Abstract
The ability to hold information in working memory (WM) is fundamental for cognition. Contrary to the longstanding view that WM depends on sustained, elevated activity, we present evidence suggesting that information can be held in WM via “activity-silent” synaptic mechanisms. Using machine learning to decode brain activity patterns, we show that the active representation of an item in WM drops to baseline when attention shifts away. A targeted pulse of transcranial magnetic stimulation produces a brief reemergence of the item in concurrently measured brain activity. This reactivation effect only occurs and influences memory performance when the item is potentially relevant later in the trial, suggesting that the representation is dynamic and modifiable via cognitive control. The results support a Synaptic Theory of Working Memory.
The ability to mentally retain information in an accessible state, to manipulate it, and to use it to guide behavior is a critical building block for cognition. It has long been assumed that the neural basis for this working memory (WM) ability is elevated and persistent neuronal firing(1). This assumption has been called into question by recent proposals that information can be held in WM via synaptic mechanisms that do not require sustained, elevated brain activity(2–4).
Building on theoretical frameworks that information can be held in WM in one of several states of activation(5, 6), we recorded neural activity while participants performed a multi-step task in which two items were presented as memoranda for each trial. A cue indicated which item would be tested by the impending recognition memory probe, followed by the probe, then by a second cue, and then a second probe (Fig. 1). There was equal probability that following the first cue, but not the second, that the uncued item might be needed for an ensuing memory judgment. This procedure moves the uncued item into a different state than the cued item, which, by definition, is in the focus of attention(7). Cognitive theories refer to the intermediate state of this unattended memory item (UMI) as “activated long-term memory” (LTM)(5, 6).
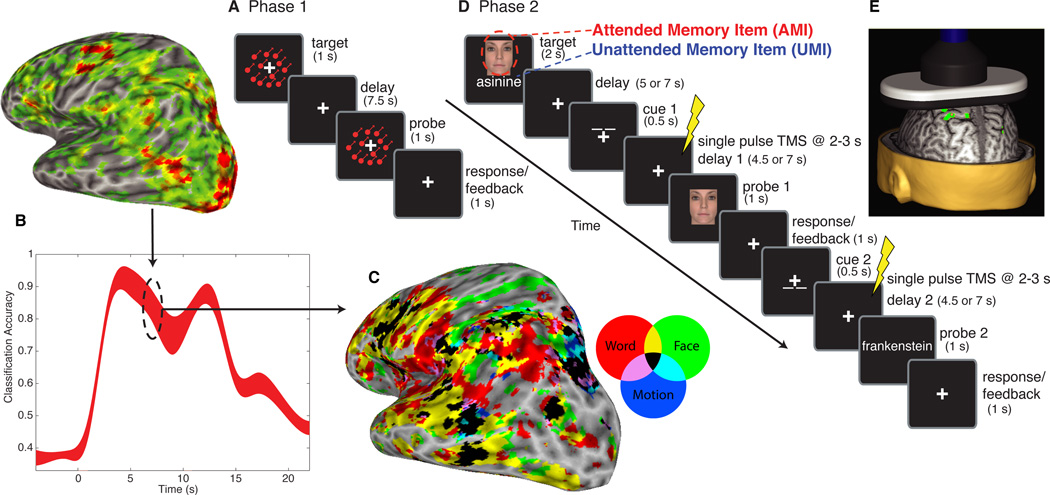
Fig. 1. General procedure.
In Phase 1, functional magnetic resonance imaging (fMRI) data were acquired while participants performed a one-item delayed-recognition task for words, faces, or directions of motion (A), and used for multivariate pattern analysis (MVPA). Classifiers trained on the delay-period (B) were used for subsequent analyses. For Experiment 1, these classifiers were used to decode fMRI activity from Phase 2 (Fig. 2). For Experiments 2 and 3, they were used in a whole-brain searchlight, conjunction-analysis to generate subject-specific maps of category-sensitive areas (C); non-overlapping areas were used for transcranial magnetic stimulation (TMS) targeting in Phase 2 (D). In Phase 2, single pulses of TMS (E) were delivered during the post-cue delay periods.
For Experiment 1, multivariate pattern analysis (MVPA) showed evidence for an active representation of the UMI that dropped to baseline levels (Fig. 2) (7–9). This suggests that information in WM (but outside of focal attention) can be maintained in a latent state, via mechanisms other than sustained, elevated activity. Although a similar drop-to-baseline pattern is observed when subjects are instructed to drop information from WM(10, 11), here the UMI remains in WM because, when so instructed by the second cue, subjects accurately reactivate it and use it to evaluate the final probe (Fig. 2B).
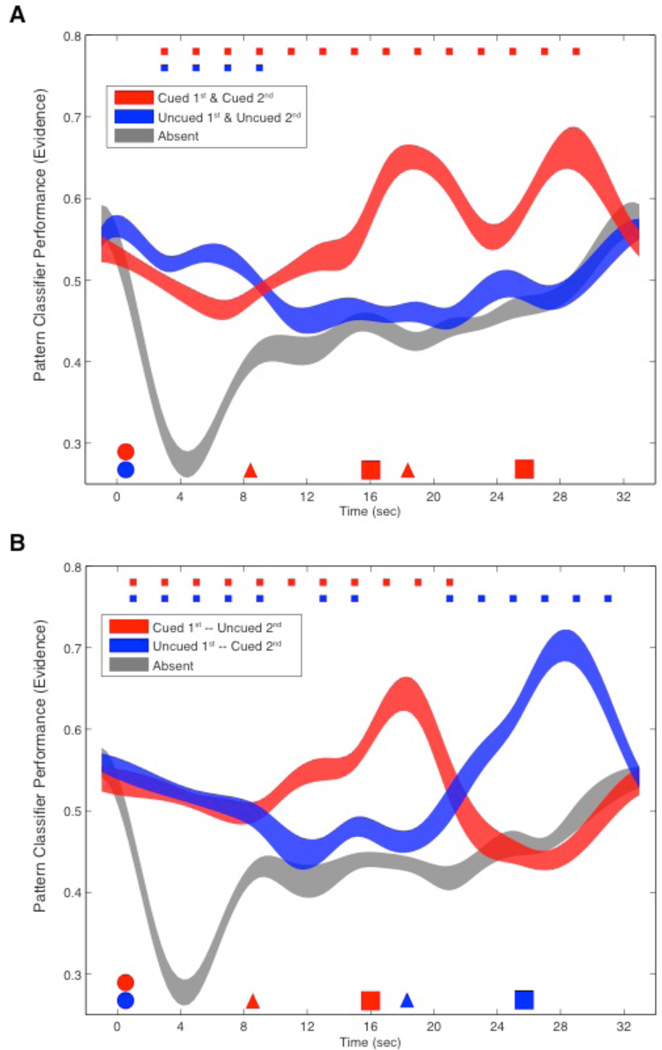
Fig. 2. Experiment 1 fMRI decoding (Train Phase 1, Test Phase 2): Classifier evidence as a function of an item’s status, collapsed across stimulus category.
After stimulus presentation (red and blue circles), delay-period classifier evidence for both items was elevated relative to the empirical baseline of evidence for the category that was not presented on that trial (“absent”, gray). Upon presentation of the first cue (red triangle), evidence for the cued category (red) remained elevated, but for the uncued category (blue) dropped to baseline. After the first probe (red square), on half the trials the second cue designated that the same item would be tested by the second probe (A), and evidence for the two categories remained the same relative to baseline. When the second cue designated the previously uncued item (B) evidence for the two categories reversed for the remainder of the trial. (Color-coded markers at the top of each plot indicate p<.01; line width reflects SEM.)
In three additional experiments we tested the hypothesis that, if a UMI is encoded in a distributed pattern of synaptic weights, and held in a state that is more accessible than trial-irrelevant information, the readout from a nonspecific burst of activity filtered through this network might reveal this latent representation(2) (Fig. S1). This would be consistent with the idea that networks in posterior cortex can be dynamically configured as matched filters to encode behaviorally relevant information(3, 4, 12, 13).
For Experiments 2 and 3, participants performed the Phase 2 WM task (Fig. 1) while we recorded electroencephalography (EEG) and applied single-pulse transcranial magnetic stimulation (TMS) 2–3 sec after the first cue. For Experiment 2 we targeted brain regions identified from the Phase 1 MRI task as preferentially supporting MVPA decoding for one category, but not the other two. MVPA of the spectrally transformed EEG data from only the Phase 2 task detected reliable evidence for an active representation of both memory items across the initial portion of the trial, until the onset of the first cue, at which point decoding accuracy remained elevated for the attended memory item (AMI), but dropped to the baseline for the UMI(14).
After a single pulse of TMS, there was a brief recovery of MVPA decoding of the UMI—a “reactivation effect”—before it returned to baseline and remained there while the cued item was tested (p=.01; Bayes Factor (BF)=3.64 against-the-null) (Fig. 3A). TMS affected neither broadband decoding of the AMI, nor recognition memory judgments (Fig. S4). When we analyzed bandpass-filtered data, the TMS reactivation effect was isolated to signal from the beta band (Fig. S5), and was associated with a transient period of above-chance decoding performance for both the UMI and the AMI. The TMS reactivation effect was specific for information that was in WM on that trial, because above-chance MVPA performance, as assessed with the area-under-the-curve (AUC) analysis, necessarily means that TMS did not activate a representation of the category that was irrelevant on that trial.
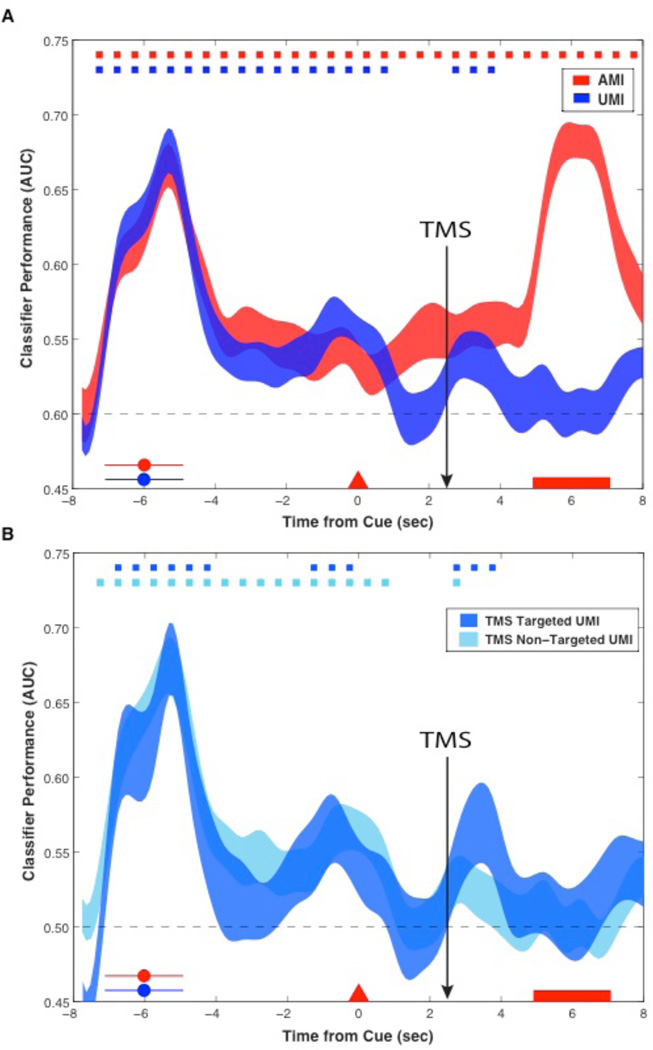
Fig. 3. Experiment 2 EEG decoding (Train and Test on Phase 2 data): Classifier accuracy (area under curve, AUC) as a function of an item’s status at the time of the first cue, collapsed across stimulus category.
AUC reflects classifier sensitivity to discriminating between evidence for the AMI or UMI relative to the absent category. (A) Classification timeseries of the AMI and UMI upon stimulus presentation (red and blue circles), the first cue (red triangle), TMS, and first probe (red rectangle), averaged over N=18 sessions, 2,952 trials (decoding ends where the AMI and UMI switched on 50% of the trials). (B) Decoding UMIs as a function of whether TMS targeted that item’s Phase 1-defined region or a different category’s region. (Color-coded markers at the top of each plot indicate p<.05, line width reflects SEM).
In Experiment 2 we administered blocks of trials with TMS targeting one of the category-selective regions, but varied, on a trial-by-trial basis, which category was the AMI and which was the UMI. Each block included trials for which the UMI belonged to the targeted region’s preferred category, and trials for which it did not. A TMS reactivation effect was observed (Fig. 3B) whether or not TMS targeted the UMI’s category-preferred region, although the effect was larger and more prolonged when it did (BF for Targeted and Non-Targeted sites were 4.02 and 1.72, respectively). This suggests that WM is supported by heightened connectivity between cortical networks that represent all trial-relevant information (AMI and UMI) relative to trial-irrelevant information(15, 16).
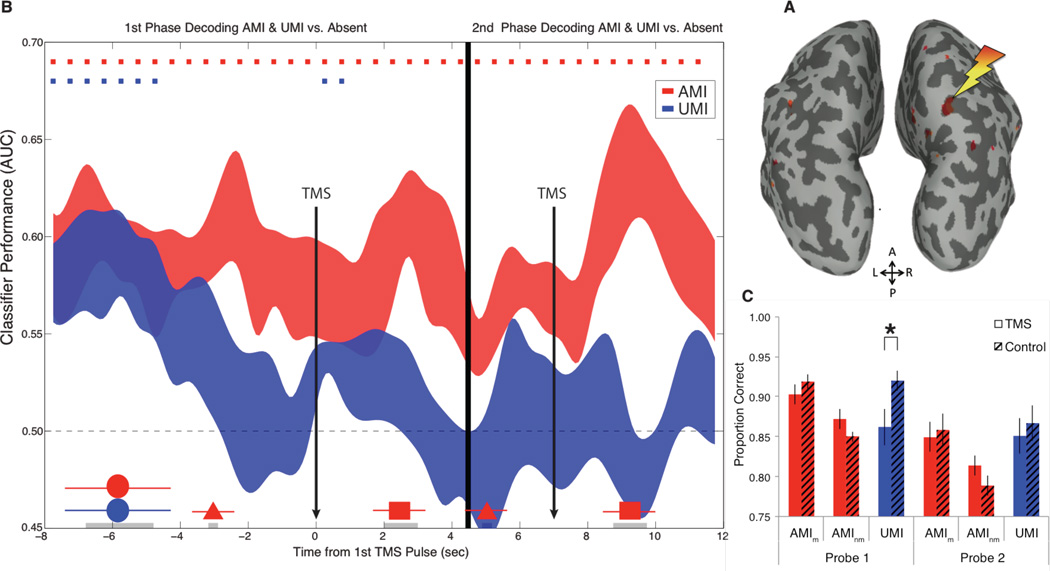
Retrocues that inform subjects that they can drop an item from memory result in a rapid loss of multivariate evidence for the no-longer-relevant item(11, 17). Nonetheless, proactive interference from stimuli presented on previous trials indicates that the brain retains a residual trace of such recent, but no-longer-relevant, information(18). An important test of state-based models of WM is whether there is a functional distinction between UMIs (putatively held in a state of activated LTM) and dropped information (no longer in WM). In Experiment 3, with a different group of participants we also administered TMS after the second cue, after which the uncued item would no longer be relevant on the trial, and at which point it should have the same status as an irrelevant item. If the TMS reactivation effect is a consequence of an item being maintained in a privileged state, it should only be observed when that item is still potentially relevant for the trial. We also jittered the onset of TMS between 2–3 seconds after the cue(14), and standardized TMS by targeting the same region across subjects and for every trial--an MVPA-defined region in the right precuneus known to be critical for the top-down control of visual attention(19) (Fig. 4A).
Fig. 4.
(A) shows the MVPA-defined TMS target for Experiments 3 and 4 (right precuneus). (B) Classification timeseries from Experiment 3 showing TMS reactivation of the UMI following the first cue, when the UMI was still relevant (left panel), but not following the second cue, when the UMI was no longer relevant on the trial (right panel) averaged over 1,152 trials. (C) Experiment 4 recognition memory for AMI match probes, AMI nonmatch probes, and UMI (nonmatch) probes (bars=SEM).
For the first half of the trial, the results from Experiment 3 replicated those from Experiment 2 (Fig. 4B), with a robust TMS reactivation effect for the UMI (BF=9.8 against-the-null). For the delay period following the second cue, however, there was no evidence for significant decoding of the uncued item following the TMS pulse (BF=3.4 in-favor-of-the-null). These results suggest that UMIs are maintained in a different state than are items that have been dropped from WM, and that the mechanisms that maintain latent representations in WM are dynamic and modifiable via cognitive control(20).
Because our design has entailed decoding at the category level, it does not rule out the possibility that the TMS reactivation effect reflects a general reinstatement of category context(21), rather than the temporary activation of the UMI itself. The idea that the representation of the UMI, itself, drives this effect would be strengthened by a demonstration that TMS can influence recognition memory decisions on this task. If the TMS reactivation effect reflects a temporary reinstatement of the UMI back into the focus of attention, participants should have more difficulty rejecting the UMI as a lure when probing their memory of the AMI.
In Experiment 4, we presented recognition memory probes that matched the AMI on 50% of trials, and of the 50% of probes that were invalid, 30% were drawn from the same category as the AMI, and a critical 20% matched the UMI(14). Subjects were instructed to reject memory probes that did not match the AMI. Critically, only for the first probe was there an increased proportion of false alarms to the UMI for TMS relative to no-TMS trials (Fig. 3C, p=.01, BF=3.48) (14).
Our results have important implications for the understanding of WM at many levels. They provide neural evidence for at least two levels of WM that are distinct from the default state of LTM representations(5, 6). They are inconsistent with models positing just one level of WM storage(22, 23). They also suggest that instead of “activated LTM”, a more apt label for the second level of WM is prioritized LTM. Information can be held in WM in latent “activity-silent” traces(11, 20). What might be the physiological bases of such representations? Computational models of WM have proposed that short-term synaptic plasticity could be the basis for the transient formation of weight-based networks that can represent information over short time-periods(2, 24).
The present results provide empirical evidence for the existence of a short-term plasticity mechanism that is likely to be fundamental to a wide range of cognitive functions involving attentional selection (25), and may provide the building blocks for long-term potentiation mechanisms that support LTM(26). Therefore, the present results introduce a potential avenue for reactivating and strengthening representations that underlie many classes of high-level cognition.
Supplementary Material
Acknowledgments
Jason Samaha, Andrew Sheldon, Bornali Kundu, Jarrod Lewis-Peacock, and Jeff Johnson provided assistance and helpful discussions. The research was supported by NIH-MH095984 to BRP. The data are stored at curate.nd.edu.
Footnotes
Supplementary Materials
Materials and Methods
Fig. S1–S6
Movie S1
Supplemental Text
Table S1
References 27–38
Author Contributions: NSR, BRP, JJL, & ACR designed the research; NSR, OG, MJS, & EMM conducted the research; NSR, JJL, & ACR analyzed the data; NSR, BRP, & JJL wrote the manuscript.
The authors declare no conflict of interest.
References and Notes
- 1.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 2.Mongillo G, Barak O, Tsodyks M. Synaptic theory of working memory. Science. 2008;319:1543–1546. doi: 10.1126/science.1150769. [DOI] [PubMed] [Google Scholar]
- 3.Sugase-Miyamoto Y, Liu Z, Wiener MC, Optican LM, Richmond BJ. Short-term memory trace in rapidly adapting synapses of inferior temporal cortex. PLoS Comput Biol. 2008;4:e1000073. doi: 10.1371/journal.pcbi.1000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolff MJ, Ding J, Myers NE, Stokes MG. Revealing hidden states in visual working memory using electroencephalography. Front Syst Neurosci. 2015;9:123. doi: 10.3389/fnsys.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowan N. An Embedded-Processes Model of Working Memory Models of Working Memory. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 6.Oberauer K. Psychology of Learning and Motivation. Vol. 51. Academic Press; 2009. pp. 45–100. [Google Scholar]
- 7.LaRocque JJ, Lewis-Peacock JA, Drysdale AT, Oberauer K, Postle BR. Decoding attended information in short-term memory: an EEG study. J Cogn Neurosci. 2013;25:127–142. doi: 10.1162/jocn_a_00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis-Peacock JA, Drysdale AT, Oberauer K, Postle BR. Neural evidence for a distinction between short-term memory and the focus of attention. J Cogn Neurosci. 2012;24:61–79. doi: 10.1162/jocn_a_00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaRocque JJ, Riggall AC, Emrich SM, Postle BR. Within-category decoding of attended vs. unattended items in short-term memory. Cereb Cortex. doi: 10.1093/cercor/bhw283. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ester EF, Anderson DE, Serences JT, Awh E. A neural measure of precision in visual working memory. J Cogn Neurosci. 2013;25:754–761. doi: 10.1162/jocn_a_00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riggall AC, Postle BR. The relationship between working memory storage and elevated activity as measured with functional magnetic resonance imaging. J Neurosci. 2012;32:12990–12998. doi: 10.1523/JNEUROSCI.1892-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayden BY, Gallant JL. Working memory and decision processes in visual area v4. Front Neurosci. 2013;7:18. doi: 10.3389/fnins.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myers NE, et al. Testing sensory evidence against mnemonic templates. Elife. 2015;4:e09000. doi: 10.7554/eLife.09000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Materials and methods are available as supplementary materials on Science Online.
- 15.Kundu B, Chang JY, Postle BR, Van Veen BD. Context-specific differences in fronto-parieto-occipital effective connectivity during short-term memory maintenance. Neuroimage. 2015;114:320–327. doi: 10.1016/j.neuroimage.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee TG, D'Esposito M. The dynamic nature of top-down signals originating from prefrontal cortex: a combined fMRI-TMS study. J Neurosci. 2012;32:15458–15466. doi: 10.1523/JNEUROSCI.0627-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christophel TB, Hebart MN, Haynes JD. Decoding the contents of visual short-term memory from human visual and parietal cortex. J Neurosci. 2012;32:12983–12989. doi: 10.1523/JNEUROSCI.0184-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monsell S. Recency, immediate recognition memory, and reaction time. Cognitive Psychology. 1978;10:465–501. [Google Scholar]
- 19.Beck DM, Muggleton N, Walsh V, Lavie N. Right parietal cortex plays a critical role in change blindness. Cereb Cortex. 2006;16:712–717. doi: 10.1093/cercor/bhj017. [DOI] [PubMed] [Google Scholar]
- 20.Stokes MG. 'Activity-silent' working memory in prefrontal cortex: a dynamic coding framework. Trends Cogn Sci. 2015;19:394–405. doi: 10.1016/j.tics.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polyn SM, Natu VS, Cohen JD, Norman KA. Category-specific cortical activity precedes retrieval during memory search. Science. 2005;310:1963–1966. doi: 10.1126/science.1117645. [DOI] [PubMed] [Google Scholar]
- 22.McElree B. Psychology of Learning and Motivation. Vol. 46. Academic Press; 2006. pp. 155–200. [Google Scholar]
- 23.Baddeley A. Working memory: theories, models, and controversies. Annu Rev Psychol. 2012;63:1–29. doi: 10.1146/annurev-psych-120710-100422. [DOI] [PubMed] [Google Scholar]
- 24.Itskov V, Hansel D, Tsodyks M. Short-Term Facilitation may Stabilize Parametric Working Memory Trace. Front Comput Neurosci. 2011;5:40. doi: 10.3389/fncom.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 26.Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- 27.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 28.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. 3-Dimensional proportional system: an approach to cerebral imaging. 1988 [Google Scholar]
- 29.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogasch NC, et al. Analysing concurrent transcranial magnetic stimulation and electroencephalographic data: a review and introduction to the open-source TESA software. Neuroimage. doi: 10.1016/j.neuroimage.2016.10.031. (in press) [DOI] [PubMed] [Google Scholar]
- 32.Morton NW, et al. Category-specific neural oscillations predict recall organization during memory search. Cereb Cortex. 2013;23:2407–2422. doi: 10.1093/cercor/bhs229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newman EL, Norman KA. Moderate excitation leads to weakening of perceptual representations. Cereb Cortex. 2010;20:2760–2770. doi: 10.1093/cercor/bhq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fawcett T. An introduction to ROC analysis. Pattern Recogn. Lett. 2006;27:861–874. [Google Scholar]
- 35.Rouder JN, Speckman PL, Sun D, Morey RD, Iverson G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon Bull Rev. 2009;16:225–237. doi: 10.3758/PBR.16.2.225. [DOI] [PubMed] [Google Scholar]
- 36.Dienes Z. Using Bayes to get the most out of non-significant results. Front Psychol. 2014;5:781. doi: 10.3389/fpsyg.2014.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson JS, Kundu B, Casali AG, Postle BR. Task-dependent changes in cortical excitability and effective connectivity: a combined TMS-EEG study. J Neurophysiol. 2012;107:2383–2392. doi: 10.1152/jn.00707.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casali AG, et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci Transl Med. 2013;5:198ra105. doi: 10.1126/scitranslmed.3006294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.