Abstract
Purpose
Macular pigment (MP) spatial distribution varies considerably among individuals. We investigated ethnic variations in MP spatial distribution in relation to foveal architecture.
Methods
We measured MP optical density (MPOD) using heterochromatic flicker photometry (MAP test, City, University of London) in 76 white, 80 South Asian and 70 black volunteers (18 to 39 years). MPOD spatial profiles were classified objectively as exponential, ring-like or central dip, based on deviations away from an exponential fit. Measurements including total retinal thickness (RT), inner retinal layer (IRL), inner and outer plexiform layer (IPL and OPL) thickness, foveal width and foveal pit slope were taken from Spectralis SD-OCT (Heidelberg, Germany) scans.
Results
Integrated MPOD up to 1.8° (MPODint) was higher in South Asian (0.84±0.26) and black (0.84±0.31) than whites (0.63±0.24, P<0.0005). Ethnicity explained around 10% of the variance while gender played no significant role. MPOD profile phenotypes were associated with ethnicity: 58% with ring profiles were South Asian and 43% with dip profiles were black (χ2(4,226) = 13.4, P = 0.009). MPODint was lower in exponential (0.66±0.21) compared to ring-like (0.96±0.26) and central dip (1.00±0.32, P<0.0005) groups. White subjects had thicker IRL at 0° (130±21μm) than South Asian (123±16μm) and blacks (116±14μm; F(2) = 12.4, P<0.0005), with comparable results for IPL (P<0.0005) and OPL (P = 0.03). There was no significant difference in IRL, IPL or OPL (from 0 to 3.8° retinal eccentricity) or foveal width between MP profile groups (P>0.05).
Conclusion
We report a significant difference in the amount and distribution of MP between ethnicities that is not explained by variations in foveal morphology.
Introduction
Increased MPOD may reduce the likelihood of age-related macular degeneration (AMD) by protecting the retina from oxidative and photochemical damage [1]. MPOD has been shown to vary among individuals [2–5] and may be affected by age [6–9], gender [10], iris colour [3, 11] and modifiable factors such as smoking status [12]. MPOD typically follows an exponential decline with eccentricity from the centre of the fovea [13–16]. Previous studies have highlighted the occurrence of atypical MP spatial profiles also known as secondary sub-peaks [17], bi-modal [18], ring-like [19, 20] and central dip [21, 22] distributions. Increased prevalence of atypical ring-like profiles has been reported in females [18], non-smokers [21], healthy subjects compared to those with AMD [19] and in ethnicities that may present lower prevalence of AMD [22, 23]. However, there are inconsistencies in the literature regarding increased prevalence of ring-like profiles among non-white ethnicities [10, 14, 16]. Nonetheless, it has been shown that white subjects demonstrate lower central MPOD than non-whites, including South Asian [22, 24, 25] and black ethnicities [23].
A possible predictor of the MPOD spatial profile may be foveal morphology. Almost thirty years ago it was suggested that the spatial distribution of MP was attributable to its position within the individual retinal layers, with maximum concentration of MP in the fovea within the fibres of Henle and in the inner and outer plexiform layers in the parafovea [13, 26]. As the fibres of Henle extend horizontally into the inner nuclear layer, MP has been found between the cell nuclei of the nuclear layer [27] and there is evidence that the Müller cells interleaved amongst the cone photoreceptors also contain macular carotenoids [28, 29]. It has been suggested that variations in foveal pit morphology play a role in the spatial distribution of MP across the retina [16–18, 30]. In white subjects, a thicker central retina has been associated with significantly higher MPOD levels [30, 31]. However, such an association has not been reported in other studies [16, 17, 32]. It has been proposed that a sharper decline in MPOD with eccentricity from the fovea is associated with a steeper incline in retinal thickness from the centre of the fovea to the periphery, possibly due to compression of the inner plexiform and cone axon layers of the retina that host MP [17]. On the other hand, wider foveas support longer cone axons and may therefore provide more storage capacity for MP [16, 31].
Variations in foveal pit morphology have been found to vary across ethnicities [33–35], whereby non-white subjects have a significantly thinner central retina and wider foveas compared to whites [33, 35–39]. Given the inter-individual differences in both MPOD and foveal morphology, the current study was designed to investigate ethnic variations in MP spatial distribution in relation to foveal architecture with specific emphasis on the inner and outer plexiform layers.
Methods
The investigation took place at the Division of Optometry and Visual Science, City University London between October 2013 and March 2015. Ethical approval for the study was obtained from the University Research & Ethics Committee and written informed consent was obtained from all subjects prior to participation, conforming to the tenets of the Declaration of Helsinki. Participants completed a health and lifestyle questionnaire, including information about general and ocular health, use of medication, nutritional supplementation, and smoking status. Exclusion criteria were visual acuity of 0.3 logMAR or worse in the test eye, ocular pathology in the test eye, previous refractive surgery, current use of carotenoid supplementation. Inclusion into the study was based on self-reported white, South Asian or black ethnicity and between 18 to 39 years of age. Classification of ethnicity was based on the criteria used by the Office of National Statistics [40]. The following ethnic groups were not included in the study: mixed/multiple ethnic groups; Asian Chinese or any other Asian background not mentioned in the inclusion criteria; and all other ethnic groups.
Macular pigment measurements
MPOD was measured using the Macular Assessment Profile (MAP) test, a VDU-based test incorporating the HFP technique. Its rationale has been described in detail elsewhere [22, 41]. The MAP test measures MPOD at 0°, 0.8°, 1.8°, 2.8° and 3.8°. The average of two measurements at 6.8° and 7.8° retinal eccentricity serves as the peripheral reference point. As with other HFP methods, the luminance of the short wavelength test beam is altered to cancel or minimize the perception of flicker. A double reversal technique is employed to give the luminance of the test beam required to cancel the reference beam (the flicker null point) at each eccentricity. The average of four low and high threshold values is used to compute MPOD for each retinal location tested (calculated by comparing the mean luminance adjustment of the test beam in the central retina to the peripheral reference point). The area under the MP spatial profile curve from 0° to 1.8° i.e. MPODint (0 to 1.8) was calculated [17]. In addition, the slope of the MP spatial profile between 0° to 0.8°, 0.8° to 1.8° and 1.8° to 2.8° was calculated based on a previously described method [17].
Foveal morphology measurements
Infrared scanning laser ophthalmoscope fundus imaging and SD-OCT (Spectralis, Heidelberg, Germany) imaging was performed on the test eye of each subject utilising the Automated Real Time eye-tracking feature. For each participant, mean keratometry and mean spherical error (MSE) measurements obtained using the TRK 1-P autorefractor (Topcon, Tokyo, Japan) were incorporated into the Spectralis SD-OCT software prior to scan acquisition to minimize errors in lateral measurements caused by ocular magnification [42, 43]. High resolution 20° x 10° volume scans (97 B-sections 30 microns apart, 16 frames including 1024 A-scans) were taken on an undilated eye in a dark room [16, 44]. Measurements of foveal morphology were obtained [45] and included:
Total retinal, IRL, IPL and OPL thickness at eccentricities corresponding to the locations where MPOD is measured by the MAP test i.e. 0°, 0.8°, 1.8°, 2.8° and 3.8°,
Total retinal volume derived from the 20° by 20° volume scan;
Foveal width; and
Foveal pit profile slope between 0° to 0.8°, 0.8° to 1.8° and 1.8° to 2.8°.
Statistical analysis
All statistical analyses were performed using SPSS version 22.0 for Windows (SPSS Inc., Chicago, USA). Values in the text and tables are presented as the mean ± SD. Two-way ANCOVA evaluated the impact of ethnicity and gender confounders on MPOD and foveal morphology parameters. One-way ANCOVA was used to explore the effect of the MP spatial profile phenotype (exponential, ring-like or central dip) on foveal parameters. Pearson's product moment correlation coefficients were calculated to examine the association between retinal architecture parameters and MPOD measures. Statistical significance was accepted at the 95% confidence level (P < 0.05).
Sample size calculation
An a priori power analysis conducted using G*Power 3.1 [46, 47] revealed that a total sample size of one hundred and ninety subjects was required. This was based on ANCOVA fixed effects, special, main effects and interactions calculated for three groups, with a power level of 80%, a statistical significance level of α = 0.05 and a medium effect size of 0.3.
Results
In total, two hundred and twenty-six volunteers participated in the study, including seventy-six white (24 males; 52 females), eighty South Asian (31 males; 49 females) and seventy black subjects (25 males; 45 females). The right eye fulfilled the inclusion criteria and was therefore used as the test eye in two hundred and eighteen (96%) subjects. The range of MSE was -8.75 to +7.50DS in the white, -13.00 to +1.25DS in the South Asian and -7.75 to +1.75DS in the black ethnic groups. Mean MSE did not significantly vary between the three ethnic groups (P > 0.05). Thirty-two volunteers reported being current or ex-smokers (20 white, 7 South-Asian, and 5 black). Smoking pack/year was 0.98 ± 2.54 for the white, 0.043 ± 0.17 for the South Asian, and 0.048 ± 0.30 for the black ethnic groups. Due to the small number of smokers in the non-white groups, this variable was only considered within the white ethnic group.
Variations in MP spatial distribution between ethnic groups
Mean central MPOD values were consistently lower in the white ethnic group (0.47 ± 0.17) compared to the South Asian (0.61 ± 0.17) and black ethnic groups (0.56 ± 0.19, P < 0.0005). This trend continued for MPOD at 0.8° and MPODint (0 to 1.8) as presented in Table 1. There were no statistically significant difference between the South Asian and black ethnic groups for any of these parameters (P > 0.05). While the main effect of gender reached statistical significance for MPOD at 0°, (F(1,226) = 4.63, P = 0.033), this was not the case for MPOD at 0.8° or MPODint (0 to 1.8) (P > 0.05). In any event the estimated effect size (partial eta squared) of gender was 0.02 or less on all MPOD variables.
Table 1. Mean ± SD MPOD at 0°, 0.8° and MPODint (0 to 1.8) per ethnic group and gender.
Results of two-way analysis of covariance between-subjects effects of ethnicity are presented.
| White | South Asian | Black | P-value | Partial eta squared | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | ±SD | Mean | ±SD | Mean | ±SD | ||||
| MPOD at 0° | Whole group | 0.47 | 0.17 | 0.61 | 0.17 | 0.56 | 0.19 | < 0.0005 | 0.08 |
| Male | 0.51 | 0.18 | 0.62 | 0.16 | 0.61 | 0.20 | 0.033 | 0.02 | |
| Female | 0.45 | 0.17 | 0.60 | 0.18 | 0.53 | 0.18 | |||
| MPOD at 0.8° | Whole group | 0.39 | 0.16 | 0.53 | 0.17 | 0.52 | 0.20 | < 0.0005 | 0.10 |
| Male | 0.42 | 0.16 | 0.52 | 0.18 | 0.56 | 0.21 | 0.12 | 0.01 | |
| Female | 0.37 | 0.16 | 0.53 | 0.17 | 0.49 | 0.20 | |||
| MPODint (0 to 1.8) | Whole group | 0.63 | 0.24 | 0.84 | 0.26 | 0.84 | 0.31 | < 0.0005 | 0.11 |
| Male | 0.68 | 0.24 | 0.84 | 0.27 | 0.92 | 0.32 | 0.06 | 0.02 | |
| Female | 0.61 | 0.23 | 0.83 | 0.25 | 0.79 | 0.29 | |||
Among the white ethnic group, MPOD at 0° was higher in never smokers (0.49 ± 0.19) compared to current or ex-smokers (0.40 ± 0.12). However this failed to reach statistical significance (t(74) = 1.76, P = 0.08). Of note, the mean smoking pack/year ranged between 0 and 12 with a median < 0.0001. A multiple regression model explored the effect of retinal thickness and smoking on central MPOD in white subjects only. The model failed to reach significance (P = 0.08) and revealed that neither smoking (beta = -0.20) nor retinal thickness (beta = 0.17) made a unique contribution to MPOD.
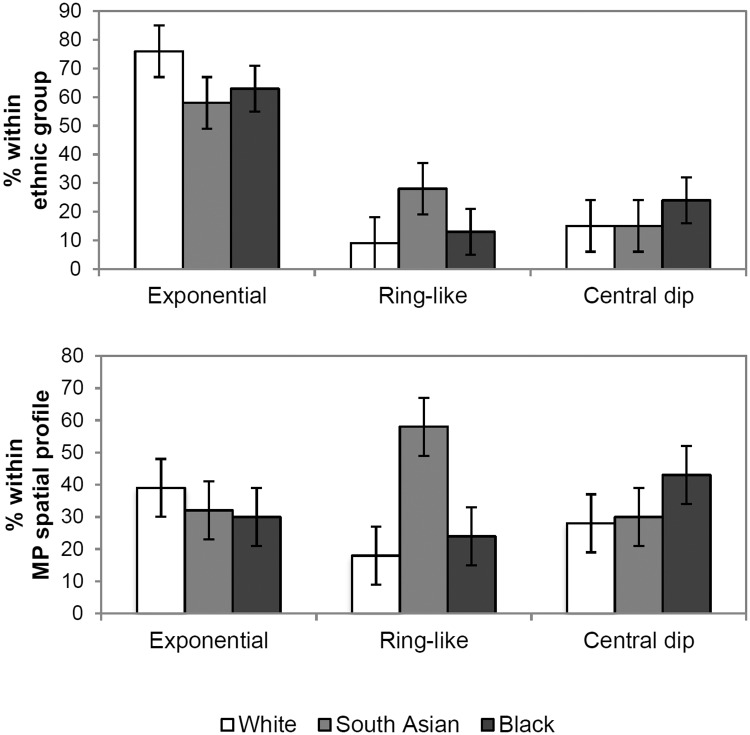
The percentage of individuals presenting each MP spatial profile phenotype within the three ethnic groups and within each MP spatial profile phenotype is provided in Fig 1A chi-square test for independence indicated a statistically significant association between ethnicity and presence of an exponential, ring or central dip MPOD spatial profile type (χ2 (4, n = 226) = 13.4, P = 0.009, Cramer's V = 0.17); the majority of subjects (58%) presenting with a ring-like MP spatial profile were of South Asian ethnicity, compared to 18% of white and 24% of black participants. Additionally more black subjects (43%) presented with a dip profile compared to whites (28%) or South Asian (30%).
Fig 1. Percentage of individuals with exponential, ring-like or central dip MP spatial profile phenotypes within each ethnic group (upper graph).
Percentage of white, South Asian and black individuals within each MP spatial profile phenotype (lower graph).
Variations in foveal architecture between ethnic groups
Mean ± SD foveal morphology parameters per ethnic group are presented in Table 2. A two way ANCOVA was conducted to investigate differences in foveal architecture between the white, South Asian and black ethnic groups, controlling for MSE. The main effects of ethnicity and gender were statistically significant for all foveal morphology dependent variables with a larger estimated effect size (partial eta squared) for ethnicity compared to gender.
Table 2. Mean ± SD retinal thickness, foveal width and foveal volume, inner and outer plexiform layers per ethnic group and results of two-way analysis of covariance showing between-subjects effects of ethnicity with mean spherical error as covariate (results for gender analysis not shown).
| White | South Asian | Black | P-value | Partial eta squared | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | ±SD | Mean | ±SD | Mean | ±SD | |||
| Retinal thickness at 0° (μm) | 229 | 20 | 220 | 14 | 215 | 14 | <0.0005 | 0.15 |
| Inner Retinal Layer at 0° (μm) | 130 | 21 | 123 | 16 | 116 | 14 | <0.0005 | 0.12 |
| Foveal width (μm) | 2282 | 225 | 2474 | 260 | 2449 | 284 | <0.0005 | 0.11 |
| Foveal volume (μm3) | 8.86 | 0.34 | 8.71 | 0.35 | 8.73 | 0.39 | 0.04 | 0.03 |
| Inner plexiform layer (μm) | ||||||||
| at 0° | 13 | 3 | 12 | 3 | 11 | 3 | <0.0005 | 0.05 |
| at 0.8° | 17 | 4 | 15 | 4 | 15 | 4 | <0.0005 | 0.11 |
| at 1.8° | 31 | 6 | 26 | 6 | 29 | 6 | <0.0005 | 0.13 |
| Outer plexiform layer (μm) | ||||||||
| at 0° | 5 | 5 | 5 | 5 | 3 | 3 | 0.032 | 0.031 |
| at 0.8° | 15 | 8 | 13 | 9 | 11 | 5 | 0.003 | 0.051 |
| at 1.8° | 30 | 6 | 28 | 9 | 27 | 10 | 0.264 | 0.012 |
Post-hoc Tukey testing indicated that IRL thickness was greater in whites compared to South Asian (P = 0.009) and blacks (P = 0.001). There was also a significant difference in IRL between South Asian and blacks (P = 0.009). These findings were replicated when the two-way ANCOVA was repeated for RT and IRL at 0.8° and 1.8°. Foveal width was increased in South Asian (P = 0.001) and blacks (P = 0.003) compared to whites. There was no significant difference between South Asian and blacks (P = 0.32).
The main effects of ethnicity and gender were statistically significant for IPL at 0°, 0.8° and 1.8°, with a small estimated effect size (partial eta squared < 0.1) for ethnicity and gender for IPL at 0° and a larger effect size for ethnicity and gender for IPL at 0.8° and 1.8°. Males had a tendency towards thicker IPL. Post hoc analysis revealed a thicker IPL in whites compared to the South Asian and black groups (P < 0.0005).
Association of MPOD with foveal architecture according to ethnicity
For whole group analysis, there was no association between MPOD and RT at 0° (r = 0.12, P = 0.07). Following separate ethnic group analysis, there was no association in the white group (r = 0.16, P = 0.16) in comparison to South Asian r = 0.23, P = 0.04; black r = 0.34, P = 0.004). Although statistically significant for whole group analysis, the correlation between MPOD and IRL at 0° was weak (r = 0.18, P = 0.01). The strength of this association increased following separate ethnic group analysis (white r = 0.24, P = 0.03; South Asian r = 0.23, P = 0.04; black r = 0.38, P = 0.001). There were no statistically significant correlations between MPOD at 0.8° and 1.8° and corresponding retinal thickness (RT, IRL, IPL and OPL) for whole group or per ethnic group (P > 0.05 for all).
There were no significant correlations between MPOD at 0° and foveal width when analysed for the whole sample and per ethnic group (P > 0.05 for all). Likewise, MPODint (0 to 1.8) was not related to foveal width or volume (P > 0.05). In addition, there were no significant associations between the MP profile slope (0 to 0.8, 0.8 to 1.8 and 1.8 to 2.8) and corresponding foveal pit profile slope when the group was analysed as a whole or per ethnic group (P > 0.05). Gender appeared to play no significant role in any of our findings.
Variations in foveal anatomy according to MP spatial profile phenotype
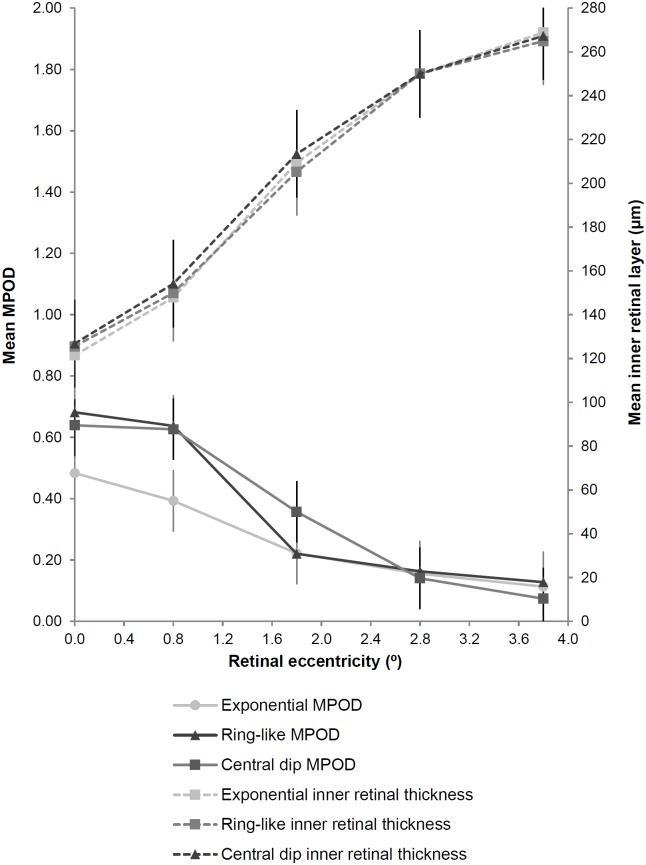
We found no significant difference in retinal thickness (RT, IRL, IPL and OPL at 0°, 0.8° and 1.8°) according to MP spatial profile phenotype (irrespective of the ethnic grouping) (P > 0.05 for all). The lack of difference in IRL thickness between the three MP profile groups, in contrast to the significant variation in MPOD at 0° and 0.8° (P > 0.0005), is presented graphically Fig 2. On the other hand foveal width was significantly increased in the ring-like MP profile group (2516 ± 295μm) compared to exponential (2389 ± 267μm) and central dip profiles (2364 ± 270μm; F(2) = 4.28, P = 0.015). However, the estimated effect size was small (partial eta squared = 0.037). No difference in foveal volume was found between the three spatial profile groups (P = 0.78).
Fig 2. Variation in mean MPOD (primary y-axis) plotted against retinal eccentricity (x-axis) according to spatial profile phenotype with corresponding inner retinal layer thickness plotted on the secondary y-axis.
Error bars indicate ±SD. Although MPOD at 0° and 0.8° is increased in the ring-like and central dip compared to the exponential spatial profile groups, there is no significant difference in inner retinal layer thickness between the groups.
Investigation of association between MPOD and foveal anatomy variables per MP spatial profile group revealed a statistically significant moderate positive correlation between MPOD and total RT at 0° (r = 0.33, P = 0.04) and IRL at 0° (r = 0.42, P = 0.007) for the central dip group only. There were no other significant associations between MPOD at 0°, 0.8° and 1.8° and corresponding retinal thickness measures (RT, IRL, IPL and OPL); likewise there were no significant associations between MPOD at 0° and foveal width; or MPODint (0 to 1.8) and volume (P > 0.05). We also report a statistically significant moderate negative association between the MP profile slope and corresponding foveal pit profile slope between 0.8° to 1.8° (r = -0.40, P = 0.01), 1.8° to 2.8° (r = -0.33, P = 0.01) and 2.8) to 3.8° (r = -0.35, P = 0.01) for the central dip group only. This result indicates that a steeper decline in MPOD is associated with a shallower i.e. flatter foveal pit gradient for the central dip profile group.
Discussion
The current study was conducted to investigate the association of MP spatial distribution and foveal architecture among young healthy white, South Asian and black subjects.
Variations in MP spatial distribution between ethnic groups
White subjects, compared to South Asian and black subjects, presented with significantly lower levels of MP as represented by MPOD at 0°, 0.8° and MPODint (0 to 1.8). No statistically significant difference was found in these MPOD variables between the non-white ethnic groups. Around 10% of the variance in the MPOD measurements could be explained by ethnicity. In addition, our results suggest that non-exponential i.e. ring-like and central dip MP spatial profile phenotypes occur more frequently in individuals of South Asian and black ethnicity respectively, compared to white ethnicity (P = 0.009). This supports the suggestion that ethnicity plays a small role in the spatial distribution of MP [23, 48]. Whilst our findings support previous reports of a significant effect of gender of MPOD [10, 18], this was only apparent for MPOD at 0°. Smoking did not have a significant effect on MPOD; however, our study population only included a small number of smokers.
Variations in foveal architecture between ethnic groups
We report that around 12–15% of the variation in retinal thickness was explained by ethnicity, while gender explained 3–5%. South Asian and black subjects presented with a statistically significant thinner central retina (RT and IRL) compared to whites, similar to earlier findings [35–39]. In contrast to a previous study [49] we found no significant difference in RT at 0° between the South Asian and black ethnic groups. Foveal width presented significant variations between the white and non-white ethnic groups in accordance with previous investigations that have shown that overall foveal morphology varies with fundus pigmentation [33, 50].
Association of MPOD with foveal architecture according to ethnicity
A significant moderate positive association between MPOD at 0° and corresponding RT was determined, but this was only present in the South Asian and black ethnic groups. This is in agreement with the work by Nolan et al. whereby a lack of association between MPOD at 0.25° (measured by HFP) and averaged central foveal thickness in white subjects was found, compared to a significant positive correlation in a non-white sample that included South Asian, black and Hispanic subjects (r = 0.59, P < 0.01; n = 18) [16]. However, other studies have reported inconsistent findings; a positive correlation between central MPOD and central foveal thickness has been demonstrated [31], whereas others have found no correlation even when taking ethnicity into account [17, 31, 32]. In contrast, a significant negative correlation between central retinal thickness and MPOD at 0.5° (measured by HFP) was determined in a young healthy Caucasian cohort (r = -0.39, P = 0.01) [51]. The role of ethnicity remains unclear. Inconsistent reports of the correlation between MPOD at 0° with corresponding retinal thickness may be due to the location of MP specifically in the inner retina [13, 26, 29]. It is conceivable that any relationship that may exist between MPOD and retinal thickness is merely due to variations in IRL thickness whereby variations in total retinal thickness offset any underlying association. Indeed, we report a significant moderate positive relationship between MPOD and IRL at 0° demonstrated among the white, South Asian and black ethnic groups.
Interestingly, given that the non-white ethnic groups presented with thinner IRL thickness but presented with increased central and integrated MPOD, one might have expected to find a negative rather than a positive association between the two parameters. Rather than a linear association with retinal thickness, it has been proposed that the relationship between MPOD and corresponding retinal thickness may be governed by the overall shape or profile of the foveal dip created by thickness of the individual retinal layers beyond the foveola towards 2° eccentricity [52]. This finding could not be confirmed in the present study (Table 2). It therefore seems there is no immediate link between increased MPOD and increased thickness of the IPL or OPL. Furthermore, the hypothesis that a wider fovea is associated with increased MPOD was not supported by our findings.
Variations in foveal anatomy according to MP spatial profile phenotype
We found that MP profile phenotypes were associated with ethnicity: 58% with ring profiles were South Asian and 43% with dip profiles were black (χ2(4,226) = 13.4, P = 0.009). In agreement with an earlier investigation [17], we found no significant variation in RT or IRL at 0° and 0.8° between subjects with exponential, ring-like or central dip MP spatial profiles. This suggests that increased retinal thickness is not responsible for the increased MPOD at 0° and 0.8° demonstrated in subjects with non-exponential MP spatial profiles. Inter-individual variations in the size and shape of the Müller cell cone may better explain the variations in MP distribution profiles. Indeed it has been postulated that the spatial arrangement of MPOD is created by the superimposition of the Henle fibre layer and the Müller cell cone [52]. Perhaps a monotonic decline of MP is due to a continuum of these structures whereby there is no superimposition of the Henle fibre layer and the Müller cell cone. Non-exponential profiles may therefore be a result of increased Müller cell cone thickness alone and a ring-like structure could be due to overlapping of the two structures.
While a positive correlation between the gradient of the MP and foveal pit has been reported [17], no association was established in a more recent investigation [52]. In the present study, no correlation between the MP profile slope and corresponding foveal pit profile slope was present for whole, ethnic or gender group testing. Interestingly, a significant negative association was present for the central dip group. Although the correlation was not strong, this finding suggests that a steeper decline in MPOD is associated with a shallower incline in the foveal pit profile slope. It would be of interest to apply more complex foveal pit modelling [53] to the current data to evaluate the sensitivity of the model to population demographic differences.
Our results imply that ethnic variations in MP and its spatial distribution cannot be explained by the differences observed in foveal morphology. Notwithstanding it is important to bear in mind that imaging of the retinal layers by SD-OCT is based on the optical properties of retinal tissue and the inbuilt algorithm to identify each layer. It has been proposed that the anatomical structures attributed to some of the hyper reflective bands may be incorrect and also may vary between devices [54]. Furthermore, not all retinal layers identified by histological studies are distinguishable on SD-OCT images. For example, the Henle layer has been visualised in vitro by histological examination [55, 56] but cannot be delineated by standard SD-OCT imaging (although a novel approach to achieve this has been described involving directionally altering the entry position of the SD-OCT beam through the subject's pupil [57, 58]). This in turn may explain the seeming lack of association of MP with foveal morphology.
Conclusion
To our best knowledge this is the first study to consider the effect of ethnicity and gender on the association between MP and its spatial profile and foveal morphology. South Asian and black individuals presented with increased integrated MPOD, thinner central retinas and wider foveal pit compared to white individuals. Additionally, increased MPOD at 0.8° in ring-like profiles did not appear to be related to increased retinal thickness at its corresponding location. The results suggest that the spatial density distribution of MP is not a direct function of foveal morphology as measured in vivo by SD-OCT methods.
Supporting Information
(XLSX)
Acknowledgments
We would like to thank Professor John Barbur for use of the MAP test, City, University of London.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Khachik F, Bernstein PS, Garland DL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci. 1997;38(9):1802–11. [PubMed] [Google Scholar]
- 2.Liew SHM, Gilbert CE, Spector TD, Mellerio J, Marshall J, van Kuijk FJ, et al. Heritability of macular pigment: a twin study. Invest Ophthalmol Vis Sci. 2005;46(12):4430–6. 10.1167/iovs.05-0519 [DOI] [PubMed] [Google Scholar]
- 3.Ciulla TA, Curran-Celantano J, Cooper DA, Hammond BR, Danis RP, Pratt LM, et al. Macular pigment optical density in a midwestern sample. Ophthalmology. 2001;108(4):730–7. [DOI] [PubMed] [Google Scholar]
- 4.Delori FC, Goger DG, Hammond BR, Snodderly DM, Burns SA. Macular pigment density measured by autofluorescence spectrometry: comparison with reflectometry and heterochromatic flicker photometry. J Opt Soc Am. 2001;18(6):1212–30. [DOI] [PubMed] [Google Scholar]
- 5.Hammond BR, Caruso-Avery M. Macular pigment optical density in a Southwestern sample. Invest Ophthalmol Vis Sci. 2000;41(6):1492–7. [PubMed] [Google Scholar]
- 6.Beatty S, Murray IJ, Henson DB, Carden D, Koh HH, Boulton ME. Macular pigment and risk for age-related macular degeneration in subjects from a Northern European population. Invest Ophthalmol Vis Sci. 2001;42(2):439–46. [PubMed] [Google Scholar]
- 7.Berendschot TTJM, van Norren D. On the age dependency of the macular pigment optical density. Exp Eye Res. 2005;81(5):602–9. 10.1016/j.exer.2005.03.019 [DOI] [PubMed] [Google Scholar]
- 8.Ciulla TA, Hammond BR. Macular pigment density and aging, assessed in the normal elderly and those with cataracts and age-related macular degeneration. Am J Ophthalmol. 2004;138(4):582–7. 10.1016/j.ajo.2004.05.057 [DOI] [PubMed] [Google Scholar]
- 9.Nolan JM, Kenny R, O'Regan C, Cronin H, Loughman J, Connolly EE, et al. Macular pigment optical density in an ageing Irish population: the Irish longitudinal study on ageing. Ophthalmic Res. 2010;44(2):131–9. 10.1159/000315531 [DOI] [PubMed] [Google Scholar]
- 10.Hammond BR, Curran-Celentano J, Judd S, Fuld K, Krinsky NI, Wooten BR, et al. Sex differences in macular pigment optical density: relation to plasma carotenoid concentrations and dietary patterns. Vision Res. 1996;36(13):2001–12. [DOI] [PubMed] [Google Scholar]
- 11.Hammond BR, Fuld K, Snodderly DM. Iris color and macular pigment optical density. Exp Eye Res. 1996;62:715–20. [DOI] [PubMed] [Google Scholar]
- 12.Hammond BR, Wooten BR, Snodderly DM. Cigarette smoking and retinal carotenoids: implications for age-related macular degeneration. Vision Res. 1996;36(18):3003–9. [DOI] [PubMed] [Google Scholar]
- 13.Snodderly DM, Auran JD, Delori FC. The macular pigment II. Spatial distribution in primate retinas. Invest Ophthalmol Vis Sci. 1984;25(6):674–85. [PubMed] [Google Scholar]
- 14.Hammond BR, Wooten BR, Snodderly DM. Individual variations in the spatial profile of human macular pigment. J Opt Soc Am. 1997;14(6):1187–96. [DOI] [PubMed] [Google Scholar]
- 15.Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci. 1988;29(6):843–9. [PubMed] [Google Scholar]
- 16.Nolan JM, Stringham JM, Beatty S, Snodderly DM. Spatial profile of macular pigment and its relationship to foveal architecture. Invest Ophthalmol Vis Sci. 2008;49(5):2134–42. 10.1167/iovs.07-0933 [DOI] [PubMed] [Google Scholar]
- 17.Kirby ML, Galea M, Loane E, Stack J, Beatty S, Nolan JM. Foveal anatomic associations with the secondary peak and the slope of the macular pigment spatial profile. Invest Ophthalmol Vis Sci. 2009;50(3):1383–91. 10.1167/iovs.08-2494 [DOI] [PubMed] [Google Scholar]
- 18.Delori FC, Goger DG, Keilhauer C, Salvetti P, Staurenghi G. Bi-modal spatial distribution of macular pigment: evidence of a gender relationship. J Opt Soc Am. 2006;23(3):521–38. [DOI] [PubMed] [Google Scholar]
- 19.Dietzel M, Zeimer M, Heimes B, Pauleikhoff D, Hense HW. The ringlike structure of macular pigment in age-related maculopathy: results from the Muenster Aging and Retina Study (MARS). Invest Ophthalmol Vis Sci. 2011;52(11):8016–24. 10.1167/iovs.11-7610 [DOI] [PubMed] [Google Scholar]
- 20.Berendschot TTJM, van Norren D. Macular pigment shows ringlike structures. Invest Ophthalmol Vis Sci. 2006;47(2):709–14. 10.1167/iovs.05-0663 [DOI] [PubMed] [Google Scholar]
- 21.Kirby ML, Beatty S, Loane E, Akkali MC, Connolly EE, Stack J, et al. A central dip in the macular pigment spatial profile is associated with age and smoking. Invest Ophthalmol Vis Sci. 2010;51(12):6722–8. 10.1167/iovs.10-5344 [DOI] [PubMed] [Google Scholar]
- 22.Huntjens B, Asaria TS, Dhanani S, Konstantakopoulou E, Ctori I. Macular pigment spatial profiles in South Asian and white subjects. Invest Ophthalmol Vis Sci. 2014;55(3):1440–6. 10.1167/iovs.13-13204 [DOI] [PubMed] [Google Scholar]
- 23.Wolf-Schnurrbusch UEK, Röösli N, Weyermann E, Heldner MR, Höhne K, Wolf S. Ethnic differences in macular pigment density and distribution. Invest Ophthalmol Vis Sci. 2007;48(8):3783–7. 10.1167/iovs.06-1218 [DOI] [PubMed] [Google Scholar]
- 24.Howells O, Eperjesi F, Bartlett H. Macular pigment optical density in young adults of South Asian origin. Invest Ophthalmol Vis Sci. 2013;54(4):2711–9. 10.1167/iovs.12-10957 [DOI] [PubMed] [Google Scholar]
- 25.Raman R, Rajan R, Biswas S, Vaitheeswaran K, Sharma T. Macular pigment optical density in a South Indian population. Invest Ophthalmol Vis Sci. 2011;52(11):7910–6. 10.1167/iovs.11-7636 [DOI] [PubMed] [Google Scholar]
- 26.Snodderly DM, Brown PK, Delori FC, Auran JD. The macular pigment I. Absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Invest Ophthalmol Vis Sci. 1984;25(6):660–73. [PubMed] [Google Scholar]
- 27.Trieschmann M, van Kuijk FJ, Alexander R, Hermans P, Luthert P, Bird AC, et al. Macular pigment in the human retina: histological evaluation of localization and distribution. Eye. 2008;22:132–7. 10.1038/sj.eye.6702780 [DOI] [PubMed] [Google Scholar]
- 28.Powner MB, Gillies MC, Tretiach M, Scott A, Guymer RH, H G.S., et al. Perifoveal muller cell depletion in a case of macular telangiectasia Type 2. Ophthalmology. 2010;117(12):2407–16. 10.1016/j.ophtha.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gass JDM. Müller cell cone, an overlooked part of the anatomy of the fovea centralis: hypotheses concerning its role in the pathogenesis of macular hole and foveomacular retinoschisis. Arch Ophthalmol. 1999;117(6):821–3. [DOI] [PubMed] [Google Scholar]
- 30.Liew SHM, Gilbert CE, Spector TD, Mellerio J, Kuijk FJV, Beatty S, et al. Central retinal thickness is positively correlated with macular pigment optical density. Exp Eye Res. 2006;82(5):915–20. 10.1016/j.exer.2005.10.014 [DOI] [PubMed] [Google Scholar]
- 31.van der Veen RLP, Ostendorf S, Hendrikse F, Berendschot TTJM. Macular pigment optical density relates to foveal thickness. Eur J Ophthalmol. 2009;19(5):836–41. [DOI] [PubMed] [Google Scholar]
- 32.Kanis MJ, Berendschot TTJM, van Norren D. Interocular agreement in melanin and macular pigment optical density. Exp Eye Res. 2007;84(5):934–8. 10.1016/j.exer.2007.01.020 [DOI] [PubMed] [Google Scholar]
- 33.Wagner-Schuman M, Dubis AM, Nordgren RN, Lei Y, Odell D, Chiao H, et al. Race- and sex-related differences in retinal thickness and foveal pit morphology. Invest Ophthalmol Vis Sci. 2011;52(1):625–34. 10.1167/iovs.10-5886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ooto S, Hangai M, Tomidokoro A, Saito H, Araie M, Otani T, et al. Effects of age, sex, and axial length on the three-dimensional profile of normal macular layer structures. Invest Ophthalmol Vis Sci. 2011;52(12):8769–79. 10.1167/iovs.11-8388 [DOI] [PubMed] [Google Scholar]
- 35.Pilat AV, Proudlock FA, Mohammad S, Gottlob I. Normal macular structure measured with optical coherence tomography across ethnicity. Br J Ophthalmol. 2014;98(7):941–5. 10.1136/bjophthalmol-2013-303119 [DOI] [PubMed] [Google Scholar]
- 36.Girkin CA, McGwin G Jr, Sinai MJ, Sekhar GC, Fingeret M, Wollstein G, et al. Variation in optic nerve and macular structure with age and race with spectral-domain optical coherence tomography. Ophthalmology. 2011;118(12):2403–8. 10.1016/j.ophtha.2011.06.013 [DOI] [PubMed] [Google Scholar]
- 37.Asefzadeh B, Cavallerano AA, Fisch BM. Racial differences in macular thickness in healthy eyes. Optom Vis Sci. 2007;84(10):E941–E5. [DOI] [PubMed] [Google Scholar]
- 38.Kashani AH, Zimmer-Galler IE, Shah SM, Dustin L, Do DV, Eliott D, et al. Retinal thickness analysis by race, gender, and age using Stratus OCT. Am J Ophthalmol. 2010;149(3):496–502. 10.1016/j.ajo.2009.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelty PJ, Payne JF, Trivedi RH, Kelty J, Bowie EM, Burger BM. Macular thickness assessment in healthy eyes based on ethnicity using Stratus OCT optical coherence tomography. Invest Ophthalmol Vis Sci. 2008;49(6):2668–72. 10.1167/iovs.07-1000 [DOI] [PubMed] [Google Scholar]
- 40.Office for National Statistics. Ethnicity and National Identity in England and Wales 2011 [Online]. http://webarchive.nationalarchives.gov.uk/20160105160709/http://www.ons.gov.uk/ons/rel/census/2011-census/key-statistics-for-local-authorities-in-england-and-wales/sty-enthnicity-in-england-and-wales.html [Accessed 15 July 2016].
- 41.Barbur JL, Konstantakopoulou E, Rodriguez-Carmona M, Harlow JA, Robson AG, Moreland JD. The Macular Assessment Profile test—a new VDU-based technique for measuring the spatial distribution of the macular pigment, lens density and rapid flicker sensitivity. Ophthalmic Physiol Opt. 2010;30(5):470–83. 10.1111/j.1475-1313.2010.00748.x [DOI] [PubMed] [Google Scholar]
- 42.Sanchez-Cano A, Baraibar B, Pablo LE, Honrubia FM. Magnification characteristics of the optical coherence tomograph Stratus OCT 3000. Ophthalmic Physiol Opt. 2008;28(1):21–8. 10.1111/j.1475-1313.2007.00527.x [DOI] [PubMed] [Google Scholar]
- 43.Ctori I, Gruppetta S, Huntjens B. The effects of ocular magnification on Spectralis spectral domain optical coherence tomography scan length. Graefes Arch Clin Exp Ophthalmol. 2014;253(5):733–8. [DOI] [PubMed] [Google Scholar]
- 44.Paunescu LA, Schuman JS, Price LL, Stark PC, Beaton S, Ishikawa H, et al. Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using Stratus OCT. Invest Ophthalmol Vis Sci. 2004;45(6):1716–24. 10.1167/iovs.03-0514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ctori I, Huntjens B. Repeatability of foveal measurements using Spectralis optical coherence tomography segmentation software. PLoS ONE. 2015;10(6):e0129005 10.1371/journal.pone.0129005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91. [DOI] [PubMed] [Google Scholar]
- 47.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–60. 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- 48.Huntjens B, Ctori I. Variations and repeatability of macular pigment and its spatial profiles in South Asian and white subjects. Acta Ophthalmol. 2014;92(s253). [DOI] [PubMed] [Google Scholar]
- 49.Grover S, Murthy RK, Brar VS, Chalam KV. Normative data for macular thickness by high-definition spectral-domain optical coherence tomography (Spectralis). Am J Ophthalmol. 2009;148(2):266–71. 10.1016/j.ajo.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 50.Tick S, Rossant F, Ghorbel I, Gaudric A, Sahel JA, Chaumet-Riffaud P, et al. Foveal shape and structure in a normal population. Invest Ophthalmol Vis Sci. 2011;52(8):5105–10. 10.1167/iovs.10-7005 [DOI] [PubMed] [Google Scholar]
- 51.Kyle-Little Z, Zele AJ, Morris P, Feigl B. The effect of BCMO1 gene variants on macular pigment optical density in young healthy Caucasians. Front Nutr. 2014;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyer zu Westrup V, Dietzel M, Pauleikhoff D, Hense H-W. The association of retinal structure and macular pigment distribution. Invest Ophthalmol Vis Sci. 2014;55(2):1169–75. 10.1167/iovs.13-12903 [DOI] [PubMed] [Google Scholar]
- 53.Ding Y, Spund B, Glazman S, Shrier EM, Miri S, Selesnick I, et al. Application of an OCT data-based mathematical model of the foveal pit in Parkinson disease. J Neural Transm. 2014;121(11):1367–76. 10.1007/s00702-014-1214-2 [DOI] [PubMed] [Google Scholar]
- 54.Spaide RF, Curcio CA. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model. Retina. 2011;31(8):1609–19. 10.1097/IAE.0b013e3182247535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hendrickson A, Possin D, Vajzovic L, Toth CA. Histologic development of the human fovea from midgestation to maturity. Am J Ophthalmol. 2012;154(5):767–78. 10.1016/j.ajo.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hendrickson A. A morphological comparison of foveal development in man and monkey. Eye. 1992;6(2):136–44. [DOI] [PubMed] [Google Scholar]
- 57.Lujan BJ, Roorda A, Croskrey JA, Dubis AM, Cooper RF, Bayabo J-K, et al. Directional optical coherence tomography provides accurate outer nuclear layer and Henle fiber layer measurements. Retina. 2015;35(8):1511–20. 10.1097/IAE.0000000000000527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lujan BJ, Roorda A, Knighton RW, Carroll J. Revealing Henle's fiber layer using spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(3):1486–92. 10.1167/iovs.10-5946 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.