Abstract
Post-operative memory changes after temporal lobe surgery have been established mainly by group analysis of cognitive outcome. This study investigated individual patient-based memory outcome in surgically-treated patients with mesial temporal lobe epilepsy (TLE). This study included 84 consecutive patients with intractable TLE caused by unilateral hippocampal sclerosis (HS) who underwent epilepsy surgery (47 females, 41 left [Lt] TLE). Memory functions were evaluated with the Wechsler Memory Scale-Revised before and at 1 year after surgery. Pre-operative memory function was classified into three patterns: verbal dominant memory impairment (Verb-D), visual dominant impairment (Vis-D), and no material-specific impairment. Post-operative changes in verbal and visual memory indices were classified into meaningful improvement, worsening, or no significant changes. Pre-operative patterns and post-operative changes in verbal and visual memory function were compared between the Lt and right (Rt) TLE groups. Pre-operatively, Verb-D was the most common type of impairment in both the Lt and Rt TLE groups (65.9 and 48.8%), and verbal memory indices were lower than visual memory indices, especially in the Lt compared with Rt TLE group. Vis-D was observed only in 11.6% of Rt and 7.3% of Lt TLE patients. Post-operatively, meaningful improvement of memory indices was observed in 23.3–36.6% of the patients, and the memory improvement was equivalent between Lt and Rt TLE groups and between verbal and visual materials. In conclusion, Verb-D is most commonly observed in patients with both the Lt and Rt TLE associated with HS. Hippocampectomy can improve memory indices in such patients regardless of the side of surgery and the function impaired.
Keywords: hippocampal sclerosis, temporal lobe epilepsy, epilepsy surgery, memory, neuropsychological function
Introduction
Neuropsychological evaluation is a very important tool to characterize cognitive abnormalities in patients with temporal lobe epilepsy (TLE).1) Memory disorders in patients with refractory TLE are believed to reflect pervasive damage in the medial temporal memory system.2) Most of the neuropsychological test batteries used in patients with intractable TLE have focused on evaluation of material-specific memory functions (verbal information in the left [Lt] temporal lobe and visual information in the right [Rt] temporal lobe) to assist in the determination of seizure lateralization/localization, as well as in the demonstration of post-surgical changes in these material-specific memory functions.3)
Material-specific memory impairment can indicate lateralized temporal lobe dysfunction. It is theoretically conceivable that verbal memory impairment suggests damage in the Lt temporal lobe, and that visual memory impairment suggests damage in the Rt temporal lobe. However, although dysfunction of the Lt temporal lobe has a strong and well-characterized relationship with impairment of verbal episodic memory, dysfunction of the Rt temporal lobe shows a far less consistent relationship with non-verbal or visuospatial memory.4,5)
When interpreting neuropsychological test in clinical situation, it is important to characterize patterns of memory deficits occurring in “individual” patients, because substantial individual variability exists in the test scores.6–8) Moreover, prediction of post-operative cognitive outcome must consider “meaningful” improvement or worsening of cognitive function in individual patients, because spurious improvement due to practice effect can occur on repeated testing.5) To overcome the problem, setting optimal cut-off scores is needed for assessing the impact of surgery on cognitive function.
This study was aimed to characterize patterns of material-specific memory impairment and their post-operative changes in individual patients with mesial TLE, and to investigate predictors for memory outcome after surgery.
Methods
This study was approved by the Tohoku University Hospital ethics committee (2010–187).
Subjects
This study included consecutive 84 patients with drug-resistant TLE caused by unilateral hippocampal sclerosis (HS) who underwent surgery at our institution between 1998 and 2012. All patients underwent comprehensive pre-surgical evaluations including brain magnetic resonance imaging (MRI), long-term video-electroencephalography (EEG) monitoring, neuropsychological evaluation, and functional imaging as necessary. All patients satisfied the clinical characteristics of mesial TLE,9,10) and pre-operative MRI findings indicated HS as the only etiology of epilepsy. Patient characteristics are summarized in Table 1. The Lt and Rt TLE groups showed no significant differences in age during surgery, sex, epilepsy onset, seizure freedom, and handedness. The histological diagnosis of HS was established in all cases.
Table 1.
Patient characteristics
| Lt TLE (n = 41) | Rt TLE (n = 43) | P value | |
|---|---|---|---|
| Sex (female:male) | 26:15 | 21:22 | n.s. |
| Handedness (Rt:Lt:Ambi) | 39:0:1 | 40:3:0 | n.s. |
| Age at surgery*, years | 38.2 ± 12.0 | 38.3 ± 9.9 | n.s. |
| Epilepsy onset*, years | 10.8 ± 5.9 | 10.9 ± 6.8 | n.s. |
| Type of surgery (ATL:SAH) | 37:4 | 38:5 | n.s. |
| Seizure freedom after surgery (%) | 68.3 | 74.4 | n.s. |
Values are means ± standard deviation. Ambi: ambidextrous, ATL: anterior temporal lobectomy, n.s.: not significant, SAH: selective amygdalohippocampectomy.
Pre-surgical evaluations
The imaging diagnosis of HS was based on the findings of obvious volume loss on the coronal section perpendicular to the hippocampal axis and increased intensity on T2-weighted and fluid-attenuated inversion recovery MRI of the unilateral hippocampus. 1.5-T and 3-T MRI systems were used in 62 and 22 patients, respectively. Patients with concomitant extra-hippocampal epileptogenic lesion (dual pathology) and/or ambiguous hippocampal pathology were excluded from the study.
Long-term video-EEG recording was performed for 4–7 days to detect at least one episode of habitual seizures. International 10–20 electrode placement and additional anterior temporal electrodes were used. Invasive evaluation with subdural grid and/or strip electrodes was performed in 12 patients. Interictal fluorodeoxyglucose positron emission tomography, interictal single-photon emission computed tomography, and magnetic source imaging were performed to confirm the diagnosis in 22 (26%), 63 (75%), and 67 (80%) patients, respectively.
Neuropsychological evaluation
All patients underwent cognitive assessments before and at 1 year after surgery. Memory and intellectual functions were evaluated with the Wechsler Memory Scale-Revised (WMS-R) and Wechsler Adult Intelligence Scale-Revised (WAIS-R), respectively. We defined significant “material-specificity” in memory function as 13 points or more difference between verbal and visual memory indices on WMS-R based on the normative data for people of age between 35 and 44 years.11) Accordingly, patients whose verbal memory index was 13 points or more lower than visual memory index and vice versa were diagnosed as having verbal dominant memory impairment (Verb-D) or visual dominant memory impairment (Vis-D), respectively. To evaluate post-operative changes in cognitive functions, we defined meaningful improvement and worsening as gains in an index of >12 and declines in an index of >7, respectively. These cut-off scores were set according to the previous studies in which practice effects with 2.15 ± 10.09 points gain were observed in repeated administration of the test.12,13)
Surgical treatment
Anterior temporal lobectomy with amygdalohippocampectomy was performed in 75 patients. The antero-inferior temporal lobe was removed by up to 35 mm in the dominant side and 45 mm in the non-dominant side from the tip of the temporal lobe, then the inferior horn of the lateral ventricle was opened. The amygdala was aspirated subpially to the level of the anterior choroidal artery or optic tract. The mesial temporal structures including the hippocampus and parahippocampal gyrus were removed en bloc, and further aspirated to the level of the superior colliculus in the anterior–posterior direction.14)
Transsylvian selective amygdalohippocampectomy was performed in nine patients treated after 2010. After standard fronto-temporal craniotomy, the proximal sylvian fissure was opened and a 15-mm long cortical incision was made parallel to the proximal middle cerebral artery, and the inferior horn of the lateral ventricle was reached through the trans-amygdala route. The mesial temporal lobe structures were removed to the level of the superior colliculus.15)
Histological examination
Hippocampal tissue sufficient for histological diagnosis was obtained from all patients. The resected specimens were qualitatively assessed for pattern of cell loss and gliosis in hippocampal subfields CA1 and CA3, and in the dentate gyrus.
Post-operative course
Patients were discharged and followed up while receiving therapeutic dosages of the same antiepileptic drugs as given pre-operatively. Termination of antiepileptic drugs was not attempted at 1 year post-operatively in all patients. Post-operative seizure outcome was evaluated using Engel’s classification. One year after surgery, 28 patients (68.3%) had achieved seizure freedom (Class I), 7 had rare seizures (Class II), and 6 had worthwhile improvement (Class III) in the Lt TLE group. Thirty-two patients achieved seizure freedom (74.4%), 4 had rare seizures, and 7 had worthwhile improvement in the Rt TLE group. No significant differences were found in the outcomes of seizure freedom between the Lt and Rt TLE groups.
Statistical evaluation
The data were analyzed using JMP software, version 10. Comparison of clinical characteristics including sex, handedness, type of surgery, and seizure outcome between the Lt and Rt TLE groups were performed using Fisher’s exact test. Comparisons between pre- and post-surgical neuropsychological performance in each group were performed using the paired t-test. Repeated measures analysis of variance (ANOVA) was used to examine the main effects of memory materials (verbal or non-verbal) and the sides of lesion/resection (Rt or Lt), and the interaction between the two factors on pre-operative memory performance and post-operative changes in memory performance. Multiple logistic regression analysis was performed to investigate the impact of pre-operative cognitive functions (WAIS-R FIQ and WMS-R general memory index), epileptogenic sides and other clinical variables (age of onset, duration of epilepsy, types of surgery, and post-operative seizure outcome) on post-operative memory improvement (post-operative gain in WMS-R general memory index >12).
Results
Table 2 summarizes the pre- and post-operative neuropsychological indices in the Lt and Rt TLE groups. Neuropsychological indices were generally significantly improved at group level except for verbal IQ after both left- and right-side surgery and verbal memory after left-side surgery.
Table 2.
Pre- and post-operative neuropsychological scores in patients with Lt and Rt TLE
| Lt TLE (n = 41) | Rt TLE (n = 43) | |||||
|---|---|---|---|---|---|---|
| Pre-operative* | Post-operative* | P value | Pre-operative* | Post-operative* | P value | |
| WAIS-R | ||||||
| Verbal IQ | 77.32 ± 15.03 | 79.37 ± 14.67 | n.s. | 81.36 ± 15.75 | 82.72 ± 16.91 | n.s. |
| Performance IQ | 81.54 ± 15.26 | 90.18 ± 18.66 | <0.01 | 85.22 ± 18.12 | 92.03 ± 20.67 | <0.01 |
| Full scale IQ | 76.02 ± 15.68 | 81.88 ± 16.45 | <0.01 | 83.05 ± 17.41 | 88.16 ± 20.18 | <0.01 |
| WMS-R | ||||||
| Verbal memory | 72.1 ± 15.67 | 75.46 ± 14.32 | n.s. | 81.77 ± 18.32 | 86.77 ± 20.6 | <0.01 |
| Visual memory | 94.22 ± 20.91 | 100.29 ± 17.98 | <0.05 | 94.02 ± 18.57 | 100.09 ± 15.59 | <0.01 |
| General memory | 74.44 ± 17.49 | 79.22 ± 15.28 | <0.05 | 83.12 ± 19.61 | 88.6 ± 20.25 | <0.01 |
Values are means ± standard deviation. n.s.: not significant, WAIS-R: Wechsler Adult Intelligence Scale-Revised.
Pre-operative patterns of memory impairment
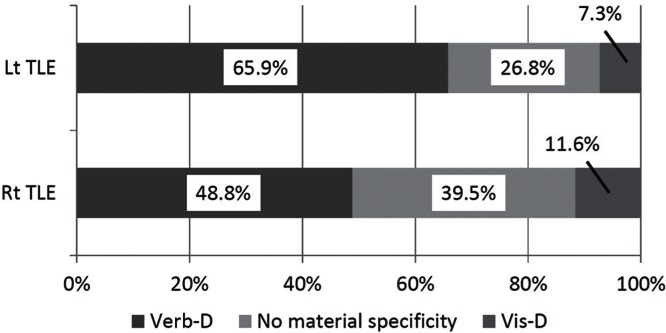
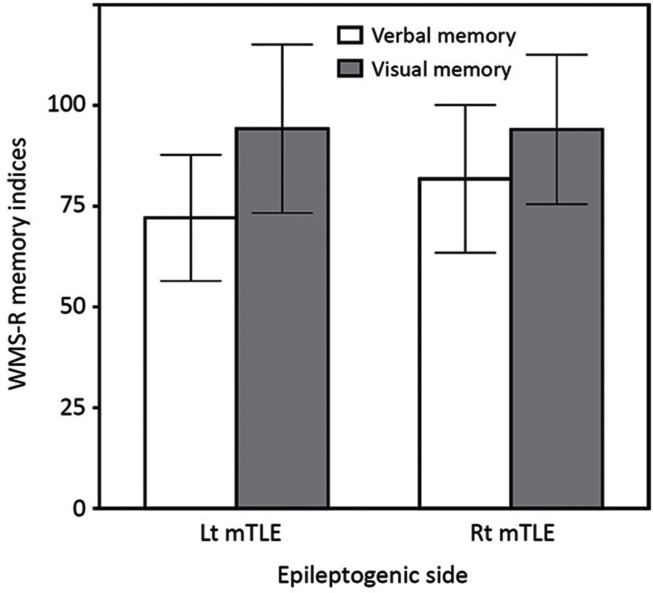
Pre-operatively, Verb-D was the most common type of impairment in both the Lt and Rt TLE groups, but was more frequent in Lt TLE group (27 patients, 65.9%) than in Rt TLE group (21 patients, 48.8%) (Fig. 1). Vis-D was infrequent in both the Rt TLE group (5 patients, 11.6%) and Lt TLE group (3 patients, 7.3%). No clear material specificities were observed in 11 patients (26.8%) of the Lt TLE group and in 17 patients (39.5%) of the Rt TLE group. Two-way repeated ANOVA demonstrated a significant effect of memory materials (F = 69.33, P < 0.0001) but not of epileptogenic sides (F = 1.887, P = 0.1732), in which verbal memory index was lower compared to visual memory index (Fig. 2). A significant interaction between memory materials and epileptogenic sides was observed (F = 5.725, P = 0.019). Post-hoc pair-wise comparisons demonstrated that verbal memory index was lower in patients with left-side epilepsy compared to those with right-side epilepsy.
Fig. 1.
Pre-operative patterns of memory impairment. Bar graphs showing the population of patients with verbal dominant memory impairment (Verb-D in dark gray), visual dominant memory impairment (Vis-D in gray), and no material-specific memory impairment (light gray). Verbal dominant memory impairment was the most common type of memory impairment in both Lt and Rt TLE groups.
Fig. 2.
Pre-operative verbal and visual memory indices in the Lt and Rt TLE groups are shown in bar charts showing means and standard deviations. Verbal memory indices are lower than visual memory indices in both groups. Two-way repeated measures ANOVA demonstrated a significant effect of memory materials, but not of epileptogenic sides. A significant interaction was found between memory materials and epileptogenic sides, in which verbal memory index was lower in the Lt TLE group than in the Rt TLE group.
Post-operative memory changes
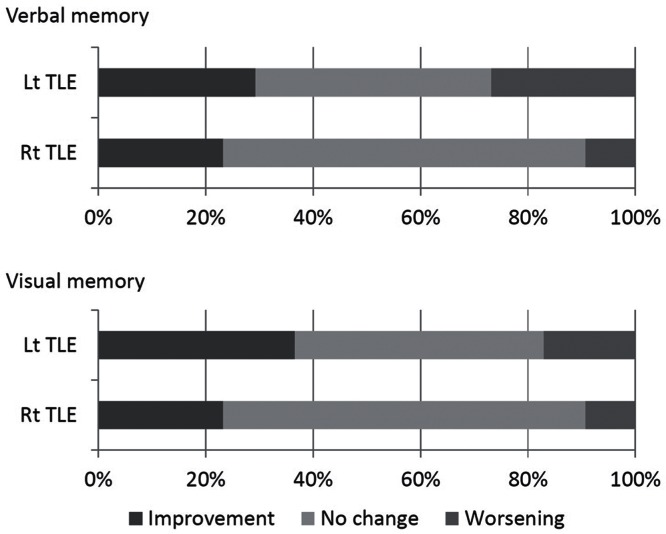
While post-operative gains in the mean verbal memory index were 3.36 in the Lt TLE group and 5.00 in the Rt TLE group (Table 2), meaningful improvement was observed in 12 patients (29.3%) and in 10 patients (23.3%), respectively, and meaningful worsening was observed in 11 patients (26.8%) and in 4 patients (9.3%), respectively (Fig. 3). While post-operative gains in the mean visual memory index were 6.07 in the Lt TLE group and 6.07 in the Rt TLE group, meaningful improvement was observed in 15 patients (36.6%) and in 10 patients (23.3%), respectively, and meaningful worsening was observed in 7 patients (17.1%) and in 4 patients (9.3%), respectively.
Fig. 3.
Patterns of post-operative memory changes. Bar graphs showing the population of patients with meaningful improvement (dark gray), meaningful worsening (gray), and no meaningful changes (light gray) in verbal memory scores (upper graphs) and visual memory scores (lower graphs). Meaningful improvement of verbal memory was observed in 29.3% of patients with Lt TLE and in 23.3% of patients with Rt TLE, and meaningful worsening was observed in 26.8% of patients with Lt TLE and in 9.3% of patients with Rt TLE. Meaningful improvement of visual memory was observed in 36.6% of patients with Lt TLE and in 23.3% of patients with Rt TLE, and meaningful worsening was observed in 17.1% of patients with Lt TLE and in 9.3% of patients with Rt TLE.
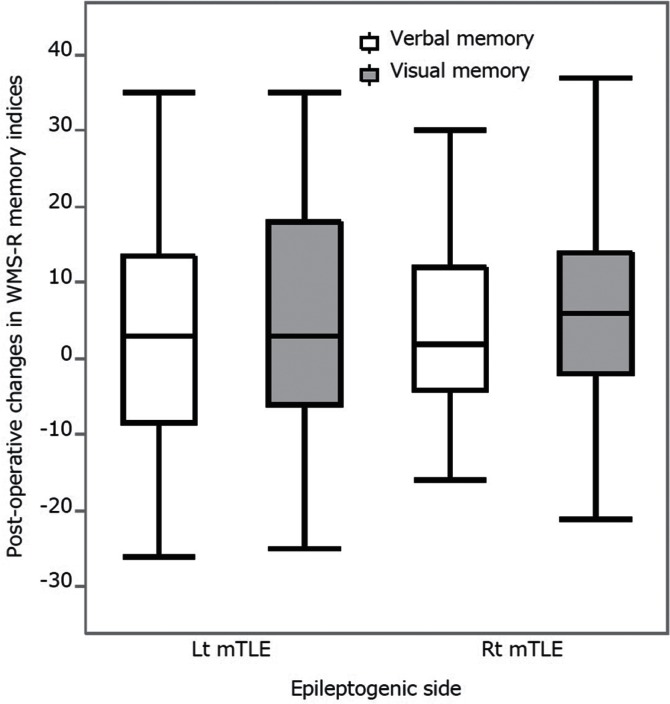
Paired t-tests demonstrated significant post-operative improvement in visual memory index in the Lt TLE group and in both verbal and visual memory indices in the Rt TLE group (Table 2). The two-way repeated ANOVA did not demonstrate any significant effects of memory materials (F = 1.22, P = 0.2727) or epileptogenic sides (F = 0.1204, P = 0.7295), indicating that post-operative memory improvement was equivalent between the Rt and Lt TLE groups and between verbal and visual materials (Fig. 4).
Fig. 4.
Post-operative changes of verbal and visual memory indices in the Lt and Rt TLE groups are shown in boxplots. Positive values indicate post-operative gains in memory indices. Two-way repeated measures ANOVA demonstrated no significant effects of memory materials and epileptogenic sides, indicating that post-operative memory changes were equivalent between the Rt and Lt TLE groups and between verbal and visual materials.
Multiple logistic regression analysis showed that only the type of surgery affected post-operative improvement in general memory (Table 3), in which selective surgery was a factor for post-operative memory improvement. However, pre-operative memory indices were lower in the patients who underwent selective amygdalohippocampcetomy compared with those who underwent anterior temporal lobectomy (data not shown), suggesting that co-linearity between pre-operative memory indices and type of surgery possibly biased the results. When the analysis was limited to the patients who underwent anterior temporal lobectomy, no clinical factors predicted post-operative improvement in general memory (Table 4).
Table 3.
Multiple logistic regression analysis of the relationship between post-operative improvement in general memory and pre-operative clinical factors
| Factors | P value |
|---|---|
| Laterality of epilepsy | 0.62 |
| Age at onset of epilepsy (year) | 0.06 |
| Duration of epilepsy (year) | 0.85 |
| Seizure freedom at 1 year | 0.32 |
| Pre-operative full scale IQ (WAIS-R) | 0.75 |
| Pre-operative general memory index (WMS-R) | 0.12 |
| Type of surgery (ATL or SAH) | 0.01* |
P < 0.05. WAIS-R: Wechsler Adult Intelligence Scale-Revised, WMS-R: Wechsler Memory Scale-Revised, ATL: anterior temporal lobectomy, SAH: selective amygdalohippocampectomy.
Table 4.
Multiple logistic regression analysis of the relationship between post-operative improvement in general memory and pre-operative clinical factors in the patients who underwent anterior temporal lobectomy
| Factors | P value |
|---|---|
| Laterality of epilepsy | 0.86 |
| Age at onset of epilepsy (year) | 0.14 |
| Duration of epilepsy (year) | 0.36 |
| Seizure freedom at 1 year | 0.44 |
| Pre-operative full scale IQ (WAIS-R) | 0.83 |
| Pre-operative general memory index (WMS-R) | 0.17 |
P < 0.05. WAIS-R: Wechsler Adult Intelligence
Scale-Revised, WMS-R: Wechsler Memory Scale-Revised.
Discussion
This study showed that verbal dominant memory impairment was most common in both Lt and Rt TLE with HS, although verbal dominant impairment was relatively more frequent in Lt TLE and visual dominant impairment was infrequent. Verbal memory index was lower than visual memory index in both Lt and Rt TLE, and more severe impairment of verbal memory was associated with Lt TLE. Post-operative memory indices showed significant improvement after surgery, except for verbal memory in Lt TLE. However, meaningful improvement in memory indices was experienced by 20% or more of patients with both Lt and Rt TLE, and laterality of epilepsy and memory materials were not associated with post-operative memory improvement.
This findings are consistent with previous reports of pre-operative memory function and post-operative change.16) In our study, the Lt TLE group had worse pre-operative verbal memory compared to the Rt TLE group. Verbal memory deficit was the most frequent finding in patients with Lt TLE, whereas, visual memory deficit was infrequent even in patients with Rt TLE. A key concept in assessing the function of each temporal lobe “independently” is the material-specificity principle, which proposes that verbal memory is a function of the Lt temporal lobe and visual or spatial memory is a function of the Rt temporal lobe.17) The material-specificity principle generally holds true, especially with respect to verbal memory,18) but has lower reliability for identifying the laterality of dysfunction.5,19) The sensitivity of verbal memory tests to Lt mesial temporal damage varies across instruments, suggesting that verbal memory is not a unitary construct. For instance, patients with Lt TLE, but not patients with Rt TLE, have impaired memory for word pairs but intact memory for prose passages.20) Some studies show a relationship between Rt TLE and visual memory,21) but other studies do not or find impairments of similar magnitude in both Lt and Rt TLE.22)
In this study, both Lt and Rt TLE groups showed significant post-operative improvement in memory indices in general, except for verbal memory after left-side surgery. As functional inadequacy of the brain to be resected is crucial to good cognitive outcome,6) the presence of HS suggests that the ipsilateral temporal lobe was adequately dysfunctional so that the removal do not carry significant and specific risks for post-operative deterioration.23) Patients who had undergone Lt anterior temporal lobectomy without significant sclerosis in the resected hippocampus had greater risk of deterioration in verbal memory, because the resected hippocampus had reasonably good function.24) Therefore, our findings suggest that patients with TLE and unilateral HS have low risk for post-operative impairment in memory indices regardless of the side of surgery.
Improvement in overall memory indices is less common and may even be less correlated with good seizure outcome.25,26) Improvement in neuropsychological function may be influenced by the reduction in seizure frequency after surgery.25) This logistic regression analysis failed to demonstrate an impact of post-surgical seizure freedom on memory improvement. This finding is probably related to the selection of the patient population. This study included patients with unilateral HS and no other epileptogenic lesion, which is associated with excellent and less variable seizure outcome after surgery. Successful removal of the epileptogenic tissue may reduce the amount of both ictal and interictal epileptic activities, resulting in preservation of the function of the surrounding tissues.25)
Most patients showed no meaningful post-operative changes in memory indices. Importantly, meaningful improvement was observed more frequently than worsening, although worsening was relatively frequent in the verbal memory after left-sided surgery. Similar outcomes were reported in patients undergoing temporal lobe surgery,27) but worsening was more frequent than improvement, possibly because patients with various etiologies were included, not limited to those with only unilateral HS. Infrequent worsening of memory function is probably related to excellent seizure control in our patient cohort. Cognitive changes should be evaluated at individual level for outcome prediction,6,7) although many previous studies focused on cognitive changes at group level.
Type of surgery may influence post-operative cognitive outcome. In our study, selective amygdalohippocampectomy was an only predictor for post-operative improvement of general memory. However, careful interpretation is necessary because only nine patients during the last study period were indicated for selective surgery, and those patients had lower pre-operative memory indices than others. Selective surgery is theoretically better for cognitive outcome, but consistent results have not been reported and its advantage is often marginal probably because of “collateral” damage made by surgical approach, i.e. cut of temporal stem fibers.16)
Our study has a limitation in the method defining meaningful differences and changes in memory indices. Significant difference and changes were defined in this study after the standard deviation obtained from the previous studies. Empirically based techniques, such as the reliable change index and standardized regression-based change scores, provide more accurate and reliable risk estimates. Reliable change indexes of WMS-R verbal memory score and visual memory score were 16.67 and 16.46 for the 90% confidence interval, respectively.8) Therefore, an individual memory score must change from baseline by 17 points to be considered significant (P < 0.05). This provides more strict criteria for meaningful changes than our study. Further correlation of the criteria to quality of life should be established.
Conclusion
Verbal dominant memory impairment is most commonly observed in patients with both Lt and Rt TLE associated with HS, although verbal dominant impairment is relatively frequent in Lt TLE and visual dominant impairment is infrequent. Hippocampectomy can improve memory indices in such patients regardless of the side of surgery and the function impaired.
Acknowledgment
The author AFK was supported by The Japan Epilepsy Research Foundation Research Grant for Inviting Overseas Researchers to Japan and by Takeda Science Foundation International Fellowship Programs for Foreign Researchers.
Footnotes
Conflicts of Interest Disclosure
The authors report no conflicts of interest concerning with the materials or methods used in this study or the findings specified in this paper. The authors MI, NN, and TT have registered online Self-reported COI Disclosure Statement Forms through the website for JNS members.
References
- 1). Lee TMC, Yip JTH, Jones-Gotman M: Memory deficits after resection from left or right anterior temporal lobe in humans: a meta-analytic review. Epilepsia 43: 283– 291, 2002. [DOI] [PubMed] [Google Scholar]
- 2). Alessio A, Bonilha L, Rorden C, Kobayashi E, Min LL, Damasceno BP, et al. : Memory and language impairments and their relationships to hippocampal and perirhinal cortex damage in patients with medial temporal lobe epilepsy. Epilepsy Behav 8: 593– 600, 2006. [DOI] [PubMed] [Google Scholar]
- 3). Milner B: Psychological aspects of focal epilepsy and its neurosurgical management. Adv Neurol 8: 299– 321, 1975. [PubMed] [Google Scholar]
- 4). Helmstaedter C, Pohl C, Elger CE: Relations between verbal and nonverbal memory performance: evidence of confounding effects particularly in patients with right temporal lobe epilepsy. Cortex 31: 345– 355, 1995. [DOI] [PubMed] [Google Scholar]
- 5). Jeyaraj MK, Menon RN, Justus S, Alexander A, Sarma PS, Radhakrishnan K: A critical evaluation of the lateralizing significance of material-specific memory deficits in patients with mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsy Behav 28: 460– 6, 2013. [DOI] [PubMed] [Google Scholar]
- 6). Baxendale S, Thompson P: Defining meaningful postoperative change in epilepsy surgery patients: measuring the unmeasurable? Epilepsy Behav 6: 207– 211, 2005. [DOI] [PubMed] [Google Scholar]
- 7). Sherman EMS, Wiebe S, Fay-McClymont TB, Tellez-Zenteno J, Metcalfe A, Hernandez-Ronquillo L, et al. : Neuropsychological outcomes after epilepsy surgery: systematic review and pooled estimates. Epilepsia 52: 857– 869, 2011. [DOI] [PubMed] [Google Scholar]
- 8). Chelune GJ, Naugle RI, Luders H, Sedlak J, Awad IA: Individual change after epilepsy surgery: practice effects and base-rate information. Neuropsychology 7: 41– 52, 1993. [Google Scholar]
- 9). Wiebe S, Blume WT, Girvin JP, Eliasziw M: A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 345: 311– 318, 2001. [DOI] [PubMed] [Google Scholar]
- 10). Wieser HG: ILAE Commission on Neurosurgery of Epilepsy: ILAE commission report: mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia 45: 695– 714, 2004. [DOI] [PubMed] [Google Scholar]
- 11). Wechsler D: Manual for the Wechsler Memory Scale-Revised. San Antonio, The Psychological Corporation, 1987. [Google Scholar]
- 12). Naugle RI, Chelune GJ, Cheek R, Lüders H, Awad IA: Detection of changes in material-specific memory following temporal lobectomy using the Wechsler Memory Scale-Revised. Arch Clin Neuropsychol 8: 381– 95, 1993. [PubMed] [Google Scholar]
- 13). Dikmen SS, Heaton RK, Grant I, Temkin NR: Test–retest reliability and practice effects of expanded Halstead–Reitan neuropsychological test battery. J Int Neuropsychol Soc 5: 346– 56, 1999. [PubMed] [Google Scholar]
- 14). Spencer DD, Spencer SS, Mattson RH, Williamson PD, Novelly RA: Access to the posterior medial temporal lobe structures in the surgical treatment of temporal lobe epilepsy. Neurosurgery 15: 667– 671, 1984. [DOI] [PubMed] [Google Scholar]
- 15). Yaşargil MG, Krayenbühl N, Roth P, Hsu SPC, Yaşargil DCH: The selective amygdalohippocampectomy for intractable temporal limbic seizures. J Neurosurg 112: 168– 185, 2010. [DOI] [PubMed] [Google Scholar]
- 16). Helmstaedter C: Cognitive outcomes of different surgical approaches in temporal lobe epilepsy. Epileptic Disord 15: 221– 39, 2013. [DOI] [PubMed] [Google Scholar]
- 17). Milner B: Intellectual function of the temporal lobes. Psychol Bull 51: 42– 62, 1954. [DOI] [PubMed] [Google Scholar]
- 18). Helmstaedter C, Grunwald T, Lehnertz K, Gleissner U, Elger CE: Differential involvement of left temporolateral and temporomesial structures in verbal declarative learning and memory: evidence from temporal lobe epilepsy. Brain Cogn 35: 110– 131, 1997. [DOI] [PubMed] [Google Scholar]
- 19). Saling MM: Verbal memory in mesial temporal lobe epilepsy: beyond material specificity. Brain 132: 570– 582, 2009. [DOI] [PubMed] [Google Scholar]
- 20). Rausch R, Babb TL: Hippocampal neuron loss and memory scores before and after temporal lobe surgery for epilepsy. Arch Neurol 50: 812– 817, 1993. [DOI] [PubMed] [Google Scholar]
- 21). Gleissner U, Helmstaedter C, Elger CE: Right hippocampal contribution to visual memory: a presurgical and postsurgical study in patients with temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 65: 665– 669, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Glikmann-Johnston Y, Saling MM, Chen J, Cooper KA, Beare RJ, Reutens DC: Structural and functional correlates of unilateral mesial temporal lobe spatial memory impairment. Brain 131: 3006– 3018, 2008. [DOI] [PubMed] [Google Scholar]
- 23). Shin MS, Lee S, Seol SH, Lim YJ, Park EH, Sergeant JA, et al. : Changes in neuropsychological functioning following temporal lobectomy in patients with temporal lobe epilepsy. Neurol Res 31: 692– 701, 2009. [DOI] [PubMed] [Google Scholar]
- 24). Davies KG, Bell BD, Bush AJ, Wyler AR: Prediction of verbal memory loss in individuals after anterior temporal lobectomy. Epilepsia 39: 820– 828, 1998. [DOI] [PubMed] [Google Scholar]
- 25). Wachi M, Tomikawa M, Fukuda M, Kameyama S, Kasahara K, Sasagawa M, et al. : Neuropsychological changes after surgical treatment for temporal lobe epilepsy. Epilepsia 42: 4– 8, 2001. [PubMed] [Google Scholar]
- 26). Leonard G: Temporal lobe surgery for epilepsy: neuropsychological variables related to surgical outcome. Can J Neurol Sci 18: 593– 597, 1991. [DOI] [PubMed] [Google Scholar]
- 27). Helmstaedter C, Kurthen M, Lux S, Reuber M, Elger CE: Chronic epilepsy and cognition: a longitudinal study in temporal lobe epilepsy. Ann Neurol 54: 425– 432, 2003. [DOI] [PubMed] [Google Scholar]