Abstract
Purpose
Cancer-related fatigue (CRF) is a disruptive symptom for many survivors. Despite promising evidence for efficacy of Mindfulness-Based Stress Reduction (MBSR) in reducing CRF, no trials comparing it to an active comparator for fatigued survivors have been published. The purpose of this trial was to compare MBSR to psychoeducation for CRF and associated symptoms.
Methods
Breast (n=60) and colorectal (n=11) cancer survivors (stage 0–III) with clinically significant CRF after completing chemotherapy and/or radiation therapy an average of 28 months prior to enrollment were randomized to MBSR or psychoeducation/support groups (PES). MBSR focused on mindfulness training; PES focused on CRF self-management. Outcomes included CRF interference (primary), CRF severity and global improvement, vitality, depression, anxiety, sleep disturbance, and pain. Outcomes were assessed at baseline (T1), post-intervention (T2), and 6-month follow-up (T3) using intent-to-treat analysis.
Results
Between-group differences in CRF interference were not significant at any time point; however, there was a trend favoring MBSR (d=−0.46, p=0.073) at T2. MBSR participants reported significantly greater improvement in vitality (d=0.53, p=0.003) and were more likely to report CRF as moderately-to-completely improved compared to the PES group (χ2 (1)=4.1765, p=0.041) at T2. MBSR participants also reported significantly greater reductions in pain at T2 (d=0.53, p=0.014). In addition, both MBSR and PES produced moderate-to-large and significant within-group improvements in all fatigue outcomes, depression, anxiety, and sleep at T2 and T3 compared to T1.
Conclusion
MBSR and PES appear efficacious for CRF and related symptoms. Larger trials including a usual care arm are warranted.
Keywords: Cancer, fatigue, mindfulness, MBSR, meditation, psychoeducation
BACKGROUND
Fatigue is the most common and distressing cancer-related symptom [1,2], affecting approximately 30% of breast and colorectal cancer survivors years into disease-free survivorship [3,4]. Cancer-related fatigue (CRF) negatively impacts survivors’ quality of life[5] and ability to work [6], and places survivors at increased risk for cancer recurrence [7]. Moreover, CRF is drastically under-treated [8], with <15% of fatigued patients reporting having received any guidance in CRF management from their health care team [9].
Clinical practice guidelines for CRF recommend symptom management [10]. Mindfulness-Based Stress Reduction (MBSR) is a non-pharmacologic intervention that has shown promise in the treatment of CRF [11–13]. MBSR is a group meditation intervention that provides experiential training in mindfulness, a non-judging mental state whereby one attends to and purposefully manages one’s awareness of what is happening in the present moment. The goal of MBSR is not necessarily to decrease symptom severity, but to enhance survivors’ ability to live with their symptoms in a non-reactive way, thereby reducing symptom-related interference with quality of life. Recent meta-analyses have established MBSR as efficacious in improving mental health outcomes in cancer [14–16]. Systematic reviews in adults with cancer [17,18,15] and in non-cancer populations [19,20] have concluded that mindfulness-based interventions are promising but require further investigation, particularly with respect to treating fatigue and its correlates [1,21]. Although clinical practice guidelines added MBSR as an evidence-based intervention for fatigue following cancer treatment in 2014 [10], to date only trials comparing MBSR to wait-list controls on fatigue outcomes have been conducted [11–13]. Of these, only one used fatigue as the primary outcome of the trial and enrolled participants based on presence of clinically significant fatigue. In that trial, Johns and colleagues reported that the MBSR group experienced large post-intervention improvements in fatigue interference, fatigue severity, and vitality compared to the wait list group, and the improvements were maintained at 1- and 6-month follow-up [11].
Because the impact of MBSR on CRF has already been evaluated using wait-list controls, a necessary step to establish the specific effects of MBSR [22] is to conduct a more rigorous trial comparing MBSR specifically targeting CRF to another active treatment. In the current trial, we employed an active treatment control group intended to help rule out the possibility that placebo or related non-specific effects might be responsible for MBSR’s beneficial impact. Therefore, the aim of the current trial was to determine the effect size of MBSR compared to an active treatment in reducing fatigue and associated symptoms among cancer survivors following chemotherapy and/or radiotherapy. Because it is recommended as a treatment for CRF [10] and prior research has shown it is efficacious in treating CRF and its correlates [23–25], psychoeducation and support (PES) was selected as the active comparator for this study.
METHODS
Design
This was a 2-arm randomized clinical pilot trial. Study procedures were approved by the Indiana University and Community Health Network institutional review boards. Written informed consent was obtained from all participants. The study was funded by the Walther Cancer Foundation and the Indiana Clinical and Translational Sciences Institute. The study is registered with ClinicalTrials.gov (NCT01919853).
Participants
Breast (n=60) and colorectal (n=11) cancer survivors (BCS and CRCS, respectively) with persistent fatigue were consecutively recruited over 5 months in 2012–2013 from clinics affiliated with a National Cancer Institute-designated cancer center and through tumor registry mailings. BCS and CRCS were chosen as populations of interest as they are two of the most prevalent cancer diagnoses within our setting.
Individuals were told the study purpose was to test two potentially helpful behavioral treatments for CRF. Individuals were eligible if they were age 18 or older, had a first-time diagnosis of non-metastatic (stages 0–III) breast or colorectal cancer treated with chemotherapy and/or radiation therapy, and had clinically significant CRF (Fatigue Symptom Inventory severity composite ≥ 4) [26] that had persisted for at least 2 months. Individuals were excluded if they had received any cancer treatment (i.e., chemotherapy, radiation therapy, or surgery) less than 3 months or more than 5 years prior to enrollment (current endocrine therapy for breast cancer was allowed), reported severe depressive symptoms (Patient Health Questionnaire-8 score ≥ 20) [27], or reported past participation in a mindfulness class and/or ongoing meditation practice.
Randomization and Blinding
Eligible participants attended a group enrollment session, including completion of baseline surveys, and randomization to MBSR or PES. The allocation ratio was 1:1, with the sequence generated by coin toss in randomly varied block sizes of 4 to 6 and concealed in opaque sequentially-numbered envelopes. Participants and research assistants were blinded to the allocation sequence. Outcomes were self-reported on study questionnaires, to which group facilitators lacked access. Participants were blinded to study hypotheses and had no knowledge of the content of the course to which they were not assigned.
Interventions
Participants in both interventions met for 2 hours each week for 8 weeks. The difference between the interventions was the active component of guided meditation in MBSR; topics related to mindfulness, meditation, and relaxation were not included in the PES intervention. Groups averaged 10 participants each to ensure equal amounts of time and attention. To limit contamination, groups met in separate locations.
Mindfulness-Based Stress Reduction (MBSR)
MBSR provides guided training during class and through audio recordings outside of class on mindfulness meditation practices (i.e., body scan, sitting meditation, hatha yoga, walking meditation, and compassion meditation). Through these practices, present-centered awareness is enhanced, facilitating non-reactive and non-judgmental acceptance of thoughts, feelings, and bodily sensations [15,18,28]. Relating to symptoms such as fatigue in this adaptive way has been shown to reduce symptom-related interference with quality of life [29]. MBSR was delivered in a manner consistent with the standard MBSR curriculum [30], tailored to the needs of fatigued survivors. Adaptations included 2-hour classes, no retreat, brief psychoeducation related to CRF, a 10-minute bedtime body scan to support rest, and shorter guided home practices (20 min). Participants tracked daily meditation practices on weekly logs. Notably, the MBSR intervention implemented in the present study was highly similar to the MBSR intervention implemented in our earlier study with fatigued post-treatment cancer survivors comparing MBSR to wait-list control [11]. MBSR instructors were a physician and a doctoral-level clinical health psychologist with 9 and 3 years of MBSR teaching experience, respectively. Further details on MBSR for CRF are published elsewhere [11]. A description of the MBSR sessions used in this study is presented in Table 1.
Table 1.
Description of Mindfulness-Based Stress Reduction Sessions
| Session Theme | Meditation Exercises | Didactic Teaching | Home Practice | |
|---|---|---|---|---|
| 1 | Awareness: Meeting ourselves where we are in honesty and kindness |
|
|
|
| 2 | Perception and creative responding: Wholeness no matter what is here |
|
|
|
| 3 | The pleasure and power of being present |
|
|
|
| 4 | Reacting on autopilot: mindfulness skills supporting healthy responsiveness |
|
|
|
| 5 | Creative ways of responding to stress |
|
|
|
| 6 | Mindful communication; cultivating compassion; responsiveness in speech and action |
|
|
|
| 7 | Taking care of yourself: Healthy living choices arising from practice |
|
|
|
| 8 | The rest of your life: Making the practice your own |
|
|
|
“Calendars” are 2-page handouts facilitating brief daily journaling.
Psychoeducation and support (PES)
To provide a rigorous test of MBSR effects, a PES group intervention was included as an active comparator. The goal of PES is to educate and support patients to better cope with the side effects of their illness. For cancer survivors, PES programs include group discussions of CRF and its impact on psychological and social functioning; sharing, listening to, and affirming patients’ CRF-related experiences; and offering evidenced-based tips and strategies for managing CRF (e.g., sleep, nutrition, exercise) [31].
The study team developed a manualized PES intervention, in accordance with PES programs used in other published studies of CRF [23]. Specific topics were addressed each week, and sessions included sharing of experiences living with CRF and strategies for CRF self-management. The details of each 8 week session are presented in Table 2. For between-session home practice, participants received supplemental readings on fatigue self-management from After Cancer Treatment: Heal Faster, Better, Stronger [32], as well as the American Cancer Society, American Society of Clinical Oncology, National Cancer Institute, and CURE Magazine websites. Participants tracked time spent doing reading assignments and other self-care strategies discussed in class on weekly logs. PES facilitators were master’s level social workers each with approximately 4 years of experience facilitating PES groups. Participants in PES were provided with information on how to access MBSR courses at the end of the study.
Table 2.
Description of Psychoeducational Support Intervention Sessions
| Session Theme | Didactic Teaching | Invited Sharing | Readings & Home Practice | |
|---|---|---|---|---|
| 1 | Orientation |
|
|
|
| 2 | Fatigue & Lingering Effects of Cancer |
|
|
|
| 3 | Restorative Sleep |
|
|
|
| 4 | Mood |
|
|
|
| 5 | Nutrition |
|
|
|
| 6 | Relationships |
|
|
|
| 7 | Exercise & Survivorship Care |
|
|
|
| 8 | Conclusion/Wrap-up |
|
|
Note. Abbreviations: FSI=Fatigue Symptom Inventory. CRF=cancer-related fatigue. ASCO=American Society of Clinical Oncology. ACS=American Cancer Society.
Treatment Fidelity
Fidelity to MBSR and PES was ensured through the use of standardized manuals and audio recordings and evaluation of sessions for adherence to the protocol using checklists created for each intervention condition. Mean treatment fidelity ratings across a randomly selected 25% of sessions were 85.1% for MBSR facilitators and 95.8% for PES facilitators.
Measures
Demographic data collected included gender, race, educational level, employment status, marital status, and income level, as determined by participants’ response to the question, “When you consider your household income from all sources today, would you say that you are comfortable, have just enough to make ends meet, or not enough to make ends meet.” Patient-reported outcomes were assessed on printed study surveys at baseline (T1), post intervention (T2), and 6 months later (T3) at the study site. Intent-to-treat participants completed T2 and T3 surveys by mail.
Primary Outcome
The Assessing the Symptoms of Cancer using Patient-Reported Outcomes (ASCPRO) group [33] recommends selecting CRF measures based upon study and intervention intent (e.g., reducing the negative impact of fatigue on functioning). Thus, fatigue interference was selected as the primary outcome, as the intent of MBSR is to reduce symptom-related functional interference. Prior studies of the effects of the active comparator have included fatigue interference among their outcomes [23] as interference tends to be of greater concern to survivors than the presence or severity of fatigue [34].
The 7-item interference subscale of the Fatigue Symptom Inventory (FSI) [35] was used in this study to assess the degree to which fatigue has interfered with functioning across multiple domains (e.g., general activity level, ability to bathe/dress, work, relationships, mood) over the past week. Items are rated on 11-point scales (0=no interference; 10=extreme interference) and then averaged.
Secondary Outcomes
Fatigue severity was assessed using the 4-item FSI severity subscale [35]. FSI severity scores ≥ 3 (range 0 – 10) are considered clinically significant [26]. Vitality was measured with the 4-item SF-36 Vitality Scale [36]. Scores range from 0 to 100, with higher scores indicating greater vitality. Vitality scores ≤ 45 are indicative of clinically-significant CRF [26]. Fatigue global improvement was assessed with a single item asking respondents to rate their CRF compared to when they started the study, with the options being worse, about the same, or a little, somewhat, moderately, a lot, or completely better [37]. Additional secondary outcome measures included the Patient Health Questionnaire 8-item depression scale (PHQ-8) [27] and 7-item Generalized Anxiety Disorder scale (GAD-7) [38], the 7-item Insomnia Severity Index (ISI) [39], and a 3-item version of the Brief Pain Inventory [40].
Additional Outcomes
At the beginning of session 2, participants were asked to complete a 5-item expectancy-credibility scale adapted from Devilly and Borkovec that measures participants’ perceptions of the expected benefits and credibility of treatment on a 0–9 scale [41]. Participants’ overall satisfaction with the study was rated at T3 on an 11-point scale (0=not at all satisfied; 10=completely satisfied). Also at T3, MBSR participants were asked to report the average number of days per week, if any, they had continued to participate in mindfulness practice, both formal (e.g., body scan, sitting practice, yoga) and informal (doing everyday activities mindfully).
Statistical Power
Because this was a pilot study, the principal aim was to estimate an effect size for a definitive phase 3 randomized clinical trial. The sample size of 71 had 80% power to detect a medium-to-large effect size (0.65–0.70) difference between means for tests of between-group efficacy.
Statistical Analysis
Intent-to-treat analyses were conducted using imputation to fill in missing data according to randomly assigned group membership regardless of degree of adherence to their intervention. Available data from all participants were included in the analyses regardless of attendance or engagement in the intervention. Groups were compared on T1 demographic and medical characteristics using Chi square and Fisher’s Exact tests for categorical variables and t-tests for continuous variables. Although there were no statistically significant differences between groups on these variables at p<0.05, we controlled for characteristics thought to be clinically/theoretically relevant in an investigation of CRF and/or those where the between group difference was p<0.10 [42], including cancer type and income.
Analysis of covariance (ANCOVA) was used to test efficacy by comparing MBSR to PES on primary and secondary outcomes at T2 and separately at T3 while adjusting for covariates and baseline scale scores for each variable. When computing scale scores, a person-specific and scale-specific mean of non-missing items was substituted for missing items if 33% or fewer of the scale’s items were missing. However, missing data occurred infrequently. Effect sizes (Cohen’s d) were calculated for each outcome variable at T2 and T3 as the standardized mean difference between the MBSR and PES groups divided by the pooled baseline standard deviation of the outcome variable. CRF global improvement (defined as those reporting their CRF as being moderately to completely better since T1) was compared at T2 and at T3 with Chi square. Between-group comparisons on expectancy-credibility and satisfaction were analyzed with t-tests. The paired t-test was used to assess within-group improvements on all outcomes for each group at T2 and T3 as compared to T1 scores on each variable. Within-group effect sizes were assessed by the standardized response mean (SRM), which is the difference between means (T1 to T2; or T1 to T3) divided by the SD of changes scores. Analyses were performed using SAS version 9.3 (SAS Institute Inc, Cary, NC).
RESULTS
Enrollment and Attrition
As shown in Figure 1, 224 consecutive BCS and CRCS were screened for eligibility, of which 79 were found ineligible and excluded. Of the remaining 145 eligible survivors, 71 (49%) agreed to participate and were enrolled in the study and randomized. Overall, retention exceeded 97% at T2 and 94% at T3 in both groups.
Fig. 1.
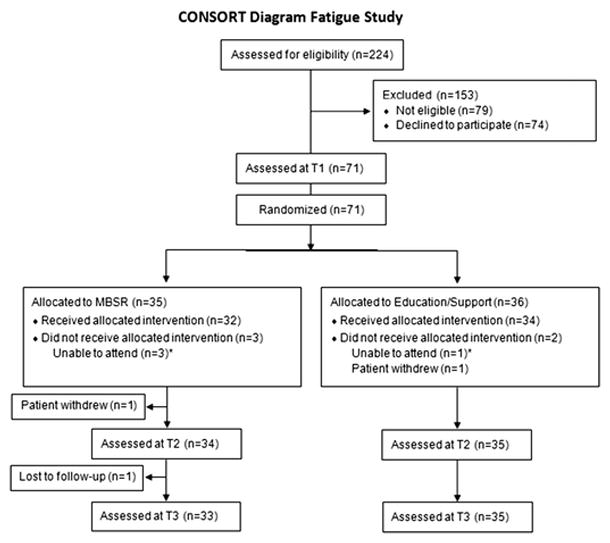
CONSORT diagram for trial accrual, intervention delivery, and data collection
Baseline Participant Characteristics
As summarized in Table 3, the sample was predominantly female (90.1%) and white (70.4%), with less than a college degree (56.3%). Approximately half were employed (52.1%), endorsed having a comfortable income (52.1%), and were married/partnered (54.9%). All had received chemotherapy and/or radiation therapy, and the average time since completion of these treatments was approximately 2.4 years. Many BCS (46%) were taking endocrine therapy at enrollment. With the exception of income (p = 0.07), there were no statistically significant group differences in baseline characteristics.
Table 3.
Demographic and Medical Characteristics
| MBSR n=35 |
Education/Support n=36 |
p-value | |
|---|---|---|---|
| Demographic Characteristics | |||
| Age, mean (SD) | 56.9 (9.9) | 56.4 (12.7) | 0.85 |
| Female, % | 94.3 | 86.1 | 0.43 |
| White, % | 77.1 | 63.9 | 0.22 |
| Married/Partnered, % | 62.9 | 47.2 | 0.19 |
| College degree, % | 42.9 | 44.4 | 0.89 |
| Employed, % | 51.4 | 52.8 | 0.91 |
| Comfortable income, % | 62.9 | 41.7 | 0.07 |
| Medical Characteristics | |||
| Cancer type | 0.35 | ||
| Breast cancer, % | 51.7 | 48.3 | |
| Colorectal cancer, % | 36.4 | 63.6 | |
| Cancer stage at diagnosis, % | 0.75 | ||
| 0 | 12.8 | 5.3 | |
| I | 41.0 | 36.8 | |
| II | 20.5 | 23.7 | |
| III | 20.5 | 29.0 | |
| Years since cancer treatment completion, mean (SD) | 2.2 (1.4) | 2.5 (1.6) | 0.48 |
| Chemotherapy, % | 65.7 | 80.6 | 0.16 |
| Radiation, % | 80.0 | 75.0 | 0.61 |
| Chemo-radiation, % | 45.7 | 55.6 | 0.41 |
| Current endocrine therapy, % | 46.0 | 46.0 | 1.00 |
| Co-morbid medical conditions in addition to cancer, mean (SD) | 1.80 (1.5 ) | 1.7(1.2) | 0.75 |
| Current Mental Health Treatment, % | 17.1 | 22.2 | 0.59 |
| Past Mental Health Treatment, % | 25.7 | 41.7 | 0.16 |
Note. MBSR=Mindfulness-Based Stress Reduction. SD=standard deviation.
Between-Group Intervention Effects
Primary Outcome
CRF interference did not significantly differ between groups at T2 or T3 (Table 4). However, there was a non-significant trend favoring MBSR (d=-0.46, p=0.073) at T2. The PES group experienced accumulating benefits over time, with a mean CRF interference score similar to the MBSR group at T3.
Table 4.
Within-group and Between-group Fatigue Outcomes
| Fatigue Outcome | Within-Group Effects | Between-Group Effects | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| MBSR (n=35)1 | Education/Support (n=36)2 | |||||||||
|
| ||||||||||
| Mean | (SD) | SRM | (95% CI) | Mean | (SD) | SRM | (95% CI) | d | (95% CI) | |
| FSI Fatigue Interference | ||||||||||
| Baseline | 4.91 | (2.17) | 5.06 | (1.50) | ||||||
| Post-intervention | 2.63 | (1.97) | −1.07*** | (−1.42, −0.73) | 3.32 | (1.83) | −0.87*** | (−1.22, −0.53) | −0.46 | (−0.93, 0.01) |
| 6-Months Post | 3.16 | (2.31) | −0.76*** | (−1.11, −0.40) | 2.93 | (2.39) | −0.86*** | (−1.20, −0.52) | 0.21 | (−0.28, 0.69) |
|
| ||||||||||
| FSI Fatigue Severity | ||||||||||
| Baseline | 5.24 | (1.57) | 5.48 | (1.30) | ||||||
| Post-intervention | 3.79 | (1.81) | −0.71*** | (−1.06, −0.37) | 4.11 | (1.57) | −0.72*** | (−1.06, −0.37) | −0.14 | (−0.65, 0.38) |
| 6-Months Post | 4.17 | (2.26) | −0.51** | (−0.87, −0.16) | 3.78 | (1.84) | −0.81*** | (−1.16, −0.47) | 0.30 | (−0.19, 0.80) |
|
| ||||||||||
| SF-36 Vitality | ||||||||||
| Baseline | 30.54 | (15.81) | 33.33 | (13.77) | ||||||
| Post-intervention | 52.21 | (17.60) | 1.11*** | (0.77, 1.46) | 43.57 | (17.25) | 0.74*** | (0.40, 1.08) | 0.53** | (0.12, 0.94) |
| 6-Months Post | 49.05 | (21.32) | 0.98*** | (0.62, 1.33) | 46.79 | (19.79) | 0.91*** | (0.57, 1.26) | 0.27 | (−0.17, 0.71) |
Note. For within-group and between-group comparisons, post-intervention was compared to baseline and 6-months post was compared to baseline for each variable. Abbreviations: FSI=Fatigue Symptom Inventory. SD=standard deviation. SRM=standardized response mean. CI=confidence interval. d=Cohen’s d effect size. Significant p-values are designated as follows:
p < .05,
p<.01,
p<.001
Secondary Outcomes
As shown in Table 4, the MBSR group demonstrated a moderate and significant effect size in vitality at T2 compared to the PES group (d=0.53, p=0.003). Although the MBSR group maintained their improvement in vitality at T3, the between-group difference was no longer significant (d=0.27, p=0.136) because of continued improvement in the PES group. On the CRF global improvement item, MBSR participants were significantly more likely than PES participants [58.8% vs. 34.3%, respectively; χ2 (1)=4.176, p=0.041] to report their CRF as being moderately to completely better at T2. Groups reported similar levels of global improvement in CRF at T3, with approximately half of each group [MBSR 45.5% vs. PES 54.3%; χ2 (1)=0.530, p=0.467] reporting their fatigue as moderately to completely better. As shown in Table 5, the MBSR group reported moderate and significant reduction at the end of the intervention in pain compared to PES participants (d=−0.50, p= 0.014). There were no significant between-group differences on any other secondary outcomes at any time point (Table 5).
Table 5.
Within-group and Between-group Secondary Symptom Outcomes
| Fatigue Outcome | Within-Group Effects | Between-Group Effects | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| MBSR (n = 35)1 | Education/Support (n = 36)2 | |||||||||
|
| ||||||||||
| Mean | (SD) | SRM | (95% CI) | Mean | (SD) | SRM | (95% CI) | d | (95% CI) | |
| Depression PHQ-8 | ||||||||||
| Baseline | 11.35 | (5.57) | 12.53 | (4.90) | ||||||
| Post-intervention | 6.27 | (3.90) | −1.05*** | (−1.40, −0.70) | 7.80 | (4.67) | −0.94*** | (−1.28, −0.59) | −0.27 | (−0.72, 0.18) |
| 6-Months Post | 6.55 | (4.67) | −0.98*** | (−1.33, −0.62) | 8.51 | (6.08) | −0.66*** | (−1.01, −0.32) | −0.16 | (−0.64, 0.31) |
|
| ||||||||||
| Anxiety GAD-7 | ||||||||||
| Baseline | 7.47 | (5.50) | 8.57 | (5.31) | ||||||
| Post-intervention | 3.21 | (3.76) | −0.89*** | (−1.24, −0.55) | 5.28 | (4.27) | −0.85*** | (−1.20, −0.51) | −0.33 | (−0.73, 0.08) |
| 6-Months Post | 3.76 | (5.14) | −0.74*** | (−1.10, −0.39) | 5.69 | (6.11) | −0.63*** | (−0.97, −0.28) | −0.07 | (−0.47, 0.34) |
|
| ||||||||||
| Sleep Disturbance ISI | ||||||||||
| Baseline | 15.34 | (6.45) | 17.33 | (6.27) | ||||||
| Post-intervention | 10.82 | (5.74) | −0.93*** | −1.27, −0.58) | 12.31 | (5.04) | −1.16*** | (−1.50, −0.81) | −0.10 | (−0.44, 0.25) |
| 6-Months Post | 9.45 | (6.01) | −0.94*** | (−1.29, −0.58) | 12.10 | (6.84) | −0.83*** | (−1.18, −0.49) | −0.20 | (−0.65, 0.26) |
|
| ||||||||||
| Pain PEG | ||||||||||
| Baseline | 3.95 | (3.09) | 3.43 | (2.80) | ||||||
| Post-intervention | 2.10 | (2.07) | −0.77*** | (−1.12, −0.42) | 2.73 | (2.44) | −0.27 | (−0.62, 0.07) | −0.50* | (−0.90, −0.10) |
| 6-Months Post | 2.56 | (3.00) | −0.53** | (−0.88, −0.17) | 2.37 | (2.68) | −0.38* | (−0.73, −0.04) | 0.08 | (−0.36, 0.51) |
Note. For within-group and between-group comparisons, post-intervention was compared to baseline and 6-months post was compared to baseline for each variable. Abbreviations: PHQ-8 = 8-item Patient Health Questionnaire depression scale (range, 0–24). GAD-7 = 7-item Generalized Anxiety Disorder anxiety scale (range, 0–21). ISI = Insomnia Severity Index (range, 0–28). PEG = 3-item abbreviated version of Brief Pain Inventory (range 0–10). SD=standard deviation. SRM= standardized response mean. CI=confidence interval. d=Cohen’s d effect size. Significant p-values are designated as follows:
p<.05,
p<.01,
p<.001
Both groups reported high expectations that their assigned intervention would be helpful and that the intervention was credible on the 0–9 expectancy-credibility scale [MBSR mean (SD)=7.6 (1.5); PES mean (SD)=7.2 (1.5); p=0.255]. Likewise, satisfaction was rated similarly high across groups, with the MBSR group reporting a mean (SD) of 8.7 (1.9) compared to 8.4 (2.1) in the PES group (p=0.54) on the 0–10 satisfaction scale.
Within-Group Intervention Effects
Moderate-to-large and significant within-group improvements on all fatigue outcomes were found at T2 and T3 compared to T1 in both MBSR and PES groups (Table 4). Participants in both interventions also experienced moderate-to-large and significant improvements at both time points on depression, anxiety, and sleep disturbance. Effects on pain were moderate and significant for the MBSR group at both time points and significant for the PES group only at T3 (Table 5).
Attendance and Home Practice
Attendance was similar between groups. MBSR participants attended an average of 5.8 (SD=2.1) sessions compared to 6.3 (SD=1.9) for PES participants [t(68)=0.96, p=0.30]. Participants in both groups also reported a similar amount of time completing home practice assignments each week. MBSR participants reported an average of 117.6 (SD=85.9) minutes per week of home practice compared to 92.5 (SD=92.1) minutes per week for PES participants [t(69)=1.19 p=0.2398)]. At 6-month follow-up, 75.8% of MBSR participants reported continued “formal” mindfulness practice; however, only 36.4% reported frequent practice (≥3 days/week). Almost all MBSR participants (84.8%) reported continued “informal” mindfulness practice after completing the MBSR course.
DISCUSSION
This pilot trial revealed several important findings regarding MBSR compared to PES for CRF. First, there was a trend favoring MBSR on fatigue interference, and a clear advantage favoring MBSR in vitality and global improvement in fatigue immediately post intervention. However, these between group differences were not evident at 6 months follow-up as PES participants experienced ongoing improvements in fatigue. Second, both MBSR and PES participants experienced significant, moderate-to-large, within-group improvements in fatigue and its correlates that were maintained 6 months post intervention. As there were no statistically significant differences in baseline scores on fatigue or its correlates, these findings suggest that both interventions are efficacious in alleviating fatigue, although MBSR might yield results sooner than PES. Lastly, both interventions proved feasible and acceptable as evidenced by high rates of retention, attendance, satisfaction, and adherence to home practice, indicating that cancer survivors are open to participating in weekly 2-hour classes and daily home practice despite their persistent fatigue. The results of this pilot trial are rather promising as they demonstrate two potentially acceptable and efficacious interventions for ameliorating fatigue.
The significant between-group differences in vitality and fatigue global improvement at T2 combined with the significant within-group fatigue improvements for MBSR at T2 and T3 support its use as a potential treatment for CRF. These results are consistent with those of a recent randomized controlled trial testing an 8-week mindfulness-based intervention in which the mindfulness group reported significant improvements in fatigue outcomes compared to participants in the wait-list control group post-intervention, and these improvements were maintained for the mindfulness group through the 6-month follow-up [43]. Although our study lacked a usual care (UC) group for comparison, results of extant studies strongly suggest that the persistent fatigue that defined our sample was unlikely to have remitted spontaneously [11,43]. These findings suggest that mindfulness may be efficacious in improving vitality and patients’ perceptions of fatigue.
The purpose of this pilot trail was to provide a rigorous test of MBSR, yet surprisingly this study also strengthened support for the use of PES interventions for CRF. In developing the PES intervention, we intended to provide a credible treatment that did not include mindfulness training and controlled for nonspecific factors (e.g., amount of social contact and attention from an empathic clinician) [22]. Although we expected psychoeducation to be helpful, we were surprised by the magnitude and durability of the within-group effect sizes of the PES intervention. The mechanisms by which PES interventions lead to reductions in fatigue and associated symptoms are unclear, but there are at least two possible explanations. First, the sharing of experiences and interacting with other cancer survivors within group sessions may have contributed to patients’ perceptions of social support and validation, a factor critical to helping patients adjust during survivorship [44]. In addition, our intervention may have fostered patients’ sense of self-efficacy, or their beliefs in their abilities to cope. By providing information about fatigue and potential strategies to manage fatigue, patients may have felt better equipped to deal with fatigue and other lingering side effects of cancer treatment [45]. Additional studies are needed to elucidate the mechanisms of PES interventions and their effects on CRF.
This pilot study had several strengths. Care was taken to match the PES intervention to MBSR in facilitator skill, class duration, and home practice expectations, which allowed for a direct comparison of the effects of MBSR relative to an active comparator. The trial also included a 6-month follow-up, which has been missing in most trials of integrative CRF treatments. A strength related to generalizability is the demographic heterogeneity of the sample.
This study also had limitations. There was a lack of power for efficacy testing. However, this pilot study was designed to estimate effect sizes for future work, which was accomplished. A larger trial is warranted that includes a UC arm to assess differential efficacy between MBSR and PES interventions compared to UC. Another limitation was the heterogeneity regarding cancer stage and time since completion of cancer treatment, as these variables could potentially contribute to differential responses to MBSR or PES. Yet, these characteristics were balanced between groups, and thus any potential effects likely were negligible. Although gender, too, was balanced across groups, the relatively low percentage of male participants may limit the generalizability of the results. Finally, MBSR’s quicker impact might have been due in part to the doctoral level training of the facilitators in that arm, as opposed to the master’s level facilitators in the PES arm. However, the fidelity checks were uniformly high, and in fact a little higher in the PES arm.
CONCLUSIONS
Our trial provides promising support for MBSR as well as PES for the treatment of CRF. This is especially important given the current lack of evidenced-based pharmacological therapies for CRF. Phase 3 trials comparing these potentially efficacious behavioral treatments compared to UC are warranted. When more than one efficacious behavioral treatment exists, patient preferences should play a central role.
Acknowledgments
This project was funded, in part, by the Walther Cancer Foundation (0106-01) and the Indiana Clinical and Translational Sciences Institute (#TR000163 and #TR000006) from the National Institutes of Health, National Center for Advancing Translational Sciences. Mentoring support was provided by Dr. Victoria Champion through the National Cancer Institute of the National Institutes of Health (#K05CA175048). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors have no financial disclosures.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Barsevick AM, Irwin MR, Hinds P, Miller A, Berger AM, Jacobsen P, Ancoli-Israel S, Reeve BB, Mustian K, O’Mara A, Lai J-S, Fisch M, Cella D. Recommendations for High-Priority Research on Cancer-Related Fatigue in Children and Adults. Journal of the National Cancer Institute. 2013;105(19):1432–1440. doi: 10.1093/jnci/djt242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroenke K, Zhong X, Theobald D, Wu J, Tu W, Carpenter JS. Somatic Symptoms in Patients With Cancer Experiencing Pain or Depression: Prevalence, Disability, and Health Care Use. Arch Intern Med. 2010;170(18):1686–1694. doi: 10.1001/archinternmed.2010.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in long-term breast carcinoma survivors. Cancer. 2006;106(4):751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 4.Schneider EC, Malin JL, Kahn KL, Ko CY, Adams J, Epstein AM. Surviving colorectal cancer : patient-reported symptoms 4 years after diagnosis. Cancer. 2007;110(9):2075–2082. doi: 10.1002/cncr.23021. [DOI] [PubMed] [Google Scholar]
- 5.Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nature reviews Clinical oncology. 2014;11(10):597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, Johnson DH, Miaskowski C, Scherr SL, Portenoy RK, Vogelzang NJ. Impact of cancer-related fatigue on the lives of patients: New findings from the Fatigue Coalition. The Oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 7.Groenvold M, Petersen MA, Idler E, Bjorner JB, Fayers PM, Mouridsen HT. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Research and Treatment. 2007;105:209–219. doi: 10.1007/s10549-006-9447-x. [DOI] [PubMed] [Google Scholar]
- 8.Blaney LM, Lowe-Strong A, Rankin-Watt J, Campbell A, Gracey JH. Cancer survivors’ exercise barriers, facilitators and preferences in the context of fatigue, quality of life and physical activity participation: A questionnaire-survey. Psycho-Oncology. 2011 doi: 10.1002/pon.2072. [DOI] [PubMed] [Google Scholar]
- 9.Stone P, Richardson A, Ream E, Smith AG, Kerr DJ, Kearney N. Cancer-related fatigue: inevitable, unimportant and untreatable? Results of a multi-centre patient survey. Cancer Fatigue Forum. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2000;11(8):971–975. doi: 10.1023/a:1008318932641. [DOI] [PubMed] [Google Scholar]
- 10.Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carepenter KM, Cella D, Cleeland CS, Dotan E, Eisenberger MA, Escalante CP, Jacobsen PB, Jankowski C, LeBlanc T, Ligibel JA, Loggers ET, Mandrell B, Murphy BA, Palesh O, Pirl WF, Plaxe SC, Riba MB, Rugo HS, Salvador C, Wagner LI, Wagner-Johnston ND, Zachariah FJ. NCCN Clinical Practice Guidelines in Oncology: Cancer-related Fatigue Version 2.2015. [Accessed January 15, 2015];National Comprehensive Cancer Network. 2015 doi: 10.6004/jnccn.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johns SA, Brown LF, Beck-Coon K, Monahan PO, Tong Y, Kroenke K. Randomized controlled pilot study of mindfulness-based stress reduction for persistently fatigued cancer survivors. Psycho-oncology. 2015;24(8):885–893. doi: 10.1002/pon.3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lengacher CA, Johnson-Mallard V, Post-White J, Moscoso MS, Jacobsen PB, Klein TW, Widen RH, Fitzgerald SG, Shelton MM, Barta M, Goodman M, Cox CE, Kip KE. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psycho-oncology. 2009;18(12):1261–1272. doi: 10.1002/pon.1529. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman CJ, Ersser SJ, Hopkinson JB, Nicholls PG, Harrington JE, Thomas PW. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(12):1335–1342. doi: 10.1200/JCO.2010.34.0331. [DOI] [PubMed] [Google Scholar]
- 14.Piet J, Wurtzen H, Zachariae R. The effect of mindfulness-based therapy on symptoms of anxiety and depression in adult cancer patients and survivors: a systematic review and meta-analysis. Journal of consulting and clinical psychology. 2012;80(6):1007–1020. doi: 10.1037/a0028329. [DOI] [PubMed] [Google Scholar]
- 15.Zainal NZ, Booth S, Huppert FA. The efficacy of mindfulness-based stress reduction on mental health of breast cancer patients: a meta-analysis. Psychooncology. 2013;22(7):1457–1465. doi: 10.1002/pon.3171. [DOI] [PubMed] [Google Scholar]
- 16.Zhang MF, Wen YS, Liu WY, Peng LF, Wu XD, Liu QW. Effectiveness of Mindfulness-based Therapy for Reducing Anxiety and Depression in Patients With Cancer: A Meta-analysis. Medicine. 2015;94(45):e0897–0890. doi: 10.1097/MD.0000000000000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shennan C, Payne S, Fenlon D. What is the evidence for the use of mindfulness-based interventions in cancer care? A review. Psychooncology. 2011;20(7):681–697. doi: 10.1002/pon.1819. [DOI] [PubMed] [Google Scholar]
- 18.Ledesma D, Kumano H. Mindfulness-based stress reduction and cancer: a meta-analysis. Psychooncology. 2009;18(6):571–579. doi: 10.1002/pon.1400. [DOI] [PubMed] [Google Scholar]
- 19.Simpson R, Booth J, Lawrence M, Byrne S, Mair F, Mercer S. Mindfulness based interventions in multiple sclerosis--a systematic review. BMC neurology. 2014;14:15. doi: 10.1186/1471-2377-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawrence M, Booth J, Mercer S, Crawford E. A systematic review of the benefits of mindfulness-based interventions following transient ischemic attack and stroke. International journal of stroke : official journal of the International Stroke Society. 2013;8(6):465–474. doi: 10.1111/ijs.12135. [DOI] [PubMed] [Google Scholar]
- 21.Sood A, Barton DL, Bauer BA, Loprinzi CL. A critical review of complementary therapies for cancer-related fatigue. Integrative cancer therapies. 2007;6(1):8–13. doi: 10.1177/1534735406298143. [DOI] [PubMed] [Google Scholar]
- 22.Mohr DC, Spring B, Freedland KE, Beckner V, Arean P, Hollon SD, Ockene J, Kaplan R. The selection and design of control conditions for randomized controlled trials of psychological interventions. Psychotherapy and Psychosomatics. 2009;78:275–284. doi: 10.1159/000228248. [DOI] [PubMed] [Google Scholar]
- 23.Reif K, de Vries U, Petermann F, Gorres S. A patient education program is effective in reducing cancer-related fatigue: a multi-centre randomised two-group waiting-list controlled intervention trial. European journal of oncology nursing : the official journal of European Oncology Nursing Society. 2013;17(2):204–213. doi: 10.1016/j.ejon.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Dolbeault S, Cayrou S, Bredart A, Viala AL, Desclaux B, Saltel P, Gauvain-Piquard A, Hardy P, Dickes P. The effectiveness of a psycho-educational group after early-stage breast cancer treatment: results of a randomized French study. Psychooncology. 2009;18(6):647–656. doi: 10.1002/pon.1440. [DOI] [PubMed] [Google Scholar]
- 25.Boesen EH, Ross L, Frederiksen K, Thomsen BL, Dahlstrom K, Schmidt G, Naested J, Krag C, Johansen C. Psychoeducational intervention for patients with cutaneous malignant melanoma: a replication study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(6):1270–1277. doi: 10.1200/JCO.2005.05.193. [DOI] [PubMed] [Google Scholar]
- 26.Donovan KA, Jacobsen PB, Small BJ, Munster PN, Andrykowski MA. Identifying clinically meaningful fatigue with the Fatigue Symptom Inventory. Journal of Pain and Symptom Management. 2008;36(5):480–487. doi: 10.1016/j.jpainsymman.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL, Williams JBW. The Patient Health Questionnaire-9: Validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits: A meta-analysis. Journal of Psychosomatic Research. 2004;57:35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- 29.Lengacher CA, Reich R, Post-White J, Moscoso M, Shelton M, Barta M, Le N, Budhrani P. Mindfulness based stress reduction in post-treatment breast cancer patients: an examination of symptoms and symptom clusters. Journal of Behavioral Medicine. 2012;35(1):86–94. doi: 10.1007/s10865-011-9346-4. [DOI] [PubMed] [Google Scholar]
- 30.Santorelli S, Kabat-Zinn J. Mindfulness-based stress reduction professional education and training resource manual: MBSR standards of practice, curriculum, and supporting materials. Center for Mindfulness in Medicine, Health Care, and Society, University of Massachusetts Medical School; Worcester, MA: 2011. [Google Scholar]
- 31.Lukens EP, McFarlane WR. Psychoeducation as evidence-based practice: Considerations for practice, research, and policy. Brief Treatment and Crisis Intervention. 2004;4(3):205–225. [Google Scholar]
- 32.Silver JK. After Cancer Treatment: Heal Faster, Better, Stronger. John Hopkins University Press; Baltimore, MD: 2006. [Google Scholar]
- 33.Barsevick AM, Cleeland CS, Manning DC, O’Mara AM, Reeve BB, Scott JA, Sloan JA, Ascpro ASCPRO recommendations for the assessment of fatigue as an outcome in clinical trials. Journal of pain and symptom management. 2010;39(6):1086–1099. doi: 10.1016/j.jpainsymman.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohamed AF, Hauber AB, Johnson FR, Coon CD. Patient preferences and linear scoring rules for patient-reported outcomes. The patient. 2010;3(4):217–227. doi: 10.2165/11537880-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 35.Hann DM, Denniston MM, Baker F. Measurement of fatigue in cancer patients: Further validation of the Fatigue Symptom Inventory. Quality of Life Research. 2000;9:847–854. doi: 10.1023/a:1008900413113. [DOI] [PubMed] [Google Scholar]
- 36.Ware J, Snow K, Kosinski M. SF-36 healthy survey manual and interpretation guide. Quality Metric Inc; Lincoln, RI: 1993. [Google Scholar]
- 37.Kroenke K, Bair M, Damush T, Hoke S, Nicholas G, Kempf C, Huffman M, Wu J, Sutherland J. Stepped Care for Affective Disorders and Musculoskeletal Pain (SCAMP) study: design and practical implications of an intervention for comorbid pain and depression. General hospital psychiatry. 2007;29(6):506–517. doi: 10.1016/j.genhosppsych.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder. Archives of Internal Medicine. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 39.Morin CM. Insomnia: Psychological assessment and management. Guilford Press; New York: 1993. [Google Scholar]
- 40.Krebs EE, Lorenz KA, Bair MJ, Damush TM, Wu J, Sutherland JM, Asch SM, Kroenke K. Development and initial validation of the PEG, a three-itme scale assessing pain intensity and interference. Journal of General Internal Medicine. 2009;24(6):733–738. doi: 10.1007/s11606-009-0981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. Journal of Behavior Therapy and Experimental Psychiatry. 2000;31(2):73–86. doi: 10.1016/s0005-7916(00)00012-4. doi: http://dx.doi.org/10.1016/S0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 42.Senn S. Testing for baseline balance in clinical trials. Statistics in medicine. 1994;13(17):1715–1726. doi: 10.1002/sim.4780131703. [DOI] [PubMed] [Google Scholar]
- 43.van der Lee ML, Garssen B. Mindfulness-based cognitive therapy reduces chronic cancer-related fatigue: a treatment study. Psycho-Oncology. 2012;21(3):264–272. doi: 10.1002/pon.1890. [DOI] [PubMed] [Google Scholar]
- 44.Knobf MT. Clinical update: psychosocial responses in breast cancer survivors. Semin Oncol Nurs. 2011;27(3):e1–e14. doi: 10.1016/j.soncn.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Scheier MF, Helgeson VS, Schulz R, Colvin S, Berga S, Bridges MW, Knapp J, Gerszten K, Pappert WS. Interventions to enhance physical and psychological functioning among younger women who are ending nonhormonal adjuvant treatment for early-stage breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(19):4298–4311. doi: 10.1200/JCO.2005.05.362. [DOI] [PubMed] [Google Scholar]


