Abstract
Many important processes in biology involve the translocation of a biopolymer through a nanometer-scale pore. Moreover, the electrophoretic transport of DNA across nanoscale pores is under intense investigation for single-molecule DNA sequencing and analysis. Here we show that the precise patterning of the entry and the middle section of the ClyA nanopore with positive charges are crucial to observe the electrophoretic translocation of DNA at physiological ionic strength. Surprisingly, the strongly electronegative 3.3 nm internal constriction of the nanopore did not require modifications. Further, DNA translocation could only be observed from the wide entry of the nanopore. Our results suggest that the engineered positive charges are important to align the DNA in order to overcome the entropic and electrostatic barriers for DNA translocation through the narrow constriction. The dependencies of nucleic acid translocations on the Debye length of the solution are consistent with a physical model where the capture of double stranded DNA is diffusion-limited while the capture of single stranded DNA is reaction-limited.
Keywords: ClyA nanopore, DNA rotaxane, protein engineering, DNA sequencing, bacteriophage DNA sliding
The translocation of DNA across specialized proteins is an important biological process. Bacteria use secretin channels in their pili to uptake or transfer DNA, while viruses such as phages use their portal proteins to either pack genomic DNA into the viral capsid or to eject it into target cells. In the three domains of life, β-clamp proteins form a toroid structure that encircles and slides along DNA to aid the function of DNA processing enzymes. Portal proteins in all known bacteriophages (λ, P22, T4, T3, T7, SPP1, P2 and ϕ29)1 and many components of secretin channels2 form dodecameric rings. The available crystal structures of portal proteins (ϕ29,3 SPP1,4 P225 and T46) have revealed the presence of a central channel through which dsDNA must both enter during packaging and exit during injection. While the interior channel lining is mostly negatively charged, a few positively charged amino acids, arranged as rings along the length of the pore, can also be found. A similar arrangement of negative and positive charges can also be observed on β-clamp DNA sliding proteins.7 It has been proposed that the net internal negative surface charge is important to allow the smooth sliding of the opposing negatively charged DNA as it passes through the connector3,8 or β-clamp7 channels and that the positively charged rings play a direct role in packaging by interacting with the negatively charged phosphate groups in the backbone of the translocating DNA.3,9
Ionic current through biological nanopores reconstituted into artificial membranes have been used to identify small molecules10 or folded proteins11, and monitor chemical12 or enzymatic13 reactions at the single-molecule level. The electrophoretic translocation of DNA across nanopores holds great promise for practical applications such as DNA sequencing14–22 and biomarker recognition.23 Although not a membrane protein per se, Φ29 portal protein was found to insert into black lipid bilayers.24–27 Such nanopores electrophoretically translocated dsDNA at 1.0/0.5 M NaCl, making it the first biological nanopore to allow the study of dsDNA. Despite this achievement, however, the exact hydrophobic modifications of the nanopore that allowed membrane insertion were not disclosed,27 and the Φ29 nanopores occasionally released from the lipid membranes,27 posing limitations in practical applications. dsDNA has shown to translocate through artificial nanopores fabricated in solid-state membranes28–31, which, with the exception of atom-thin materials such as graphene32–34 or bilayer of molybdenum disulfide35–37, mostly have a negative internal surface charge.31 In such nanopores, with radii comparable to the Debye length of the solution, the diffusive layer on the inner nanopore walls overlaps, resulting in a large electrostatic barrier for the entry of DNA into the nanopore. As a consequence, the translocation of DNA across solid-state nanopores at physiological ionic strength has only been observed using large nanopores (10 nm)38 or using small nanopores (~3.5 nm) in 340 mM salt39 or under asymmetric salt concentrations.40
Recently, we have described the ClyA nanopore, a dodecameric protein with an internal constriction of ~3.3 nm (Fig. 1A), as an effective tool to investigate folded proteins.11,41–44 Although dsDNA translocation across the nanopore was observed at 2.5 M NaCl solutions,45 the strong negative interior of the nanopore (Fig. 1A) prevented DNA translocation at lower ionic strengths. In this work we engineered the ClyA nanopore, enabling it to translocate DNA at physiological ionic strengths. This is useful in many applications where electrostatic interactions between molecules and DNA are important, for example in DNA sequencing or mapping where enzymes are used to control the translocation of DNA across the nanopore, or to study DNA-protein interactions. We could observe the translocation of DNA after two rings of positive charges were introduced at the wider cis-side of the nanopore, while modification of the more constricted trans-entrance of the nanopore did not improve the efficiency of DNA translocation. Interestingly, many proteins that slide on DNA display a surface charge similar to the engineered ClyA nanopores,3 suggesting that the alternation of positive and negative charges might provide a general mechanism for improving the translocation of DNA across nanoscale structures.
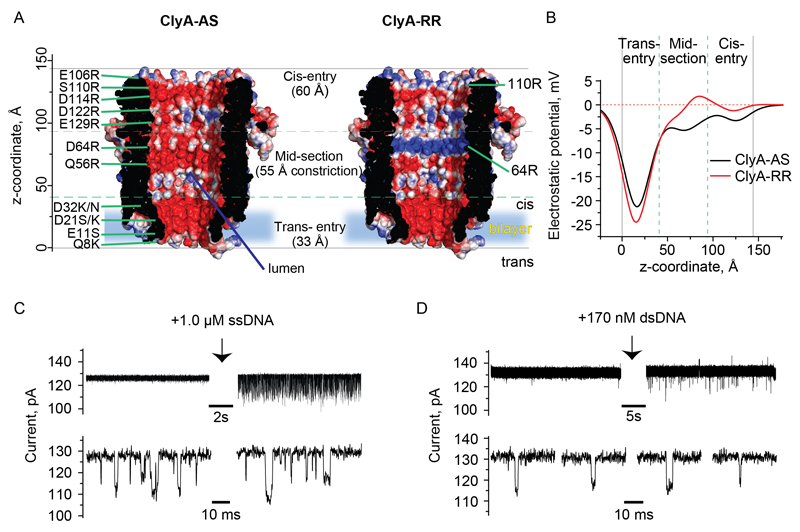
Fig. 1. Engineering the ClyA nanopore for DNA translocation.
(A) Cross sections of the ClyA-AS (left) and ClyA-RR (right) nanopores imbedded into a lipid bilayer (blue) constructed by homology modeling from the E. coli ClyA structure using VMD49 and NAMD50 (PDB: 2WCD, 90% sequence identity). The inner pore lumen is shown using the solvent accessible surface area as calculated by PyMOL (Version 1.8 Schrödinger, LLC) and colored according to the electrostatic potential in a 150 mM NaCl solution as calculated by APBS.51–53 Red and blue regions correspond to negative and positive potentials (range -2 to +2 kBT/e or -51.4 to + 51.4 mV), respectively. (B) Electrostatic potential at the center of ClyA-AS (black line) and ClyA-RR (red line) nanopores at 150 mM NaCl concentration. (C) ssDNA (1a, 1.0 μM) and dsDNA (1, 170 nM) translocation through ClyA-RR nanopores at physiological ionic strength at +70 mV. DNA was added in the cis compartment. The bottom current traces show a magnification of the DNA translocation events. The current signal was acquired at 10kHz applying a 2 kHz low-pass Bessel filter. The buffer contained 150 mM NaCl and 15 mM Tris-HCl (pH 7.5) at 22 °C.
Results
Engineering ClyA nanopores to capture DNA
In this work we used ClyA-AS (Fig. 1A), an engineered ClyA version selected for its favorable proprieties in planar lipid bilayers11 and in which the translocation of ssDNA or dsDNA is only observed above 2.0 M NaCl ionic strengths.45 Most likely, at low ionic strengths the strong negative electrostatic potential inside the nanopore (Fig. 1B) prevents DNA entry and translocation, while at high ionic strengths the charges of the nanopore surface are effectively screened. To induce the capture of DNA by the nanopores at physiological ionic strengths, inspired by previous work with the αHL46–48 and MspA19 nanopores, we modified the internal charges of the ClyA-AS nanopore (Table S1, Fig. 1A). We first introduced a single ring of positive charges in the form of arginine residues at the cis-entrance of ClyA-AS (S110R, ClyA-R, Fig. 1A) and then we proceeded to modify three sections of the nanopore: the cis entry, the mid-section and the trans constriction (Fig. 1A). The substitution of neutral residues with positive charges at the cis opening of ClyA-R showed no DNA translocation in 150 mM NaCl (Table S1). Arginine rings in the mid-section of the ClyA-R nanopore induced ssDNA (Fig. 1C) and dsDNA (Fig. 1D) translocation when the negatively charged glutamate residues at position 64 were replaced by arginine (D64R, ClyA-RR), but not when a neutral side chain at a nearby position was substituted with arginine (Q56R). The exchange of negatively charged residues in the transmembrane region with a neutral (ClyA-R-E11S) or a positively charged residue (ClyA-R-Q8K) induced no DNA translocation events in 150 mM NaCl solutions (Table S1 and Fig. S1). Surprisingly, additional positively charged residue in both the mid-section and trans entry of ClyA-R (ClyA-R-Q56R-Q8K) also did not induce DNA translocation events (Fig. S1). Despite the observation that only ClyA-RR allowed DNA translocation, ClyA-RR, ClyA-R and ClyA-AS showed the same ion selectivity (PNa+/PCl-=1.9±0.7, 2.0±1.6, 1.9±0.9, respectively, Table S2), indicating that DNA translocation was not induced by an enhanced electro-osmotic flow through the nanopore.
In order to obtain a greater insight into the changes of the electrostatic potential caused by the two additional arginine rings, full-atom homology models of ClyA-AS and ClyA-RR were constructed using VMD49 and NAMD50 starting from the E. coli ClyA crystal structure. The Adaptive Poisson-Boltzmann Solver (APBS)51–53 was employed to calculate the electrical potential distribution of both pores in 150 mM NaCl (Fig. 1B). In ClyA-AS, the potential at the center of the pore was found to be increasingly negative moving from the cis-entry, through he mid-section, and to the trans-entry (-2.6, -4.8 and -15.2 mV, respectively). In the case of ClyA-RR, a rise in the potential could be observed at both the cis-entrance and mid-section of the pore (-0.3 and -1.1 mV, respectively). The potential in the trans constriction appeared to decrease further to -17.3 mV. Notice that these values are calculated when no external bias is applied.
A DNA rotaxane as a proof of DNA translocation
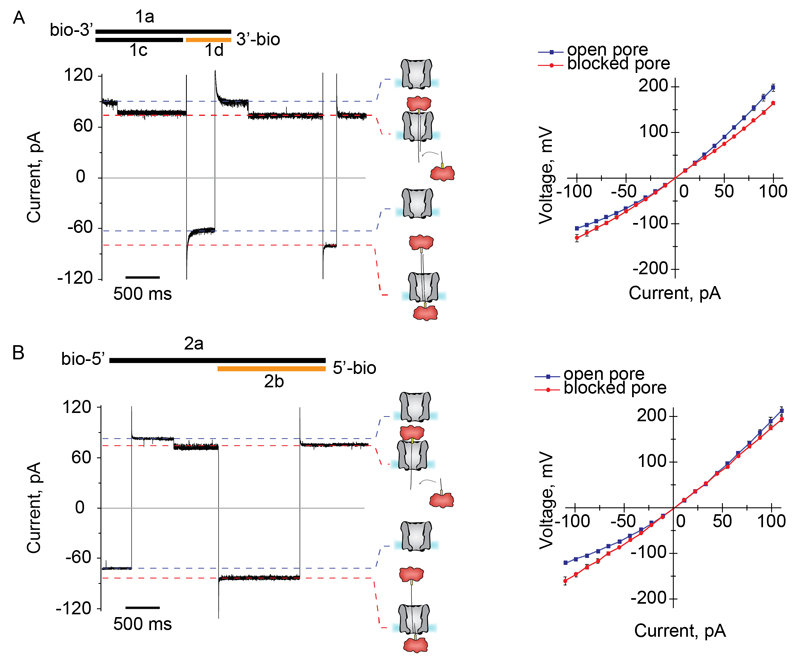
A rotaxane is a dumbbell shaped molecule formed by a macrocycle that encircles a thread locked by two stoppers. Here we formed two nanopore/DNA rotaxanes to prove the translocation of ssDNA and dsDNA through the nanopore. The first rotaxane was formed using a 3’-biotinylated 59 base pairs dsDNA molecule extended with a 31 bases overhang at the 5’ of the biotinylated strand as the initial thread (1a:1c, Table S3). The second rotaxane was formed using a 100mer 5’-biotinylated ssDNA molecule (2a, Table S3) added to the cis compartment as the initial thread. The rotaxanes were locked by adding on the opposite side of the nanopore another biotinylated ssDNA molecule, 1d (31mer, 3’-biotinylated) or 2b (50mer, 5’-biotinylated) designed to hybridize with the overhangs of 1a:1c or 2a, respectively. Both cis and trans solutions contained NeutrAvidin (NA, 0.3 µM), which in complex with biotin prevented the full translocation of the DNA strand across the nanopore.
At negative applied potentials, the ionic current in the rotaxane configuration was higher than the open pore current (IRES-50 = 1.16±0.03 and 1.11±0.06, for ssDNA and dsDNA/ssDNA threads, respectively, N=3 independent nanopore experiments, Fig. 2). This effect was previously observed for the translocation of DNA through 10 nm solid-state nanopores at low ionic strengths,38 and was explained by the accumulation of counterions inside the DNA blocked pore.38 Intriguingly, however, at positive applied potentials the open pore current was higher than the blocked current (Fig. 1C-D and Fig. 2), suggesting that the accumulation of counterions on the DNA differs at the cis- and trans sides of the nanopore.
Fig. 2. DNA rotaxane formation in 150 mM NaCl solutions at +50 mV.
(A) A dsDNA rotaxane was formed by adding 1a:1c (1.0 µM, black lines) and 1d (1.0 µM, orange line) to the cis and trans compartments, respectively. NeutrAvidin (NA, 0.3 µM, tetramer, red) was also added in both solutions. (B) A ssDNA/dsDNA hybrid rotaxane was formed by addition of a 5’ biotinylated ssDNA thread 2a (1.0 µM, black line) to the cis compartment and a 5’ biotinylated ssDNA molecule complementary to the 3’ end of 2a (2b, 1.0 µM, orange line) to the trans compartment. NeutrAvidin (0.3 µM, tetramer) was present on both sides. The graphs on the right-hand side of the current traces show the voltage relationship (IV curve) for ClyA-RR (blue line) and ClyA-RR in a rotaxane configuration (red line). Experiments were carried out in a buffer contained 150 mM NaCl and 15 mM Tris-HCl (pH 7.5) at 22 °C. The DNA sequences are shown in Table S2.
DNA capture and threading depends on the ionic strength of the solution
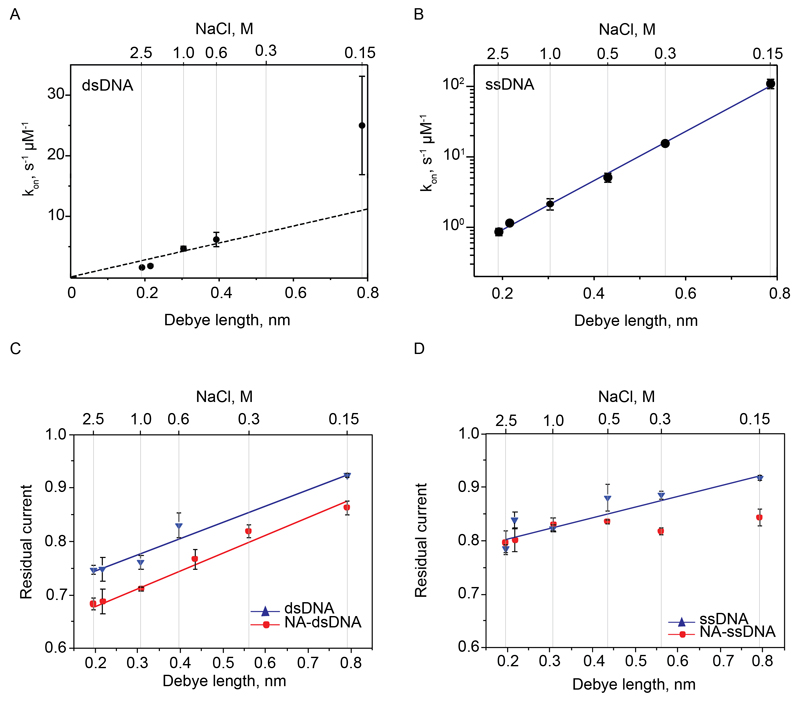
The capture rate kon, which is the inverse of the interevent time τon (Table S4, +70mV, 1 μM DNA), increased with the Debye length of the solution (λD) for both ssDNA and dsDNA (Fig. 3). However, while the dsDNA capture rate increased linearly with λD (Fig. 3A), ssDNA capture rate increased exponentially with λD (Fig. 3B). This suggests, therefore, different capture mechanisms for these two different forms of DNA. As reported before with solid-state nanopores, the residual current of DNA blocked nanopores increased as the ionic strength of the solution decreased38 (e.g. from 0.78±0.09 in 2.5 M NaCl to 0.92±0.02 in 150 mM NaCl for dsDNA). Interestingly, we found a linear relationship between the IRES of the DNA blockades and the Debye length of the solution (Fig. 3C and 3D). For dsDNA in complex with NeutrAvidin the residual current was ~10% lower than during free DNA translocation, suggesting that NA contributed to the overall ionic current of the blockade, most likely by interacting with the nanopore lumen. At 150 mM NaCl, ssDNA molecules in complex with NeutrAvidin showed permanent blockades to ClyA-RR nanopores, while at 1 M NaCl or higher, the blockades were transient (Fig. S2). A likely explanation to this data is that at high ionic strengths ssDNA enters and escapes the pore from the cis side. Confirming this interpretation, at ionic strengths ≥1 M the IRES values for ssDNA and ssDNA:NeutrAvidin were the same (Fig. 3D and Fig. S2), suggesting that under these conditions ssDNA might not fully thread the nanopore, preventing NeutrAvidin from interacting with the lumen of ClyA.
Fig. 3. Ionic strength dependency of DNA translocation and threading under +70 mV.
(A-B) Debye length dependency of the frequency of dsDNA (A) and ssDNA (B) translocation per 1 µM DNA. The dotted line depicts the theoretical prediction of translocation frequencies for a diffusion-limited process. The blue line is an exponential regression indicating a barrier-limited process. (C) Debye length dependency of residual current of dsDNA (blue circles) and NeutrAvidin:dsDNA (NA-dsDNA) complex (red circles) blockades. The lines represent linear regressions. (D) Same as in C but for ssDNA. (D) Ionic strength dependency of DNA threading. ssDNA (1a, 1 µM) was first added to the cis side. The electrical recordings were carried out in buffers containing the given NaCl concentrations and 15 mM Tris-HCl (pH 7.5) at 22 °C.
Unidirectional entry of DNA into ClyA nanopores
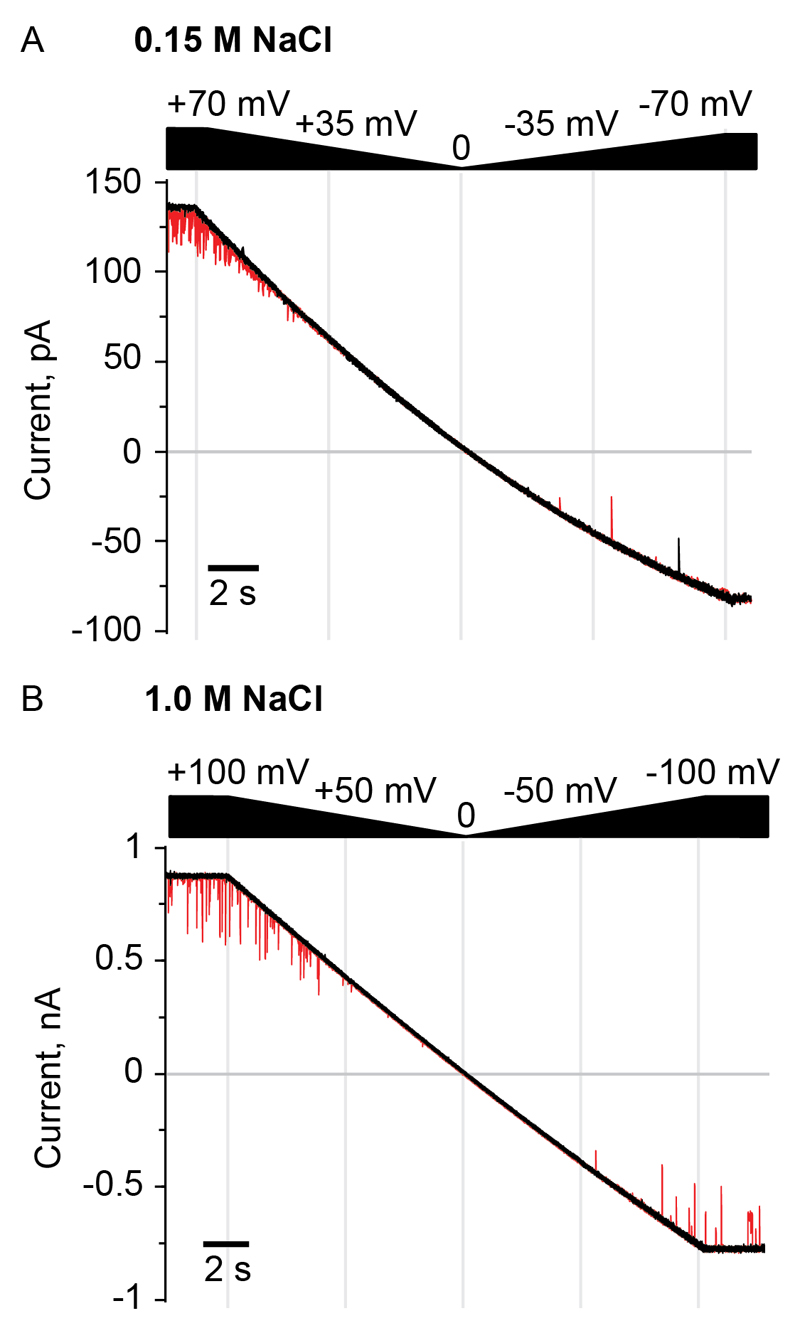
In 150 mM NaCl solutions the addition of 1 µM of ssDNA or dsDNA to the cis and trans compartments of ClyA-RR induced DNA blockades only under positive applied potentials (higher than ~+50 mV, Fig. 4A), indicating that 1) DNA cannot enter the nanopore from the trans entrance of ClyA and 2) there is a voltage threshold for the translocation of ssDNA from the cis side of the nanopore. The entry (Fig. 4B) and translocation (Fig. S3) of DNA from the trans compartment, however, was observed in 1 M NaCl solutions. In order to induce the entry of DNA from the trans compartment under physiological ionic strengths, we remodeled the charges of the transmembrane region of ClyA-RR nanopores (Table S1). Although DNA induced current blockades to the modified ClyA-RR pores were occasionally observed upon the addition of 1 µM of dsDNA 1 to the trans chamber under negatively applied potentials (Table S1, Fig. S4), DNA rotaxanes could not be formed, suggesting that dsDNA might not fully translocate through ClyA-RR nanopores under these conditions.
Fig. 4. Unidirectional DNA translocation through ClyA-RR nanopores.
(A) In 150 mM NaCl solutions, the addition of 3.0 µM of dsDNA 1 to both the cis and trans sides of a ClyA-RR nanopores induced transient current blockades (red lines) only under positive applied potentials. (B) In 1.0 M NaCl solutions the DNA blockades were observed under both applied potentials. DNA induced blockades are shown in red. The applied potential was automatically changed from +70 to -70 mV (A) or from +100 to -100 mV (B) in 21 seconds. The electrical recordings were carried out in buffer solutions containing the given NaCl concentrations and 15 mM Tris-HCl (pH 7.5) at 22 °C. Data were recorded by applying a 2-kHz (A) and 10 kHz (B) low-pass Bessel filter and using a 10 kHz (A) and 50 kHz (B) sampling rate.
Discussion
Precise nanopore engineering is required for DNA translocation at physiological ionic strength
In this work we engineered ClyA nanopores to allow the electrophoretic translocation of DNA at physiological ionic strengths. DNA translocation was observed when two sets of positive charges were introduced at the entry and in the mid-section of the ClyA nanopore (Fig. 1A). Surprisingly, the trans entrances of the nanopore, which provides the highest entropic (Fig. 1A) and electrostatic (Fig. 1B) barriers for DNA translocation, did not require modification. Further, despite extensive modification to the trans entrance of ClyA (Table S1), DNA translocation could be observed only when initiated from the wider cis entry of the nanopore. These data suggest, therefore, that the cis lumen of the nanopore is important to initiate the translocation of DNA through the constriction of the nanopore. Moreover, the frequency of dsDNA translocation through ClyA-RR nanopores increased with the Debye length of the solution (Fig. 3A), confirming that the favorable electrostatic interactions of dsDNA with the cis entry of ClyA-RR dominate over the unfavorable electrostatic repulsion of the DNA with the nanopore constriction. Worth of note is that the stiffness of dsDNA does not change significantly over the range of ionic strength tested.54 Further, the increased electro-osmotic flow as the ionic strength is lowered cannot account for the increased frequency of DNA translocation, because the electro-osmotic flow opposes DNA entry and translocation.
dsDNA capture is diffusion-limited, while ssDNA capture is reaction-limited
The DNA translocation experiments at different salt concentrations revealed two different capture mechanisms for dsDNA and ssDNA (Fig. 3A and 3B, respectively, Fig. 5A and 5B, respectively). The behavior of dsDNA is consistent with a diffusion-limited capture process.55 This can be explained because the dsDNA used in this work is shorter than its persistence length (150bp) and behaves as a rigid uniformly charged rod. Within the capture radius (about 50 nm from the nanopore center for a λD of 0.5 nm, supporting information), the electric field attracts the DNA towards the pore and aligns it along the field lines so that it hits the pore entrance with one end (Fig. 5A-i). Once inside the pore, the engineered charges interact with the DNA, preventing the retraction back to the cis solution (Fig. 5A-ii to 5A-iv). Therefore, the dynamics of DNA capture can be approximated by that of a diffusing particle in a purely attractive potential of electrophoretic origin. In this case the electrophoretic mobility of the dsDNA is proportional to the Debye length of the solution and the corresponding drift-diffusion equation can be solved exactly55 (supporting information). By approximating the geometry of the ClyA nanopore with a cylinder of length l = 13 nm and a capture diameter d = 6 nm the capture frequency can be estimated55 by (supporting information for more details):
| (1) |
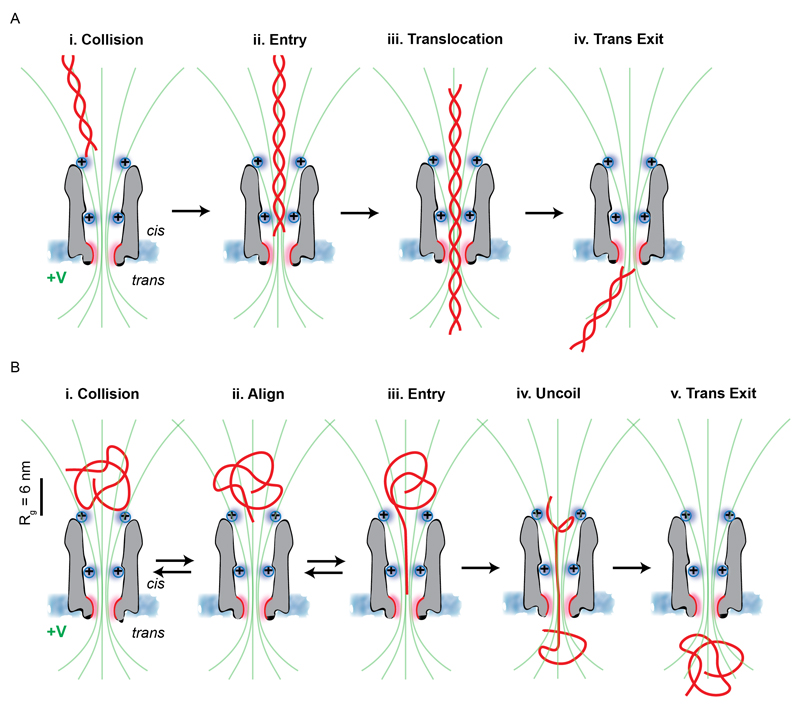
Fig. 5. Mechanism of dsDNA and ssDNA translocation through ClyA-RR nanopores.
(A) dsDNA translocation is diffusion-limited. (i) dsDNA, which under the experimental conditions is a rigid rod, is aligned by the electric field lines (green) and enters the nanopore with a defined orientation. (ii) dsDNA penetrates inside the nanopore where it interacts with the second layer of engineered charges. (iii) The dsDNA can then translocate the constriction and (iv) exit the pore. The charges at the cis entrance of the nanopore aid in the initial capture. (B) ssDNA translocation is reaction-limited. (i) ssDNA has a coiled structure with a gyration radius (Rg ≈ 6 nm), which is about twice the radius of the nanopore. (ii) ssDNA is not yet in the pore and it searches for the entry. (iii) One end of ssDNA finds the entry of the cis lumen and starts to uncoil. Because there is an entropic energy barrier to enter the nanopore, several attempts can be made before a successful translocation event. (iv) In order to translocate the constriction, ssDNA needs to fully uncoil. (v) DNA exits the nanopore and then recoils. The additional charges at the cis entrance most likely mediate the efficient capture of the DNA inside the nanopore. The DNA molecules and the nanopore are drawn to scale.
This is in remarkably good agreement with the experimental data for λD (at high salt concentrations, Fig. 3A). This is striking, because no fitting parameters are used. However, some care should be taken in this comparison, as the choice of the pore parameters is to some extent arbitrary since ClyA’s geometry deviates significantly from a perfect cylinder. At low salt concentrations (0.15 M NaCl, λD = 0.8 nm), the capture rate is higher than predicted by equation 1 (Fig. 3A). Likely, the positive charges at the ClyA-RR entrance, which are not taken into account in the model, speed up the capture at low salt concentrations, while at higher salt concentrations these charges are more effectively screened.
For ssDNA, the relation between kon and λD is exponential, which is consistent with a barrier crossing (reaction-limited process). In solution, the ssDNA assumes a coiled conformation while it is pulled towards the nanopore by the electrophoretic force as DNA (Fig. 5B-i). In the vicinity of the entry of the pore, however, a successful translocation event can only take place if one end of the strand faces the pore entry (Fig. 5B-ii), and if the ssDNA is uncoiled (Fig. 5B-iii to 5B-iv). This additional repulsive force of entropic origin effectively results in an additional energy barrier that must be crossed prior to translocation. The theory of such barrier-limited translocation has been discussed recently,56 and on general grounds the capture rate is given by:
| (2) |
Here, ΔFb is the barrier height and ω is a characteristic attempt rate for barrier crossing. The exponential factor gives the probability of a successful crossing event. Although estimating ΔFb from model inputs is difficult,56 it was shown that the probability of successful translocation contains a term proportional to the electrophoretic mobility which in turn is proportional to λD (supporting information). This would explain the exponential dependence of kon on λD (Fig. 3B). It should be noticed that while kon is obtained from the inverse interevent time, and not all measured current blockades necessarily describe a translocation event. Part of these blockades may be due to the entry of a DNA strand followed by a retraction back to the cis-side (Fig. 5B-iii to 5B-i). Nevertheless, the formation of rotaxanes shows that at least some molecules successfully translocate. In any case, the argument leading to an exponential dependence of kon on λD remains valid.
Biological significance
In bacteriophages, DNA is transferred into the procapsid by packing proteins that align and push the DNA through portal proteins. Such proteins have similar dimensions, stoichiometry, internal surface charge, and internal diameters to ClyA nanopores.9 A negative internal surface charge appears to be important for the smooth translocation of DNA across the portal proteins,3 and it is observed as well in other proteins that encircle and slide along DNA such as β clamp proteins.7 Portal proteins and β clamp proteins also have positively charged rings that have been proposed to play a direct role in genomic DNA packaging by interacting with the negatively charged phosphate backbone of the translocating DNA.3,9 In this work, we showed that the electrophoretic translocation of DNA through ClyA nanopores was observed when two rings of positive charged residues were introduced at the cis entrance and mid-section of the nanopore, effectively aligning the DNA for the passage through the narrow and very electronegative constriction. In the absence of such interactions, i.e. during the threading from the trans side, DNA translocation is not observed. Our results suggest, therefore, that in connector proteins such rings of positive charges might be important to help initiating the ejection of the DNA out of the capsid into the infected cell.
Conclusion
In this work we have engineered a ClyA nanopore, named ClyA-RR, to translocate dsDNA and ssDNA at physiological ionic strengths. ClyA-RR might be used to study protein-DNA interactions at the single-molecule level and could be employed in DNA mapping and sequencing applications, where an enzyme controls the translocation of the nucleic acid through the nanopore. We found that the introduction of rings of positive charges at the wider entry (the cis side) and mid-section of the nanopore is crucial to induce DNA translocation through the negatively charged trans constriction. Surprisingly, the constriction itself did not require modifications. These results suggest that attractive interactions at the entry and in the middle of the nanopore are important to ‘grab’ and orient the DNA for effective sliding through the narrow and negatively charged trans constriction. Interestingly, the charge distribution in ClyA-RR is mirrored in viral portal proteins, suggesting that the precise engineering of biological nanopores is important for the efficient packing and ejection of DNA in and out the viral capsid. Finally, the linear and exponential ionic strength dependencies of the frequency of dsDNA and ssDNA translocations, respectively, suggest a likely mechanism where the dsDNA capture follows a diffusion-limited process, while the ssDNA capture a reaction-limited process.
Materials and Methods
DNA was purchased from Integrated DNA Technologies (IDT), NeutrAvidin from Thermo Fisher and 1,2-diphytanoyl-sn-glycero-3-phosphocholine from Avanti Polar Lipids. β-Dodecyl maltoside (DDM) was purchased from GLYCON Biochemicals GmbH. Enzymes were bought from Fermentas and all other materials from Sigma.
Protein preparation
Single point mutations to the ClyA-AS gene were performed by using the “mega primer” method.44,57 ClyA was expressed in E. cloni® EXPRESS BL21 (DE3) cells by using a pT7 plasmid. Monomers containing a C-terminal oligo-histidine tag were expressed in E. coli BL21 cells, purified by using Ni-NTA affinity chromatography and oligomerized in the presence of 0.5% β-dodecyl maltoside (GLYCON Biochemicals GmbH) as extensively explained before.37
DNA preparation
dsDNA 1 was prepared by mixing equimolar concentrations of 1a and 1b (Table S3). The mix was brought to 95 °C and the temperature stepped down at regular intervals. The DNA was then purified from the excess of ssDNA with affinity chromatography using a biotin-binding column containing monomeric avidin immobilized on agarose beads (Thermo Scientific Pierce). The dsDNA was then eluted from the column according to the manufacturer protocol. The elution fraction was concentrated and further purified using a PCR quick purification kit (QIAGEN). Typically, a DNA concentration of 0.2 ug/mL was obtained. 1a:1c duplex was annealed as explained for 1, but not further purified.
Ion permeability
IV curves under asymmetric conditions (Table S5) were collected by adding ClyA to the cis chamber under symmetric conditions (150 mM NaCl, 15 mM Tris-HCl pH 7.5 in both cis and trans solutions). The electrodes were then balanced and the electrolyte concentration in cis was increased up to 1M, by adding aliquots of 5 M NaCl stock solutions to the cis compartment. The volume of the trans chamber was adjusted by adding the same volume added to the cis side using the same buffer of the cis solution (150 mM NaCl).
Permeability ratios (, Table S2) were calculated using the Goldman-Hodgkin-Katz (GHK) equation (below) using the measured reverse potential (Vr) values (Table S2), which were extrapolated from the IV curves.
| (3) |
R is the universal gas constant (8.314 J K-1 mol-1), T the temperature in Kelvin F the Faraday’s constant (96485 C mol-1), are the relative membrane permeability for the ions Na+ or Cl-, while are their respective activities. The cis chamber was the ground. Ag/AgCl electrodes with 2.5% agarose bridges containing 2.5 M NaCl were used to perform all the experiments.
Electrical recordings
Ionic currents were measured by recording from planar bilayers formed from diphytanoyl-sn-glycero-3-phosphocholine (Avanti Polar Lipids, Alabaster, AL). The electrical potential was applied by using Ag/AgCl electrodes submerged in agar bridges (3% w/v low melt agarose in 2.5 M NaCl buffer) using a patch-clamp amplifier (Axopatch 200B, Axon Instruments, Foster City, CA) as described previously.13,58 Single channels were characterized by measuring the current versus applied voltage relationship, (IV curve, the potential was applied in 10 mV steps from -100 to +100 mV in 21s, Fig. S1, Fig. S4, Table S6). In 0.15 M NaCl, ionic currents were recorded by applying a 2-kHz low-pass Bessel filter and using a 10 kHz sampling rate. At higher salt concentrations ionic currents were sampled at 50 kHz and the low-pass Bessel filter was set at 10 kHz. Current traces at 0.3 and 0.5 M NaCl were filtered post-acquisition with a 4-kHz Bessel digital filter (Fig. S5 and Fig. S6). The use of different filtering frequencies influence the overall number of detected events. For example, a specific trace where the translocation of dsDNA (0.17 µM, 1 M NaCl) was sampled at 50 kHz while applying a 10 kHz Bessel filter. Under these conditions, the interevent time is 221 ms (with an average dwell time of 0.12 ms). If a 2 kHz digital Gaussian filter is applied to such trace, the interevent time is increased to 311 ms. To test the effect of excessive filtering on the Debye length dependence of the DNA capture frequency, we plotted the data described in Figure 3A after applying a 1 kHz Gaussian filter to all current traces (Fig. S7). We found that the ssDNA and dsDNA blockades still fitted better to an exponential and a linear regression, respectively (Fig. S7).
Data analysis
Current blockade events were collected individually by using the “single channel search” function of the Clampfit software (Molecular devices) using a data acquisition threshold of 0.05 ms. Open and blocked pore current values were obtained from Gaussian fitting to all-point histograms. Residual currents were calculated by dividing the blocked pore current values for the open pore current values. The DNA translocation dwell time values (τoff) were calculated from a single exponential fit from event histograms of DNA blockade dwell-times, while interevent time (τon) values were calculated using an exponential logarithmic probability fits from logarithmic histograms of the interevent times (Fig. 3, Table S3, Fig. S5 and Fig. S6). The errors indicate the standard deviation from the average from at least three independent nanopore experiments, the number of which is indicated by ‘N’.
Supplementary Material
Supporting Information Available: Additional text explaining the derivation of Eqs. (1) and (2), additional tables which show the electrical properties of engineered ClyA variants, and additional figures showing the details of the translocation of ssDNA and dsDNA across ClyA nanopores. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We thank Dr. Misha Soskine for suggesting the use of ClyA-R. We thank the European Research Council and IWT for financial support.
References
- (1).Casjens SR. Nat Rev Microbiol. 2011;9:647. doi: 10.1038/nrmicro2632. [DOI] [PubMed] [Google Scholar]
- (2).Korotkov KV, Gonen T, Hol WGJ. Trends in Biochemical Sciences. 2011;36:433. doi: 10.1016/j.tibs.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Guasch A, Pous J, Ibarra B, Gomis-Rüth FX, Valpuesta JMa, Sousa N, Carrascosa JL, Coll M. Journal of Molecular Biology. 2002;315:663. doi: 10.1006/jmbi.2001.5278. [DOI] [PubMed] [Google Scholar]
- (4).Lebedev AA, Krause MH, Isidro AL, Vagin AA, Orlova EV, Turner J, Dodson EJ, Tavares P, Antson AA. EMBO J. 2007;26:1984. doi: 10.1038/sj.emboj.7601643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Olia AS, Prevelig PE, Jr, Johnson JE, Cingolani G. Nat Struct Mol Biol. 2011;18:597. doi: 10.1038/nsmb.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Sun L, Zhang X, Gao S, Rao PA, Padilla-Sanchez V, Chen Z, Sun S, Xiang Y, Subramaniam S, Rao VB, Rossmann MG. Nat Commun. 2015;6 doi: 10.1038/ncomms8548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Georgescu RE, Kim S-S, Yurieva O, Kuriyan J, Kong X-P, O'Donnell M. Cell. 2008;132:43. doi: 10.1016/j.cell.2007.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Simpson AA, Tao Y, Leiman PG, Badasso MO, He Y, Jardine PJ, Olson NH, Morais MC, Grimes S, Anderson DL, Baker TS, et al. Nature. 2000;408:745. doi: 10.1038/35047129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Rao VB, Feiss M. Annual Review of Virology. 2015;2:351. doi: 10.1146/annurev-virology-100114-055212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Gu LQ, Braha O, Conlan S, Cheley S, Bayley H. Nature. 1999;398:686. doi: 10.1038/19491. [DOI] [PubMed] [Google Scholar]
- (11).Soskine M, Biesemans A, Moeyaert B, Cheley S, Bayley H, Maglia G. Nano Letters. 2012;12:4895. doi: 10.1021/nl3024438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Shin SH, Luchian T, Cheley S, Braha O, Bayley H. Angewandte Chemie-International Edition. 2002;41:3707. doi: 10.1002/1521-3773(20021004)41:19<3707::AID-ANIE3707>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- (13).Ho C-W, Van Meervelt V, Tsai K-C, De Temmerman P-J, Mast J, Maglia G. Science Advances. 2015;1 doi: 10.1126/sciadv.1500905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Stoddart D, Heron AJ, Mikhailova E, Maglia G, Bayley H. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7702. doi: 10.1073/pnas.0901054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Stoddart D, Heron AJ, Klingelhoefer J, Mikhailova E, Maglia G, Bayley H. Nano Letters. 2010;10:3633. doi: 10.1021/nl101955a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Wallace EV, Stoddart D, Heron AJ, Mikhailova E, Maglia G, Donohoe TJ, Bayley H. Chem Commun (Camb) 2010;46:8195. doi: 10.1039/c0cc02864a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Franceschini L, Mikhailova E, Bayley H, Maglia G. Chem Commun (Camb) 2012;48:1520. doi: 10.1039/c1cc16124e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Cockroft SL, Chu J, Amorin M, Ghadiri MR. Journal of the American Chemical Society. 2008;130:818. doi: 10.1021/ja077082c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Butler TZ, Pavlenok M, Derrington IM, Niederweis M, Gundlach JH. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20647. doi: 10.1073/pnas.0807514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Manrao EA, Derrington IM, Pavlenok M, Niederweis M, Gundlach JH. PLoS One. 2011;6:e25723. doi: 10.1371/journal.pone.0025723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Benner S, Chen RJ, Wilson NA, Abu-Shumays R, Hurt N, Lieberman KR, Deamer DW, Dunbar WB, Akeson M. Nat Nanotechnol. 2007;2:718. doi: 10.1038/nnano.2007.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Hurt N, Wang H, Akeson M, Lieberman KR. J Am Chem Soc. 2009;131:3772. doi: 10.1021/ja809663f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Wang Y, Zheng D, Tan Q, Wang MX, Gu LQ. Nat Nanotechnol. 2011;6:668. doi: 10.1038/nnano.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Geng J, Wang S, Fang H, Guo P. ACS nano. 2013;7:3315. doi: 10.1021/nn400020z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Jing P, Haque F, Vonderheide AP, Montemagno C, Guo P. Molecular Biosystems. 2010;6:1844. doi: 10.1039/c003010d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Jing P, Haque F, Shu D, Montemagno C, Guo P. Nano Lett. 2010;10:3620. doi: 10.1021/nl101939e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Wendell D, Jing P, Geng J, Subramaniam V, Lee TJ, Montemagno C, Guo PX. Nature Nanotechnology. 2009;4:765. doi: 10.1038/nnano.2009.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Li J, Stein D, McMullan C, Branton D, Aziz MJ, Golovchenko JA. Nature. 2001;412:166. doi: 10.1038/35084037. [DOI] [PubMed] [Google Scholar]
- (29).Storm A, Chen J, Zandbergen H, Dekker C. Physical Review E. 2005;71 doi: 10.1103/PhysRevE.71.051903. [DOI] [PubMed] [Google Scholar]
- (30).Heng JB, Ho C, Kim T, Timp R, Aksimentiev A, Grinkova YV, Sligar S, Schulten K, Timp G. Biophys J. 2004;87:2905. doi: 10.1529/biophysj.104.041814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Dekker C. Nature Nanotechnology. 2007;2:209. doi: 10.1038/nnano.2007.27. [DOI] [PubMed] [Google Scholar]
- (32).Schneider GF, Kowalczyk SW, Calado VE, Pandraud G, Zandbergen HW, Vandersypen LM, Dekker C. Nano Letters. 2010;10:3163. doi: 10.1021/nl102069z. [DOI] [PubMed] [Google Scholar]
- (33).Merchant CA, Healy K, Wanunu M, Ray V, Peterman N, Bartel J, Fischbein MD, Venta K, Luo Z, Johnson AT, Drndic M. Nano Letters. 2010;10:2915. doi: 10.1021/nl101046t. [DOI] [PubMed] [Google Scholar]
- (34).Garaj S, Hubbard W, Reina A, Kong J, Branton D, Golovchenko JA. Nature. 2010;467:190. doi: 10.1038/nature09379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Liu K, Feng J, Kis A, Radenovic A. ACS nano. 2014;8:2504. doi: 10.1021/nn406102h. [DOI] [PubMed] [Google Scholar]
- (36).Feng J, Liu K, Graf M, Lihter M, Bulushev RD, Dumcenco D, Alexander DT, Krasnozhon D, Vuletic T, Kis A, Radenovic A. Nano Lett. 2015;15:3431. doi: 10.1021/acs.nanolett.5b00768. [DOI] [PubMed] [Google Scholar]
- (37).Waduge P, Bilgin I, Larkin J, Henley RY, Goodfellow K, Graham AC, Bell DC, Vamivakas N, Kar S, Wanunu M. ACS nano. 2015;9:7352. doi: 10.1021/acsnano.5b02369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Smeets RMM, Keyser UF, Krapf D, Wu MY, Dekker NH, Dekker C. Nano Letters. 2006;6:89. doi: 10.1021/nl052107w. [DOI] [PubMed] [Google Scholar]
- (39).Ivankin A, Carson S, Kinney SRM, Wanunu M. Journal of the American Chemical Society. 2013;135:15350. doi: 10.1021/ja408354s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Wanunu M, Morrison W, Rabin Y, Grosberg AY, Meller A. Nat Nanotechnol. 2010;5:160. doi: 10.1038/nnano.2009.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Soskine M, Biesemans A, Maglia G. J Am Chem Soc. 2015;137:5793. doi: 10.1021/jacs.5b01520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Biesemans A, Soskine M, Maglia G. Nano Letters. 2015;15:6076. doi: 10.1021/acs.nanolett.5b02309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Van Meervelt V, Soskine M, Maglia G. ACS nano. 2014;8:12826. doi: 10.1021/nn506077e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Soskine M, Biesemans A, De Maeyer M, Maglia G. J Am Chem Soc. 2013;135:13456. doi: 10.1021/ja4053398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Franceschini L, Soskine M, Biesemans A, Maglia G. Nature Communications. 2013;4:2415. doi: 10.1038/ncomms3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Rincon-Restrepo M, Mikhailova E, Bayley H, Maglia G. Nano Letters. 2011;11:746. doi: 10.1021/nl1038874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Maglia G, Rincon Restrepo M, Mikhailova E, Bayley H. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19720. doi: 10.1073/pnas.0808296105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Maglia G, Henricus M, Wyss R, Li Q, Cheley S, Bayley H. Nano Lett. 2009;9:3831. doi: 10.1021/nl9020232. [DOI] [PubMed] [Google Scholar]
- (49).Humphrey W, Dalke A, Schulten K. Journal of Molecular Graphics. 1996;14:33. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- (50).Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kalé L, Schulten K. Journal of Computational Chemistry. 2005;26:1781. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Proceedings of the National Academy of Sciences. 2001;98:10037. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Dolinsky TJ, Nielsen JE, McCammon JA, Baker NA. Nucleic Acids Research. 2004;32:W665. doi: 10.1093/nar/gkh381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Dolinsky TJ, Czodrowski P, Li H, Nielsen JE, Jensen JH, Klebe G, Baker NA. Nucleic Acids Research. 2007;35:W522. doi: 10.1093/nar/gkm276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Baumann CG, Smith SB, Bloomfield VA, Bustamante C. Proceedings of the National Academy of Sciences. 1997;94:6185. doi: 10.1073/pnas.94.12.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Grosberg AY, Rabin Y. The Journal of Chemical Physics. 2010;133:165102. doi: 10.1063/1.3495481. [DOI] [PubMed] [Google Scholar]
- (56).Rowghanian P, Grosberg AY. Phys Rev E Stat Nonlin Soft Matter Phys. 2013;87:042722. doi: 10.1103/PhysRevE.87.042722. [DOI] [PubMed] [Google Scholar]
- (57).Miyazaki K. Methods Enzymol. 2011;498:399. doi: 10.1016/B978-0-12-385120-8.00017-6. [DOI] [PubMed] [Google Scholar]
- (58).Maglia G, Heron AJ, Stoddart D, Japrung D, Bayley H. Methods Enzymol. 2010;475:591. doi: 10.1016/S0076-6879(10)75022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.