Abstract
The pretargeting system based on the inverse electron demand Diels-Alder reaction (IEDDA) between trans-cyclooctene (TCO) and tetrazine (Tz) combines the favorable pharmacokinetic properties of radiolabeled small molecules with the affinity and specificity of antibodies. This strategy has proven to be an efficient method for the molecularly targeted delivery of pharmaceuticals, including isotopes for radiological imaging. Despite encouraging results from in vivo PET imaging studies, this promising system has yet to be thoroughly evaluated for pretargeted radioimmunotherapy (PRIT). Towards that end, we synthesized two novel 177Lu-labeled tetrazine-bearing radioligands. Next we compared the usefulness of our ligands for PRIT when paired with TCO-modified 5B1— a human, anti-CA19.9 mAb — in preclinical murine models of pancreatic cancer. The exemplary ligand, 177Lu-DOTA-PEG7-Tz, showed rapid (4.6 ± 0.8 %ID/g at 4h) and persistent (16.8 ± 3.9 %ID/g at 120h) uptake in tumors while concurrently clearing from blood and non-target tissues. Single-dose therapy studies using 5B1-TCO and varying amounts of 177Lu-DOTA-PEG7-Tz (400, 800, and 1200µCi) showed that our system elicits a dose-dependent therapeutic response in mice bearing human xenografts. Furthermore, dosimetry calculations suggest that our approach is amenable to clinical applications with its excellent dosimetric profile in organs of clearance (i.e. liver and kidneys) as well as in dose-limiting tissues, such as red marrow. This study established that a pretargeted methodology utilizing the IEDDA reaction can rapidly and specifically deliver a radiotherapeutic payload to tumor tissue, thus illustrating its excellent potential for clinical translation.
Keywords: pretargeting, radioimmunotherapy, pancreatic cancer
Introduction
For over three decades, the remarkable selectivity and affinity of antibodies for tumor biomarkers have made them attractive vectors for the selective delivery of therapeutic radiation to cancer cells. (1, 2) A wide variety of antibodies have been labeled with a wide variety of therapeutic isotopes. Indeed, a number of therapeutic radioimmunoconjugates are currently the subject of clinical trials, and two constructs — 90Y-ibritumomab tiuxetan and 131I-tositumomab — have been approved for the treatment of non-Hodgkin lymphoma. (3, 4) However, an important limitation compromising targeted radioimmunotherapy (RIT) is that immunoglobulins can take multiple days to reach their optimal biodistribution in vivo. This relatively slow pharmacokinetic profile mandates the use of therapeutic isotopes with long physical half-lives, such as 90Y (t1/2 ~ 2.7 d), 177Lu (t1/2 ~ 6.6 days), and 131I (t1/2 ~ 8.0 days). This combination of slow pharmacokinetics, long physical half-lives, and therapeutic radiations can potentially can result in prohibitively high radiation doses to healthy organs, particularly bone marrow. (5) An effective radioimmunoconjugate must therefore strike a balance between delivering a therapeutic dose of radiation to the tumor while avoiding, or at least minimizing, radiation-related side effects. This issue becomes particularly important in the context of solid lesions, for which effective RIT has long proven elusive due to slow uptake, radiation resistance and poor tumor penetration. (6, 7) In principle, these obstacles could be overcome by injecting higher activities; however, the suboptimal tumor-to-tissue ratios of traditional radioimmunoconjugates often preclude this course, as dose-limiting radiation toxicities would be reached before effective therapy could be achieved.
In response to these issues, considerable attention has been dedicated to the creation of RIT strategies that reduce the radiation burden to healthy tissues, most notably the development of radiolabeled antibody fragments. (8, 9) Another promising approach is pretargeted radioimmunotherapy (PRIT). (10) The aim of PRIT is to harness the tumor-targeting properties of antibodies while avoiding their pharmacokinetic drawbacks by decoupling the targeting vector from the radioisotope. More specifically, PRIT strategies typically employ four steps (Fig. 1A): (i) the administration of an unlabeled antibody with the ability to bind both an antigen and a radioligand; (ii) the slow accumulation of the antibody in the tumor and its concomitant clearance from the blood; (iii) the subsequent administration of a small-molecule radioligand; and (iv) the rapid binding of the radioligand to the antibody at the tumor site and the rapid excretion of any excess radioligand. Administration of the radioisotope in the form of a small molecule with rapid pharmacokinetics rather than a large molecule (i.e., the antibody) with slow kinetics is the key feature of this paradigm. As a result, PRIT yield high activity concentrations in tumors while keeping radiation doses to healthy organs low. Until recently, three approaches to in vivo pretargeting have dominated the field: bispecific antibodies engineered to bind both an antigen and a radiolabeled hapten, streptavidin-labeled antibodies with biotin-based radioligands, and oligonucleotide-bearing antibodies with radioligands modified with complementary sequences. All three strategies have proven effective in preclinical studies, yet each has been limited somewhat by various obstacles, such as the immunogenicity of streptavidin-based immunoconjugates. (11–15)
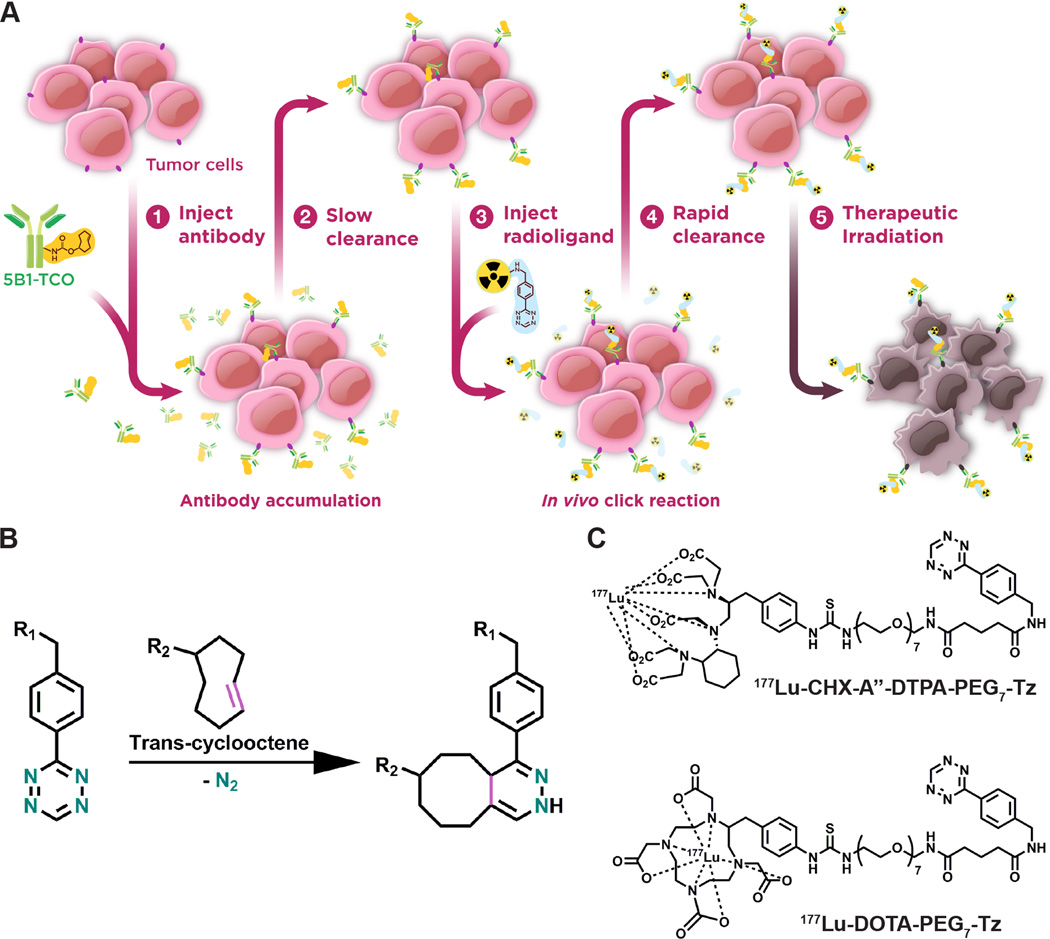
Figure 1.
A cartoon depicting the four basic steps of the pretargeted radioimmunotherapy approach used in these studies is shown (A). Also depicted is the reaction between the two components (TCO and Tz) on which the system is based (B) and the two 177Lu-labeled ligands used in these studies (C).
The past five years have witnessed the emergence of a new approach to in vivo pretargeting based on the inverse electron demand Diels-Alder (IEDDA) reaction between tetrazine (Tz) and trans-cyclooctene (TCO; Fig. 1B). (16, 17) This straightforward and modular methodology is predicated on the extraordinary selectivity and rapidity (k1 > 30,000 M−1s−1) of the bioorthgonal cycloaddition between a TCO-modified antibody and a tetrazine-bearing radioligand (Fig. 1A). (18–21) The earliest reports of this methodology focused on pretargeted SPECT imaging using an 111In-labeled radioligand and a TAG72-targeting CC49-TCO immunoconjugate. (22) More recently, our laboratories have developed highly effective pretargeted PET imaging strategies for both colorectal carcinoma and pancreatic ductal adenocarcinoma (PDAC) using 64Cu- and 18F-labeled tetrazine radioligands. (23–25) In both cases, the pretargeting approaches identify tumor tissue extremely well, yielding images with high tumor-to-background contrast at only a fraction of the radiation dose produced by directly-labeled radioimmunoconjugates. Given the success of these pretargeted imaging modalities, the logical next step is to leverage this technology for the development of a safe and effective approach to PRIT. (26) Notably, while Rossin, et al. have successfully demonstrated the feasibility of 177Lu-based PRIT utilizing this methodology, to the best our knowledge no in vivo therapy studies have yet been published. (27, 28)
In the current manuscript, we present the development, optimization, and in vivo validation of a pretargeted approach for the RIT of PDAC based on bioorthogonal click chemistry. We have employed 177Lu-labeled tetrazine radioligands (Fig. 1C) and a TCO-modified immunoconjugate of 5B1, a fully human antibody that targets the PDAC biomarker carbohydrate antigen 19.9 (CA19.9). (23, 24, 29–31) We demonstrate that this approach elicits a dose-dependent therapeutic response in mice bearing CA19.9-expressing BxPC3 human PDAC xenografts. Moreover, this strategy has proven effective while delivering only a small fraction of the radiation dose to healthy tissues delivered by traditional RIT with a directly-labeled 177Lu-5B1 construct.
Materials and methods
Cell lines and animal models
All studies were performed in accordance with Memorial Sloan Kettering Institutional Animal Care and Use Committee. All in vivo studies were carried out in female, athymic nude mice [Crl:NU(NCr)-Foxn1nu, 6–8 weeks) bearing subcutaneous BxPC3 xenografts that were inoculated on the right flank, as previously described. (29, 31) BxPC3 cells obtained from ATCC (Manassas, VA) were obtained in November 2014, validated via STR analysis, and used within 4 weeks of resuscitation. BxPC3 cells were grown in RPMI medium modified to contain 4.5 g/L glucose, 1.5g/L sodium bicarbonate and supplemented with 10% (vol/vol) heat-inactivated fetal calf serum, 100 IU penicillin, 100 µg/mL streptomycin, 10mM HEPES and 10 cc/L non-essential amino acids. Mice were xenografted subcutaneously with 5×106 cells, suspended in 150 µL of a solution containing a 1:1 mixture of Matrigel (Becton Dickinson, Bedford, MA) and cell culture medium. BxPC3 tumors were grown for 21–28 days post-implantation prior to imaging or biodistribution.
Preparation and characterization of 5B1-TCO
The N-hydroxysuccinyl ester of TCO [(E)-cyclooct-4-enyl 2,5-dioxo-1-pyrrolidinyl carbonate; TCO-NHS)] was conjugated to 5B1 as previously described. (23) The average number of TCO molecules per antibody was estimated by incubating the 5B1-TCO with >150-fold excess of a Tz-functionalized AlexaFluor 680™ (Tz-AF680) and quantifying the ratio of Tz-AF680 per antibody, after purification by gel filtration, via UV-Vis as previously described. (23–25) Further details are provided in the supporting information.
Synthesis and characterization of CHX-A˝-DTPA-PEG7-Tz (1) and DOTA-PEG7-Tz (2)
Both DOTA-PEG7-Tz and CHX-A˝-DTPA-PEG7-Tz were prepared via a three-step synthesis that is fully described in the supporting information (Fig. S1). In short, a commercially available, amine-reactive N-hydroxysuccinyl tetrazine (Tz-NHS) was coupled to a mono-Boc protected, bis-amino PEG7 molecule to form Tz-PEG7-NHBoc (1) which was subsequently deprotected in a mixture of methylene chloride and trifluoroacetic acid to form a common intermediate, Tz-PEG7-NH2 (2). Then, the p-isothiocyanate of DOTA or CHX-A˝-DTPA was coupled to yield the products; DOTA-PEG7-Tz and CHX-A˝-DTPA-PEG7-Tz. All products and intermediates were characterized by 1H-NMR (Fig. S2), 13C-NMR, ESI-MS, and high-resolution mass spectrometry (HR-MS).
Radiolabeling of DOTA-Tz and CHX-A˝-DTPA-Tz
DOTA-Tz and CHX-A˝-DTPA-Tz were radiolabeled (Fig. S3) by diluting stock solution (10 mg/mL in DMSO) into ammonia acetate buffer (200 mM, pH 5.4, 100 µL) prior to addition of 177LuCl3 in a 0.05M HCl solution (0.5 – 5 µL). The labeling solution was incubated at room temperature (RT) for 20 min before quenching with EDTA (50 mM, pH 5.5, 10 µL). The products were isolated via HPLC (0–100% MeCN, 15 min), and the solvent was removed on a rotary evaporator before reconstituting in 0.9% saline for injection.
Functional characterization of radioligands
The partition coefficient (log D) for both radioligands was determined in PBS and 1-octanol. In short, a small amount of radioligand (~100 µCi) was added to a 1:1 mixture of PBS and 1-octanol and the sample was vortexed for 10 min, and the layers separated by centrifugation (1000 rpm for 10 min). The layers were then placed into separate tubes for quantification by gamma counting. Additionally, the in vitro stability of each radioligand was analyzed in both PBS (pH 7.4) and human serum at 37 °C up to 48 h (Table S1). Aliquots of radiolabeled ligands (1.0 mCi) were added to PBS or serum and incubated at 37 °C. At various time points an aliquot was removed and analyzed by radio-iTLC. All experiments were performed in triplicate; additional details may be found in the supporting information.
Biodistribution
Tumor-bearing mice were injected via the lateral tail vein with 5B1-TCO (100 µg or 200 µg). For all biodistribution studies, mice that received 5B1-TCO were administered 40 – 50 µCi of 177Lu-DOTA-Tz or 177Lu-CHX-A˝-DTPA-Tz in an approximately 1.5:1 ratio of radioligand to 5B1-TCO (either 1nmol or 2nmol), which was delivered 72h after administration of the 5B1-TCO (Figs. 2, SI4–6, and Table S2). Additionally, one cohort was administered 1200 µCi to ensure that increasing the specific activity did not cause a deviation in total the biodistribution (Fig. This method was established as the optimal dosing strategy based on the in vivo behavior of 5B1 in previous studies. (23, 24) Tissues were collected and analyzed as previously described between 4 and 120 h. Further details are outlined in the supporting information.
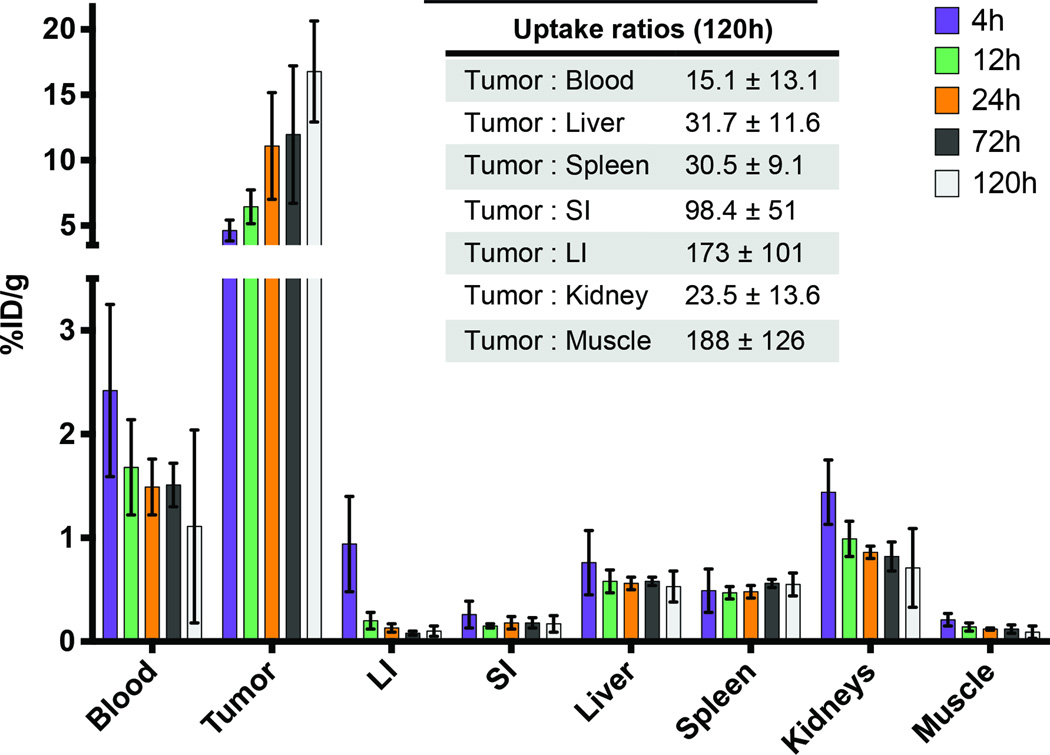
Figure 2.
Biodistribution data obtained from an in vivo pretargeting experiment in which 177Lu-DOTA-PEG7-Tz (2 nmol) was injected 72 h after the administration of 5B1-TCO (1.3 nmol). The activity concentrations in selected tissues at time points ranging from 4 to 120 h after the injection of 177Lu-DOTA-PEG7-Tz were determined along with selected tumor-to-tissue activity concentration ratios (inset).
Dosimetry
The biodistribution data for the 177Lu-Tz-PEG7-DOTA-based pretargeting system were expressed as normal-organ mean standard uptake values (SUVs) versus time post-administration. Assuming, in first order, that SUVs are independent of body mass and thus the same among species, the mean SUV in mouse organ i, SUVi, was converted to the fraction of the injected dose in the corresponding human organ I, FIDI, using the organ and total-body masses of the 70-kg Standard Man anatomic model and the following formula:
. These data (corrected for radioactive decay) were fit to exponential time-activity functions. The cumulated activity, or residence time, in human organ i (ti, in µCi-h/µCi) was calculated by integrating the time-activity function in organ i, replacing the biological clearance constant, (λb)x for each component, x, of the fitted exponential function with the corresponding effective clearance constant, (λe)x [(λe)x = (λb)x + λp, where λp is the physical decay constant of the radionuclide]. The resulting organ residence times were entered into the OLINDA computer program to yield the mean organ absorbed doses and effective dose in rad/mCi and rem/mCi, respectively (Tables 1 and 2).(32)
Table 1.
Absorbed doses and therapeutic index calculated using the biodistribution data for 177Lu-DOTA-PEG7-Tz + 5B1-TCO.
| Target Organ |
rad/mCi† | cGy/MBq | Therapeutic Index |
|---|---|---|---|
| Tumor | 20,571 | 556.0 | - |
| Blood | 1,804 | 48.8 | 11.4 |
|
Small Intestine |
126 | 3.4 | 163 |
| Stomach | 69.2 | 1.9 | 297 |
|
Large Intestine |
74.9 | 4.0 | 275 |
| Heart | 253 | 6.8 | 81.4 |
| Kidneys | 978 | 26.4 | 21.0 |
| Liver | 794 | 21.5 | 25.9 |
| Lungs | 835 | 22.6 | 24.6 |
| Muscle | 138 | 3.7 | 149 |
| Bone | 307 | 8.3 | 67.0 |
Table 2.
Dosimetry and absorbed dose estimations for 70kg Standard Man model calculated from the biodistribution of 177Lu-DOTA-PEG7-Tz + 5B1-TCO in a murine xenograft model.
| Organ | rad/mCi† | cGy/MBq | Absorbed dose (rad) – 70kg human | |
|---|---|---|---|---|
| 150mCi | 700mCi | |||
| Adrenals | 0.229 | 0.00619 | 34 | 160 |
| Brain | 0.222 | 0.00600 | 33 | 155 |
| Breasts | 0.215 | 0.00581 | 32 | 151 |
| Gallbladder Wall | 0.231 | 0.00624 | 35 | 162 |
| Lower Lg. Int. Wall | 0.234 | 0.00632 | 35 | 164 |
| Small Intestine | 0.282 | 0.00762 | 42 | 197 |
| Stomach Wall | 0.235 | 0.00635 | 35 | 165 |
| Upper Lg. Int. Wall | 0.231 | 0.00624 | 35 | 162 |
| Heart Wall | 0.263 | 0.00711 | 39 | 184 |
| Kidneys | 0.188 | 0.00508 | 28 | 132 |
| Liver | 0.229 | 0.00619 | 34 | 160 |
| Lungs | 0.223 | 0.00603 | 33 | 156 |
| Muscle | 0.156 | 0.00422 | 23 | 109 |
| Ovaries | 0.230 | 0.00622 | 35 | 161 |
| Pancreas | 0.230 | 0.00622 | 35 | 161 |
| Red Marrow | 0.213 | 0.00576 | 32 | 149* |
| Osteogenic Cells | 0.982 | 0.02654 | 147 | 687 |
| Skin | 0.211 | 0.00570 | 32 | 148 |
| Spleen | 0.146 | 0.00395 | 22 | 102 |
| Testes | 0.220 | 0.00595 | 33 | 154 |
| Thymus | 0.223 | 0.00603 | 33 | 156 |
| Thyroid | 0.223 | 0.00603 | 33 | 156 |
| Bladder Wall | 0.228 | 0.00616 | 34 | 160 |
| Uterus | 0.232 | 0.00627 | 35 | 162 |
| Total Body | 0.280 | 0.00757 | 42 | 196 |
| Effective Dose | 0.222 | 0.00600 | 33 | 155 |
Mean organ absorbed doses and effective dose are expressed in rad/mCi and rem/mCi.
Projected dose limiting organ for PRIT is bone marrow (MTD ~ 150 rad).
In vivo therapy
Tumor volumes for all mice were determined via caliper measurements of the longest dimension (x) and shortest dimension (y) and using the equation tumor volume (mm3) = x/2 * y2. After initial tumor measurements, mice were randomized into 5 cohorts (n = 7 – 8 per cohort) ensuring all cohort had approximately equal average tumor volumes. Seventy-two hours prior to administration of the radioligands, the three cohorts that were selected to receive therapy were administered 5B1-TCO (200 µg; 1.3 nmol), whereas the two control cohorts were administered vehicle only (0.9% sterile saline). Seventy-two hours later (Day 1) the tumors dimensions were measured again, and those resulting volumes set as the initial tumor volumes to which the relative growth was compared throughout the study. Immediately after Day 1 tumor measurements, mice in the therapy cohorts were then administered 400, 800, or 1200 µCi of 177Lu-DOTA-Tz (1.92 ± 0.06 nmol, 1.44 ± 0.05 Tz:mAb ratio). The control cohorts that had not been previously administered 5B1-TCO were injected with either vehicle (0.9% sterile saline) or 1200 µCi of 177Lu-DOTA-Tz (1.83 nmol). Tumor volume was monitored via caliper measurement every 3 to 7 days up to the predetermined endpoint of tumor volume (1000 mm3) or 51 Days (Fig. 3). All mice were assessed twice per week throughout the study for outward signs of toxicity, including lethargy, loss of appetite, and decreasing body weight. The endpoint for individual mice was defined as the point at which the volume of the xenograft was measured to be ≥1000 mm3, and the study was terminated when 50% of both control groups had reached the predetermined endpoint.
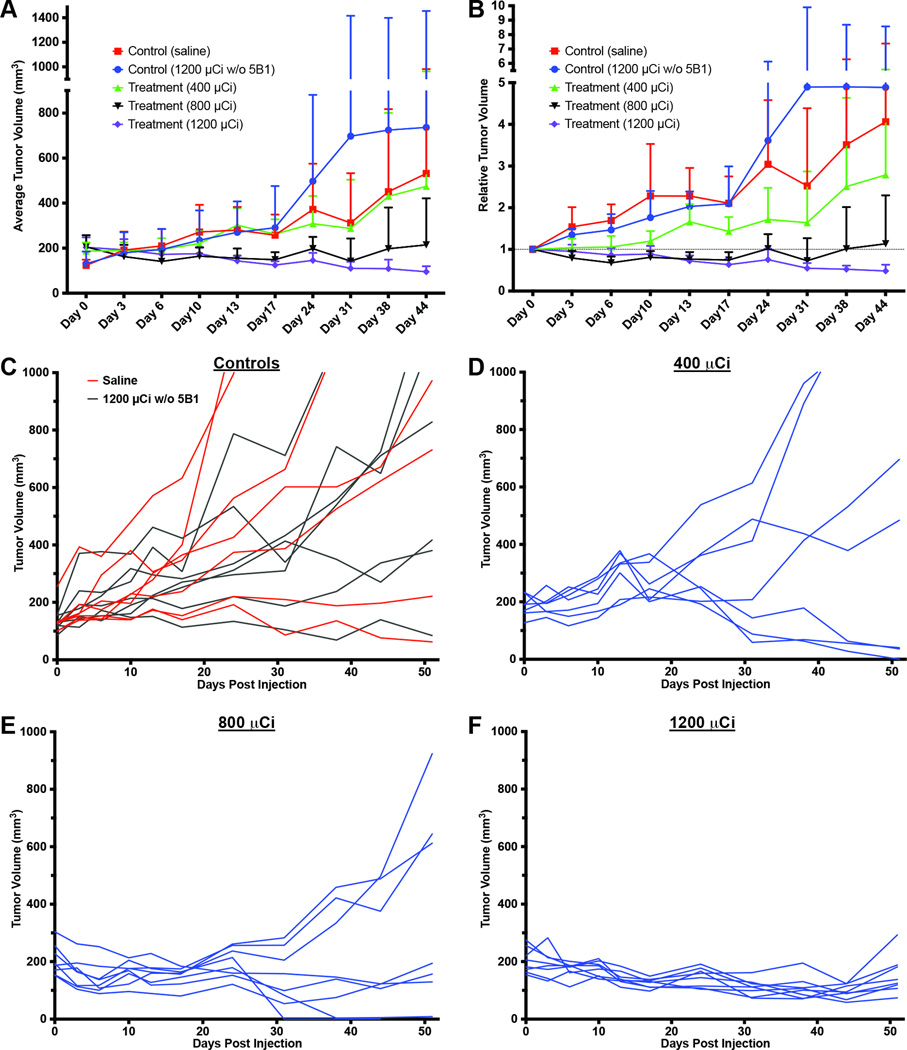
Figure 3.
Plots of the average tumor volume (A) and relative tumor volume (B) for each cohort of mice during the first 31 days of the PRIT study are shown with error bars denoting standard deviation. Line graphs mapping the response of each individual mouse over entire course of the PRIT study are shown for the control groups (C) as well as each therapy cohort (D–F).
PET imaging
89Zr-DFO-5B1 (89Zr-5B1) was prepared as previously described. (29) For this study, mice that still harbored viable tumor tissue of approximately the same size (200–450 mm3) were selected, including one from each control group and two each from the cohorts that received 800 or 1200 µCi in the in vivo PRIT study. The selected mice were injected with 89Zr-5B1 (118 ± 3 µCi, ~35 µg, ~0.23 nmol), and PET images were acquired at 120 h p.i. as previously described (Fig. 4A–B and S7). (29, 31) Further details regarding image acquisition, reconstruction, and processing are provided in the supporting information.
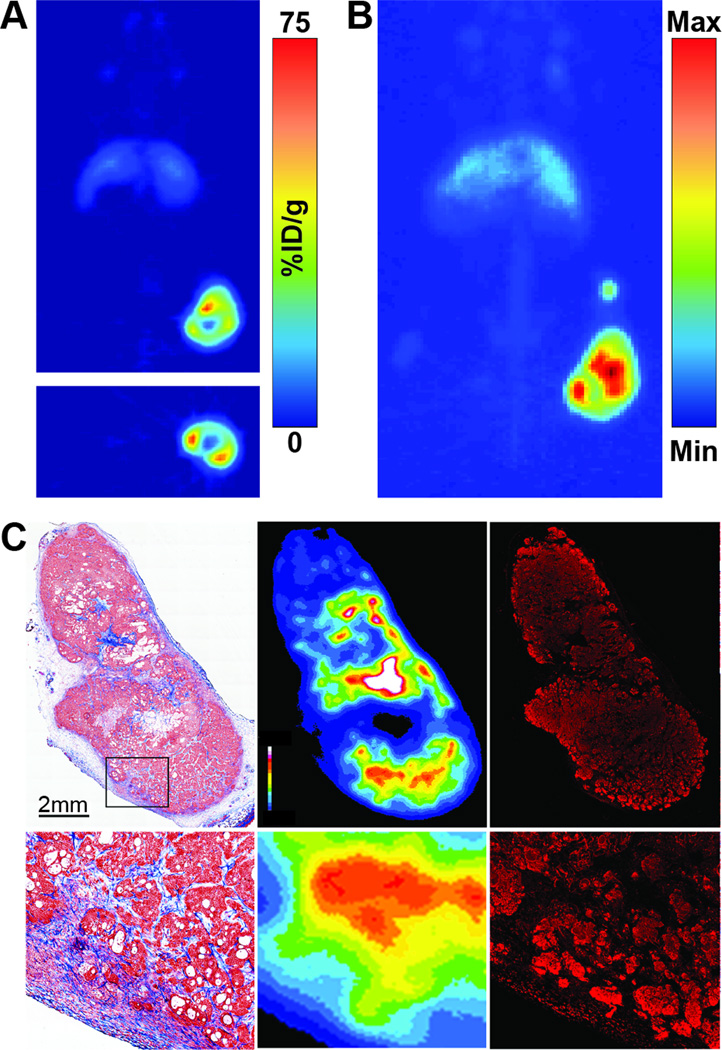
Figure 4.
Tomographic slices (A) and maximum intensity projections (B) of PET images (120 h post-injection) of a mouse from the 800 µCi therapy cohort that was injected with 89Zr-5B1 at the conclusion of the therapy study. Masson’s trichrome (C, left), autoradiography (C, middle), and anti-CA19.9 immunofluorescence (C, right) on the excised tumor of the same mouse at the conclusion of the imaging study.
Ex vivo analysis
BxPC3 xenografts were excised and one each was either embedded in Tissue–Plus OCT compound and frozen on dry ice or fixed with 4% paraformaldehyde (PFA) solution and subsequently sectioned (10 µm). Masson’s trichrome staining, autoradiography, and anti-CA19.9 immunofluorescence staining were performed on sequential sections as previously described (Fig. 4C). (29, 31) Further details may be found in the supporting information.
Statistical analysis
To analyze the effect of therapy within each cohort, the therapeutic response was assessed using a random effect model.(33) In this model it is assumed that the log volume-time profile of each mouse deviates in a random fashion from an average profile. The model for mouse i, in which μ is the average intercept, αi is a random intercept (accounting for random effect) for each mouse, β is the slope (treatment effect over time), and ci is a random error term, is:
Using a Likelihood Ratio Test, the above model was used to test for a significant change in log tumor volume over time, within each cohort and for pair-wise comparisons between cohorts. For each cohort, we use the above model to estimate the doubling or halving time and the delta method to calculate a 95% confidence interval for our estimate.(34) All models were fit using the lme4 package in R version 3.1.1.
Results
Preparation and characterization of PRIT components
For this investigation, 5B1-TCO was synthesized via conjugation of the antibody with TCO-NHS and characterized in vitro as previously reported. (23)
Radioligand precursors were successfully synthesized in three steps: the coupling of Tz-NHS and O-(2-aminoethyl)-O’-[2-(bocamino)ethyl]hexaethylene glycol to form Tz-PEG7-NHBoc, the removal of the protecting group with TFA/CH2Cl2, and the ligation of Tz-PEG7-NH2 with either p-NCS-Bn-DOTA or p-NCS-Bn-CHX-A′-DTPA (Fig. S1). In this way, Tz-PEG7-CHX-A′-DTPA (1) and Tz-PEG7-DOTA (2) and were synthesized in 87% and 73% yield, respectively. In both cases, the chemical structure of all compounds was confirmed via 1H-NMR (Fig. S2), 13C-NMR, ESI-MS, and high-resolution mass spectrometry.
The chelator-modified tetrazines were radiolabeled via incubation with [177Lu]-LuCl3 for 10 minutes at 37 °C in 0.25 mM NH4OAc, pH 5.5 (Fig. S3). 177Lu-CHX-A′-DTPA-PEG7-Tz (177Lu-1) was produced in >99% radionuclidic purity, 99.5% radiochemical yield, and a specific activity of 383 mCi/µmol (n=3), while 177Lu-DOTA-PEG7-Tz (177Lu-2) was produced in >96% radionuclidic purity, 96.9 ± 0.74% radiochemical yield, and a specific activity of 466 ± 155 mCi/µmol (n = 3).
Functional characterization of radioligands
The partition coefficients of the radioligands were determined using 1-octanol and PBS (pH 7.4), yielding values for the two hydrophilic compounds of −3.7 ± 0.1 for 177Lu-DOTA-PEG7-Tz and −3.2 ± 0.2 for 177Lu- CHX-A′-DTPA-PEG7-Tz. These values suggest that there is little difference in the hydrophobicity of the ligands. Stability assays revealed that both radioligands are quite stable, with >85% and >80% of each radioligand remaining intact after an incubation of 48 h in PBS and serum, respectively (Table S1). These rates of decomposition are unlikely to hamper the in vivo performance of the system, as the clearance half-times of the radioligands are short. Ultimately, owing to the similar physical properties and the relative ease of synthesis and purification, we chose to evaluate both radioligands in preliminary in vivo investigations.
Biodistribution
Biodistribution studies were performed with three goals in mind: (1) the identification of the appropriate dose of 5B1-TCO to give for PRIT in this xenograft model; (2) a side-by-side comparison of both radioligands to determine the best candidate for in vivo PRIT studies; and (3) the quantification of the uptake and clearance of the preferred radioligand for dosimetry calculations. We first performed a pilot study in which mice with BxPC3 xenograft-bearing mice were administered either 100µg or 200µg of 5B1-TCO, followed by 1.5 molar equivalents of 177Lu-CHX-A′-DTPA-PEG7-Tz. We chose to analyze only one radioligand for this study in order to reduce the number of animals needed. We found that administration of 200 µg of 5B1-TCO led to significantly better tumor accumulation and similar or even better tumor-to-tissue activity concentration ratios (Fig. S4A–B), consistent with previously reported PET imaging studies using 5B1-TCO as a targeting vector. (23, 24)
Next, we compared the two radioligands using the optimal amount of 5B1-TCO. This investigation showed that pretargeting with 177Lu-DOTA-PEG7-Tz produced significantly higher tumor activity concentrations than pretargeting with 177Lu-CHX-A′-DTPA-PEG7-Tz: 12.0 ± 5.3 %ID/g and 4.3 ± 1.8 %ID/g at 72 h post-injection, respectively (Figs. 2 and S4C–D). This led us to choose the former for in vivo therapy studies. A final biodistribution was performed by administering 200 µg of 5B1-TCO followed 72 h later by 1.5 molar equivalents of 177Lu-DOTA-PEG7-Tz and collecting tissues at 5 time points between 4 and 120 h. The activity concentrations observed in the tumor tissue were high, reaching 16.8 ± 3.87 %ID/g at 120 h post-injection (Fig. 2, S5, and Table S2). Clearance from background tissue was rapid, with all tumor-to-tissue activity concentration ratios exceeding 15:1 at 120h (Fig. 2, inset).
Dosimetry
The mean organ absorbed doses for mice and the extrapolation of this data to the 70kg Standard Man model are reported in Tables 1 and 2, respectively. The ratio of the mean organ absorbed dose in mice relative to that to the tumor (‘therapeutic index’) is also reported. Calculating the absorbed dose in humans using the dosimetry data suggests that a 150 mCi administration of radioligand would not exceed the MTD in off-target tissues in humans. Assuming a MTD for red marrow of 150 rad, the maximum tolerated administered activity would be approximately 700mCi (Table 2). (7, 35)
In vivo therapy
In order to establish dose response using the optimized PRIT protocol, mice (n = 7–8 per cohort) were administered 5B1-TCO 72 h prior to the injection of 400, 800, or 1200 µCi of 177Lu-DOTA-Tz. Control groups were not administered 5B1-TCO and were instead injected either with vehicle or 1200 µCi of 177Lu-DOTA-Tz. One mouse from the 800 µCi therapy cohort was euthanized after 24 days due to an infection, although it is unclear whether this was event was related to the therapy study. No other mice showed any outward signs of toxicity, and all mice maintained an acceptable body weight (>80% original weight) throughout the course of the study. Average weight loss for any cohort never exceeded 6%, and there was not any significant difference among any of the therapy or control cohorts at any time point. These results suggest that the treatment was well tolerated up to the highest administered therapeutic dose of 1200 µCi.
Following the average tumor volume for the first 31 days after treatment clearly shows a differential response that improves (more dramatic growth delay or shrinkage) with increasing 177Lu activity in the therapy cohorts (Fig. 3A–B). Analysis of the growth-delay effects across each cohort becomes convoluted after that point, as mice with tumors >1000 mm3 are removed from that point on. As of 51 days post-treatment, 75% (6 of 8) of the mice from the therapy cohort receiving 1200 µCi of 177Lu-DOTA-Tz had tumors that were smaller than they had been on Day 1.
A subjective analysis of the average tumor volume and relative tumor volume measurements (Fig. 3A–B) suggested therapeutic efficacy in both the 800 µCi and 1200 µCi therapy cohorts, which was sustained until the endpoint of the experiment. Some heterogeneity in response within cohorts was observed, especially for the 800-µCi cohort, as depicted by the line graphs following each individual mouse (Fig. 3C–F). This may be attributed to variations in the CA19.9 expression among tumors or differences in tumor volumes at the beginning of the study. To account for that variance and objectively examine the data over the course of the entire study, the data were analyzed using a random effect model (Table S3). The statistical analysis showed that the therapeutic response was statistically significant for 1200-µCi cohort relative to both controls and the 400-µCi cohort.
PET imaging
89Zr-5B1 PET images acquired in 6 selected mice at the conclusion of the in vivo PRIT study all showed demonstrable uptake of the tracer in tumor (Fig. 4A–B and S7). The mice that received PRIT showed marginally higher uptake of the tracer in the tumor at 120 h post-injection relative to the controls, though no conclusions as to the cause of that increase can be drawn due to the small sample size.
Ex vivo analysis
Ex vivo analysis of 89Zr-5B1 distribution via autoradiography revealed a non-uniform microscopic distribution of the tracer within the tumor. The areas of highest activity concentration were observed to be close to regions with a higher density of tumor stroma (Fig. 4C), which most likely indicates delivery of the radiotracer via the tumor vasculature. Diffusion of 89Zr-5B1 from these sites is also observed, and association with regions of 5B1 expression (Fig. 4C) is also seen. This uptake pattern of the radiotracer can be explained by a combination of slow diffusion from the site of vascular delivery and the high affinity binding of 89Zr-5B1 to local sites of high CA19.9 that prevents further tumor penetration. Minimal uptake of 89Zr-5B1 was observed in regions of central tumor necrosis.
Discussion
Our approach to PRIT is based on our previously reported strategy for the pretargeted PET imaging of PDAC. (23) The system employed two components, a 64Cu-labeled tetrazine radioligand (64Cu-Tz-PEG7-NOTA) and a trans-cyclooctene-conjugated 5B1 antibody (5B1-TCO). 5B1 is a fully human monoclonal antibody that targets carbohydrate antigen 19.9 (CA19.9), a cell surface antigen and a biomarker that has shown clinical value for the diagnosis, staging, and prognostic evaluation of pancreatic cancer. Critically, our results demonstrate that the delineation of CA19.9-expressing tissues via pretargeted PET is possible despite the shedding of CA19.9 from cells and the moderate internalization rates of 5B1 after binding its antigen. (23) Not surprisingly, however, a shift in the structure of the radioligand was needed. The β-emitting lanthanide 177Lu3+ (t1/2 ~ 6.7 d) requires a different chelator than the divalent 64Cu2+ (t1/2 ~ 12.7 h) that was used for the PET imaging system. As a result, two novel tetrazine-bearing radioligands were designed for the current project, 177Lu-DOTA-PEG7-Tz and 177Lu-CHX-A′-DTPA-PEG7-Tz (Fig. 1C). These two constructs each employ a well-characterized and clinically validated chelator for 177Lu3+, DOTA and CHX-A′-DTPA, as well as a PEG7 linker and Tz functionality. The inclusion of the PEG7 linker in this type of molecule has been shown to expedite the hepatobiliary clearance while maintaining the capability of similar radioligands to target TCO-conjugated biomolecules in tumor tissues.
The two radioligands that we assessed, 177Lu-DOTA-PEG7-Tz and 177Lu-CHX-A′-DTPA-PEG7-Tz, did not produce equal tumor activity concentrations in pretargeting experiments with 5B1-TCO. Indeed, our pilot biodistribution studies in mice with BxPC3 xenografts indicated that pretargeting with 177Lu-DOTA-PEG7-Tz and 5B1-TCO produced higher tumor activity concentrations at 72 h post-injection than pretargeting with 177Lu-CHX-A′-DTPA-PEG7-Tz and 5B1-TCO (Fig. S3). The trends in the biodistribution data do not offer a definitive explanation for the superior performance of the DOTA-bearing radioligand compared to its CHX-A′-DTPA-modified counterpart, but the latter did show a modest increase in retention in the large intestine and kidneys (Fig. S4). Furthermore, the stability and log D studies do little to shed any additional light on the differing uptake of the two ligands. As may have been expected, the radioligand with the cyclic chelator DOTA showed slightly higher stability in serum (Table S1), but this would likely not account for the >2.5-fold increase in tumor activity concentrations observed in the pretargeting biodistribution experiments. Despite the lack of a cogent explanation for the differential behavior of these two radioligands,177Lu-DOTA-PEG7-Tz was chosen for more thorough in vivo biodistribution, dosimetry, and, most importantly, therapy studies.
Further studies in which the biodistribution of the 177Lu-DOTA-PEG7-Tz 72 h after administration of 5B1-TCO confirmed the high uptake and, perhaps more importantly, long retention in tumor tissue up to 5 days post-injection (Fig. 2). Clearance from non-target tissues was rapid and led to remarkably high tumor-to-normal tissue activity concentration ratios at 120 h (Fig. 2, inset). The gradually increasing uptake and long retention seen with this system are an advantage over other PRIT systems that rely solely on non-covalent interactions between radioligands and immunoconjugates. Ligands that rely on such interactions may clear from tumor tissue in a matter of hours after reaching maximal uptake. Of course, this is not ideal when using an isotope (e.g. 177Lu) that delivers its radioactive payload over the course of weeks. By covalently linking the radioligand to the targeting vector — in this case 5B1-TCO — and achieving a rapid clearance from non-target tissues, a higher proportion of the injected activity may be delivered to the tumor tissue while minimizing the risk of radiogneic toxicity in non-target tissues.
The dosimetry estimations for a 70kg Standard Man model suggest that our PRIT approach may efficiently deliver cytotoxic radiation to tumor tissues without causing significant side-effects in humans. The most common dose-limiting tissue in radioimmunotherapy is the red marrow, the maximum tolerated dose being approximately 150cGy. (7) Organs that are typically associated with the clearance of antibody-based agents are the kidneys, liver, and spleen and those organs can typically tolerate doses of greater than 1500cGy. (7, 35) The data indicate that with a single administration of 200 mCi (7400MBq) of radioligand the predicted maximum tolerated dose in any off-target tissue would not be reached (Table 2). In our estimation, the MTD in humans would be reached at a dose of approximately 700 mCi (25,900 MBq), with the dose-limiting organ most likely being the red marrow (Table 2). Although it is not possible to reliably predict the radiation dose and resulting therapeutic response in human tumors, a retrospective evaluation of previous RIT approaches, both directly targeted and pretargeted, indicates that our approach meets or exceeds the dose benchmarks in preclinical murine models that would likely be necessary to achieve therapeutic response in humans.
Previously reported PRIT systems have introduced a clearing agent via an additional injection between those of the targeting vector and the radioligand. The function of the clearing agent is to reduce the amount of accessible binding or reactive sites on the targeting vector that is still circulating in the blood at the time of radioligand injection, thus reducing the dose to sensitive, non-target organs such as the red marrow from the subsequent injection of the radioligand. Based on our biodistribution and dosimetry results, our system may not require the use of a clearing agent, an added advantage over other systems. Previous studies have nonetheless shown that clearing agents are effective in the context of TCO- and Tz-based imaging systems (26), and this approach could certainly be adopted in the future if necessary.
PRIT studies confirmed that our approach was effective in causing significant growth delay and even regression of BxPC3 xenografts at the two highest activities tested, whereas the growth delay observed with the lowest activity (400 µCi) was not statistically significant. While detailed statistical analysis of each cohort is complicated as mice from each cohort began to reach the endpoint, the trend is clear, and statistical significance was rapidly achieved in the control groups and both the 800- and the 1200-µCi cohorts.
Interestingly, line graphs (Fig. 3C–F) depicting the tumor size of each individual mouse within a cohort show that, perhaps with the exception of the 1200 µCi therapy cohort, not all tumors within a particular cohort responded in a similar manner. This is likely due to differences in the initial uptake of the 5B1-TCO, the heterogeneity of CA19.9 expression, and the tumor sizes. We have observed this same variation in uptake in BxPC3 xenografts with a number of 5B1-based radiotracers in the past. (23, 29, 31) In fact, heterogeneity is a common characteristic of many types of human malignancies, especially PDAC. While no quantitative PET imaging was carried out prior to the initiation PRIT in this study, we believe that 89Zr-5B1, which is currently being evaluated in clinical trials for patients with PDAC, may be useful as a diagnostic tool.
The results of the post-PRIT PET imaging study with 89Zr-5B1 (Figs. 4A and S7) suggest that CA19.9 expression persists post-therapy in tumors that remain viable after treatment. This suggests that 89Zr-5B1 PET imaging could be used to monitor therapeutic response as well as to determine whether a patient may potentially be responsive to multiple administrations of fractionated PRIT. Pretargeted PET imaging with recently reported 18F- or 64Cu-labeled Tz ligands would likely also be effective. Using pretargeted imaging with short-lived isotopes would rapidly provide high-quality images while reducing the radiation. Using the pretargeted imaging approach would also provide a more straightforward assessment of the possibility of delivering additional cytotoxic radiation to the tumors via additional rounds of PRIT. Each of these potential theranostic approaches are currently being investigated in murine models of PDAC.
Ex vivo analysis showed that delivery of 5B1-based imaging and therapy agents may be limited by diffusion and trapping of the agents by areas of high CA19.9 density near sites of vascular delivery. It is likely that adjustments to the specific activity and/or injected mass of 5B1 could influence this distribution, as could the length of time allowed between administration and assessment. PDAC tumors are known for their dense desmoplastic stroma, and tumor penetration may well be a concern in the translation of antibody-based PRIT. However, this constraint may be alleviated by the use of compounds that act to increase stromal permeability (36). Further studies to evaluate the effectiveness of such an approach are currently underway in our laboratory.
In conclusion, our in vivo therapy results have shown that our bioorthogonal pretargeting system utilizing Tz-based radioligands and TCO-modified immunoconjugates is a promising platform for the development of PRIT systems. It is important to note that the inherent complexity of pretargeted immunotherapy can create complications as we move toward the clinic. Indeed, the translation and clinical optimization of a multi-component system will undoubtedly be more cumbersome than analogous approaches that utilize a single agent. Nonetheless, we are confident that the benefits of our PRIT strategy — and especially future generations — will outweigh any logistical drawbacks. Along these lines, we believe that this platform warrants further optimization and a number of prospective routes could lead to improved results. For example, previous studies on PRIT systems that employ a fractionated dosing schedule have shown promise, with some demonstrating equal absorbed doses in non-target organs and significant improvements in response rates compared to a single administration of the radioligand. Our laboratories are currently working to establish MTD in preparation for fractioned PRIT studies using the system reported herein. Additionally, in future experiments we will assess the ability of 5B1-based PET imaging to quantify CA19.9 levels prior to therapy in order to evaluate 89Zr-5B1 as a theranostic tool for predicting response to 5B1-based PRIT.
Supplementary Material
Acknowledgments
Conflict of Interest Statement: Woflgang W. Scholz is an employee of MabVax Therepeutics.
Financial support: The MSKCC Small Animal Imaging Core Facility as well as the Radiochemistry and Molecular Imaging Probe core were supported in part by NIH grant P30 CA08748. J.L. Houghton received 1F32CA180452-01A1 and 5R25CA096945-09. W.S. Scholz received 2R42CA128362 and HHSN261201300060C. B.M. Zeglis received 1K99CA178205-01A1 and 4R00CA178205-02. All experiments were supported by The Experimental Therapeutics Center at MSKCC.
We would like to thank the staff of the Small Animal Imaging Core Facility, the Radiochemistry and Molecular Imaging Probe Core Facility, and The Center for Experimental Therapeutics at MSKCC.
References
- 1.Goldenberg DM. Targeted therapy of cancer with radiolabeled antibodies. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2002;43:693–713. [PubMed] [Google Scholar]
- 2.Jurcic JG, Larson SM, Sgouros G, McDevitt MR, Finn RD, Divgi CR, et al. Targeted α particle immunotherapy for myeloid leukemia. Blood. 2002;100:1233–1239. [PubMed] [Google Scholar]
- 3.Kaminski MS, Estes J Fau, Zasadny KR, Zasadny Kr Fau, Francis IR, Francis Ir Fau, Ross CW, Ross Cw Fau, Tuck M, Tuck M Fau, Regan D, et al. Radioimmunotherapy with iodine (131)I tositumomab for relapsed or refractory B-cell non-Hodgkin lymphoma: updated results and long-term follow-up of the University of Michigan experience. Blood. 2000;96:1259–1266. [PubMed] [Google Scholar]
- 4.Witzig TE, Gordon LI, Cabanillas F, Czuczman MS, Emmanouilides C, Joyce R, et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:2453–2463. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- 5.Ugur O, Kostakoglu L, Hui ET, Fisher DR, Garmestani K, Gansow OA, et al. Comparison of the targeting characteristics of various radioimmunoconjugates for radioimmunotherapy of neuroblastoma: dosimetry calculations incorporating cross-organ beta doses. Nuclear medicine and biology. 1996;23:1–8. doi: 10.1016/0969-8051(95)02001-2. [DOI] [PubMed] [Google Scholar]
- 6.Huang CY, Pourgholami Mh Fau, Allen BJ, Allen BJ. Optimizing radioimmunoconjugate delivery in the treatment of solid tumor. Cancer Treat Rev. 2012;38:854–860. doi: 10.1016/j.ctrv.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Larson SM, Carrasquillo JA, Cheung NK, Press OW. Radioimmunotherapy of human tumours. Nature reviews Cancer. 2015;15:347–360. doi: 10.1038/nrc3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viola-Villegas NT, Sevak KK, Carlin SD, Doran MG, Evans HW, Bartlett DW, et al. Noninvasive Imaging of PSMA in prostate tumors with (89)Zr-Labeled huJ591 engineered antibody fragments: the faster alternatives. Mol Pharm. 2014;11:3965–3973. doi: 10.1021/mp500164r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe R, Hanaoka H, Sato K, Nagaya T, Harada T, Mitsunaga M, et al. Photoimmunotherapy targeting prostate-specific membrane antigen: are antibody fragments as effective as antibodies? Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2015;56:140–144. doi: 10.2967/jnumed.114.149526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frampas E, Rousseau C, Bodet-Milin C, Barbet J, Chatal JF, Kraeber-Bodere F. Improvement of radioimmunotherapy using pretargeting. Frontiers in oncology. 2013;3:159. doi: 10.3389/fonc.2013.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheal SM, Xu H, Guo HF, Zanzonico PB, Larson SM, Cheung NK. Preclinical evaluation of multistep targeting of diasialoganglioside GD2 using an IgG-scFv bispecific antibody with high affinity for GD2 and DOTA metal complex. Molecular cancer therapeutics. 2014;13:1803–1812. doi: 10.1158/1535-7163.MCT-13-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraeber-Bodere F, Rousseau C, Bodet-Milin C, Ferrer L, Faivre-Chauvet A, Campion L, et al. Targeting, toxicity, and efficacy of 2-step, pretargeted radioimmunotherapy using a chimeric bispecific antibody and 131I-labeled bivalent hapten in a phase I optimization clinical trial. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2006;47:247–255. [PubMed] [Google Scholar]
- 13.Mallikaratchy P, Gardner J, Nordstrom LU, Veomett NJ, McDevitt MR, Heaney ML, et al. A self-assembling short oligonucleotide duplex suitable for pretargeting. Nucleic acid therapeutics. 2013;23:289–299. doi: 10.1089/nat.2013.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohsin H, Jia F, Bryan JN, Sivaguru G, Cutler CS, Ketring AR, et al. Comparison of pretargeted and conventional CC49 radioimmunotherapy using 149Pm, 166Ho, and 177Lu. Bioconjugate chemistry. 2011;22:2444–2452. doi: 10.1021/bc200258x. [DOI] [PubMed] [Google Scholar]
- 15.Pagel JM, Kenoyer AL, Back T, Hamlin DK, Wilbur DS, Fisher DR, et al. Anti-CD45 pretargeted radioimmunotherapy using bismuth-213: high rates of complete remission and long-term survival in a mouse myeloid leukemia xenograft model. Blood. 2011;118:703–711. doi: 10.1182/blood-2011-04-347039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blackman ML, Royzen M, Fox JM. Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels-Alder Reactivity. Journal of the American Chemical Society. 2008;130:13518–13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiner T, Zeglis BM. The inverse electron demand Diels-Alder click reaction in radiochemistry. Journal of labelled compounds & radiopharmaceuticals. 2014;57:285–290. doi: 10.1002/jlcr.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devaraj NK, Upadhyay R, Haun JB, Hilderbrand SA, Weissleder R. Fast and sensitive pretargeted labeling of cancer cells through a tetrazine/trans-cyclooctene cycloaddition. Angewandte Chemie (International ed in English) 2009;48:7013–7016. doi: 10.1002/anie.200903233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossin R, van den Bosch SM, Ten Hoeve W, Carvelli M, Versteegen RM, Lub J, et al. Highly reactive trans-cyclooctene tags with improved stability for Diels-Alder chemistry in living systems. Bioconjugate chemistry. 2013;24:1210–1217. doi: 10.1021/bc400153y. [DOI] [PubMed] [Google Scholar]
- 20.Rossin R, van Duijnhoven SM, Lappchen T, van den Bosch SM, Robillard MS. Trans-cyclooctene tag with improved properties for tumor pretargeting with the diels-alder reaction. Mol Pharm. 2014;11:3090–3096. doi: 10.1021/mp500275a. [DOI] [PubMed] [Google Scholar]
- 21.Zeglis BM, Sevak KK, Reiner T, Mohindra P, Carlin SD, Zanzonico P, et al. A pretargeted PET imaging strategy based on bioorthogonal Diels-Alder click chemistry. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2013;54:1389–1396. doi: 10.2967/jnumed.112.115840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossin R, Verkerk PR, van den Bosch SM, Vulders RC, Verel I, Lub J, et al. In vivo chemistry for pretargeted tumor imaging in live mice. Angewandte Chemie (International ed in English) 2010;49:3375–3378. doi: 10.1002/anie.200906294. [DOI] [PubMed] [Google Scholar]
- 23.Houghton JL, Zeglis BM, Abdel-Atti D, Sawada R, Scholz WW, Lewis JS. Pretargeted Immuno-PET of Pancreatic Cancer: Overcoming Circulating Antigen and Internalized Antibody to Reduce Radiation Doses. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016;57:453–459. doi: 10.2967/jnumed.115.163824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer JP, Houghton JL, Kozlowski P, Abdel-Atti D, Reiner T, Pillarsetty NV, et al. (18)F-Based Pretargeted PET Imaging Based on Bioorthogonal Diels-Alder Click Chemistry. Bioconjugate chemistry. 2016;27:298–301. doi: 10.1021/acs.bioconjchem.5b00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeglis BM, Brand C, Abdel-Atti D, Carnazza KE, Cook BE, Carlin S, et al. Optimization of a Pretargeted Strategy for the PET Imaging of Colorectal Carcinoma via the Modulation of Radioligand Pharmacokinetics. Mol Pharm. 2015;12:3575–3587. doi: 10.1021/acs.molpharmaceut.5b00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossin R, Lappchen T, van den Bosch SM, Laforest R, Robillard MS. Diels-Alder reaction for tumor pretargeting: in vivo chemistry can boost tumor radiation dose compared with directly labeled antibody. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2013;54:1989–1995. doi: 10.2967/jnumed.113.123745. [DOI] [PubMed] [Google Scholar]
- 27.Rossin R, Robillard MS. Pretargeted imaging using bioorthogonal chemistry in mice. Curr Opin Chem Biol. 2014;21:161–169. doi: 10.1016/j.cbpa.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 28.van Duijnhoven SM, Rossin R, van den Bosch SM, Wheatcroft MP, Hudson PJ, Robillard MS. Diabody Pretargeting with Click Chemistry In Vivo. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2015;56:1422–1428. doi: 10.2967/jnumed.115.159145. [DOI] [PubMed] [Google Scholar]
- 29.Viola-Villegas NT, Rice SL, Carlin S, Wu X, Evans MJ, Sevak KK, et al. Applying PET to broaden the diagnostic utility of the clinically validated CA19.9 serum biomarker for oncology. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2013;54:1876–1882. doi: 10.2967/jnumed.113.119867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girgis MD, Kenanova V, Olafsen T, McCabe KE, Wu AM, Tomlinson JS. Anti-CA19-9 diabody as a PET imaging probe for pancreas cancer. The Journal of surgical research. 2011;170:169–178. doi: 10.1016/j.jss.2011.03.065. [DOI] [PubMed] [Google Scholar]
- 31.Houghton JL, Zeglis BM, Abdel-Atti D, Aggeler R, Sawada R, Agnew BJ, et al. Site-specifically labeled CA19.9-targeted immunoconjugates for the PET, NIRF, and multimodal PET/NIRF imaging of pancreatic cancer. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:15850–15855. doi: 10.1073/pnas.1506542112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2005;46:1023–1027. [PubMed] [Google Scholar]
- 33.Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. New York: Springer; 2000. [Google Scholar]
- 34.Casella G, Berger RL. Statistical inference. 2nd. Australia; Pacific Grove, CA: Thomson Learning; 2002. [Google Scholar]
- 35.Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. International journal of radiation oncology, biology, physics. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 36.Whatcott CJ, Han H, Posner RG, Hostetter G, Von Hoff DD. Targeting the tumor microenvironment in cancer: why hyaluronidase deserves a second look. Cancer Discov. 2011;1:291–296. doi: 10.1158/2159-8290.CD-11-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.