Abstract
This study was carried out in order to investigate the spatial variation of algal toxin (microcystin) concentrations along the shoreline of Lake Victoria. A total of 16 nearshore stations differing in connectivity to the main lake basin were categorized as either closed bays (ratio of bay area to bay opening < 1) or open bays (ratio ≥ 1) and sampled during November and December 2009. Water samples were analyzed for total phosphorus (TP), chlorophyll a, phytoplankton community composition and concentrations of microcystin (MC). Open and closed bays were significantly different for phytoplankton abundance and composition: Average phytoplankton biovolume was higher for closed bays (45 mm3 L-1 ± 11 SE) than open bays (5 ± 2 mm3 L-1). Cyanobacterial biovolume (mainly Microcystis spp., Anabaena spp. and Planktolyngbya spp.) also was significantly higher in closed bays (82 ± 9% of total biovolume) than in open bays (44 ± 5%). In contrast, diatom biovolume was lower in closed bays (7 ± 1%) than in open bays (36 ± 6%). MCs were found only among sites from closed bays and concentrations ranged from 0.4 to 13 μg L-1 MC-LR equiv. and coincided with high abundance of Microcystis spp. It is concluded that the level of water exchange from individual bays to the main basin is an important factor influencing eutrophication and microcystin production in nearshore habitats of Lake Victoria.
Keywords: bay morphometry, eutrophication, Microcystis, Anabaena, microcystin
Introduction
During the last four decades Lake Victoria has faced eutrophication resulting in a general increase of bloom-forming and potentially toxin-producing cyanobacteria throughout the year (Ochumba and Kibaara, 1989; Hecky, 1993; Lung’aiya et al., 2000; Kling et al., 2001; Mbonde et al., 2004; Ngupula et al., 2011). High levels of total phosphorus (> 100 μg L-1) have been recorded nearshore (Sekadende et al., 2005; Ngupula et al., 2012; Sitoki et al., 2013). The resulting increase in algal biomass has caused fish kills, deoxygenation of deeper waters in the lake (Ochumba, 1990) and has reduced the quality of drinking water for human consumption. Despite an overall eutrophic state, spatial variation in phytoplankton species composition and abundance exist (Haande et al., 2011; Ngupula et al., 2011). Several studies have suggested that spatial variation in physicochemical conditions and phytoplankton density in bays of Lake Victoria is due to water exchange with the main basin, driven by surface seiches and wind activity (Haande, 2008; Okely et al., 2010; Haande et al., 2011). Other factors that can influence the variation of nearshore phytoplankton include river inflow, direct discharge of wastewater, deforestation and agricultural activities, among others (Huisman et al., 2005).
During the dry season (May-September), the Tanzanian shoreline of the lake experiences the strong seasonal South-Eastern winds (Akiyama et al., 1977), which cause upwelling of colder water from the hypolimnion and which are important in embayment dilutions (Sekadende et al., 2005). At the shore, waves induced by seiches may cause water movement in and out of shallow bays (Haande et al., 2011). Haande (2008) obtained data on the daily variation in water levels at Murchison Bay that were used to calculate water volume entering the bay. By comparing the concentrations of both lake and bay waters it was possible to calculate a dilution factor. This horizontal mixing of embayment waters with the main lake has the potential to reduce the growth of toxigenic cyanobacteria such as Microcystis or Anabaena.
In Lake Victoria, occurrence of toxins produced by cyanobacteria, particularly microcystin (MC), has been documented from Kenyan and Ugandan waters (Krienitz et al., 2002; Semyalo et al., 2010; Okello et al., 2009; Sitoki et al., 2012). At the Tanzanian side of the lake, only Sekadende et al. (2005) have reported occurrence of MC in Mwanza Gulf. MC is a hepatotoxic cyclic peptide that was repeatedly involved in toxic outbreaks of livestock and humans (Chorus and Bartram, 1999). Besides it’s acute toxicity it has been considered to be carcinogenic under chronic exposure conditions at the sublethal level (Svircev et al., 2009).
In order to understand the spatial variation in phytoplankton and MC concentration in the nearshore waters of Lake Victoria, 16 study sites located along the Tanzanian shoreline were categorized as closed and open bays (see below and Fig. 1) and sampled during November and December 2009. During this time maxima in water stratification and cyanobacteria biovolume have been observed in Mwanza Gulf (Akiyama et al., 1977) and more recently, in Nyanza Gulf (Sitoki et al., 2012). Some & Omurwa (1994) estimated through interviews that in the Lake Victoria region, 4% of all households in the Kisumu district use lake water for drinking purposes. Taking into account the local population density (National Bureau of Statistics, 2013) along the study area it can be estimated that 360,000 people depend on the lake water for their domestic use. Since these nearshore embayments experience similar climatic conditions, similar anthropogenic activities and similar eutrophication, spatial variation of phytoplankton is possibly a function of water exchange through the above indicated physical processes. It was hypothesized that the reduced exchange of closed bays with the main basin of Lake Victoria relative to open bays leads to increased biovolume of phytoplankton possibly resulting in microcystin occurrence.
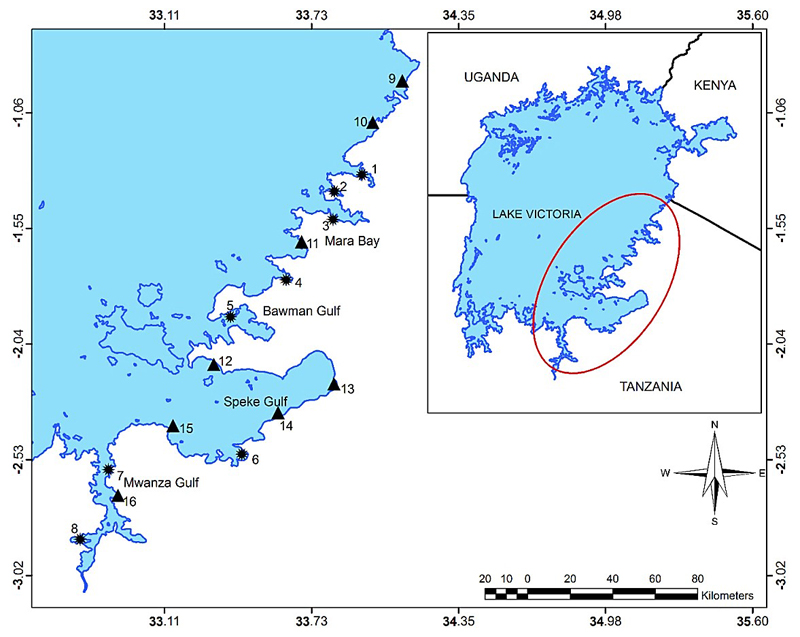
Figure 1.
Map of Lake Victoria (Southern part) showing the sampling sites on its Eastern side near the shore. Numbers 1-8 (✹) are closed bays, and 9 -16 (▲) are open bays. Inset shows Lake Victoria and national borderlines.
Materials and Methods
Study sites
The study sites comprised 16 shoreline stations with water depths below 10 m (Figure 1) that were sampled in November and December, 2009. All sites had human population density between 10,000 and 47,000 except of sites no. 1, 5 and 12 (< 10,000). Sites 5, 8, 11 and 12 were located in remote areas with less industrial but moderate agricultural activities. Depending on the width of the channel opening to the main basin the sampling stations were divided into bays that showed a large surface area and narrow opening (referred to as closed bays) and consequently were considered to have a more limited water exchange to the main lake (1. Mori Bay, 2. Mugubya Bay, 3. Mara Bay, 4. Suguti Bay, 5. Bawman Gulf, 6. Magu Bay, 7. Nyegezi, 8., Chole Bay), and those that showed a smaller surface area and a more wide opening (open bays), which were considered to have significant water exchange with the main lake (9. Kirongwe Bay, 10. Shirati Bay, 11. Kiemba, 12. Bulamba, 13. Lamadi, 14. Nyamikoma, 15. Nassa Bay, 16. Luchelele Bay). By this working definition, a closed bay had a ratio of bay surface area to bay opening below one. The closed and open bays showed a significant difference in their width of bay opening (p = 0.003), their areas (p = 0.028), and their ratios of area to openings (p < 0.001), (Appendix 1: available at http:// … ).
Sampling
At each station, depth-integrated water samples (from surface to the bottom) were taken using a van Dorn sampler for the quantification of total phosphorus (TP), phytoplankton abundance and biovolume, and MC analysis. From this sample, 50 ml aliquots were fixed with Lugol’s solution for quantitative phytoplankton analysis. In addition, a phytoplankton net (30 μm mesh size) was used to collect phytoplankton for qualitative analysis of phytoplankton and MC analysis. On-site measurements of water transparency (using Secchi disk), conductivity, temperature and pH were performed at the water surface using a multiprobe (HQ40d18, HACH Co.).
Chemical analysis
Chlorophyll a was extracted according to ISO (1992) using hot (78°C) ethanol. TP was hydrolysed by a potassium persulphate digestion and subsequently the total phosphate concentration was analyzed by the ammonium molybdate method (Wetzel and Likens, 2000). MCs were extracted in aqueous methanol according to Fastner et al. (1998) and analyzed using high performance liquid chromatography with diode array detection as described (Lawton et al., 1994). All peaks identified as putative MCs were collected manually for confirmation that was done using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) by Anagnostec GmbH (Potsdam Golm, Germany) as described by Kurmayer et al. (2004).
Phytoplankton species analysis
Identification and counting of phytoplankton species were done under an inverted microscope (400× magnification) according to Utermöhl (1958). Phytoplankton species were differentiated by morphological criteria using standard morphological keys (e.g. Büdel et al., 2007; Ettl et al., 1999) and according to earlier descriptions (Talling, 1987; Komarek and Kling, 1991). Typically, species were counted as individual cells, except the cyanobacterial genera Planktolyngbya and Romeria which were counted as filaments. Calculations of phytoplankton abundance and biovolume were done according to Wetzel and Likens (2000). Sample data were compared between closed and open bays using a non-parametric rank sum test (Mann-Whitney U test). If the assumption of normal distribution was met, the Student’s t-test was used. For all tests Sigma Plot 10.0 software was used.
Results
Difference in physicochemical parameters between closed and open bays
Overall the differences in depth and in physicochemical parameters between the two sample categories were rather minor. Only water temperature and conductivity showed a slight, albeit significant, increase among samples from closed bays when compared with open bay samples (Table 1). There was a positive correlation between conductivity and chlorophyll a (R = 0.59, P = 0.016) and a significant positive relationship was observed between temperature and phytoplankton biovolume both within the closed bay samples (R = 0.78, p = 0.02) and within all samples combined (R = 0.75, p < 0.001).
Table 1.
Mean (± SE) of limnological parameters recorded nearshore in Lake Victoria from either closed or open bays (n.d = not determined). P value calculated from pairwise comparison using Mann-Whitney Rank Sum test or Students t-test if normality and equal variance tests were passed.
| Bay Morphometry | Depth (m) | Temp. (°C) | Conductivity (μS cm-1) | pH | Secchi Depth (m) | TP (μgL-1) | Chl a (μgL-1) | Biovolume (mm3 L-1) |
|---|---|---|---|---|---|---|---|---|
| Nov 2009 | ||||||||
| Closed Bays (n=8) | ||||||||
| Mean | 2.9±0.4 | 27.1±0.8 | 118.7±5.3 | 8.5±0.3 | 1.2±0.2 | 81.6±0.7 | 24.6±5.7 | 36.3±12 |
| Median | 2.6 | 26.9 | 116.2 | 8.8 | 1.0 | 87.9 | 21.4 | 28 |
| Open Bays (n=8) | ||||||||
| Mean | 2.5±0.5 | 26.3±2.2 | 106.3±3.8 | 8.4±0.3 | 1.3±0.2 | 78.9±10 | 9.6±2.3 | 5.1±1.3 |
| Median | 2.0 | 25.8 | 105.2 | 8.1 | 1.3 | 71.1 | 6.9 | 5.1 |
| P value | 0.2 | 0.46 | 0.066 | 0.53 | 0.43 | 0.49 | 0.03 | 0.01 |
| Dec 2009 | ||||||||
| Closed Bays (n=6) | ||||||||
| Mean | 2.2±0.4 | 28.4±0.6 | n.d | 7.9±0.4 | 0.8±0.2 | 116.3±26 | n.d | 63±29 |
| Median | 2.0 | 28.5 | 8.2 | - | 104.8 | 38 | ||
| Open Bays (n=4) | ||||||||
| Mean | 1.9±0.1 | 26.6±0.2 | n.d | 7.4±0.3 | 0.5±0 | 120.4±33.3 | n.d | 7.4±4 |
| Median | 13.0 | 26.6 | 7.3 | - | 95.8 | 5.6 | ||
| P value | 0.82 | 0.04 | 0.3 | - | 0.9 | 0.26 | ||
| Data combined | ||||||||
| Closed bays | 2.6±0.3 | 27.4±0.5 | 118.7±5.3 | 8.4±0.3 | 1.0±0.1 | 97.0±5.9 | 24.6±5.7 | 45.4±10.9 |
| Open bays | 2.5±0.5 | 26.3±0.3 | 106.3±3.8 | 8.1±0.2 | 1.2±0.2 | 85.8±14.3 | 9.6±2.3 | 5.4±1.5 |
| P value | 0.4 | 0.14 | 0.066 | 0.4 | 0.46 | 0.05 | 0.03 | 0.003 |
Difference in phytoplankton abundance and species composition between closed and open bays
In total 88 species were observed (77 in closed bays and 67 in open bays), constituting six classes, i.e. the Bacillariophyceae (23 taxa), Chlorophyceae (29), Cryptophyceae (1), Cyanobacteria (29), Dinophyceae (2), and Euglenophyceae (4). The Shannon diversity index indicated higher phytoplankton species diversity and equitability (evenness) in open bays (H’=1.05, EH = 0.60) than in closed bays (H’=0.71, EH = 0.38). A few species (20 taxa) constituted more than 1% of total phytoplankton biovolume while 15 taxa constituted more than 5%.
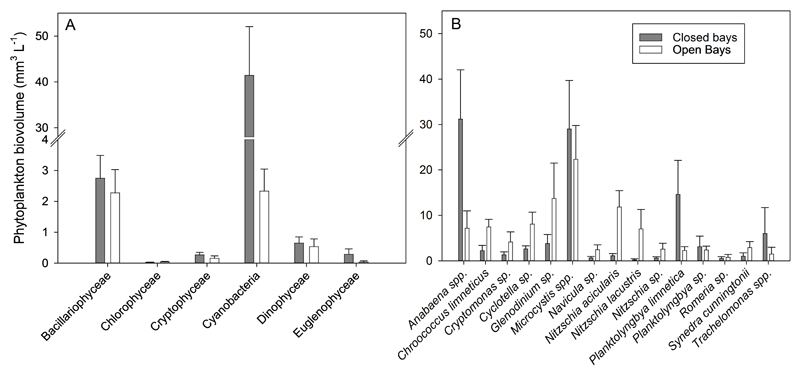
Although TP concentrations were similar in closed and open bays, the differences in chlorophyll a, total phytoplankton abundance and biovolume between the two sample sets were pronounced: 3.4×108 ± 6.1×107 ind. L-1 and 45 ± 11 mm3 L-1 in closed bays vs. 4.1×107 ± 0.3×106 ind. L-1 and 5.4 ± 1.5 mm3 L-1 in open bays (Fig. 2A), (Table 1, p= 0.003). On average, the phytoplankton biovolume recorded from closed bays was eight times higher when compared with biovolume recorded from open bays. Cyanobacteria were abundant in both closed and open bays, however, the proportion of cyanobacterial biovolume was significantly higher in closed bays (82 ± 9% of total biovolume) when compared with open bays (44 ± 5%), (p = 0.007). In contrast, the proportion of diatoms in total biovolume was lower in closed bays (6 ± 1.4%) than in open bays (36 ± 6%), (p < 0.001). Among the cyanobacteria, Anabaena spp. (closed: 31 ± 11%; open: 7.2 ± 4%), Planktolyngbya spp. (closed: 19 ± 9%, open: 6 ± 2%) and Microcystis spp. (closed: 29 ± 11%; open: 22 ± 7%) dominated. Within diatoms Nitzschia spp. (closed: 2 ± 0.5%; open: 21 ± 4%) and Cyclotella sp. (closed: 2.6 ± 0.7; open: 8.1 ± 2.6%) dominated. Taking all data together, both the biovolume of cyanobacteria (R = 0.73, p = 0.001) and diatoms (R = 0.58, p = 0.02) were found positively related to water temperature.
Figure 2.
Comparison of phytoplankton biovolumes (± SE) of the main phytoplankton classes (A) and dominant taxa (B) between closed and open bays. Only taxa contributing > 5% to total biovolume are shown.
Difference in microcystin occurrence between closed and open bays
MCs were detected at five stations all being closed bays: No. 1, 2, 3, 4, 7. The MC structural variants found in different stations were identified as MC-LR (protonated mass 995.5), MC–RR (1038.4), MC–YR (1045.2), MC-AR (953.2) and di-desmethyl-MC-RR (1010.7) by the respective masses as determined by MALDI-TOF MS. The MC concentrations ranged from 0.4 to 13 μg L-1 MC-LR equiv (the highest concentration occurred among samples from No.4, Suguti). Stations No. 3, 4, 7 had high abundance of Microcystis spp. (≥ 3.03×108 cells L-1) and also showed MC occurrence. At some stations (No. 6, 9, 12) relatively high Microcystis cell numbers (6.8×107 – 1.8×108 cells L-1) did not result in MC production. However, since Anabaena spp. co-occurred with Microcystis spp. (except at station 4 in November) the MC producer could not be identified by correlation analysis.
Discussion
The null hypothesis of this study was that phytoplankton abundance/biovolume was rather evenly distributed across the different stations in the lake. However, substantial differences between closed and open bays were observed. The much higher dominance of cyanobacteria in closed bays relative to open bays suggests that closed bays provided a more suitable habitat for cyanobacteria, such as Anabaena or Microcystis. In addition to reduced water exchange many of the closed bays were surrounded by hills that can protect the bays from winds resulting in a further reduction of water mixing. Large colony-forming cyanobacteria generally have lower growth rates when compared with diatoms and are sensitive to flushing (Reynolds et al., 2002). During calm conditions buoyant cyanobacteria like Anabaena and Microcystis accumulate on the water surface allowing for efficient light harvesting. The continued access to light through the formation of surface scums is thought to be one of the main factors contributing to the competitive success of those cyanobacteria when compared with non-buoyant phytoplankton.
In contrast diatoms flourish under turbulent conditions reducing sinking losses to a minimum. Diatoms are also most efficient in light harvesting (Reynolds et al., 2002) which enables them to use lowest irradiances under more turbulent conditions. Particularly in the open bays wind might be able to increase the mixing further in addition to the mixing caused by the horizontal water exchange with the main basin.
Both water temperature and conductivity were found increased among samples from closed bays. The increase in conductivity may indicate a reduced water exchange with the main basin as observed previously in the course of a sample gradient through the Nyanza Gulf (e.g. Sitoki et al., 2012). The higher temperature among closed bays may be related to the more sheltered location of these waterbodies. It is generally accepted that the optimal temperature for cyanobacterial growth often is 25°C or higher, for example, Microcystis strains have been shown to flourish at > 25°C (Robarts and Zohary, 1987). In this study the variation range in temperature (24.9 - 32.3°C) exceeded the variation in temperature that was observed across transects in Lake Victoria earlier (Talling, 1966). However, more data are needed to verify this nearshore variation in water temperature and its potential consequences on phytoplankton growth.
During the study period, water samples of closed bay stations of Lake Victoria (Tanzania) had detectable levels of MCs. According to earlier studies the major MC producers in Lake Victoria are Microcystis and Anabaena species (Krienitz et al., 2002; Okello et al., 2010; Semyalo et al., 2010). Okello et al. (2010) used PCR and restriction fragment length polymorphism to identify the MC producers in various freshwater lakes including Lake Victoria and found only Microcystis to contain the genes of the MC biosynthesis gene cluster. Nevertheless, correlations between Microcystis/Anabaena cell numbers and MC concentrations have been frequently observed.
In this study the abundance of Microcystis was not always associated with the detection of MC. In general, MC is produced when growth conditions are favorable for the cyanobacteria (Orr and Jones, 1998). Such growth conditions directly affect cyanobacteria growth but indirectly affect MC concentrations. The environmental factors related to cellular growth and bloom formation and MC net production included high temperatures that is possibly favored by the more sheltered location of the closed bays. For example, high temperature (30.3°C), high MC concentrations and high Microcystis biovolume (10 mm3 L-1) were recorded at Suguti Bay (No.4) in December. Likewise, Mori Bay (no. 1) in November had the highest biovolume of Microcystis (61 mm3 L-1) at 26.9°C resulting in relatively high concentrations of MCs.
All the five sites in closed bays that showed MCs are located near towns (like Musoma and Mwanza, Fig. 1) and untreated wastewater from the towns and agricultural activities into the lake is discharged via rivers like Mara River and Mori River. Occurrences of MCs in these areas can be a potential threat to people and their livestock health as majority of them use the near shore water for drinking before any treatment. Since these MC contaminated sites like Suguti, Mori Bay and Mara Bay are located near towns that continue to discharge nutrients into the lake, the problems of MCs may be more serious with increasing eutrophication in the near future.
When comparing the results of this study to those reported from the Kenyan and Ugandan areas (e.g. Sitoki et al., 2012, Okello et al., 2010) that have been obtained using the same system in the same laboratory (Kurmayer et al., 2004), there is some indication that toxin levels recorded in Tanzania were generally lower. Sekadende et al. (2005) also reported low MC concentrations in Mwanza Gulf (Tanzania). The higher concentrations of MCs from other parts of the lake such as Nyanza Gulf in Kenya may be explained by the characteristic morphometry of these inshore waters. Nyanza Gulf is only connected to the main basin by a narrow channel, and maximum MC concentrations >100 μg MC L-1 have been found (Sitoki et al., 2012). In general the intensity of human activities (industrialization) and hence nutrient loading gradually declines along the shore of Lake Victoria from the north to the south. Eutrophication and closed morphometry of the bays in the northern part of Lake Victoria may result in even higher growth of MC-producing colony-forming cyanobacteria such as Microcystis and Anabaena than described in this study.
Conclusion and recommendation
Spatial variation in phytoplankton biovolume and MC concentration nearshore in Lake Victoria depend on morphometry and connectivity to the main basin rather than eutrophication alone. During this survey, human activities were frequently observed nearshore or directly in the water. The WHO drinking water guideline recommends less than 1.0 μg MC-LR equivalents L-1 and 0.2 mm3 L-1 biovolume or 1 μg L-1 chlorophyll a (Chorus and Bartram, 1999; WHO, 2006) for safe water use. Since this threshold was exceeded, the nearshore water of MC-positive sites of Lake Victoria (Tanzania) is not recommended to be used as drinking water without treatment.
Since eutrophication in the lake is still ongoing, the installation of wastewater treatment plants to reduce eutrophication particularly at those closed bay sites showing MC occurrence is highly recommended. Such wastewater treatment systems may include also the extension of wetlands along the shoreline. The effort to construct wastewater treatment systems should go hand in hand with reducing direct human waste disposal and poor agricultural practices. Regular monitoring of phytoplankton abundance and phytoplankton composition along the shoreline by remote techniques or using multiparameter probes is highly recommended to identify all problematic sites along the Tanzanian shoreline.
Supplementary Material
Acknowledgements
We are grateful to Gerold Winkler and Regina Brandstätter for their continuous assistance. Two anonymous reviewers provided comments that substantially improved the current manuscript. We are thankful to Tanzania Fisheries Research Institute for their cooperation during sample collection. We are indebted to Eugen Rott (Institute for Botany, University of Innsbruck, Austria) for assisting in identification of various phytoplankton species and Dr. I. Kimirei (TAFIRI-Kigoma) and Mr. C. Mashafi (TAFIRI Mwanza) for their valuable assistance during figure constructions. We are grateful to the Netherlands Fellowship Programme for funding this research work and the International Postgraduate course of Limnology (IPGL) administered by the Austrian Academy of Sciences for financial support. The data analysis was financially supported by the Austrian Science Fund (P24070). This study is a contribution to the EU network “European Cooperation in Science and Technology” (COST) Action ES1105 ‘CYANOCOST’ (http://cyanocost.com).
References
- Akiyama T, Kajumulo A, Olsen S. Seasonal variations of plankton and physicochemical condition in Mwanza Gulf, Lake Victoria. Bull Freshw Fish Res Lab. 1977;27:49–60. [Google Scholar]
- Büdel B, Gärtner G, Krienitz L, Schagerl M, editors. Süswasserflora von Mitteleuropa Cyanoprokaryota (Oscillatoriales) Elsevier Gmbh München; Germany: 2007. [Google Scholar]
- Chorus I, Bartram J. Toxic cyanobacteria in water. A guide to their public health consequences, Monitoring and Management. World Health Organization, E & FN Spon; London and New York, USA: 1999. [Google Scholar]
- Ettl E, Gärtner G, Heynig H, Mollenhauer D, editors. Süswasserflora von Mitteleuropa Cyanoprokaryota (Chloroococales) Gustav Fischer Verlag; Jena, Germany: 1999. [Google Scholar]
- Fastner J, Flieger I, Neumann U. Optimised extraction of microcystins from field samples - a comparison of different solvents and procedures. Water Res. 1998;32:3177–3181. [Google Scholar]
- Haande S. Dissertation presented for the degree of Doctor Scientiarum. University of Bergen; Norway: 2008. On the ecology, toxicology, and phylogeny of cyanobacteria in Murchison Bay of Lake Victoria, Uganda. [Google Scholar]
- Haande S, Rohrlack T, Semyalo R, Brettum P, Edvardsen B, Lyche-Solheim A, Sorensen K, Larsson P. Phytoplankton dynamics and cyanobacterial dominance in Murchison Bay of Lake Victoria (Uganda) in relation to environmental conditions. Limnologica. 2011;41:20–29. [Google Scholar]
- Hecky RE. The eutrophication of Lake Victoria. Verh Internat Verein Limnol. 1993;25:39–48. [Google Scholar]
- Huisman J, Matthijs CP, Visser PM, editors. Harmful cyanobacteria. Springer; The Netherlands: 2005. p. 241. [Google Scholar]
- ISO. International Organisation for Standardization. Geneva: 1992. Water quality. Measurement of biochemical parameters, Spectrometric determination of the chlorophyll-a concentration, ISO 10260. [Google Scholar]
- Komárek J, Kling H. Variation in six planktonic cyanophyte genera in Lake Victoria (East Africa) Algol Stud. 1991;61:21–45. [Google Scholar]
- Kling HJ, Muggide R, Hecky RE. Recent changes in the phytoplankton community of Lake Victoria in response to eutrophication. In: Munawar M, Hecky RE, editors. The Great Lakes of the World (GLOW): Food web, health and integrity, Ecovision World Monograph series. Backhuys publishers; Leiden, The Netherlands: 2001. pp. 47–65. [Google Scholar]
- Krienitz L, Ballot A, Wiegand C, Kotut K, Codd GA, Pflugmacher S. Cyanotoxin producing bloom of Anabaena flos-aquae, Anabaena discoidea and Microcystis aeruginosa (cyanobacteria) in Nyanza Gulf of Lake Victoria, Kenya. J Appl Bot. 2002;76:179–183. [Google Scholar]
- Kurmayer R, Christiansen G, Fastner J, Börner T. Abundance of active and inactive microcystin genotypes in populations of the toxic cyanobacterium Planktothrix spp. Environ Microbiol. 2004;6:831–841. doi: 10.1111/j.1462-2920.2004.00626.x. [DOI] [PubMed] [Google Scholar]
- Lawton LA, Edwards C, Codd GA. Extraction and high-performance liquid chromatographic method for the determination of microcystins in raw and treated waters. Analyst. 1994;119:1525–1530. doi: 10.1039/an9941901525. [DOI] [PubMed] [Google Scholar]
- Lung’aiya HBO, M’harzi A, Tackx M, Gichuki J, Symoens J. Phytoplankton community structure and environment in the Kenyan waters of Lake Victoria. Freshw Biol. 2000;43:529–543. [Google Scholar]
- Mbonde ASE, Shayo S, Sekadende BS, Lyimo TJ. Phytoplankton species diversity and abundance in the nearshore waters of Tanzanian side of Lake Victoria. Tanz J Sci. 2004;30:71–82. [Google Scholar]
- National Bureau of Statistics. Population Distribution by Administrative Units. 2012 Population and Housing Census. The United Republic of Tanzania, Dar es Salaam, Zanzibar, Tanzania: 2013. [Google Scholar]
- Ngupula GW, Mbonde ASE, Ezekiel CN. Spatial and temporal patterns of phytoplankton abundance and composition in three ecological zones in the Tanzanian waters of Lake Victoria. Afr J Aqu Sci. 2011;36:197–206. [Google Scholar]
- Ngupula GW, Ezekiel CN, Kimirei IA, Mboni E, Kashindye BB. Physical and chemical characteristics of the Tanzanian inshore and offshore waters of Lake Victoria in 2005–2008. Afr J Aqu Sci. 2012;37:339–345. [Google Scholar]
- Ochumba PBO, Kibaara DI. Observations on blue-green algal blooms in the open waters of Lake Victoria, Kenya. Afr J Ecol. 1989;27:23–24. [Google Scholar]
- Ochumba PBO. Massive fish kills within the Nyanza Gulf of Lake Victoria, Kenya. Hydrobiologia. 1990;208:93–99. [Google Scholar]
- Okello W, Portmann C, Erhard M, Gademann K, Kurmayer R. Occurrence of microcystin-producing cyanobacteria in Ugandan freshwater habitats. Environ Toxicol. 2010;25:367–380. doi: 10.1002/tox.20522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okely P, Imberger J, Antenucci JP. Processes affecting horizontal mixing and dispersion in Winam Gulf, Lake Victoria. Limnol Oceanogr. 2010;55:1865–1880. [Google Scholar]
- Orr PT, Jones GJ. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnol Oceanogr. 1998;43:1604–1614. [Google Scholar]
- Reynolds CS, Huszar V, Kruk C, Naselli-Flores L, Melo S. Towards a functional classification of the freshwater phytoplankton. J Plankton Res. 2002;24:417–428. [Google Scholar]
- Robarts RD, Zohary T. Temperature effects on photosynthetic capacity, respiration, and growth rates of bloom-forming cyanobacteria. New Zealand J Mar Freshw Res. 1987;21:391–399. [Google Scholar]
- Sekadende BC, Lyimo TJ, Kurmayer R. Microcystin production by cyanobacteria in the Mwanza Gulf (Lake Victoria, Tanzania) Hydrobiologia. 2005;543:299–304. [Google Scholar]
- Semyalo R, Rohrlack T, Naggawa C, Nyakairu GW. Microcystin concentrations in Nile tilapia (Oreochromis niloticus) caught from Murchison Bay, Lake Victoria and Lake Mburo: Uganda. Hydrobiologia. 2010;638:235–244. [Google Scholar]
- Some E, Omurwa T. Seasonality and community's satisfaction with sources of domestic water in the Lake Victoria basin. East Afr Med J. 1994;71:39–41. [PubMed] [Google Scholar]
- Sitoki L, Kurmayer R, Rott E. Spatial variation of phytoplankton composition, biovolume, and resulting microcystin concentrations in the Nyanza Gulf (Lake Victoria, Kenya) Hydrobiologia. 2012;691:109–122. doi: 10.1007/s10750-012-1062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitoki L, Kofler W, Rott E. Planktonic needle-shaped Nitzschia species from Lake Victoria, Africa, revisited. Diatom Research. 2013;28:165–174. [Google Scholar]
- Svircev Z, Krstic S, Miladinov-Mikov M, Baltic V, Vidovic M. Freshwater cyanobacterial blooms and primary liver cancer epidemiological studies in Serbia. J Environ Sci Health Part C-Environ Carcinogen & Ecotox Rev. 2009;27:36–55. doi: 10.1080/10590500802668016. [DOI] [PubMed] [Google Scholar]
- Talling J. The annual cycle of stratification and phytoplankton growth in Lake Victoria (East Africa) Int Revue ges Hydrobiol. 1966;51:545–621. [Google Scholar]
- Talling JF. The phytoplankton of Lake Victoria (East Africa) Arch Hydrobiol Beih Ergebn Limnol. 1987;25:229–256. [Google Scholar]
- Utermöhl H. Zur Vervollkommnung der quantitativen Phytoplanktonmethodik. Mitt Int Verein Theoret Angew Limnol. 1958;2:1–38. [Google Scholar]
- Wetzel RA, Likens GE. Limnological analysis. Third Edition. Springer Verlag; New York: 2000. [Google Scholar]
- WHO. Guidelines for drinking-water quality. First addendum to third edition. Third edition. Vol. 1. World Health Organization; Geneva, Switzerland: 2006. recommendations. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.