Abstract
Background
Head injury is reported to be associated with increased risks of dementia and Alzheimer’s disease (AD) in many but not all the epidemiological studies. We conducted a systematic review and meta-analysis to estimate the relative effect of head injury on dementia and AD risks.
Methods
Relevant cohort and case-control studies published between Jan 1, 1990, and Mar 31, 2015 were searched in PubMed, Web of Science, Scopus, and ScienceDirect. We used the random-effect model in this meta-analysis to take into account heterogeneity among studies.
Results
Data from 32 studies, representing 2,013,197 individuals, 13,866 dementia events and 8,166 AD events, were included in the analysis. Overall, the pooled relative risk (RR) estimates showed that head injury significantly increased the risks of any dementia (RR = 1.63, 95% CI 1.34–1.99) and AD (RR = 1.51, 95% CI 1.26–1.80), with no evidence of publication bias. However, when considering the status of unconsciousness, head injury with loss of consciousness did not show significant association with dementia (RR = 0.92, 95% CI 0.67–1.27) and AD (RR = 1.49, 95% CI 0.91–2.43). Additionally, this positive association did not reach statistical significance in female participants.
Conclusions
The findings from this meta-analysis indicate that head injury is associated with increased risks of dementia and AD.
Introduction
The estimated annual global incidence of head injury requiring medical attention or resulting in hospitalization or death is over 10 million, and the risk of subsequent morbidity, mortality and disability is high[1].Head injury is also the major cause of loss of years of productive life and is a social problem to which governments do not pay sufficient attention[2].Neurodegenerative diseases, including amyotrophic lateral sclerosis, Parkinson’s disease and Alzheimer’s disease (AD), have frequently been reported to develop in patients with head injury[3,4]. AD is the most common neurodegenerative disorder of modern societies accounting for 50–60% of all-cause dementia[5].The possibility that head injury may predispose a person to developing AD has significant social and medical implications.
An association between head injury and AD is biologically plausible. Head injury can cause over-expression of the β-amyloid precursor protein, leading to the accumulation of β-amyloid deposits in the brain, similar to that seen in brains of AD patients[6]. Franzblau et al.[7]also suggest that the pathological link between head injury and AD may be due to the vascular damage, in that people with history of head injury are predisposed to AD symptoms due to altered brain vasculature; vice versa, the progression of AD pathology may be accelerated by head injury especially when the brain insult worsens hippocampal degeneration. However, the association between head injury and the risk of dementia has been debated in the epidemiological studies. Although some reports supported a positive relation with AD[8–10], other studies could not confirm head injury as a risk factor for dementia or AD[11–13].Therefore, there is no conclusive evidence to suggest a relationship between head injury and the risk of dementia.
Given the inconsistency in the literature on the role of head injury and risk of dementia and AD, we conducted a meta-analysis to quantitatively assess the relation and the strength between head injury and the risk of dementia and AD.
Materials and Methods
Search strategy and selection criteria
We followed the guidelines published by the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group (S1 Table)[14] and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) group (S1 Appendix) to complete the meta-analysis.We systematically searched PubMed, Web of Science, Scopus, and ScienceDirect for reports published between Jan 1, 1990, and Mar 31, 2015, using a combined text and MeSH heading search strategy with the terms: “head injuries”, “head injury”, “brain injuries”, “brain injury”, “head trauma”, “brain trauma”, “traumatic brain injury”, “brain damage”, “dementia”, “Alzheimer's disease”, “Alzheimer disease”, “AD”, “Alzheimer's”, “cognitive decline”, and “neurocognitive impairment”. We also checked the reference lists of identified reports for other potentially relevant studies. We included studies after 1990, since we sought to examine evidence from the period most applicable to the present status of dementia and AD risks associated with head injury. The search strategy was limited to cohort studies and case-control studies. No attempt was made to find articles in languages other than English. We contacted the authors of the included studies to ask them for additional information and unpublished data as needed.
A study was eligible for inclusion if the following criteria were met: (1) examination of head injury or traumatic brain injury (TBI) as the variable of interest; (2) determination of incidence of AD or other types of dementias as the outcome of interest; and (3) reporting the RRs or odds ratios (ORs) of dementia or AD with their 95% confidence intervals (CIs). The studies about animal experiment, mechanistic research and review research were excluded.
Data extraction and study quality evaluation
We extracted the characteristics of each included study, including author, study region, study design, sample size, mean age of the sample, exposure ascertainment, exposure variable, outcome (any dementia or AD), disease ascertainment, RRs or ORs with CIs, and factors adjusted for. The most adjusted estimate was included when a study reported more than one risk estimate, and crude risk estimate was included if a study did not adjust for other factors. For the purpose of sensitivity analysis, we also extracted information on minimally adjusted (crude or adjusted for sex and age) risk estimates from each study when available. The quality of each study was assessed by the Newcastle-Ottawa Scale recommended by Wells and colleagues[15].
Statistical analysis
A previous study indicated that OR is close to RR when the outcome is relatively uncommon (less than 20%)[16]. Thus, in our pooled analyses, ORs were considered equivalent to RRs since the incidence of dementia and AD was uncommon among the population (obviously below 20%) in the included studies. Pooled RRs were used as summary estimates throughout the procedure to simplify reporting[17].We used the random effects model to estimate the pooled RRs of dementia and AD associated with head injury to take into account heterogeneity among studies, since the study design and measuring time were varied across studies. The I-squared (I2) statistic and Q-statistic were used to explore the heterogeneity among studies. Large I2 (>50%) or P<0.10 for Q-statistic suggests substantial heterogeneity among studies. Subgroup analyses were performed according to the status of unconsciousness, including head injury regardless of status of unconsciousness, head injury with loss of consciousness (LOC), and head injury without LOC. We did several sensitivity analyses: mean age of the participants (≥65 year vs<65 years), sex, geographic location (Europe, North America, or Asia & Pacific), study design (cohort or case-control), exposure type (TBI or other types of head injury), disease ascertainment methods, study quality score (full marks or not full marks), and year of publication (pre-2000 or 2000 onwards). We also performed sensitivity analyses by removing each individual study from the meta-analysis. We used funnel plots to examine the presence of publication bias (ie, by plotting the natural log of the odds ratio against its standard error). Egger’s regression test and Begg-Mazumdar test were used to further assess publication bias. All statistical analyses were done with Stata Version 12.0 software (Stata Corp, College Station, TX).
Results
Study characteristics
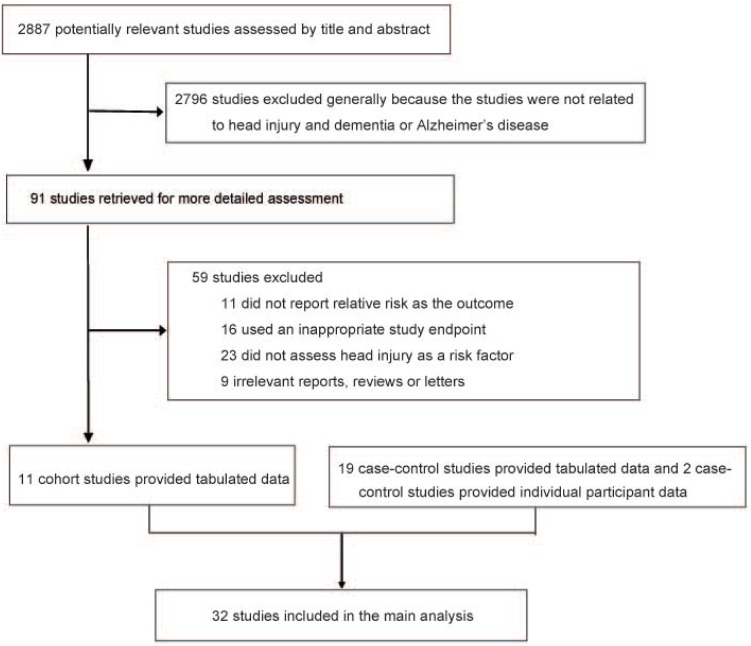
The systematic search identified 2887 articles, which were assessed by title and abstract. Of these, 91 articles were qualified for selection (Fig 1, S2 Appendix). After full-text assessment, a total of 32 articles met the inclusion criteria and were included in the meta-analysis, including 11 cohort studies[10,12,18–26] and 21 case-control studies[8,9,11,13,27–43]. Table 1 shows the baseline characteristics of all 32 included studies. Of these studies, eleven reported on dementia incidence and twenty-eight on AD incidence. The sum number of individual studies was more than 32 because some studies reported both dementia and AD outcomes. Overall, data were available from 2,013,197 individuals, of whom 13,866 developed dementia and 8,166 developed AD. Head injury/TBI ascertainment was mainly based on detailed/structured interview with multiple questions or International Classification of Diseases codes. Thus, possible occurrence of memory bias may exist but was considered limited. The quality assessment of the included studies was presented in detail in the supplementary material (S2 and S3 Tables).
Fig 1. Flowchart for the selection of eligible studies.
Table 1. Characteristics of included studies.
| Author | Region | Study design | Sample size | Age, mean/range (years) | Exposure ascertainment | Exposure variable | Outcome | Disease ascertainment | RR (95% CI) | Adjustment |
|---|---|---|---|---|---|---|---|---|---|---|
| Abneret al, 2014 | The United States | Cohort | 649 | 72.9/≥60 | Single question | Head injury | AD | CERAD | 1.47(1.03–2.09)* | APOE-ε4, sex, age at death, presence of at least mild cerebral amyloid angiopathy, and whether AD was observed before death |
| 1.18(0.83–1.68)** | ||||||||||
| Bachmanet al, 2003 | The United States | Case-control | 2779 | 70.6/≥50 | Detailed interview | Head trauma | AD | NINCDS-ADRDA | 2.40(1.80–3.10) | Age, sex, education, head trauma, alcohol, and smoking |
| Boston et al, 1999 | The United Kingdom | Case-control | 396 | 82.9/≥75 | Not reported | Head injury | Dementia | MMSE and CAMDEX | 0.49(0.14–1.75) | Age, social class, age of left school, family history of dementia, history of falls, history of heart attack, history of hypertension, blood pressure, smoking, drinking, psychiatric history, cholesterol, and HDL |
| AD | 0.80(0.44–1.43) | |||||||||
| Broeet al, 1990 | Australia | Case-control | 340 | 78.1/52-96 | Detailed interview | Any head injury | AD | NINCDS-ADRDA | 1.33(0.46–3.83) | Age and sex |
| 1.75(0.52–5.88)* | ||||||||||
| Early head injury | AD | 1.60(0.53–4.84) | ||||||||
| 2.33(0.63–8.67)* | ||||||||||
| Dams-O'Connor et al, 2013 | The United States | Cohort | 4225 | 74.9/≥65 | Single question | TBI with LOC | Dementia | DSM-IV | 0.87(0.60–1.27) | Age, age-squared, gender, and education |
| AD | NINCDS-ADRDA | 0.95(0.65–1.38) | ||||||||
| Ferini-Strambiet al, 1990 | Italy | Case-control | 189 | 58.9/Not reported | Structured interview | Head injury | AD | Full-scale WAIS IQ and Blessed-Tomlinson-Roth Dementia Scale | 1.00(0.32–3.10) | Age, sex, residential area, education and social status |
| Fischer et al, 2008 | Austria | Cohort | 479 | 75.8/75-76 | Structured interview | Head trauma | AD | NINCDS-ADRDA | 0.46(0.16–1.31) | None |
| Forster et al, 1995 | England | Case-control | 218 | Not reported/<65 | Standardised interview | Any head injury | AD | NINCDS-ADRDA | 1.20(0.57–2.56) | Age and sex |
| Adult head injury | 1.50(0.68–3.41) | |||||||||
| Childhoodhead injury | 0.70(0.14–2.81) | |||||||||
| Fratiglioniet al, 1993 | Sweden | Case-control | 314 | Not reported/≥75 | Structured interview | TBI with LOC | AD | DSM-III-R and CDR | 0.30(0.10–1.20) | Age, sex, education, type of informant, and alcohol consumption |
| Gardner et al, 2014 | The United States | Cohort | 164661 | 71.6/≥55 | ICD-9 | TBI | Dementia | ICD-9 codes for the diagnosis of dementia | 1.26(1.21–1.32) | Age, sex, race, comorbidities, trauma mechanism, health care use, and trauma severity |
| Graves et al, 1990 | The United States | Case-control | 260 | 64.9/Not reported | Structured interview | Any head trauma | AD | DSM-III and NINCDS-ADRDA | 3.50(1.50–8.30) | Age and family history of AD |
| Head traumawith LOC | 2.90(1.10–7.53) | |||||||||
| Head trauma without LOC | 5.50(1.35–22.50) | |||||||||
| Guoet al, 2000 | The United States, Canada, and Germany | Case-control | 16901 | Not reported | Structured interview | Any head injury | AD | NINCDS-ADRDA | 2.70(2.20–3.30) | Gender and kinship |
| Head injury with LOC | 4.00(2.90–5.50) | |||||||||
| Head injury without LOC | 2.00(1.50–2.70) | |||||||||
| Lee et al, 2013 | Taiwan | Cohort | 720933 | Not reported/≥18 | ICD-9 | Mild TBI | Dementia | ICD-9-CM | 3.26(2.69–3.94) | Age, gender, urbanization level, socio-economic status, diabetes, hyperlipidemia coronary artery disease, history of alcohol intoxication, ischemic stroke, intracranial hemorrhage and Charlson comorbidity index |
| Li et al, 1992 | China | Case-control | 210 | 65.3/Not reported | Structured interview | Head injury with LOC | AD | NINCDS-ADRDA and ICD-10 | 1.00(0.09–11.03) | Age and sex |
| Lindsay et al, 2002 | Canada | Case-control | 4088 | 73.3/≥65 | Structured interview | Head injury | AD | NINCDS-ADRDA | 0.87(0.56–1.36) | Age, sex and education |
| Luukinenet al, 2005 | Finland | Cohort | 152 | 75.1/≥70 | Medical examination | TBI | Dementia | DSM-IV and the MMSE | 2.80(1.35–5.81) | Low educational status and sex |
| Mayeuxet al, 1993 | The United States | Case-control | 331 | 78.1/Not reported | Structured interview | Head injury with LOC | AD | National Institutes of Neurological Disorders and Stroke criteria | 3.70(1.40–9.70) | Gender, age, ethnic group, years of education, and head injury |
| McDowell et al, 1994 | Canada | Case-control | 793 | 80.9/≥65 | Structured interview | Head injury | AD | DSM-III-R and NINCDS-ADRDA | 1.66(0.97–2.84) | Age, sex, residence in community or institution, and education |
| Mehta et al, 1999 | The Netherlands | Cohort | 6645 | 68.9/≥55 | Detailed interview | Head trauma with LOC | Dementia | DSM-III-R and NINCDS-ADRDA | 1.00(0.50–2.00) | Age, education, and if applicable, gender |
| AD | 0.80(0.40–1.90) | |||||||||
| Dementia | 0.70(0.20–2.40)* | |||||||||
| AD | 0.90(0.20–4.00)* | |||||||||
| Dementia | 1.30(0.60–2.80)** | |||||||||
| AD | 0.90(0.30–2.40)** | |||||||||
| Nordstrom et al, 2014 | Sweden | Cohort | 811622 | 18.0/Not reported | ICD-8,9,10 | One mild TBI | Dementia | ICD-8, 9, 10 | 1.50(1.10–2.00)* | Age, place and year of conscription, overall cognitive function, alcohol intoxication, weight, height, knee extension strength, TBI in parents, dementia in parents, income, educational level, systolic blood pressure, drug intoxication, depression, and cerebrovascular disease |
| AD | 1.00(0.50–2.00)* | |||||||||
| At least two mild TBI | Dementia | 1.80(1.10–3.00)* | ||||||||
| AD | 2.50(0.80–8.10)* | |||||||||
| One severe TBI | Dementia | 2.30(1.50–3.60)* | ||||||||
| AD | 0.70(0.10–5.20)* | |||||||||
| O'Meara et al, 1997 | The United States | Case-control | 691 | 78.0/≥60 | Detailed interview | Head injury with LOC | AD | DSM-III-R and NINCDS-ADRDA | 2.10(1.10–3.80) | None |
| 4.20(1.50–11.50)* | ||||||||||
| 1.10(0.50–2.60)** | ||||||||||
| Ogunniyiet al, 2006 | The United States | Case-control | 523 | 77.9/Not reported | Screening interview | Head injury | AD | NINCDS-ADRDA | 0.55(0.21–1.45)) | Age and gender |
| Nigeria | 470 | 79.2/Not reported | 1.36(0.30–6.30)) | |||||||
| Plassmanet al, 2000 | The United States | Cohort | 1776 | 72.9/Not reported | Medical record | Head injury | Dementia | DSM-III-R and NINCDS-ADRDA | 2.46(1.43–4.24)* | Years of education and age |
| AD | 2.16(1.10–4.23)* | |||||||||
| Rasmussonet al, 1995 | The United States | Case-control | 102 | 71.0/Not reported | Standardized interview | Head injury | AD | Not reported | 13.75(1.76–107.52) | None |
| Rippon et al, 2006 | The United States | Case-control | 1498 | 68.2/Not-reported | Standardized interview | Head injury | AD | NINCDS-ADRDA | 1.00(0.70–1.60) | ε4 status, age, gender, and education |
| Salibet al, 1997 | The United Kingdom | Case-control | 538 | 75.1/>65 | Head injury | DementiaAD | NINCDS-ADRDA | 2.46(1.42–4.10) | Age, sex, time lag between head injury and onset, duration of condition and family history of dementia | |
| 1.52(0.98–2.35) | ||||||||||
| 2.10(1.10–4.10)* | ||||||||||
| 1.38(0.74–2.60)** | ||||||||||
| Schofield et al, 1997 | The United States | Cohort | 271 | 75.3/≥60 | Detailed interview | Head injury with LOC | AD | NINCDS-ADRDA | 1.05(0.34–3.22) | Sex and education |
| Suhanovet al, 2006 | Russia | Case-control | 520 | 69.3/40-89 | Structured interview | Head injury with LOC | AD | NINCDS-ADRDA | 1.70(1.00–2.80) | Family history of dementia, family history of parkinsonism, and hypertension |
| Sundstromet al, 2007 | Sweden | Case-control | 543 | 72.8/40-85 | Medical record or interview | Mild head injury | Dementia | DSM-IV | 0.90(0.40–1.80) | Age and gender |
| Tsolakiet al, 1997 | Greece | Case-control | 134 | Not reported/>70 | Structured interview | Head trauma | AD | DSM-IV and NINCDS-ADRDA | 1.07(0.47–2.45) | Age and gender |
| vanDuijnet al, 1992 | The Netherlands | Case-control | 396 | 56.8/Not reported | Structured interview | Head trauma with LOC | AD | NINCDS-ADRDA | 1.60(0.80–3.40) | Age, sex, dementia in first-degree relatives and education |
| 2.50(0.90–7.00)* | ||||||||||
| 0.90(0.30–2.80)** | ||||||||||
| Wang et al, 2012 | Taiwan | Cohort | 269550 | 40.8/≥15 | ICD-9 | TBI | Vascular dementia | ICD-9-CM | 1.32(1.06–1.65) | Sex, age group, year of index healthcare use, stroke, diabetes, hyperlipidaemia, hypertension, coronary heart disease, heart failure and arterial fibrillation |
| Unspecific dementia | 1.74(1.62–1.88) | |||||||||
| AD | 1.49(1.08–2.07) | |||||||||
RR = relative risk. CI = confidence interval. AD = Alzheimer’s disease. CERAD = The Consortium to Establish a Registry for Alzheimer's Disease. MMSE = Mini-Mental State Examination test. CAMDEX = Cambridge Examination for Mental Disorders of the Elderly. TBI = traumatic brain injury. LOC = loss of consciousness. DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. NINCDS-ADRDA = National Institute of Neurological and Communicative Diseases and Stroke-Alzheimer Disease and Related Disorders Association. DSM-III-R = Diagnostic and Statistical Manual of Mental Disorders, third edition revised. ICD-9 = International Classification of Diseases, Ninth Revision. CDR = The Clinical Dementia Rating scale.
* Relative risks and 95% CIs for males.
** Relative risks and 95% CIs for females.
Head injury and risk of dementia
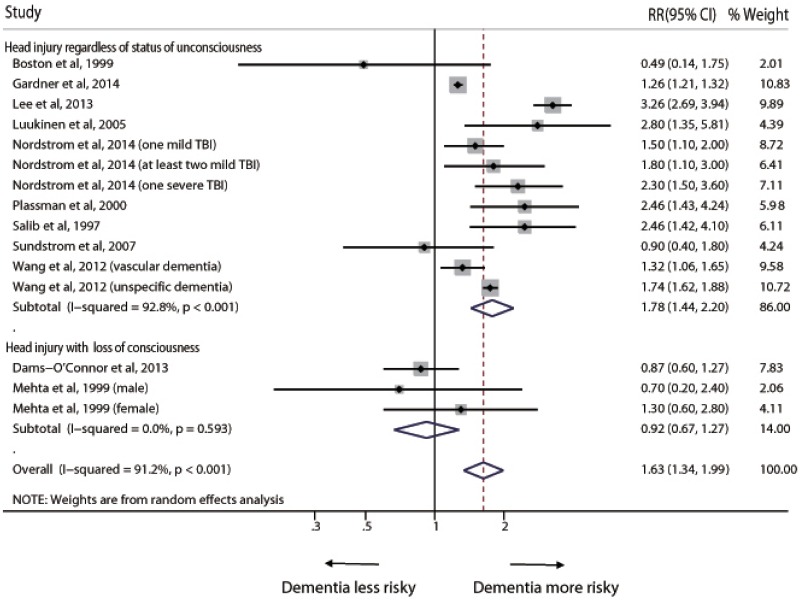
The overall pooled RR based on all available data for any dementia risk associated with head injury was 1.63 (95% CI 1.34–1.99) (Fig 2). The I2 statistic for heterogeneity between studies was 91.2%, with p value for the Q test <0.001, suggesting substantial between-study heterogeneity. In the sub-group analyses, when regardless of status of unconsciousness, the pooled RR was 1.78 (95% CI 1.44–2.20); however, head injury with LOC did not show significant association with risk of dementia (RR = 0.92, 95% CI 0.67–1.27) (Fig 2).
Fig 2. Pooled relative risk for any dementia, comparing individuals with head injury to those without head injury.
Box sizes are in proportion to study weights. TBI = traumatic brain injury.
Head injury and risk of AD
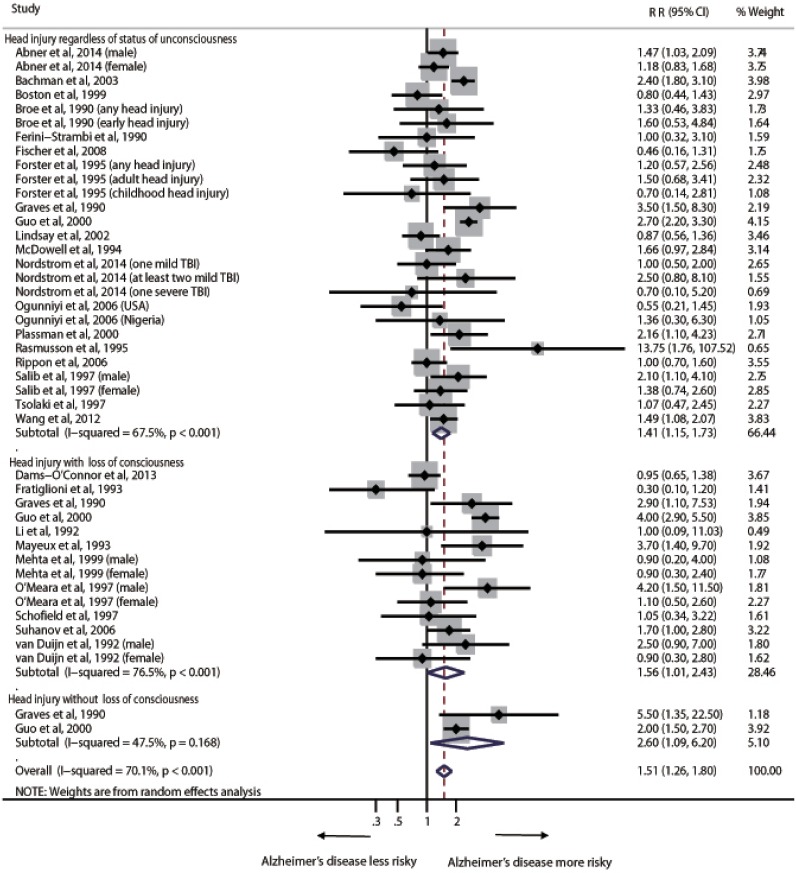
The pooled RR estimates for risk of AD associated with head injury was 1.51(95% CI 1.26–1.80) (Fig 3). The I2 statistic for heterogeneity between studies was 70.1%, with p value for the Q test <0.001, suggesting substantial between-study heterogeneity. In the subgroup analyses, head injury regardless of status of unconsciousness (RR = 1.41, 95% CI 1.15–1.73), head injury with LOC (RR = 1.56, 95% CI 1.01–2.43) and head injury without LOC (RR = 2.60, 95% CI 1.09–6.20) were all associated with increased risk of AD (Fig 3). Exclusion of the three studies with results not adjusted for other factors did not change the RR estimates (1.50 [1.26–1.80]) and did not reduce the between–study heterogeneity (I2 = 69.9%, p<0.001) (S1 Fig). However, the results did not show significant association between head injury with LOC and risk of AD (RR = 1.49, 95% CI 0.91–2.43) (S1 Fig).
Fig 3. Pooled relative risk for Alzheimer’s disease, comparing individuals with head injury to those without head injury.
Box sizes are in proportion to study weights. TBI = traumatic brain injury.
Sensitivity analyses
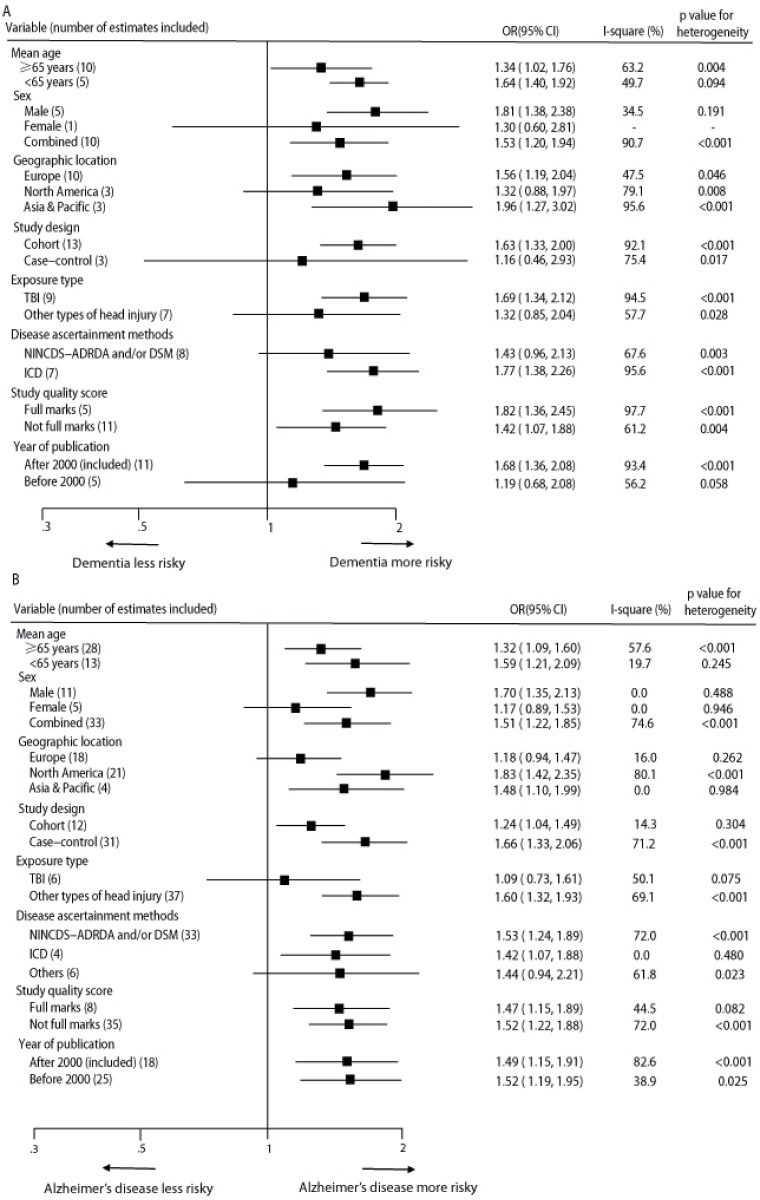
In the sensitivity analyses, the pooled RRs for any dementia and AD did not differ significantly by mean age of the participants, sex, geographic location, study design, exposure type of head injury, disease ascertainment methods, study quality score, or year of publication (Fig 4). However, the RRs of any dementia and AD associated with head injury did not reach statistical significances in female participants, although the point estimates were over 1 (RR = 1.30, 95% CI 0.60–2.81, and RR = 1.17, 95% CI 0.89–1.53, respectively). When considering the types of head injury, TBI rather than other types of head injury showed significant association with increased risk of any dementia (RR = 1.69, 95% CI 1.34–2.12, and RR = 1.32, 95% CI 0.85–2.04, respectively), while other types of head injury rather than TBI showed significant association with increased risk of AD (RR = 1.60, 95% CI 1.32–1.93, and RR = 1.09, 95% CI 0.73–1.61, respectively). Additionally, the positive associations were not materially changed in the leave-one-out analyses by omitting one study in turn, with a pooled RR of any dementia range from 1.51 (95% CI 1.27–1.79) to 1.72 (95% CI 1.40–2.11) (S2 Fig), and a pooled RR of AD range from 1.45 (95% CI 1.23–1.72) to 1.54 (95% CI 1.29–1.84) (S3 Fig).In the sensitivity analyses, 11 risk estimates for dementia from 7 studies and 36 estimates for AD from 21 studies originally reporting minimally adjusted RRs with 95% CIs were included. The results were in line with the pooled estimates found in meta-analyses (data not shown).
Fig 4. Sensitivity analyses for estimated risks of any dementia (A) and Alzheimer’s disease (B).
TBI = traumatic brain injury. NINCDS-ADRDA = National Institute of Neurological and Communicative Diseases and Stroke-Alzheimer Disease and Related Disorders Association. DSM = Diagnostic and Statistical Manual of Mental Disorders.
Publication bias
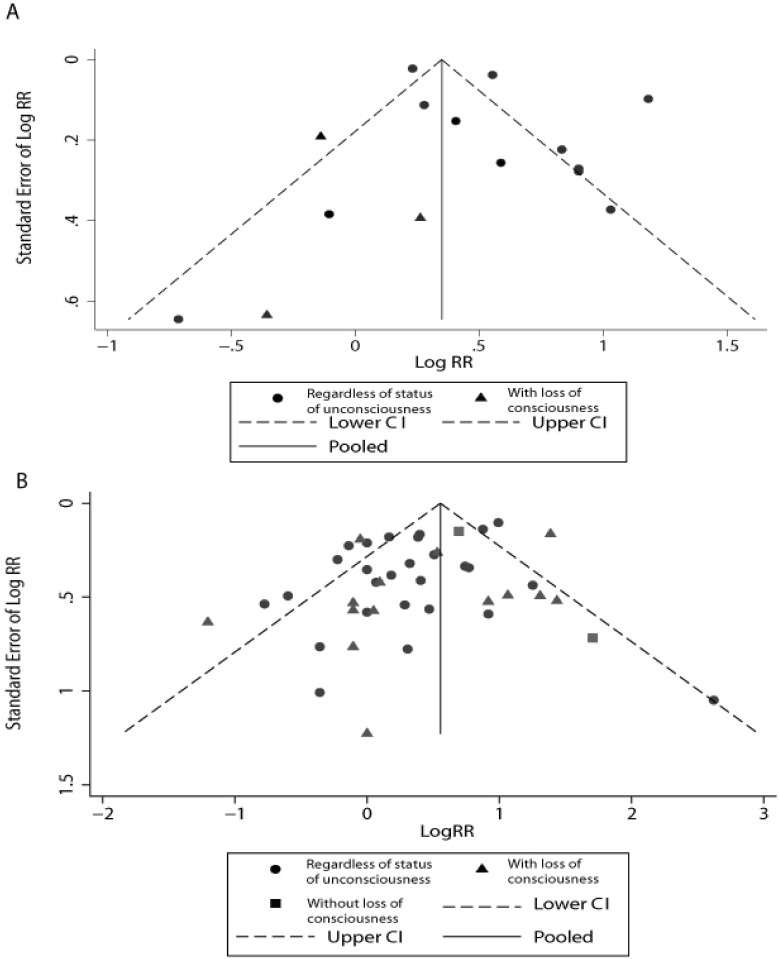
Visual assessment of funnel plots showed that the studies were distributed fairly symmetrically about the combined effect size in both meta-analyses (Fig 5), which suggests little publication bias in our meta-analyses. Egger’s regression test (P = 0.327 and P = 0.139, respectively) and Begg-Mazumdar test (P = 0.255 and P = 0.958, respectively) further confirmed that there was no potential publication bias in both meta-analyses.
Fig 5. Funnel plot to explore publication bias in the estimates of any dementia (A) and Alzheimer’s disease (B).
The vertical line is at the mean effect size.
Discussion
In this pooled analysis of 32 observational studies, with data for more than two million individuals and more than 13,000 dementia events and 8,000 AD events, head injury was a risk factor for any dementia and AD. Indeed, we observed an overall 63% increase in any dementia risk and 51% increase in AD, comparing individuals with head injury to those without head injury. These findings add to the accumulating evidence that head injury may lead to neurodegenerative diseases, although current evidence from the literature is mixed.
The findings of this pooled analysis are partially consistent with the positive association observed between head injury and AD observed in a meta-analysis that combined case-control studies that were conducted up to the year 2001[44]. The previous meta-analysis did not assess the relationship between head injury and risk of any dementia, and only included case-control studies that published before 2001. Our analysis included more studies than did the previous meta-analysis (11 compared with none and 28 compared with 15 for studies examining any dementia and AD, respectively), especially included many studies that published after 2001. Moreover, in our meta-analysis, we considered the status of unconsciousness and conducted sensitivity analyses for more variables in addition to gender, and the pooled estimate of the AD risk (RR = 1.51) was more precise (95% CI 1.26–1.80) than that of the other study. Perry et al assessed the relationship between TBI and subsequent neurological and psychiatric disease in a meta-analysis[45], using dementia and AD as subgroups of the overall analysis. Similarly, the studies relevant to dementia and AD were not enough comprehensive in their study (only included seven studies for dementia and nineteen for AD) and the relationship between TBI and dementia/AD was not well discussed. The relationship between any head injury and dementia/AD was not assessed in their meta-analysis either. In addition, Perry et al’s study did not find significant association between TBI and dementia (OR = 1.36, 95% CI 0.84–2.19), which is inconsistent with our result. This might be due to the limited number of studies that included in their meta-analysis.
Our meta-analysis included not only retrospective case-control studies but also prospective cohort studies. An important strength of prospective studies is that recruitment takes place and information about head injury is recorded before participants know whether they will develop dementia or AD. The robustness of prospective data is demonstrated by the stability of the findings in the sensitivity analyses. When the retrospective studies were assessed in isolation in the sensitivity analyses, their aggregate findings differed from those of the prospective studies, which may be caused by biases in some retrospective studies. Many retrospective study results could have been somewhat biased by selective participation of head injury patients, and in all retrospective studies information about head injury was recorded after dementia or AD diagnosis, so there might have been differential recall of head injury.
An interesting finding in our subgroup analyses indicated that head injury with LOC had no significant association with dementia, and had no significant association with AD in the meta-analysis that excluded studies with results not adjusted for other factors. However, head injury without LOC showed consistently positive association with AD. This finding conflicted with the preconceived opinion that more severe injury induces more serious complications. One primary possible explanation was that most included studies did not distinguish head injury with and without LOC. Thus, there were very limited studies in the head injury with LOC or without LOC subgroup, making the results of subgroup low of statistical power. More studies are needed to further assess the relative risk of dementia/AD induced by head injury with LOC and without LOC separately. Another explanation was that head injuries without LOC would be susceptible to greater recall bias, and if that were so, one might observe a greater risk for AD among head injured persons without than those with LOC[31]. Also, there may be a survivor bias, where people with history of more severe head injury who later enrolled in studies or survived into old age were the best able to recover from those injuries. In addition, the idea of the early pre clinical minor motor features of dementia leading to falls and minor head injury seems a much more probable explanation for our findings. Moreover, residual or unmeasured confounding factors, such as alcohol consumption, misuse prescribed opiates, and other psychiatric illnesses such as depression may also contribute to this anomalous result. Although this finding is consistent with the result of a large EURODEM pooled analysis of four European population-based studies which showed head trauma with unconsciousness was not associated with AD[46], the mechanism that the influence of head injury severity on dementia and AD need to be further clarified.
In the sensitivity analyses by sex, the positive association between head injury and dementia/AD did not reach statistical significance in female participants. The previous meta-analysis also indicated that the excess risk of head injury in those with AD is only found in males[44]. The sex difference in the risk of dementia and AD following head injury may contribute to the role of the female hormones, oestrogen and progesterone. Animal models of stroke and TBI have suggested that these hormones may confer a neuroprotective and neuroregenerative effect[47,48]. In the animal model conducted by Bramlett and Dietrich[49],neuropathological protection effect after TBI was found in intact female rats versus males or ovariectomized females. Their results provided evidence for endogenous hormonal histopathological protection following brain injury. Moreover, oestrogen has been reported as a protective factor in the development of AD.
The strengths of the present meta-analysis include that almost all the worldwide evidence from eligible epidemiological studies was included, as well as the acceptable methodologic quality of the studies on which the analysis is based. One limitation of our study is that all types of “head injury” were treated equally in the pooled analyses. Although we did sensitivity analyses by exposure type (TBI or other types of head injury), the results show that other types of head injury didn’t show significant association with increased risk of any dementia and TBI didn’t show significant association with increased risk of AD. This may be due to the limited number of studies included in the sensitivity analyses. More studies are needed to further clarify the relationship between a true TBI and the risk of dementia and AD. Other possible limitations of the study include the heterogeneity between the studies, including studies in which outcomes were recorded with different scales. Defining the status of head injury at baseline (cohort studies) or during the reference period (case-control studies) possibly caused the heterogeneity between the included studies, because the follow-up periods and the reference dates varied between the studies. Moreover, there were numerous differences in the analytic methods used to estimate the ORs and RRs among different studies, which may also contribute to the heterogeneity in the results. Therefore, our results must be interpreted with caution. We assumed that the true effect would vary between the studies because of potential additional heterogeneity in the populations, designs, and analyses of the various studies, in addition to the usual sampling variation in the estimates within studies. To account for the heterogeneity, we used the random effects model to combine the results of the original studies. The random-effect approach provides some allowance for heterogeneity in studies beyond sampling error. One can expect a very limited influence of heterogeneity by using the random effects model, although this does not necessarily rule out the effect of heterogeneity between the studies. Sensitivity analyses by some study-level factors were performed to further explore the sources of heterogeneity, but heterogeneity persisted after we performed relevant sensitivity analyses. Although the studies included in our meta-analysis were heterogeneous, the relation is largely consistent.
Our meta-analysis is based on observational studies, and the possibility of selection bias, misclassification bias related to exposure, and failure to consider residual or unmeasured confounding cannot be ruled out. The assessment methods for head injury may also vary between the studies. The assessment in several included studies was based on self-reported questionnaire and medical records documenting the severity of injury were not accessed for all participants, and such data are subject to recall bias, especially for the patients with dementia and AD. Moreover, the present data do not address the important issues of whether a single head injury and repeated head injury can both increase the risk of dementia and AD, and whether a recent injury and an earlier injury on head or whether mild TBI and serious TBI have different impact on the risk of dementia and AD. Unfortunately, the data available in the current study do not allow for more refined categorisations of head injury. The time elapsed between head injury and dementia symptoms starting was reported rarely among the included studies, and this may be another limitation of this study. Besides, without autopsy confirmation, clinical diagnosis of AD is suspect due to all the “AD mimics”(hippocampal sclerosis, primary age-related lalopathy, Lewy body disease, vascular dementia, etc).
Limitations aside, this meta-analysis remains the most comprehensive study to date that addressed the association between head injury and risk of dementia and AD. The diversity of location, ethnicity, age of participants, and head injury status reported in these studies also allows for increased generalisability of these results to other populations. However, studies included in our meta-analysis are mainly from Europe and The United States, thus, the results of our study must be interpreted with caution when generalized to populations from other regions. Although we recognize the methodological limitations of the studies included in this meta-analysis, our study does serve as a comprehensive review of this literature.
Conclusion
On the basis of epidemiologic evidence, we found that head injury was associated with an increased risk of dementia and AD. For further studies, based on our findings, we suggest that the investigators should improve the standardization of various assessment methods of head injury, dementia and AD. Furthermore, this study adds to the existing evidence that head injury may lead to neurodegenerative diseases, and the use of genetic and biological makers as surrogate end points in the future studies should help to clarify the case and effect relationship that links head injury and dementia and AD.
Supporting Information
(DOC)
(TIF)
Box sizes are in proportion to study weight. TBI = traumatic brain injury.
(TIF)
Pooled relative risks for any dementia associated with head injury by omitting one study in turn.
(TIF)
Pooled relative risks for Alzheimer’s disease associated with head injury by omitting one study in turn.
(TIF)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Key Project of Postgraduate Innovation, Jiamusi University (LZZ2015-011). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. 2007; 22 (5): 341–353. [PubMed] [Google Scholar]
- 2.Basso A, Previgliano I, Duarte JM, Ferrari N. Advances in management of neurosurgical trauma in different continents. World J Surg. 2001; 25 (9): 1174–1178. [DOI] [PubMed] [Google Scholar]
- 3.Rugbjerg K, Ritz B, Korbo L, Martinussen N, Olsen JH. Risk of Parkinson's disease after hospital contact for head injury: population based case-control study. BMJ. 2008; 337: a2494 10.1136/bmj.a2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nemetz PN, Leibson C, Naessens JM, Beard M, Kokmen E, Annegers JF, et al. Traumatic brain injury and time to onset of Alzheimer's disease: a population-based study. Am J Epidemiol. 1999; 149 (1): 32–40. [DOI] [PubMed] [Google Scholar]
- 5.Andersen OM, Schmidt V, Spoelgen R, Gliemann J, Behlke J, Galatis D, et al. Molecular dissection of the interaction between amyloid precursor protein and its neuronal trafficking receptor SorLA/LR11. Biochemistry. 2006; 45 (8): 2618–2628. 10.1021/bi052120v [DOI] [PubMed] [Google Scholar]
- 6.Graham DI, Gentleman SM, Nicoll JA, Royston MC, McKenzie JE, Roberts GW, et al. Altered beta-APP metabolism after head injury and its relationship to the aetiology of Alzheimer's disease. Acta Neurochir Suppl. 1996; 66: 96–102. [DOI] [PubMed] [Google Scholar]
- 7.Franzblau M, Gonzales-Portillo C, Gonzales-Portillo GS, Diamandis T, Borlongan MC, Tajiri N, et al. Vascular damage: a persisting pathology common to Alzheimer's disease and traumatic brain injury. Med Hypotheses. 2013; 81 (5): 842–845. 10.1016/j.mehy.2013.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasmusson DX, Brandt J, Martin DB, Folstein MF. Head injury as a risk factor in Alzheimer's disease. Brain Inj. 1995; 9 (3): 213–219. [DOI] [PubMed] [Google Scholar]
- 9.O'Meara ES, Kukull WA, Sheppard L, Bowen JD, McCormick WC, Teri L, et al. Head injury and risk of Alzheimer's disease by apolipoprotein E genotype. Am J Epidemiol. 1997; 146 (5): 373–384. [DOI] [PubMed] [Google Scholar]
- 10.Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, et al. Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology. 2000; 55 (8): 1158–1166. [DOI] [PubMed] [Google Scholar]
- 11.Fratiglioni L, Ahlbom A, Viitanen M, Winblad B. Risk factors for late-onset Alzheimer's disease: a population-based, case-control study. Ann Neurol. 1993; 33 (3): 258–266. 10.1002/ana.410330306 [DOI] [PubMed] [Google Scholar]
- 12.Mehta KM, Ott A, Kalmijn S, Slooter AJ, van Duijn CM, Hofman A, et al. Head trauma and risk of dementia and Alzheimer's disease: The Rotterdam Study. Neurology. 1999; 53 (9): 1959–1962. [DOI] [PubMed] [Google Scholar]
- 13.Li G, Shen YC, Li YT, Chen CH, Zhau YW, Silverman JM. A case-control study of Alzheimer's disease in China. Neurology. 1992; 42 (8): 1481–1488. [DOI] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000; 283 (15): 2008–2012. [DOI] [PubMed] [Google Scholar]
- 15.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. (2011) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: University of Ottawa. [Google Scholar]
- 16.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997; 315 (7121): 1533–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987; 9: 1–30. [DOI] [PubMed] [Google Scholar]
- 18.Abner EL, Nelson PT, Schmitt FA, Browning SR, Fardo DW, Wan L, et al. Self-reported head injury and risk of late-life impairment and AD pathology in an AD center cohort. Dement Geriatr Cogn Disord. 2014; 37 (5–6): 294–306. 10.1159/000355478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dams-O'Connor K, Gibbons LE, Bowen JD, McCurry SM, Larson EB, Crane PK. Risk for late-life re-injury, dementia and death among individuals with traumatic brain injury: a population-based study. J Neurol Neurosurg Psychiatry. 2013; 84 (2): 177–182. 10.1136/jnnp-2012-303938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol. 2014; 71 (12): 1490–1497. 10.1001/jamaneurol.2014.2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YK, Hou SW, Lee CC, Hsu CY, Huang YS, Su YC. Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PLoS One. 2013; 8 (5): e62422 10.1371/journal.pone.0062422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luukinen H, Viramo P, Herala M, Kervinen K, Kesaniemi YA, Savola O, et al. Fall-related brain injuries and the risk of dementia in elderly people: a population-based study. Eur J Neurol. 2005; 12 (2): 86–92. 10.1111/j.1468-1331.2004.00953.x [DOI] [PubMed] [Google Scholar]
- 23.Nordstrom P, Michaelsson K, Gustafson Y, Nordstrom A. Traumatic brain injury and young onset dementia: a nationwide cohort study. Ann Neurol. 2014; 75 (3): 374–381. [DOI] [PubMed] [Google Scholar]
- 24.Wang HK, Lin SH, Sung PS, Wu MH, Hung KW, Wang LC, et al. Population based study on patients with traumatic brain injury suggests increased risk of dementia. J Neurol Neurosurg Psychiatry. 2012; 83 (11): 1080–1085. 10.1136/jnnp-2012-302633 [DOI] [PubMed] [Google Scholar]
- 25.Fischer P, Zehetmayer S, Jungwirth S, Weissgram S, Krampla W, Hinterberger M, et al. Risk factors for Alzheimer dementia in a community-based birth cohort at the age of 75 years. Dement Geriatr Cogn Disord. 2008; 25 (6): 501–507. 10.1159/000128577 [DOI] [PubMed] [Google Scholar]
- 26.Schofield PW, Tang M, Marder K, Bell K, Dooneief G, Chun M, et al. Alzheimer's disease after remote head injury: an incidence study. J Neurol Neurosurg Psychiatry. 1997; 62 (2): 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broe GA, Henderson AS, Creasey H, McCusker E, Korten AE, Jorm AF, et al. A case-control study of Alzheimer's disease in Australia. Neurology. 1990; 40 (11): 1698–1707. [DOI] [PubMed] [Google Scholar]
- 28.Ferini-Strambi L, Smirne S, Garancini P, Pinto P, Franceschi M. Clinical and epidemiological aspects of Alzheimer's disease with presenile onset: a case control study. Neuroepidemiology. 1990; 9 (1): 39–49. [DOI] [PubMed] [Google Scholar]
- 29.Forster DP, Newens AJ, Kay DW, Edwardson JA. Risk factors in clinically diagnosed presenile dementia of the Alzheimer type: a case-control study in northern England. J Epidemiol Community Health. 1995; 49 (3): 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graves AB, White E, Koepsell TD, Reifler BV, van Belle G, Larson EB, et al. The association between head trauma and Alzheimer's disease. Am J Epidemiol. 1990; 131 (3): 491–501. [DOI] [PubMed] [Google Scholar]
- 31.Guo Z, Cupples LA, Kurz A, Auerbach SH, Volicer L, Chui H, et al. Head injury and the risk of AD in the MIRAGE study. Neurology. 2000; 54 (6): 1316–1323. [DOI] [PubMed] [Google Scholar]
- 32.Mayeux R, Ottman R, Tang MX, Noboa-Bauza L, Marder K, Gurland B, et al. Genetic susceptibility and head injury as risk factors for Alzheimer's disease among community-dwelling elderly persons and their first-degree relatives. Ann Neurol. 1993; 33 (5): 494–501. 10.1002/ana.410330513 [DOI] [PubMed] [Google Scholar]
- 33.McDowell I, Hill G, Lindsay J, Helliwell B, Costa L, Beattie L, et al. The Canadian Study of Health and Aging: risk factors for Alzheimer's disease in Canada. Neurology. 1994; 44 (11): 2073–2080. [DOI] [PubMed] [Google Scholar]
- 34.Salib E, Hillier V. Head injury and the risk of Alzheimer's disease: a case control study. Int J Geriatr Psychiatry. 1997; 12 (3): 363–368. [DOI] [PubMed] [Google Scholar]
- 35.Sundstrom A, Nilsson LG, Cruts M, Adolfsson R, Van Broeckhoven C, Nyberg L. Increased risk of dementia following mild head injury for carriers but not for non-carriers of the APOE epsilon4 allele. Int Psychogeriatr. 2007; 19 (1): 159–165. 10.1017/S1041610206003498 [DOI] [PubMed] [Google Scholar]
- 36.van Duijn CM, Tanja TA, Haaxma R, Schulte W, Saan RJ, Lameris AJ, et al. Head trauma and the risk of Alzheimer's disease. Am J Epidemiol. 1992; 135 (7): 775–782. [DOI] [PubMed] [Google Scholar]
- 37.Bachman DL, Green RC, Benke KS, Cupples LA, Farrer LA. Comparison of Alzheimer's disease risk factors in white and African American families. Neurology. 2003; 60 (8): 1372–1374. [DOI] [PubMed] [Google Scholar]
- 38.Boston PF, Dennis MS, Jagger C. Factors associated with vascular dementia in an elderly community population. Int J Geriatr Psychiatry. 1999; 14 (9): 761–766. [DOI] [PubMed] [Google Scholar]
- 39.Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, et al. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002; 156 (5): 445–453. [DOI] [PubMed] [Google Scholar]
- 40.Ogunniyi A, Hall KS, Gureje O, Baiyewu O, Gao S, Unverzagt FW, et al. Risk factors for incident Alzheimer's disease in African Americans and Yoruba. Metab Brain Dis. 2006; 21 (2–3): 235–240. 10.1007/s11011-006-9017-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rippon GA, Tang MX, Lee JH, Lantigua R, Medrano M, Mayeux R. Familial Alzheimer disease in Latinos: interaction between APOE, stroke, and estrogen replacement. Neurology. 2006; 66 (1): 35–40. 10.1212/01.wnl.0000191300.38571.3e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suhanov AV, Pilipenko PI, Korczyn AD, Hofman A, Voevoda MI, Shishkin SV, et al. Risk factors for Alzheimer's disease in Russia: a case-control study. Eur J Neurol. 2006; 13 (9): 990–995. 10.1111/j.1468-1331.2006.01391.x [DOI] [PubMed] [Google Scholar]
- 43.Tsolaki M, Fountoulakis K, Chantzi E, Kazis A. Risk factors for clinically diagnosed Alzheimer's disease: a case-control study of a Greek population. Int Psychogeriatr. 1997; 9 (3): 327–341. [DOI] [PubMed] [Google Scholar]
- 44.Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer's disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry. 2003; 74 (7): 857–862. 10.1136/jnnp.74.7.857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perry DC, Sturm VE, Peterson MJ, Pieper CF, Bullock T, Boeve BF, et al. Association of traumatic brain injury with subsequent neurological and psychiatric disease: a meta-analysis. J Neurosurg. 2015: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A, Amaducci LA, et al. Rates and risk factors for dementia and Alzheimer's disease: results from EURODEM pooled analyses. EURODEM Incidence Research Group and Work Groups. European Studies of Dementia. Neurology. 1999; 52 (1): 78–84. [DOI] [PubMed] [Google Scholar]
- 47.Stein DG. Brain damage, sex hormones and recovery: a new role for progesterone and estrogen? Trends Neurosci. 2001; 24 (7): 386–391. [DOI] [PubMed] [Google Scholar]
- 48.Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma. 2000; 17 (5): 367–388. 10.1089/neu.2000.17.367 [DOI] [PubMed] [Google Scholar]
- 49.Bramlett HM, Dietrich WD. Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J Neurotrauma. 2001; 18 (9): 891–900. 10.1089/089771501750451811 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(TIF)
Box sizes are in proportion to study weight. TBI = traumatic brain injury.
(TIF)
Pooled relative risks for any dementia associated with head injury by omitting one study in turn.
(TIF)
Pooled relative risks for Alzheimer’s disease associated with head injury by omitting one study in turn.
(TIF)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.