Abstract
Introduction:
First-in-class drugs use a unique mechanism of action. This study assessed the therapeutic innovativeness and safety of these drugs approved by Health Canada from 1997–2012.
Methods:
A list of new drugs was compiled and a database from the Food and Drug Administration was used to determine first-in-class status. Post-market safety warnings and drugs withdrawn for safety reasons were identified from the MedEffect Canada website. Therapeutic innovation evaluations came from the Patented Medicine Prices Review Board (PMPRB) and Prescrire International. The proportion of first-in-class drugs that were innovative was compared to the proportion of non-first-in-class drugs that were innovative. Kaplan–Meier survival curves assessed safety.
Results:
In all, 462 drugs were approved by Health Canada during the period under study. Among these, 345 were evaluated by PMPRB and/or Prescrire, and first-in-class data were available for 292. Ninety-eight of the 292 were first-in-class and 16 were innovative compared to 9 of 194 drugs that were not-first-in-class. There was no difference in safety between the two groups.
Discussion:
Overall, the benefit-to-harm ratio of first-in-class drugs, as measured by post-market safety warnings/withdrawals, is better than those that were not-first-in-class.
Abstract
Introduction:
Les nouvelles classes de médicaments utilisent des mécanismes d'action uniques. Cette étude évalue l'aspect innovant et l'innocuité de ces médicaments approuvés par Santé Canada entre 1997–2012.
Méthodes:
Une liste de nouveaux médicaments a été compilée et une base de données de la Food and Drug Administration a été utilisée afin de déterminer le statut de ces nouvelles classes de médicaments. Les mises en garde diffusées après la commercialisation et le retrait de médicaments pour des raisons de santé ont été établis à partir du site Web MedEffet Canada. L'évaluation des aspects thérapeutiques et innovants provient du Conseil d'examen du prix des médicaments brevetés (CEPMB) et de Prescrire International. La proportion des nouvelles classes de médicaments innovants a été comparée à la proportion des médicaments innovants qui n'appartenaient pas à de nouvelles classes. L'innocuité a été évaluée grâce aux courbes d'estimation de Kaplan–Meier pour la fonction de survie.
Résultats:
En tout, 462 médicaments ont été approuvés par Santé Canada pendant la période soumise à l'étude. Parmi ceux-ci, 345 ont été évalués par le CEPMB et Prescrire International, ou l'un des deux, et des données pour de nouvelles classes de médicament étaient disponibles pour 292 d'entre eux. Quatre-vingt-dix-huit de ces 292 médicaments appartenaient à de nouvelles classes et 16 de ceux-ci étaient innovants, comparés aux 9 des 194 médicaments qui n'appartenaient pas à de nouvelles classes. Il n'y avait pas de différence sur le plan de l'innocuité entre les deux groupes.
Discussion:
En général, le ratio bienfait/méfait des nouvelles classes de médicaments, tel que mesuré selon les mises en garde et les retraits, est mieux que celui des médicaments n'appartenant pas à de nouvelles classes.
Introduction
First-in-class drugs are ones that use a new and unique mechanism of action for treating a medical condition. These products are often referred to as innovative and cited as offering new treatment options for patients (Lanthier et al. 2013; Pharmaceutical Research and Manufacturers of America 2015b). However, the new mechanism of action can also mean that unexpected safety problems can develop with these products. The first glitazone for treatment of Type II diabetes, troglitazone, had to be removed from the market because of hepatotoxicity (Rawson and Kaitin 2003) and sibutramine, an oral anorexiant, was withdrawn because of cardiovascular toxicity (Lexchin 2014a).
The purpose of this study was to assess the therapeutic innovativeness of first-in-class drugs approved by Health Canada and to compare their safety with drugs that were not-first-in-class. Second, this study examines the review status that Health Canada assigned to both groups of products and whether there is any difference in the number of first-in-class drugs introduced into the Canadian market over time.
Methods
Data sources
A list of all new drugs approved from January 1, 1997 to December 31, 2012, their dates of approval and their review status (priority or standard) was compiled from the annual reports of the Therapeutic Products Directorate and the Biologics and Genetic Therapies Directorate of Health Canada. The 1997 reports from these directorates were the first ones to indicate which products received a priority review. The reports are available by directly contacting the directorates at publications@hc-sc.gc.ca. The priority review pathway is used for drugs under two conditions: (1) for drugs that treat “a serious, life-threatening or severely debilitating disease or condition for which there is substantial evidence of clinical effectiveness that the drug provides … effective treatment … [and] for which no drug is presently marketed in Canada” and (2) for drugs that represent “a significant increase in efficacy and/or significant decrease in risk, such that the overall benefit/ risk profile is improved over existing therapies … for a disease or condition that is not adequately managed by a drug marketed in Canada” (Health Canada: Health Products and Food Branch 2009). The timeline for priority reviews is 180 days and for standard reviews, it is 300 days.
Health Canada does not indicate which products are first-in-class, so this determination was based on an analysis of 645 new drugs approved by the US Food and Drug Administration from 1987 to 2011 (Lanthier et al. 2013). In defining a drug as first-in-class, Lanthier et al. based their definition on a combination of factors including FDA-established pharmacologic class designations, approved indications and supplementary sources (for example, commercial databases such as Drug Facts and Comparisons and Pharmaprojects).
Post-market safety warnings and drug withdrawals, hereafter collectively referred to as post-market safety warnings, for the period January 1, 1997 to December 31, 2012, were identified through advisories for health professionals in the Recalls and Safety Alerts Database on the MedEffect Canada website at www.hc-sc.gc.ca/dhp-mps/medeff/advisories-avis/prof/index-eng.php. According to Health Canada, this database is a comprehensive list of recalls, advisories and safety alerts. For each safety advisory or notice of withdrawal of a product, the date was recorded. All serious safety advisories (those using bold black print and/or boxed warnings) were included except for those dealing with the withdrawal of a specific batch, or lot number due to manufacturing problems, or those issued because a drug was being used for an unapproved indication or because of medication errors (e.g., a warning about remembering to remove a transdermal patch before applying a second one). If a drug received more than one serious post-market safety warning, only the time of the first warning was used. When necessary, notices on the MedEffect website were supplemented by searching for the product's name in the Drug Product Database (DPD) at http://webprod3.hc-sc.gc.ca/dpd-bdpp/index-eng.jsp. The DPD website states that it contains product-specific information on drugs approved for use in Canada as well as all products discontinued since 1996, but it does not show changes to the Product Monograph.
The Patented Medicine Prices Review Board (PMPRB) is a federal agency that is responsible for calculating the maximum introductory price for all new patented medications introduced into the Canadian market. It is important to note that the PMPRB is not a payer and, therefore, its decisions about therapeutic value are not influenced by how much it might have to pay for the product. As part of the process of determining the price, the PMPRB's independent Human Drug Advisory Panel (HDAP) determines the therapeutic value of each product it reviews and these evaluations are published in its annual reports available online from 2003 to 2012 at www.pmprb-cepmb.gc.ca/english/View.asp?x=91 and for previous years by directly contacting the PMPRB at pmprb@pmprb-cepmb.gc.ca. HDAP determines the ratings for the drugs before the maximum price is established. For the purpose of this study, products that were deemed as breakthroughs and substantial improvement were termed “innovative” and products in other categories were termed “not innovative.” In deciding on the level of therapeutic innovation, HDAP considers two primary factors – increased efficacy and reduction in incidence or grade of important adverse reactions – and nine secondary factors – route of administration, patient convenience, compliance improvements leading to improved therapeutic efficacy, caregiver convenience, time required to achieve the optimal therapeutic effect, duration of usual treatment course, success rate, percentage of affected population treated effectively and disability avoidance/ savings. The primary factors are given the greatest weight, followed by an assessment of any additional improvement as a result of the secondary factors (PMPRB 2014). In some cases, the PMPRB annual reports indicated that the therapeutic value of the product was still being determined and in those cases, the PMPRB was contacted directly to determine the final classification.
Prescrire (http://english.prescrire.org/en/Summary.aspx, subscription required) assesses the therapeutic value of medicines through a multistep process. First, it “examines the condition or clinical setting for which the drug is proposed; then, the natural course of the disease, the efficacy and safety of existing treatments, and the most relevant outcome measures. This is followed by a systematic search for clinical data on the efficacy and adverse effects of the new drug, and an assessment of the level of evidence. Based on [its] independent analysis of clinical data, [it] form[s] a judgement as to whether or not the new drug is beneficial for patients or whether or not its harmful effects outweigh the benefit” (Prescrire Editorial Staff 2011). Based on its analysis, it rates products using the following categories: bravo (major therapeutic innovation in an area where previously no treatment was available); a real advance (important therapeutic innovation but has limitations); offers an advantage (some value but does not fundamentally change the present therapeutic practice); possibly helpful (minimal additional value and should not change prescribing habits except in rare circumstances); nothing new (may be new molecule but is superfluous because it does not add to clinical possibilities offered by previously available products); not acceptable (without evident benefit but with potential or real disadvantages); judgment reserved (decision postponed until better data and more thorough evaluation) (Prescrire Editorial Staff 2002). The first 2 Prescrire categories were defined as innovative and the other Prescrire categories were defined as not innovative. If Prescrire put the drug into the judgment reserved category, then no Prescrire evaluation was recorded.
Drugs were categorized using the first level of the World Health Organization (WHO) Anatomical Therapeutic Chemical (ATC) classification system into one of 14 main anatomical groups (WHO Collaborating Centre for Drug Statistics Methodology & Norwegian Institute of Public Health 2011).
Data analyses
If a drug was judged innovative by either the PMPRB and/or Prescrire, it was rated as innovative. If both organizations evaluated the drug and the ratings were discordant, i.e., one said it was not innovative and one said it was, the drug was still considered innovative. The proportion of first-in-class and non-first-in-class drugs rated as innovative was calculated and the proportions were compared with a z-ratio.
Kaplan–Meier survival curves, i.e., time to event curves, were calculated for the period from approval until a first post-market safety warning for first-in-class and non-first-in-class drugs and curves were compared using a Log rank (Mantel–Cox) test. A Kaplan–Meier analysis accounts for the fact that drugs were on the market for variable periods and, therefore, some drugs were more likely to have received a post-market safety warning by the end of the study period (December 31, 2012). The analyses combined drugs with post-market safety warnings and those withdrawn from the market to increase statistical power to detect differences. Previous work has shown that there are relatively few safety withdrawals (Lexchin 2014a).
Finally, the proportion of first-in-class and non-first-in-class drugs that received a priority review was calculated and the proportions were compared with a z-ratio. The z-ratio calculates the significance of the difference between two independent proportions (http://vassarstats.net).
The latter two analyses were repeated for just the first-in-class group of drugs. Comparisons were made for the proportion of innovative and non-innovative drugs in this group that received a priority review and for the time to a first post-market safety warning for innovative and non-innovative drugs.
The total number of first-in-class drugs and innovative first-in-class drugs introduced annually from 1997 to 2012 was plotted and the curves were analyzed using linear regression to determine any trends over time.
All analyses were done with Prism 6.0 (GraphPad Software, www.graphpad.com) and p < 0.05 was considered statistically significant.
Results
A total of 426 drugs were approved by Health Canada between 1997 and 2012 and 345 of these drugs were evaluated by PMPRB/Prescrire. Data on first-in-class status was available for 292 of these 345 drugs and the analyses were based on this group of 292. Appendix 1 lists all of the drugs by their first-in-class and innovation status and by ATC group. Ninety-eight drugs were first-in-class and 194 were not-first-in-class. Sixteen of 98 (16.3%, 95% CI: [10.3, 24.9]) were innovative compared to 9 of 194 (4.6%, 95% CI: [2.5, 8.6]), z-ratio = 3.371 (p = 0.0004). Table 1 shows the breakdown of first-in-class and non-first-in-class drugs by ATC group and innovation status, as determined by PMPRB/Prescrire, for 290 of the drugs. (Two drugs, neither first-in-class nor with a post-market safety warning, were not listed in the WHO ATC database.) Seventy of the 98 first-in-class drugs came from 4 of the 14 drug groups – antineoplastic and immunomodulating agents (35), alimentary tract and metabolism (14), anti-infectives for systemic use (12) and nervous system (9). Although the plurality of non-first-in-class drugs were in the antineoplastic and immunomodulating group, drugs were more evenly distributed throughout the various anatomical groups.
Table 1.
First-in-class and non-first-in-class drugs grouped by innovation status and Anatomic Therapeutic Chemical classification*
| Anatomic Therapeutic Chemical classification (first level) | First-in-class | Non-first-in-class | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Innovative | Not Innovative | Total number in group and per cent of total first-in-class | Post-market safety warning/withdrawal | Innovative | Not Innovative | Total number in group and per cent of total not-first-in-class | Post-market safety warning/withdrawal | |||||
| Number | Per cent of drugs in group (%) | Per cent of all post-market safety warnings/withdrawals (%) | Number | Per cent of drugs in group (%) | Per cent of all post-market safety warnings/withdrawals (%) | |||||||
| Alimentary tract and metabolism | 4 | 10 | 14 (14.3) | 4 | 28.6 | 12.1 | 1 | 14 | 15 (7.8) | 4 | 26.7 | 8.2 |
| Anti-infectives for systemic use | 1 | 11 | 12 (12.2) | 2 | 16.7 | 6.1 | 1 | 31 | 32 (16.7) | 12 | 37.5 | 24.5 |
| Antineoplastic and immunomodulating agents | 4 | 31 | 35 (35.7) | 16 | 45.7 | 48.5 | 5 | 30 | 35 (18.2) | 12 | 34.3 | 24.5 |
| Blood and blood-forming agents | 1 | 3 | 4 (4.1) | 1 | 25 | 3.0 | 0 | 12 | 12 (6.3) | 4 | 33.3 | 8.2 |
| Cardiovascular system | 0 | 3 | 3 (3.1) | 1 | 33.3 | 3.0 | 0 | 16 | 16 (8.3) | 6 | 37.5 | 12.2 |
| Dermatologicals | 0 | 3 | 3 (3.1) | 0 | 0 | 0 | 0 | 2 | 2 (1) | 0 | 0 | 0 |
| Genitourinary system and sex hormones | 1 | 1 | 2 (2) | 0 | 0 | 0 | 0 | 14 | 14 (7.3) | 1 | 7.1 | 2.0 |
| Musculoskeletal system | 0 | 4 | 4 (4.1) | 2 | 50 | 6.1 | 0 | 8 | 8 (4.2) | 3 | 37.5 | 6.1 |
| Nervous system | 1 | 8 | 9 (9.2) | 4 | 36.4 | 12.1 | 1 | 33 | 34 (17.7) | 5 | 14.7 | 10.2 |
| Respiratory system | 0 | 3 | 3 (3.1) | 1 | 33.3 | 3.0 | 0 | 8 | 8 (4.2) | 1 | 12.5 | 2.0 |
| Sensory organs | 2 | 1 | 3 (3.1) | 1 | 33.3 | 3.0 | 1 | 8 | 9 (4.7) | 0 | 0 | 0 |
| Systemic hormonal preparations, excluding sex hormones and insulins | 1 | 2 | 3 (3.1) | 1 | 33.3 | 3.0 | 0 | 4 | 4 (2.1) | 0 | 0 | 0 |
| Various | 1 | 2 | 3 (3.1) | 0 | 0 | 0 | 0 | 3 | 3 (1.6) | 1 | 33.3 | 2.0 |
| Total | 16 | 82 | 98 | 33 | 33.7 | 100.0 | 9 | 183 | 192 | 49 | 25.5 | 100.0 |
Two drugs, neither first-in-class nor with a post-market safety warning, were not listed in the World Health Organization Anatomical Therapeutic Chemical classification system.
Almost half of the drugs in the antineoplastic group (45.7%) had a post-market safety warning, and similarly, almost half of all of the post-market safety warnings (48.5%) in first-in-class drugs were in this group. For non-first-in-class drugs, the antineoplastic and anti-infective groups each had 24.5% of the total number of post-market safety warnings. Of all of the non-first-in-class antineoplastic drugs, 34.3% had a post-market safety warning (Table 1). Out of the 98 first-in-class drugs, 33 had a serious post-market safety warning compared to 49 of the 194 non-first-in-class group. Figure 1 compares the time between approval and post-market safety warning for the two groups. There was no statistically significant difference between the curves, p = 0.0799, Log rank (Mantel–Cox) test. An analysis of just the antineoplastic drugs in the first-in-class and non-first-in-class groups shows no statistically significant difference in post-market safety warnings, p = 0.1816, Log rank (Mantel–Cox) test (curves not shown). Eleven drugs were withdrawn for safety reasons. Five were first-in-class and one of these was innovative. Of the six non-first-in-class, none were innovative (Table 2).
Figure 1.
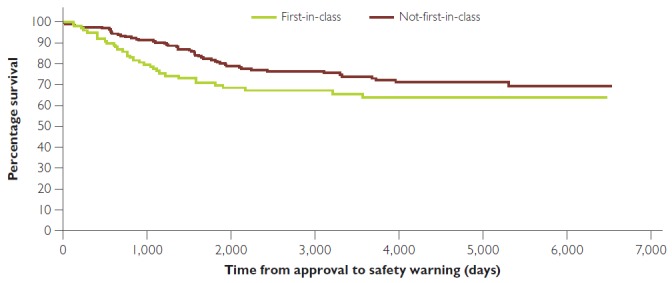
Kaplan–Meier curve showing time to first serious safety warning or removal from market for first-in-class and non-first-in-class drugs
No significant difference between curves, p = 0.0799, Log rank (Mantel–Cox) test.
Table 2.
Drugs withdrawn for safety reasons by first-in-class and innovation status
| Drug | First-in-class status | Innovation status |
|---|---|---|
| Cerivastatin | No | No |
| Drotrecogin alfa | Yes | Yes |
| Efalizumab | Yes | No |
| Gatifloxacin | No | No |
| Grepafloxacin | No | No |
| Rofecoxib | No | No |
| Sibutramine | Yes | No |
| Tegaserod | Yes | No |
| Tolcapone | Yes | No |
| Trovafloxacin | No | No |
| Valdecoxib | No | No |
Forty-one of the 98 first-in-class drugs received a priority review (41.8%, 95% CI: [32.6, 51.7]) compared to 33 of the 194 non-first-in-class drugs (17%, 95% CI: [12.4, 22.9]), z-ratio = 4.605 (p < 0.0002).
Figure 2 compares the time between approval and post-market safety warning for the innovative and non-innovative first-in-class drugs. There was no statistically significant difference between the curves, p = 0.1734, Log rank (Mantel–Cox) test. Of the 16 first-in-class drugs that were innovative, 14 had a priority review (87.5%, 95% CI: [64.0, 96.5]) compared to 27 of the 82 that were non-innovative (32.9%, 95% CI: [23.7, 43.7]), z-ratio = 4.048 (p < 0.0002).
Figure 2.
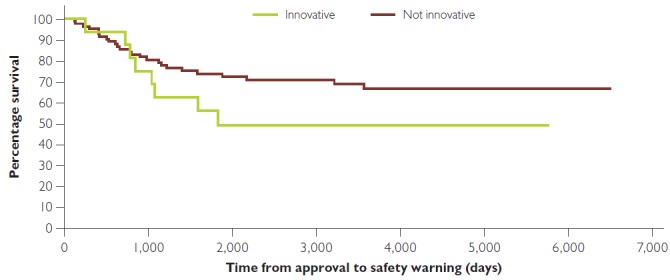
Kaplan–Meier curve showing time to first serious safety warning or removal from market for innovation and not innovative first-in-class drugs
No significant difference between curves, p = 0.1734, Log rank (Mantel–Cox) test.
Figure 3 plots the total number of first-in-class drugs and the number of those that were innovative introduced from 1997 to 2012. Linear regression analysis shows no time trend for either group.
Figure 3.
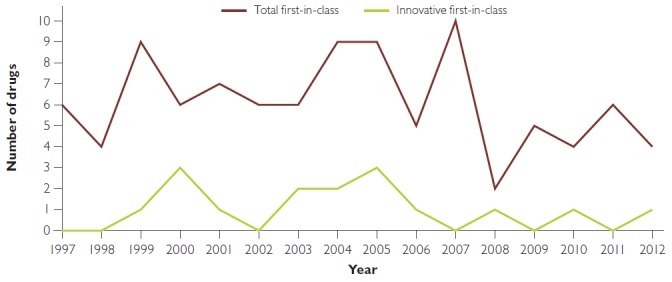
Number of (total and innovative) first-in-class drugs, 1997–2012
Discussion
Just over 16% of the drugs that were first-in-class were judged as therapeutically innovative compared to under 5% of those that were not-first-in-class. There was no difference in the safety of these two groups as judged by the marker used for safety in this study, that is, the time from approval to the first serious post-market safety warning. Therefore, overall, first-in-class drugs have a better benefit-to-harm ratio, as measured by post-market safety warnings, than drugs that were not-first-in-class. At the same time, it is also important to note that more than five in six of the drugs that were first-in-class were not innovative. Health Canada was much more likely to give a priority review to first-in-class drugs compared to those that were not-first-in-class, but its accuracy in predicting which first-in-class drugs are going to be therapeutically innovative is relatively weak, as only 16 out of 41 drugs with a priority review (39.0%) were rated as innovative.
Among the first-in-class drugs, there was no difference in safety between those that were and were not innovative as measured by the time to a first serious post-market safety warning. This finding reaffirms the conclusion that innovative first-in-class drugs do not have additional safety concerns because of their new mechanisms of action. In this subgroup, Health Canada made much better use of its priority review process, with almost 87.5% of the innovative group getting a priority review compared to 32.9% of the non-innovative group. Overall though, Health Canada is much more accurate in assigning first-in-class drugs to a standard review than it is to a priority review as shown by a negative predictive value of 91.2% versus a positive predictive value of 46.3% (Lexchin 2015). The positive predictive value measures the number of drugs evaluated as innovative by the PMPRB/Prescrire as a percentage of all drugs given a priority review by Health Canada, and the negative predictive value is the number of drugs rated as not therapeutically innovative as a percentage of all drugs given a standard review by Health Canada. This present study shows that, in addition to review status, the mechanism of action, i.e., being first-in-class, is also not a good indicator of significant therapeutic innovation. It reinforces the point that the ability to predict which products will turn out to be major therapeutic innovations needs to be determined by clinical trials and real-world experience, not based on surrogate measures such as review and first-in-class status.
The antineoplastic and immunomodulating group had the largest number of first-in-class drugs, although only 11.4% (4 of 35) were innovative. Drugs in this group, both first-in-class and non-first-in-class, were also the ones most likely to have a post-market serious safety warning, although the chances of this happening were higher in first-in-class drugs, 45.7% versus 34.3%.
As in the US (Lanthier et al. 2013), there is no trend, positive or negative, in the overall number of first-in-class drugs introduced into the Canadian market. Similarly, the number of innovative first-in-class drugs introduced is stable over time. The increasing amount of money being spent on research and development (Pharmaceutical Research and Manufacturers of America 2015a) does not seem to be leading to more therapeutic innovation.
The detection of safety problems with drugs may have improved over the period analyzed. Between 2004 and 2010, Health Canada increased the number of people and resources devoted to post-market safety monitoring (Wiktorowicz et al. 2010). However, better monitoring is likely to have affected the ability to detect safety problems for both first-in-class and non-first-in-class products and, therefore, should not have affected the results of this study.
This study has five significant limitations. First, because of data limitations, only 292 (68.5%) of the 426 approved new drugs could be analyzed. Whether the conclusions would be different if more of the drugs could have been included is unknown. Second is the assumption that the evaluations by PMPRB/Prescrire represent a gold standard in the evaluation of a drug's therapeutic value. While there is always a legitimate debate about therapeutic value, the rigorous processes that these organizations use to arrive at their conclusions and their independence give strong face validity to their assessments. Third, the definition of a serious post-market safety warning was based on the way that Health Canada displayed the information (bold black print and/or boxed text), but the criteria that Health Canada used to develop its safety warnings and the emphasis that it placed on any particular safety issue are extremely vague. One Health Canada document states: “Regulatory actions … are taken according to the regulatory framework in place. This implies an evaluation of the signal and the appropriate benefit-risk review of the information available” (Marketed Health Products Directorate 2004). Fourth, the metric, serious safety warnings, is only an indirect measure of safety; it does not measure the number of people potentially affected by safety problems nor the seriousness of the harms that the drugs cause. Finally, previous work has shown that the median time between approval and a post-market safety warning or safety withdrawal is about 3 to 3.5 years (Lexchin 2014a, 2014b) and, therefore, some drugs may not have been on the market long enough for one of these events to have taken place.
In summary, there does not appear to be any greater concern about the safety of first-in-class drugs than with non-first-in-class ones despite their novel mechanism of action, and first-in-class drugs are more likely to be therapeutically innovative. However, only a minority of first-in-class drugs (16%) were found to be therapeutically innovative, and the improved benefit-to-harm ratio among first-in-class drugs only applies to this subgroup.
References
- Health Canada: Health Products and Food Branch. 2009. Guidance for Industry: Priority Review of Drug Submissions. Ottawa, ON: Minister of Public Works and Government Services Canada. [Google Scholar]
- Lanthier M., Miller K., Nardinelli C., Woodcock J. 2013. “An Improved Approach to Measuring Drug Innovation Finds Steady Rates of First-in-Class Pharmaceuticals.” Health Affairs 32(8): 1433–39. [DOI] [PubMed] [Google Scholar]
- Lexchin J. 2014a. “How Safe Are New Drugs? Market Withdrawal of Drugs Approved in Canada between 1990 and 2009.” Open Medicine 8(1): e14–e19. [PMC free article] [PubMed] [Google Scholar]
- Lexchin J. 2014b. “Post-Market Safety Warnings for Drugs Approved in Canada under the Notice of Compliance with Conditions Policy.” British Journal of Clinical Pharmacology 79(5): 847–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexchin J. 2015. “Health Canada's Use of Its Priority Review Process for New Drugs: A Cohort Study.” BMJ Open 5: e006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marketed Health Products Directorate. 2004. How Adverse Reaction Information on Health Products Is Used. Ottawa, ON: Health Canada. [Google Scholar]
- Patented Medicine Prices Review Board (PMPRB). 2014. Compendium of Policies, Guidelines and Procedures – Reissued June 2013. Retrieved July 20, 2014. <www.pmprb-cepmb.gc.ca/view.asp?ccid=492>. Pharmaceutical Research and Manufacturers of America. 2015a. 2015 Biopharmaceutical Research Industry Profile. Washington, DC: Author. [Google Scholar]
- Pharmaceutical Research and Manufacturers of America. 2015b. Innovative Oncology Medicines Have Led to Impressive Gains in Patient Survivorship. Retrieved August 26, 2015. <www.phrma.org/innovative-oncology-medicines-have-led-to-impressive-gains-in-survivorship>.
- Prescrire Editorial Staff. 2002. “Prescrire's Rating System.” Prescrire International 11: 43. [Google Scholar]
- Prescrire Editorial Staff. 2011. “Prescrire's Ratings System: Gauge the Usefulness of New Products at a Glance.” Retrieved August 26, 2015. <http://english.prescrire.org/en/81/168/46800/0/NewsDetails.aspx>.
- Rawson N., Kaitin K. 2003. “Canadian and US Drug Approval Times and Safety Considerations.” Annals of Pharmacotherapy 37(10): 1403–08. [DOI] [PubMed] [Google Scholar]
- WHO Collaborating Centre for Drug Statistics Methodology & Norwegian Institute of Public Health. 2011. “Structure and Principles.” Retrieved December 15, 2015. <www.whocc.no/atc/structure_and_principles/>.
- Wiktorowicz M., Lexchin J., Moscou K., Silversides A., Eggertson L. 2010. Keeping an Eye on Prescription Drugs … Keeping Canadians Safe: Active Monitoring Systems for Drug Safety and Effectiveness in Canada and Internationally. Toronto, ON: Health Council of Canada. [Google Scholar]


