Abstract
Medulloblastoma (MB) is one of the most frequent malignant brain tumors in children. The current standard treatment regimen consists of surgical resection, craniospinal irradiation, and adjuvant chemotherapy. Although these treatments have the potential to increase the survival of 70–80% of patients with MB, they are also associated with serious treatment-induced morbidity. The current risk stratification of MB is based on clinical factors, including age at presentation, metastatic status, and the presence of residual tumor following resection. In addition, recent genomic studies indicate that MB consists of at least four distinct molecular subgroups: WNT, sonic hedgehog (SHH), Group 3, and Group 4. WNT and SHH MBs are characterized by aberrations in the WNT and SHH signaling pathways, respectively. WNT MB has the best prognosis compared to the other MBs, while SHH MB has an intermediate prognosis. The underlying signaling pathways associated with Group 3 and 4 MBs have not been identified. Group 3 MB is frequently associated with metastasis, resulting in a poor prognosis, while Group 4 is sometimes associated with metastasis and has an intermediate prognosis. Group 4 is the most frequent MB and represents 35% of all MBs. These findings suggest that MB is a heterogeneous disease, and that MB subgroups have distinct molecular, demographic, and clinical characteristics. The molecular classification of MBs is redefining the risk stratification of patients with MB, and has the potential to identify new therapeutic strategies for the treatment of MB.
Keywords: medulloblastoma, molecular subgroups, prognostic factors, SHH, WNT
Introduction
Medulloblastoma (MB) is one of the most common pediatric malignant brain tumors, representing up to 20% of newly diagnosed central nervous system tumors in children.1) Current standard treatments include surgical resection, craniospinal irradiation with a posterior fossa boost, and adjuvant chemotherapy.2) Although these strategies have the potential to increase the survival of 70–80% of patients with MB, they are associated with serious treatment-induced morbidity.3,4) Current treatment protocols stratify patients into high and average risk groups according to their age, metastatic status, and the presence of residual tumor following resection.5,6) However, the disadvantage of this risk stratification system is that it fails to take MB heterogeneity into account. The recent studies indicate that MB consists of at least four distinct molecular subgroups: WNT, sonic hedgehog (SHH), Group 3, and Group 4.7) Each subgroup has distinct molecular, demographic, and clinical characteristics.7–11) Moreover, the molecular classification system is an important prognostic tool that has the potential to improve the treatment of patients with MB.12)
In this study, we review the molecular classification of MB, its prognostic value, and its clinical importance.
Current Risk Stratification and Histological Classification of MB
The patients with MB are currently stratified into two risk groups on the basis of the following criteria: extent of resection, age at diagnosis, and metastatic status.5,6) Patients with residual tumors (>1.5 cm2), who are <3 years of age at diagnosis, and/or exhibit the presence of metastatic disease are classified as high-risk patients, while the remainders are classified as average-risk patients. After treatment consisting of maximum surgical resection, craniospinal irradiation, and adjuvant chemotherapy, the cure rates of average- and high-risk patients are 85 and 70%, respectively.2,13,14)
MB is classified into five histological subtypes: classic, desmoplastic, anaplastic, large cell, and MB with extensive nodularity (MBEN). The classic subtype is most prevalent, while the desmoplastic and MBEN subtypes have the best outcomes.15,16) However, even though more than 70% of patients have classic MBs, their response to treatment is highly variable, suggesting that classic MBs are highly heterogeneous and that histological classification many have limited prognostic value.
Molecular Subgroups of MB
Recently, the integrated genomic profiling of MBs was conducted,1,7,17–19) and the results indicate that MB consists of at least four distinct molecular subgroups: WNT, SHH, Group 3, and Group 4.7) The demographic, transcriptional, genetic, and clinical differences among these four subgroups have important implications (Table 1).12,20,21)
Table 1.
Characteristics of each subgroup of MB
| WNT | SHH | Group 3 | Group 4 | |
|---|---|---|---|---|
| Prevalence | 10% | 30% | 25% | 35% |
| Age | Children, teens | Infants, adults | Infants, children | Infants, children, adults |
| Sex (M:F) | 1:1 | 1:1 | 2:1 | 3:1 |
| Histology | Classic | Nodular desmoplastic histology, classic, LCA | Classic, LCA | Classic, LCA |
| Metastasis | Low | Low | High | High |
| Recurrence | Rare | Local | Metastasis | Metastasis |
| Prognosis | Best | Intermediate | Poor | Intermediate |
| 5-Year OS | 95% | 75% | 50% | 75% |
| Genetics | CTNNB1 DDX3X SMARCA4 | MYCN, GLI2, PTCH1, SUFU, MLL2, SMO, TP53, BCOR1, LDB1, GABRG1 | MYC, PVT1, OTX2, MLL2, SMARCA4, CHD7 | OTX2, DDX31, CHD7, SNCAIP, MYCN, CDK6 GFI1/GFI1B, MLL2, KDM6A, MLL3, ZMYM3 |
| Chromosome | chr 6 loss | chr 3q gain, chr 9q loss, 10q loss | chr 1q gain, chr 5q loss, 10q loss | Isochromosome 17q chr X loss, 17p loss |
| Cells of origin | Lower rhombic lip progenitors | Cerebellar granule neuron precursors | Neural stem cells? | Upper rhombic lip progenitors |
LCA: large cell anaplastic.
WNT subgroup
The WNT MB is the rarest subgroup, accounting for ∼10% of all MBs, and is the subgroup with the best prognosis. Germline mutations in the gene encoding the WNT pathway inhibitor, APC, predispose individuals to develop Turcot syndrome, which increases the risk of developing MB. The loss of chromosome 6 and activating mutations in the gene encoding β-catenin are commonly found in WNT MBs.12) Other recurrent somatic mutations are also found in the genes encoding p53, DDX3X (the dead-box RNA helicase, which is involved in cell growth and proliferation), and SMARCA4, a chromatin modifier. WNT tumors frequently exhibit classic histology.22–24) However, some also exhibit large cell and anaplastic histologies, and these tumors also have a good long-term prognosis. Notably, WNT MBs develop from progenitor cells of the lower rhombic lip.25) WNT MBs are typically located at the midline of the brain, and occupy the fourth ventricle. They typically infiltrate the brain stem.20)
WNT MB rarely presents with metastasis. The sex ratio for WNT MBs is 1:1,21) and these MBs are most commonly found in older children and teenagers, and are rarely found in infants. Outcomes for patients with WNT MB are good, with 5-year overall survival rates exceeding 90%.22,26) Thus, current plans for WNT MB clinical trials are focused on developing therapy de-escalation protocols that maintain the high-cure rates, while diminishing the adverse effects of therapy. These protocols are expected to include dose-reduced craniospinal radiation and/or decreased chemotherapy regimens.
SHH subgroup
The SHH subgroup accounts for ∼30% of all MBs, and is characterized by aberrations in the SHH signaling pathway.27–30) Individuals with germline mutations in the gene encoding the SHH receptor PTCH have Gorlin syndrome, which predisposes them to MB.31,32) Similarly, individuals with germline mutations in the gene encoding the SHH inhibitor SUFU are predisposed to infantile MB.28,33) In addition, somatic mutations in the genes encoding PTCH, SUFU, and the SHH co-receptor, SMO (smoothened homolog), as well as amplification of the genes encoding the GLI1 and GLI2 transcription factors have been found in sporadic MB.27,28,30) Other somatic mutations in the genes encoding TP53 (p53) and MLL2 (or KMT2D), a lysine-specific methyltransferase, are found, respectively, in 14 and 12% of the patients with SHH MBs.34) Furthermore, SHH MBs are frequently associated with a loss of chromosome 9q, and less frequently associated with a loss of 17p or 10q, or a gain of 3q.12)
Transcriptome analysis indicates that adult and pediatric SHH MBs are transcriptionally distinct.27) Most infant SHH MBs carry PTCH1 or SUFU mutations, which are present in the patient’s germline. In children, SHH MBs harbor broader genetic heterogeneity, including SHH, GLI2, and MYCN amplifications, as well as somatic and germline TP53 mutations, together with PTCH1 mutations.20) Adult SHH MBs are typically characterized by PTCH1 and SMO mutations. Furthermore, whole genome sequencing of SHH MBs indicated the presence of recurrent somatic mutations in KMT2D, TP53, DDX3X, and the genes encoding the BCL6 corepressor, BCOR, the LIM domain-binding protein, LDB1, and the GABA(A) receptor alpha 1 subunit, GABRG1.9–11,20)
SHH MBs are frequently present with a nodular desmoplastic histology, although this histology is found in <50% of all SHH MBs, while the remaining SHH MBs exhibit classic histology.21) The large cell anaplastic (LCA) histology has also been found in SHH MBs, but it is unclear if this histology is a prognostic indicator for SHH MB.
Most of the mouse models of MB represent the SHH MB subgroup (Table 2).35–37) In these models, MB arises from the cerebellar granule neuron precursors, suggesting that SHH tumors originate in the external granule layer cells of the cerebellum.
Table 2.
Summary of preclinical models of MB
| Classification | MB subgroup | MB incidence |
|---|---|---|
| Genetically engineered mouse models (GEMMs) | ||
| Ptc+/− | SHH | 14% |
| Ptc+/− p53−/− | SHH | 95% |
| Ptc+/− Ink4c+/− or −/− | SHH | 30% |
| Ptc+/− Kip1+/− or −/− | SHH | 60–70% |
| Ptc+/− Hic1−/− | SHH | ∼40% |
| Math1–Cre/Ptc C/C | SHH | 100% |
| Gfap–Cre/Ptc C/C | SHH | 100% |
| Sufu+/−/P53−/− | SHH | 58% |
| Hemizygous ND2–SmoA | SHH | 48% |
| Homozygous ND2–SmoA | SHH | 94% |
| Gfap–Cre/Rb loxp/loxp/Tp53 −/− or loxp/loxp | SHH | >84% |
| Lig4−/−/p53−/− | SHH | 100% |
| Nestin–Cre/Xrcc4 loxp/loxp/p53−/− | SHH | 87% |
| Nestin–Cre/Xrcc2 loxp/loxp/p53−/− | SHH | >90% |
| Nestin–Cre/Lig4 loxp/loxp/p53−/− | SHH | >90% |
| Nestin–Cre/Brca2 loxp/loxp/p53−/− | SHH | >90% |
| Parp1−/−/p53−/− | SHH | 49% |
| GTML | Classic or LCA | 75% |
| Blbp–Cre/Ctnnb1+/lox(ex3)/Tp53 flx/flx | WNT | 15% |
| Retroviral mouse model | ||
| SHH expressing retrovirus | SHH | 76% |
| Transplanted transduced cells or RCAS/tv-a system | ||
| Neural progenitors with RCAS (SHH + AKT) | SHH | 48% |
| Neural progenitors with RCAS (SHH + IGF2) | SHH | 39% |
| Neural progenitors with RCAS (SHH + MYC) | SHH? | 23% |
| Neural progenitors with RCAS (SHH + MYCN T50A) | SHH | 78% |
| p53 null neural progenitors with RCAS (MYC) | Group 3? | 50% |
| Neural progenitors with RCAS (MYC + Bcl2) | Group 3? | 18% |
| p53 null cerebellar progenitor cells transduced with MYC | Group 3? | 100% |
| Cerebellar stem cells expressing MYC and mutant p53 | Group 3? | 33% |
| Patient-derived xenograft models (PDXs) | ||
| Icb-1192 | WNT | N/A |
| Icb-1140 | WNT | N/A |
| Icb-1338 | SHH | N/A |
| Ic-984 | SHH | N/A |
| HD-MB03 | Group 3 | N/A |
| MB3W1 | Group 3 | 100% |
| Icb-1572 | Group 3 | N/A |
| Icb-1494 | Group 3 | N/A |
| Icb-1595 | Group 3 | N/A |
| Icb-1197 | Group 3 | N/A |
| Icb-1299 | Group 4 | N/A |
| Icb-1078 | Group 4 | N/A |
| Icb-1487 | Group 4 | N/A |
LCA: large cell anaplastic, N/A: not available.
Similar to the WNT subgroup, the sex ratio in SHH MB is 1:1. The tumors are found at the brain midline in infants or in the cerebellar hemispheres in teenagers and adults. Metastasis at tumor presentation is not common. Furthermore, SHH MB exhibits a bimodal age distribution, predominantly affecting infants and adults, and rarely affecting children. As mentioned above, the SHH MBs in infants and adults are clinically and molecularly distinct.27) Notably, metastasis at presentation is a prognostic indicator in adult but not infant SHH MB.27)
The prognosis of SHH MB appears to be similar to that of Group 4 MB, and intermediate between that of WNT and Group 3 MB,1) and the 5-year overall survival of patients with SHH MB is ∼75% when treated with the current standard therapy.19,20) A recent finding indicates that p53 status is the most important risk factor for SHH MB. The 5-year overall survival rate is 41 and 81% for patients with SHH MBs with and without TP53 mutations, respectively, suggesting that SHH MBs are heterogeneous tumors.38)
Certain SHH MBs can be cured without radiotherapy, whereas others, associated with MYCN and GLI2 amplifications or TP53 mutations, have a poor prognosis, even when patients are treated with high-dose craniospinal radiation and adjuvant chemotherapy.39) The most attractive therapeutic target for these tumors is the SHH pathway. Small molecule inhibitors of SMO have recently been synthesized and extensively studied in patients with SHH MB.40,41) The results of these studies are promising, suggesting that SHH inhibition represents a new treatment strategy for SHH tumors and that the SHH subgroup is likely to be the first to benefit from targeted therapy.
Group 3
Group 3 tumors account for ∼25% of all MB cases and have the poorest prognosis of the MB subgroups. Group 3 tumors are frequently associated with genomic instability and MYC amplification, and present with large-cell/anaplastic histology.7,12,34,42) While SHH subgroup tumors express high MYCN levels, Group 3 tumors express high MYC levels, and Group 4 tumors express relatively low levels of both MYC and MYCN.7,18) MYC amplification appears to be restricted to Group 3 tumors, while the amplification of OTX2, a MB oncogene, occurs in both Group 3 and 4 tumors.7) The MYC and OTX2 amplicons are mutually exclusive, suggesting that two distinct pathways contribute to the neoplasia in Group 3 MB.8) Group 3 tumors are much more likely to show a gain of chromosome 1q or a loss of chromosomes 5q or 10q. Furthermore, translocations between MYC and PVT, a long non-coding RNA, are frequently found in Group 3 tumors,8) suggesting that MYC has complex functions in tumorigenesis. Group 3 MB has also been associated with recurrent mutations in the genes encoding the chromatin remodeling proteins, SMARCA4, KMT2D, and CHD7 and a variety of mutations in the KDM gene family.8,11,34) Natriuretic peptide receptor 3 (NRP3) is detected immunohistochemically in these tumors and is a candidate Group 3 MB marker.7)
Approximately, 50% of Group 3 patients with MB present with metastasis.43) The 5-year overall survival rates are 45 and 58% for infants and children with Group 3 MB, respectively.12,34) Group 3 MB is more common in males than in females, and is observed in infants and children, rarely in teenagers, and never in adults.21) Indeed, while pediatric MBs consist of four distinct subgroups, adult MBs consist of only three.7,44)
The high rate of metastasis associated with Group 3 MBs and their poor prognosis suggest that more effective therapies are needed for patients with these tumors. However, existing protocols for the treatment of patients with high-risk MB are already associated with considerable morbidity. Thus, further research is required to clarify the underlying mechanisms associated with Group 3 MB and develop novel therapeutic strategies. Group 3 tumors are associated with TGF-beta signaling,8) suggesting that strategies targeting TGF-beta signaling pathways might provide safer and more effective treatment options for Group 3 MB.
Group 4
Group 4 tumors account for ∼35% of all MBs, and are the most frequent subgroup. These tumors exhibit an intermediate prognosis, similar to that of the SHH subgroup. However, the underlying biology of Group 4 tumors is less understood than that of the other subgroups.
Genetic alterations that are frequently found in Group 4 tumors overlap with those associated with Group 3 MB, including mutations in the KDM gene family, OTX2 amplicons, DDX31 deletions, CHD7 mutations, GFI1/GFI1B amplification, and KMT2D and KMT2C mutations. However, in contrast to Group 3 MB, MYCN rather than MYC amplification is observed in Group 4 MB.8–11,34 )
Group 4 tumors are typically characterized by the amplification of MYCN and isochromosome 17q.7,18) Although isochromosome 17q is also present in Group 3 tumors, it is more common in Group 4 tumors.7,18) Similarly, the isolated 17p deletion is seen in both Group 3 and 4, but is almost never found in WNT or SHH tumors.19) Previous publications indicate that Group 4 tumors exhibit neuronal gene expression signatures1,45) that include mutations in the genes encoding KDM6A, MLL3, and the zinc finger MYM-type protein, ZMYM3. Notably, mutations in KDM6A are the most common mutations in Group 4 MB.20) Another recurrent genetic alteration in Group 4 tumors is a tandem duplication of the gene encoding synuclein alpha interacting protein (SNCAIP),8) which is also duplicated in Parkinson’s disease. Finally, X chromosome loss is also associated with Group 4 tumors, and is seen in ∼80% of the tumors in females.
Group 4 MB is found in all age groups, although rarely in infants, and the sex ratio is 3:1 (male:female).21) Metastasis at presentation is reported in ∼35–40% of the cases.12,34) Patients with standard-risk Group 4 MB have a 5-year overall survival rate that exceeds 80%, while patients with high-risk Group 4 MB have a 5-year overall survival of ∼60%.39)
Group 4 tumors usually exhibit classic histology, although in some cases they exhibit LCA histology. Only a minority of Group 4 cases present with metastasis. The potassium channel KCNA1 is detected immunohistochemically in these tumors, and is a candidate Group 4 MB marker.7)
Similar to other subgroups, Group 4 MB may have distinct subtypes. Group 4 MBs that express follistatin-like 5 (FSTL5) have a poor prognosis, whereas Group 4 MBs that do not express FSTL5 have a better prognosis.21,46)
Since the current standard therapy can cure a high proportion of patients with average risk Group 4, MB therapy de-escalation may be possible for these patients.20) However, for patients with high-risk disease, the prognosis is still poor, suggesting that more effective therapies are needed. Further research on Group 4 MBs is required to identify Group 4-specific molecular targets.
The identification and characterization of the four MB subgroups have revolutionized MB research and clinical activities. A meta-analysis of 550 MBs from seven independent studies concluded that the subgrouping of MB is the most important prognostic factor independent of the current risk stratifications.12,34) These studies suggest that molecular subgroups will be a mainstay in the risk stratification of patients with MB that will complement the clinical and histological stratification protocols.
MB Recurrence and Metastasis
Recent studies examining the MB subgroup composition of primary and matched recurrent tumors or matched metastatic compartments found that the subgroups remain the same across the various compartments.47,48) Interestingly, however, the MB recurrence patterns are different in the various subgroups. In those cases of tumor recurrence, SHH MBs primarily exhibit local recurrence, whereas Group 3 and 4 MBs exhibit metastatic recurrence.48) These findings have important ramifications for treatment; increased local treatment should be considered for patients with SHH MB, while treatments focused on controlling metastasis should be considered for patients with Group 3 or 4 MB. These findings also indicate that subgroup-specific strategies may be required for the treatment of recurrent MB. Further research is needed to identify the molecular mechanisms involved in local tumor recurrence and metastasis in the various MB subgroups.
Clinical Importance of MB Subgroups
The identification and characterization of MB subgroups over the past few years has dramatically changed our perspective of MB. It is now clear that MB, which was previously considered to be a single disease entity, consists of at least four distinct molecular subgroups, and these subgroups are clinically relevant. As shown in this review, the four subgroups have unique demographic, transcriptional, genetic, and clinical features, and the prognosis of patients with MB is highly associated with their subgroup classification. Thus, the molecular subgrouping of MB is expected to add significantly to outcome predictions, more than any of the established clinical prognostic markers, such as, age, metastatic stage at diagnosis, extent of resection, and World Health Organization (WHO) classification. In addition to impacting patients with MB stratification and treatment strategies, the molecular subgrouping of MB will contribute to the identification of subgroup-specific targeted therapies. As discussed earlier, SMO inhibitors have recently been developed and tested on patients with SHH MB, with promising results. Subgroup-specific therapeutics has the potential to be more effective and to have significantly improved safety profiles compared to current standard therapies.
New Risk Stratification Systems for MB
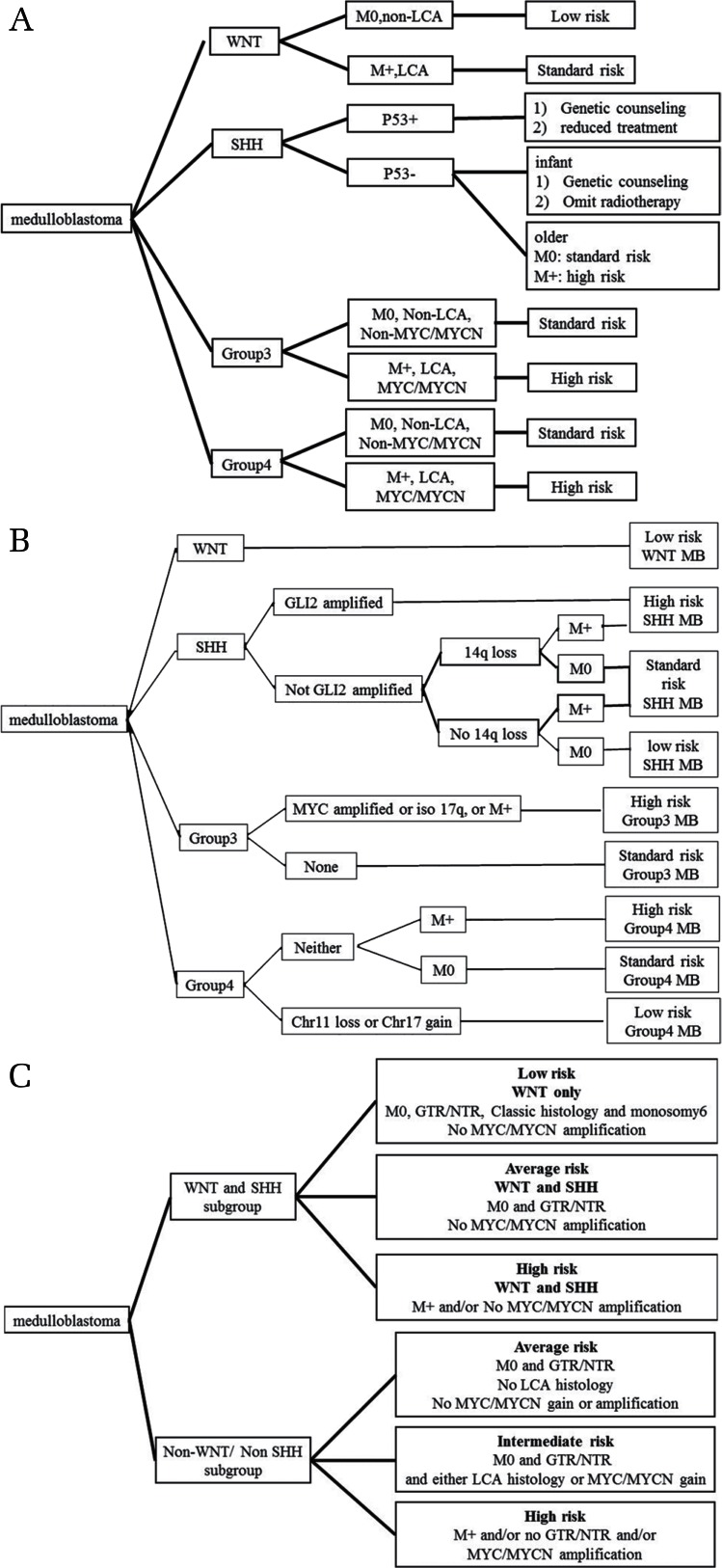
The current risk stratification for MB is based on the patient age, metastatic stage, and extent of resection. Because it is now recognized that MB consists of at least four molecular subgroups with different demographics, transcriptomes, genetics, and clinical outcomes, recent studies have proposed new risk stratification systems for MB based on clinical, histological, and molecular variables.39,49,50) These studies suggest that determining the clinical stage (residual tumor, metastasis, and age), histopathological subtype, and molecular subgroup are critical prerequisites for patient-risk stratification and risk-adapted therapeutic strategies. The new proposed risk stratification systems for MB are summarized in Fig. 1.
Fig. 1.
Newly proposed risk stratification systems for MB patients. (A) Risk stratification proposed by Pietsch et al.49) (B) Risk stratification proposed by Shih et al.39) (C) Risk stratification proposed by Gottardo et al.50) LCA: large cell anaplastic, M: metastatic status, GTR: gross total resection, NTR: near total resection.
Cells of Origin of MB
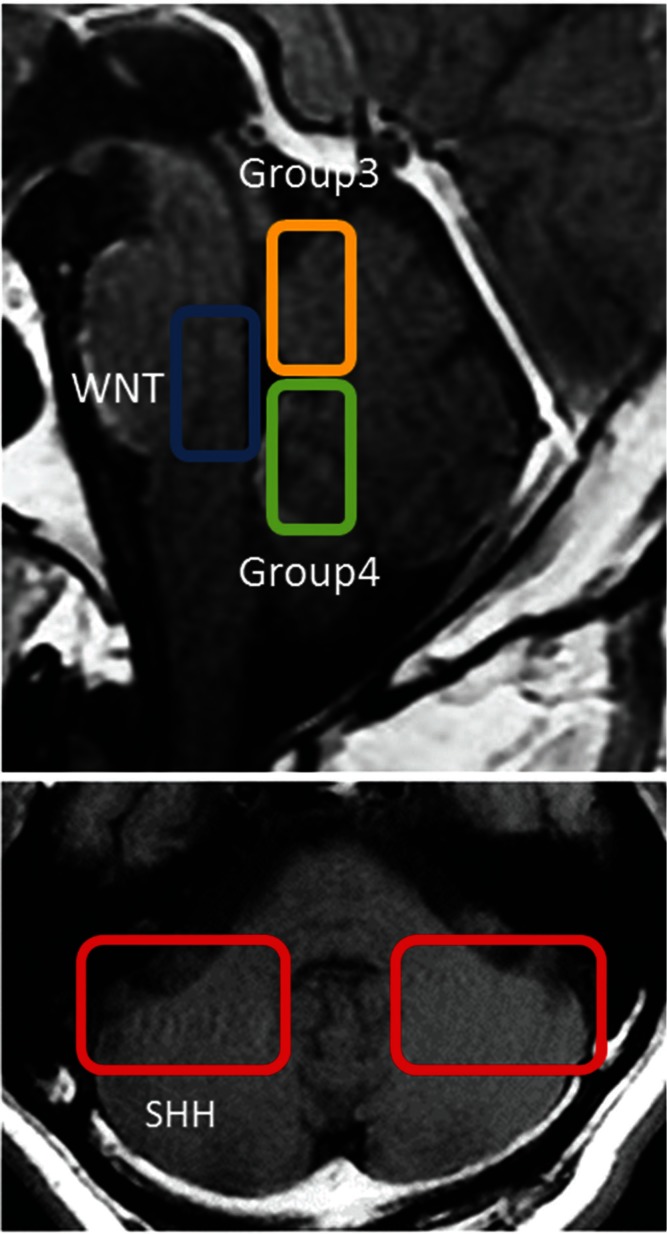
Recent studies have sought to identify the cells of origin for each subgroup of MB (Table 1). WNT MB is reported to develop from progenitor cells of the lower rhombic lip,25) and SHH MB from cerebellar granule neuron precursors.35–37) Group 3 MB is proposed to originate from neural stem cells. The cells of origin of Group 4 MB have long been unknown, but a very recent study suggests that they are progenitors of the upper rhombic lip (uRL).51) The characteristic MRI findings of patients with MB from each subgroup were also recently reported.52,53) Most of the Group 3 and Group 4 tumors grow in the vermis and infiltrate the fourth ventricle. WNT MB contacts the brain stem and expands into the fourth ventricle. Conversely, SHH MB grows predominantly in the rostral cerebellar hemisphere. These locations may be related to the cells of origin of each MB subgroup52,53) These MRI findings are summarized in Fig. 2.
Fig. 2.
MB localization of the various MB subgroups.
Preclinical Models of MB
Genetically engineered mouse models and patient-derived xenograft models are the most common preclinical models used in cancer biology studies. These preclinical models are indispensable tools for the basic biology and translational research of MB. However, many of the previously reported genetically engineered mouse models of MB represent the SHH subgroup (Table 2).54) Now that MB is known to consist of four distinct subgroups, preclinical models representing each subgroup are essential for future MB research. Recently, mouse models of MB subgroups other than SHH were established by transplanting p53-null cerebellar progenitor cells transduced with MYC55) or cerebellar stem cells expressing MYC and mutant p5356) into mouse brain. These models appear to represent Group 3 MB. In addition, many efforts are underway to establish patient-derived xenograft models of MB representing all four subgroups of MB;57–59) however, many of the MB tumor cells that can be passaged in vitro or in vivo are Group 3 MB, and only a few xenograft models are of Group 4 MB. Because most of the genetically engineered mouse models of MB are of the SHH subgroup, these xenograft models representing other subgroups are essential for MB research.
Another preclinical model of MB is a retroviral mouse model generated by injecting an SHH-expressing retrovirus into the embryonic mouse cerebellum.60) The RCAS/tv-a system similarly generates mouse models by orthotopic cell transplantation into a transgenic mouse. In this system, RCAS, replication-competent avian leucosis virus splice-acceptor subgroup-A (ALV-A) vectors carrying transgenes are transferred into cells, and the virus-producing cells are then injected into the brain of a transgenic mouse. The RCAS/tv-a system has been used to simultaneously transfer multiple transgenes, including MYCN, MYC, IGF2, Akt, Gli1, Bcl2 into nestin-expressing cell populations in the cerebellum of newborn mice, to induce MB in the neural progenitors.61–64) These mouse models generated using the RCAS/tv-a system represent SHH and probably Group 3 MB. The previously reported preclinical models of MB are summarized in Table 2.
Future Prospects
Future studies are needed to clarify the underlying biology of each subgroup and to contribute to the development of new MB treatment strategies and new molecular therapeutic targets for Group 3 and Group 4 MBs. Recent studies indicate that the four MB subgroups are most likely comprised of multiple distinct subtypes. More detailed genomic analyses, including whole transcriptome sequencing and epigenomic analysis may be useful for clarifying all of the MB subtypes. The results of these studies will further improve patient stratification and treatment strategies, and lead to the development of safer and more effective treatments for MB.
Footnotes
Conflicts of Interest Disclosure
The authors have no conflict of interest.
References
- 1). Cho YJ, Tsherniak A, Tamayo P, Santagata S, Ligon A, Greulich H, Berhoukim R, Amani V, Goumnerova L, Eberhart CG, Lau CC, Olson JM, Gilbertson RJ, Gajjar A, Delattre O, Kool M, Ligon K, Meyerson M, Mesirov JP, Pomeroy SL: Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol 29: 1424– 1430, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, Woo S, Wheeler G, Ahern V, Krasin MJ, Fouladi M, Broniscer A, Krance R, Hale GA, Stewart CF, Dauser R, Sanford RA, Fuller C, Lau C, Boyett JM, Wallace D, Gilbertson RJ: Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol 7: 813– 820, 2006. [DOI] [PubMed] [Google Scholar]
- 3). Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ: Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol 22: 706– 713, 2004. [DOI] [PubMed] [Google Scholar]
- 4). Zeltzer LK, Recklitis C, Buchbinder D, Zebrack B, Casillas J, Tsao JC, Lu Q, Krull K: Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Clin Oncol 27: 2396– 2404, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Gajjar A, Hernan R, Kocak M, Fuller C, Lee Y, McKinnon PJ, Wallace D, Lau C, Chintagumpala M, Ashley DM, Kellie SJ, Kun L, Gilbertson RJ: Clinical, histopathologic, and molecular markers of prognosis: toward a new disease risk stratification system for medulloblastoma. J Clin Oncol 22: 984– 993, 2004. [DOI] [PubMed] [Google Scholar]
- 6). Packer RJ, Rood BR, MacDonald TJ: Medulloblastoma: present concepts of stratification into risk groups. Pediatr Neurosurg 39: 60– 67, 2003. [DOI] [PubMed] [Google Scholar]
- 7). Northcott PA, Korshunov A Witt H, Hielscher T, Eberhart CG, Mack S, Bouffet E, Clifford SC, Hawkins CE, French P, Rutka JT, Pfister S, Taylor MD: Medulloblastoma comprises four distinct molecular variants. J Clin Oncol 29: 1408– 1414, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Northcott PA, Shih DJ, Peacock J, Garzia L, Morrissy AS, Zichner T, Stütz AM, Korshunov A, Reimand J, Schumacher SE, Beroukhim R, Ellison DW, Marshall CR, Lionel AC, Mack S, Dubuc A, Yao Y, Ramaswamy V, Luu B, Rolider A, Cavalli FM, Wang X, Remke M, Wu X, Chiu RY, Chu A, Chuah E, Corbett RD, Hoad GR, Jackman SD, Li Y, Lo A, Mungall KL, Nip KM, Qian JQ, Raymond AG, Thiessen NT, Varhol RJ, Birol I, Moore RA, Mungall AJ, Holt R, Kawauchi D, Roussel MF, Kool M, Jones DT, Witt H, Fernandez-L A, Kenney AM, Wechsler-Reya RJ, Dirks P, Aviv T, Grajkowska WA, Perek-Polnik M, Haberler CC, Delattre O, Reynaud SS, Doz FF, Pernet-Fattet SS, Cho BK, Kim SK, Wang KC, Scheurlen W, Eberhart CG, Fèvre-Montange M, Jouvet A, Pollack IF, Fan X, Muraszko KM, Gillespie GY, Di Rocco C, Massimi L, Michiels EM, Kloosterhof NK, French PJ, Kros JM, Olson JM, Ellenbogen RG, Zitterbart K, Kren L, Thompson RC, Cooper MK, Lach B, McLendon RE, Bigner DD, Fontebasso A, Albrecht S, Jabado N, Lindsey JC, Bailey S, Gupta N, Weiss WA, Bognár L, Klekner A, Van Meter TE, Kumabe T, Tominaga T, Elbabaa SK, Leonard JR, Rubin JB, Liau LM, Van Meir EG, Fouladi M, Nakamura H, Cinalli G, Garami M, Hauser P, Saad AG, Iolascon A, Jung S, Carlotti CG, Vibhakar R, Ra YS, Robinson S, Zollo M, Faria CC, Chan JA, Levy ML, Sorensen PH, Meyerson M, Pomeroy SL, Cho YJ, Bader GD, Tabori U, Hawkins CE, Bouffet E, Scherer SW, Rutka JT, Malkin D, Clifford SC, Jones SJ, Korbel JO, Pfister SM, Marra MA, Taylor MD: Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature 488: 49– 56, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Pugh TJ, Weeraratne SD, Archer TC, Pomeranz Krummel DA, Auclair D, Bochicchio J, Carneiro MO, Carter SL, Cibulskis K, Erlich RL, Greulich H, Lawrence MS, Lennon NJ, McKenna A, Meldrim J, Ramos AH, Ross MG, Russ C, Shefler E, Sivachenko A, Sogoloff B, Stojanov P, Tamayo P, Mesirov JP, Amani V, Teider N, Sengupta S, Francois JP, Northcott PA, Taylor MD, Yu F, Crabtree GR, Kautzman AG, Gabriel SB, Getz G, Jäger N, Jones DT, Lichter P, Pfister SM, Roberts TM, Meyerson M, Pomeroy SL, Cho YJ: Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature 488: 106– 110, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Jones DT, Jäger N, Kool M, Zichner T, Hutter B, Sultan M, Cho YJ, Pugh TJ, Hovestadt V, Stütz AM, Rausch T, Warnatz HJ, Ryzhova M, Bender S, Sturm D, Pleier S, Cin H, Pfaff E, Sieber L, Wittmann A, Remke M, Witt H, Hutter S, Tzaridis T, Weischenfeldt J, Raeder B, Avci M, Amstislavskiy V, Zapatka M, Weber UD, Wang Q, Lasitschka B, Bartholomae CC, Schmidt M, von Kalle C, Ast V, Lawerenz C, Eils J, Kabbe R, Benes V, van Sluis P, Koster J, Volckmann R, Shih D, Betts MJ, Russell RB, Coco S, Tonini GP, Schüller U, Hans V, Graf N, Kim YJ, Monoranu C, Roggendorf W, Unterberg A, Herold-Mende C, Milde T, Kulozik AE, von Deimling A, Witt O, Maass E, Rössler J, Ebinger M, Schuhmann MU, Frühwald MC, Hasselblatt M, Jabado N, Rutkowski S, von Bueren AO, Williamson D, Clifford SC, McCabe MG, Collins VP, Wolf S, Wiemann S, Lehrach H, Brors B, Scheurlen W, Felsberg J, Reifenberger G, Northcott PA, Taylor MD, Meyerson M, Pomeroy SL, Yaspo ML, Korbel JO, Korshunov A, Eils R, Pfister SM, Lichter P: Dissecting the genomic complexity underlying medulloblastoma. Nature 488: 100– 105, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Robinson G, Parker M, Kranenburg TA, Lu C, Chen X, Ding L, Phoenix TN, Hedlund E, Wei L, Zhu X, Chalhoub N, Baker SJ, Huether R, Kriwacki R, Curley N, Thiruvenkatam R, Wang J, Wu G, Rusch M, Hong X, Becksfort J, Gupta P, Ma J, Easton J, Vadodaria B, Onar-Thomas A, Lin T, Li S, Pounds S, Paugh S, Zhao D, Kawauchi D, Roussel MF, Finkelstein D, Ellison DW, Lau CC, Bouffet E, Hassall T, Gururangan S, Cohn R, Fulton RS, Fulton LL, Dooling DJ, Ochoa K, Gajjar A, Mardis ER, Wilson RK, Downing JR, Zhang J, Gilbertson RJ: Novel mutations target distinct subgroups of medulloblastoma. Nature 488: 43– 48, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Kool M, Korshunov A, Remke M, Jones DT, Schlanstein M, Northcott PA, Cho YJ, Koster J, Schouten-van Meeteren A, van Vuurden D, Clifford SC, Pietsch T, von Bueren AO, Rutkowski S, McCabe M, Collins VP, Bäcklund ML, Haberler C, Bourdeaut F, Delattre O, Doz F, Ellison DW, Gilbertson RJ, Pomeroy SL, Taylor MD, Lichter P, Pfister SM: Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol 123: 473– 484, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Crawford JR, MacDonald TJ, Packer RJ: Medulloblastoma in childhood: new biological advances. Lancet Neurol 6: 1073– 1085, 2007. [DOI] [PubMed] [Google Scholar]
- 14). Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, Bayer L, LaFond D, Donahue BR, Marymont MH, Muraszko K, Langston J, Sposto R: Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol 24: 4202– 4208, 2006. [DOI] [PubMed] [Google Scholar]
- 15). Grill J, Dufour C, Kalifa C: High-dose chemotherapy in children with newly-diagnosed medulloblastoma. Lancet Oncol 7: 787– 789, 2006. [DOI] [PubMed] [Google Scholar]
- 16). Rutkowski S, Bode U, Deinlein F, Ottensmeier H, Warmuth-Metz M, Soerensen N, Graf N, Emser A, Pietsch T, Wolff JE, Kortmann RD, Kuehl J: Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med 352: 978– 986, 2005. [DOI] [PubMed] [Google Scholar]
- 17). Thompson MC, Fuller C, Hogg TL, Dalton J, Finkelstein D, Lau CC, Chintagumpala M, Adesina A, Ashley DM, Kellie SJ, Taylor MD, Curran T, Gajjar A, Gilbertson RJ: Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol 24: 1924– 1931, 2006. [DOI] [PubMed] [Google Scholar]
- 18). Kool M, Koster J, Bunt J, Hasselt NE, Lakeman A, van Sluis P, Troost D, Meeteren NS, Caron HN, Cloos J, Mrsić A, Ylstra B, Grajkowska W, Hartmann W, Pietsch T, Ellison D, Clifford SC, Versteeg R: Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One 3: e3088, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, Ellison DW, Lichter P, Gilbertson RJ, Pomeroy SL, Kool M, Pfister SM: Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol 123: 465– 472, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Gajjar AJ, Robinson GW: Medulloblastoma-translating discoveries from the bench to the bedside. Nat Rev Clin Oncol 11: 714– 722, 2014. [DOI] [PubMed] [Google Scholar]
- 21). Northcott PA, Korshunov A, Pfister SM, Taylor MD: The clinical implications of medulloblastoma subgroups. Nat Rev Neurol 8: 340– 351, 2012. [DOI] [PubMed] [Google Scholar]
- 22). Ellison DW, Dalton J, Kocak M, Nicholson SL, Fraga C, Neale G, Kenney AM, Brat DJ, Perry A, Yong WH, Taylor RE, Bailey S, Clifford SC, Gilbertson RJ: Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol 121: 381– 396, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Clifford SC, Lusher ME, Lindsey JC, Langdon JA, Gilbertson RJ, Straughton D, Ellison DW: Wnt/Wingless pathway activation and chromosome 6 loss characterize a distinct molecular sub-group of medulloblastomas associated with a favorable prognosis. Cell Cycle 5: 2666– 2670, 2006. [DOI] [PubMed] [Google Scholar]
- 24). Lindsey JC, Hill RM, Megahed H, Lusher ME, Schwalbe EC, Cole M, Hogg TL, Gilbertson RJ, Ellison DW, Bailey S, Clifford SC: TP53 mutations in favorable-risk Wnt/Wingless-subtype medulloblastomas. J Clin Oncol 29: e344– e346, 2011. [DOI] [PubMed] [Google Scholar]
- 25). Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C, Kranenburg TA, Hogg T, Poppleton H, Martin J, Finkelstein D, Pounds S, Weiss A, Patay Z, Scoggins M, Ogg R, Pei Y, Yang ZJ, Brun S, Lee Y, Zindy F, Lindsey JC, Taketo MM, Boop FA, Sanford RA, Gajjar A, Clifford SC, Roussel MF, McKinnon PJ, Gutmann DH, Ellison DW, Wechsler-Reya R, Gilbertson RJ: Subtypes of medulloblastoma have distinct developmental origins. Nature 468: 1095– 1099, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Ellison DW, Kocak M, Dalton J, Megahed H, Lusher ME, Ryan SL, Zhao W, Nicholson SL, Taylor RE, Bailey S, Clifford SC: Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol 29: 1400– 1407, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Northcott PA, Hielscher T, Dubuc A, Mack S, Shih D, Remke M, Al-Halabi H, Albrecht S, Jabado N, Eberhart CG, Grajkowska W, Weiss WA, Clifford SC, Bouffet E, Rutka JT, Korshunov A, Pfister S, Taylor MD: Pediatric and adult sonic hedgehog medulloblastomas are clinically and molecularly distinct. Acta Neuropathol 122: 231– 240, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Taylor MD, Liu L, Raffel C, Hui CC, Mainprize TG, Zhang X, Agatep R, Chiappa S, Gao L, Lowrance A, Hao A, Goldstein AM, Stavrou T, Scherer SW, Dura WT, Wainwright B, Squire JA, Rutka JT, Hogg D: Mutations in SUFU predispose to medulloblastoma. Nat Genet 31: 306– 310, 2002. [DOI] [PubMed] [Google Scholar]
- 29). Slade I, Murray A, Hanks S, Kumar A, Walker L, Hargrave D, Douglas J, Stiller C, Izatt L, Rahman N: Heterogeneity of familial medulloblastoma and contribution of germline PTCH1 and SUFU mutations to sporadic medulloblastoma. Fam Cancer 10: 337– 342, 2011. [DOI] [PubMed] [Google Scholar]
- 30). Northcott PA, Nakahara Y, Wu X, Feuk L, Ellison DW, Croul S, Mack S, Kongkham PN, Peacock J, Dubuc A, Ra YS, Zilberberg K, McLeod J, Scherer SW, Sunil Rao J, Eberhart CG, Grajkowska W, Gillespie Y, Lach B, Grundy R, Pollack IF, Hamilton RL, Van Meter T, Carlotti CG, Boop F, Bigner D, Gilbertson RJ, Rutka JT, Taylor MD: Multiple recurrent genetic events converge on control of histone lysine methylation in medulloblastoma. Nat Genet 41: 465– 472, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Bale SJ, Falk RT, Rogers GR: Patching together the genetics of Gorlin syndrome. J Cutan Med Surg 3: 31– 34, 1998. [DOI] [PubMed] [Google Scholar]
- 32). Taylor MD, Mainprize TG, Rutka JT: Molecular insight into medulloblastoma and central nervous system primitive neuroectodermal tumor biology from hereditary syndromes: a review. Neurosurgery 47: 888– 901, 2000. [DOI] [PubMed] [Google Scholar]
- 33). Pastorino L, Ghiorzo P, Nasti S, Battistuzzi L, Cusano R, Marzocchi C, Garrè ML, Clementi M, Scarrà GB: Identification of a SUFU germline mutation in a family with Gorlin syndrome. Am J Med Genet A 149A: 1539– 1543, 2009. [DOI] [PubMed] [Google Scholar]
- 34). Northcott PA, Jones DT, Kool M, Robinson GW, Gilbertson RJ, Cho YJ, Pomeroy SL, Korshunov A, Lichter P, Taylor MD, Pfister SM: Medulloblastomics: the end of the beginning. Nat Rev Cancer 12: 818– 834, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Hallahan AR, Pritchard JI, Hansen S, Benson M, Stoeck J, Hatton BA, Russell TL, Ellenbogen RG, Bernstein ID, Beachy PA, Olson JM: The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res 64: 7794– 7800, 2004. [DOI] [PubMed] [Google Scholar]
- 36). Oliver TG, Read TA, Kessler JD, Mehmeti A, Wells JF, Huynh TT, Lin SM, Wechsler-Reya RJ: Loss of patched and disruption of granule cell development in a pre-neoplastic stage of medulloblastoma. Development 132: 2425– 2439, 2005. [DOI] [PubMed] [Google Scholar]
- 37). Grammel D, Warmuth-Metz M, von Bueren AO, Kool M, Pietsch T, Kretzschmar HA, Rowitch DH, Rutkowski S, Pfister SM, Schüller U: Sonic hedgehog-associated medulloblastoma arising from the cochlear nuclei of the brainstem. Acta Neuropathol 123: 601– 614, 2012. [DOI] [PubMed] [Google Scholar]
- 38). Zhukova N, Ramaswamy V, Remke M, Pfaff E, Shih DJ, Martin DC, Castelo-Branco P, Baskin B, Ray PN, Bouffet E, von Bueren AO, Jones DT, Northcott PA, Kool M, Sturm D, Pugh TJ, Pomeroy SL, Cho YJ, Pietsch T, Gessi M, Rutkowski S, Bognar L, Klekner A, Cho BK, Kim SK, Wang KC, Eberhart CG, Fevre-Montange M, Fouladi M, French PJ, Kros M, Grajkowska WA, Gupta N, Weiss WA, Hauser P, Jabado N, Jouvet A, Jung S, Kumabe T, Lach B, Leonard JR, Rubin JB, Liau LM, Massimi L, Pollack IF, Shin Ra Y, Van Meir EG, Zitterbart K, Schüller U, Hill RM, Lindsey JC, Schwalbe EC, Bailey S, Ellison DW, Hawkins C, Malkin D, Clifford SC, Korshunov A, Pfister S, Taylor MD, Tabori U: Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J Clin Oncol 31: 2927– 2935, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Shih DJ, Northcott PA, Remke M, Korshunov A, Ramaswamy V, Kool M, Luu B, Yao Y, Wang X, Dubuc AM, Garzia L, Peacock J, Mack SC, Wu X, Rolider A, Morrissy AS, Cavalli FM, Jones DT, Zitterbart K, Faria CC, Schüller U, Kren L, Kumabe T, Tominaga T, Shin Ra Y, Garami M, Hauser P, Chan JA, Robinson S, Bognár L, Klekner A, Saad AG, Liau LM, Albrecht S, Fontebasso A, Cinalli G, De Antonellis P, Zollo M, Cooper MK, Thompson RC, Bailey S, Lindsey JC, Di Rocco C, Massimi L, Michiels EM, Scherer SW, Phillips JJ, Gupta N, Fan X, Muraszko KM, Vibhakar R, Eberhart CG, Fouladi M, Lach B, Jung S, Wechsler-Reya RJ, Fèvre-Montange M, Jouvet A, Jabado N, Pollack IF, Weiss WA, Lee JY, Cho BK, Kim SK, Wang KC, Leonard JR, Rubin JB, de Torres C, Lavarino C, Mora J, Cho YJ, Tabori U, Olson JM, Gajjar A, Packer RJ, Rutkowski S, Pomeroy SL, French PJ, Kloosterhof NK, Kros JM, Van Meir EG, Clifford SC, Bourdeaut F, Delattre O, Doz FF, Hawkins CE, Malkin D, Grajkowska WA, Perek-Polnik M, Bouffet E, Rutka JT, Pfister SM, Taylor MD: Cytogenetic prognostication within medulloblastoma subgroups. J Clin Oncol 32: 886– 896, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Robinson GW, Orr BA, Wu G, Gururangan S, Lin T, Qaddoumi I, Packer RJ, Goldman S, Prados MD, Desjardins A, Chintagumpala M, Takebe N, Kaste SC, Rusch M, Allen SJ, Onar-Thomas A, Stewart CF, Fouladi M, Boyett JM, Gilbertson RJ, Curran T, Ellison DW, Gajjar A: Vismodegib exerts targeted efficacy against recurrent sonic hedgehog-subgroup medulloblastoma: results from phase II pediatric brain tumor consortium studies PBTC-025B and PBTC-032. J Clin Oncol 33: 2646– 2654, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, Holcomb T, Stinson J, Gould SE, Coleman B, LoRusso PM, Von Hoff DD, de Sauvage FJ, Low JA. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med 361: 1173– 1178, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Northcott PA, Shih DJ, Remke M, Cho YJ, Kool M, Hawkins C, Eberhart CG, Dubuc A, Guettouche T, Cardentey Y, Bouffet E, Pomeroy SL, Marra M, Malkin D, Rutka JT, Korshunov A, Pfister S, Taylor MD: Rapid, reliable, and reproducible molecular sub-grouping of clinical medulloblastoma samples. Acta Neuropathol 123: 615– 626, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Wang X, Ramaswamy V, Remke M, Mack SC, Dubuc AM, Northcott PA, Taylor MD: Intertumoral and intratumoral heterogeneity as a barrier for effective treatment of medulloblastoma. Neurosurgery 60 Suppl 1: 57– 63, 2013. [DOI] [PubMed] [Google Scholar]
- 44). Remke M, Hielscher T, Northcott PA, Witt H, Ryzhova M, Wittmann A, Benner A, von Deimling A, Scheurlen W, Perry A, Croul S, Kulozik AE, Lichter P, Taylor MD, Pfister SM, Korshunov A: Adult medulloblastoma comprises three major molecular variants. J Clin Oncol 29: 2717– 2723, 2011. [DOI] [PubMed] [Google Scholar]
- 45). Korshunov A, Remke M, Kool M, Hielscher T, Northcott PA, Williamson D, Pfaff E, Witt H, Jones DT, Ryzhova M, Cho YJ, Wittmann A, Benner A, Weiss WA, von Deimling A, Scheurlen W, Kulozik AE, Clifford SC, Peter Collins V, Westermann F, Taylor MD, Lichter P, Pfister SM: Biological and clinical heterogeneity of MYCN-amplified medulloblastoma. Acta Neuropathol 123: 515– 527, 2012. [DOI] [PubMed] [Google Scholar]
- 46). Remke M, Hielscher T, Korshunov A, Northcott PA, Bender S, Kool M, Westermann F, Benner A, Cin H, Ryzhova M, Sturm D, Witt H, Haag D, Toedt G, Wittmann A, Schöttler A, von Bueren AO, von Deimling A, Rutkowski S, Scheurlen W, Kulozik AE, Taylor MD, Lichter P, Pfister SM: FSTL5 is a marker of poor prognosis in non-WNT/non-SHH medulloblastoma. J Clin Oncol 29: 3852– 3861, 2011. [DOI] [PubMed] [Google Scholar]
- 47). Wang X DA, Ramaswamy V, Mack S, Gendoo DM, Remke M, Wu X, Garzia L, Luu B, Cavalli F, Peacock J, López B, Skowron P, Zagzag D, Lyden D, Hoffman C, Cho YJ, Eberhart C, MacDonald T, Li XN, Van Meter T, Northcott PA, Haibe-Kains B, Hawkins C, Rutka JT, Bouffet E, Pfister SM, Korshunov A, Taylor MD: Medulloblastoma subgroups remain stable across primary and metastatic compartments. Acta Neuropathol 129: 449– 457, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Ramaswamy V, Remke M, Bouffet E, Faria CC, Perreault S, Cho YJ, Shih DJ, Luu B, Dubuc AM, Northcott PA, Schüller U, Gururangan S, McLendon R, Bigner D, Fouladi M, Ligon KL, Pomeroy SL, Dunn S, Triscott J, Jabado N, Fontebasso A, Jones DT, Kool M, Karajannis MA, Gardner SL, Zagzag D, Nunes S, Pimentel J, Mora J, Lipp E, Walter AW, Ryzhova M, Zheludkova O, Kumirova E, Alshami J, Croul SE, Rutka JT, Hawkins C, Tabori U, Codispoti KE, Packer RJ, Pfister SM, Korshunov A, Taylor MD: Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol 14: 1200– 1207, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49). Pietsch T, Schmidt R, Remke M, Korshunov A, Hovestadt V, Jones DT, Felsberg J, Kaulich K, Goschzik T, Kool M, Northcott PA, von Hoff K, von Bueren AO, Friedrich C, Mynarek M, Skladny H, Fleischhack G, Taylor MD, Cremer F, Lichter P, Faldum A, Reifenberger G, Rutkowski S, Pfister SM: Prognostic significance of clinical, histopathological, and molecular characteristics of medulloblastomas in the prospective HIT2000 multicenter clinical trial cohort. Acta Neuropathol 128: 137– 149, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50). Gottardo NG, Hansford JR, McGlade JP, Alvaro F, Ashley DM, Bailey S, Baker DL, Bourdeaut F, Cho YJ, Clay M, Clifford SC, Cohn RJ, Cole CH, Dallas PB, Downie P, Doz F, Ellison DW, Endersby R, Fisher PG, Hassall T, Heath JA, Hii HL, Jones DT, Junckerstorff R, Kellie S, Kool M, Kotecha RS, Lichter P, Laughton SJ, Lee S, McCowage G, Northcott PA, Olson JM, Packer RJ, Pfister SM, Pietsch T, Pizer B, Pomeroy SL, Remke M, Robinson GW, Rutkowski S, Schoep T, Shelat AA, Stewart CF, Sullivan M, Taylor MD, Wainwright B, Walwyn T, Weiss WA, Williamson D, Gajjar A: Medulloblastoma down under 2013: a report from the third annual meeting of the International Medulloblastoma Working Group. Acta Neuropathol 127: 189– 201, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Lin CY, Erkek S, Tong Y, Yin L, Federation AJ, Zapatka M, Haldipur P, Kawauchi D, Risch T, Warnatz HJ, Worst BC, Ju B, Orr BA, Zeid R, Polaski DR, Segura-Wang M, Waszak SM, Jones DT, Kool M, Hovestadt V, Buchhalter I, Sieber L, Johann P, Chavez L, Gröschel S, Ryzhova M, Korshunov A, Chen W, Chizhikov VV, Millen KJ, Amstislavskiy V, Lehrach H, Yaspo ML, Eils R, Lichter P, Korbel JO, Pfister SM, Bradner JE, Northcott PA: Active medulloblastoma enhancers reveal subgroup-specific cellular origins. Nature 530: 57– 62, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52). Wefers AK, Warmuth-Metz M, Pöschl J, von Bueren AO, Monoranu CM, Seelos K, Peraud A, Tonn JC, Koch A, Pietsch T, Herold-Mende C, Mawrin C, Schouten-van Meeteren A, van Vuurden D, von Hoff K, Rutkowski S, Pfister SM, Kool M, Schüller U: Subgroup-specific localization of human medulloblastoma based on pre-operative MRI. Acta Neuropathol 127: 931– 933, 2014. [DOI] [PubMed] [Google Scholar]
- 53). Patay Z, DeSain LA, Hwang SN, Coan A, Li Y, Ellison DW: MR imaging characteristics of wingless-type-subgroup pediatric medulloblastoma. Am J Neuroradiol 36: 2386– 2393, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54). Wu X, Northcott PA, Croul S, Taylor MD: Mouse models of medulloblastoma. Chin J Cancer 30: 442– 449, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55). Kawauchi D, Robinson G, Uziel T, Gibson P, Rehg J, Gao C, Finkelstein D, Qu C, Pounds S, Ellison DW, Gilbertson RJ, Roussel MF: A mouse model of the most aggressive subgroup of human medulloblastoma. Cancer Cell 21: 168– 180, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56). Pei Y, Moore CE, Wang J, Tewari AK, Eroshkin A, Cho YJ, Witt H, Korshunov A, Read TA, Sun JL, Schmitt EM, Miller CR, Buckley AF, McLendon RE, Westbrook TF, Northcott PA, Taylor MD, Pfister SM, Febbo PG, Wechsler-Reya RJ: An animal model of MYC-driven medulloblastoma. Cancer Cell 21: 155– 167, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57). Zhao X, Liu Z, Yu L, Zhang Y, Baxter P, Voicu H, Gurusiddappa S, Luan J, Su JM, Leung HC, Li XN: Global gene expression profiling confirms the molecular fidelity of primary tumor-based orthotopic xenograft mouse models of medulloblastoma. Neuro Oncol 14: 574– 583, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58). Milde T, Lodrini M, Savelyeva L, Korshunov A, Kool M, Brueckner LM, Antunes AS, Oehme I, Pekrun A, Pfister SM, Kulozik AE, Witt O, Deubzer HE: HD-MB03 is a novel Group 3 medulloblastoma model demonstrating sensitivity to histone deacetylase inhibitor treatment. J Neurooncol 110: 335– 348, 2012. [DOI] [PubMed] [Google Scholar]
- 59). Dietl S, Schwinn S, Dietl S, Riedel S, Deinlein F, Rutkowski S, von Bueren AO, Krauss J, Schweitzer T, Vince GH, Picard D, Eyrich M, Rosenwald A, Ramaswamy V, Taylor MD, Remke M, Monoranu CM, Beilhack A, Schlegel PG, Wölfl M: MB3W1 is an orthotopic xenograft model for anaplastic medulloblastoma displaying cancer stem cell- and Group 3-properties. BMC Cancer 16: 115, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60). Weiner HL, Bakst R, Hurlbert MS, Ruggiero J, Ahn E, Lee WS, Stephen D, Zagzag D, Joyner AL, Turnbull DH: Induction of medulloblastomas in mice by sonic hedgehog, independent of Gli1. Cancer Res 62: 6385– 6389, 2002. [PubMed] [Google Scholar]
- 61). Browd SR, Kenney AM, Gottfried ON, Yoon JW, Walterhouse D, Pedone CA, Fults DW: N-myc can substitute for insulin-like growth factor signaling in a mouse model of sonic hedgehog-induced medulloblastoma. Cancer Res 66: 2666– 2672, 2006. [DOI] [PubMed] [Google Scholar]
- 62). Rao G, Pedone CA, Coffin CM, Holland EC, Fults DW: c-Myc enhances sonic hedgehog-induced medulloblastoma formation from nestin-expressing neural progenitors in mice. Neoplasia 5: 198– 204, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63). Rao G, Pedone CA, Del Valle L, Reiss K, Holland EC, Fults DW: Sonic hedgehog and insulin-like growth factor signaling synergize to induce medulloblastoma formation from nestin-expressing neural progenitors in mice. Oncogene 23: 6156– 6162, 2004. [DOI] [PubMed] [Google Scholar]
- 64). Jenkins NC, Rao G, Eberhart CG, Pedone CA, Dubuc AM, Fults DW: Somatic cell transfer of c-Myc and Bcl-2 induces large-cell anaplastic medulloblastomas in mice. J Neurooncol 126: 415– 424, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]