Abstract
Hsp90 inhibitors have been investigated as cancer therapeutics in mono-therapy and to augment radiotherapy, however serious adverse effects of early generation Hsp90 inhibitors limited their development. TAS-116 is a novel Hsp90 inhibitor with lower adverse effects than other Hsp90 inhibitors, and here we investigated the radio-sensitizing effects of TAS-116 in low LET X-ray, and high LET carbon ion irradiated human cancer cells and mouse tumor xenografts. TAS-116 decreased cell survival of both X-ray and carbon ion-irradiated human cancer cell lines (HeLa and H1299 cells), and similar to other Hsp90 inhibitors, it did not affect radiosensitivity of non-cancerous human fibroblasts. TAS-116 increased the number of radiation-induced γ-H2AX foci, and delayed the repair of DNA double-strand breaks (DSBs). TAS-116 reduced the expression of proteins that mediate repair of DSBs by homologous recombination (RAD51) and non-homologous end joining (Ku, DNA-PKcs), and suppressed formation of RAD51 foci and phosphorylation/activation of DNA-PKcs. TAS-116 also decreased expression of the cdc25 cell cycle progression marker, markedly increasing G2/M arrest. Combined treatment of mouse tumor xenografts with carbon ions and TAS-116 showed promising delay in tumor growth compared to either individual treatment. These results demonstrate that TAS-116 radio-sensitizes human cancer cells to both X rays and carbon ions by inhibiting the two major DSB repair pathways, and these effects were accompanied by marked cell cycle arrest. The promising results of combination TAS-116 + carbon ion radiation therapy of tumor xenografts justify further exploration of TAS-116 as an adjunct to radiotherapy using low or high LET radiation.
Keywords: TAS-116, Hsp90 inhibitor, radio-sensitization, X- rays, carbon ions
Introduction
Heat shock protein 90 (Hsp90) is a chaperone protein that regulates the function of tumor growth-related proteins, and Hsp90 inhibition is considered a useful strategy for treating cancer (1). Importantly, Hsp90 inhibition selectively affects tumor cells (1–3), because Hsp90 proteins in tumor cells are present in complexes with higher ATPase activity than those in normal cells (4, 5). Hsp90 inhibition suppresses levels of key DNA double-strand break (DSB) repair proteins (1–3). Thus, Hsp90 inhibitors are promising agents for cancer therapy, including combined treatment with radiation (1, 6). As adverse side effects have been reported with early Hsp90 inhibitors (1, 7), development of novel Hsp90 inhibitors is urgently needed to overcome these limitations. One of the side effects induced by early generation Hsp90 inhibitors is visual impairment (8, 9). TAS-116, a novel Hsp90 inhibitor, has shown less ocular toxicity than conventional Hsp90 inhibitors (10), so we explored whether TAS-116 presents a useful alternative with reduced adverse effects in chemo-radiotherapy with X-rays or carbon ion radiation. There are only three prior reports of TAS-116 effects, showing antitumor activity against multiple myeloma and lung tumors (10–12). The biological effects of TAS-116 are poorly understood, and the effects of combined TAS-116 and radiation therapy has not been previously investigated.
Radiotherapy using carbon ions has received great attention because it offers improved tumor targeting and increased biological effectiveness (13, 14). Unlike low LET radiation, high LET radiation such as carbon ions overcomes radio-resistance of S-phase and hypoxic cells (15, 16), and it induces more complex DSBs that are repaired more slowly than X-ray induced DSBs (14, 17). Due to these and other differences, radio-sensitizing agents may differentially influence cellular responses to low and high LET radiation, including DNA repair, DNA damage signaling, cell cycle arrest, and activation of cell death pathways. These processes are critical determinants of cell and tumor responses to radiotherapy, thus it is important to study the mechanistic basis of radio-sensitization in cells irradiated with low and high LET radiation.
DSB repair is an important determinant of cellular radio-sensitivity (18, 19). DSBs are repaired by homologous recombination (HR) and non-homologous end joining (NHEJ), and cells with defects in HR or NHEJ repair show increased radio-sensitivity (20–22). The Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin (17-AAG) was found to inhibit HR and enhance radio-sensitivity (2, 3). DSB repair pathways and other processes fluctuate during the cell cycle, hence cell cycle distribution is another determinant of radio-sensitivity (23). Hsp90 inhibitors have been shown to alter cell cycle distribution by regulating cell cycle checkpoints. The Hsp90 inhibitors NVP-AUY922 and 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG) induce G2/M arrest in A549 cells by downregulating Cdk1 and Cdk4 (24), and geldanamycin (GA) and 17-AAG down-regulate the cdc2 and cdc25c cell cycle regulators in glioblastoma cell lines (25).
Here we investigate the mechanistic basis for the radio-sensitizing effects of TAS-116 to low and high LET irradiation by focusing on DSB repair pathways and cell cycle distribution. Our results indicate that TAS-116 enhances cell death induced by X rays or carbon ions in vitro, and that it enhances tumor control by carbon ion radiotherapy in a mouse xenograft model. Radio-sensitization resulted from TAS-116 inhibition of both HR and NHEJ, and it was associated with marked G2/M arrest.
Materials and Methods
Cell lines and culture
All cell lines were authenticated by short tandem repeat profiling, and cells were thawed upon arrival, expanded, and stored in a liquid nitrogen tank. Each stocked cell line was thawed, expanded, and used within 3 months after thawing. Human cervical carcinoma cells (HeLa) were obtained from Cell Resource Center for Biomedical Research at Tohoku University in 2013 and cultured in alpha MEM (Wako, Osaka, Japan) supplemented with 10% fetal bovine serum (FBS). Human non-small cell lung carcinoma cells (H1299) were purchased from ATCC in 2014 and cultured in RPMI-1640 (Wako) supplemented with 10% FBS. HFL1 human fibroblasts were purchased from RIKEN BioResource Center in 2002 and cultured in alpha MEM supplemented with 15% FBS. The HFL1 cell line is non-transformed and non-tumorigenic, and this cell line was used as a normal control as previously reported (26–28). All cells were grown in a humidified incubator at 37 °C and 5% CO2.
Drug treatment and irradiation
TAS-116 (Supplementary figure S1) was purchased from Active Biochem (Maplewood, NJ) and dissolved into dimethyl sulfoxide (DMSO). Cells were pretreated with TAS-116 or DMSO for 24 hr, and irradiated with X rays or carbon ions. Cells were irradiated with a TITAN-320 X-ray generator (200 kV, 20 mA, Shimadzu, Kyoto, Japan) or with 290 MeV/n carbon ions (6 cm spread-out Bragg peak (SOBP), ~50 keV/μm) accelerated by the Heavy Ion Medical Accelerator in Chiba (HIMAC) at the National Institute of Radiological Sciences (NIRS). After irradiation, cell culture medium was replaced with fresh medium without drug.
Colony formation assay
Cell survival was evaluated by colony formation assay. After irradiation, cells were trypsinized and seeded into cell culture dishes at appropriate cell densities. Cells were cultured for 10~14 days, and they were fixed with ethanol and stained with 0.2% crystal violet. Colonies with more than 50 cells were counted. Surviving fractions (SF) were calculated based on the plating efficiencies of corresponding non-irradiated cells.
γ-H2AX and RAD51 foci formation and resolution
Cells were grown on 4-well chamber slides (Nunc, Rochester, NY). At various time points after irradiation, cells were fixed in 4% paraformaldehyde, permeabilized in 0.5% Triton X-100, and blocked in 3% bovine serum albumin. γ-H2AX and RAD51 foci were detected with primary and secondary antibodies: anti-phospho-histone H2AX (Ser139) (Millipore, Billerica, MA), anti-RAD51 (Santa Cruz Biotechnology, Santa Cruz, CA), Alexa 488-anti-mouse secondary antibody and Alexa 594-anti-rabbit secondary antibody (Thermo Fisher Scientific, Rockford, IL). The cells were stained and mounted with ProLong Gold antifade agent with DAPI (Life Technologies, Grand Island, NY). γ-H2AX and RAD51 foci were scored in at least 50 cells per condition with an Olympus BX51 fluorescence microscope.
Flow cytometric analysis
Cell cycle distributions and the percentage of sub-G1 cells were determined by fixing cells in 70% cold ethanol 24 or 48 hr after irradiation. Fixed cells were washed in PBS and stained with 50 μg/ml propidium iodide (Sigma-Aldrich, St. Louis, MO) in the presence of RNase (Wako). Cellular DNA content was measured with a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA), and the data were analyzed with CellQuestPro Software (BD Biosciences) and ModFit Software (Verity Software House, Topsham, ME). At least 10,000 cells were analyzed per sample.
Western blotting
Protein levels were determined in whole cell lysates prepared by washing cells in cold PBS and lysing with RIPA buffer (Pierce, Rockford, IL). Protein concentrations of cell lysates were determined by BCA Protein assay kit (Pierce). Equal amounts of proteins were loaded onto SDS-PAGE gels, separated by electrophoresis, and transferred onto PVDF membranes. The membranes were blocked with 0.2% I-Block (Tropix, Bedford, MA) and incubated with following primary and secondary antibodies: RAD51 (Santa Cruz Biotechnology, CA), Ku70 (Thermo Fisher Scientific), DNA-PKcs (Thermo Fisher Scientific), phospho DNA-PKcs (S2056, Abcam, Cambridge, MA), cdc25c (Cell Signaling Technology, Dancers, MA), cleaved caspase 3 (Cell Signaling Technology), PARP (Cell signaling Technology), β-actin (Cell Signaling Technology), GAPDH (Cell Signaling Technology), and anti-mouse/rabbit secondary antibodies (Sigma-Aldrich). Protein expression levels were quantified with an ImageQuant LAS-4000 system (Fuji Film, Tokyo, Japan).
Carbon ion radiotherapy of tumor xenografts
All experimental procedures were approved by the Institutional Animal Care and Use Committees at NIRS, conforming to the U.S. National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (29). HeLa cells (106 cells) were subcutaneously injected into right hind legs of BALB/c-nu/nu mice (male, 7 weeks old). One week after cell inoculation, TAS-116 was administered via intraperitoneal injection at a dose of 20 mg/kg three times daily before irradiation. The tumors were irradiated locally with carbon ions, and tumor size and mice body weight were measured twice a week. Tumor volume (mm3) was calculated by [length × width × height × π/6], and tumor volume and body weight following treatments were determined relative to measurements performed before drug injection.
Statistical analyses
Statistical analysis was performed by Student's t-tests to evaluate significant differences between TAS-116 treated and mock treated groups using SAS 9.3 (SAS Institute Inc., Cary, NC).
Results
TAS-116 sensitizes human cancer cell lines to both low and high LET radiation
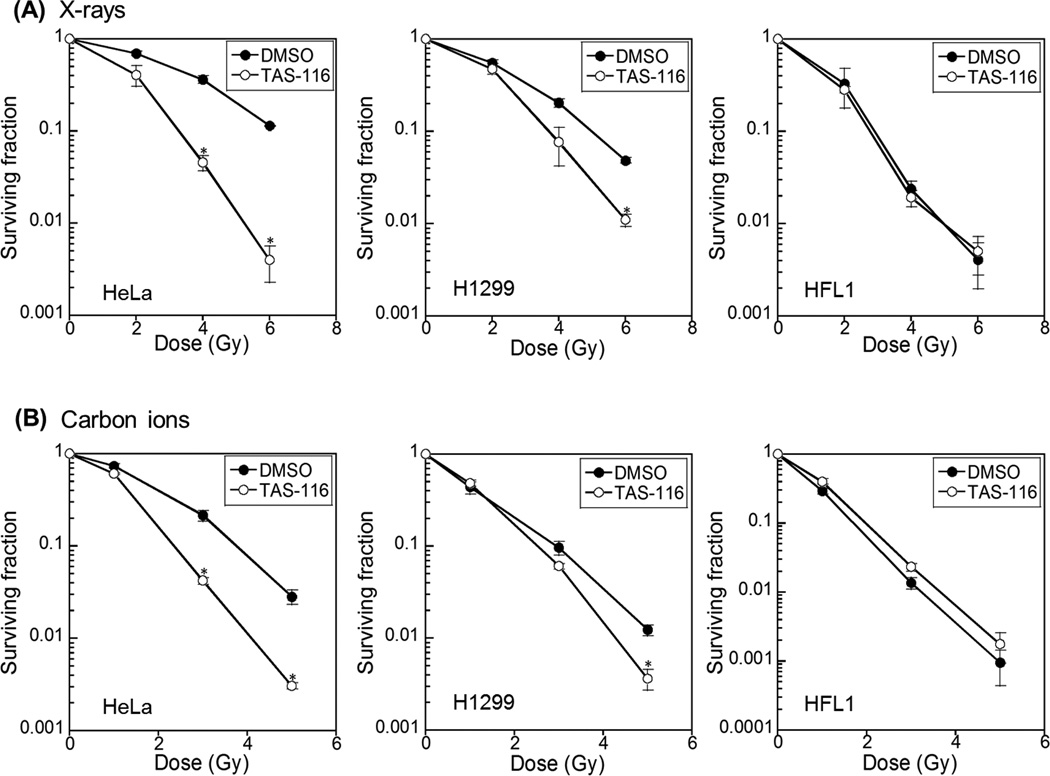
We performed colony formation assays to evaluate the radio-sensitizing effects of TAS-116. As shown in Figure 1A, pretreatment of human cancer cells with 1 μM TAS-116 significantly enhanced sensitivity to X rays. The sensitizing enhancement ratio (SER) of TAS-116 (1 μM) calculated at the D10 value (dose required to reduce surviving fraction to 10%) was 1.87 and 1.34 for HeLa and H1299 cells, respectively. Importantly, TAS-116 did not radio-sensitize non-cancerous human fibroblast HFL1 cells. The relative biological effectiveness (RBE) of carbon ions compared to X rays calculated based on the D10 value was 1.67 and 1.77 for mock-treated HeLa and H1299 cells, and 1.36 and 1.46 for TAS-116-treated HeLa and H1299 cells. Similar to X-irradiated cells, TAS-116 also enhanced cell death in carbon ion irradiated cancer cells (Figure 1B); in these cases, SER values calculated from D10 values were 1.53 and 1.11 for HeLa and H1299 cells, respectively. As with X rays, TAS-116 did not sensitize non-cancerous HFL1 cells to carbon ions. The cancer-selective effects of TAS-116 are consistent with prior studies of other Hsp90 inhibitors which are known to have lower toxicity in normal cells than cancer cells, and do not affect radio-sensitivity of normal cells (3, 30). Because TAS-116 sensitized cancer cells to both X rays and carbon ions, this agent is a promising radio-sensitizer to augment cancer therapy with low or high LET radiation, and its minimal toxicity to normal cells will serve to increase the therapeutic index.
Figure 1.
TAS-116 sensitizes human cancer cells to radiation. HeLa, H1299, and HFL1 cells were pretreated with 1 µM TAS-116 for 24 hr, and irradiated with X rays (A) or carbon ions (B). Colonies with more than 50 cells were scored. Data represent mean ± SEM of two independent experiments. Asterisks indicate significant differences between TAS-116 treated and mock treated cells (Student’s t-test, p < 0.05).
TAS-116 inhibits DSB repair in irradiated cancer cells
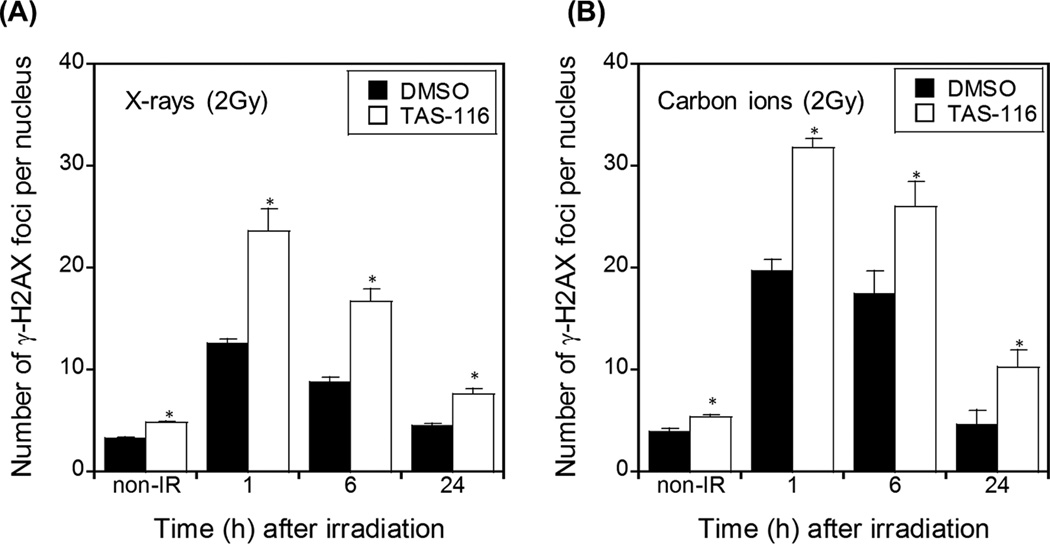
In order to determine the mechanisms by which TAS-116 achieves its effects, we first assessed the ability of this drug to inhibit DSB repair. The number of γ-H2AX foci per nucleus, a marker of DSBs, was determined in HeLa and H1299 cells irradiated with X rays or carbon ion with or without pre-treatment with TAS-116. As shown in Figure 2 and Supplementary figure S2, TAS-116 significantly increased the number of γ-H2AX foci per nucleus compared to mock (DMSO)-treated cells. In mock-treated cells γ-H2AX foci were at maximum levels 1 hr after irradiation, returning to background levels of non-irradiated control cells within 24 hr. In contrast, γ-H2AX foci persisted for longer periods in TAS-116 treated cells. Although TAS-116 treatment alone slightly increased γ-H2AX foci, TAS-116 clearly increased radiation-induced γ-H2AX foci whether measured as absolute values (Figure 2 and Supplementary figure S2) or when background levels were subtracted from each measurement (data not shown). Inhibition of DSB repair is a well-known cause of radio-sensitization (19), and these results suggest that TAS-116 inhibition of DSB repair is one mechanism by which it sensitizes cancer cells to X rays and carbon ion radiation.
Figure 2.
TAS-116 inhibits DSB repair in irradiated HeLa cells. HeLa cells were pretreated with 1 µM TAS-116 for 24 hr, and irradiated with 2 Gy X-rays (A) or carbon ions (B). Cells were fixed and stained with γ-H2AX antibodies and DAPI at indicated times. γ-H2AX foci were counted in at least 50 nuclei per condition. Data represent mean ± SEM of more than two independent experiments. Asterisks indicate significant differences between TAS-116 treated and mock treated cells (Student’s t-test, p < 0.05).
TAS-116 suppresses RAD51 expression and radiation-induced RAD51 foci in HeLa cells
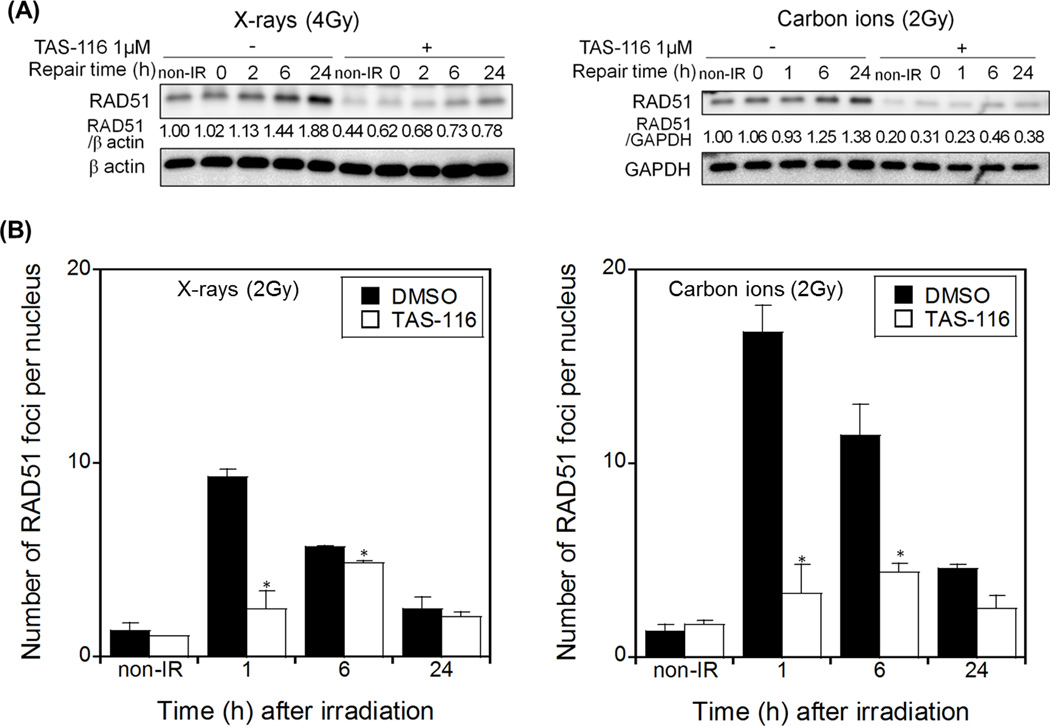
To further investigate how TAS-116 inhibits DSB repair, we tested whether it affects RAD51, a key protein involved in DSB repair by HR. We pre-treated HeLa cells with TAS-116 prior to irradiation, then replaced growth medium with drug-free medium to mimic the conditions used in colony forming assays. Treatment of HeLa cells with TAS-116 alone suppressed RAD51 levels, and it also suppressed RAD51 induction in response to radiation at various time points after treatment with X rays or carbon ions (Figure 3A). We scored the number of RAD51 foci per nucleus after irradiation in HeLa cells with or without pre-treatment with TAS-116. The number of RAD51 foci increased dramatically within 1 hr of X ray or carbon ion irradiation in mock treated cells, and then decreased to near background levels after 24 hr. Consistent with TAS-116 suppression of RAD51, radiation-induced RAD51 foci were sharply reduced by TAS-116 treatment, and RAD51 recruitment to damage was delayed (Figure 3B). These results indicate that TAS-116 represses RAD51 expression and recruitment to DNA damage, suggesting that TAS-116 inhibits RAD51-mediated DSB repair by the HR pathway.
Figure 3.
TAS-116 impairs RAD51-mediated HR repair in irradiated HeLa cells. HeLa cells were pretreated with 1 µM TAS-116 for 24 hr, and irradiated with 4 Gy X-rays or 2 Gy carbon ions (A). Proteins were immunoblotted with RAD51, and with GAPDH or β actin antibodies as loading controls. RAD51 foci were counted in TAS-116 treated or mock treated HeLa cells at indicated times after irradiation (2 Gy X rays or 2 Gy carbon ions, B). Data represent mean ± SEM of two independent experiments. Asterisks indicate significant differences between TAS-116 treated and mock treated cells (Student’s t-test, p < 0.05).
TAS-116 inhibits Ku70/DNA-PKcs-mediated NHEJ repair in HeLa cells
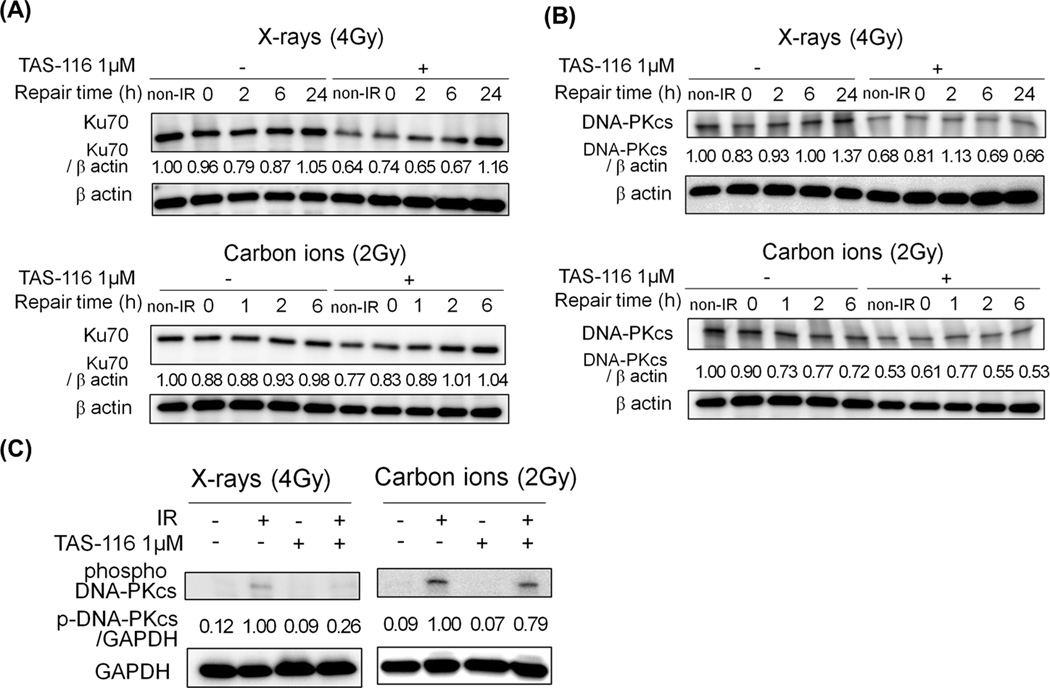
We next examined the effects of TAS-116 on the expression of NHEJ repair proteins. In the NHEJ repair pathway, DNA-PKcs is recruited to DSBs by the Ku70/Ku80 heterodimer and activated to facilitate repair DSBs. As above, growth medium was replaced with drug-free medium following irradiation. We found that TAS-116 treatment alone decreased Ku70 and DNA-PKcs expression, and it also suppressed DNA-PKcs levels after irradiation (Figure 4A, B). In addition, TAS-116 inhibited activation of DNA-PKcs induced by X rays or carbon ions, as revealed with phospho-specific antibodies (Figure 4C). A more substantial reduction in phosphorylated DNA-PKcs was observed with X-rays than carbon ions (Figure 4C). Together these results indicate that TAS-116 suppresses two key NHEJ proteins, consistent with the significant inhibition of DSB repair in irradiated tumor cells.
Figure 4.
TAS-116 decreases the expression levels and activation of NHEJ proteins in HeLa cells. HeLa cells were pretreated with 1 µM TAS-116 for 24 hr, and irradiated with X-rays (4 Gy) or carbon ions (2 Gy). Cells were collected at indicated times after irradiation, and proteins were immunoblotted with Ku70 (A), DNA-PKcs (B) and β actin antibodies. To measure the expression level of phospho-DNA-PKcs (S2056), cells were collected 30 min after X-irradiation and 1 hr after carbon irradiation. Proteins were immunoblotted with phospho-S2056 DNA-PKcs and GAPDH antibodies (C). GAPDH and β actin served as loading controls.
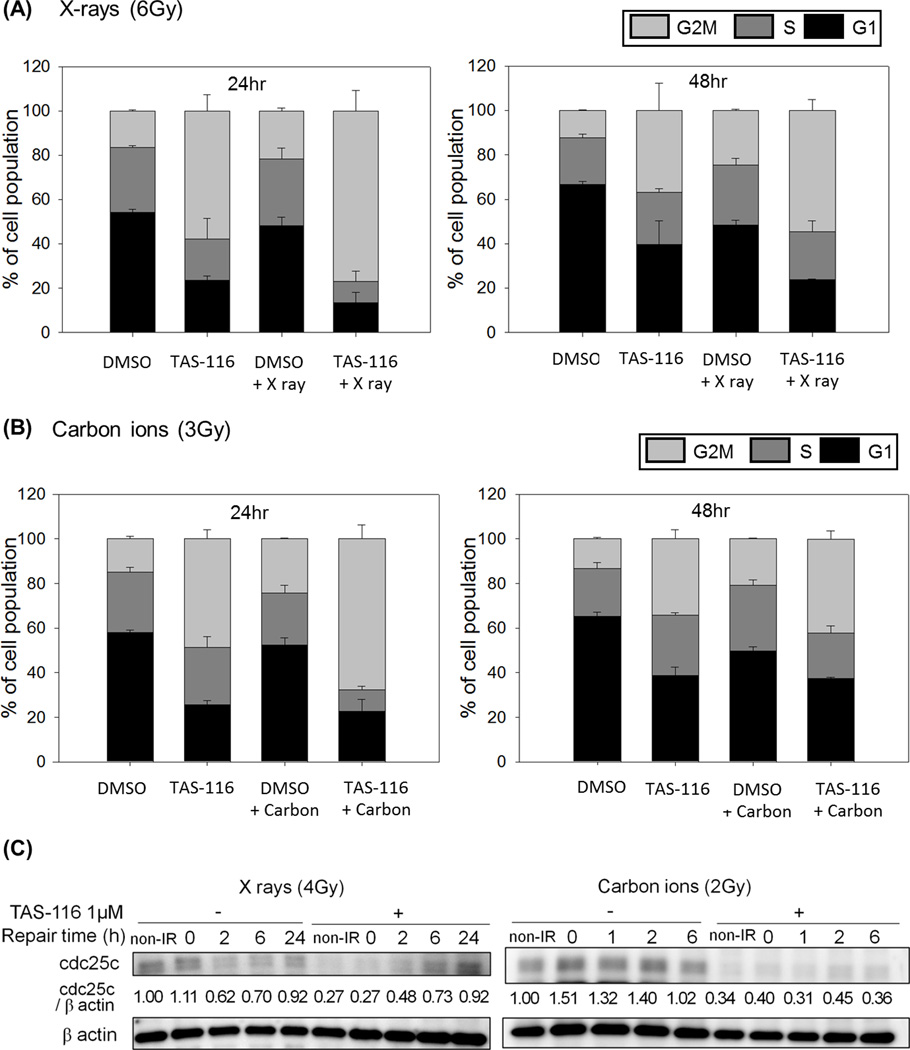
TAS-116 induces G2/M arrest in cancer cells
DNA repair efficiency varies during the cell cycle, thus we investigated whether TAS-116 altered cell cycle distributions of HeLa and H1299 cells to further investigate potential mechanisms of TAS-116 induced radio-sensitization. We found that TAS-116 treatment alone increased the percentage of cells in G2/M phase, and that combined treatment with TAS-116 and irradiation sharply increased G2/M arrest compared to either treatment alone (Figure 5A, B and Supplementary figure S3, S4). G2/M arrest was particularly prominent 24 hr after cells were transferred to drug-free medium, and it was still apparent even 48 hr after drug removal. Cdc25c plays an important role in G2/M progression, so we examined cdc25c protein expression levels in response to TAS-116. As with the DSB repair proteins above, TAS-116 suppressed cdc25c, and this effect was apparent in both non-irradiated cells and in cells irradiated with X-rays or carbon ions (Figure 5C). It appears that cdc25c expression recovered as a function of time after cells were transferred to drug-free medium, consistent with the reductions in the percentage of G2/M cells at later time points. These results indicate that TAS-116 suppresses cdc25c expression, causing G2/M arrest, and this is additional mechanism by which TAS-116 might confer radio-sensitivity to cancer cells.
Figure 5.
TAS-116 induces G2/M arrest in HeLa cells. HeLa cells were pretreated with 1 µM TAS-116 for 24 hr, and irradiated with X rays (6 Gy, A) or carbon ions (3 Gy, B). Cells were fixed and stained with propidium iodide solution at indicated times after irradiation, and cell cycle distributions were analyzed by using flow cytometry. TAS-116 or mock treated HeLa cells were collected after X-irradiation (4 Gy) or carbon (2 Gy) irradiation, and proteins were immunoblotted with cdc25c and β actin antibodies (C). β actin served as a loading control.
In order to investigate mode of cell death by TAS-116 treatment, we evaluated the percentage of sub-G1 cells, and expression of apoptosis-related proteins. TAS-116 increased the percentage of sub-G1 cells, and increased expression of cleaved PARP and cleaved caspase 3 (Supplementary figure S5 and S6), indicating that TAS-116 induces apoptotic cell death. However, there appeared to be no synergistic increase in these apoptosis markers after combined treatment with TAS-116 and irradiation (Supplementary figure S5 and S6). Thus, apoptosis may not be the principal mode of cell death in combined treated cells, and other modes such as mitotic catastrophe might account for the radio-sensitization by TAS-116.
Evidence that TAS-116 suppresses growth of HeLa tumor xenografts treated with carbon ions
The radio-sensitizing effects of TAS-116 on cancer cells prompted us to investigate whether this drug could be useful as an adjunct to radiotherapy. We established HeLa tumor xenografts in mice to study TAS-116 effects in an in vivo tumor model. Mice were assigned to 4 groups: DMSO treated and non-irradiated (n=6); DMSO treated and carbon irradiated (n=6); TAS-116 treated and non-irradiated (n=6); and TAS-116 treated and carbon irradiated (n=7). The mice were treated with TAS-116 and/or irradiated with carbon ions by using the protocol diagrammed in Figure 6A, and tumor volume and body weight were measured twice a week for ~6 weeks. Treatment with TAS-116 alone did not affect tumor growth, but combined treatment with TAS-116 and carbon ions inhibited tumor growth to a significantly greater extent than carbon ions alone within 26 days after tumor injection (Figure 6B, p < 0.05). Beyond 29 days after tumor injection this trend continued, but was not statistically significant with these sample sizes. Representative tumors at 26 and 45 days after tumor injection with mono- and combination therapy are shown in Supplementary Figure S7. These promising preliminary radiotherapy results justify further studies aimed at enhancing therapeutic efficacy by optimizing drug and radiation doses. It is reasonable to expect that a study with larger sample sizes, higher TAS-116 concentrations, and/or a more frequent dosing schedule, will confirm that TAS-116 radio-sensitization is a useful strategy to enhance the efficacy of radiotherapy.
Figure 6.
TAS-116 delays tumor growth in carbon irradiated HeLa xenografts. Mice were treated with TAS-116 (20 mg/kg/day) delivered intraperitoneally three times before carbon irradiation (A). Tumor volume (B) and body weight (C) were measured twice a week. Relative values were calculated based on the tumor volume and body weight of each mouse before drug injection. Six or seven mice were used for each treatment, and data represent mean ± SEM (*, p < 0.05; †, p < 0.10 for comparisons of mock-treated mice and TAS-116 treated mice).
We also measured mouse body weight to determine whether TAS-116 induced severe side effects. No significant changes were observed in body weights of TAS-116 treated groups (Figure 6C), thus it is likely that TAS-116 has minimal effect on normal tissue, consistent with our finding that TAS-116 did not radio-sensitize non-transformed HFL1 cells. The ability of TAS-116 to induce radio-sensitivity to tumors without causing noticeable damage to host mice supports the use of this drug as a safe and effective adjunct to radiotherapy.
Discussion
Hsp90 inhibitors have shown promise as monotherapy anti-tumor agents (31, 32). Hsp90 inhibitors modulate oncogenic/growth regulatory proteins (32), and DNA damage response proteins which enhances radio-sensitivity and suggests their use as adjuncts to radiotherapy (6, 19, 33–35). It has been proposed that by targeting multiple cancer relevant pathways, Hsp90 inhibitors might be effective against multiple cancer types and highly heterogeneous cancers (19, 32). However, use of early Hsp90 inhibitors has been limited because of serious side effects, including ocular toxicity, spurring the development of new Hsp90 inhibitors with improved bioavailability, greater specificity to Hsp90 in cancer cells, and reduced side effects. TAS-116 is a recently developed Hsp90 inhibitor with lower ocular toxicity than other Hsp90 inhibitors (10). The present study is the first to investigate the radio-sensitizing effects of TAS-116, and we demonstrate that TAS-116 sensitizes cancer cells to both low and high LET radiation.
Moreover, TAS-116 has little or no effect on radio-sensitivity of non-cancerous cells. Although we used non-transformed HFL1 cells as a normal cell control, rather than primary human cells, the selective effects of TAS-116 we observed are consistent with previously reported cancer-specific effects of Hsp90 inhibitors (3, 30). This selectivity of Hsp90 inhibitors has been traced to their high binding affinity to the altered conformation of Hsp90 expressed in cancer cells (4, 5, 31). Thus, TAS-116 follows this pattern as it did not affect the radio-sensitivity of non-cancerous cells, suggesting TAS-116 as a promising radio-sensitizer with selective effects towards cancer cells. DSB repair inhibition is an important mechanism for radio-sensitization, and prior studies with other Hsp90 inhibitors have demonstrated their effects on DSB repair pathways. For example, 17-AAG decreased RAD51 expression following irradiation of cancer cells with X rays or carbon ions (2, 3), and 17DMAG attenuated X ray-induced DNA-PKcs activation (34). It is well-established that impairment of these proteins significantly inhibits DSB repair and enhances ionizing radiation-induced cell death (18, 19). Most research on DSB repair inhibition by Hsp90 inhibitors has focused on either HR or NHEJ, not both pathways. Takahashi et al. (22), however, suggested that inhibition of both HR and NHEJ repair pathways will cause greater radio-sensitization than impairment of either repair pathway alone (22). A unique feature of the current study is the demonstration that TAS-116 selectively radio-sensitizes cancer cells by down-regulating expression of RAD51, Ku70, and DNA-PKcs, three proteins with critical roles in DSB repair by HR and NHEJ repair pathways. Thus, TAS-116 is a very promising candidate radio-sensitizer that is likely to be more effective than agents that affect only one DSB repair pathway.
Another mechanism by which Hsp90 inhibitors may radio-sensitize tumor cells is by altering cell cycle distributions. Hsp90 inhibitors are known to affect cell cycle distributions by impairing cell cycle checkpoint proteins (24, 36). The Hsp90 inhibitors GA and 17-AAG were shown to down-regulate cdc2 and cdc25c, induce G2/M arrest, and inhibit cell growth of glioblastoma and lung cancer cell lines (25, 37). These studies demonstrated that cdc25c and cdc2 are client proteins of Hsp90, and that GA, and its derivative 17-AAG, alters chaperone complexes, leading to degradation of these key cell cycle proteins. Consistent with these reports, we found that TAS-116 decreases cdc25c expression in HeLa cells, leading to G2/M arrest and inhibition of cell growth. Treatment with X-rays or carbon ions also induced G2/M arrest, and combined treatment with TAS-116 and radiation caused more pronounced G2/M arrest than either individual treatment. These results suggest another potential mechanism by which TAS-116 radio-sensitizes tumor cells by altering cell cycle distribution and enhancing radiation-induced growth arrest.
Because low and high LET radiation induce different types of DNA damage that appear to differentially engage DSB repair pathways (17, 38, 39), it is not surprising that radio-sensitizing agents sometimes show differential effects with low vs. high LET radiation. For example, gemcitabine sensitizes human myeloma cell lines to low LET (60Co) γ-rays, but not high-LET alpha particles (40). This result is consistent with the finding that gemcitabine only affects HR, not NHEJ repair of DSBs. High LET radiation induces more complex DSB damage than low LET radiation, and repair of complex DSB damage appears to require the interplay of both HR and NHEJ repair pathways (41). Importantly, we found that TAS-116 sensitizes cancer cells to the cytotoxic effects of both low LET X rays and high LET carbon ions, consistent with its suppression of key HR and NHEJ repair proteins. These results indicate that TAS-116 is an excellent candidate for tumor radio-sensitization as an adjunct to low and high LET radiotherapy.
In summary, we demonstrated that the novel Hsp90 inhibitor TAS-116 sensitizes cancer cells to carbon ions as well as X-rays, and that it has no radio-sensitizing effect on non-cancerous cells. TAS-116 suppressed the two major DSB repair pathways that are central to tumor radio-resistance, by decreasing expression of the key HR and NHEJ proteins RAD51, Ku70, DNA-PKcs, by suppressing phosphorylation/activation of DNA-PKcs, and by stimulating G2/M arrest via suppression of cdc25c. TAS-116 gave favorable results in a preliminary in vivo study of tumor radiotherapy response, justifying further optimization of this promising radio-sensitizer to augment radiotherapy with low or high LET radiation.
Supplementary Material
Acknowledgments
This work was supported by JSPS KAKENHI grants to R.Okayasu (nos. 24249067 and 23390301), NIH R01 GM084020 to J.A.Nickoloff and was also a part of Research Project with Heavy Ions at NIRS-HIMAC.
We thank Drs. Shuichi Ohkubo and Hiromi Muraoka for valuable discussions during the preparation of this manuscript. We are thankful to the HIMAC operators for their support during carbon ion irradiation.
Footnotes
Conflict of Interest; none.
References
- 1.Garcia-Carbonero R, Carnero A, Paz-Ares L. Inhibition of HSP90 molecular chaperones: moving into the clinic. Lancet Oncol. 2013;14:e358–e369. doi: 10.1016/S1470-2045(13)70169-4. [DOI] [PubMed] [Google Scholar]
- 2.Noguchi M, Yu D, Hirayama R, Ninomiya Y, Sekine E, Kubota N, et al. Inhibition of homologous recombination repair in irradiated tumor cells pretreated with Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Biochem Biophys Res Commun. 2006;351:658–663. doi: 10.1016/j.bbrc.2006.10.094. [DOI] [PubMed] [Google Scholar]
- 3.Hirakawa H, Fujisawa H, Masaoka A, Noguchi M, Hirayama R, Takahashi M, et al. The combination of Hsp90 inhibitor 17AAG and heavy-ion irradiation provides effective tumor control in human lung cancer cells. Cancer Med. 2015;4:426–436. doi: 10.1002/cam4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 5.Moulick K, Ahn JH, Zong H, Rodina A, Cerchietti L, Gomes DaGama EM, et al. Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat Chem Biol. 2011;7:818–826. doi: 10.1038/nchembio.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kabakov AE, Kudryavtsev VA, Gabai VL. Hsp90 inhibitors as promising agents for radiotherapy. J Mol Med (Berl) 2010;88:241–247. doi: 10.1007/s00109-009-0562-0. [DOI] [PubMed] [Google Scholar]
- 7.Jhaveri K, Taldone T, Modi S, Chiosis G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim Biophys Acta. 2012;1823:742–755. doi: 10.1016/j.bbamcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sessa C, Shapiro GI, Bhalla KN, Britten C, Jacks KS, Mita M, et al. First-in-human phase I dose-escalation study of the HSP90 inhibitor AUY922 in patients with advanced solid tumors. Clin Cancer Res. 2013;19:3671–3680. doi: 10.1158/1078-0432.CCR-12-3404. [DOI] [PubMed] [Google Scholar]
- 9.Renouf DJ, Velazquez-Martin JP, Simpson R, Siu LL, Bedard PL. Ocular toxicity of targeted therapies. J Clin Oncol. 2012;30:3277–3286. doi: 10.1200/JCO.2011.41.5851. [DOI] [PubMed] [Google Scholar]
- 10.Ohkubo S, Kodama Y, Muraoka H, Hitotsumachi H, Yoshimura C, Kitade M, et al. TAS-116, a highly selective inhibitor of heat shock protein 90alpha and beta, demonstrates potent antitumor activity and minimal ocular toxicity in preclinical models. Mol Cancer Ther. 2015;14:14–22. doi: 10.1158/1535-7163.MCT-14-0219. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki R, Hideshima T, Mimura N, Minami J, Ohguchi H, Kikuchi S, et al. Anti-tumor activities of selective HSP90alpha/beta inhibitor, TAS-116, in combination with bortezomib in multiple myeloma. Leukemia. 2015;29:510–514. doi: 10.1038/leu.2014.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki R, Kikuchi S, Harada T, Mimura N, Minami J, Ohguchi H, et al. Combination of a Selective HSP90alpha/beta Inhibitor and a RAS-RAF-MEK-ERK Signaling Pathway Inhibitor Triggers Synergistic Cytotoxicity in Multiple Myeloma Cells. PLoS One. 2015;10:e0143847. doi: 10.1371/journal.pone.0143847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen C, Borak TB, Tsujii H, Nickoloff JA. Heavy charged particle radiobiology: using enhanced biological effectiveness and improved beam focusing to advance cancer therapy. Mutat Res. 2011;711:150–157. doi: 10.1016/j.mrfmmm.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okayasu R. Repair of DNA damage induced by accelerated heavy ions--a mini review. Int J Cancer. 2012;130:991–1000. doi: 10.1002/ijc.26445. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Liu S, Zhang P, Zhang S, Naidu M, Wang H, et al. S-phase cells are more sensitive to high-linear energy transfer radiation. Int J Radiat Oncol Biol Phys. 2009;74:1236–1241. doi: 10.1016/j.ijrobp.2008.12.089. [DOI] [PubMed] [Google Scholar]
- 16.Bird RP, Burki HJ. Survival of synchronized Chinese hamster cells exposed to radiation of different linear-energy transfer. Int J Radiat Biol Relat Stud Phys Chem Med. 1975;27:105–120. doi: 10.1080/09553007514550121. [DOI] [PubMed] [Google Scholar]
- 17.Okayasu R, Okada M, Okabe A, Noguchi M, Takakura K, Takahashi S. Repair of DNA damage induced by accelerated heavy ions in mammalian cells proficient and deficient in the non-homologous end-joining pathway. Radiat Res. 2006;165:59–67. doi: 10.1667/rr3489.1. [DOI] [PubMed] [Google Scholar]
- 18.Mladenov E, Magin S, Soni A, Iliakis G. DNA double-strand break repair as determinant of cellular radiosensitivity to killing and target in radiation therapy. Front Oncol. 2013;3:113. doi: 10.3389/fonc.2013.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camphausen K, Tofilon PJ. Inhibition of Hsp90: a multitarget approach to radiosensitization. Clin Cancer Res. 2007;13:4326–4330. doi: 10.1158/1078-0432.CCR-07-0632. [DOI] [PubMed] [Google Scholar]
- 20.Vandersickel V, Mancini M, Slabbert J, Marras E, Thierens H, Perletti G, et al. The radiosensitizing effect of Ku70/80 knockdown in MCF10A cells irradiated with X-rays and p(66)+Be(40) neutrons. Radiat Oncol. 2010;5:30. doi: 10.1186/1748-717X-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zafar F, Seidler SB, Kronenberg A, Schild D, Wiese C. Homologous recombination contributes to the repair of DNA double-strand breaks induced by high-energy iron ions. Radiat Res. 2010;173:27–39. doi: 10.1667/RR1910.1. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi A, Kubo M, Ma H, Nakagawa A, Yoshida Y, Isono M, et al. Nonhomologous end-joining repair plays a more important role than homologous recombination repair in defining radiosensitivity after exposure to high-LET radiation. Radiat Res. 2014;182:338–344. doi: 10.1667/RR13782.1. [DOI] [PubMed] [Google Scholar]
- 23.Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:928–942. doi: 10.1016/j.ijrobp.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Stingl L, Stuhmer T, Chatterjee M, Jensen MR, Flentje M, Djuzenova CS. Novel HSP90 inhibitors, NVP-AUY922 and NVP-BEP800, radiosensitise tumour cells through cell-cycle impairment, increased DNA damage and repair protraction. Br J Cancer. 2010;102:1578–1591. doi: 10.1038/sj.bjc.6605683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Morales P, Carrasco-Garcia E, Ruiz-Rico P, Martinez-Mira R, Menendez-Gutierrez MP, Ferragut JA, et al. Inhibition of Hsp90 function by ansamycins causes downregulation of cdc2 and cdc25c and G(2)/M arrest in glioblastoma cell lines. Oncogene. 2007;26:7185–7193. doi: 10.1038/sj.onc.1210534. [DOI] [PubMed] [Google Scholar]
- 26.Fokas E, Prevo R, Pollard JR, Reaper PM, Charlton PA, Cornelissen B, et al. Targeting ATR in vivo using the novel inhibitor VE-822 results in selective sensitization of pancreatic tumors to radiation. Cell Death Dis. 2012;3:e441. doi: 10.1038/cddis.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu XR, Liu L, Zhang ZF, Zhang B, Sun H, Chan GL, et al. Selective protection of normal cells during chemotherapy by RY4 peptides. Mol Cancer Res. 2014;12:1365–1376. doi: 10.1158/1541-7786.MCR-13-0425. [DOI] [PubMed] [Google Scholar]
- 28.Reaper PM, Griffiths MR, Long JM, Charrier JD, Maccormick S, Charlton PA, et al. Selective killing of ATM- or p53-deficient cancer cells through inhibition of ATR. Nat Chem Biol. 2011;7:428–430. doi: 10.1038/nchembio.573. [DOI] [PubMed] [Google Scholar]
- 29.National Research Council (US) Guide for the care and use of laboratory animals. Washington: National Academies Press; 2011. Available from: http://www.ncbi.nlm.nih.gov/books/NBK54050. [Google Scholar]
- 30.Segawa T, Fujii Y, Tanaka A, Bando S, Okayasu R, Ohnishi K, et al. Radiosensitization of human lung cancer cells by the novel purine-scaffold Hsp90 inhibitor, PU-H71. Int J Mol Med. 2014;33:559–564. doi: 10.3892/ijmm.2013.1594. [DOI] [PubMed] [Google Scholar]
- 31.He H, Zatorska D, Kim J, Aguirre J, Llauger L, She Y, et al. Identification of potent water soluble purine-scaffold inhibitors of the heat shock protein 90. J Med Chem. 2006;49:381–390. doi: 10.1021/jm0508078. [DOI] [PubMed] [Google Scholar]
- 32.Page P, Yang LX. Novel chemoradiosensitizers for cancer therapy. Anticancer Res. 2010;30:3675–3682. [PubMed] [Google Scholar]
- 33.Koll TT, Feis SS, Wright MH, Teniola MM, Richardson MM, Robles AI, et al. HSP90 inhibitor, DMAG, synergizes with radiation of lung cancer cells by interfering with base excision and ATM-mediated DNA repair. Mol Cancer Ther. 2008;7:1985–1992. doi: 10.1158/1535-7163.MCT-07-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dote H, Burgan WE, Camphausen K, Tofilon PJ. Inhibition of hsp90 compromises the DNA damage response to radiation. Cancer Res. 2006;66:9211–9220. doi: 10.1158/0008-5472.CAN-06-2181. [DOI] [PubMed] [Google Scholar]
- 35.Sauvageot CM, Weatherbee JL, Kesari S, Winters SE, Barnes J, Dellagatta J, et al. Efficacy of the HSP90 inhibitor 17-AAG in human glioma cell lines and tumorigenic glioma stem cells. Neuro Oncol. 2009;11:109–121. doi: 10.1215/15228517-2008-060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burrows F, Zhang H, Kamal A. Hsp90 activation and cell cycle regulation. Cell Cycle. 2004;3:1530–1536. doi: 10.4161/cc.3.12.1277. [DOI] [PubMed] [Google Scholar]
- 37.Senju M, Sueoka N, Sato A, Iwanaga K, Sakao Y, Tomimitsu S, et al. Hsp90 inhibitors cause G2/M arrest associated with the reduction of Cdc25C and Cdc2 in lung cancer cell lines. J Cancer Res Clin Oncol. 2006;132:150–158. doi: 10.1007/s00432-005-0047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Zhang X, Wang P, Yu X, Essers J, Chen D, et al. Characteristics of DNA-binding proteins determine the biological sensitivity to high-linear energy transfer radiation. Nucleic Acids Res. 2010;38:3245–3251. doi: 10.1093/nar/gkq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hada M, Sutherland BM. Spectrum of complex DNA damages depends on the incident radiation. Radiat Res. 2006;165:223–230. doi: 10.1667/rr3498.1. [DOI] [PubMed] [Google Scholar]
- 40.Supiot S, Thillays F, Rio E, Gouard S, Morgenstern A, Bruchertseifer F, et al. Gemcitabine radiosensitizes multiple myeloma cells to low let, but not high let, irradiation. Radiother Oncol. 2007;83:97–101. doi: 10.1016/j.radonc.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Anderson JA, Harper JV, Cucinotta FA, O'Neill P. Participation of DNA-PKcs in DSB repair after exposure to high- and low-LET radiation. Radiat Res. 2010;174:195–205. doi: 10.1667/RR2071.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.