Cigarette smoke (CS) increases up-regulation of TLR4-mediated signaling and induces TLR4-dependent inflammation in lungs. CS exposure–induced HMGB1 translocation and release of HMGB1 controls CS-induced inflammatory response. MGB1 induces TLR4-mediated proinflammatory cytokine production and activates NF-κB and JNK/p38 pathways.
Abstract
We performed studies to determine the role of high-mobility group box 1 (HMGB1) in cigarette smoke (CS)–induced pulmonary inflammation. After mice were exposed to five cigarettes four times a day for 3 d, toll-like receptor 4 (TLR4) expression and TLR4-mediated signaling were significantly up-regulated, and HMGB1 had translocated from the nucleus to the cytoplasm in lung epithelial cells and then been released into the extracellular lung space. On CS exposure, inflammatory cell recruitment and proinflammatory cytokine production were significantly increased in lung tissue and bronchoalveolar lavage, and these effects depended on the TLR4 signaling pathway. HMGB1 inhibition decreased the CS-induced inflammatory response, whereas treatment with exogenous HMGB1 aggravated the damage and increased the phosphorylation of JNK, p38, and IκBα in the lungs of wild-type mice but not in TLR4-knockout mice. Blockade of TLR4 action or TLR4 knockout significantly inhibited HMGB1-induced proinflammatory cytokine production in mouse tracheal epithelial (MTE) cells and lung tissues. In addition, a MyD88 deficiency inhibited JNK, p38, and IκBα phosphorylation, and this effect was associated with the suppressed production of TNF-α and IL-1β in MTE cells and lung tissues in response to CS stimulation. Thus HMGB1 activates the NF-κB and JNK/p38 pathways through TLR4/MyD88-dependent signaling and induces an inflammatory response in lungs exposed to CS.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) accounts for an overwhelming proportion of the morbidity and mortality suffered by patients exposed to chronic cigarette smoke (CS; Sopori, 2002). CS-induced pulmonary inflammation, which is characterized by the activation of resident cells and the recruitment of inflammatory cells that release proinflammatory cytokines, chemokines, oxygen radicals, and proteases into the lungs, substantially promotes the abnormalities observed in COPD and plays a critical role in its development (Sopori, 2002; D’hulst et al., 2005; Karimi et al., 2006). Therefore clarification of the mechanisms responsible for CS-induced pulmonary inflammation in COPD and the identification of therapeutic interventions that may inhibit pulmonary inflammation hold great potential for reducing morbidity and mortality in patients with COPD.
High-mobility group box 1 (HMGB1), a highly conserved nonhistone chromosomal protein, was originally identified as a DNA-binding protein involved in the maintenance of nucleosome structure and the regulation of gene transcription (Huang et al., 2010). The translocation of HMGB1 from the intracellular environment to the extracellular environment is a critical event in host defense and inflammatory responses (Schierbeck et al., 2011). HMGB1 was shown to act as a potent proinflammatory cytokine and as an early driver of inflammation that participates in the development of the systemic inflammatory response (Zhu et al., 2011). The addition of purified recombinant HMGB1 to human monocyte cultures significantly stimulated the release of cytokines, including tumor necrosis factor α (TNF-α), interleukin 1α (IL-1α), IL-1β, IL-6, and IL-8 (Andersson et al., 2000). HMGB1 is either actively released by activated immune cells or passively released from damaged/necrotic cells (Scaffidi et al., 2002; Dumitriu et al., 2005). HMGB1 triggers inflammatory responses and tissue injury pathology in various organ systems, including enteritis (Maeda et al., 2007; Davé et al., 2009; Nadatani et al., 2012), pancreatitis (Sawa et al., 2006), atherosclerosis (Qin et al., 2015), myelodysplastic syndromes (Velegraki et al., 2013), and ischemia-reperfusion (I-R) injuries of the liver (Tsung et al., 2005), heart (Andrassy et al., 2008), and kidney (Wu et al., 2007).
The biological effects of extracellular HMGB1 may be mediated by the activation of signaling pathways coupled to toll-like receptors (TLRs; Tian et al., 2007; Mazarati et al., 2011; Yang et al., 2012). TLRs comprise a family of pattern recognition receptors that, upon ligand engagement, activate signaling pathways that stimulate the production of numerous cytokines and inflammatory mediators (Aderem and Ulevitch, 2000; Akira et al., 2001). TLR2 and TLR4 trigger MyD88-dependent intracellular signaling cascades involving the activation of nuclear factor-κB (NF-κB), mitogen-activated protein kinase (MAPK) p38, extracellular signal-regulated kinase (ERK), and c-Jun NH(2)-terminal kinase (JNK; Park et al., 2004; Andrassy et al., 2008; Mogensen, 2009). This signaling leads to the release of proinflammatory cytokines, including TNF-α and IL-1β (Chen et al., 2012). Based on strong evidence from numerous clinical studies, TLR4 activation may contribute to CS-induced pulmonary inflammation in patients exposed to CS (Maes et al., 2006; Doz et al., 2008), resulting in the up-regulation of adhesion molecules and inflammatory mediators, such as cytokines and chemokines, through the activation of NF-κB (Medzhitov and Janeway et al., 2000; Takeda et al., 2003).
Little information is available regarding the potential receptors and signaling mechanisms of HMGB1 that underlie its immunological functions in CS-induced pulmonary inflammation. In the present study, we probed the roles of HMGB1 in proinflammatory cytokine production and pulmonary inflammation after CS exposure. Specifically, we studied the potential receptors and signaling pathways of HMGB1 and the degree of activation of TLR4-related signal transduction pathways in the lungs of mice with CS-induced pulmonary inflammation.
RESULTS
CS increases TLR4 expression and up-regulates TLR4-mediated signaling in the lungs
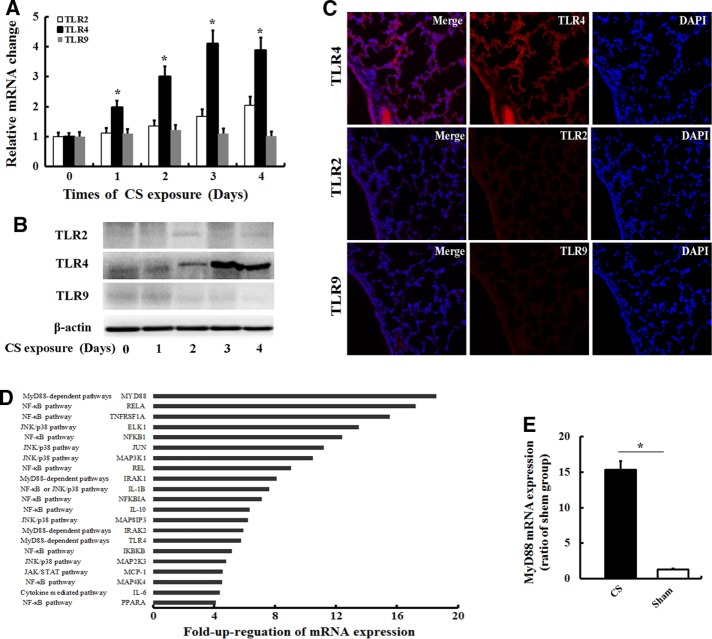
We first examined whether CS exposure induces changes in TLR2, TLR4, and TLR9 expression in the lungs. After C57BL/6J mice were exposed to five cigarettes four times a day for various time periods (0, 1, 2, 3, and 4 d), real-time PCR revealed that CS exposure increased the expression levels of the TLR4 and TLR2 mRNAs in a time-dependent manner. The levels of the TLR4 mRNA peaked after 3 d, but CS did not affect the expression of the TLR9 mRNA (Figure 1A). Consistent with the real-time PCR study, Western blot analysis showed the same changes in TLR4 expression in the lungs, but changes in the expression of the TLR2 and TLR9 proteins were not observed (Figure 1B). Immunofluorescence studies revealed a high level of TLR4 expression in epithelial cells and inflammatory cells in the lungs after 3 d of CS exposure (Figure 1C), whereas TLR2 and TLR9 were expressed at low levels in the lungs. Based on these data, the lungs of CS-exposed mice displayed a degree of TLR up-regulation, with a prominent increase in TLR4 expression.
FIGURE 1:
CS exposure induced the expression of genes and protein involved in TLR signaling. (A) After C57BL/6J mice (n = 5) were exposed to five cigarettes four times a day for various times (0, 1, 2, 3, and 4 d), the expression levels of the TLR2, TLR4, and TLR9 mRNAs were determined by real-time PCR. Values are expressed as the means ± SD from three independent experiments. *p < 0.05 vs. 0-d treatment. In addition, the expression of the TLR2, TLR4, and TLR9 proteins in the lungs of mice exposed to CS for 3 d was determined by (B) Western and (C) immunofluorescence analyses. The gels were run under the same experimental conditions. (D) Fold increase in the expression of genes involved in TLR signaling in the lungs of mice that had been exposed to CS for 3 d compared with the sham controls using a real-time PCR array. Genes exhibiting at least a fourfold increase in expression in the lungs in response to CS exposure compared with normal samples. (E) The relative expression of the MYD88 mRNA in lungs exposed to CS for 3 d and sham controls was assessed by individual real-time PCR assays to validate the array data. Values are expressed as means ± SD from three independent experiments. *p < 0.05
We screened 78 TLR-associated genes in mice that had been exposed to CS for 3 d to determine whether the increased TLR4 expression in the lungs of CS-exposed mice was associated with up-regulation of TLR4-mediated signaling. As shown in Figure 1D, 21 of the 78 TLR-related genes displayed an at least fourfold increase in mRNA expression in CS-exposed mice compared with the controls. Of interest, a number of genes related to NF-κB signaling and the JNK/p38 pathway were up-regulated in CS-exposed mice, suggesting that the increased TLR4 expression in the lungs of CS-exposed mice was associated with the downstream activation of the NF-κB and JNK/p38 pathways. Furthermore, we evaluated the expression of the MyD88 mRNA, a key adaptor molecule for TLR4/MyD88-dependent signaling, using quantitative real-time PCR. The relative expression of the MyD88 mRNA in lung tissues was significantly increased in CS-exposed mice compared with the controls (Figure 1E). Thus CS increased TLR4 expression and up-regulated TLR4-mediated signaling in the lungs.
CS exposure induces TLR4-dependent cellular inflammation and proinflammatory cytokine production in the lungs
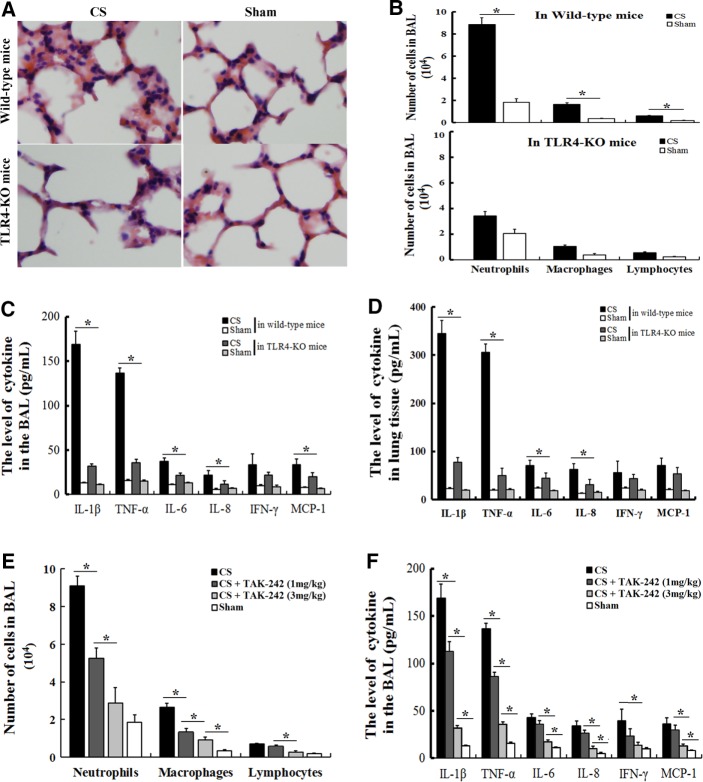
C57BL/6 mice that were exposed to CS for 3 d developed marked pulmonary inflammation and alveolar destruction (Figure 2A). Moreover, bronchoalveolar lavage (BAL) analysis (Figure 2A) also showed a significant increase in the number of inflammatory cells, consisting of neutrophils and, to a lesser extent, macrophages and lymphocytes, in the BAL of C57BL/6 mice that had been exposed to CS for 3 d. In contrast, CS-exposed TLR4-deficient mice displayed a reduction in inflammatory cell recruitment into the airways (Figure 2B) and lung parenchyma (Figure 2A), suggesting that the cellular inflammation observed in mice in response to smoke exposure depended on TLR4. Investigations of the proinflammatory cytokines and chemokines confirmed the presence of cellular inflammation in the lungs of mice exposed to CS, which was less pronounced in the TLR4-deficient mice. The production of the inflammatory cytokines IL-1β and TNF-α was significantly elevated in the BAL fluids (Figure 2C) and the lung tissue homogenates (Figure 2D) after 3 d of smoke exposure, but their levels were reduced in TLR4-deficient mice. In an attempt to identify a mechanistic explanation for the TLR4-dependent inflammatory response, we searched for known selective TLR4 signal transduction inhibitors, such as TAK-242. When administered to C57BL/6J mice, TAK-242 significantly decreased the recruitment of inflammatory cells (Figure 2E) and the production of proinflammatory cytokines (Figure 2F) in BAL fluid in response to CS exposure in a dose-dependent manner. Therefore CS-induced cellular inflammation and proinflammatory cytokine production depended on TLR4 signaling pathways.
FIGURE 2:
Cellular inflammation and proinflammatory cytokine production are induced by CS exposure and depend on the TLR4 signaling pathway. Wild-type mice (n = 7) and TLR4-KO mice (n = 7) received an acute (3 d) exposure to CS or air (sham group, n = 6). Three days later, the lungs and BAL fluid were obtained and used to (A) perform hematoxylin and eosin staining, (B) perform differential cell counts, or (C, D) measure the differences in cytokine or chemokine concentrations. C57BL/6 mice received 0, 1, or 3 mg/kg TAK-242 (n = 5 for each group) during the acute (3 d) exposure to CS or air (sham group, n = 5). Three days later, BAL fluid was obtained and used to measure (E) the differential cell counts and (F) the differences in the proinflammatory cytokine concentrations. The numbers of monocytes/macrophages, neutrophils, and lymphocytes were determined based on morphological criteria (cytospin). The levels of IL-1β, IL-6, IL-8, IFN-γ, TNF-α, and MCP-1 in the BAL fluid and lungs were measured using a multiplex cytokine assay. The results are displayed as means ± SD. from three independent experiments. *p < 0.05.
CS exposure induces HMGB1 translocation and release
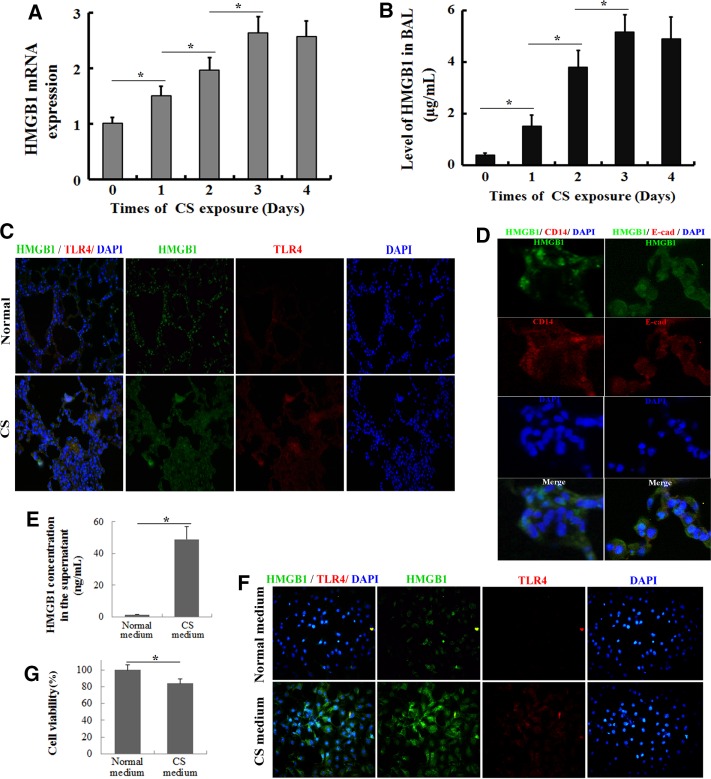
Based on recent evidence, HMGB1 is released extracellularly and may induce proinflammatory cytokine production upon binding to TLR4 by activating the NF-κB and JNK/p38 pathways, in addition to its intracellular actions of stabilizing nucleosomes and facilitating transcription (Chen et al., 2012; Nadatani et al., 2012). As a nuclear protein, the translocation of HMGB1 from the intracellular environment to the extracellular environment plays a critical role in the inflammatory response (Andersson et al., 2000; Huang et al., 2010; Nadatani et al., 2012). After C57BL/6 mice were exposed to CS for 3 d, HMGB1 expression in the lung tissues and the BAL fluids was assessed by real-time PCR, enzyme-linked immunosorbent assay (ELISA), and immunofluorescence. The levels of the HMGB1 mRNA in the lung tissues peaked after 3 d of CS exposure (Figure 3A), and similar dynamics of the HMGB1 levels was observed in the BAL fluids (Figure 3B). As shown in the immunofluorescence analysis, HMGB1 was limited to the nuclei of epithelial cells and interstitial cells in normal lung tissue (Figure 3C). However, CS exposure induced prominent cytoplasmic staining of HMGB1 by day 3 (Figure 3C). Of interest, inflammatory cells did not show significant HMGB1 translocation; in contrast, the lung epithelial cells showed marked HMGB1 translocation after CS exposure (Figure 3D). We then confirmed these results in vitro. After mouse tracheal epithelial (MTE) cells were exposed to medium containing CS, HMGB1 expression in the culture supernatant and cells was assessed by ELISA and immunofluorescence, respectively. As expected, almost no HMGB1 was released from cells exposed to normal medium, whereas CS-containing medium significantly increased the concentration of HMGB1 in the supernatant (Figure 3E). HMGB1 was mainly located in the nuclei of cells exposed to normal medium but translocated to the cytoplasm in cells treated with CS-containing medium (Figure 3F). In addition, CS-containing medium altered MTE cell viability in the MTT (3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl-2H-tetrazolium bromide) assay (Figure 3G). Thus CS exposure induced HMGB1 translocation and release.
FIGURE 3:
HMGB1 translocation and release in the lung after CS exposure. The lungs and BAL fluid were obtained from C57BL/6J mice that had been exposed to the smoke from five cigarettes four times per day for various times (0, 1, 2, 3, and 4 d). (A) HMGB1 mRNA expression levels were determined by quantitative real-time PCR. (B) The concentrations of HMGB1 in the BAL fluid were determined by ELISA. Values are expressed as means ± SD from three independent experiments. *p < 0.05. (C) Double immunohistochemical staining for HMGB1 and TLR4 in the lung tissue after a 3-d CS exposure. (D) Double immunohistochemical staining for HMGB1 and CD14 (a marker of inflammatory cells) or E-cadherin (a marker of epithelial cells) in the lung tissue after a 3-d CS exposure. After MTE cells were stimulated with the CS-containing medium for 24 h, the HMGB1 content in the supernatant and HMGB1 translocation were determined by (E) ELISA and (F) immunofluorescence, respectively. (G) Cell viability was determined as a percentage of the levels in the nontreated control cells using the MTT assay. The results are displayed as means ± SD. from three independent experiments. *p < 0.05.
HMGB1 controls the CS-induced inflammatory response
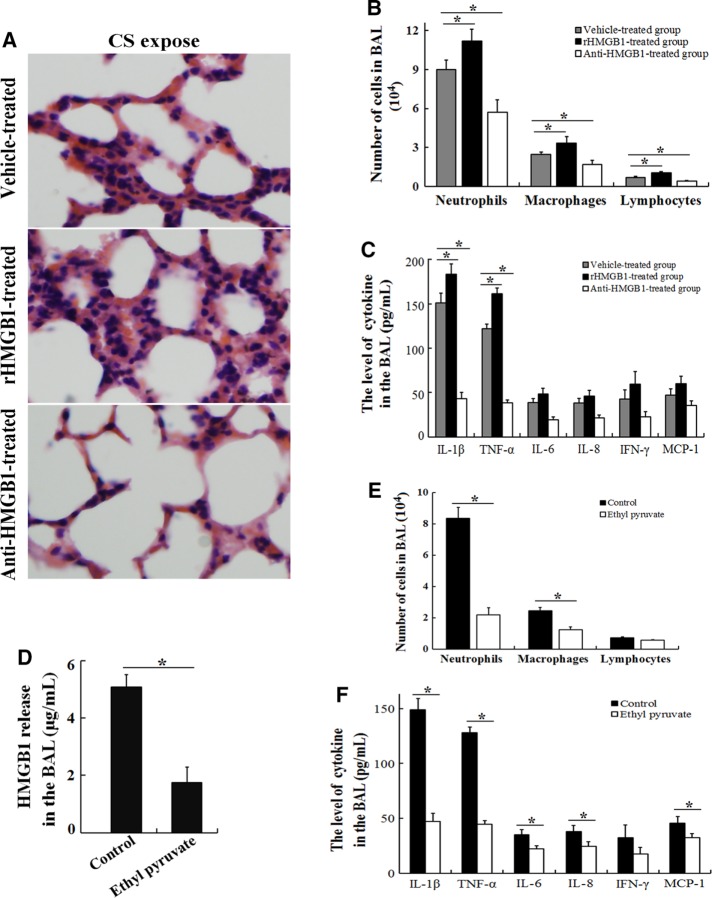
C57BL/6 mice were treated with 200 μg/kg rHMGB1, neutralizing antibodies to HMGB1, or vehicle, and the response to CS exposure was assessed to confirm our hypothesis that CS induces inflammatory responses via HMGB1. Three days of CS exposure induced inflammatory cell infiltration (Figure 2, A and B) and the production of proinflammatory cytokines (Figure 2, D and E). In the rHMGB1-treated group, CS exposure caused more severe injuries, with more inflammatory cell infiltration (Figure 4A) in lung tissues compared with the vehicle-treated group, whereas it caused less inflammation in the anti-HMGB1 antibody–treated group (Figure 4A). Similarly, the BAL analysis revealed that the recruitment of inflammatory cells (Figure 4B) and the release of proinflammatory cytokines (Figure 4C) were significantly higher in the rHMGB1-treated group after CS exposure. In contrast, CS exposure for three consecutive days induced less inflammatory cell recruitment (Figure 4B) and proinflammatory cytokine release (Figure 4C) in the BAL fluid of the anti-HMGB1 antibody–treated group. Furthermore, the administration of ethyl pyruvate, which is an inhibitor of HMGB1 release (Figure 4D), markedly inhibited CS-induced pulmonary inflammation (Figure 4, E and F). Based on these results, HMGB1 controls the CS-induced inflammatory response.
FIGURE 4:
HMGB1 controls the CS-induced inflammatory response in the lungs. C57BL/6 mice received an intravenous injection of 200 μg/kg rHMGB1, a neutralizing anti-HMGB1 antibody (10 mg/kg), or vehicle (PBS) via the tail vein each day (n = 6 for each group) during acute (3 d) exposure to CS. (A) The lungs were collected for hematoxylin and eosin staining, and the BAL fluid was obtained and used to measure (B) differential cell counts and (C) the differences in the proinflammatory cytokine concentrations. C57BL/6 mice were intravenously administered ethyl pyruvate (20 mg/kg; an inhibitor of HMGB1 release) or vehicle (PBS) during acute (3 d) exposure to CS (n = 5 for each group). Three days later, the BAL fluid was obtained and used to measure (D) HMGB1 release in the BAL, (E) differential cell counts, and (F) differences in the proinflammatory cytokine concentrations. The results are displayed as means ± SD from three independent experiments. *p < 0.05.
HMGB1 induces TLR4-mediated proinflammatory cytokine production
The production of proinflammatory cytokines (TNF-α and IL-1β) in the BAL fluid (Figure 4B) of CS-exposed mice was significantly increased after rHMGB1 stimulation. Therefore subsequent analyses of the mechanisms of proinflammatory cytokine production were performed with HMGB1-stimulated MTE cells harvested from C57BL/6 mice. We first investigated whether TLR4 signaling was involved in the rHMGB1-induced expression of TNF-α and IL-1β in MTE cells. MTE cells were treated with anti-TLR4 antibodies 2 h before rHMGB1 (100 ng/ml, 24 h) stimulation. As shown in the real-time PCR analysis, the TNF-α and IL-1β mRNA levels were significantly increased by the 24-h stimulation with rHMGB1 compared with the unstimulated controls (Figure 5, A and B). However, preincubation with the anti-TLR4 antibody significantly inhibited the HMGB1-induced expression of the TNF-α and IL-1β mRNAs in MTE cells (Figure 5, A and B) compared with cells that had been preincubated with an isotype control antibody. In addition, the anti-TLR4 antibody dramatically inhibited rHMGB1-induced TNF-α and IL-1β production in the supernatant of MTE cells (Figure 5C). TLR4-knockout (KO) mice and wild-type mice that had been exposed to CS for 3 d were treated with 200 μg/kg rHMGB1 to assess whether TLR4 signaling was responsible for HMBG1-induced proinflammatory cytokine production in the lung tissues of CS-exposed mice. Exogenous HMGB1 increased proinflammatory cytokine production in wild-type mice but did not affect proinflammatory cytokine production in TLR4-KO mice after CS exposure (Figure 5, D and E). Therefore TLR4 signaling is responsible for HMGB1-induced proinflammatory cytokine production in the lung tissue after CS exposure.
FIGURE 5:
HMGB1 induces TLR4-mediated TNF-α and IL-1β production. MTE cells were preincubated (2 h at 37°C) with an anti-TLR4 monoclonal antibody (20 mg/ml) or an isotype control antibody and then stimulated with human rHMGB1 (100 ng/ml) for 24 h. After stimulation, the culture supernatant and cells were collected. (A, B) TNF-α and IL-1β mRNA expression levels were determined by quantitative real-time PCR, and (C) concentrations of TNF-α and IL-1β in the supernatant were determined using a multiplex cytokine assay. Wild-type mice and TLR4-KO mice received an intravenous injection of 200 μg/kg rHMGB1 or vehicle (PBS) via the tail vein each day (n = 5 for each group) during acute (3 d) exposure to CS. (D) The BAL fluid was obtained and used to measure the differences in the proinflammatory cytokine concentrations. (E) Double immunohistochemical staining for TLR4 and TNF-α or IL-1β in the lung tissues from wild-type mice and TLR4-KO mice. The results are displayed as means ± SD from three independent experiments. *p < 0.05.
HMGB1 activates the NF-κB and JNK/p38 pathways through TLR4/MyD88 signaling
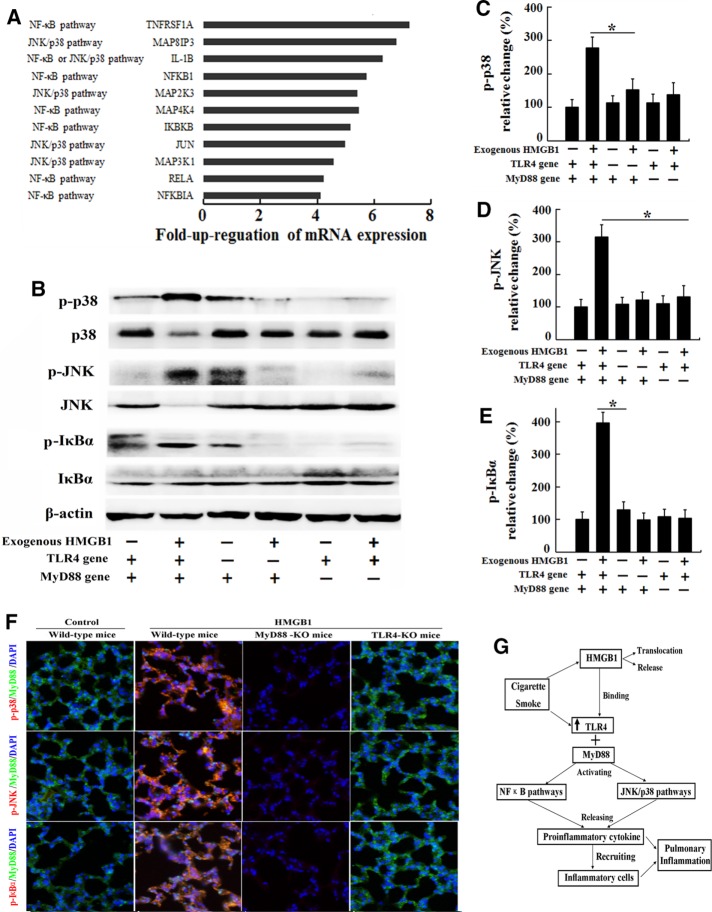
We then compared the mRNA levels of TLR4 signaling molecules associated with specific downstream pathways, such as the NF-κB pathway, the JNK/p38 pathway, the JAK/STAT pathway, and cytokine-mediated pathways, in the lung tissue of CS-exposed C57BL/6 mice treated with 200 μg/kg rHMGB1 or vehicle. CS-exposed mice treated with rHMGB1 displayed increased expression of mRNAs related to the JNK, p38, and NF-κB pathways compared with CS-exposed mice treated with vehicle (Figure 6A). Because NF-κB and MAPK play pivotal roles in intracellular TLR4 signaling events and NF-κB is involved in regulating the expression of genes that encode inflammatory cytokines such as TNF-α and IL-1β (Park et al., 2004; Chen et al., 2012; Nadatani et al., 2012), we further investigated the effects of HMGB1 on the activation of the p38, JNK, and NF-κB pathways in MTE cells harvested from TLR4-KO mice, MyD88-KO mice, and wild-type mice. After MTE cells were treated with HMGB1 (100 ng/ml, 24 h), the levels of phosphorylated p38, JNK, and IκBα were detected by Western blot assays (Figure 6B). The levels of phosphorylated JNK, p38, and IκBα were significantly increased in HMGB1-stimulated cells compared with control MTE cells harvested from wild-type mice, with increases of up to 283, 321, and 388%, respectively (Figure 6, C–E). There was no significant difference in the levels of phosphorylated JNK, p38, and IκBα between the HMGB1-stimulated and control groups of MTE cells harvested from TLR4-KO mice or MyD88-KO mice. Based on these results, HMGB1 activates the p38, JNK, and NF-κB pathways through TLR4/MyD88 signaling in the lungs of CS-exposed mice. We evaluated the levels of phosphorylated JNK, p38, and IκBα in the lung tissues of CS-exposed mice treated with 200 μg/kg rHMGB1 or vehicle to confirm these results. Exogenous HMGB1 aggravated the increased phosphorylation of JNK, p38, and IκBα in lung tissues of CS-exposed wild-type mice, whereas TLR4 or MyD88 deficiency resulted in inhibition of JNK, p38, and IκBα phosphorylation in lung tissues of TLR4-KO or MyD88-KO mice after CS exposure (Figure 6F). These findings further support the hypothesis that HMGB1 activates the p38, JNK, and NF-κB pathways through TLR4/MyD88 signaling in lungs exposed to the inflammatory milieu of CS.
FIGURE 6:
Signal transduction is activated in lungs exposed to the inflammatory CS milieu. C57BL/6 mice received an intravenous injection of 200 μg/kg rHMGB1 or vehicle (PBS) via the tail vein each day (n = 5 for each group) during acute (3 d) exposure to CS. Three days later, the lungs were collected for the real-time PCR assay. (A) Genes involved in TLR4 signaling and molecules associated with specific downstream pathways that exhibited an at least fourfold increase in expression in the lungs from the rHMGB1-treated group compared with the vehicle group. MTE cells harvested from wild-type mice, TLR4-KO mice, or MyD88-KO mice were stimulated with human rHMGB1 (100 ng/ml) or vehicle (PBS) for 24 h. After stimulation, the cells were collected. (B) The levels of phosphorylated MAPKs p38, JNK, and IκBα were evaluated by Western blotting. The gels were run under the same experimental conditions. The expression levels of (C) phospho-p38, (D) phospho-JNK (D), and (E) phospho-IκBα were normalized to the levels of total p38, total JNK, and total IκBα, respectively. Wild-type mice, TLR4-KO mice, and MyD88-KO mice received an intravenous injection of 200 μg/kg rHMGB1 or control (PBS) via the tail vein each day during acute (3 d) exposure to CS (n = 5 for each group). (F) Immunohistochemical staining for phospho-p38, phospho-JNK, and phospho-IκBα in the lung tissue. (G) Diagram summarizing the mechanism by which HMGB1 mediates CS-induced pulmonary inflammation in the mouse. The results are displayed as means ± SD. from three independent experiments. *p < 0.05.
DISCUSSION
In the present study, CS increased the expression of TLR4, up-regulated TLR4-mediated signaling in the lungs, and induced the translocation of HMGB1 from the nucleus to the cytoplasm, followed by its extracellular release. HMGB1 activates the p38, JNK, and NF-κB pathways through TLR4/MyD88-dependent signaling pathways and induces cellular inflammation and proinflammatory cytokine production in lungs exposed to the inflammatory milieu of CS (Figure 6G).
Smoking induces the activation of resident cells and the recruitment of inflammatory cells into the lungs, which leads to the release of proinflammatory cytokines, chemotactic factors, oxygen radicals, and proteases. As reported in previous studies, CS plays a critical role in the pathogenesis of COPD (Sopori, 2002; D’hulst et al., 2005; Karimi et al., 2006). CS components may be recognized by molecular pattern recognition receptors of the innate immune system, such as the TLRs (Karimi et al., 2006; Maes et al., 2006; Doz et al., 2008). We initially determined whether CS exposure changes the expression of TLR2, TLR4, and TLR9 in the lungs. Only TLR4 expression and TLR4-mediated signaling were significantly up-regulated in the lungs after 3 d of CS exposure. In contrast, the expression of TLR2, TLR4, and TLR9 was significantly increased in the lungs after endotoxin exposure (Supplemental Figure S1), suggesting that CS exposure induces TLR-dependent lung inflammation in a manner different from endotoxin exposure. On acute CS exposure, the lung tissues develop inflammation resulting from the activation of TLR4 and subsequent signaling leading to macrophage differentiation, the mobilization of neutrophils, and the stimulation of lymphocytes. TLR4 activation also increases the levels of the inflammatory cytokines TNF-α, IL-6, IL-8, IFN-γ, IL-1β, and MCP-1 and the chemokine RANTES (Yang et al., 2006; Gaschler et al., 2008).
Reactive oxygen species produced in response to inhaling CS cause cell necrosis and apoptosis of both the parenchyma and alveolar septae (Dekhuijzen, 2004). Dying cells produce danger signals, such as HMGB1 protein (Lotze and Tracey, 2005), which may activate TLR2 and TLR4 and/or the receptor for advanced glycation end products (Park et al., 2006) and induce apoptosis (Nadatani et al., 2012; Velegraki et al., 2013). HMGB1 is a nonhistone DNA-binding protein that acts as a cytokine when released into the extracellular milieu (Mitola et al., 2006). Extracellular HMGB1 is considered a signaling molecule that induces tissue injury and a mediator of inflammation (Sims et al., 2010). HMGB1 was believed to be primarily secreted by immune cells, including macrophages. A recent study reported the effects of tobacco smoke on HMGB1 translocation and release in macrophages (Chen et al., 2016). In this study, CS exposure was shown to induce inflammatory cell infiltration in the lungs. However, we did not observe HMGB1 translocation in inflammatory cells. Instead, CS exposure induced the translocation of HMGB1 from the nucleus to cytoplasm in epithelial cells, leading to extracellular release and the accompanying inflammatory response. HMGB1 inhibition with neutralizing antibodies or ethyl pyruvate decreased the CS-induced inflammatory response, including the infiltration of inflammatory cells and the production of proinflammatory cytokines in the lung tissues and BAL fluid, whereas exogenous HMGB1 aggravated the inflammatory response. These results suggest a critical role for HMGB1 in CS-induced pulmonary inflammation. In addition, CS does not require TLR4 to induce HMGB1 release in MTE cells (Supplemental Figure S2). Increased extracellular HMGB1 levels have been described in a number of inflammatory and malignant diseases (Sims et al., 2010; Nadatani et al., 2012; Velegraki et al., 2013); however, an association with CS-induced pulmonary inflammation has not been recognized. In this study, we provide the first evidence that HMGB1 translocation and release in the lungs in response to CS exposure strongly enhances the inflammatory response.
Once HMGB1 is released into the extracellular environment, it acts as an inflammatory cytokine (an alarmin; Wang et al., 1999). HMGB1 exerts these proinflammatory effects through TLR2, which is a receptor for components of the Gram-positive bacterial cell wall, and through TLR4, which is a multiligand receptor belonging to the immunoglobulin superfamily (Yu et al., 2006). TLR4 was one of the few receptors for HMGB1 that was up-regulated in the lungs of CS-exposed mice, suggesting that TLR4 might be activated by HMGB1. Consistent with the up-regulation of TLR4, similar dynamics in HMGB1 levels was observed in the lungs in response to CS exposure, indicating a possible association between HMGB1 and TLR4 activation in CS-exposed mice.
Regarding TLR4 ligands, TLR4 is activated by both microorganism-associated molecular patterns and endogenous ligands, such as the HMGB1 protein (Lotze and Tracey, 2005; Park et al., 2006). Therefore we infer that HMGB1 triggers TLR4-mediated signaling in the lungs of CS-exposed mice. MTE cells harvested from C57BL/6 mice treated with HMGB1 (100 ng/ml, 24 h) exhibited increased TNF-α and IL-1β production, whereas preincubation with anti-TLR4 antibodies significantly inhibited this effect. On the basis of the aforementioned results on the release and action of HMGB1, one could hypothesize that the HMGB1-induced TNF-α and IL-1β production in lung tissues and MTE cells exposed to CS might result from the active release of HMGB1 from necrotic cells, which then exerts these proinflammatory effects by triggering TLR4. Exogenous HMGB1 administration aggravated proinflammatory cytokine production in the lungs of CS-exposed mice but failed to affect proinflammatory cytokine production in TLR4-KO mice in response to CS exposure. These results validate our hypothesis.
Although in the present study, HMGB1 was shown to exert its proinflammatory effects by triggering the TLR4 receptor in response to CS exposure, similar to its effects in other organs, including the intestine, pancreas, blood vessels, marrow, liver, heart, and kidneys, during inflammation and injury (Tsung et al., 2005; Sawa et al., 2006; Maeda et al., 2007; Wu et al., 2007; Andrassy et al., 2008; Davé et al., 2009; Nadatani et al., 2012; Velegraki et al., 2013; Qin et al., 2015), the mechanisms underlying these actions remain unknown, limiting our understanding of the potential therapies for CS-induced pulmonary inflammation. Based on substantial experimental evidence, binding of HMGB1 to TLR4 activates NF-κB and stimulates the phosphorylation of p38, ERK, and JNK in inflammatory cells through MyD88-dependent signaling pathways (Park et al., 2004; Andrassy et al., 2008; Mogensen, 2009). The activation of MAPKs through the HMGB1-TLR4 signaling pathway has been reported in several models of tissue injury. HMGB1 stimulates p38 and JNK phosphorylation in I-R liver injury (Tsung et al., 2005) and ERK phosphorylation in I-R myocardial injury (Andrassy et al., 2008). Activated NF-κB regulates the expression of the mRNAs encoding the proinflammatory cytokines that recruit monocytes, drive monocyte differentiation into macrophages, and stimulate T lymphocytes, resulting in inflammatory cell proliferation and the release of additional cytokines (Yang et al., 2006). These cytokines include IFN-γ, IL-6, and TNF-α, all of which further activate the NF-κB pathway, thus establishing a positive autoregulatory loop that can amplify the inflammatory response and increase the duration of chronic inflammation (Yamamoto and Gaynor, 2001).
Gene expression microarray technology has been used to identify the genes/molecular pathways regulated by TLR4 that are associated with specific downstream pathways activated as the inflammatory response evolves. A number of genes associated with the NF-κB pathway and the JNK/p38 pathway exhibit increased expression in the lung tissue of CS-exposed mice treated with rHMGB1 compared with CS-exposed control mice. The HMGB1 treatment increased the levels of phosphorylated JNK, p38, and IκBα in TBE cells and lung tissues in response to CS stimulation, whereas TLR4 or MyD88 deficiency significantly inhibited this phosphorylation and reduced the expression of inflammatory cytokines in CS-induced lung injury. Thus HMGB1 exerts its proinflammatory effects by triggering TLR4/MyD88 to activate the p38, JNK, and NF-κB pathways in the lungs in response to CS exposure. TLR4 can also signal through toll-interleukin 1 receptor domain-containing adapter-inducing interferon-β (TRIF); therefore we explored the role of TRIF in the inflammatory responses measured in response to CS. However, CS and HMGB1 induce TNF-α and IL-1β production in a TRIF-independent manner (Supplemental Figure S3).
In conclusion, HMGB1 activates NF-κB and MAPKs through TLR4/MyD88-dependent signaling pathways, leading to the production of proinflammatory cytokines and the aggravation of damage in CS-induced pulmonary inflammation. Because HMGB1 is a major endogenous ligand of TLR4, as well as a complicating factor mediating inflammation in response to tissue damage induced by pollution and smoke, blocking TLR4 or inhibiting MyD88-dependent signaling pathways may be useful therapeutic options for CS-induced pulmonary inflammation.
MATERIALS AND METHODS
Animals
This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of West China Hospital, Sichuan University.
CS exposure and experimental treatment
See Suplemental Extended Experimental Procedures. The animals (including wild-type mice, TLR4-KO, mice and MyD88-KO mice) received the smoke from five cigarettes (harmful components and tar reduction in the cigarettes) per exposure in four exposures per day with 30-min smoke-free intervals for 3 d. During the CS exposure, mice (five to seven in each group) received an intravenous injection of 1.0 or 3.0 mg/kg TAK-242, 200 μg/kg human recombinant HMGB1 (reduced rHMGB1; Sigma-Aldrich, St. Louis, MO), or vehicle (phosphate-buffered saline [PBS]) via the tail vein per day. In addition, mice (five to seven in each group) were intravenously injected with a neutralizing chicken anti-HMGB1 polyclonal antibody (10 mg/kg; Shino-Test, Tokyo, Japan), normal chicken immunoglobulin Y (10 mg/kg; Sigma-Aldrich), or ethyl pyruvate (20 mg/kg; Sigma-Aldrich), an inhibitor of HMGB1 release, during the CS exposure.
Mouse tracheal epithelial cell stimulation in vitro
See Supplemental Extended Experimental Procedures. Primary MTE cells were obtained from naive TLR4-deficient mice, MyD88-deficient mice, and C57BL/6 mice as previously described (You et al., 2002). MTE cells were plated in 96-well microculture plates (at 105 cells/well) and stimulated with CS condensate (20 μg/ml) for 24 h. The isolated MTE cells were preincubated (2 h at 37°C) with the anti-TLR4 monoclonal antibody (20 mg/ml; InvivoGen, San Diego, CA) or isotype control antibody and then stimulated with human HMGB1 (100 ng/ml; Sigma-Aldrich) for 24 h to examine the effects of HMGB1 on the secretion of proinflammatory cytokines from MTE cells. After stimulation, the culture supernatant and cells were collected.
Statistical analysis
All of the treatments were performed in triplicate, and the results are displayed as means ± SD. The data were subjected to one-way analysis of variance. Probabilities of ≤0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
The project was funded by the Natural Science Foundation of China (81500590) and the Natural Science Foundation of Chongqing Science and Technology Commission (Grant cstc2012jjA10033).
Abbreviations used:
- BAL
bronchoalveolar lavage
- JNK
c-Jun NH(2)-terminal kinase
- COPD
chronic obstructive pulmonary disease
- CS
cigarette smoke
- ELISA
enzyme-linked immunosorbent assay
- ERK
extracellular signal-regulated kinase
- HMGB1
high-mobility group box 1
- IL-1α
interleukin 1α
- KO
knockout
- MAPK
mitogen-activated protein kinase
- MTE
mouse tracheal epithelial
- NF-κB
nuclear factor-κB
- PBS
phosphate-buffered saline
- TLR4
toll-like receptor 4
- TLRs
toll-like receptors
- TNF-α
tumor necrosis factor α.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E16-02-0126) on November 2, 2016.
REFERENCES
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK, et al. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117:3216–3226. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- Chen XL, Sun L, Guo F, Wang F, Liu S, Liang X, Wang RS, Wang YJ, Sun YX. High-mobility group box-1 induces proinflammatory cytokines production of Kupffercells through TLRs-dependent signaling pathway after burn injury. PLoS One. 2012;7:e50668. doi: 10.1371/journal.pone.0050668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Li G, Liu Y, Werth VP, Williams KJ, Liu ML. Translocation of endogenous danger signal HMGB1 from nucleus to membrane microvesicles in macrophages. J Cell Physiol. 2016;231:2319–2326. doi: 10.1002/jcp.25352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davé SH, Tilstra JS, Matsuoka K, Li F, DeMarco RA, Beer-Stolz D, Sepulveda AR, Fink MP, Lotze MT, Plevy SE. Ethyl pyruvate decreases HMGB1 release and ameliorates murine colitis. J Leukoc Biol. 2009;86:633–643. doi: 10.1189/jlb.1008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekhuijzen PN. Antioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary disease. Eur Respir J. 2004;23:629–636. doi: 10.1183/09031936.04.00016804. [DOI] [PubMed] [Google Scholar]
- D’hulst AI, Vermaelen KY, Brusselle GG, Joos GF, Pauwels RA. Time course of cigarette smoke-induced pulmonary inflammation in mice. Eur Respir J. 2005;26:204–213. doi: 10.1183/09031936.05.00095204. [DOI] [PubMed] [Google Scholar]
- Doz E, Noulin N, Boichot E, Guénon I, Fick L, Le Bert M, Lagente V, Ryffel B, Schnyder B, Quesniaux VF, et al. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol. 2008;180:1169–1178. doi: 10.4049/jimmunol.180.2.1169. [DOI] [PubMed] [Google Scholar]
- Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P. Release of high mobility group box 1 by dendritic cells controls T cell activation viathe receptor for advanced glycation end products. J Immunol. 2005;174:7506–7515. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- Gaschler GJ, Zavitz CC, Bauer CM, Skrtic M, Lindahl M, Robbins CS, Chen B, Stämpfli MR. Cigarette smoke exposure attenuates cytokine production by mouse alveolar macrophages. Am J Respir Cell Mol Biol. 2008;38:218–226. doi: 10.1165/rcmb.2007-0053OC. [DOI] [PubMed] [Google Scholar]
- Huang W, Tang Y, Li L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine. 2010;51:119–126. doi: 10.1016/j.cyto.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Karimi K, Sarir H, Mortaz E, Smit JJ, Hosseini H, De Kimpe SJ, Nijkamp FP, Folkerts G. Toll-like receptor-4 mediates cigarette smoke-induced cytokine production by human macrophages. Respir Res. 2006;7:66. doi: 10.1186/1465-9921-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- Maeda S, Hikiba Y, Shibata W, Ohmae T, Yanai A, Ogura K, Yamada S, Omata M. Essential roles of high-mobility group box 1 in the development of murine colitis and colitis-associated cancer. Biochem Biophys Res Commun. 2007;360:394–400. doi: 10.1016/j.bbrc.2007.06.065. [DOI] [PubMed] [Google Scholar]
- Maes T, Bracke KR, Vermaelen KY, Demedts IK, Joos GF, Pauwels RA, Brusselle GG. Murine TLR4 is implicated in cigarette smoke-induced pulmonary inflammation. Int Arch Allergy Immunol. 2006;141:354–368. doi: 10.1159/000095462. [DOI] [PubMed] [Google Scholar]
- Mazarati A, Maroso M, Iori V, Vezzani A, Carli M. High-mobility group box-1 impairs memory in mice through both toll-like receptor 4 and receptor for advanced glycation end products. Exp Neurol. 2011;232:143–148. doi: 10.1016/j.expneurol.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Janeway C. The Toll receptor family and microbial recognition. Trends Microbiol. 2000;8:452–456. doi: 10.1016/s0966-842x(00)01845-x. [DOI] [PubMed] [Google Scholar]
- Mitola S, Belleri M, Urbinati C, Coltrini D, Sparatore B, Pedrazzi M. Cutting edge: extracellular high mobility group box-1 protein is a proangiogenic cytokine. J Immunol. 2006;176:12–15. doi: 10.4049/jimmunol.176.1.12. [DOI] [PubMed] [Google Scholar]
- Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadatani Y, Watanabe T, Tanigawa T, Machida H, Okazaki H, Yamagami H, Watanabe K, Tominaga K, Fujiwara Y, Arakawa T. High mobility group box 1 promotes small intestinal damage induced by nonsteroidal anti-inflammatory drugs through Toll-like receptor 4. Am J Pathol. 2012;181:98–110. doi: 10.1016/j.ajpath.2012.03.039. [DOI] [PubMed] [Google Scholar]
- Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A. High mobility group box 1 protein interacts with multiple toll-like receptors. Am J Physiol. 2006;290:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of Toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- Qin WD, Mi SH, Li C1, Wang GX, Zhang JN, Wang H, Zhang F, Ma Y, Wu DW, Zhang M. Low shear stress induced HMGB1 translocation and release via PECAM-1/PARP-1pathway to induce inflammation response. PLoS One. 2015;10:e0120586. doi: 10.1371/journal.pone.0120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa H, Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Nakajima T, Kuroda Y. Blockade of high mobility group box-1 protein attenuates experimental severe acute pancreatitis. World J Gastroenterol. 2006;12:7666–7670. doi: 10.3748/wjg.v12.i47.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggersinflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Schierbeck H, Lundbäck P, Palmblad K, Klevenvall L, Erlandsson-Harris H, Andersson U, Ottosson L. Monoclonal anti-HMGB1 (high mobility group box chromosomal protein 1) antibody protection in two experimental arthritis models. Mol Med. 2011;17:1039–1044. doi: 10.2119/molmed.2010.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2:372–377. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velegraki M, Papakonstanti E, Mavroudi I, Psyllaki M, Tsatsanis C, Oulas A, Iliopoulos I, Katonis P, Papadaki HA. Impaired clearance of apoptotic cells leads to HMGB1 release in the bone marrow of patients with myelodysplastic syndromes and induces TLR4-mediated cytokine production. Haematologica. 2013;98:1206–1215. doi: 10.3324/haematol.2012.064642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova LH, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Chen L, Yang J, Ding J, Rong H, Dong W, Li X. High mobility group box-1 induces migration of vascular smooth muscle cells viaTLR4-dependent PI3K/Akt pathway activation. Mol Biol Rep. 2012;39:3361–3367. doi: 10.1007/s11033-011-1106-6. [DOI] [PubMed] [Google Scholar]
- Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291:L46–L57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol. 2002;283:1315–1321. doi: 10.1152/ajplung.00169.2002. [DOI] [PubMed] [Google Scholar]
- Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H. HMGB1 signals through Toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- Zhu XM, Yao YM, Liang HP, Xu CT, Dong N, Yu Y, Sheng ZY. High mobility group box-1 protein regulate immunosuppression of regulatory T cells through toll-like receptor 4. Cytokine. 2011;54:296–304. doi: 10.1016/j.cyto.2011.02.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.