Abstract
Thrombus growth at the site of vascular injury is mediated by the sequential events of platelet recruitment, activation and aggregation concomitant with the initiation of the coagulation cascade, resulting in local thrombin generation and fibrin formation. While the biorheology of a localized thrombus formation has been well studied, it is unclear whether local sites of thrombin generation propagate platelet activation within the bloodstream. In order to study the physical biology of platelet activation downstream of sites of thrombus formation, we developed a platform to measure platelet activation and microaggregate formation in the bloodstream. Our results show that thrombi formed on collagen and tissue factor promote activation and aggregation of platelets in the bloodstream in a convection-dependent manner. Pharmacological inhibition of the coagulation factors (F) X, XI or thrombin dramatically reduced the degree of distal platelet activation and microaggregate formation in the bloodstream without affecting the degree of local platelet deposition and aggregation on a surface of immobilized collagen. Herein we describe the development and an example of the utility of a platform to study platelet activation and microaggregate formation in the bloodstream (convection-limited regime) relative to the local site of thrombus formation.
Keywords: platelets, factor XI, factor X, thrombin, shear, biophysics, physical biology
INTRODUCTION
Platelets and coagulation factors contribute to both hemostasis, a physiological response to staunch blood loss from an injured vessel, and thrombosis, a pathological development of a clot that obstructs the vessel lumen. At sites of vessel injury, circulating platelets are rapidly recruited to the exposed extracellular matrix under shear flow, followed by platelet activation.2 Platelet activation triggers the surface expression of CD62P (P-selectin) from α-granules, and expression of active glycoprotein (GP) IIbIIIa, by which platelets can form homotypic aggregates via fibrinogen binding. In parallel, the exposure of blood to tissue factor (TF) or negatively charged surfaces induces activation of extrinsic and intrinsic coagulation pathways, respectively, leading to thrombin generation and fibrin formation. Contact with negatively charged surfaces induces autoactivation of the zymogen coagulation factor XII (FXII).20 Activated FXII (FXIIa) is a serine protease that converts coagulation factor XI (FXI) to the activated form of FXI (FXIa). Subsequently, FXIa activates factor IX (FIX), which in turn activates factor X (FX). Activated FX (FXa) then converts prothrombin to thrombin. In addition to fibrin formation and platelet activation, thrombin can mediate feedback activation of FXI to perpetuate its own generation.13 Thrombin converts fibrinogen to fibrin, and cleaves platelet protease-activated receptors (PARs). The complex mass of fibrin and activated platelets forms the basis of a hemostatic plug or, under pathological conditions, a thrombus that obstructs blood flow.
The rate of platelet activation, aggregation and fibrin formation in a fluid shear environment (i.e., flowing blood) is a balance between the delivery of reactants (platelets and coagulation factors) to the local site of vascular injury relative to the rate of assembly and (in)activation of coagulation factors on the activated platelet surface.15 The biorheology of local thrombus formation has been extensively studied in silico, in vitro and in vivo.11 However, the process by which local generation of thrombin at sites of thrombus formation promotes the activation of the coagulation cascade and platelets in the bloodstream is ill-defined. The aim of this study was to develop a platform to define the physical biology of platelet activation and microaggregate formation in the bloodstream distal to sites of local thrombus formation. An improved understanding of platelet activation and aggregate formation in the bloodstream may lead to the identification of antithrombotic targets that prevent distal platelet activation in the bloodstream without affecting local hemostasis.
MATERIALS AND METHODS
Reagents
Fibrillar collagen reagent was purchased from Nycomed Austria GmbH (Linz, Austria). The tetrapeptide Gly-Pro-Arg-Pro-OH fibrin polymerization inhibitor, Pefabloc® FG (GPRP) was purchased from Pentapharm (Basel, Switzerland). Serine protease inhibitor Phe-Pro-Argchloromethylketone (PPACK) was purchased from Santa Cruz and tissue factor (TF, Innovin) was purchased from Siemens. Murine monoclonal anti-FXI antibodies 1A6 and 14E11 were cloned, expressed and purified as described.13 The antibody 14E11 blocks the kininogen-binding region on the apple 2 (A2) domain and inhibits the activation of FXI by FXIIa.6 The antibody 1A6 blocks the FIX-binding region on the A3 domain of FXI and inhibits FIX activation by FXIa.6 The direct inhibitor of activated factor X (FXa), rivaroxaban, and thrombin inhibitor, melagatran, were provided from the manufacturer Bayer HealthCare AG. Rivaroxaban binds reversibly to FXa via the S1 and S4 pockets and inhibits FXa serine protease activity.19 PE-conjugated mouse anti-human CD41a and CD61a, and APC-conjugated mouse human anti-CD62P was purchased from BD Biosciences (Heidelberg, Germany). Thrombin receptor activator peptide-6 (TRAP-6) was purchased from Bachem (Bubendorf, Switzerland). The partial thromboplastin time (aPTT) reagent, STA® C. K. Prest® 5 was purchased from Diagnostica STAGO (Asnières-sur-Seine, France). TriniCLOT calcium chloride was purchased from Trinity Biotech (Bray, Ireland). All other reagents were from Sigma-Aldrich (St. Louis, MO, USA) or previously named sources.10,22
Collection of human blood
Human venous blood was drawn by venipuncture from healthy adult volunteers in accordance with the Oregon Health & Science University institutional review board. Blood was taken into 3.8% (w/v) sodium citrate using a ratio of 1 part citrate: 9 parts blood and immediately used for experiments.
Clotting time assay
The activated partial thromboplastin time (aPTT) of human plasma were measured with a Trinity AMAX200 coagulation analyzer (Trinity Biotech, Bray, Ireland). Pooled human plasma was pretreated at 37°C for 10 min with 1A6, 14E11 or rivaroxaban, followed by incubation with aPTT reagent for 3 min at 37°C. Coagulation was then initiated by the addition of CaCl2 (8.3 mM final), and clotting times were recorded.
Flow chamber assays
The volume and surface area of platelet proximal aggregates were measured as previously described.4 Briefly, glass capillary tubes (0.2 × 2 mm, VitroCom, Mountain Lakes, NJ, USA) were coated with collagen (150 μg/ml) for 1 hr at room temperature, washed and incubated with tissues factor (TF, 0.1 nM) for additional hour, when indicated. Surfaces were blocked with 5 mg/ml denatured bovine serum albumin (BSA) for 1 hr prior to assembly into a flow system on the stage of a Zeiss microscope (Carl Zeiss, Thornwood, NY). Citrate-anticoagulated whole blood was incubated with the fibrin polymerization inhibitor, tetrapeptide Gly-Pro-Arg-Pro-OH (GPRP; 3 mM final) prior to the incubation with vehicle, 1A6 (1-50 μg/ml), 14E11 (1-50 μg/ml), or rivaroxaban (30-300 nM) for 10 min at 37°C. Blood was re-calcified to final of 7.5 mM CaCl2, 3.5 mM MgCl2 immediately prior to perfusion over the collagen/TF-coated slide at a set initial shear for 5-10 min. The capillary tubes containing platelet aggregates were washed for 5 min with modified Hepes/Tyrode buffer (129 mM NaCl, 12 mM NaHCO3, 2.9 mM KCl, 20 mM HEPES, 1 mM MgCl2, 0.34 mM Na2HPO4·12H2O, 5.6 mM glucose; pH 7.3) at the same shear rate to remove unbound blood components. The samples were fixed with 4% paraformaldehyde for image analysis. Z-stack images from three random fields of view (215 μm × 160 μm) were processed for each sample and taken from the surface of the slide to 5 μm above the platelet aggregate. For the data presentation, the volume histograms used 150 μm3 size bins, while the surface area histogram used 40 μm2 size bins, as previously described.3 For the measurement of distal platelet activation in the presence of pharmacological antagonists, glass slides (Menzel-Gläser SUPERFROST 76 × 26 mm; Gerhard Menzel GmbH, Braunschweig, Germany) were coated with collagen (150 μg/ml) overnight at 4°C, followed by blocking with BSA prior to assembly into a flow system on the stage of a Zeiss microscope. Whole blood downstream of the flow chamber was collected into modified Hepes/Tyrode buffer containing 100 mM PPACK and 3.8% (v/w) sodium citrate at 1 min intervals. In parallel, whole blood was sampled from the upstream of the flow chamber and treated with buffer control (vehicle) or TRAP-6 (10 μg/ml) for 5 min.
Fluorescence Activated Cell Sorting (FACS)-analysis
Blood samples collected from the upstream and downstream of the flow chamber were diluted with modified Hepes/Tyrode buffer containing 100 mM PPACK and 3.8% (v/w) sodium citrate and incubated with antibodies for 20 min. To stop antibody-labeling reactions, samples were diluted 1:10 with CellWash/Permafix (BD Biosciences). 10,000 single platelets were determined by a PE-conjugated platelet marker (CD41a or CD61a) and the characteristic forward and side-scatter patterns via flow cytometry (FACS). Platelet CD62P expression levels, microaggregate formation and single platelet consumption were determined as previously described.16
Simulation of thrombin mass transfer in flow distal to thrombus formation
The distribution of thrombin in the bloodstream distal to the local site of thrombus formation was modeled using COMSOL with a single activated platelet as a source of initial thrombin concentration. The activated platelet is assumed to generate thrombin flux with a constant surface concertation of 0.5 mol/m3.5 Platelets’ diameter was set to 3.6 μm and platelets were assumed to move with the bulk blood flow at approximately 10-15 μm from the wall surface of a 1.0 mm diameter channel. Our model assumes that the neighboring platelets get rapidly activated and degranulate immediately due to the change in thrombin flux.12 This phenomena was incorporated in the model by giving a time delay in thrombin generation from the adjacent platelets. The time delay is estimated by calculating the time taken for the thrombin to reach the adjacent platelets, less than 1 second. The activated platelets also contribute to the overall thrombin concentration with the same boundary condition as the first platelet. The equation used to obtain the thrombin concentration profiles in flow was:
The boundary condition on the first activated platelet (p1) was set as: and for the rest of the platelets the same boundary condition was applied after a delay (i.e. ). In the above equation, Di is the diffusivity of thrombin and is taken as 10 μm2/s and u is the velocity profile in the network.14 This equation along with the Navier Stokes equation and continuity were solved simultaneously to obtain thrombin concentration and velocity profiles at 62.25, 250, 1000 and 4000 s−1 shear rates. Peclet number (LU/D) of this flow was >> 1, which implies that mass transfer was dominated by convection compared to diffusion resulting in thrombin distribution mostly along the stream lines of the bulk flow. Based on this hypothesis it can be predicated that lower flow rates will have thicker ‘plumes’ of thrombin near the walls of the channel compared to higher flow rates, resulting in a higher level of platelet aggregation in the bloodstream at lower flow rates.
RESULTS
Development of a platform for the study of local and distal platelet activation
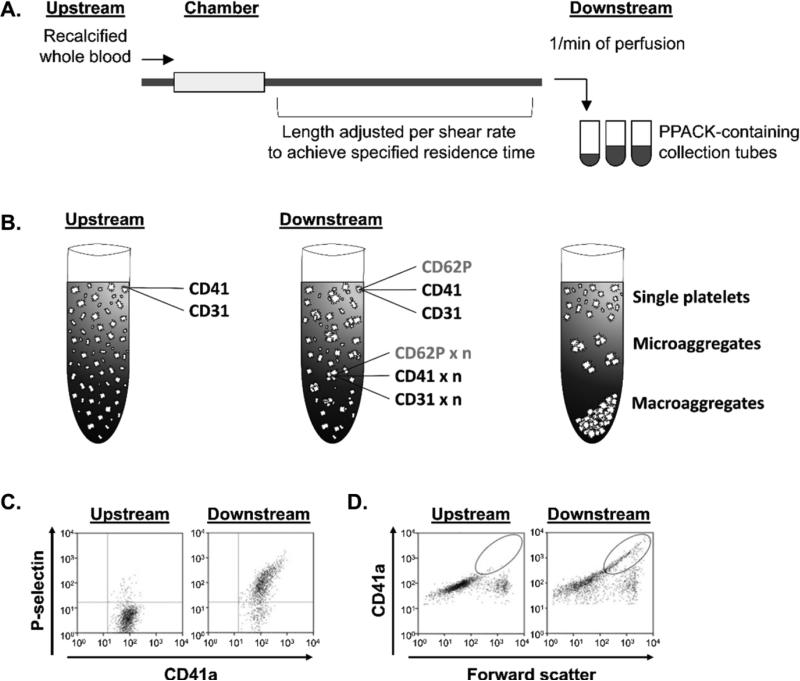
To investigate and characterize the biorheology of platelet activation in the bloodstream distal to sites of thrombus formation on collagen and tissue factor (collagen/TF)-coated surfaces as compared to control surfaces coated with bovine serum albumin (BSA control), we developed a FACS-based assay to measure platelet activation and microaggregate formation in downstream samples (Figure 1A). Following perfusion of recalcified whole-blood through the flow chamber, downstream samples were collected at 1 min intervals and analyzed for platelet P-selectin (CD62P) expression as well as platelet-platelet aggregation. We utilized the fibrinpolymerization inhibitor, GPRP, in order to focus our current study on single platelet recruitment and platelet-platelet aggregation in the absence of extensive fibrin formation. This is a limitation of the current study, as fibrin is known to serve as a sink for thrombin. Single platelets in the bloodstream samples were detected by the combination of light scattering and PE-CD41/CD61 (GPIIbIIIa) fluorescence. Quantification of the percentage of platelet activation was achieved by creating a gate around platelets with CD62P expression above the threshold on CD62P/CD41a scatter plots and normalizing events within gate to total CD41a-positive events (Figure 1B). To quantify platelet microaggregate formation in the blood downstream of the collagen/TF or BSA-coated flow chamber, CD41a/forward scatter plots were generated and CD41a-positive events with increased forward scatter and mean fluorescence intensity were defined as platelet microaggregates and reported as events versus 104 single platelets (Figure 1C). This measurement platform allowed for the study of the effect of the local activation of the coagulation cascade on the activation of platelets in the bloodstream.
Figure 1. The schematic of the flow chamber and FACS analysis.
Whole blood samples were taken before the blood perfusion (upstream) or at distal site to a collagen and tissue factor-coated flow chamber (downstream) and analyzed by FACS. (B) Platelet activation and microaggregate formation as well as total single platelet consumption (single platelet integration into forming microaggregates and precipitating out of blood macroaggregates) were assessed. Samples were labeled for constitutively expressed platelet CD41a or CD31 and P-selectin (CD62P) expressed on activated platelets. (C) Percent platelet activation was determined by a dot plot with PE-CD41a and APC-CD62P fluorescence. (D) Platelet microaggregate formation was defined by CD41a mean fluorescence intensity and size (forward scatter) shift, as indicated by the region marked with the circle. Gated platelets before (upstream; left) and after (downstream; right) perfusion over a collagen-coated surface are shown.
Characterization of the effect of shear on platelet aggregation in the bloodstream
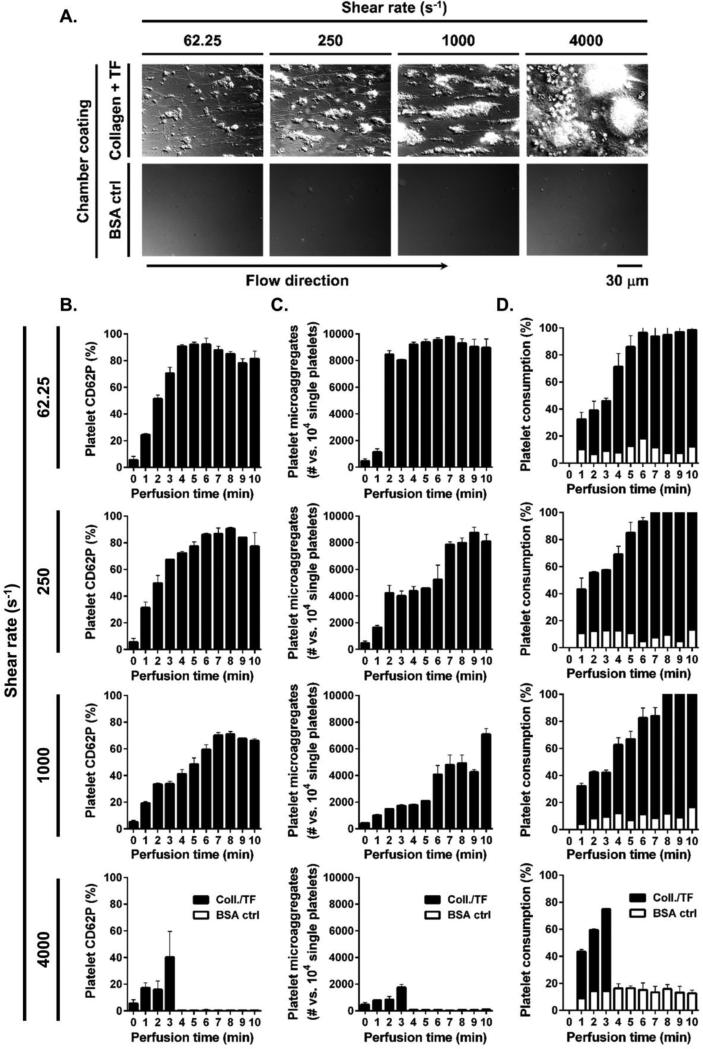
To investigate and characterize the effect of shear on the biorheology of platelet aggregation in the bloodstream, we first looked at local thrombus formation on surfaces of immobilized collagen and tissue factor (collagen/TF) as compared to control surfaces coated with bovine serum albumin (BSA control). We observed increased platelet recruitment to collagen/TF-coated surfaces as compared to BSA control-coated surfaces. Platelet aggregation within 10 minutes of blood perfusion increased as a function of shear rate, with the lowest degree of local platelet aggregation observed at 62.25 s−1 and the greatest degree of local platelet aggregation observed at 1000 s−1. Of note, flow chambers coated with collagen/TF completely occluded after 3 minutes of blood perfusion at a shear rate of 4000 s−1 (Figure 2A).
Figure 2. Effect of shear on local platelet aggregate formation and distal platelet activation.
Recalcified whole blood was perfused over collagen/TF or BSA-coated control surfaces for 10 min at 62.25, 250 or 1000 s−1 shear rate and for 3 min (collagen/TF-coated chambers occluded by 3 min of perfusion) at 4000 s−1 shear rate. (A) Final local platelet aggregates formed were evaluated by differential interference contrast (DIC) microscopy. Samples were collected distally at each minute of perfusion after 15 s of residence time in flow and (B) distal platelet activation, (C) microaggregate formation and (D) single platelet consumption were quantified by FACS. Images and histograms are representative from each 3 fields of view from two different blood donors.
Our goal was to develop a measurement platform to study platelet activation and microaggregate formation in the bloodstream relative to the local site of thrombus formation. We found that platelet CD62P expression and aggregation dramatically increased in whole blood samples collected 15 seconds (residence time in flow) downstream of the site of thrombus formation on collagen/TF as compared to BSA control-coated surfaces at all shear rates tested (62.25, 250, 1000 and 4000 s−1; Figure 2B-D). Maximal platelet activation was achieved sooner at lower shear rates, with 92±1.9% platelets activated by 5 min at 62.25 s−1, 90±1.2% by 8 min at 250 s−1 and 71±2.0% by 8 min at 1000 s−1, while 40±19.4% of platelets were activated by 3 min at 4000 s−1 just prior to chamber occlusion. Per 10000 single platelets, 9714±27 platelets formed microaggregates by 7 min at 62.25 s−1, 8714±393 by 9 min at 250 s−1, 7050±421 by 10 min at 1000 s−1 and 1689±220 by 3 min at 4000 s−1. Total single platelet consumption was achieved by 7 min at 62.25 s−1, while both 250 s−1 and 1000 s−1 had 2±0.4% of single platelets remaining by 10 min of perfusion; 40±0.6% of single platelets remained in samples distal to local thrombus formation at 4000 s−1 prior to chamber occlusion by 3 min.
Effect of distal residence time in flow on platelet activation in the bloodstream
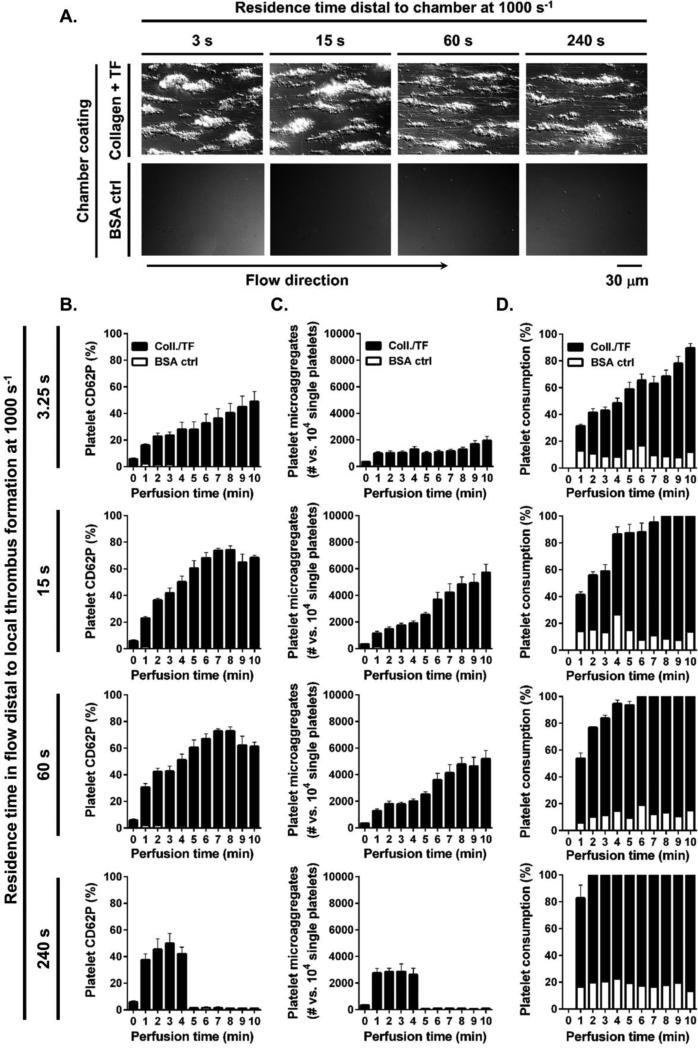
We next investigated the effect of blood residence time in flow distal to the local site of thrombus formation on platelet activation and aggregation in the bloodstream. We found that the local thrombus formation on surfaces of immobilized collagen and tissue factor (collagen/TF) and BSA control surfaces at 1000 s−1 shear rate were not affected by addition of tubing downstream of the chambers (Figure 3A). We found that platelet CD62P expression and aggregation dramatically increased in whole blood as a function of residence time in flow downstream of the site of thrombus formation on collagen/TF as compared to BSA control surface (Figure 3B-D). Maximal platelet activation was achieved faster at longer residence times. For a distal residence time of 3.25 s, 10 min of local thrombus formation (perfusion time) was required to achieve activation of 47±7.7% of the platelets in the bloodstream. For a distal residence time of 15, 60 and 240 s, 8, 7 and 3 min of perfusion time was required to achieve activation of 73±3.0%, 72±1.6%, and 49±7.5% of the platelets in the bloodstream, respectively. Per 10000 single platelets, 1874±312 platelets formed microaggregates following 10 min of perfusion for a residence time of 3.25 s, 5671±625 platelets formed microaggregates by 10 min for a residence time of 15 s, 5164±606 platelets formed microaggregates by 10 min for a residence time of 60 s, and 2782±582 platelets formed microaggregates by 3 min for a residence time of 240 s of blood residence in flow. Single platelet consumption increased as a function of residence time, with 78±3.3% of single platelets being consumed following 10 min of perfusion for a residence time of 3.25 s, whereas 99±0.1% of single platelets were consumed following 10 min of perfusion for residence times of either 15 s or 60 s, and 100±0.5% of single platelets were consumed by 4 min after 240 s of residence time.
Figure 3. Effect of distal residence time in flow on platelet activation in the bloodstream.
Recalcified whole blood was perfused over collagen/TF or BSA-coated control surfaces for 10 min at 1000 s−1 shear rate. (A) Local platelet aggregates formed after 10 min of blood perfusion were evaluated by differential interference contrast (DIC) microscopy. Samples were collected distally to local thrombus formation after 3.25, 15, 60 or 240 s of residence time in flow and (B) distal platelet activation, (C) microaggregate formation and (D) single platelet consumption over perfusion time (min) were assessed by FACS. Images and histograms are representative from each 3 fields of view from four different blood donors.
Thrombin mass transfer in flow distal to thrombus formation
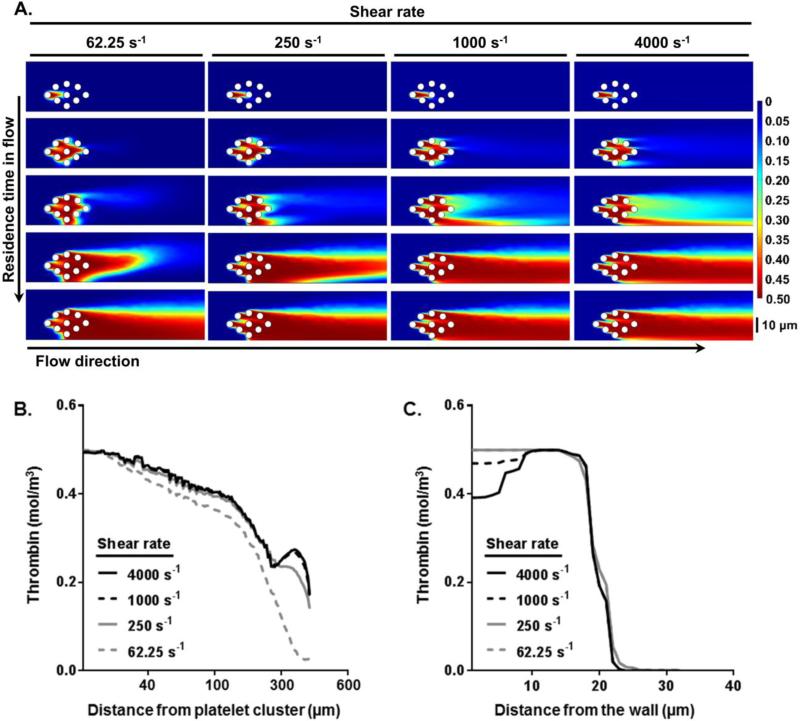
Finite element simulations of thrombin mass transfer using COMSOL was used to study the effect of inlet shear rate and residence time on thrombin generation in the bloodstream distal to sites of local thrombus formation (Figure 4). We hypothesized that the level of platelet aggregate formation in the bloodstream would increase as a function of residence time. Our model incorporated the following steps: (i) thrombin mass transfer within the bloodstream from a single activated platelet towards unactivated platelets, and (ii) the transport of non-activated platelets towards increasing concentrations of thrombin within the bloodstream, resulting in platelet activation. Therefore, our model predicts that platelet aggregation in the bloodstream is proportional to the area of thrombin concentration plume generated by a cluster of activated platelets present in the blood flow along with the residence time of this cluster. Our model predicted that thrombin concentrations reach steady state both axially and radially within 15 seconds of residence time in flow at different shear rates (Figure 4A). Our simulations predicted that thrombin mass transfer in the bloodstream is dominated by convection and follows bulk flow. Thus, after 15 seconds of residence time in flow, the thrombin concentration profile in the axial direction starting from the platelet cluster would distribute farther at higher shear rates (Figure 4B). We used our model to estimate the mass transfer of thrombin 20 μm downstream of platelet clusters as a function of radius from the wall to the center of the channel. Our simulations predicted that higher shear rates lead to a moderately lower initial radial diffusion profile compared to lower shear rates (Figure 4C). Our model predicted that the probability of platelet activation in the bloodstream increases as a function of residence time.
Figure 4. Thrombin mass transfer in flow distal to thrombus formation.
Thrombin distribution (mol/m3) in the bloodstream distal to local thrombus formation at increasing shear rates was simulated using COMSOL (A) over different residence time in flow; final residence time shown is 15 s. Predictions of (B) axial thrombin distribution from a platelet cluster in flow and (C) radial thrombin distribution from the wall to the channel center at different set shear rates after 15 s of residence time in flow.
Characterization of inhibitors of the coagulation cascade
We first verified the anticoagulant activity of pharmacological inhibitors of the coagulation cascade in an aPTT clotting assay using pooled platelet-poor human plasma. In this study we utilized the function-blocking FXI antibodies, 14E11 and 1A6, and the direct FXa inhibitor, rivaroxaban. 14E11 inhibits FXI activation by FXIIa, and 1A6 blocks FIX activation by FXIa and FXI activation by FXIIa.18 Rivaroxaban reversibly binds to FXa via the S1 and S4 pockets and inhibits FXa serine protease activity.19 The maximum aPTT prolongation with 14E11 and 1A6 was observed at the concentrations above 3 μg/ml (Figure 5A,B). Both 14E11 and 1A6 doubled the clotting time at a concentration of 1-2 μg/ml. The maximal inhibition of clotting time observed with 1A6 (95 s) was somewhat longer than with 14E11 (85 s). The FXa inhibitor rivaroxaban inhibited clotting in a concentration-dependent manner, doubling the aPTT at 300 nM (Figure 5C). No saturating effect was observed for rivaroxaban up to a concentration of 800 nM.
Figure 5. 14E11, 1A6, and rivaroxaban prolonged the clotting time of human plasma.
Pooled human plasma was incubated with increasing concentrations of (A) 14E11, (B) 1A6 or (C) rivaroxaban for 10 min and aPTT was recorded, as described in Methods.
Study of local platelet deposition and aggregation on immobilized collagen
Next, we investigated platelet adhesion and aggregation on immobilized collagen in a flow chamber system in the presence of pharmacological agents targeting intrinsic or extrinsic coagulation pathway. We observed platelet recruitment to collagen-coated surfaces under arterial shear conditions (1000 s−1; Figure 6A). As expected, the presence of the anti-FXI antibodies, 14E11 or 1A6, or FXa-inhibitor rivaroxaban did not alter the volume or surface coverage of platelet aggregates as compared to vehicle-treated controls (Figure 6B).
Figure 6. 14E11, 1A6, and rivaroxaban did not affect local platelet aggregate formation under flow.
Recalcified whole blood was perfused over collagen-coated surfaces at 1000 s−1 shear rate. (A) Platelet aggregates formed on collagen surfaces in the presence of 14E11 (50 μg/ml), 1A6 (50 μg/ml) or rivaroxaban (100 nM). (B) Aggregate volume (left) and surface area (right) in the presence of 14E11 (top), 1A6 (middle) or rivaroxaban (bottom) at indicated concentrations. Images and histograms are representative from each 3 fields of view from two different blood donors. Scale bar = 20 μm.
Study of distal platelet activation and microaggregate formation in the blood downstream of sites of thrombus formation
We found that platelet CD62P expression dramatically increased in whole blood downstream of the site of thrombus formation on surfaces of immobilized collagen with maximal CD62P expression reaching 7.8±1.0% upstream vs. 75.1±4.5% downstream of the flow (Figure 7A). We next investigated the role of contact activation of the coagulation cascade in promoting distal platelet activation. Treatment of blood with the function-blocking FXI-antibody, 14E11, inhibited CD62P expression, with the maximum inhibitory effect of 14E11 recorded at 10 μg/ml (75.1±4.5% vs. 25.6±6.7% in the presence of PBS or 14E11, respectively). The function-blocking FXI-antibody, 1A6, reduced CD62P expression in a concentration-dependent manner and reached a maximum inhibition at 50 μg/ml (76.3±3.5% vs. 24.8±2.6% in the presence of PBS or 1A6, respectively). Platelet CD62P levels were reduced in the presence of the FXa-inhibitor 100 nM rivaroxban by over 50% (42.4±6.6% vs. 17.8±0.67% in the presence of DMSO or rivaroxaban, respectively). Moreover, treatment of blood with the direct thrombin inhibitor, melagatran, was found to inhibit platelet CD62P expression to basal levels (45.0±4.9% vs. 13.7±2.2% in the presence of DMSO or melagatran, respectively). Treatment of whole blood with the TRAP-6 induced comparable levels of platelet CD62P expression in the presence of vehicle, 14E11, 1A6, rivaroxaban, or melagatran.
Figure 7. FXI and FXa inhibitors abrogated distal platelet CD62P expression and microaggregate formation under shear.
Whole blood, treated with 14E11, 1A6, rivaroxaban or melagatran at indicated concentrations, was perfused over a collagen-coated chambers immediately after recalcification; PBS or DMSO was used as vehicle. In parallel, whole blood samples were collected after treatment with vehicle or inhibitor, and incubated with TRAP-6 (10 μg/ml) or vehicle for 5 min. Data are reported as (A) mean ± SEM percentage of CD62P-positive platelets, or (B) mean ± SEM platelet microaggregate count versus 104 gated single platelets in gated population of at least three experiments. The maximal CD62P expression levels or microaggregate counts after 5 mins of perfusion are shown in the graphs for each treatment.
We next analyzed downstream samples for the presence of platelet microaggregates. Previous studies have shown that FACS-analysis of whole blood samples can detect platelet microaggregates based on their characteristic forward scatter and fluorescence profiles,1,17 although these and other studies were confined to the study of platelet activation and aggregation in closed systems. As shown in Figure 7, our platform was used to measure the formation of platelet microaggregates in the bloodstream sampled distal to the site of thrombus formation (~1500 or 850 aggregates/104 single platelets in PBS or DMSO, respectively). We found that platelet microaggregate formation was significantly decreased by over 50% in the presence of the function-blocking FXI antibodies, 14E11 or 1A6, and by over 70% in the presence of the FXa inhibitor rivaroxaban or direct thrombin inhibitor melagatran as compared to vehicle (Figure 7B). Taken together, we demonstrate the utility of a measurement platform to study the effects of local activation of the coagulation cascade on the promotion of distal platelet activation and microaggregate formation in the bloodstream.
DISCUSSION
At sites of vessel injury, thrombin generation is reaction rate limited by the conversation of zymogens to active enzymes following the assembly of coagulation factor complexes on the activated platelet surface.7 The supply of reactants in the form of unactivated platelets and coagulation factors is in excess due to blood flow to the growing hemostatic plug. Thus, the ratio of the reaction rate to the convective mass transport rate, as defined by the Dahmköhler number (Da), is high (Da>1).15 Thus, for reactions catalyzed by the activated platelet surface within the growing hemostatic plug, such as the FXa and thrombin generation, the flow time scale is the rate limiting step as compared to the chemical time scale. Model systems have shown that the activation of FX under shear flow increases with the one-third power of shear rate, in agreement with a convection-limited reaction.9 Meanwhile, the process of local thrombus formation at sites of vessel injury is sensitive to the presence of cofactors that form the tenase and prothrombinase complexes to drive thrombin generation, such as the coagulation (F) factors IX and V, respectively, as evidenced by the bleeding phenotype observed for patients with a deficiency or dysfunction of FXI or FV.8
In contrast, free-flowing platelets within the bloodstream present a unique 3-dimensional spatial distribution as compared to platelets recruited and bound to a growing thrombus on the blood vessel wall. We have previously shown that the physical parameter of spatial separation, defined as the cubic root of the volume per cell, provides a measure of the average linear separation between nearest neighbor cells, and dominates the control of coagulation kinetics for cells in flow.21 Our previous work demonstrated that the spatial separation of procoagulant surfaces in the circulation, as calculated from carrier count of either TF-coated beads or TF-expressing cells, strongly correlated with their procoagulant and prothrombotic activity.21 The results presented in the current study suggest that the probability of platelet activation in the bloodstream increases as a function of residence time distal to sites of thrombus formation. Moreover, our work suggests that activation of the coagulation factors XI and X play a key role in promoting thrombin generation, platelet activation and microaggregate formation in the bloodstream.23
In summary, this study demonstrates the utility of a measurement platform to assess platelet activation and microaggregate formation in solution downstream of sites of thrombus formation under shear flow. Further understanding of the distal effects of thrombus formation may lead to the development of safe and effective antithrombotic agents.
ACKNOWLEDGEMENTS
We thank Cristina Puy for insightful comments and Kevin Phillips for technical assistance. This work was supported by grants from the National Institutes of Health (R01HL101972 and R01GM116184) and the Oregon Health and Science University School of Medicine MD/PhD program. O.J.T. McCarty is an American Heart Association Established Investigator (13EIA12630000). A.I. is a Bayer International Fellow and S.M.B. is a Whitaker International Fellow.
ABBREVIATIONS
- BSA
bovine serum albumin
- PBS
phosphate buffered saline
- DIC
differential interference contrast
- FACS
fluorescence activated cell sorting
- FX
coagulation factor X
- FXI
coagulation factor XI
- Da
Dahmköhler number
Footnotes
CONFLICTS OF INTEREST
R. Vetter and C. Gerdes are employees of Bayer Pharma AG. A. Gruber, E.I. Tucker, and Oregon Health & Science University have a significant financial interest in Aronora Inc., a company that may have a commercial interest in the results of this research. This potential conflict of interest has been reviewed and managed by the Oregon Health & Science University Conflict of Interest in Research Committee. J. Zilberman-Rudenko, A. Itakura, J. Maddala, S.M. Baker-Groberg and O.J.T. McCarty declare no competing financial interests.
ETHICAL STANDARDS
All human subject research was carried out in accordance with institutional guidelines approved by the Oregon Health & Science University Institutional Review Board. No animal studies were carried out by the authors for this article.
REFERENCES
- 1.Abulencia JP, Tien N, McCarty OJ, Plymire D, Mousa SA, Konstantopoulos K. Comparative antiplatelet efficacy of a novel, nonpeptide GPIIb/IIIa antagonist (XV454) and abciximab (c7E3) in flow models of thrombosis. Arterioscler. Thromb. Vasc. Biol. 2001;21:149–156. doi: 10.1161/01.atv.21.1.149. [DOI] [PubMed] [Google Scholar]
- 2.Andrews RK, Berndt MC. Platelet physiology and thrombosis. Thromb. Res. 2004;114:447–453. doi: 10.1016/j.thromres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Baker-Groberg SM, Cianchetti FA, Phillips KG, McCarty OJ. Development of a method to quantify platelet adhesion and aggregation under static conditions. Cell. Mol. Bioeng. 2014;7:285–290. doi: 10.1007/s12195-014-0328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker SM, Phillips KG, McCarty OJT. Development of a label-free imaging technique for the quantification of thrombus formation. Cell. Mol. Bioeng. 2012;5:488–492. doi: 10.1007/s12195-012-0249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang JY. Thrombin specificity. Requirement for apolar amino acids adjacent to the thrombin cleavage site of polypeptide substrate. Eur. J. Biochem. FEBS. 1985;151:217–224. doi: 10.1111/j.1432-1033.1985.tb09091.x. [DOI] [PubMed] [Google Scholar]
- 6.Cheng Q, et al. A role for factor XIIa–mediated factor XI activation in thrombus formation in vivo. Blood. 2010;116:3981–3989. doi: 10.1182/blood-2010-02-270918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fogelson AL, Neeves KB. Fluid Mechanics of Blood Clot Formation. Annu. Rev. Fluid Mech. 2015;47:377–403. doi: 10.1146/annurev-fluid-010814-014513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodeve AC, Pavlova A, Oldenburg J. Genomics of bleeding disorders. Haemoph. Off. J. World Fed. Hemoph. 2014;20(Suppl 4):50–53. doi: 10.1111/hae.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hathcock JJ. Flow effects on coagulation and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2006;26:1729–1737. doi: 10.1161/01.ATV.0000229658.76797.30. [DOI] [PubMed] [Google Scholar]
- 10.Itakura A, et al. Activated factor XI inhibits chemotaxis of polymorphonuclear leukocytes. J. Leukoc. Biol. 2011;90:923–7. doi: 10.1189/jlb.0411182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones CM, et al. Measurement science in the circulatory system. Cell. Mol. Bioeng. 2014;7:1–14. doi: 10.1007/s12195-013-0317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonnalagadda D, Izu LT, Whiteheart SW. Platelet secretion is kinetically heterogeneous in an agonist-responsive manner. Blood. 2012;120:5209–5216. doi: 10.1182/blood-2012-07-445080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kravtsov DV, et al. Factor XI contributes to thrombin generation in the absence of factor XII. Blood. 2009;114:452–458. doi: 10.1182/blood-2009-02-203604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee AM, Tormoen GW, Kanso E, McCarty OJ, Newton PK. Modeling and simulation of procoagulant circulating tumor cells in flow. Front. Oncol. 2012;2:108. doi: 10.3389/fonc.2012.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarty OJ, et al. Dimensional analysis and scaling relevant to flow models of thrombus formation: communication from the SSC of the ISTH. J. Thromb. Haemost. 2016;14:619–622. doi: 10.1111/jth.13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarty OJ, Abulencia JP, Mousa SA, Konstantopoulos K. Evaluation of platelet antagonists in in vitro flow models of thrombosis. Methods Mol. Med. 2004;93:21–34. doi: 10.1385/1-59259-658-4:21. [DOI] [PubMed] [Google Scholar]
- 17.Mousa SA, Abulencia JP, McCarty OJ, Turner NA, Konstantopoulos K. Comparative efficacy between the glycoprotein IIb/IIIa antagonists roxifiban and orbofiban in inhibiting platelet responses in flow models of thrombosis. J. Cardiovasc. Pharmacol. 2002;39:552–560. doi: 10.1097/00005344-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Puy C, et al. Factor XII promotes blood coagulation independent of factor XI in the presence of long-chain polyphosphates. J. Thromb. Haemost. 2013;11:1341–1352. doi: 10.1111/jth.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roehrig S, et al. Discovery of the novel antithrombotic agent 5-chloro-N-({(5S)-2-oxo-3- [4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene- 2-carboxamide (BAY 59-7939): an oral, direct factor Xa inhibitor. J. Med. Chem. 2005;48:5900–5908. doi: 10.1021/jm050101d. [DOI] [PubMed] [Google Scholar]
- 20.Samuel M, Pixley RA, Villanueva MA, Colman RW, Villanueva GB. Human factor XII (Hageman factor) autoactivation by dextran sulfate. Circular dichroism, fluorescence, and ultraviolet difference spectroscopic studies. J. Biol. Chem. 1992;267:19691–19697. [PubMed] [Google Scholar]
- 21.Tormoen GW, Rugonyi S, Gruber A, McCarty OJ. The role of carrier number on the procoagulant activity of tissue factor in blood and plasma. Phys. Biol. 2011;8:066005. doi: 10.1088/1478-3975/8/6/066005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White-Adams TC, et al. Laminin promotes coagulation and thrombus formation in a factor XII-dependent manner. J. Thromb. Haemost. 2010;8:1295–1301. doi: 10.1111/j.1538-7836.2010.03850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zilberman-Rudenko J, et al. Coagulation factor XI promotes distal platelet activation and single platelet consumption in the bloodstream under shear flow. Arterioscler. Thromb. Vasc. Biol. 2016;36:510–517. doi: 10.1161/ATVBAHA.115.307034. [DOI] [PMC free article] [PubMed] [Google Scholar]