end. Once released from the centrosome, the minus ends of MTs are stabilized by the CAMSAP/Patronin family of proteins (Marcette et al., 2014; Richardson et al., 2014; Yau et al., 2014). New research also indicates that MTs can be nucleated throughout the neuron via the augmin and γ-tubulin ring complexes (Sanchez-Huertas et al., 2016). In axons, MTs are oriented with the plus ends away from the cell body, whereas in dendrites, MTs are of a mixed polarity, with both minus and plus ends oriented toward the cell body (Baas et al., 1988; Figure 1). Neurons are postmitotic, highly polarized cells that are maintained throughout the life of an organism. In addition, neurons are particularly rich in structural MAPs that stabilize and bundle MTs (Dehmelt and Halpain, 2005; Halpain and Dehmelt, 2006). Hence it has generally been believed that the MT cytoskeleton in neurons was stable, in order to provide both structural stability and tracks on which to transport important cargoes long distances in axons and dendrites.
FIGURE 1:
Orientation of microtubules in CNS neurons. A group of neurons (cortical or hippocampal) showing apical dendrites, basal dendrites, and axons. Insets show microtubule orientation in dendrites and axons. Microtubules are composed of stable (purple) and dynamic (pink) regions. Dynamic regions undergo polymerization and depolymerization, termed dynamic instability. Arrows indicate that microtubules are oriented antiparallel in dendrites (plus and minus ends distal) and parallel in axons (plus ends distal).
Nevertheless, studies from two decades ago by Black and colleagues indicated that both dendrites and axons contain a dynamic fraction of MTs, with dendrites having preferentially more dynamic MTs (Baas et al., 1991; Brown et al., 1993). Although the plus ends of MTs were shown to be the exclusive end of the MT that incorporated tubulin dimers (Baas and Ahmad, 1992), it was not until the discovery and fluorescent labeling of +TIP proteins, which specifically label the growing ends of MTs, that we truly appreciated just how dynamic MTs could be in cells (Perez et al., 1999). The latter study, however, was conducted with an immortalized cell line. Therefore it was not obvious that MTs in primary neurons would behave in a manner similar to this cell line because of the morphological and physiological constraints of highly polarized neurons. In the first report of labeled +TIPs in neurons, an EB3–green fluorescent protein (GFP) fusion protein was used to track polymerizing MTs. Results indicated similar velocities of MT polymerization in axons and dendrites of 2– to 6–d in vitro (DIV) primary hippocampal neurons, 10- to 17-DIV Purkinje neurons, and Cos1 cells (Stepanova et al., 2003). Further studies in mature cultured primary hippocampal and cortical neurons (63DIV) demonstrated substantial EB3-GFP–labeled polymerizing MTs in dendrites (Hu et al., 2008). Of importance, recent studies showed that EB3-labeled MTs are also dynamic in cortical neuron dendrites in living brains of adult mice (Kleele et al., 2014; Yau et al., 2016). Together these data indicate that CNS neurons in culture and in vivo maintain a proportion of their dendritic MTs in a dynamic state.
None of these studies quantified the percentage of MTs in dendrites that are dynamic, which cannot be accomplished with EB3 labeling because EB3 labels only growing MTs, not stable or depolymerizing MTs. In addition, the packing of MTs within the cylindrical dendrite makes it difficult to tease out individual MTs even with superresolution microscopy techniques. Nevertheless, based on the foregoing work, it is likely that most, if not all, individual MTs in dendrites (and axons) have a stable minus end and a dynamic plus end but may differ in the length of stable and dynamic regions along individual MTs (reviewed in Baas et al., 2016). Further advances in superresolution imaging, computational methods, and correlative electron microscopy may have to be implemented to determine the dynamic/stable state of the population of MTs in mature neurons.
MICROTUBULE DYNAMICS IN LEARNING AND MEMORY
The fact that MTs are dynamic in mature neurons raises the question of what function their dynamics might serve in a highly polarized and relatively stable neuronal structure. One possibility is that neurons, like all cells in the body, must respond to changes that occur within the organism, and MT dynamics may be an important factor in this cellular plasticity. Plasticity is especially important for neurons because they must maintain their extreme polarity but also undergo changes throughout the life span of the organism. Given that most neurons in the mammalian brain are postmitotic, one area where plastic changes occur is through the synapse, the junction between the presynaptic axonal bouton and the postsynaptic dendritic spine (Figure 2). Morphological and molecular changes to the synapse, both presynaptically and postsynaptically, are widely regarded as the substrate for learning and memory (Xu et al., 2009; Yang et al., 2009; Hubener and Bonhoeffer, 2010). Thus the plasticity of dendritic spines undoubtedly plays a key role in the proper functioning of the brain, and defects in spine plasticity are often associated with disease and neurodegeneration (Sala and Segal, 2014).
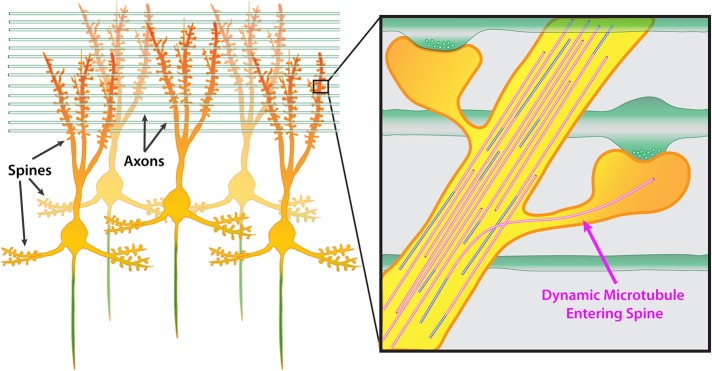
FIGURE 2:
Microtubules are capable of polymerizing into dendritic spines. A group of mature CNS neurons showing dendritic spines located along both apical and basal dendrites. The axon is colored green. Green horizontal rods indicate axons from other neurons growing perpendicular to apical dendrites. Inset shows two spines along a dendrite synapsing onto two perpendicular axons. Presynaptic vesicles are shown in axons. In this example, one dynamic microtubule polymerizes into the right dendritic spine, extending well into the head of the spine.
Several recent studies have begun to suggest that MT dynamics in dendrites play important roles in brain function and disease. Long-term potentiation (LTP), a stimulation protocol that mimics memory formation ex vivo and can modify memories in living mice (Nabavi et al., 2014), is affected by changes in MT dynamics. Pharmacological stabilization of MTs with paclitaxel (Shumyatsky et al., 2005) or inhibition of MT polymerization with nanomolar (Jaworski et al., 2009) or micromolar (Barnes et al., 2010) concentrations of nocodazole abrogates LTP. One important caveat of these studies is that they all used bath application of MT drugs, which affects both axonal (presynaptic) and dendritic (postsynaptic) MTs, as well as glial MTs. It is therefore not possible to determine exactly what MT-dependent processes affect LTP. Nevertheless, these data suggest that MTs might play a key role in memory formation or retention.
Studying MT dynamics directly in living animals is exceedingly difficult; however, ingenious methods have been used to determine whether MT dynamics function in learning and memory in the intact organism. By knocking out stathmin, a protein that binds tubulin and inhibits MT polymerization, Shumyatsky et al. (2005) demonstrated that affecting MT stability results in deficits in LTP, in addition to both learned and innate fear in mice. A subsequent study by another group implemented a stable isotope method to label newly synthesized tubulin, which is incorporated into MTs undergoing dynamic instability (Fanara et al., 2010). By purifying the tau-associated (a proxy for axonal MTs) and MAP2-associated (a proxy for dendritic MTs) MT fractions, as well as cold-stable MT fractions (hyperstable MTs generally only present in neurons), this group showed that contextual fear conditioning resulted in increased turnover of the MAP2 and cold-stable fractions (Fanara et al., 2010). Inhibition of MT polymerization by nocodazole injection inhibited freezing in the contextual fear paradigm, whereas inclusion of paclitaxel (to stabilize MTs) or brain-derived neurotrophic factor (BDNF) rescued freezing. Similarly, nocodazole inhibited the increase in spine density in hippocampus and cortex after contextual fear conditioning, and these effects were rescued by paclitaxel or BDNF. Although it is not possible to pinpoint directly which cells and what subcellular changes in MT stability occur in this study, it is still intriguing that disrupting MTs can affect learning and memory.
More recent evidence, focused on a specific MT-associated protein, showed that learning can induce a stathmin phosphorylation–dependent biphasic change in MT stability (Uchida et al., 2014). Using contextual fear conditioning, followed by synaptosomal preparations and quantitative blotting, this group showed that there was an increase in tyrosinated tubulin, indicative of new/dynamic MTs, 30 min after training, followed by an increase in detyrosinated tubulin, indicative of older/stable MTs, 8 h after training. Of interest, they could pharmacologically disrupt memory formation by intrahippocampal injection of paclitaxel in the early phase and inhibit or enhance the late phase of MT hyperstability by injecting nocodazole or paclitaxel, respectively. Furthermore, they showed that stathmin regulates transport of the GluA2 subunit of the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor via KIF5, resulting in increased GluA2 at synaptic sites, which promoted long-term memory. This idea of an early phase in which MTs are hyperdynamic, followed several hours later by a phase in which MTs are hyperstable is intriguing and suggests, like the Fanara et al. (2010) study, that manipulating MT dynamics can affect learning and memory.
MICROUBULE DYNAMICS AND DENDRITIC SPINES
If dendritic spine plasticity and MT dynamics are both important for learning and memory, is there a direct connection between MTs and spines? It is well established that MTs form a dense, overlapping array in the dendrite shaft. In fact, one would be hard pressed to find a single MT entering a dendritic spine in fixed cultures either by fluorescence light microscopy or electron microscopy (EM), whereas actin filaments are highly concentrated within dendritic spines but sparse within dendrite shafts. Previous research corroborated the idea that these cytoskeletal elements maintain separate domains within the dendrite. Early studies using fluorescently labeled actin and the MT-associated protein MAP2C in both live and fixed hippocampal neurons found that these two cytoskeletal elements did not overlap (Kaech et al., 1997, 2001). Similarly, EM studies demonstrated that MTs could enter the complex—branched CA3 spines that synapse onto mossy fibers in the hippocampus (Chicurel and Harris, 1992)—but this seemed to be the exception rather than the rule. In addition, brains from perfused animals never contained MTs in dendritic spines (Fiala et al., 2003). Early EM studies did document the presence of MTs in dendritic spines of cortical neurons, but these studies were discounted based on the unusual technique of dissecting out brain sections into 20% bovine serum albumin/water before fixation (Westrum et al., 1980, 1983; Gray et al., 1982). Thus the bulk of data favored the interpretation that MTs and actin filaments did not overlap, suggesting the transport of any cargo by kinesin and dynein along MTs in the dendrite shaft must be “handed off” to actin filaments below the base of spines to be transported via myosin into the spine (Ryan et al., 2005; Guillaud et al., 2008).
Could these studies have missed otherwise dynamic MTs interacting with actin filaments in dendritic spines? Recent data from several labs suggest that this is in fact the case. Four independent studies published within a year of one another demonstrated that MTs indeed enter spines in a synaptic activity–dependent manner (Gu et al., 2008; Hu et al., 2008; Mitsuyama et al., 2008; Jaworski et al., 2009). It is also clear, as demonstrated by live-cell microscopy, that all of the MT polymerization events into spines are transitory, averaging only a few minutes at a time (Hu et al., 2008, 2011; Jaworski et al., 2009; Kapitein et al., 2011; Merriam et al., 2011, 2013; Wagner et al., 2011; McVicker et al., 2016). Moreover, the presence of MTs in spines in fixed neurons required rapid fixation and the use of MT-stabilizing buffer (Gu et al., 2008; Hu et al., 2008; Mitsuyama et al., 2008). Thus the prevalence of dynamic MTs in dendrites allows them to specifically polymerize into individual spines throughout the life of the neuron. Such behavior by dendritic MTs opens up the exciting possibility that they could be directly influencing selected dendritic spines through the host of proteins that associate with actively polymerizing MTs and through the cargo transported along these MTs into and out of dendritic spines.
Why did previous studies fail to detect these dynamic MTs? In regard to the live-cell imaging studies mentioned earlier, overexpression of MAP2C may have stabilized dendritic MTs. Furthermore, the sensitivity of the camera and the infrequent imaging interval that was used also may have limited the likelihood of detecting these dynamic MTs (Kaech et al., 1997, 2001). The more recent studies used GFP-labeled +TIP proteins (EB3) and/or tubulin, which provided enhanced signal-to-noise ratio to detect MT dynamics, and, unlike classical MAPs, would not overstabilize MTs. In addition, standard EM techniques may be insufficient for detecting MTs in dendritic spines. As mentioned earlier, each MT in a mature dendrite is likely to have a stable region at the minus end and a dynamic region at the plus end. Perfusion of brains in preparation for EM requires time for the fixative to perfuse through the capillaries and into brain tissue. It is likely that during this time, the most dynamic regions of MTs will depolymerize. Thus standard animal perfusion protocols will cause depolymerization of the most dynamic ends of MTs and result in the lack of MTs in spines in electron micrographs, even though the more stable sections of MTs appear in the dendritic shaft. It is unclear whether perfusing brains with MT-stabilizing fixative would be sufficient to preserve dynamic MTs entering spines. Future studies using two-photon confocal microscopy and cranial window imaging of fluorescently labeled MTs in neurons in the intact brain will have to be performed to determine whether MTs enter dendritic spines in living animals.
HOW DO MICROTUBULES ENTER SPINES?
The dynamicity of MTs in dendrites does not necessarily predict that they would polymerize into dendritic spines. After all, dendritic spines are generally oriented perpendicular to the dendrite shaft, and the necks of spines are an order of magnitude thinner than the parent dendrite, with diameters ranging from 50 to 250 nm (Bourne and Harris, 2008; Tonnesen et al., 2014). Moreover, MTs generally polymerize in a relatively straight line, unless they encounter organelles or other noncompliant structures within cells. It is likely that MTs that enter dendritic spines polymerize from a stable portion of an existing MT. However, this would require the correct orientation (perpendicular to the dendrite shaft) of the polymerization event. When Merriam et al. (2013) recorded plus-end MT dynamics at rapid intervals with the +TIP protein EB3, they found that most of the MTs that polymerized into dendritic spines originated within 2 μm of the spine they invaded, suggesting that their entry into spines was locally regulated. Indeed, both a spike of calcium within the spine head and neck and subsequent actin polymerization were required for MT polymerization into spines (Merriam et al., 2013; Figure 3). Therefore MTs appear to specifically target spines that are undergoing activity-dependent changes in a regulated manner rather than polymerizing into a small subset of spines at random.
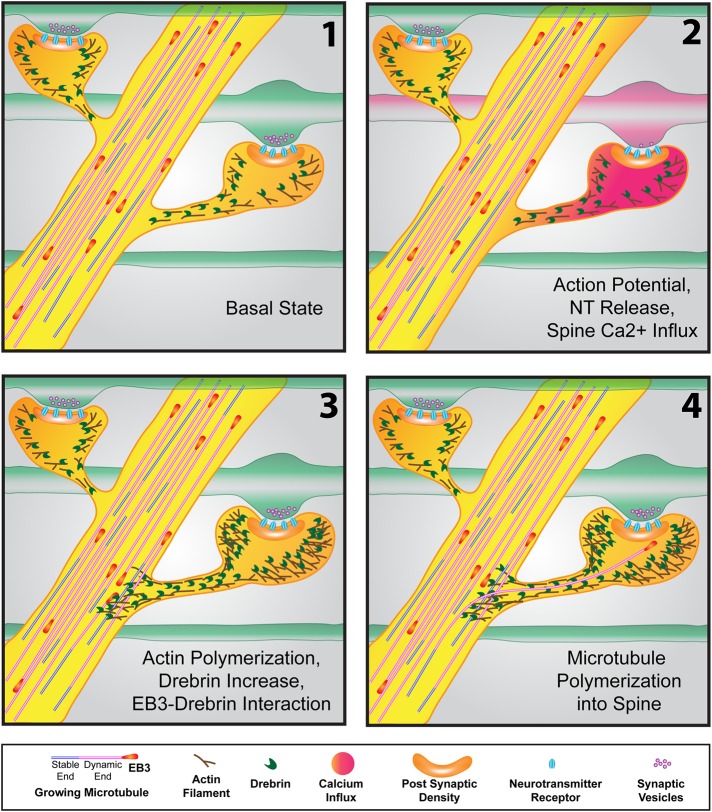
FIGURE 3:
Events resulting in microtubule polymerization into a dendritic spine. (1) In the basal state, microtubules, tipped by “comets” of +TIP proteins (EB3 shown here), actively polymerize throughout the dendrite (as well as the axon; not shown). Actin and the actin-binding protein drebrin are concentrated in spines. (2) As the action potential makes its way down an axon (red in second frame), resulting in neurotransmitter release at the synapse, calcium influx in the postsynaptic spine occurs. (3) Within seconds to minutes after calcium spikes in the spine, actin polymerization occurs in the spine head and neck and can extend into the dendritic shaft. The spine head increases in size due to the increased actin polymerization. Increased actin also concentrates drebrin, which interacts with EB3 protein at the tips of polymerizing microtubules in the vicinity of the spine. (4) This drebrin–EB3 interaction results in the increased probability that the polymerizing microtubule will enter the spine (on the right). The microtubule can extend well into the spine head, often to the postsynaptic density.
Actin polymerization in the spine neck and protrusion into the dendrite shaft might provide a sufficient “gate” to allow MTs polymerizing near a spine to follow the actin filaments into the spine (Figure 3). There is precedent for actin filaments interdigitating with dendritic MTs in platinum replica electron micrographs (Korobova and Svitkina, 2010), potentially providing a substrate upon which MTs could enter spines. Although MTs may polymerize along actin filaments, the interaction is unlikely to be direct. Instead, actin–MT interactions occur through many different actin- and MT-associated proteins (Coles and Bradke, 2015; Cammarata et al., 2016). The +TIP protein EB3, upon entering spines at the tip of polymerizing MTs, stabilizes the actin-associated protein p140Cap in the postsynaptic density (Jaworski et al., 2009). In a separate study, EB3 and the actin-bundling protein drebrin were found to interact in developing axonal growth cones (Geraldo et al., 2008). Drebrin is highly expressed in dendritic spines and is enhanced in the hippocampus after LTP (Fukazawa et al., 2003; Ivanov et al., 2009). Recent experiments in dendrites confirmed that drebrin was a key actin-associated protein involved in MT polymerization into spines (Merriam et al., 2013; Figure 3). Thus there is likely to be a complex interplay among actin- and MT-associated proteins in the spine neck and head. Of importance, polymerizing MTs must be recruited into specific dendritic spines rather than stochastically polymerizing into any spine. This recruitment of MTs into spines involves N-methyl-d-aspartate–dependent calcium influx, signaling cascades resulting in actin polymerization, and interaction of MT +TIP proteins (EB3) with actin-associated proteins in the spine neck (drebrin) and head (p140Cap; Jaworski et al., 2009; Merriam et al., 2011, 2013; McVicker et al., 2015). Further research will be required to determine whether other proteins associated with actin–MT interactions are involved in MT entry of spines.
Once the MT enters a spine, it is only present for a short period of time, depolymerizing out of the spine on the order of a few minutes (Hu et al., 2008). MTs can target the same spine multiple times, but each entry is transient. Moreover, MTs target spines of different shapes, including mushroom, thin, and stubby spines, as well as dendritic filopodia (Gu et al., 2008; Hu et al., 2008; Jaworski et al., 2009). It is not known whether MTs can polymerize into all spines on a dendritic tree or certain spines are specifically targeted but others are not. Nevertheless, synaptic activity influences MT polymerization into dendritic spines in a direct manner. More synaptic activity results in more spines targeted by MTs (Hu et al., 2008). Moreover, the time that MTs remain in spines can be lengthened by addition of BDNF (Hu et al., 2011), and the frequency of polymerization into spines and the percentage of spines targeted increases after the induction of LTP (Merriam et al., 2011) and decreases after induction of long-term depression (LTD; Kapitein et al., 2011). Because MTs are the primary cytoskeletal polymers that transport cargo within cells and LTP increases spine size but LTD decreases spine size (Tada and Sheng, 2006), it is possible that MTs may transport specific cargoes into or out of dendritic spines during synaptic plasticity.
WHAT CARGOES ARE MICROTUBULES TRANSPORTING INTO OR OUT OF SPINES?
There are a number of ways in which material could enter dendritic spines (Figure 4). Transmembrane receptors or proteins that are associated with these receptors can be exocytosed in the dendrite shaft and diffuse into the spine in the plane of the membrane (Gray et al., 2006; Huganir and Nicoll, 2013). Cytoplasmic proteins can diffuse into spines from the dendrite shaft (Rose et al., 2009). Both of these mechanisms would be efficient for entry of material into spines but may not provide a very targeted way for cargo to enter particular spines that are undergoing plastic changes versus adjacent spines that are not being potentiated.
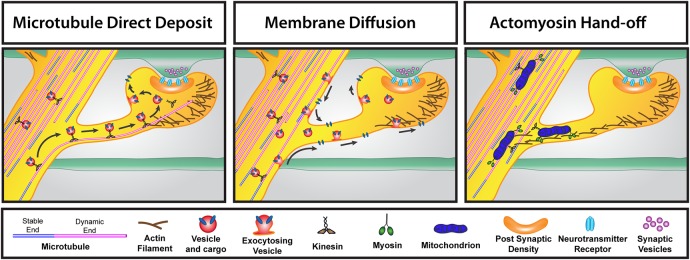
FIGURE 4:
Three different routes for transporting material into dendritic spines. Left, the microtubule direct deposit model. During the time that a microtubule has polymerized into a dendritic spine, kinesin-based transport of vesicles containing cargo enter the spine along the microtubule. Release of the motor from the microtubule leads to exocytosis of the vesicle and cargo in the spine head. Middle, the membrane diffusion model. Kinesin-based transport results in movement of vesicles and cargo in the dendrite. Vesicles exocytose in the dendrite shaft and spine and diffuse in the plane of the membrane throughout the dendritic spine. Right, the actomyosin-based hand-off model of transport into spines. In this example, mitochondria are transported throughout the dendrite via kinesin-based transport but also have myosin motors attached. A hand-off occurs between kinesin–microtubule transport to actin-myosin–based transport, resulting in transport of mitochondria into the dendritic spine. Endoplasmic reticulum is transported into spines via this mechanism as well.
To circumvent this lack of specificity, it is possible that cargo could associate with both MT- and actin-based motor proteins. For example, cargo could be shuttled anterogradely in a dendrite via the motor protein kinesin or dynein, given that dendrites contain MTs of mixed polarity (Baas et al., 1988). However, once the cargo reaches the spine for which it is destined, the MT-based motor could “hand off” the cargo for transport via a myosin-based motor along the actin filaments at the base or in the neck of the spine (Figure 4). The cargo would then use myosin motors to enter the dendritic spine. In fact, this type of hand-off is likely to occur for the entry of endoplasmic reticulum (ER) into dendritic spines. A study showed that in cerebellar Purkinje neurons, ER enters dendritic spines via myosin-V–based transport (Wagner et al., 2011). Surprisingly, although the ER is intimately associated with MTs in the dendrite shaft, MT entry of Purkinje spines was not associated with ER entry, whereas knockdown of myosin-V abolished ER entry (Wagner et al., 2011). Studies in our lab using hippocampal neurons confirmed that ER entry of spines is not a MT-based process (unpublished data). Moreover, our recent work suggests that mitochondria may also be using this actomyosin hand-off model to enter dendritic spines (McVicker et al., 2016; Figure 4). This raises a question: if MTs are not transporting ER into spines, then what are the potential cargoes entering spines along MT tracks?
Given that one of the primary functions of MTs in all cells is to act as railways for the transport of cargo throughout the cell, it is likely that transient MT polymerization into dendritic spines acts as a path for the delivery and/or removal of cargo that play a role in spine plasticity. However, MT polymerization into spines is transient, with a dwell time of only a few minutes. Is this enough time to transport cargo into or out of spines? The likely answer is yes. Both kinesin and dynein motor proteins can move at speeds of ∼1 μm/s (Hammond et al., 2009), and dendritic spines are only a few micrometers long. Thus, if a MT is present in a spine for only a few seconds, the speed of dynein and kinesin-based transport would be sufficient to transport material either into or out of spines along a MT.
What cargoes might use MTs to enter or exit spines? PSD-95 is an important postsynaptic scaffolding protein that is directly associated with synaptic strength (El-Husseini et al., 2000; Ehrlich et al., 2007) and is increased in spines after BDNF treatment (Yoshii and Constantine-Paton, 2007). Hu et al. (2011) showed that although PSD-95 was not directly trafficked along MTs into dendritic spines, polymerization of MTs into spines was necessary for the increase in PSD-95 after BDNF treatment. A more recent study showed that recycling endosomes could use MTs to enter dendritic spines, but their primary method of entry was through myosin-V–based transport (Esteves da Silva et al., 2015). Thus a particular cargo has yet to be demonstrated to specifically use MTs to enter dendritic spines.
Given that MTs polymerize into spines plus end leading, it is likely that kinesin motor proteins will convey cargo into spines and cytoplasmic dynein will convey cargo out of spines along MTs. One study showed cytoplasmic dynein and neuroligin leaving a dendritic spine together, but MTs were not imaged simultaneously (Schapitz et al., 2010). These data suggest that dynein can transport neuroligin out of spines along MTs, but definitive proof of motor/cargo transport out of spines along MTs will require simultaneous imaging with tubulin. However, a recent study documented a motor/cargo pair that uses MTs for entering dendritic spines. McVicker et al. (2016) showed that the kinesin 3 motor KIF1A transports the synaptotagmin (syt) family member syt4 directly into spines along dynamic MTs. Once the syt4-containing vesicle enters a spine, it can exocytose in the spine head (Figure 4). Entry of this KIF1A/syt4 motor/cargo pair is dependent on synaptic activity and is regulated by homeostatic plasticity. Moreover, this work showed that knockdown of KIF1A paradoxically results in increased syt4 entry and exocytosis in dendritic spines, as well as in the dendrite shaft. This increase in cargo exocytosis appears to occur because the syt4-containing vesicles are no longer tethered to the MTs in the dendrite shaft via KIF1A. The increased exocytosis that occurs throughout the dendritic arbor is followed by movement of exocytosed syt4 within the plane of the membrane. Together these results indicate that MTs are indeed capable of transporting specific cargo into targeted dendritic spines, and, of importance, this directed targeting of cargo is highly disrupted in the absence of a particular motor protein, KIF1A (McVicker et al., 2016). Thus not only are MTs important for transporting material throughout the dendritic arbor, but they are also instrumental in sequestering vesicles and cargo away from the plasma membrane while it is en route to its destination.
IMPLICATIONS FOR DISEASE AND NEURODEGENERATION
The aforementioned studies suggest that MTs change their dynamics in dendrites during episodes of learning and memory and that pharmacological manipulation of MT dynamics can have a pronounced effect by either enhancing or disrupting the process of learning and memory. If this is the case, then it follows that diseases that are known to affect MTs or microtubule-associated proteins (MAPs) in dendrites may be amenable to therapeutics that target MT dynamics. Indeed, paclitaxel has been used extensively in treatment of multiple types of cancers and is beginning to be used for nerve injury (Baas and Ahmad, 2013). Unfortunately, paclitaxel treatment results in peripheral neuropathy (Gornstein and Schwarz, 2014) and does not easily cross the blood–brain barrier (Fellner et al., 2002). However, new MT-stabilizing agents have been developed with more desirable properties (Brunden et al., 2014).
One drug that has been tested in a number of animal models is epothilone D (EpoD; BMS-231027; Kolman, 2004). EpoD has been shown to be much more brain penetrant than paclitaxel (Andrieux et al., 2006; Brunden et al., 2010). Moreover, since several neurodegenerative and psychiatric diseases, including Alzheimer’s, Parkinson’s, amyotrophic lateral sclerosis, schizophrenia, and depression, appear to have etiologies associated with destabilization of MTs, drugs that are capable of entering the brain and stabilizing MTs might have a use as therapeutic agents (Brunden et al., 2014). For example, several recent studies have shown that EpoD is effective in ameliorating axonal dysfunction, neurotoxicity, cognitive deficits, and pathology in two different Alzheimer’s mouse models (Brunden et al., 2010; Barten et al., 2012; Zhang et al., 2012). These results suggest that EpoD may provide important therapeutic benefits for human patients.
To test the therapeutic potential of EpoD, a phase 1 clinical trial was conducted in 2012–2013 by Bristol-Myers Squibb to evaluate the effects of EpoD on cerebral spinal fluid (CSF) biomarkers in patients with mild Alzheimer’s disease (clinicaltrials.gov/ct2/show/NCT01492374). One of the issues with EpoD and other MT-stabilizing drugs such as paclitaxel concerns the adverse side effects of the drug when given at doses effective for cancer treatment, which include neutropenia and peripheral neuropathy (Brogdon et al., 2014). However, for this study, EpoD was dosed less frequently and at lower concentrations than in previous cancer therapy trials and was generally well tolerated by patients. Unfortunately, EpoD showed little change in CSF biomarkers (tau N-terminal fragments) during the 9-wk dosing regimen, and further clinical trials were abandoned. In studies with Alzheimer’s model mice, EpoD took 11 wk to inhibit incorporation of newly synthesized tubulin into MTs (an indirect measure of MT dynamics) and reduce tau pathology (Barten et al., 2012). Thus it is unlikely that efficacy in humans would have been detected in only 9 wk in the clinical trial of EpoD. Several other MT-stabilizing compounds, including CNDR-51657 (Kovalevich et al., 2016) and dictyostatin (Makani et al., 2016), continue to be evaluated in Alzheimer’s disease mouse and cell models, with some encouraging results. However, it is unclear whether stabilizing MTs will prove to be an effective therapy for diseases such as Alzheimer’s.
Although stabilizing otherwise hyperdynamic MTs in Alzheimer’s disease may prove to be an effective treatment in the future, it is important to consider that MT dynamics in all cell types in the body will be affected by systemic administration of a pharmacological compound like EpoD. Therefore systemic treatments need to be well tolerated and not induce other toxic side effects (e.g., peripheral neuropathy, neutropenia; Baas and Ahmad, 2013). Moreover, it is unclear how MT-stabilizing compounds affect MT dynamics in other cells of the brain, including astrocytes, oligodendrocytes, and microglia. Because MTs in glial cells polymerize faster than neurons (Stepanova et al., 2003) and have fewer markers for long-lived MTs, such as detyrosination and acetylation (unpublished data), it is likely that MT dynamics in glial cells will be substantially affected by such drugs, which may also affect brain function.
CONCLUSIONS
This is an exciting time in the field of cytoskeletal dynamics. Given that a portion of MTs remain dynamic for the life of the neuron and are capable of polymerizing into dendritic spines raises new questions of how MT dynamics may affect fundamental processes in the brain, such as learning and memory. Moreover, data indicating that MT dynamics is compromised in developmental or neurodegenerative diseases suggest novel avenues for intervention. Nevertheless, we should proceed cautiously when administering potential therapeutic agents that affect MT dynamics. Although neuronal MTs may be more labile in diseases such as Alzheimer’s, stabilization of MTs must be carefully controlled so as not to overstabilize these polymers, which will likely compromise learning and memory. Going forward, it will also be important to understand how such pharmacological agents affect MT dynamics in other cells of the brain and how stabilizing MTs in these cells affects their functions. Although MTs and their inherent dynamics may prove to be useful targets for therapeutic agents, MT dynamic instability may prove to be too broad of a target. Instead, it might be more prudent to devote time and effort to designing compounds that target specific MT-associated proteins, which may provide more precise control and have fewer side effects. Clearly, a more thorough understanding of the interplay between MT dynamics, MAPs, and other cellular components will provide an essential foundation on which to build our knowledge and pursue the most efficacious interventions.
Acknowledgments
I thank members of the Dent lab for insightful discussions and critiques. This work was supported by grants from the National Institutes of Health (R01NS080928, R01NS098372).
Abbreviations used:
- BDNF
brain-derived neurotrophic factor
- DIV
days in vitro
- EpoD
epothilone D, BMS-241027
- ER
endoplasmic reticulum
- LTD
long-term depression
- LTP
long-term potentiation
- MAP
microtubule-associated protein
- MT
microtubule.
Footnotes
REFERENCES
- Akhmanova A, Steinmetz MO. Control of microtubule organization and dynamics: two ends in the limelight. Nat Rev Mol Cell Biol. 2015;16:711–726. doi: 10.1038/nrm4084. [DOI] [PubMed] [Google Scholar]
- Andrieux A, Salin P, Schweitzer A, Begou M, Pachoud B, Brun P, Gory-Faure S, Kujala P, Suaud-Chagny MF, Hofle G, Job D. Microtubule stabilizer ameliorates synaptic function and behavior in a mouse model for schizophrenia. Biol Psychiatry. 2006;60:1224–1230. doi: 10.1016/j.biopsych.2006.03.048. [DOI] [PubMed] [Google Scholar]
- Baas PW, Ahmad FJ. The plus ends of stable microtubules are the exclusive nucleating structures for microtubules in the axon. J Cell Biol. 1992;116:1231–1241. doi: 10.1083/jcb.116.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Ahmad FJ. Beyond taxol: microtubule-based treatment of disease and injury of the nervous system. Brain. 2013;136:2937–2951. doi: 10.1093/brain/awt153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Deitch JS, Black MM, Banker GA. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci USA. 1988;85:8335–8339. doi: 10.1073/pnas.85.21.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Rao AN, Matamoros AJ, Leo L. Stability properties of neuronal microtubules. Cytoskeleton (Hoboken) 2016;73:442–460. doi: 10.1002/cm.21286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Slaughter T, Brown A, Black MM. Microtubule dynamics in axons and dendrites. J Neurosci Res. 1991;30:134–153. doi: 10.1002/jnr.490300115. [DOI] [PubMed] [Google Scholar]
- Barnes SJ, Opitz T, Merkens M, Kelly T, von der Brelie C, Krueppel R, Beck H. Stable mossy fiber long-term potentiation requires calcium influx at the granule cell soma, protein synthesis, and microtubule-dependent axonal transport. J Neurosci. 2010;30:12996–13004. doi: 10.1523/JNEUROSCI.1847-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barten DM, Fanara P, Andorfer C, Hoque N, Wong PY, Husted KH, Cadelina GW, Decarr LB, Yang L, Liu V, et al. Hyperdynamic microtubules, cognitive deficits, and pathology are improved in tau transgenic mice with low doses of the microtubule-stabilizing agent BMS-241027. J Neurosci. 2012;32:7137–7145. doi: 10.1523/JNEUROSCI.0188-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowne-Anderson H, Hibbel A, Howard J. Regulation of microtubule growth and catastrophe: unifying theory and experiment. Trends Cell Biol. 2015;25:769–779. doi: 10.1016/j.tcb.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogdon CF, Lee FY, Canetta RM. Development of other microtubule-stabilizer families: the epothilones and their derivatives. Anticancer Drugs. 2014;25:599–609. doi: 10.1097/CAD.0000000000000071. [DOI] [PubMed] [Google Scholar]
- Brown A, Li Y, Slaughter T, Black MM. Composite microtubules of the axon: quantitative analysis of tyrosinated and acetylated tubulin along individual axonal microtubules. J Cell Sci. 1993;104:339–352. doi: 10.1242/jcs.104.2.339. [DOI] [PubMed] [Google Scholar]
- Brunden KR, Trojanowski JQ, Smith AB, 3rd, Lee VM, Ballatore C. Microtubule-stabilizing agents as potential therapeutics for neurodegenerative disease. Bioorg Med Chem. 2014;22:5040–5049. doi: 10.1016/j.bmc.2013.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunden KR, Zhang B, Carroll J, Yao Y, Potuzak JS, Hogan AM, Iba M, James MJ, Xie SX, Ballatore C, et al. Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J Neurosci. 2010;30:13861–13866. doi: 10.1523/JNEUROSCI.3059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarata GM, Bearce EA, Lowery LA. Cytoskeletal social networking in the growth cone: how +TIPs mediate microtubule-actin cross-linking to drive axon outgrowth and guidance. Cytoskeleton (Hoboken) 2016;73:461–476. doi: 10.1002/cm.21272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicurel ME, Harris KM. Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. J Comp Neurol. 1992;325:169–182. doi: 10.1002/cne.903250204. [DOI] [PubMed] [Google Scholar]
- Cianfrocco MA, DeSantis ME, Leschziner AE, Reck-Peterson SL. Mechanism and regulation of cytoplasmic dynein. Annu Rev Cell Dev Biol. 2015;31:83–108. doi: 10.1146/annurev-cellbio-100814-125438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CH, Bradke F. Coordinating neuronal actin-microtubule dynamics. Curr Biol. 2015;25:R677–R691. doi: 10.1016/j.cub.2015.06.020. [DOI] [PubMed] [Google Scholar]
- Dehmelt L, Halpain S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005;6:204. doi: 10.1186/gb-2004-6-1-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Klein M, Rumpel S, Malinow R. PSD-95 is required for activity-driven synapse stabilization. Proc Natl Acad Sci USA. 2007;104:4176–4181. doi: 10.1073/pnas.0609307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Esteves da Silva M, Adrian M, Schatzle P, Lipka J, Watanabe T, Cho S, Futai K, Wierenga CJ, Kapitein LC, Hoogenraad CC. Positioning of AMPA receptor-containing endosomes regulates synapse architecture. Cell Rep. 2015;13:933–943. doi: 10.1016/j.celrep.2015.09.062. [DOI] [PubMed] [Google Scholar]
- Fanara P, Husted KH, Selle K, Wong PY, Banerjee J, Brandt R, Hellerstein MK. Changes in microtubule turnover accompany synaptic plasticity and memory formation in response to contextual fear conditioning in mice. Neuroscience. 2010;168:167–178. doi: 10.1016/j.neuroscience.2010.03.031. [DOI] [PubMed] [Google Scholar]
- Fellner S, Bauer B, Miller DS, Schaffrik M, Fankhanel M, Spruss T, Bernhardt G, Graeff C, Farber L, Gschaidmeier H, et al. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J Clin Invest. 2002;110:1309–1318. doi: 10.1172/JCI15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Kirov SA, Feinberg MD, Petrak LJ, George P, Goddard CA, Harris KM. Timing of neuronal and glial ultrastructure disruption during brain slice preparation and recovery in vitro. J Comp Neurol. 2003;465:90–103. doi: 10.1002/cne.10825. [DOI] [PubMed] [Google Scholar]
- Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38:447–460. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- Geraldo S, Khanzada UK, Parsons M, Chilton JK, Gordon-Weeks PR. Targeting of the F-actin-binding protein drebrin by the microtubule plus-tip protein EB3 is required for neuritogenesis. Nat Cell Biol. 2008;10:1181–1189. doi: 10.1038/ncb1778. [DOI] [PubMed] [Google Scholar]
- Gornstein E, Schwarz TL. The paradox of paclitaxel neurotoxicity: mechanisms and unanswered questions. Neuropharmacology. 2014;76:175–183. doi: 10.1016/j.neuropharm.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Gray EG, Westrum LE, Burgoyne RD, Barron J. Synaptic organisation and neuron microtubule distribution. Cell Tissue Res. 1982;226:579–588. doi: 10.1007/BF00214786. [DOI] [PubMed] [Google Scholar]
- Gray NW, Weimer RM, Bureau I, Svoboda K. Rapid redistribution of synaptic PSD-95 in the neocortex in vivo. PLoS Biol. 2006;4:e370. doi: 10.1371/journal.pbio.0040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Firestein BL, Zheng JQ. Microtubules in dendritic spine development. J Neurosci. 2008;28:12120–12124. doi: 10.1523/JNEUROSCI.2509-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaud L, Wong R, Hirokawa N. Disruption of KIF17-Mint1 interaction by CaMKII-dependent phosphorylation: a molecular model of kinesin-cargo release. Nat Cell Biol. 2008;10:19–29. doi: 10.1038/ncb1665. [DOI] [PubMed] [Google Scholar]
- Halpain S, Dehmelt L. The MAP1 family of microtubule-associated proteins. Genome Biol. 2006;7:224. doi: 10.1186/gb-2006-7-6-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JW, Cai D, Blasius TL, Li Z, Jiang Y, Jih GT, Meyhofer E, Verhey KJ. Mammalian kinesin-3 motors are dimeric in vivo and move by processive motility upon release of autoinhibition. PLoS Biol. 2009;7:e72. doi: 10.1371/journal.pbio.1000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Ballo L, Pietila L, Viesselmann C, Ballweg J, Lumbard D, Stevenson M, Merriam E, Dent EW. BDNF-induced increase of PSD-95 in dendritic spines requires dynamic microtubule invasions. J Neurosci. 2011;31:15597–15603. doi: 10.1523/JNEUROSCI.2445-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Viesselmann C, Nam S, Merriam E, Dent EW. Activity-dependent dynamic microtubule invasion of dendritic spines. J Neurosci. 2008;28:13094–13105. doi: 10.1523/JNEUROSCI.3074-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubener M, Bonhoeffer T. Searching for engrams. Neuron. 2010;67:363–371. doi: 10.1016/j.neuron.2010.06.033. [DOI] [PubMed] [Google Scholar]
- Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80:704–717. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, Esclapez M, Pellegrino C, Shirao T, Ferhat L. Drebrin A regulates dendritic spine plasticity and synaptic function in mature cultured hippocampal neurons. J Cell Sci. 2009;122:524-534. doi: 10.1242/jcs.033464. [DOI] [PubMed] [Google Scholar]
- Jaworski J, Kapitein LC, Gouveia SM, Dortland BR, Wulf PS, Grigoriev I, Camera P, Spangler SA, Di Stefano P, Demmers J, et al. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron. 2009;61:85–100. doi: 10.1016/j.neuron.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Kaech S, Fischer M, Doll T, Matus A. Isoform specificity in the relationship of actin to dendritic spines. J Neurosci. 1997;17:9565–9572. doi: 10.1523/JNEUROSCI.17-24-09565.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S, Parmar H, Roelandse M, Bornmann C, Matus A. Cytoskeletal microdifferentiation: a mechanism for organizing morphological plasticity in dendrites. Proc Natl Acad Sci USA. 2001;98:7086–7092. doi: 10.1073/pnas.111146798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitein LC, Yau KW, Gouveia SM, van der Zwan WA, Wulf PS, Keijzer N, Demmers J, Jaworski J, Akhmanova A, Hoogenraad CC. NMDA receptor activation suppresses microtubule growth and spine entry. J Neurosci. 2011;31:8194–8209. doi: 10.1523/JNEUROSCI.6215-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleele T, Marinkovic P, Williams PR, Stern S, Weigand EE, Engerer P, Naumann R, Hartmann J, Karl RM, Bradke F, et al. An assay to image neuronal microtubule dynamics in mice. Nat Commun. 2014;5:4827. doi: 10.1038/ncomms5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolman A. Epothilone D (Kosan/Roche) Curr Opin Investig Drugs. 2004;5:657–667. [PubMed] [Google Scholar]
- Korobova F, Svitkina T. Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Mol Biol Cell. 2010;21:165–176. doi: 10.1091/mbc.E09-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalevich J, Cornec AS, Yao Y, James M, Crowe A, Lee VM, Trojanowski JQ, Smith AB, 3rd, Ballatore C, Brunden KR. Characterization of brain-penetrant pyrimidine-containing molecules with differential microtubule-stabilizing activities developed as potential therapeutic agents for Alzheimer’s disease and related tauopathies. J Pharmacol Exp Ther. 2016;357:432–450. doi: 10.1124/jpet.115.231175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makani V, Zhang B, Han H, Yao Y, Lassalas P, Lou K, Paterson I, Lee VM, Trojanowski JQ, Ballatore C, et al. Evaluation of the brain-penetrant microtubule-stabilizing agent, dictyostatin, in the PS19 tau transgenic mouse model of tauopathy. Acta Neuropathol Commun. 2016;4:106. doi: 10.1186/s40478-016-0378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcette JD, Chen JJ, Nonet ML. The Caenorhabditis elegans microtubule minus-end binding homolog PTRN-1 stabilizes synapses and neurites. Elife. 2014;3:e01637. doi: 10.7554/eLife.01637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVicker DP, Awe AM, Richters KE, Wilson RL, Cowdrey DA, Hu X, Chapman ER, Dent EW. Transport of a kinesin-cargo pair along microtubules into dendritic spines undergoing synaptic plasticity. Nat Commun. 2016;7:12741. doi: 10.1038/ncomms12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVicker DP, Millette MM, Dent EW. Signaling to the microtubule cytoskeleton: an unconventional role for CaMKII. Dev Neurobiol. 2015;75:423–434. doi: 10.1002/dneu.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam EB, Lumbard DC, Viesselmann C, Ballweg J, Stevenson M, Pietila L, Hu X, Dent EW. Dynamic microtubules promote synaptic NMDA receptor-dependent spine enlargement. PLoS One. 2011;6:e27688. doi: 10.1371/journal.pone.0027688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam EB, Millette M, Lumbard DC, Saengsawang W, Fothergill T, Hu X, Ferhat L, Dent EW. Synaptic regulation of microtubule dynamics in dendritic spines by calcium, f-actin, and drebrin. J Neurosci. 2013;33:16471–16482. doi: 10.1523/JNEUROSCI.0661-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Mitsuyama F, Niimi G, Kato K, Hirosawa K, Mikoshiba K, Okuya M, Karagiozov K, Kato Y, Kanno T, Sanoe H, Koide T. Redistribution of microtubules in dendrites of hippocampal CA1 neurons after tetanic stimulation during long-term potentiation. Ital J Anat Embryol. 2008;113:17–27. [PubMed] [Google Scholar]
- Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R. Engineering a memory with LTD and LTP. Nature. 2014;511:348–352. doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley BR, Paolillo V, Zheng Y. gamma-Tubulin complexes in microtubule nucleation and beyond. Mol Biol Cell. 2015;26:2957–2962. doi: 10.1091/mbc.E14-11-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez F, Diamantopoulos GS, Stalder R, Kreis TE. CLIP-170 highlights growing microtubule ends in vivo. Cell. 1999;96:517–527. doi: 10.1016/s0092-8674(00)80656-x. [DOI] [PubMed] [Google Scholar]
- Richardson CE, Spilker KA, Cueva JG, Perrino J, Goodman MB, Shen K. PTRN-1, a microtubule minus end-binding CAMSAP homolog, promotes microtubule function in Caenorhabditis elegans neurons. Elife. 2014;3:e01498. doi: 10.7554/eLife.01498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll-Mecak A, McNally FJ. Microtubule-severing enzymes. Curr Opin Cell Biol. 2010;22:96–103. doi: 10.1016/j.ceb.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J, Jin SX, Craig AM. Heterosynaptic molecular dynamics: locally induced propagating synaptic accumulation of CaM kinase II. Neuron. 2009;61:351–358. doi: 10.1016/j.neuron.2008.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan XP, Alldritt J, Svenningsson P, Allen PB, Wu GY, Nairn AC, Greengard P. The Rho-specific GEF Lfc interacts with neurabin and spinophilin to regulate dendritic spine morphology. Neuron. 2005;47:85–100. doi: 10.1016/j.neuron.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Sala C, Segal M. Dendritic spines: the locus of structural and functional plasticity. Physiol Rev. 2014;94:141–188. doi: 10.1152/physrev.00012.2013. [DOI] [PubMed] [Google Scholar]
- Sanchez-Huertas C, Freixo F, Viais R, Lacasa C, Soriano E, Luders J. Non-centrosomal nucleation mediated by augmin organizes microtubules in post-mitotic neurons and controls axonal microtubule polarity. Nat Commun. 2016;7:12187. doi: 10.1038/ncomms12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapitz IU, Behrend B, Pechmann Y, Lappe-Siefke C, Kneussel SJ, Wallace KE, Stempel AV, Buck F, Grant SG, Schweizer M, et al. Neuroligin 1 is dynamically exchanged at postsynaptic sites. J Neurosci. 2010;30:12733–12744. doi: 10.1523/JNEUROSCI.0896-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumyatsky GP, Malleret G, Shin RM, Takizawa S, Tully K, Tsvetkov E, Zakharenko SS, Joseph J, Vronskaya S, Yin D, et al. stathmin, a gene enriched in the amygdala, controls both learned and innate fear. Cell. 2005;123:697–709. doi: 10.1016/j.cell.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Stepanova T, Slemmer J, Hoogenraad CC, Lansbergen G, Dortland B, De Zeeuw CI, Grosveld F, van Cappellen G, Akhmanova A, Galjart N. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein) J Neurosci. 2003;23:2655–2664. doi: 10.1523/JNEUROSCI.23-07-02655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16:95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Tonnesen J, Katona G, Rozsa B, Nagerl UV. Spine neck plasticity regulates compartmentalization of synapses. Nat Neurosci. 2014;17:678–685. doi: 10.1038/nn.3682. [DOI] [PubMed] [Google Scholar]
- Uchida S, Martel G, Pavlowsky A, Takizawa S, Hevi C, Watanabe Y, Kandel ER, Alarcon JM, Shumyatsky GP. Learning-induced and stathmin-dependent changes in microtubule stability are critical for memory and disrupted in ageing. Nat Commun. 2014;5:4389. doi: 10.1038/ncomms5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, Shumyatsky GP. Deceivingly dynamic: learning-dependent changes in stathmin and microtubules. Neurobiol Learn Mem. 2015;124:52–61. doi: 10.1016/j.nlm.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhey KJ, Kaul N, Soppina V. Kinesin assembly and movement in cells. Annu Rev Biophys. 2011;40:267–288. doi: 10.1146/annurev-biophys-042910-155310. [DOI] [PubMed] [Google Scholar]
- Wagner W, Brenowitz SD, Hammer JA., 3rd Myosin-Va transports the endoplasmic reticulum into the dendritic spines of Purkinje neurons. Nat Cell Biol. 2011;13:40–48. doi: 10.1038/ncb2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westrum LE, Gray EG, Burgoyne RD, Barron J. Synaptic development and microtubule organization. Cell Tissue Res. 1983;231:93–102. doi: 10.1007/BF00215777. [DOI] [PubMed] [Google Scholar]
- Westrum LE, Jones DH, Gray EG, Barron J. Microtubules, dendritic spines and spine appratuses. Cell Tissue Res. 1980;208:171–181. doi: 10.1007/BF00234868. [DOI] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau KW, Schatzle P, Tortosa E, Pages S, Holtmaat A, Kapitein LC, Hoogenraad CC. Dendrites in vitro and in vivo contain microtubules of opposite polarity and axon formation correlates with uniform plus-end-out microtubule orientation. J Neurosci. 2016;36:1071–1085. doi: 10.1523/JNEUROSCI.2430-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau KW, van Beuningen SF, Cunha-Ferreira I, Cloin BM, van Battum EY, Will L, Schatzle P, Tas RP, van Krugten J, Katrukha EA, et al. Microtubule minus-end binding protein CAMSAP2 controls axon specification and dendrite development. Neuron. 2014;82:1058–1073. doi: 10.1016/j.neuron.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat Neurosci. 2007;10:702–711. doi: 10.1038/nn1903. [DOI] [PubMed] [Google Scholar]
- Yu W, Centonze VE, Ahmad FJ, Baas PW. Microtubule nucleation and release from the neuronal centrosome. J Cell Biol. 1993;122:349–359. doi: 10.1083/jcb.122.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Carroll J, Trojanowski JQ, Yao Y, Iba M, Potuzak JS, Hogan AM, Xie SX, Ballatore C, Smith AB, 3rd, et al. The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J Neurosci. 2012;32:3601–3611. doi: 10.1523/JNEUROSCI.4922-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]