Abstract
From the embarrassing character commonly infiltrating eloquent brain regions, the surgical resection of glioma remains challenging. Owing to the recent development of in vivo visualization techniques for the human brain, white matter regions can be delineated using diffusion tensor imaging (DTI) as a routine clinical practice in neurosurgery. In confirmation of the results of DTI tractography, a direct electrical stimulation (DES) substantially influences the investigation of cortico-subcortical networks, which can be identified via specific symptoms elicited in the concerned white matter tracts (eg., the arcuate fascicle, superior longitudinal fascicles, inferior fronto-occipital fascicle, inferior longitudinal fascicle, frontal aslant tract, sensori-motor tracts, optic radiation, and so forth). During awake surgery for glioma using DES, it is important to identify the anatomo-functional structure of white matter tracts to identify the surgical boundaries of brain regions not only to achieve maximal resection of the glioma but also to maximally preserve quality of life. However, the risk exists that neurosurgeons may be misled by the inability of DTI to visualize the actual anatomy of the white matter fibers, resulting in inappropriate decisions regarding surgical boundaries. This review article provides information of the critical neuronal network that is necessary to identify and understand in awake surgery for glioma, with special references to white matter tracts and the author’s experiences.
Keywords: glioma, awake surgery, white matter tract, tractography
Introduction
Owing to recent developments in functional brain imaging, human cerebral neuronal and functional networks have been investigated, and this knowledge has contributed to neurosurgical fields, including brain tumor surgery.1) A glioma, which arises from glial cells, is a brain primary neoplasm invading the central nervous system. The tendency of this tumor type to infiltrate cortical and subcortical functional structures renders its treatment is challenging, and treatment has therefore been widely debated. The extended surgical resection of the glioma has been suggested to contribute to improvement of the prognosis;2) however, the functional connectivity, which is complex and composed of both cortical and subcortical networks, should be preserved to maintain functional outcomes.3) In order to take maximal advantage of the plasticity of the brain, “hodotopy,” named for the Greek “hodos” (path, referring to the subcortical fibers) and “topos” (place, referring to the cortex) is critically important to the surgical strategy for the treatment of gliomas.4)
It has been recently elucidated that cerebral functional cortical regions, which constitute a so-called “eloquent area,” have the potential to move and change dynamically, facilitated by subcortical white matter pathways.5) Direct electrical stimulation (DES) is a useful tool for brain mapping to investigate the cortical and subcortical functional complexity associated with brain plasticity. Stimulation under appropriate conditions interferes locally and transiently with a small cortical or axonal site, which might be connected only to a part of the network.1,6) Using this technique, an awake surgery for glioma in eloquent areas is now the gold standard, developed by the contribution not only of advancements in anesthesiology and electrophysiology but also of dedicated clinical neurosurgical experience.7–9) In addition to classic anatomical information regarding cerebral white matter connectivity, subcortical networks have been extensively elucidated by additional contributions from the awake brain mapping undertaken in glioma surgeries. Furthermore, the original methodology has provided new insights into the functional connectivity underlying not only sensorimotor and visuospatial language systems but also sociocognitive and multimodal systems such as working memory, attention, executive functions, and even consciousness.1)
In this study, we review reports of the critical neural networks investigated and identified via awake surgery for gliomas, with special reference to white matter tracts and our clinical experiences.
Clinical Procedures
Awake surgery and functional mapping
All surgical procedures were performed using an asleep–awake–asleep technique with DES mapping.10–12) A bipolar electrode with 5-mm spaced tips delivering a biphasic current (pulse frequency 60 Hz; single-pulse phase duration 1 ms; amplitude 1.5–5 mA) was used in all cases. During the awake phase, the intensity threshold was set either by evoking speech arrest when stimulating the ventral premotor cortex, or by evoking motor disorder via the stimulation of the primary motor cortex within the pre-central gyrus. Complete cortical mapping was achieved using the picture-naming, motor, passive sensory, line bisection, spatial 2-back, and facial expression and emotional tasks, after which resection was begun. Subcortical resection was stopped when DES reproduced specific findings and disorders during task execution. As shown in Table 1, task selection was determined by which white matter tracts and structures that needed to be preserved to maintain tract-specific functions were present near the resection site. Positive mapping areas induced by DES on cortical and subcortical regions were marked via number tags and video-recorded.
Table 1.
Subcortical tracts, mapping tasks, and symptoms induced by the direct electrical stimulation
| Subcortical tracts | Tasks | Symptoms*) | |
|---|---|---|---|
| Arcuate fascicle |
|
||
| IFOF |
|
||
| Fronto-striatal tract |
|
||
| SLF | I |
|
|
| II |
|
|
|
| III |
|
||
| Frontal aslant tract |
|
||
| Optic radiation |
|
||
| ILF (posterior part) |
|
||
| Pyramidal tract |
|
|
|
| Somatosensory tract |
|
|
|
IFOF: inferior fronto-occipital fascicle, ILF: inferior longitudinal fascicle, PPTT: pyramids and palm trees test, SLF: superior longitudinal fascicle. An asterisk shows reference numbers.
Tractography
Diffusion weighted (DW)-MR images were acquired preoperatively using a 3.0 Tesla MRI scanner (Signa Excite HDx 3.0T, General Electric Medical Systems). A series of diffusion-weighted axial images with (b-value = 1,000 s/mm2) and without (b-value = 0) a diffusion-sensitizing gradient along 30 directions were obtained. The other diffusion parameters were as following: time of repetition (TR) = 14,000 ms, time of echo (TE) = 69.6 ms, number of excitations (NEX) = 2, 60 axial slices with slice thickness = 2.5 mm with no interslice gap, and field of view (FOV) = 220 × 220 mm with a matrix = 88 × 88, resulting in an effective resolution of 2.5 mm3 isotropic voxels. The DW-MR images were transferred to a workstation using iPlan Cranial 3.0 software (BrainLab, Feldkirchen, Germany), which reconstructed qualitative maps. Regions of interest targeting each white-matter tract were selected manually by referring to the diffusion tensor imaging (DTI)-tractography atlas and to results of previous studies.13,14) All three-dimensional tracts were reconstructed with fiber propagation stopped at a fractional anisotropy threshold of <0.18. An illustrative case with the tractography of a healthy adult is presented in Fig. 1.
Fig. 1.
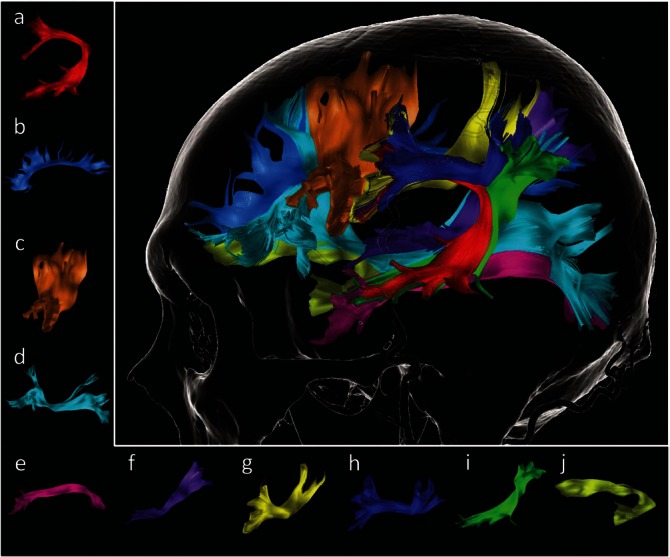
An illustrative case of diffusion tensor imaging tractography in the left cerebral hemisphere of a healthy adult. Each tract is shown in a–j: a, arcuate fascicle (red); b, cingulate fascicle (sky blue); c, frontal aslant tract (brown); d, inferior fronto-occipital fascicle (cyan); e, inferior longitude fascicle (magenta); f, middle longitude fascicle (purple); g, superior longitudinal fascicle II (yellow); h, superior longitudinal fascicle III (blue); i, superior longitudinal fascicle-temporoparietal component (green); j, uncinate fascicle (yellowish green).
White Matter Tracts and DESs
Specific symptoms could be transiently elicited by subcortical DES on the relevant subcortical white matter regions using appropriate tasks (Table 1). In this section, we describe the anatomo-functional characters of each white matter tract with reference to DES. Four illustrative cases of patients who underwent awake surgery for gliomas are presented with pre- and post-operative MRI, tractography, and intraoperative photographs in Figs. 1–4.
Fig. 4.
A case of high-grade glioma located in left parietal lobe. A: Preoperative contrast enhanced-T1 axial MRI showing an enhanced lesion in the left postcentral gyrus. B: Three-dimensional cortical image in operative view. C: Tractography showing the tumor (beige) near the pyramidal tract (yellow), somato-sensory tract (blue), the superior longitudinal fascicle III (green), and the arcuate fascicle (red). D: Postoperative FLAIR axial MRI. E and F show an intraoperative photo and preoperative tractography within the white square in B. E: An intraoperative photo taken after surgical resection of tumor with preservation of positive mapping areas elicited by cortical and subcortical electrical stimulations. Cortical mappings: Dysarthria was elicited in the precentral gyrus (tag 1), and dysesthesia in the postcentral gyrus (tags 2 and 3). Subcortical mappings: Involuntary movements of right arm (tag 4) and leg (tag 6) were reproduced on the pyramidal tract, and dysesthesias of right hand (tag 5) and fingers (tag 7) on the somatosensory tract. F: Subcortical positive mapping areas are overlapped on the preoperative tractography. A and P indicate the anterior and the posterior side, respectively.
Arcuate fascicle
Burdach (1822) was the first to designate the peri-sylvian tract, a group of fibers running deeply around the sylvian fissure, collectively as the “Fasciculus Arcutas” based on the shape of the long arch.15) The classic arcuate fascicle had been believed to connect Broca’s area in the frontal lobe to Wernicke’s area in the temporal lobe. Recent information regarding the arcuate fascicle suggest that the frontal terminations broadly include the pars opercularis and triangularis of the inferior frontal gyrus and the ventral premotor cortex, although most of the arcuate fascicle terminates in the premotor area, as indicated by the results of recent postmortem fiber dissection and DTI/diffusion spectrum imaging (DSI) tractography studies.16–19) In contrast, the major temporal termination of the arcuate fascicle is in the posterior superior temporal gyrus and middle temporal gyrus; furthermore, cortex-sparing fiber dissection and DTI studies suggest that the arcuate fascicle may have extensions to the caudal inferior temporal gyrus (Fig. 2).20,21)
Fig. 2.
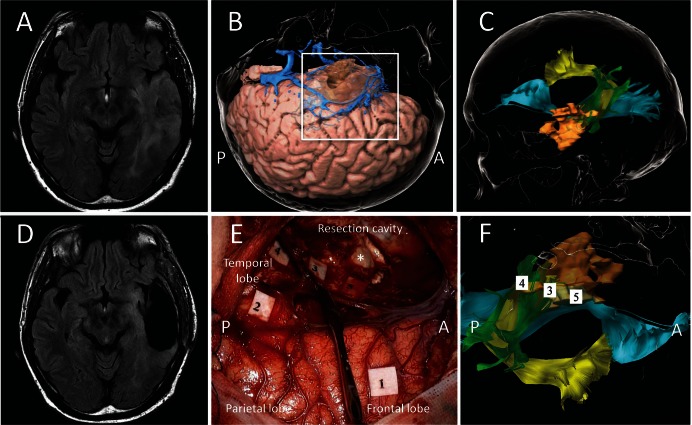
A case of left temporal low-grade glioma with intraoperative direct electrical stimulations. A: Preoperative FLAIR axial MRI showing a recurrent hyper lesion in left temporal lobe. B: Three-dimensional cortical image in operative view. C: Tractography showing the tumor (beige) infiltrating into the inferior fronto-occipital fascicle (cyan), the superior longitudinal fascicle-temporoparietal compartment (green), and the arcuate fascicle (yellow). D: Postoperative FLAIR axial MRI. E and F show an intraoperative photo and preoperative tractography within the white square in B. E: An intraoperative photo taken after surgical resection of tumor with preservation of positive mapping areas elicited by cortical and subcortical electrical stimulations. Anarthria was elicited in ventral region of precentral gyrus (tag 1), and anomia in the angular gyrus (tag 2); phosphene in right inferior quadrant area (tag 3) and right half area (tag 4) on the left optic radiation; semantic paraphasia on the inferior fronto-occipital fascicle (tag 5). An asterisk shows the head of hippocampus. F: Tags 3–5 in the subcortical positive mapping areas are overlapped on the preoperative tractography. A and P indicate the anterior and the posterior side, respectively.
A dual-stream model of speech processing has been postulated, one stream of which is the dorsal steam involved in mapping phonological representation onto articulatory motor representations by connecting cortical regions in the frontal, temporal, and the parietal lobes.22) The left arcuate fascicle has been proposed to be the white matter fiber system most prominently related to auditory-motor integration in the dorsal stream. The disconnection syndrome of this bundle is thought to lead to conduction aphasia, characterized as a deficit in the ability to encode phonological information for production.23) During intra-operative subcortical mappings using the picture-naming task, DES along the arcuate fascicle exactly reproduces transient phonological paraphasia and repetition disturbance, rather than the articulatory disorders demonstrated via the stimulation of the superior longitudinal fascicle (SLF) III described below.24–26) An anomia, a symptom characteristic of conduction as reported in some studies, is also induced (Fig. 2).24,25)
Superior longitudinal fascicle (SLF)
Dejerine, in 1852, was the first to use the term SLF and arcuate fascicle interchangeably.27) According to recent interpretations, however, the SLF does not appear to accurately coincide with the arcuate fascicle. The first tractography studies revealed that the “classic” arcuate fascicle was composed of three subcomponents—the anterior short segment, posterior short segment, and long segment.19,28) These are now called, respectively, SLF III, SLF-tp (temporo-parietal component), and the arcuate fascicle in the narrow sense. In the most recent anatomical conception of the peri-sylvian network, the SLF is a large tract composed of four subparts—SLF I, II, and III and SLF-tp.29) They run parallel with each other longitudinally from the parietal to the frontal lobe, except for SLF-tp, which vertically connects the temporal and parietal lobes (Fig. 2).
SLF III is the most lateral subpart of the SLF (also called the lateral, horizontal, or anterior segment of the SLF), which connects the supra-marginal gyrus and the ventral prefrontal cortex.19,30) The stimulation of the left SLF III causes dysarthria and other impairments in articulatory processing in coordination with SLF II.14,24,29,31) Repetition errors could also be interpreted as perturbations of articulatory and phonological processing caused by DES of SLF III and SLF-tp, respectively.29,32)
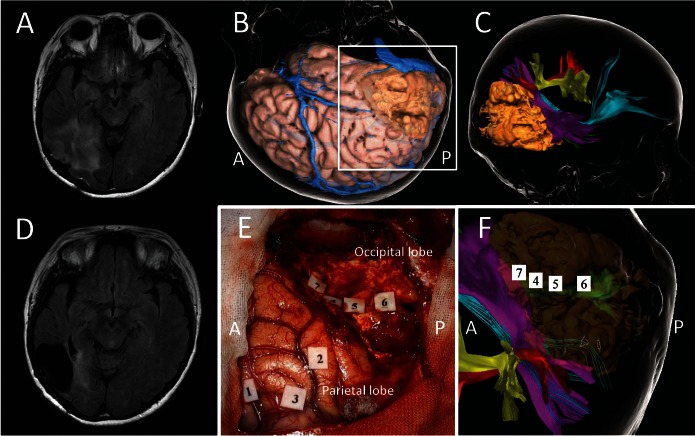
SLF II connects the dorsal and prefrontal areas to the angular gyrus. The most prominent role of SLF II is in the processing of spatial awareness in the right hemisphere. Anatomical disconnections of the fronto-parietal network result in chronic left spatial neglect, especially in cases with damage to SLF II, which is the best predictor of this morbidity.33,34) It is also possible to identify the subcortical SLF II in vivo by DES during awake craniotomy using a line bisection task (Fig. 3).35) Stimulation of SLF II can cause rightward deviations when DES transiently induces a left spatial unawareness. Successful examinations could spare postoperative neglect by detecting not only rightward but also leftward deviations even in the right hemisphere.36)
Fig. 3.
A case of recurrent low-grade glioma in the right temporal lobe. A: Preoperative FLAIR axial MRI showing a hyper lesion in right temporo-occipital area. B: Three-dimensional cortical image in operative view. C: Tractography with a recurrent tumor (beige); the inferior fronto-occipital fascicle (cyan), the superior longitudinal fascicle II (red) and III (yellow), and middle longitudinal fascicle (purple). D: Postoperative FLAIR axial MRI. E and F show an intraoperative photo and preoperative tractography within the white square in B. E: An intraoperative photo taken after surgical resection of tumor with preservation of positive mapping areas elicited by cortical and subcortical electrical stimulations. Cortical mappings: Dysesthesia of left hand in the postcentral gyrus (tag 1), and rightward deviation in the line bisection task (tags 2 and 3). Subcortical mappings: phosphene in the left superior quadrant area (tags 4–6) and topographical disorientation (tag 7). F: Tags 4–6 in the subcortical positive mapping areas are overlapped on the optic radiation (green) in the preoperative tractography. A and P indicate the anterior and the posterior side, respectively.
Not only the function of SLF I but also its existence encompass some unverified issues. In a fiber dissection study, the SLF, including segments of I and II, could be dissected, with the exception of the SLF I.37) However, DSI and spherical deconvolution tractography studies could demonstrate that the SLF I connects the precuneus with the superior frontal and anterior cingulate areas on the dorsal aspect of the main cingulum fibers, in a paracingulate or supracingulate location.21,37) Our previous study suggested that spatial working memory would be performed via parieto-frontal networks, especially with the right SLF I and II, damage to which caused chronic impairment of this function.38)
The SLF-tp, which is also called the vertical or posterior segment of the classic arcuate fascicle, connects the posterior temporal and inferior parietal lobes. This bundle may not be included in SLF subcomponents based on the anatomical feature of the “superior longitudinal” fascicle; however, it plays a role in phonological processing along with the arcuate fascicle as a part of dorsal stream of speech processing (Fig. 2).11,32,39,40) Some authors designate the arcuate fascicle as SLF-IV.40,41) These differing interpretations of peri-sylvian white matter pathways have caused unresolved confusion without enough integration, also due to recent rapid advancements in white matter tractography studies.
Inferior fronto-occipital fascicle (IFOF)
The IFOF is an anterior–posterior white matter tract consisting of two layers; the superficial and deep layers. The former connects the temporo-basal and posterior occipital lobes with the inferior frontal gyrus, and the latter connects the middle frontal and dorso-lateral prefrontal cortex. Based on its wide distribution to the frontal cortices, the IFOF can be considered a “multi-function” bundle.42) In contrast to the dorsal stream of speech subserving phonological processing, the ventral stream, composed of the IFOF, predominates in language semantics.43)
Interestingly, the IFOF does not seem to exist in non-human primates, and could thus be considered the most important bundle in humans.1) In a picture-naming task, DES to this tract generates semantic paraphasia or anomia (Fig. 2), which can be confirmed to be disturbances of comprehension, including non-verbal semantic processing, using a semantic task such as the Pyramids and Palm Trees Test (PPTT).44,45) Multimodal participation in verbal and non-verbal semantic processing originates in the deep and superficial layers of the IFOF, respectively.46) As observed in the head of the caudate, verbal perseveration could also be induced by stimulation of the IFOF, but this does not persist until 3 months.47) In addition to language functions, furthermore, Duffau and his colleagues have made the interesting suggestion that the IFOF might play a crucial role in the awareness of amodal semantic knowledge, which contributes to make the human what he is.1,45)
Inferior longitudinal fascicle (ILF)
The ILF runs parallel to a direct pathway of the ventral stream as an indirect pathway with the uncinate fascicle (UF). A predominant part of the terminations lies in the occipito-parietal region and the ventral aspect of the temporal lobes. In the posterior region, the fibers, which are bound medially by the IFOF and laterally by the subcortical U fibers, run vertically from the posterior lingual and fusiform gyri and cuneus. At the ventral aspect of the occipito-temporal junction, the ILF changes direction and runs horizontally in the white matter of the inferior temporal lobe, amygdala, and parahippocampal gyrus, and then connects to the origin of the uncinate fasciculus.28,41 ,48,49) The posterior part of the ILF is implicated in reading,50) and face and object recognition,51) whereas both the anterior and posterior parts of the ILF are involved in the processing of names,52) reading,53) and spoken language.54) In conclusion, the posterior “visual” part is involved in visual processing of objects or pictures, whereas the anterior part is involved in linking object representations to their lexical labels.54)
Intraoperative DES can induce alexia in the posterior part of ILF, frequently with anomia or phonemic paraphasia caused by simultaneous stimulation of the posterior segment of the arcuate fascicle (SLF-tp).50) The ILF does not seem to be essential in language semantic processing because the IFOF running above the ILF was shown to mainly function as a direct pathway in other intraoperative DES studies.55,56)
Uncinate fascicle (UF)
The UF is one of the indirect pathways of the semantic ventral stream. It originates in the temporal pole, aggregates lateral to the ventral part of the claustrum, moves medially through the limen insula, and then relays information from the visual object form area via ILF to the pars orbitalis of the inferior frontal gyrus.28,49) The results of several studies suggested that the UF plays a potential role not only in semantics57) but also in auditory stimuli, recognition memory,58) and emotion.59) Nonetheless, the UF does not seem to be essential for language, and does not generate semantic paraphasia in DES due to IFOF compensation.60)
Middle longitudinal fascicle
A temporo-parietal fiber bundle was first identified in a rhesus monkey in 1984 by using an in vivo technique with radioisotope injections, and named the middle longitudinal fasciculus (MdLF).61) The MdLF connects the angular gyrus with the superior temporal gyrus up to the temporal pole (Fig. 3). The tract could be a part of the ventral semantic route, but its functional role has not been elucidated despite recent evidence showing its existence by using fiber dissection.62) In addition, intraoperative DES on MdLF does not seem to generate any disorders.63)
Optic radiation (OR)
The OR comprises three bundles of visual fibers (direct, central, and Meyer loop) emerging from the lateral geniculate body, running around the roof of the occipital horn, and terminating at the primary visual cortex. This bundle runs through the sagittal stratum, which is a sheet-like structure located lateral to the atrium of the lateral ventricle, and passes close to the IFOF and ILF. The OR is located just medially and above the ILF, which runs laterally and inferiorly to the lateral wall of the temporal horn, and deeply and inferiorly to the IFOF.64) Intraoperative stimulation of the OR can elicit transient phosphene, blurred vision, or visual loss in the contralateral visual field (Fig. 3).65) A 4-screen picture-naming task is very useful to detect which part of the OR would be stimulated up to details with quadrant visual level. The disturbance of visual perception is very different from a disorder of visual recognition, which can be elicited by DES of the ILF connecting the visual cortex and visual object form area.1)
Sensori-motor tracts
The sensori-motor system is composed mainly of projection fibers including pyramidal cortico-spinal and somato-sensory thalamo-cortical tracts. All spontaneous body movements are controlled by initiation, execution, and inhibition. Notably, this motor control is enabled by a complex circuit, which comprises multiple complex networks including paracentral U fibers and short frontal association fibers (frontal aslant tract [FAT]),66) and other projection fibers (fronto-striatal tract [FST]).67) Especially, the supplementary motor area (SMA), which is located rostral to the medial region of primary motor area, has an important role as a hub of these fibers described below.
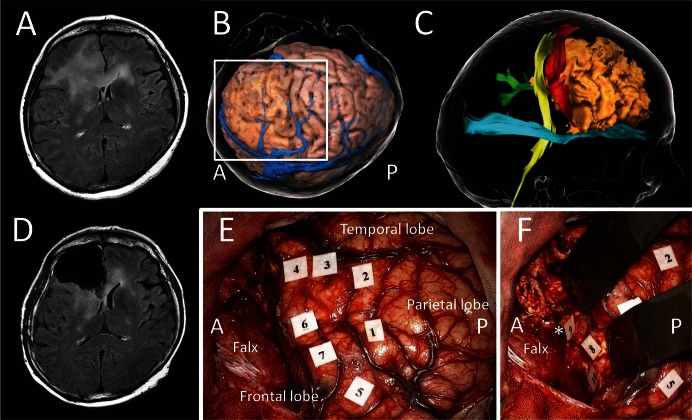
DESs of the pyramidal tract induce involuntary muscle contraction (Fig. 4),68) which should be distinguished from the negative motor response (NMR) that is reproduced on negative motor areas (Fig. 5). Motor-evoked potentials using single-pulse electrical cortical stimulation can be useful for distinguishing primary motor area and negative motor area represented by SMA.69) NMR caused by DES with high-frequency stimulation is a disorder of motor initiation and control, which ranges from complete arrest of movement to involuntary acceleration of movement without loss of consciousness.70) The negative motor network has been reported by some DES studies using cortico-cortical evoked potential71) and subcortical DES mapping.67,70) The FST, which is also called the anterior short fiber of subcallosal fascicle, forms a veil-like stream running from the SMA to the head of the caudate nucleus.72) The FAT, which connects the posterior part of the inferior frontal gyrus with the pre-SMA, runs slightly anterior and lateral to the FST. DESs can reproduce NMR contralaterally or in both upper and/or lower limbs in the FST (Fig. 5), and NMR of speech in the FAT.72,73) Stimulations of both these tracts converging in deep regions can generate cessation of combined movements with a specific somatotopic feature.67)
Fig. 5.
A case of high-grade glioma originating from right frontal lobe. A: Preoperative FLAIR axial MRI showing diffuse hyper lesion in bilateral frontal lobes. B: Three-dimensional cortical image in operative view. C: Tractography showing the tumor (beige) near the pyramidal tract (yellow), the frontal aslant tract (red), the cingulate fascicle (green), and the inferior fronto-occipital fascicle (cyan). D: Postoperative FLAIR axial MRI showing no residual-enhanced lesion. E and F show intraoperative photos within the white square in B, and were taken after surgical resection of tumor with preservation of positive mapping areas elicited by cortical and subcortical electrical stimulations. E: Cortical mappings; anarthria was elicited in ventral part of the precentral gyrus (tags 1 and 2), negative motor response in the pars triangularis (tag 3), errors in emotional task on the pars orbitalis (tag 4) and superior frontal gyrus (tag 5), and rightward deviation in line bisection task on the posterior region of the middle frontal gyrus (tags 6 and 7). An asterisk shows the anterior horn of lateral ventricle. F: Subcortical mappings; rightward deviation in line bisection task on the posterior boundary of resection cavity (tag 8), and motor inhibition of left upper-limb on the fronto-striatal tract (tags 9 and 10). A and P indicate the anterior and the posterior side, respectively.
Frontal aslant tract (FAT)
The FAT is a frontal association white matter tract recently named after the structural characteristic of running in the “aslant (oblique)” direction at coronal section. This pathway connects the inferior frontal gyrus with the superior frontal gyrus including both medial and dorsolateral parts,66,72,74) and likely subsumes the subcallosal fascicle.29) Its function has been recently described by a DES study; it seems to have a role in the control of language especially the planning of articulation, disruption of which results in disorders of initiation of speech (Fig. 5).72,75) This is also demonstrated by previous reports indicating that the disturbance of verbal fluency and errors in initiation of speech were involved in primary progression aphasia, Foix–Chavany–Marie syndrome, and stuttering, in which the FAT was shown to be damaged in tractographic studies.76–78) These data indicate that FAT may be also a part of the negative motor network, especially with regard to speech function.
Vertical occipital fascicle
Due to modern in vivo tractographic techniques, the vertical occipital fascicle (VOF) has recently reappeared after a century, stirring debate among some of the most prominent neuroanatomists of the 19th century.79,80) The bundle, previously named Wernicke’s perpendicular fasciculus, connects the occipito-temporal sulcus with the lateral portion of the occipito-parietal junction.81) VOF likely plays a role in the integration of perception of visual categories at the ventral termination, and the control of eye movements, attention, and motion perception in the dorsal termination.79) Disorders due to DESs of this tract have not been well studies; therefore, this bundle should be investigated further in awake mappings.
Cingulate fascicle
The cingulate fascicle is the largest white matter tract of the limbic system after the cingulate and the parahippocampal gyri. Many studies have investigated its function but few have examined the effects of DES of the cingulate fascicle in awake craniotomy. Intraoperative DES of the anterior cingulate cortex could reproduce executive errors in the Stroop test during awake surgery for frontal glioma.82) Similarly, in the posterior cingulate cortex, DES induced a breakdown in conscious experience characterized by a transient behavioral unresponsiveness with loss of external connectedness.83) The author suggests that the cingulate network is involved in high-level mentalization and consciousness of environment from his experiences of intraoperative DES.1) Further research is needed to elucidate this aspect of the multimodal system.
Tractography: Light and Darkness
Recent developments in DTI tractography have enabled a better understanding of subcortical networks not only in the field of neuroscience but also in neurosurgical practice. This visualization technique is especially beneficial for preoperative planning for brain tumor surgery as a noninvasive and simple method by using established software. However, despite rapid adoption of this method in clinical practice, DTI should not be used as the basis of any decisions in neurosurgery because of the inaccurate profile it provides.84)
First, DTI tractography is not a tool to directly visualize the actual anatomy of fibers, but only provides an indirect reconstruction (“shadow” in other words) based on measuring the diffusion of water molecules. Tractography does not show the actual size of the fiber bundle evaluated in the cortico-spinal tract.85) Second, the degree of white matter tracts depends on data acquired parameters (MR scanner, magnetic field, and scan parameter) and the kind of algorithms used for the analysis of the data.8) Third, DTI has technical limitations, although the fiber-tracking software used in this report is one of the most accurate methods.86) It is difficult to visualize some subcortical tracts in some conditions: 1) tracts running within glioma-infiltrating lesions and edema (e.g., OR unable to be visualized in case 1); 2) Sharply curving tracts like the Meyer loop; and 3) tracts crossing in a complex manner with other fibers (e.g., lateral parts of the sensori-motor tracts, which direct to the upper-limb and facial area, intersected by SLFs in case 4). In particular, the strong intersection of fibers at the frontal operculum would explain the controversy regarding the results of DTI studies considering the terminations of the peri-sylvian white matter tracts.16) In our experiences, the SLF I and II, Meyer loop, lateral part of the sensori-motor tracts, FST, and VOF are difficult to visualize using popular DTI methodology alone. From the limitation of DTI visualization and unverified characters of these subcortical tracts, there are still some problems remained in identification of those pathways only by intraoperative DESs.
In order to solve the micro-anatomical problems, newly developed methods including Q-ball imaging,87) DSI,88) and spherical deconvolution tractography89) have been used and have consequently contributed to recent neuroscience and brain studies investigating white matter networks and disorders. However, the main drawback of these methods is the extensive amount of data generated and scanning times. These issues will have to be resolved to enable clinical applications in the future.
Preservation of White Matter Tracts
Acquired knowledge about white matter connectivity has enabled neurosurgeons to avoid a permanent neurofunctional deficit according to functional and structural boundaries of glioma excision. For the purpose of maximal resection of the lesion, however, it is unreasonable to preserve all of those white matter tracts, considering neuronal plasticity and compensation.
There is a dynamic functional redistribution that allows extended surgical excision of brain regions in patients with slow-growing lesions such as low-grade glioma.5) This neuroplastic phenomenon also makes it possible to remove broadly the residual lesion in the second surgery of a glioma that had seemed unresectable in the previous operation.90) The functional reshaping process might be elaborated by complimentary compensation of white matter networks such as a relationship between the direct IFOF pathway and the indirect ILF-UF pathway.91) On the other hand, according to a probabilistic atlas of functional resectability of low-grade glioma, there are crucial common regions, that is, “minimal common brain” that need to be preserved to retain hodotopical functions even if involved by the tumor.92)
The dilemma regarding maximal tumor resection and functional preservation is always present in glioma surgery. From the perspective of quality of life (QOL), postoperative transient dysfunction such as akinesia due to SMA syndrome, most of which could completely improve by 1–3 months even after excision of SMA lesion, should be avoided to enable patients to return to their life as soon as possible.1) Dysfunction of negative motor network, partial defect of visual field, and hemi-spatial agnosia are also factors that might prevent patients from going back to their professional work completely. On the other hand, however, the transient nature of SMA syndrome might permit more removal of the tumor infiltrating to the SMA. A concept of “onco-functional balance” is important in the neuro-oncological treatment to accommodate better outcomes of overall survival and functional morbidity, and QOL.93) Therefore, a tailor-made surgical strategy should be systematically proposed especially in slow-growing low-grade glioma.
Conclusion
A DES during awake surgery for the resection of glioma can identify cortical and subcortical eloquent structures by eliciting specific neurological symptoms, which originate from the transient disruption of cortico-subcortical axonal networks. Owing to the recent development of visualization techniques for white matter tracts, most of these bundles can be reconstructed and clearly visualized using DTI. Thus, DTI tractography provides neurosurgeons with knowledge that considerably aids preoperative planning for glioma surgery; however, the risk exists that neurosurgeons may be misled into inappropriate decisions during the surgical resection of brain structures, as DTI cannot visualize the actual anatomy of the white matter fibers. In addition to the critical information acquired via intraoperative DES, it is important to identify the surgical boundaries of brain regions not only with a view to achieve the maximal incision of the glioma but also with consideration to the concept of onco-functional balance.
Footnotes
Conflicts of Interest (COI) Disclosure
The authors declare that they have no conflict of interest. All authors who are the members of The Japan Neurosurgical Society (JNS) have registered online Self-reported COI Disclosure Statement Forms through the website for JNS members.
References
- 1). Duffau H: Stimulation mapping of white matter tracts to study brain functional connectivity. Nat Rev Neurol 11: 255– 265, 2015. [DOI] [PubMed] [Google Scholar]
- 2). Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, Tihan T, Vandenberg S, McDermott MW, Berger MS: Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 26: 1338– 1345, 2008. [DOI] [PubMed] [Google Scholar]
- 3). De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS: Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol 30: 2559– 2565, 2012. [DOI] [PubMed] [Google Scholar]
- 4). De Benedictis A, Duffau H: Brain hodotopy: from esoteric concept to practical surgical applications. Neurosurgery 68: 1709– 1723; discussion 1723, 2011. [DOI] [PubMed] [Google Scholar]
- 5). Duffau H: Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity. Lancet Neurol 4: 476– 486, 2005. [DOI] [PubMed] [Google Scholar]
- 6). Logothetis NK, Augath M, Murayama Y, Rauch A, Sultan F, Goense J, Oeltermann A, Merkle H: The effects of electrical microstimulation on cortical signal propagation. Nat Neurosci 13: 1283– 1291, 2010. [DOI] [PubMed] [Google Scholar]
- 7). Saito T, Muragaki Y, Maruyama T, Tamura M, Nitta M, Okada Y: Intraoperative functional mapping and monitoring during glioma surgery. Neurol Med Chir (Tokyo) 55 Suppl 1: 1– 13, 2015. [PubMed] [Google Scholar]
- 8). Tamura M, Muragaki Y, Saito T, Maruyama T, Nitta M, Tsuzuki S, Iseki H, Okada Y: Strategy of surgical resection for glioma based on intraoperative functional mapping and monitoring. Neurol Med Chir (Tokyo) 55: 383– 398, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Hayashi Y, Nakada M, Kinoshita M, Hamada J: Surgical strategies for nonenhancing slow-growing gliomas with special reference to functional reorganization: review with own experience. Neurol Med Chir (Tokyo) 53: 438– 446, 2013. [DOI] [PubMed] [Google Scholar]
- 10). Duffau H: Brain mapping: from neural basis of cognition to surgical applications. Wien New York, Springer, 2011. [Google Scholar]
- 11). Duffau H, Capelle L, Sichez N, Denvil D, Lopes M, Sichez JP, Bitar A, Fohanno D: Intraoperative mapping of the subcortical language pathways using direct stimulations: an anatomo-functional study. Brain 125: 199– 214, 2002. [DOI] [PubMed] [Google Scholar]
- 12). Duffau H, Lopes M, Arthuis F, Bitar A, Sichez JP, Van Effenterre R, Capelle L: Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: a comparative study between two series without (1985–96) and with (1996–2003) functional mapping in the same institution. J Neurol Neurosurg Psychiatr 76: 845– 851, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Catani M, Thiebaut de Schotten M: Atlas of Human brain connections. New York, Oxford University Press, 2012. [Google Scholar]
- 14). Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr., Pandya DN: Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex 15: 854– 869, 2005. [DOI] [PubMed] [Google Scholar]
- 15). Burdach K: Vom bau und leben des gehirns und rückenmarks. Leipzig, In der dyk'schen buchandlung, Vol. 2, 1822. [Google Scholar]
- 16). Martino J, De Witt Hamer PC, Berger MS, Lawton MT, Arnold CM, de Lucas EM, Duffau H: Analysis of the subcomponents and cortical terminations of the perisylvian superior longitudinal fasciculus: a fiber dissection and DTI tractography study. Brain Struct Funct 218: 105– 121, 2013. [DOI] [PubMed] [Google Scholar]
- 17). Fernandez-Miranda JC, Wang Y, Pathak S, Stefaneau L, Verstynen T, Yeh FC: Asymmetry, connectivity, and segmentation of the arcuate fascicle in the human brain. Brain Struct Funct 220: 1665– 1680, 2015. [DOI] [PubMed] [Google Scholar]
- 18). Bernal B, Ardila A: The role of the arcuate fasciculus in conduction aphasia. Brain 132: 2309– 2316, 2009. [DOI] [PubMed] [Google Scholar]
- 19). Catani M, Jones DK, ffytche DH: Perisylvian language networks of the human brain. Ann Neurol 57: 8– 16, 2005. [DOI] [PubMed] [Google Scholar]
- 20). Martino J, De Witt Hamer PC, Vergani F, Brogna C, de Lucas EM, Vazquez-Barquero A, Garcia-Porrero JA, Duffau H: Cortex-sparing fiber dissection: an improved method for the study of white matter anatomy in the human brain. J Anat 219: 531– 541, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Thiebaut de Schotten M, Dell'Acqua F, Valabregue R, Catani M: Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex 48: 82– 96, 2012. [DOI] [PubMed] [Google Scholar]
- 22). Hickok G, Poeppel D: The cortical organization of speech processing. Nat Rev Neurosci 8: 393– 402, 2007. [DOI] [PubMed] [Google Scholar]
- 23). Wilshire CE, McCarthy RA: Experimental investigations of an impairement in phonological encoding. Cogn Neuropsychol 13: 1059– 1098, 1996. [Google Scholar]
- 24). Maldonado IL, Moritz-Gasser S, de Champfleur NM, Bertram L, Moulinie G, Duffau H: Surgery for gliomas involving the left inferior parietal lobule: new insights into the functional anatomy provided by stimulation mapping in awake patients. J Neurosurg 115: 770– 779, 2011. [DOI] [PubMed] [Google Scholar]
- 25). Maldonado IL, Moritz-Gasser S, Duffau H: Does the left superior longitudinal fascicle subserve language semantics? A brain electrostimulation study. Brain Struct Funct 216: 263– 274, 2011. [DOI] [PubMed] [Google Scholar]
- 26). Moritz-Gasser S, Duffau H: The anatomo-functional connectivity of word repetition: insights provided by awake brain tumor surgery. Front Hum Neurosci 7: 405, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Dejerine J: Anatomie des Centres Nerveux. Paris, Rueff et Cie, Vol. 1, 1895. [Google Scholar]
- 28). Catani M, Howard RJ, Pajevic S, Jones DK: Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 17: 77– 94, 2002. [DOI] [PubMed] [Google Scholar]
- 29). Chang EF, Raygor KP, Berger MS: Contemporary model of language organization: an overview for neurosurgeons. J Neurosurg 122: 250– 261, 2015. [DOI] [PubMed] [Google Scholar]
- 30). Duffau H: The anatomo-functional connectivity of language revisited: new insights provided by electrostimulation and tractography. Neuropsychologia 46: 927– 934, 2008. [DOI] [PubMed] [Google Scholar]
- 31). Leclercq D, Duffau H, Delmaire C, Capelle L, Gatignol P, Ducros M, Chiras J, Lehericy S: Comparison of diffusion tensor imaging tractography of language tracts and intraoperative subcortical stimulations. J Neurosurg 112: 503– 511, 2010. [DOI] [PubMed] [Google Scholar]
- 32). Fridriksson J, Kjartansson O, Morgan PS, Hjaltason H, Magnusdottir S, Bonilha L, Rorden C: Impaired speech repetition and left parietal lobe damage. J Neurosci 30: 11057– 11061, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Thiebaut de Schotten M, Dell'Acqua F, Forkel SJ, Simmons A, Vergani F, Murphy DG, Catani M: A lateralized brain network for visuospatial attention. Nat Neurosci 14: 1245– 1246, 2011. [DOI] [PubMed] [Google Scholar]
- 34). Thiebaut de Schotten M, Tomaiuolo F, Aiello M, Merola S, Silvetti M, Lecce F, Bartolomeo P, Doricchi F: Damage to white matter pathways in subacute and chronic spatial neglect: a group study and 2 single-case studies with complete virtual ‘in vivo’ tractography dissection. Cereb Cortex 24: 691– 706, 2014. [DOI] [PubMed] [Google Scholar]
- 35). Thiebaut de Schotten M, Urbanski M, Duffau H, Volle E, Levy R, Dubois B, Bartolomeo P: Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science 309: 2226– 2228, 2005. [DOI] [PubMed] [Google Scholar]
- 36). Roux FE, Dufor O, Lauwers-Cances V, Boukhatem L, Brauge D, Draper L, Lotterie JA, Demonet JF: Electrostimulation mapping of spatial neglect. Neurosurgery 69: 1218– 1231, 2011. [DOI] [PubMed] [Google Scholar]
- 37). Wang X, Pathak S, Stefaneanu L, Yeh FC, Li S, Fernandez-Miranda JC: Subcomponents and connectivity of the superior longitudinal fasciculus in the human brain. Brain Struct Funct [Epub ahead of print], 2015. [DOI] [PubMed] [Google Scholar]
- 38). Kinoshita M, Nakajima R, Shinohara H, Miyashita K, Tanaka S, Okita H, Nakada M, Hayashi Y: Chronic spatial working memory deficit associated with the superior longitudinal fasciculus: a study using voxel-based lesion-symptom mapping and intraoperative direct stimulation in right prefrontal glioma surgery. J Neurosurg [Epub ahead of print], 2016. [DOI] [PubMed] [Google Scholar]
- 39). Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, Umarova R, Musso M, Glauche V, Abel S, Huber W, Rijntjes M, Hennig J, Weiller C: Ventral and dorsal pathways for language. Proc Natl Acad Sci U S A 105: 18035– 18040, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Galantucci S, Tartaglia MC, Wilson SM, Henry ML, Filippi M, Agosta F, Dronkers NF, Henry RG, Ogar JM, Miller BL, Gorno-Tempini ML: White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain 134: 3011– 3029, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Faro SH, Mohamed FB: Functional neuroradiology: principles and clinical applications. New York, Springer, 2011. [Google Scholar]
- 42). Sarubbo S, De Benedictis A, Maldonado IL, Basso G, Duffau H: Frontal terminations for the inferior fronto-occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Struct Funct 218: 21– 37, 2013. [DOI] [PubMed] [Google Scholar]
- 43). Almairac F, Herbet G, Moritz-Gasser S, de Champfleur NM, Duffau H: The left inferior fronto-occipital fasciculus subserves language semantics: a multilevel lesion study. Brain Struct Funct 220: 1983– 1995, 2015. [DOI] [PubMed] [Google Scholar]
- 44). Gatignol P, Capelle L, Le Bihan R, Duffau H: Double dissociation between picture naming and comprehension: an electrostimulation study. Neuroreport 15: 191– 195, 2004. [DOI] [PubMed] [Google Scholar]
- 45). Moritz-Gasser S, Herbet G, Duffau H: Mapping the connectivity underlying multimodal (verbal and non-verbal) semantic processing: a brain electrostimulation study. Neuropsychologia 51: 1814– 1822, 2013. [DOI] [PubMed] [Google Scholar]
- 46). Moritz-Gasser S, Herbet G, Duffau H: Integrating emotional valence and semantics in the human ventral stream: a hodological account. Front Psychol 6: 32, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Khan OH, Herbet G, Moritz-Gasser S, Duffau H: The role of left inferior fronto-occipital fascicle in verbal perseveration: a brain electrostimulation mapping study. Brain Topogr 27: 403– 411, 2014. [DOI] [PubMed] [Google Scholar]
- 48). Nieuwenhuys R, Voogd J, Huijzen Cv: The human central nervous system: a synopsis and atlas, ed 3 Berlin; New York, Springer-Verlag, 1988. [Google Scholar]
- 49). Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, Wedeen VJ: Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain 130: 630– 653, 2007. [DOI] [PubMed] [Google Scholar]
- 50). Zemmoura I, Herbet G, Moritz-Gasser S, Duffau H: New insights into the neural network mediating reading processes provided by cortico-subcortical electrical mapping. Hum Brain Mapp 36: 2215– 2230, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Fox CJ, Iaria G, Barton JJ: Disconnection in prosopagnosia and face processing. Cortex 44: 996– 1009, 2008. [DOI] [PubMed] [Google Scholar]
- 52). Catani M, Mesulam M: What is a disconnection syndrome? Cortex 44: 911– 913, 2008. [DOI] [PubMed] [Google Scholar]
- 53). Epelbaum S, Pinel P, Gaillard R, Delmaire C, Perrin M, Dupont S, Dehaene S, Cohen L: Pure alexia as a disconnection syndrome: new diffusion imaging evidence for an old concept. Cortex 44: 962– 974, 2008. [DOI] [PubMed] [Google Scholar]
- 54). Catani M, Mesulam M: The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex 44: 953– 961, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55). Mandonnet E, Nouet A, Gatignol P, Capelle L, Duffau H: Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain 130: 623– 629, 2007. [DOI] [PubMed] [Google Scholar]
- 56). Gil-Robles S, Carvallo A, Jimenez Mdel M, Gomez Caicoya A, Martinez R, Ruiz-Ocana C, Duffau H: Double dissociation between visual recognition and picture naming: a study of the visual language connectivity using tractography and brain stimulation. Neurosurgery 72: 678– 686, 2013. [DOI] [PubMed] [Google Scholar]
- 57). Han Z, Ma Y, Gong G, He Y, Caramazza A, Bi Y: White matter structural connectivity underlying semantic processing: evidence from brain damaged patients. Brain 136: 2952– 2965, 2013. [DOI] [PubMed] [Google Scholar]
- 58). Hamberger MJ, Drake EB: Cognitive functioning following epilepsy surgery. Curr Neurol Neurosci Rep 6: 319– 326, 2006. [DOI] [PubMed] [Google Scholar]
- 59). Herbet G, Lafargue G, Moritz-Gasser S, Menjot de Champfleur N, Costi E, Bonnetblanc F, Duffau H: A disconnection account of subjective empathy impairments in diffuse low-grade glioma patients. Neuropsychologia 70: 165– 176, 2015. [DOI] [PubMed] [Google Scholar]
- 60). Duffau H, Gatignol P, Moritz-Gasser S, Mandonnet E: Is the left uncinate fasciculus essential for language? A cerebral stimulation study. J Neurol 256: 382– 389, 2009. [DOI] [PubMed] [Google Scholar]
- 61). Seltzer B, Pandya DN: Further observations on parieto-temporal connections in the rhesus monkey. Exp Brain Res 55: 301– 312, 1984. [DOI] [PubMed] [Google Scholar]
- 62). Maldonado IL, de Champfleur NM, Velut S, Destrieux C, Zemmoura I, Duffau H: Evidence of a middle longitudinal fasciculus in the human brain from fiber dissection. J Anat 223: 38– 45, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63). De Witt Hamer PC, Moritz-Gasser S, Gatignol P, Duffau H: Is the human left middle longitudinal fascicle essential for language? A brain electrostimulation study. Hum Brain Mapp 32: 962– 973, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64). Chan-Seng E, Moritz-Gasser S, Duffau H: Awake mapping for low-grade gliomas involving the left sagittal stratum: anatomofunctional and surgical considerations. J Neurosurg 120: 1069– 1077, 2014. [DOI] [PubMed] [Google Scholar]
- 65). Gras-Combe G, Moritz-Gasser S, Herbet G, Duffau H: Intraoperative subcortical electrical mapping of optic radiations in awake surgery for glioma involving visual pathways. J Neurosurg 117: 466– 473, 2012. [DOI] [PubMed] [Google Scholar]
- 66). Catani M, Dell'Acqua F, Vergani F, Malik F, Hodge H, Roy P, Valabregue R, Thiebaut de Schotten M: Short frontal lobe connections of the human brain. Cortex 48: 273– 291, 2012. [DOI] [PubMed] [Google Scholar]
- 67). Rech F, Herbet G, Moritz-Gasser S, Duffau H: Somatotopic organization of the white matter tracts underpinning motor control in humans: an electrical stimulation study. Brain Struct Funct [Epub ahead of print], 2015. [DOI] [PubMed] [Google Scholar]
- 68). Duffau H, Capelle L, Denvil D, Sichez N, Gatignol P, Taillandier L, Lopes M, Mitchell MC, Roche S, Muller JC, Bitar A, Sichez JP, van Effenterre R: Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg 98: 764– 778, 2003. [DOI] [PubMed] [Google Scholar]
- 69). Kikuchi T, Matsumoto R, Mikuni N, Yokoyama Y, Matsumoto A, Ikeda A, Fukuyama H, Miyamoto S, Hashimoto N: Asymmetric bilateral effect of the supplementary motor area proper in the human motor system. Clin Neurophysiol 123: 324– 334, 2012. [DOI] [PubMed] [Google Scholar]
- 70). Schucht P, Moritz-Gasser S, Herbet G, Raabe A, Duffau H: Subcortical electrostimulation to identify network subserving motor control. Hum Brain Mapp 34: 3023– 3030, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71). Enatsu R, Matsumoto R, Piao Z, O'Connor T, Horning K, Burgess RC, Bulacio J, Bingaman W, Nair DR: Cortical negative motor network in comparison with sensorimotor network: a cortico-cortical evoked potential study. Cortex 49: 2080– 2096, 2013. [DOI] [PubMed] [Google Scholar]
- 72). Kinoshita M, de Champfleur NM, Deverdun J, Moritz-Gasser S, Herbet G, Duffau H: Role of fronto-striatal tract and frontal aslant tract in movement and speech: an axonal mapping study. Brain Struct Funct 220: 3399– 3412, 2015. [DOI] [PubMed] [Google Scholar]
- 73). Rech F, Herbet G, Moritz-Gasser S, Duffau H: Disruption of bimanual movement by unilateral subcortical electrostimulation. Hum Brain Mapp 35: 3439– 3445, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74). Kinoshita M, Shinohara H, Hori O, Ozaki N, Ueda F, Nakada M, Hamada J, Hayashi Y: Association fibers connecting the Broca center and the lateral superior frontal gyrus: a microsurgical and tractographic anatomy. J Neurosurg 116: 323– 330, 2012. [DOI] [PubMed] [Google Scholar]
- 75). Fujii M, Maesawa S, Motomura K, Futamura M, Hayashi Y, Koba I, Wakabayashi T: Intraoperative subcortical mapping of a language-associated deep frontal tract connecting the superior frontal gyrus to Broca's area in the dominant hemisphere of patients with glioma. J Neurosurg 122: 1390– 1396, 2015. [DOI] [PubMed] [Google Scholar]
- 76). Catani M, Mesulam MM, Jakobsen E, Malik F, Martersteck A, Wieneke C, Thompson CK, Thiebaut de Schotten M, Dell'Acqua F, Weintraub S, Rogalski E: A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain 136: 2619– 2628, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77). Martino J, de Lucas EM, Ibanez-Plagaro FJ, Valle-Folgueral JM, Vazquez-Barquero A: Foix–Chavany–Marie syndrome caused by a disconnection between the right pars opercularis of the inferior frontal gyrus and the supplementary motor area. J Neurosurg 117: 844– 850, 2012. [DOI] [PubMed] [Google Scholar]
- 78). Kronfeld-Duenias V, Amir O, Ezrati-Vinacour R, Civier O, Ben-Shachar M: The frontal aslant tract underlies speech fluency in persistent developmental stuttering. Brain Struct Funct 221: 365– 381, 2016. [DOI] [PubMed] [Google Scholar]
- 79). Yeatman JD, Weiner KS, Pestilli F, Rokem A, Mezer A, Wandell BA: The vertical occipital fasciculus: a century of controversy resolved by in vivo measurements. Proc Natl Acad Sci U S A 111: E5214– E5223, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80). Wernicke C: Lehrbuch der Gehirnkrankheiten für Ärzte und Studierende. Verlag von Theodor Fischer, Kassel, 1881. [Google Scholar]
- 81). Martino J, Garcia-Porrero JA: Wernicke perpendicular fasciculus and vertical portion of the superior longitudinal fasciculus: in reply. Neurosurgery 73: E382– E383, 2013. [DOI] [PubMed] [Google Scholar]
- 82). Wager M, Du Boisgueheneuc F, Pluchon C, Bouyer C, Stal V, Bataille B, Guillevin CM, Gil R: Intraoperative monitoring of an aspect of executive functions: administration of the Stroop test in 9 adult patients during awake surgery for resection of frontal glioma. Neurosurgery 72: ons169– ons180; discussion ons180–ons181, 2013. [DOI] [PubMed] [Google Scholar]
- 83). Herbet G, Lafargue G, de Champfleur NM, Moritz-Gasser S, le Bars E, Bonnetblanc F, Duffau H: Disrupting posterior cingulate connectivity disconnects consciousness from the external environment. Neuropsychologia 56: 239– 244, 2014. [DOI] [PubMed] [Google Scholar]
- 84). Duffau H: The dangers of magnetic resonance imaging diffusion tensor tractography in brain surgery. World Neurosurg 81: 56– 58, 2014. [DOI] [PubMed] [Google Scholar]
- 85). Kinoshita M, Yamada K, Hashimoto N, Kato A, Izumoto S, Baba T, Maruno M, Nishimura T, Yoshimine T: Fiber-tracking does not accurately estimate size of fiber bundle in pathological condition: initial neurosurgical experience using neuronavigation and subcortical white matter stimulation. Neuroimage 25: 424– 429, 2005. [DOI] [PubMed] [Google Scholar]
- 86). Feigl GC, Hiergeist W, Fellner C, Schebesch KM, Doenitz C, Finkenzeller T, Brawanski A, Schlaier J: Magnetic resonance imaging diffusion tensor tractography: evaluation of anatomic accuracy of different fiber tracking software packages. World Neurosurg 81: 144– 150, 2014. [DOI] [PubMed] [Google Scholar]
- 87). Tuch DS, Reese TG, Wiegell MR, Wedeen VJ: Diffusion MRI of complex neural architecture. Neuron 40: 885– 895, 2003. [DOI] [PubMed] [Google Scholar]
- 88). Wedeen VJ, Rosene DL, Wang R, Dai G, Mortazavi F, Hagmann P, Kaas JH, Tseng WY: The geometric structure of the brain fiber pathways. Science 335: 1628– 1634, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89). Tournier JD, Calamante F, Gadian DG, Connelly A: Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neuroimage 23: 1176– 1185, 2004. [DOI] [PubMed] [Google Scholar]
- 90). Desmurget M, Bonnetblanc F, Duffau H: Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain 130: 898– 914, 2007. [DOI] [PubMed] [Google Scholar]
- 91). Duffau H, Thiebaut de Schotten M, Mandonnet E: White matter functional connectivity as an additional landmark for dominant temporal lobectomy. J Neurol Neurosurg Psychiatr 79: 492– 495, 2008. [DOI] [PubMed] [Google Scholar]
- 92). Ius T, Angelini E, Thiebaut de Schotten M, Mandonnet E, Duffau H: Evidence for potentials and limitations of brain plasticity using an atlas of functional resectability of WHO grade II gliomas: towards a ‘minimal common brain’. Neuroimage 56: 992– 1000, 2011. [DOI] [PubMed] [Google Scholar]
- 93). Duffau H, Mandonnet E: The ‘onco-functional balance’ in surgery for diffuse low-grade glioma: integrating the extent of resection with quality of life. Acta Neurochir (Wien) 155: 951– 957, 2013. [DOI] [PubMed] [Google Scholar]