Abstract
Objective
Maternal stress in humans influences behavior of children and can be assessed using biological markers. Mothers and their one-month-old infants were recruited from an existing study to examine baseline maternal serum oxytocin and hypothalamic-pituitary-adrenal (HPA) axis response to infant blood heel stick stress as measured by salivary cortisol in the dyads. Objectives were to explore (1) relationships between mother and infant cortisol levels, (2) gender differences in infant biologic cortisol response, and (3) the association of cortisol levels in the dyads and maternal oxytocin levels.
Methods
Forty-two mother-infant dyads provided biologic samples and self-report data. Initial salivary cortisol was assessed in both the mother and infant, followed by a heel stick blood draw. Twenty minutes later, salivary cortisol was collected again from dyads.
Results
Self-report measures were negative for depression and risk for childhood neglect. Although oxytocin and baseline cortisol in the infants was higher in mothers that did some breast feeding, there was no statistically significant difference (p=0.2; p=0.1, respectively). Analyses showed (a) higher baseline cortisol in mothers was related to higher baseline cortisol in infants (p<0.0001), (b) following the stressor, female infants had a larger positive change in cortisol, after adjusting for baseline cortisol (p=0.045), and (c) there was no relationship between dyad cortisol levels and maternal oxytocin.
Conclusions
Maternal and infant biologic stress measures are related. Female infants have a larger HPA response to a blood draw stressor as measured by salivary cortisol than male infants.
Keywords: Oxytocin, Cortisol, HPA Axis, Gender, Infants, Parent-Child Relations, Stress, Psychological
INTRODUCTION
The parent-infant bond provides a foundation for future adaptation, relationships, and mental health for children and adults. Early life experiences are known to shape neurologic, psychosocial, and physical health [1]. Brain development is especially receptive to nurturing care and environmental stimulation during critical early periods of development. Animal research models have shown that the in utero and infancy environment as well as parental behavior can influence the responsiveness of the hypothalamic-pituitary-adrenal (HPA) axis which has been shown to have epigenetic effects [2-4]. An inverse relationship between oxytocin and cortisol in breastfeeding non-depressed mothers has been found [5]. Maternal well-being impacts parenting behavior, which in turn, influences the psychosocial environment of the infant, as well as neurobiologic responses to stress, neurologic pruning, and thus brain development [6]. An improved understanding of the relationship of infant-maternal-biological systems to normal and abnormal stress may help us better understand how maternal behavioral responses influence future child behavior [7].
The effects of cortisol are adaptive because they prepare an individual to meet the energy demands associated with a stressful event, including preparedness and vigilance to threats [8]. Research has shown the higher the quality of maternal responsiveness the faster the cortisol recovery to a bathing stressor [9]. Genetic as well as environmental factors are known to influence an individual's response to stress. Although there is limited research on human dyads, animal studies have shown maternal levels of cortisol in breast milk influence temperament and eating behavior of infants. Meaney et al. have demonstrated that nurturing maternal care of rat pups has led to a lower HPA stress response [10-12]. In a study of 108 rhesus monkeys, mothers who had high levels of cortisol in their breast milk had more fearful, nervous offspring that put on weight faster than the offspring who drank breast milk with lower cortisol levels [13].
While some stress is healthy, chronic stress can dysregulate the normal physiological HPA-axis [14-16]. This dysregulation of the HPA-axis predisposes an individual to psychiatric vulnerability and physical illnesses later in life [17, 18]. Studies have connected this dysregulation to depression, post-traumatic stress disorder, panic disorder, generalized anxiety, and other stress-related bodily complaints [14, 15, 19, 20]. Chronic HPA activation can contribute to medical pathogeneses, including neurodegeneration of the hippocampus, development of cognitive, affective, and medical disorders, cardiovascular disease, immune deficiency, and/or autoimmune disorders [14].
Previous studies have shown in utero and maternal cortisol level to predict infant cortisol response to a stressor. One study found that amniotic fluid cortisol predicted infant cortisol response to mother/infant separation, [21] independent of prenatal, obstetric, socioeconomic factors, and child-parent attachment. Improved sensitivity of mothers to their infants, as measured by the Ainsworth sensitivity scale, led to a smaller infant salivary cortisol response to a diaper change stressor [22]. In addition, secure attachments appear to buffer cortisol elevations [23] while a more anxious and ambivalent maternal attachment style [24] is associated with higher cortisol levels [25]. There is some evidence that the presence of oxytocin which is involved in breast feeding, the birthing process and bonding may help to buffer elevations in cortisol [31]
Although, most infant neuroendocrinology research has not focused on gender differences, previous research supports that sex differences in stress response exist. However, the effect has varied across studies as a variety of stressors have been tested. Gender differences in HPA axis functioning may be present as early as in utero [14, 26]. Gender differences in neurobiology responsiveness to childhood maltreatment have been found as early as infancy [27]. Davis and Emory found that male infants had significantly higher salivary cortisol levels and a higher mean change in cortisol levels following a stressor [28]. In another study, a different stressor of parental still-face versus normal interactive response [29] showed greater overall increase in cortisol levels for the still-face task in 6 month olds females from baseline than males [30].
Some research indicates that oxytocin can potentially buffer the human stress response. The primary role of maternal oxytocin is to assist the birth process and breastfeeding. It is also released in response to positive social interactions [31] promoting social cognition and empathy [32], and plays a role in mediating social attachment, maternal behavior, and provides protection against stress and anxiety [33, 34] by attenuating the endocrine and autonomic responses[32]. Although it is not known how much oxytocin release is modified by bonding or breast feeding in mothers with new infants, breastfeeding mothers have been shown to have higher plasma oxytocin than formula-feeding [35] mothers. In humans, lactation appears to reduce maternal responses to physical and psychosocial stress exposure [36]. Thus post-breastfeeding mothers have reduced levels of cortisol most likely influenced by increased oxytocin [37-40].
It was hypothesized that (1) mother and one month old infants would have similar rates of cortisol increase in response to an infant heel stick blood draw stressor, (2) gender differences would not be evident, (3) mothers who breastfed all or some of the time would have higher levels of oxytocin and lower levels of cortisol than mothers who exclusively bottle-fed, and (4) and infants who were breastfed would have lower cortisol levels than infants who were exclusively bottle-fed.
METHODS
Obtaining Samples
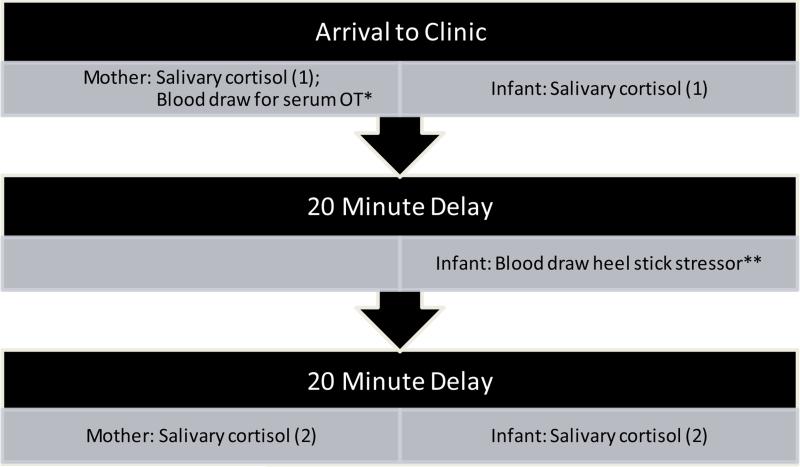
Mothers and one month old infants were recruited from morning participants for an existing study on vitamin D metabolism. Informed consent was obtained and dyad enrolled. All procedures completed in the South Carolina Clinical and Translational Research Center (SCTR) at the Medical University of South Carolina (MUSC). Baseline data were collected regarding breastfeeding or bottle-feeding practices, behavior and mood, and the mothers’ baseline serum oxytocin , and the mothers’ and infants’ salivary cortisol samples were collected. Some mothers were partially or exclusively breastfeeding and some were infants were exclusively bottle-fed. All mothers completed an Edinburgh Postnatal Depression Scale (EPDS) [41] and a child neglect SEEK screen [42]. At the baseline visit, mothers and infants provided samples of saliva for cortisol analysis. At the 20 minute mark, the heel stick procedure was completed, and approximately 15 mL of blood was collected from the mother and 4 mL of blood was collected from the infant. Mother and infant provided a second sample of saliva to measure cortisol levels 20 minutes after the heel stick stressor (See Figure 1). Serum oxytocin was analyzed at the Emory Lab, GA, and salivary cortisol was analyzed at the General Clinical Research Center (GCRC) Lab, MUSC.
Figure 1.
Study Procedure of Biologic Measures.
*Serum oxytocin was analyzed in mothers only.
**Infant heel stick blood sample was utilized for the vitamin D metabolism study
Statistical Methods
Simple descriptive statistics including median and interquartile range (IQR), were used to describe the outcomes of interest. Medians and IQRs were reported, as opposed to means and standard deviations, due to the relatively small sample size (n=42) and skewed distributions.
The primary biologic outcomes of interest were (1) infant cortisol level measured at baseline and (2) the change from baseline in infant cortisol level 20 minutes post-heel stick blood draw stressor. Several variables including gender, maternal oxytocin, feeding technique (partial or exclusive breast vs. exclusive bottle), and whether mom was present at the time of the heel stick were evaluated in models to examine the relationship with the biologic outcome measures.
Generalized linear models were used to evaluate the relationships between the outcomes and other variables of interest (feeding technique, gender, and information gathered from the moms). Univariate models explored the relationships between each of these variables and infant cortisol at baseline. For change in infant cortisol from baseline, models were adjusted for baseline infant cortisol level. For categorical variables such as gender and feeding technique, the mean and standard error for each outcome were reported between groups. For models adjusting for baseline cortisol level, the means were the adjusted means. For continuous variables, Pearson correlation coefficients were reported. Wilcoxon rank-sum tests were used to compare mother and infant variables between bottle-feeding only moms to breast-feeding moms.
RESULTS
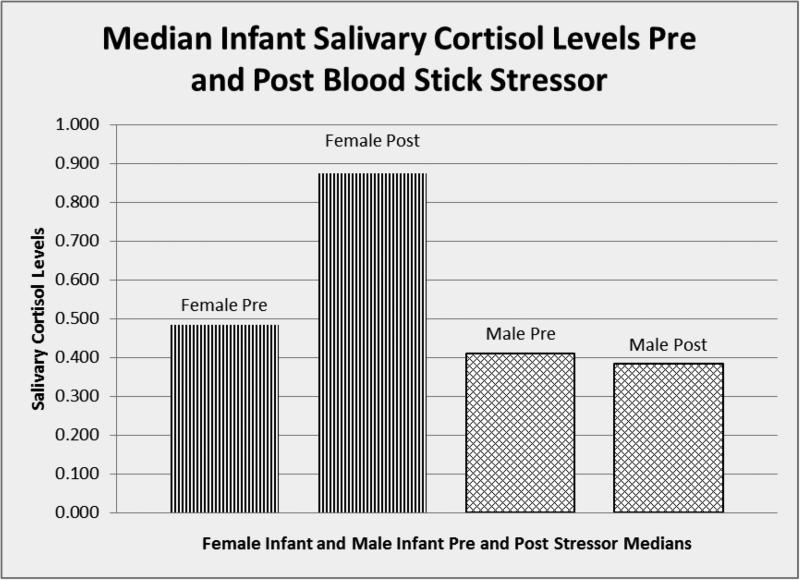
Table 1 displays the demographics for both the moms and the infants in the study. The median age for mothers was 26.6 years and the median age for infants was 34.5 days. No mothers included in this study had clinically significant depression by the EPDS, and no children were at risk for neglect per the SEEK screening questionnaire.. Table 2 describes the results of the univariate models. A statistically significant positive relationship between mom's baseline cortisol measure and infant's baseline cortisol measure was found (rho=0.68, p<0.0001). The relationship between infant's gender and the change in infant cortisol level was moderately significant. Females had a significant positive change in cortisol, after adjusting for the baseline cortisol value (adjusted difference = 0.55ug/dL, p=0.045) (See Figure 2). Fifty-five percent of the infants’ mothers were present at the time of the heel stick, and 45% chose to step out of the room. Presence or absence of mom at blood draw was not statistically significant in terms of change in cortisol levels in the infants (p=0.47).
Table 1.
Demographics
| Moms (n=42) | Infants (n=42) | |||
|---|---|---|---|---|
| Median | IQR | median | IQR | |
| Age* | 26.62 | (22.1, 31.4) | 34.50 | (31.5, 40.0) |
| Visit 1a Cortisol | 0.21 | (0.12, 0.39) | 0.45 | (0.22, 0.95) |
| Visit 1b cortisol | 0.19 | (0.12, 0.35) | 0.50 | (0.29, 1.12) |
| Change (1b-1a) | −0.02 | (−0.09, 0.08) | 0.05 | (−0.24, 0.49) |
| Oxytocin | 248.73 | (199.7, 380.6) | ||
| N | % | N | % | ||
|---|---|---|---|---|---|
| Gender | Female | 15 | 37.5 | ||
| Male | 25 | 62.5 | |||
| Race | White | 14 | 33 .3 | 9 | 23.7 |
| Black | 10 | 23.8 | 11 | 28.9 | |
| Hispanic | 18 | 42.9 | 18 | 47.4 | |
| Feeding Type | Breast | 24 | 58.5 | 24 | 58.5 |
| Bottle | 17 | 41.5 | 17 | 41.5 | |
| Mom present for blood draw | Yes | 21 | 55.3 | 21 | 55.3 |
| No | 17 | 44.7 | 17 | 44.7 |
years for moms; days for infants
Table 2.
Infant Cortisol at Baseline and Change in Infant Cortisol Post-Stressor
| Outcome | Variable | Statistica | p-value | |
|---|---|---|---|---|
| Infant cortisol at baseline | Feeding technique | Breast fed Bottle fed |
0.85(0.15) 0.51(0.17) |
0.14 |
| Presence | Mom present for blood draw Mom not present |
0.87(0.16) 0.51(0.17) |
0.13 | |
| Gender | Female Male |
0.82(0.18) 0.61(0.14) |
0.38 | |
| Mom's oxytocin | −0.12 | 0.51 | ||
| Mom's cortisol | 0.68 | <0.0001 | ||
| Change in infant cortisol* | Baseline cortisol | −0.09 | 0.61 | |
| Feeding technique | Breast fed Bottle fed |
0.33(0.19) 0.09(0.20) |
0.39 | |
| Mom present for feeding | Mom present for blood draw Mom not present |
0.32(0.20) 0.11(0.20) |
0.47 | |
| Gender | Female Male |
0.58(0.21) 0.03(0.16) |
0.045 | |
| Mom's oxytocin | −0.06 | 0.69 | ||
| Mom's cortisol | −0.02 | 0.73 | ||
| Mom's change in cortisol | −0.14 | 0.36 |
models adjusted for baseline cortisol
for categorical variables, mean(SE) is reported for groups; for continuous variables, Pearson's correlation coefficients are reported
Figure 2.
Median Salivary Cortisol Levels Pre and Post Blood Stick Stressor for Female and Male Infants
Oxytocin and baseline cortisol in the infants was slightly higher in breastfeeding mothers than bottle-feeding mothers, however, there was not a statistical significant difference (p=0.2, p=0.1, respectively) (Table 3). And although not statistically significant, it was interesting to find that infants who were exclusively bottle-fed had lower cortisol levels at baseline (median=0.40) than infants who were at least partially breastfed (median=0.51).
Table 3.
Baseline and Change from Baseline in Mother and Infant Biologic Measures, Breastfed vs. Bottlefed
| Breast-fed (n=24) | Bottle-fed (n=17) | |||||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | p-value* | ||
| Mothers | ||||||
| oxytocin | 251.76 | (191.8, 520.2) | 240.77 | (199.7, 265.7) | 0.2 | |
| cortisol at baseline | 0.22 | (0.09, 0.41) | 0.18 | (0.16, 0.31) | >0.9 | |
| change in cortisol | −0.02 | (−0.11, 0.08) | −0.01 | (−0.07, 0.07) | 0.4 | |
| Infants | cortisol at baseline | 0.51 | (0.38, 1.08) | 0.40 | (0.18, 0.86) | 0.1 |
| change in cortisol | 0.002 | (−0.38, 0.59) | 0.05 | (−0.19, 0.35) | >0.9 | |
from a Wilcoxon rank sum test
DISCUSSION
The shaping of the human brain is influenced by prenatal and childhood experiences that are influenced by neurobiology that appears as early as in utero. Previous research indicates that the human individual stress response may begin to be determined in utero. The findings of this study appear to support other research that maternal stress hormones influence infant stress hormones. An improved understanding of the neurobiologic influence of early infant-parent relationships will advance the understanding of healthy brain development. In this study, a positive relationship was found between mother and infant, as measured by a blood draw and salivary cortisol response. Although gender differences were not anticipated, female infants had a larger HPA response to the heel stick stressor than male infants.
Contrary to the hypotheses for this study, infants who were breastfed did not have lower cortisol levels than infants who were only bottle-fed. In mothers who breastfed, higher salivary cortisol levels were found compared to those who bottle-fed. These findings agree with previous research by Uvnäs-Moberg [43]. Additionally, there was no significant difference in cortisol levels between infants whose mothers were present at the time of the blood draw and those whose mothers were present at the time of the blood draw.
Although baseline cortisol levels did not differ significantly between genders, the stress response was higher in female infants than in male infants. Animal research supports some of the findings in this study, demonstrating that stressing the mother leads to increased cortisol in the offspring [44]. Emerging research has shown that in humans, females and males may respond differently to physical stressors, such as a blood stick, compared to a social stressor [15]. Also in humans, it has been observed that stressed mothers, such as those who are emotionally exhausted, working, or depressed, have children with higher cortisol levels [45].
Several limitations of the study should be noted. This research include a convenience sample taken from a study on vitamin D metabolism. The primary stressor used in the study was a heel stick blood draw. As other research has shown, variations in stressor types have demonstrated varying HPA-axis responses. The small sample size may have limited ability to see statistically significant differences in biologic measures. It is also known that individuals may reach their peak cortisol level at varying times following a stressor. Although 20 minutes was chosen given other research, having more than one post-stressor cortisol sample could help us know more about peak rate of rise as well as recovery time. Timing may be critical as biologic measures are a part of a dynamic state based on interactions and environment.
Gender differences in response to different stress paradigms and more complete phenotypic and behavioral descriptions of maternal and paternal caregivers are needed to better understand the biologic factors and care-giving relationships. Better characterizing these relationships in future studies will help clinicians develop defined interventions that will potentially improve infant-mother bonding and decrease the risk of poor mental health outcomes.
Acknowledgements
This research project was supported by Award Number UL1RR029882 from the National Center for Research Resources and from grant K23 NIH/NIMH K25MH064111 (PI: Eve G. Spratt), and the South Carolina Clinical & Translational Research Institute, Medical University of South Carolina's CTSA, NIH/NCATS Grant Number UL1TR000062. Additional resources were obtained from grant NIH/NIDA K24DA00435: Midcareer Investigator Award in Patient-Oriented Research (PI: Kathleen T. Brady). Thank you to SCTR Unit and Study personnel, MUSC's Drug Abuse Research Training (DART) Program, Dr. Eve Spratt, Courtney Marsh, Dr. Carol Wagner, Amy Wahlquist, Dr. Paul Nietert, and Dr. Kathleen Brady.
Footnotes
The authors have declared that no competing interests exist.
REFERENCES
- 1.Anda RF, Felitti VJ, Bremner JD, et al. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256(3):174–86. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter CS, Boone EM, Pournajafi-Nazarloo H, et al. Consequences of early experiences and exposure to oxytocin and vasopressin are sexually dimorphic. Dev Neurosci. 2009;31(4):332–41. doi: 10.1159/000216544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kappeler L, Meaney MJ. Epigenetics and parental effects. BioEssays. 2010;32(9):818–27. doi: 10.1002/bies.201000015. [DOI] [PubMed] [Google Scholar]
- 4.Natt D, Johansson I, Faresjo T, et al. High cortisol in 5-year-old children causes loss of DNA methylation in sine retrotransposons: A possible role for znf263 in stress-related diseases. Clin Epigenetics. 2015;7(1):91. doi: 10.1186/s13148-015-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox EQ, Stuebe A, Pearson B, et al. Oxytocin and hpa stress axis reactivity in postpartum women. Psychoneuroendocrinology. 2015;55:164–72. doi: 10.1016/j.psyneuen.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Center on the Developing Child The impact of early adversity on child development (inbrief) 2007 Available from: www.developingchild.harvard.edu.
- 7.Child CoPAo, Family Health CoEC, Adoption,, Dependent Care et al. Early childhood adversity, toxic stress, and the role of the pediatrician: Translating developmental science into lifelong health. Pediatrics. 2012;129(1):e224–e31. doi: 10.1542/peds.2011-2662. [DOI] [PubMed] [Google Scholar]
- 8.McCrory E, De Brito SA, Viding E. Research review: The neurobiology and genetics of maltreatment and adversity. J Child Psychol Psychiatry. 2010;51(10):1079–95. doi: 10.1111/j.1469-7610.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- 9.Albers EM, Riksen-Walraven JM, Sweep FC, et al. Maternal behavior predicts infant cortisol recovery from a mild everyday stressor. J Child Psychol Psychiatry. 2008;49(1):97–103. doi: 10.1111/j.1469-7610.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- 10.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–92. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 11.Meaney MJ, Viau V, Aitken DH, et al. Glucocorticoid receptors in brain and pituitary of the lactating rat. Physiol Behav. 1989;45(1):209–12. doi: 10.1016/0031-9384(89)90187-x. [DOI] [PubMed] [Google Scholar]
- 12.Meaney MJ, Aitken DH, Bodnoff SR, et al. The effects of postnatal handling on the development of the glucocorticoid receptor systems and stress recovery in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1985;9(5-6):731–4. doi: 10.1016/0278-5846(85)90050-8. [DOI] [PubMed] [Google Scholar]
- 13.Hinde K, Skibiel AL, Foster AB, et al. Cortisol in mother's milk across lactation reflects maternal life history and predicts infant temperament. Behavioral Ecology. 2014 doi: 10.1093/beheco/aru186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paris JJ, Franco C, Sodano R, et al. Sex differences in salivary cortisol in response to acute stressors among healthy participants, in recreational or pathological gamblers, and in those with posttraumatic stress disorder. Horm Behav. 2010;57(1):35–45. doi: 10.1016/j.yhbeh.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: Social rejection versus achievement stress. Biol Psychiatry. 2002;52(4):318–27. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- 16.Bublitz MH, Stroud LR. Maternal history of child abuse moderates the association between daily stress and diurnal cortisol in pregnancy: A pilot study. Stress. 2013;16(6):706–10. doi: 10.3109/10253890.2013.825768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Goozen SH, Fairchild G. Neuroendocrine and neurotransmitter correlates in children with antisocial behavior. Horm Behav. 2006;50(4):647–54. doi: 10.1016/j.yhbeh.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 18.van Goozen SH, Fairchild G. How can the study of biological processes help design new interventions for children with severe antisocial behavior? Dev Psychopathol. 2008;20(3):941–73. doi: 10.1017/S095457940800045X. [DOI] [PubMed] [Google Scholar]
- 19.Carrion VG, Weems CF, Reiss AL. Stress predicts brain changes in children: A pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics. 2007;119(3):509–16. doi: 10.1542/peds.2006-2028. [DOI] [PubMed] [Google Scholar]
- 20.Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31(2):151–78. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 21.O'Connor TG, Bergman K, Sarkar P, et al. Prenatal cortisol exposure predicts infant cortisol response to acute stress. Dev Psychobiol. 2013;55(2):145–55. doi: 10.1002/dev.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morelius E, Nelson N, Gustafsson PA. Salivary cortisol response in mother-infant dyads at high psychosocial risk. Child Care Health Dev. 2007;33(2):128–36. doi: 10.1111/j.1365-2214.2006.00637.x. [DOI] [PubMed] [Google Scholar]
- 23.Gunnar MR, Connors J, Isensee J, et al. Adrenocortical activity and behavioral distress in human newborns. Dev Psychobiol. 1988;21(4):297–310. doi: 10.1002/dev.420210402. [DOI] [PubMed] [Google Scholar]
- 24.Finzi R, Cohen O, Sapir Y, et al. Attachment styles in maltreated children: A comparative study. Child Psychiatry Hum Dev. 2000;31(2):113–28. doi: 10.1023/a:1001944509409. [DOI] [PubMed] [Google Scholar]
- 25.Hertsgaard L, Gunnar M, Erickson MF, et al. Adrenocortical responses to the strange situation in infants with disorganized/disoriented attachment relationships. Child Dev. 1995;66(4):1100–6. [PubMed] [Google Scholar]
- 26.Kajantie E, Raikkonen K. Early life predictors of the physiological stress response later in life. Neurosci Biobehav Rev. 2010;35(1):23–32. doi: 10.1016/j.neubiorev.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Teicher MH, Andersen SL, Polcari A, et al. The neurobiological consequences of early stress and childhood maltreatment. Neuroscience & Biobehavioral Reviews. 2003;27(1–2):33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 28.Davis M, Emory E. Sex differences in neonatal stress reactivity. Child Dev. 1995;66(1):14–27. doi: 10.1111/j.1467-8624.1995.tb00852.x. [DOI] [PubMed] [Google Scholar]
- 29.Tronick E, Als H, Adamson L, et al. The infant's response to entrapment between contradictory messages in face-to-face interaction. J Am Acad Child Psychiatry. 1978;17(1):1–13. doi: 10.1016/s0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]
- 30.Haley DW. Relationship disruption stress in human infants: A validation study with experimental and control groups. Stress. 2011;14(5):530–6. doi: 10.3109/10253890.2011.560308. [DOI] [PubMed] [Google Scholar]
- 31.Bell AF, Erickson EN, Carter CS. Beyond labor: The role of natural and synthetic oxytocin in the transition to motherhood. J Midwifery Womens Health. 2014;59(1):35–42. doi: 10.1111/jmwh.12101. quiz 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinrichs M, Domes G. Neuropeptides and social behaviour: Effects of oxytocin and vasopressin in humans. Prog Brain Res. 2008;170:337–50. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- 33.Heinrichs M, Baumgartner T, Kirschbaum C, et al. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54(12):1389–98. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 34.Heim C, Young LJ, Newport DJ, et al. Lower csf oxytocin concentrations in women with a history of childhood abuse. Mol Psychiatry. 2009;14(10):954–8. doi: 10.1038/mp.2008.112. [DOI] [PubMed] [Google Scholar]
- 35.Grewen KM, Davenport RE, Light KC. An investigation of plasma and salivary oxytocin responses in breast- and formula-feeding mothers of infants. Psychophysiology. 2010;47(4):625–32. doi: 10.1111/j.1469-8986.2009.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altemus M, Deuster PA, Galliven E, et al. Suppression of hypothalmic-pituitary-adrenal axis responses to stress in lactating women. J Clin Endocrinol Metab. 1995;80(10):2954–9. doi: 10.1210/jcem.80.10.7559880. [DOI] [PubMed] [Google Scholar]
- 37.Heinrichs M, Neumann I, Ehlert U. Lactation and stress: Protective effects of breast-feeding in humans. Stress. 2002;5(3):195–203. doi: 10.1080/1025389021000010530. [DOI] [PubMed] [Google Scholar]
- 38.Amico JA, Johnston JM, Vagnucci AH. Suckling-induced attenuation of plasma cortisol concentrations in postpartum lactating women. Endocr Res. 1994;20(1):79–87. doi: 10.3109/07435809409035858. [DOI] [PubMed] [Google Scholar]
- 39.Chiodera P, Salvarani C, Bacchi-Modena A, et al. Relationship between plasma profiles of oxytocin and adrenocorticotropic hormone during suckling or breast stimulation in women. Horm Res. 1991;35(3-4):119–23. doi: 10.1159/000181886. [DOI] [PubMed] [Google Scholar]
- 40.Nissen E, Uvnas-Moberg K, Svensson K, et al. Different patterns of oxytocin, prolactin but not cortisol release during breastfeeding in women delivered by caesarean section or by the vaginal route. Early Hum Dev. 1996;45(1-2):103–18. doi: 10.1016/0378-3782(96)01725-2. [DOI] [PubMed] [Google Scholar]
- 41.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item edinburgh postnatal depression scale. Br J Psychiatry. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 42.Dubowitz H, Lane WG, Semiatin JN, et al. The seek model of pediatric primary care: Can child maltreatment be prevented in a low-risk population? Academic Pediatrics. 2012;12(4):259–68. doi: 10.1016/j.acap.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uvnas-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23(8):819–35. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- 44.Putnam FW. The impact of trauma on child development. Juv Fam Court J. 2006;57:1–11. [Google Scholar]
- 45.Chryssanthopoulou CC, Turner-Cobb JM, Lucas A, et al. Childcare as a stabilizing influence on hpa axis functioning: A reevaluation of maternal occupational patterns and familial relations. Dev Psychobiol. 2005;47(4):354–68. doi: 10.1002/dev.20100. [DOI] [PubMed] [Google Scholar]