Abstract
Patients with poor-grade aneurysmal subarachnoid hemorrhage (SAH) are likely to die due to irreversible acute-stage primary brain damage. However, the mechanism(s) and pathology responsible for their high mortality rate remain unclear. We report our findings on the brains of individuals who died in the acute stage of SAH. An autopsy was performed on the brains of 11 SAH patients (World Federation of Neurosurgical Societies grade 5) who died within 3 days of admission and who did not receive respiratory assistance. All brains were free of intracranial hematoma and hydrocephalus; all harbored ruptured aneurysms. In all brains, multiple infarcts with perifocal edema were scattered throughout the cortex and subcortical white matter of the whole brain. Infarcts with a patchy – were more often seen than infarcts with a wedge-shaped pattern. Microscopic examination revealed multiple areas with cytotoxic edema and neuronal death indicative of acute ischemic changes. Edema and congestion were more obvious in areas where the subarachnoid clot tightly adhered to the pia mater. Pathologically, the brains of deceased patients with acute poor-grade SAH were characterized by edema and multifocal infarcts spread throughout the whole brain; they were thought to be attributable to venous ischemia. Diffuse disturbance in venous drainage attributable to an abrupt increase in the intracranial pressure and focal disturbances due to tight adhesion of the subarachnoid clot to the pia mater, may contribute strongly to irreversible brain damage in the acute stage of SAH.
Keywords: histopathology of the brain, poor grade SAH, early brain injury
Introduction
Aneurysmal subarachnoid hemorrhage (SAH) is life-threatening; its mortality rate is 32–67%. The severity of early brain injury (EBI), reflected in consciousness disturbance immediately after the ictus, is well correlated with the prognosis. In rats, EBI elicited cerebral ischemic complications.1) The infarcted area due to acute arterial occlusion is characterized a wedge shape and the involved gray and white matter corresponds with the region previously supplied by the occluded vessel. Venous infarcts, on the other hand, tend to be visualized as patchy, edematous areas.2)
Proposed pathophysiological mechanisms underlying venous infarcts are increased cerebral venous pressure due to cerebral venous occlusion eliciting dilated venous and capillary bed dilation, interstitial edema, increased cerebrospinal fluid (CSF) production, and decreased CSF absorption.3) The patients with poor-grade aneurysmal SAH are likely to die due to acute-stage, irreversible primary brain damage. As the involved pathological factors remain unclear, we studied the brains of individuals who died in the acute stage of SAH and recorded our pathological findings.
Materials and Methods
We studied the brains of 11 patients who died within 3 days from the onset of SAH (World Federation of Neurosurgical Societies grade 5). They were five females and six males ranging in age from 5 to 72 years (median 58 years). All were admitted to the Research Institute for Brain and Blood Vessels-Akita between 1981 and 2002. None had received respiratory assistance.
The ruptured aneurysms were located at the anterior communicating artery (AcomA, n = 5), the middle cerebral artery (MCA), the internal carotid-posterior communicating artery (IC-PC), the terminus of the internal carotid artery (ICA), the distal posterior inferior cerebellar artery (PICA), the vertebral-posterior inferior cerebellar artery (VA-PICA), or the basilar artery-superior cerebellar artery (BA-SCA) (Table 1).
Table 1.
Summary of 11 SAH (WFNS grade 5) patients who died within 3 days post-ictus
| Case | Age | Gender | Aneurysm site | Days post-ictus |
|---|---|---|---|---|
| 1 | 71 | female | left BA SCA | 0.3 |
| 2 | 45 | female | AcomA | 2 |
| 3 | 58 | female | right IC-PC | 0.2 |
| 4 | 50 | male | right VA PICA | 2 |
| 5 | 67 | male | AcomA | 0.5 |
| 6 | 54 | female | AcomA | 2 |
| 7 | 5 | male | AcomA | 0.7 |
| 8 | 59 | male | AcomA | 0.4 |
| 9 | 63 | male | left PICA distal | 2 |
| 10 | 36 | female | right ICA terminal | 3 |
| 11 | 72 | male | left MCA | 0.6 |
AcomA: anterior communicating artery, BA: basilar artery, ICA: internal carotid artery, MCA: middle cerebral artery, PICA: posterior inferior cerebellar artery, SCA: superior cerebellar artery.
Coronal sections of paraffin-fixed whole brains were stained with hematoxylin-eosin for morphological assessment of the brain damage. Macroscopic and microscopic examinations were performed to identify the infarct type and the infarct pattern (patchy or wedge-shaped) and to clarify the association between brain damage and SAH.
Results
All brains were free of intracranial hematoma or hydrocephalus. Subarachnoid hemorrhage was observed in 73 areas (12 areas in case 1, 7 in case 2, 7 in case 3, 11 in case 4, 10 in case 5, 9 in case 6, 6 in case 7, 5 in case 8, 1 in case 9, 3 in case 10, 2 in case 11). Multiple infarcts with perifocal edema were scattered throughout the cortex and subcortical white matter of the whole brain including the cerebrum, cerebellum, and brain stem (Fig. 1). Patchy was seen more often than wedge-shaped infarcts (80% vs. 20%) (Fig. 2).
Fig. 1.
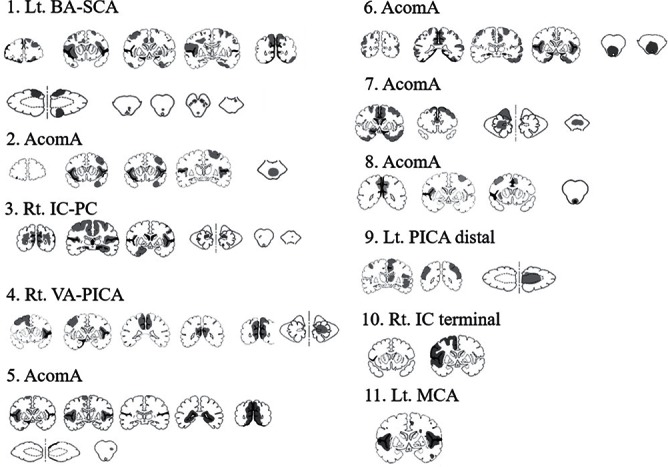
Distribution of fresh infarcts (gray) and subarachnoid hemorrhage (black) due to the rupture of a cerebral aneurysm.
Fig. 2.
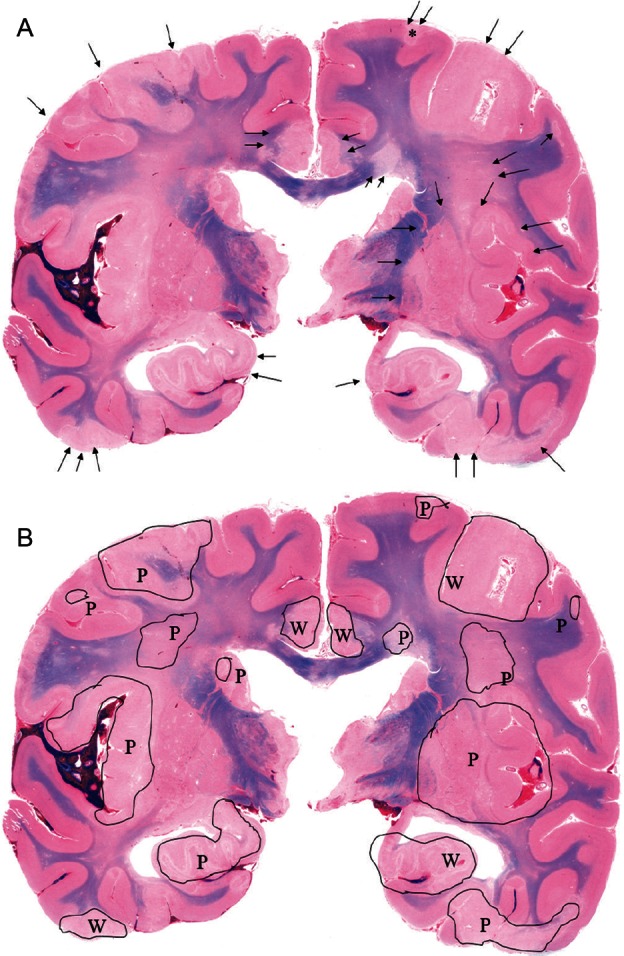
A, B - Case 1: Representative macroscopic coronal section of the cerebrum. Multiple infarcts with perifocal edema are scattered throughout the cortex and subcortical white matter (arrow). Note the flattened gyri with an unclear corticomedullary junction (Fig. 2A). Infarcts are demonstrated in black-edged area, P as patchy-shaped pattern and W as wedge-shaped pattern, respectively (Fig. 2B).
The microscopic study showed multiple foci of cytotoxic edema and neuronal death, indicative of acute ischemic changes. Edema and congestion were more obvious in all areas where the subarachnoid clot adhered tightly to the pia mater (75% area adjacent to subarachnoid hemorrhage) (Figs. 3 and 4). On the other hand, macroscopic sections showing that the subarachnoid clot existed apart from the pia mater (25% area adjacent to subarachnoid hemorrhage) revealed neither cytotoxic edema nor neuronal death, indicating that the brain tissue was normal (Fig. 5).
Fig. 3.
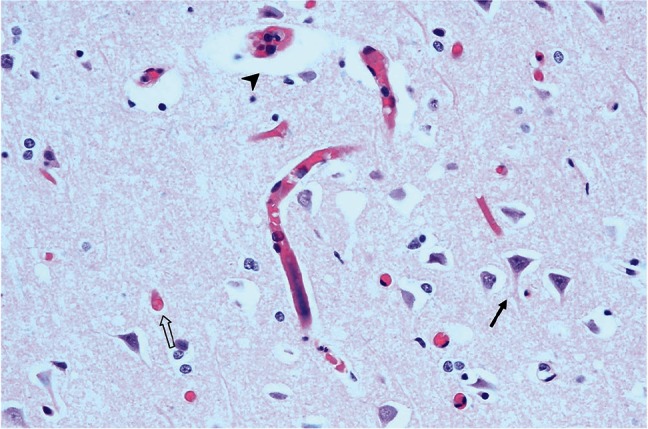
Case 1: Microscopic section of the area (*) shown in Fig. 2A. Perivascular (arrow head) and perineuronal (arrow) cytotoxic edema manifesting as astrocytic swelling and neuronal death also known as the red neuron (white arrow).
Fig. 4.
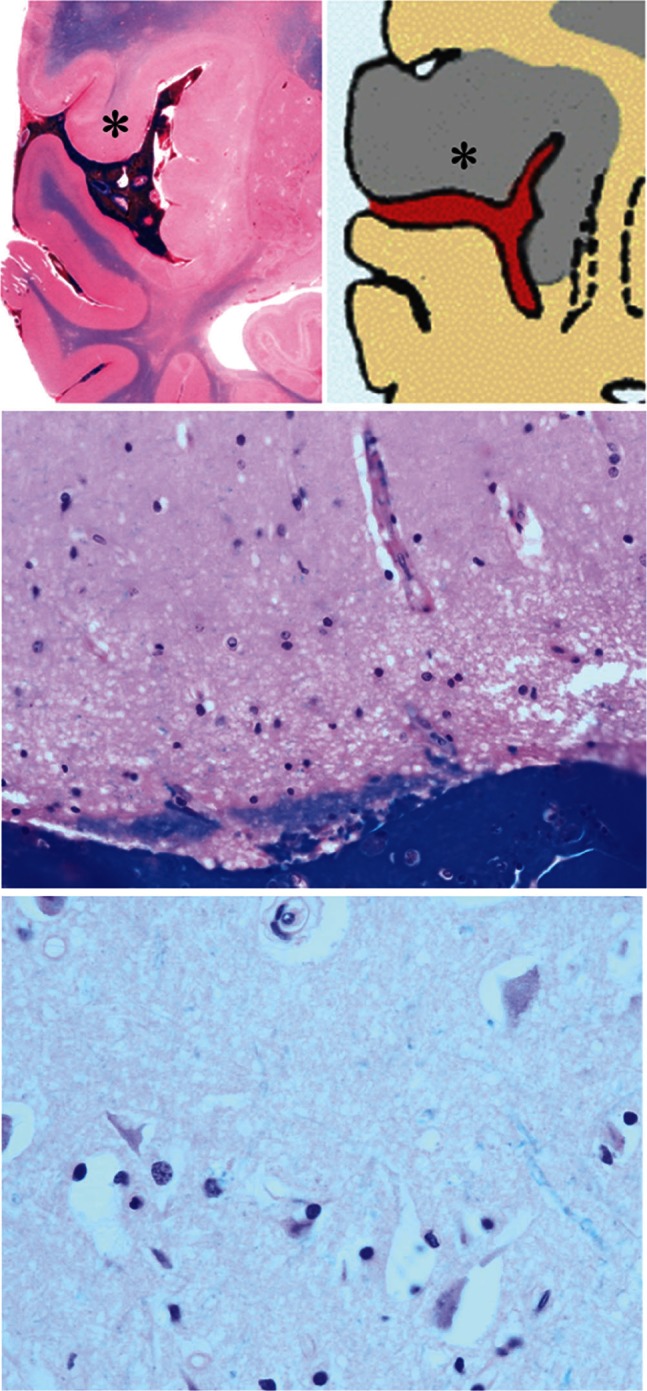
Case 1: Upper left: Macroscopic section. Note the unclear corticomedullary junction in the area with gyral swelling (*). Upper and lower right: Microscopic section of the area (*) where the subarachnoid clot tightly adhered to the pia mater. Note multiple cytotoxic edema and neuronal death indicative of acute ischemic changes.
Fig. 5.
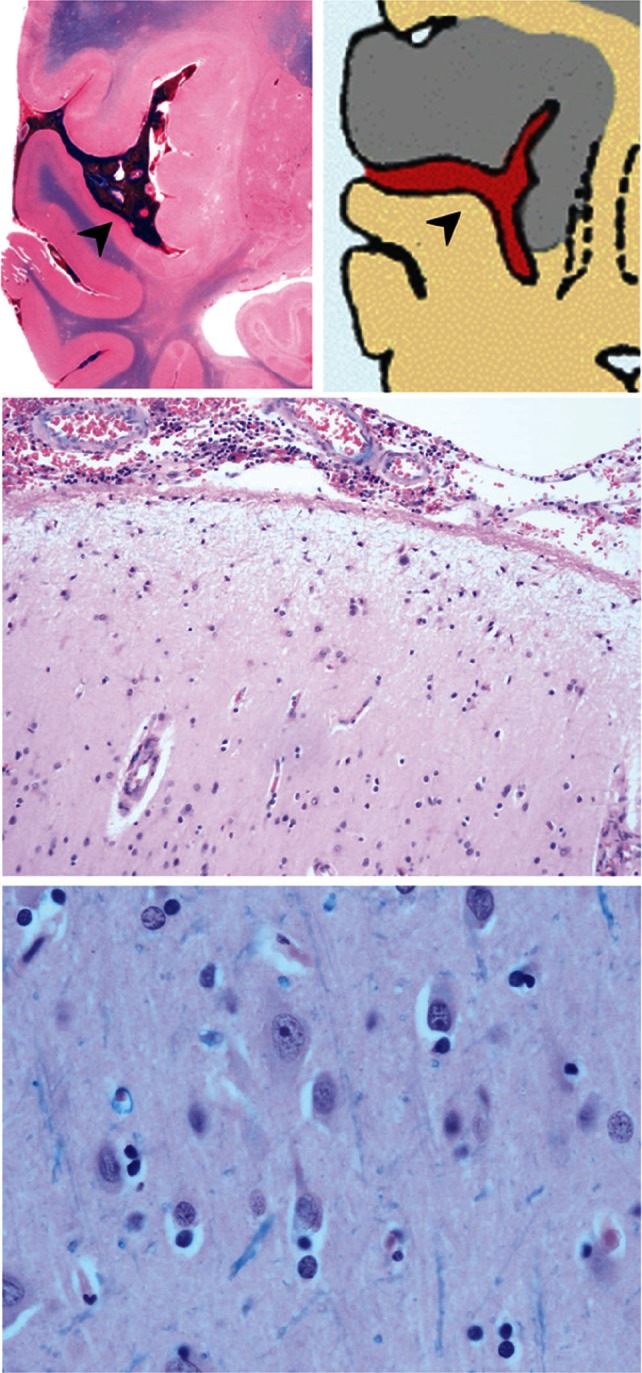
Case 1: Upper left: Macroscopic section. Note the clear corticomedullary junction in the area without gyral swelling despite the presence of a subarachnoid clot (arrowhead). Upper and lower right: Microscopic section of an area where the subarachnoid clot is not adhered to the pia mater. Neither cytotoxic edema nor neuronal death is observed, indicating normal brain tissue.
Discussion
Factors associated with an unfavorable outcome in patients with aneurysmal SAH are EBI, rebleeding, vasospasm, delayed cerebral ischemia, hydrocephalus, and systemic co-morbidities. Among them, severe EBI at onset that affects the consciousness level immediately after the ictus is strongly correlated with the prognosis. EBI can elicit cerebral ischemic complications4,5) acute stage were primarily performed in animal models.1)
The patients with poor-grade aneurysmal SAH tend to die due to acute-stage irreversible primary brain damage. The direct effect of aneurysmal SAH is thought to be due to an abrupt increase in intracranial pressure or to the disturbance of the cerebral blood flow and microcirculation.6) Death after aneurysmal rupture tends to occur sooner in patients with SAH than intracerebral hemorrhage.7) The pathological mechanism at play within 72 hours after aneurysmal SAH is thought to be the increase in the intracranial pressure following abrupt hemorrhage. It leads to a decrease in the cerebral blood flow and subsequent transient hypo-metabolism or stasis in brain function. Venous infarction has been attributed to a disturbance in the venous return. Increased cerebral venous pressure due to cerebral venous occlusion can elicit dilation of the venous and capillary bed, interstitial edema, increased CSF production, and decreased CSF absorption.3) However, the histopathology in the acute stage of poor-grade SAH remains unclear.
Factors related to acute ischemic neurological damage after aneurysmal SAH are early vasospasm8) and the no re-flow phenomenon. While autopsy studies reported a high incidence of focal infarcts,7,9,10) those findings may reflect a mixture of acute and delayed ischemic changes. Rabinstein et al.11) reported that on computed tomography scans, the two most common patterns of delayed cerebral ischemia after aneurysmal SAH are single cortical infarcts typically near the ruptured aneurysm, and multiple widespread lesions in areas including subcortical locations that are often unrelated to the aneurysmal rupture site. These findings suggest different pathophysiological mechanisms or different degrees of severity in anomalies in the same vascular process.11) However, it can be difficult to determine the stage, acute or subacute, that played a key role in eliciting the ischemic changes.
Hadeishi et al.12) reported that in patients with poor-grade SAH, diffusion-weighted magnetic resonance imaging (MRI) scans acquired in the acute stage clearly demonstrated widespread multifocal lesions in the supratentorial cerebral parenchyma that were not associated with the site of aneurysmal rupture. The infarcted area due to acute arterial occlusion is characterized by a wedge-shaped pattern with involvement of both the grey and white matter. These areas correspond with areas previously perfused by the occluded vessel. Venous- are much less common than arterial infarcts and they are often associated with thrombosis of a dural sinus. Venous infarcts usually demonstrate patchy areas with edema; in some cases petechial hemorrhages are seen.2) We found that multiple infarcts were scattered throughout the cortex and subcortical white matter of the whole brain including the cerebrum, cerebellum, and brain stem.
We posit that the infarcted areas in our series involved both supra- and infratentorial sites because our patients died within 3 days post-ictus while the patients reported by in Hadeishi et al.12) survived with neurological improvement. Furthermore, neuropathological assessment can identify the area of neuronal damage as precisely as MRI studies. Nau et al.13) who studied the brains of 20 patients who died in the acute stage (0–33 days post-SAH, median one day) demonstrated apoptosis of dentate granule cells and necrosis in the hippocampus.
We found that pathologically, the brains of patients who died in the acute stage of poor-grade aneurysmal SAH were characterized by multifocal fresh infarcts with edema spreading throughout the whole brain. Astrocytic swelling followed by eosinophilic degeneration of the cytoplasm and nuclear pyknosis are consistent with cerebral infarction in the acute stage. Our patients did not receive respiratory assistance that can result in neuronal- besides the primary brain damage attributable to SAH.14–16) Our observation that more infarcts exhibited a patchy- than a wedge-shaped pattern indicates that the majority were not associated with the arterial territory. We posit that they were due to venous ischemia as a consequence of intracranial disturbances in venous drainage after an abrupt increase in the intracranial pressure.11,12) In addition, our highly magnified microscopic findings suggested that tight adhesion of the subarachnoid clot to the pia mater contributes to focal venous circulation disturbances that elicit irreversible brain damage in the acute stage of aneurysmal SAH.
Conclusions
Pathologically, the brains of patients who died in the acute stage of poor-grade SAH were characterized by multifocal acute ischemic changes spreading throughout the whole brain. Irreversible brain damage in the acute stage of SAH may be due to diffuse disturbance in venous drainage after an abrupt increase in the intracranial pressure while focal disturbances may be attributable to the tight adhesion of the subarachnoid clot to the pia mater.
Footnotes
Conflicts of Interest Disclosure
The author has no conflicts of interest with regard to this manuscript and has registered online Self-reported COI Disclosure Statement Forms through the website for The Japan Neurosurgical Society members.
References
- 1). Lee JY, Sagher O, Keep R, Hua Y, Xi G. Comparison of experimental rat models of early brain injury after subarachnoid hemorrhage. Neurosurgery 65: 331– 343; discussion 343, 2009. [DOI] [PubMed] [Google Scholar]
- 2). Osborn A. Diagnostic neuroradiology. St. Louis: Mosby-Year Book, Inc.; 1994. [Google Scholar]
- 3). Schaller B, Graf R. Cerebral venous infarction: The pathophysiological concept. Cerebrovasc Dis 18: 179– 188, 2004. [DOI] [PubMed] [Google Scholar]
- 4). Fu C, Yu W, Sun L, Li D, Zhao C. Early cerebral infarction following aneurysmal subarachnoid hemorrhage: Frequency, risk factors, patterns, and prognosis. Curr Neurovasc Res 10: 316– 324, 2013. [DOI] [PubMed] [Google Scholar]
- 5). Kumar A, Brown R, Dhar R, Sampson T, Derdeyn CP, Moran CJ, Diringer MN. Early vs. Delayed cerebral infarction after aneurysm repair after subarachnoid hemorrhage. Neurosurgery 73: 617– 623; discussion 623, 2013. [DOI] [PubMed] [Google Scholar]
- 6). Kassell NF, Torner JC. The international cooperative study on timing of aneurysm surgery—an update. Stroke 15: 566– 570, 1984. [DOI] [PubMed] [Google Scholar]
- 7). Birse SH, Tom MI. Incidence of cerebral infarction associated with ruptured intracranial aneurysms. A study of 8 unoperated cases of anterior cerebral aneurysm. Neurology 10: 101– 106, 1960. [DOI] [PubMed] [Google Scholar]
- 8). Bederson JB, Levy AL, Ding WH, Kahn R, DiPerna CA, Jenkins AL, 3rd, Vallabhajosyula P. Acute vasoconstriction after subarachnoid hemorrhage. Neurosurgery 42: 352– 360; discussion 360–352, 1998. [DOI] [PubMed] [Google Scholar]
- 9). Crompton MR. The pathogenesis of cerebral infarction following the rupture of cerebral berry aneurysms. Brain 87: 491– 510, 1964. [DOI] [PubMed] [Google Scholar]
- 10). Smith B. Cerebral pathology in subarachnoid haemorrhage. J Neurol Neurosurg and Psychiatry 26: 535– 539, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Rabinstein AA, Weigand S, Atkinson JL, Wijdicks EF. Patterns of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke 36: 992– 997, 2005. [DOI] [PubMed] [Google Scholar]
- 12). Hadeishi H, Suzuki A, Yasui N, Hatazawa J, Shimosegawa E. Diffusion-weighted magnetic resonance imaging in patients with subarachnoid hemorrhage. Neurosurgery 50: 741– 747; discussion 747–748, 2002. [DOI] [PubMed] [Google Scholar]
- 13). Nau R, Haase S, Bunkowski S, Bruck W. Neuronal apoptosis in the dentate gyrus in humans with subarachnoid hemorrhage and cerebral hypoxia. Brain Pathol 12: 329– 336, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Wijdicks EF, Pfeifer EA. Neuropathology of brain death in the modern transplant era. Neurology 70: 1234– 1237, 2008. [DOI] [PubMed] [Google Scholar]
- 15). Leestma JE, Hughes JR, Diamond ER. Temporal correlates in brain death. Eeg and clinical relationships to the respirator brain. Arch Neurol 41: 147– 152, 1984. [DOI] [PubMed] [Google Scholar]
- 16). Moseley JI, Molinari GF, Walker AE. Respirator brain. Report of a survey and review of current concepts. Arch Pathol Lab Med 100: 61– 64, 1976. [PubMed] [Google Scholar]


