Abstract
Concomitant use of temozolomide (TMZ) and radiotherapy, which is the standard therapy for patients with high-grade glioma, involves a unique regimen with multiple-day, long-term administration. In a previous study, we showed not only higher incidence rates of chemotherapy-induced nausea and vomiting (CINV) during the overall study period, but also substantially higher incidence rates of moderate/severe nausea and particularly severe appetite suppression during the late phase of the treatment. Here, we prospectively evaluated the efficacy of a combination of palonosetron, aprepitant, and dexamethasone for CINV in patients treated with concomitant TMZ and radiotherapy. Twenty-one consecutive patients with newly diagnosed high-grade glioma were enrolled. CINV was recorded using a daily diary and included nausea assessment, emetic episodes, degree of appetite suppression, and use of antiemetic medication. The percentage of patients with a complete response in the overall period was 76.2%. The percentages of patients with no moderate/severe nausea were 90.5, 100, and 90.5% in the early phase, late phase, and overall period, respectively. Severe appetite suppression throughout the overall period completely disappeared. The combination of palonosetron, aprepitant, and dexamethasone was highly effective and well tolerated in patients treated with concomitant TMZ and radiotherapy. This combination of antiemetic therapy focused on delayed as well as acute CINV and may have the potential to overcome CINV associated with a multiple-day, long-term chemotherapy regimen.
Keywords: palonosetron, aprepitant, temozolomide, chemotherapy-induced nausea and vomiting
Introduction
Concomitant use of temozolomide (TMZ) and radiotherapy, which requires a unique regimen with multiple-day, long-term administration, has recently been established as the standard postoperative treatment for patients with newly diagnosed malignant gliomas.1) We previously revealed unexpectedly high rates of chemotherapy-induced nausea and vomiting (CINV) associated with concomitant TMZ and radiotherapy in a prospective analysis that focused specifically on the incidence rates of CINV.2) This previous study showed that even with appropriate prophylactic antiemetic therapy, approximately 90% of patients treated with concomitant TMZ still suffer from various degrees of nausea (Common Terminology Criteria grades 1–3).2) Regarding the timing of CINV, moderate/severe nausea and particularly severe appetite suppression tend to develop during the late phase of the treatment, indicating a substantial need for further improvement in antiemetic therapy that is particularly focused on the late phase.2) Although limited evidence is available for the management of CINV in patients receiving multiple-day chemotherapy regimens, current updated antiemetic guidelines recommend a combination antiemetic regimen that targets multiple molecular pathways that are associated with emesis.3–6) Palonosetron is a second-generation 5-HT3 receptor antagonist with a prolonged half-life that is more effective than first-generation 5-HT3 receptor antagonists for preventing acute and even delayed CINV.7) Aprepitant is a potent and selective oral non-peptide antagonist of the neurokinin-1 (NK-1) receptor that prevents delayed emesis.8) Accordingly, we used multiple-dose administration of palonosetron and multiple-cycle administration of aprepitant as prophylactic antiemetic therapy for concomitant TMZ and radiotherapy, which involve a multiple-day, long-term regimen. This study was designed to prospectively evaluate the efficacy of the combination of palonosetron, aprepitant, and dexamethasone for CINV in patients treated with concomitant TMZ and radiotherapy.
Materials and Methods
We enrolled 21 consecutive patients with newly diagnosed supratentorial high-grade glioma (grade III-IV) who were treated with concomitant TMZ and radiotherapy at the University of Tsukuba Hospital from March 2013 to January 2015 during the registration period of 2 years. Patients were eligible if they were adults (>18 years old) and had a Karnofsky performance status (KPS) of 60 or more. Patients were not eligible for participation in the study if they could not record notes in a self-reported diary due to neurological deficits such as consciousness disturbances or aphasia, if they experienced vomiting during the 24 h before the first administration of TMZ, or if they had any of the following abnormal laboratory values: absolute neutrocyte count <1000/μl, platelet count <100,000/μl, aspartate aminotransferase >2.5 × the upper limit of normal, alanine aminotransferase >2.5 × the upper limit of normal, bilirubin >1.5 × the upper limit of normal, or creatinine >1.5 × the upper limit of normal.
The radiation schedule for patients with high-grade glioma treated at our facilities consisted of two protocols. For standard radiotherapy, daily conventional fractionated photon radiotherapy of 2 Gy was administered five times per week, for a total dose of 60 Gy. For selected patients, proton therapy for a total dose of 96.6 GyE in 56 fractions was administered.9) Concomitant chemotherapy consisted of TMZ at a daily dose of 75 mg/m2 from the first until the last day of radiotherapy. Accordingly, TMZ administration varied from 42 to 48 days depending on the radiation modalities used and the days when radiotherapy was not given. Discontinuation of TMZ was decided according to a slightly modified standard protocol (absolute neutrocyte count <1500/μl, platelet count <100,000/μl, and prolonged lymphopenia <200/μl).10)
All patients in the study received intravenous palonosetron 0.75 mg and oral dexamethasone 4 mg before the TMZ administration on Day 1, followed by intravenous palonosetron 0.75 mg every 7 days. All patients also received oral aprepitant 125 mg before TMZ administration on Days 1 and 22, followed by oral aprepitant 80 mg daily on Days 2–5 and 23–26. Patients completed a daily diary in which the degree of nausea, number of emetic episodes, and degree of appetite suppression were recorded based on Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. In this study, the degree of CINV was reported as mild, moderate, or severe and corresponded to CTC grades 1, 2, and 3, respectively. Patients also recorded all uses of rescue antiemetic medication. The daily diary was recorded until the last day of chemoradiotherapy. The study was approved by the institutional ethics committees. Written and signed informed consent was obtained from all patients before study entry.
The primary endpoint was the percentage of patients with complete response (CR: no emesis, no rescue medication) during the overall study period after TMZ administration. The secondary endpoints were the percentage of patients with CR in the early phase (the first half of the treatment period, i.e., ≤Day 20) and in the late phase (the second half of the treatment period, i.e., ≥Day 21). The secondary endpoints also included the percentage of patients with no nausea, no moderate/severe nausea (CTC grades 2, 3), no appetite suppression, and no severe appetite suppression (CTC grade 3) during the early and late phases and the overall study period.
Statistical analyses were performed using SPSS software (version 22; SPSS, Inc.). The Fisher’s exact test was used to evaluate the difference in categorical variables. A value of P < 0.05 was considered to be statistically significant in all analyses.
Results
The characteristics of the 21 patients we studied are summarized in Table 1. We studied 12 males and nine females aged 33 to 76 years (mean, 56.7 years). Five (23.8%) patients had a KPS of 100, 10 (47.6%) had a KPS of 90, three (14.3%) had a KPS of 80, one (4.8%) had a KPS of 70, and two (9.5%) had a KPS of 60. According to the 2007 WHO classification, five patients had grade III glioma and 16 had grade IV glioma. Surgical resection resulted in gross total resection of the tumor in 11 patients (52.4%), subtotal resection in five (23.8%), partial resection in three (14.3%), and biopsy in two (9.5%). Twenty patients (95.2%) received conventional fractionated photon radiotherapy, and one (4.8%) received proton therapy.
Table 1.
Patient characteristics
| Characteristics | No. of patients | % |
|---|---|---|
| Age (yrs) | ||
| Mean ± SD | 56.7 ± 12.6 | |
| Range | 33–76 | |
| Gender | ||
| Male | 12 | 57.1 |
| Female | 9 | 42.9 |
| KPS | ||
| 100 | 5 | 23.8 |
| 90 | 10 | 47.6 |
| 80 | 3 | 14.3 |
| 70 | 1 | 4.8 |
| 60 | 2 | 9.5 |
| Pathology | ||
| WHO grade 4 glioma | 16 | 76.2 |
| WHO grade 3 glioma | 5 | 23.8 |
| Extent of resection | ||
| GTR | 11 | 52.4 |
| STR | 5 | 23.8 |
| PR | 3 | 14.3 |
| B | 2 | 9.5 |
| Radiotherapy | ||
| CRT | 20 | 95.2 |
| PT | 1 | 4.8 |
SD: standard deviation, KPS: Karnofsky performance status, GTR: gross total resection, STR: subtotal resection, PR partial resection, B: biopsy, CRT: conventional radiotherapy, PT: proton therapy.
The antiemetic effects in the current study are summarized in Table 2. The percentage of patients with CR in the overall period was 76.2%, whereas CRs in the early and late phases were 81.0 and 81.0%, respectively. The percentages of patients with no emesis were 90.5, 100, and 90.5% in the early phase, late phase, and overall period, respectively. The percentages of patients with no nausea were 61.9, 76.2, and 57.1% in the early phase, late phase, and overall period, respectively. The percentages of patients with no moderate/severe nausea were 90.5, 100, and 90.5% in the early phase, late phase, and overall period, respectively. The percentages of patients with no appetite suppression were 47.6, 38.1, and 23.8% in the early phase, late phase, and overall period, respectively. The percentage of patients with no severe appetite suppression was 100% for each period.
Table 2.
Response to antiemetic therapy
| Prior study(2) | Present study | P -value | |
|---|---|---|---|
| CR | |||
| Anterior | 44.4% | 81.0% | 0.024 |
| Latter | 50.0% | 81.0% | 0.087 |
| Overall | 38.9% | 76.2% | 0.025 |
| No emesis | |||
| Anterior | 77.8% | 90.5% | 0.387 |
| Latter | 61.1% | 100% | 0.002 |
| Overall | 61.1% | 90.5% | 0.055 |
| No nausea | |||
| Anterior | 22.2% | 61.9% | 0.023 |
| Latter | 33.3% | 76.2% | 0.011 |
| Overall | 11.1% | 57.1% | 0.006 |
| No moderate/severe nausea | |||
| Anterior | 77.8% | 90.5% | 0.387 |
| Latter | 66.7% | 100% | 0.006 |
| Overall | 61.1% | 90.5% | 0.055 |
| No appetite suppression | |||
| Anterior | 22.2% | 47.6% | 0.180 |
| Latter | 22.2% | 38.1% | 0.734 |
| Overall | 16.7% | 23.8% | 0.464 |
| No severe appetite suppression | |||
| Anterior | 66.7% | 100% | 0.006 |
| Latter | 50.0% | 100% | 0.000 |
| Overall | 44.4% | 100% | 0.000 |
CR: complete response
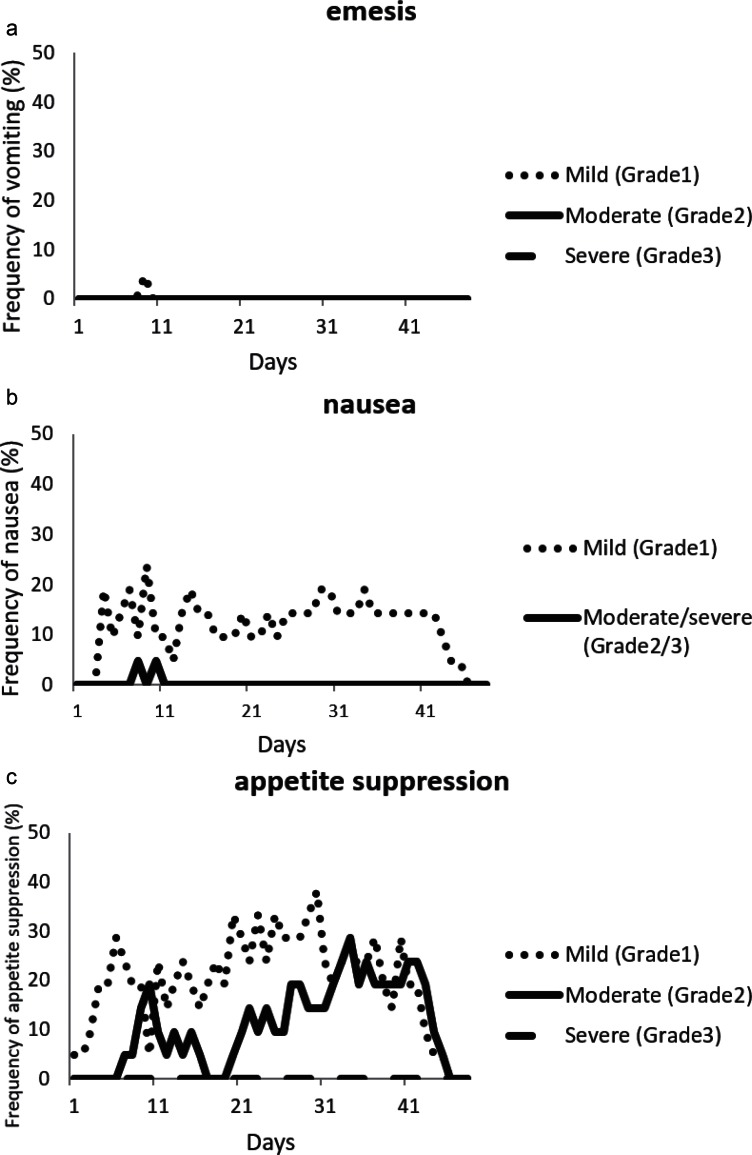
The daily incidence of emesis and its prevalence, the daily incidence and severity of nausea, and the daily incidence and severity of appetite suppression are shown in Fig. 1. The incidences of emetic episodes, moderate/severe nausea, and severe appetite suppression were markedly low throughout the overall period, although the incidence of moderate appetite suppression was relatively high during the late phase.
Fig. 1.
(a) Frequency of emesis during concomitant chemoradiotherapy including TMZ. Dotted line: CTC grade 1; solid line: CTC grade 2; dashed line: CTC grade 3. (b) Frequency of nausea during concomitant chemoradiotherapy including TMZ. Dotted line: CTC grade 1; solid line: CTC grade 2/3. (c) Frequency of appetite suppression during concomitant chemoradiotherapy including TMZ. Dotted line: CTC grade 1; solid line: CTC grade 2; dashed line: CTC grade 3. The degree of CINV was evaluated based on CTCAE version 4.0.
The combination of palonosetron, aprepitant, and dexamethasone showed good tolerability throughout the overall study period, with no reports of serious adverse events that were related to study medication.
Discussion
We evaluated the efficacy of the combination of palonosetron, aprepitant, and dexamethasone for CINV in patients treated with concomitant TMZ and radiotherapy. We found that this combination of antiemetic drugs achieved high CR rates including during the late phase of the treatment.
In the previous study that focused specifically on the incidence rates of CINV associated with concomitant TMZ and radiotherapy with prophylactic antiemetic therapy consisting of ramosetron (first-generation 5-HT3 receptor antagonist), single-cycle administration of aprepitant, and dexamethasone, we observed not only high incidence rates of CINV during the overall study period but also substantially high incidence rates of moderate/severe nausea and particularly severe appetite suppression during the late phase of the treatment.2) In general, studies designed to specifically analyze adverse events using patient self-reports are considered more likely to identify an elevated rate of such events compared to studies focused on treatment efficacy such as clinical trials.11,12) In the present study, we employed multiple-dose administration of palonosetron, multiple-cycle administration of aprepitant, and dexamethasone and found that CR rates were significantly higher compared with our previous study during the early and overall phases. Of note, the incidence rate of moderate/severe nausea during the late phase was significantly lower compared with our previous study, and the incidence of severe appetite suppression during the late phase completely disappeared. Regarding the predictive factors identified in our previous study such as female gender and lymphocyte count before beginning chemoradiotherapy, we found no significant difference in patient characteristics between the previous and present study.2)
Current updated antiemetic guidelines such as the Multinational Association of Supportive Care in Cancer guidelines, the American Society of Clinical Oncologists guidelines, and the National Comprehensive Cancer Network guidelines recommend the combination of palonosetron and dexamethasone for prevention of CINV associated with moderate emetogenic agents such as TMZ.3–5) However, in contrast to the adjuvant regimen, the concomitant regimen of TMZ consists of multiple-day, long-term chemotherapy. In the setting of multiple-day chemotherapy, daily, continuous emetogenic stimuli result in an overlap of acute and delayed CINV, particularly during the late phase of the treatment, leading to difficulty in determining the optimal strategy.6,13) The efficacy of antiemetic therapies as observed in studies of single-day chemotherapy may not be applicable to multiple-day chemotherapy because the patterns and mechanisms of CINV associated with multiple-day chemotherapy may differ from those associated with single-day chemotherapy. On the basis of the high rates of CINV associated with concomitant TMZ and radiotherapy reported by our previous study, the combination of palonosetron, aprepitant, and dexamethasone can be a routine prophylactic antiemetic therapy for concomitant TMZ and radiotherapy which involve a multiple-day, long-term regimen.
Palonosetron has a prolonged half-life of about 40 h (four to five times longer than other 5-HT3 receptor antagonists), making it effective for delayed CINV as well as acute CINV. Aprepitant is a selective NK-1 receptor antagonist that is effective for both acute and delayed CINV.7,8) The combination of palonosetron and an NK-1 receptor antagonist works synergistically because palonosetron inhibits cross-talk between the 5-HT3 and NK-1 receptor signaling pathways.14) The combination of multiple-dose administration of palonosetron and multiple-cycle administration of aprepitant employed in the present study may function synergistically to control CINV in patients treated with concomitant TMZ and radiotherapy, which involves a multiple-day, long-term regimen.
In the present study, emetic episodes, moderate/severe nausea, and severe appetite suppression were sufficiently controlled throughout the study period. Only moderate appetite suppression that was observed relatively frequently during the late phase of treatment remains to be resolved. To further improve the control of CINV, another dosing schedule of palonosetron should perhaps be introduced. In this study, palonosetron was administered at a dose of 0.75 mg every 7 days, which is the recommended dose in Japan based on the results of domestic clinical trials.15) Currently, high efficacy of multiple-day dosing of palonosetron such as administration at a dose of 0.25 mg daily or every other day in patients treated with multiple-day chemotherapy has been reported.16,17) Thus, more frequent dosing of palonosetron may be useful.
In conclusion, the present study has demonstrated that the combination of palonosetron, aprepitant, and dexamethasone sufficiently controlled CINV in patients treated with concomitant TMZ and radiotherapy. This combination of antiemetic therapy focused on delayed as well as acute CINV and may be able to overcome CINV associated with a multiple-day, long-term chemotherapy regimen.
Footnotes
Conflicts of Interest Disclosure
The authors have no personal, financial, or institutional interest in any of the drugs, materials or devices used in the article. All authors who are members of The Japan Neurosurgical Society (JNS) have registered online Self-reported COI Disclosure Statement Forms through the website for JNS members.
References
- 1). Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO: Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987– 996, 2005. [DOI] [PubMed] [Google Scholar]
- 2). Matsuda M, Yamamoto T, Ishikawa E, Nakai K, Akutsu H, Onuma K, Matsumura A: Profile analysis of chemotherapy-induced nausea and vomiting in patients treated with concomitant temozolomide and radiotherapy: results of a prospective study. Neurol Med Chir (Tokyo) 55: 749– 755, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, Chesney M, Clark-Snow RA, Flaherty AM, Freundlich B, Morrow G, Rao KV, Schwartz RN, Lyman GH: Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 29: 4189– 4198, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, Bria E, Clark-Snow RA, Espersen BT, Feyer P, Grunberg SM, Hesketh PJ, Jordan K, Kris MG, Maranzano E, Molassiotis A, Morrow G, Olver I, Rapoport BL, Rittenberg C, Saito M, Tonato M, Warr D: Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 21 Suppl 5: v232– v243, 2010. [DOI] [PubMed] [Google Scholar]
- 5). National Comprehensive Cancer Network : Antiemesis. NCCN Clinical Practice Guidelines in Oncology v.1.2015. April 1, 2015. [Google Scholar]
- 6). Affronti ML, Bubalo J: Palonosetron in the management of chemotherapy-induced nausea and vomiting in patients receiving multiple-day chemotherapy. Cancer Manag Res 6: 329– 337, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Botrel TE, Clark OA, Clark L, Paladini L, Faleiros E, Pegoretti B: Efficacy of palonosetron (PAL) compared to other serotonin inhibitors (5-HT3R) in preventing chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetogenic (MoHE) treatment: systematic review and meta-analysis. Support Care Cancer 19: 823– 832, 2011. [DOI] [PubMed] [Google Scholar]
- 8). Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, Julie Ma G, Eldridge K, Hipple A, Evans JK, Horgan KJ, Lawson F: Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 97: 3090– 3098, 2003. [DOI] [PubMed] [Google Scholar]
- 9). Matsuda M, Yamamoto T, Ishikawa E, Nakai K, Zaboronok A, Takano S, Matsumura A: Prognostic factors in glioblastoma multiforme patients receiving high-dose particle radiotherapy or conventional radiotherapy. Br J Radiol 84 Spec No 1: S54– S60, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Ishikawa E, Yamamoto T, Sakamoto N, Nakai K, Akutsu H, Tsuboi K, Takano S, Matsumura A: Low peripheral lymphocyte count before focal radiotherapy plus concomitant temozolomide predicts severe lymphopenia during malignant glioma treatment. Neurol Med Chir (Tokyo) 50: 638– 644, 2010. [DOI] [PubMed] [Google Scholar]
- 11). Bock HC, Puchner MJ, Lohmann F, Schutze M, Koll S, Ketter R, Buchalla R, Rainov N, Kantelhardt SR, Rohde V, Giese A: First-line treatment of malignant glioma with carmustine implants followed by concomitant radiochemotherapy: a multicenter experience. Neurosurg Rev 33: 441– 449, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Basch E: The missing voice of patients in drug-safety reporting. N Engl J Med 362: 865– 869, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Navari RM: Prevention of emesis from multiple-day and high-dose chemotherapy regimens. J Natl Compr Canc Netw 5: 51– 59, 2007. [DOI] [PubMed] [Google Scholar]
- 14). Stathis M, Pietra C, Rojas C, Slusher BS: Inhibition of substance P-mediated responses in NG108-15 cells by netupitant and palonosetron exhibit synergistic effects. Eur J Pharmacol 689: 25– 30, 2012. [DOI] [PubMed] [Google Scholar]
- 15). Saito M, Aogi K, Sekine I, Yoshizawa H, Yanagita Y, Sakai H, Inoue K, Kitagawa C, Ogura T, Mitsuhashi S: Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 10: 115– 124, 2009. [DOI] [PubMed] [Google Scholar]
- 16). Mattiuzzi GN, Cortes JE, Blamble DA, Bekele BN, Xiao L, Cabanillas M, Borthakur G, O'Brien S, Kantarjian H: Daily palonosetron is superior to ondansetron in the prevention of delayed chemotherapy-induced nausea and vomiting in patients with acute myelogenous leukemia. Cancer 116: 5659– 5666, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Giralt SA, Mangan KF, Maziarz RT, Bubalo JS, Beveridge R, Hurd DD, Mendoza FL, Rubenstein EB, DeGroot TJ, Schuster MW: Three palonosetron regimens to prevent CINV in myeloma patients receiving multiple-day high-dose melphalan and hematopoietic stem cell transplantation. Ann Oncol 22: 939– 946, 2011. [DOI] [PubMed] [Google Scholar]