Abstract
Chronic kidney disease is a major public health problem that continues to show an unrelenting global increase in prevalence. The prevalence of chronic kidney disease has been predicted to grow the fastest in low- to middle-income countries (LMICs). There is evidence that people living in LMICs have the highest need for renal replacement therapy (RRT) despite the lowest access to various modalities of treatment. As continuous ambulatory peritoneal dialysis (CAPD) does not require advanced technologies, much infrastructure, or need for dialysis staff support, it should be an ideal form of RRT in LMICs, particularly for those living in remote areas. However, CAPD is scarcely available in many LMICs, and even where available, there are several hurdles to be confronted regarding patient selection for this modality. High cost of CAPD due to unavailability of fluids, low patient education and motivation, low remuneration for nephrologists, lack of expertise/experience for catheter insertion and management of complications, presence of associated comorbid diseases, and various socio-demographic factors contribute significantly toward reduced patient selection for CAPD. Cost of CAPD fluids seems to be a major constraint given that many countries do not have the capacity to manufacture fluids but instead rely heavily on fluids imported from developed countries. There is need to invest in fluid manufacturing (either nationally or regionally) in LMICs to improve uptake of patients treated with CAPD. Workforce training and retraining will be necessary to ensure that there is coordination of CAPD programs and increase the use of protocols designed to improve CAPD outcomes such as insertion of catheters, treatment of peritonitis, and treatment of complications associated with CAPD. Training of nephrology workforce in CAPD will increase workforce experience and make CAPD a more acceptable RRT modality with improved outcomes.
Keywords: dialysis cost, dialysis fluid, peritoneal dialysis, peritonitis, nephrology workforce
Introduction
Chronic kidney disease (CKD) is a major burden on health care, with an estimated worldwide prevalence of 8%–16%, with growth predicted to become fastest in the poorest parts of the world.1 CKD in Africa is 3–4 times more common than in developed countries1 with the impact of rising rates of diabetes2 and hypertension3 expected to contribute significantly to the CKD burden not only in Africa but also worldwide.4 Unfortunately, lack of availability of dialysis to accommodate the global CKD burden is alarming. In 2010, it was estimated that between 2 and 7 million people died prematurely due to lack of access to renal replacement therapies (RRTs).4
Continuous ambulatory peritoneal dialysis (CAPD) should be an ideal form of RRT in low- to middle-income countries (LMICs), particularly for those living in rural areas. Apart from fewer hospital visits, CAPD allows for greater flexibility with employment and schooling. It is ideal for children and adolescents and those caring for others. It enables those living far from dialysis centers the convenience of home dialysis. Evidence also supports CAPD first policy prior to hemodialysis (HD) as there are known benefits for the preservation of residual renal function5 and protection of vascular access sites. Over the past two decades, the survival of patients treated with CAPD has steadily improved, both in absolute terms and in comparison to that of patients receiving HD.6–9 However, most studies showing survival benefit with CAPD have taken place in developed countries. Despite this, HD remains the most common RRT (Table 1)10–15 with most centers located in big cities and therefore inaccessible to vast numbers of the population living in remote areas. The aim of this review was to assess factors linked with patient selection, understand the reasons for poor utilization of CAPD in LMICs, especially in Africa, and suggest ways to improve CAPD use for end-stage renal disease (ESRD) treatment in developing countries.
Table 1.
Worldwide utilization of RRT (HD and PD only) in selected countries
| Countries | Prevalent maintenance HD capacity (pmp) | Prevalent maintenance PD capacity (pmp) | % PD use among all dialysis patients |
|---|---|---|---|
| Developed countries10–12 | |||
| USA | 1,165 | 87.1 | 7.0 |
| UK | 365 | 61 | 17.0 |
| Japan | 2,148.4 | 71.9 | 3.3 |
| Germany | 768.1 | 38.8 | 4.8 |
| Italy | 738.8 | 78.3 | 9.6 |
| Austria | 449.7 | 43.3 | 8.8 |
| Singapore | 684 | 158 | 19 |
| Developing countries13–15 | |||
| South Africa | 45 | 25 | 32 |
| Sudan | 46 | 85 | 3.5 |
| Kenya | 7.5 | 1.2 | 12 |
| Egypt | 421 | 0.3 | 0.0 |
| Nigeria | 8 | 0 | 0.0 |
| Ghana | 2 | 0 | 0.0 |
| Senegal | 4.1 | 1 | 18 |
| Cameroon | 5.9 | 0 | 0.0 |
| Algeria | 381 | 11.1 | 6.3 |
| Nepal | 10.1 | 1.5 | 13.5 |
| India | 18.0 | 5.8 | 24.5 |
| Brazil | 396.3 | 47 | 10.6 |
Role of cost and availability of CAPD services
RRT represents an expensive form of health care technology. The cost of RRT consumes a significant proportion of health care budgets in developed countries, while the cost of care remains unattainable in most developing countries. Data from the United States Renal Data System (USRDS)16 show that Medicare expenditure for all CKD rose from $41.2 billion in 2010 to $50.4 billion in 2014 representing a 22.3% increase in cost. The total cost of care in the US in 2013 is in excess of the national budgets of many countries in sub-Saharan Africa, Latin America, and Central and East Asia. The cost ratio of HD to CAPD is much lower in LMICs (Table 2).17–25 Also, dialysis availability in LMICs has thus been challenging given the double burden of communicable and noncommunicable diseases (NCDs) that many LMICs are faced with along with the added pressure to distribute health care resources to attend to all health challenges. The high cost of dialysis arises from the different components of service provision. It is generally agreed that CAPD is technologically less challenging than HD and hence provides an attractive strategy. Indeed, the governments of Thailand and Hong Kong have adopted a CAPD first policy to deal with its growing ESRD population.26–29
Table 2.
Annual cost of HD and PD in selected developed and developing countries
| Countries | Annual cost of HD (USD) | Annual cost of PD (USD) | HD/PD cost ratio | Year |
|---|---|---|---|---|
| Developed countries | ||||
| USA | 68,253 | 56,807 | 1.2 | 2005 |
| UK | 42,679 | 26,389 | 1.6 | 2008 |
| Sweden | 70,796 | 46,018 | 1.5 | 2007 |
| Canada | 51,252 | 26,959 | 1.9 | 2002 |
| Australia | 21,633 | 36,140 | 0.6 | 2007 |
| Developing countries | ||||
| Sudan | 10,500 | 11,500 | 0.9 | 2010 |
| India | 8,160 | 4,800 | 1.7 | 2009 |
| Malaysia | 23,549 | 23,431 | 1.0 | 2005 |
| South Africa | 7,000 | 12,000 | 0.6 | 2010 |
| Sri Lanka | 3,888 | 9,600 | 0.4 | 2001 |
| Namibia | 24,500 | 24,500 | 1.0 | 2010 |
| Kenya | 16,000 | 12,000 | 1.3 | 2010 |
Cost comparison between CAPD and HD has been a subject of debate, even though CAPD is more affordable in most places.30,31 In places with developed CAPD services like South America, CAPD costs are lower compared to HD. As an example, the HD/CAPD ratio in Egypt is 0.22 compared to 1.5 in Mexico.30 The difference in CAPD costs can be mainly explained by ability or otherwise of fluid manufacturing within a country and by government policies that support use of CAPD as a first option for RRT. In countries with CAPD first policy, cost and utilization have been noted to have improved.32 Similarly, countries that are able to manufacture CAPD fluids are able to cut out cost of international transportation, various tariffs, and cost of storage, thus massively reducing the cost of CAPD.33,34 Unfortunately, due to the lack of infrastructure and appropriate technologies, most LMICs depend entirely on imported CAPD fluids which make the cost excessively high.
In Africa, the prevalence of CAPD varies from 0 to 45 per million population (pmp) compared with 90 pmp in the USA.35 Limited CAPD utilization is also significantly related to the availability of adequately trained health care workers.33 Human resource for health continues to be inadequate for primary health care in several LMICs where there has been a significant and demonstrable inequality in urban–rural distribution of health care workers, with the majority residing in urban settings36,37 (Table 3).14,38–40 Low workforce impacts on various aspects of CAPD usage including catheter insertion, management of catheter malfunction, treatment of peritonitis, and assessment of membrane function. Inadequate workforce may also influence the number of people available to provide the much required patient education for CAPD use.32
Table 3.
Global health workforce for physicians, nephrologists, and nurses
| World region | Number of physicians | Physician density* | Nurses density** | Nephrologists density*** |
|---|---|---|---|---|
| WHO region | ||||
| Africa | 33,183 | 2.7 | 1.16 | 1 |
| America | 1,981,621 | 21.5 | 3.05 | 22 (North America); 8 (South America) |
| Europe | 2,356,671 | 32.1 | 8.19 | 31 |
| South-East Asia | 1,128,508 | 5.9 | 1.79 | 1 |
| Western Pacific | 2,435,023 | 14 | 4.74 | 9 (Australia and New Zealand) |
| Eastern | 532,486 | 10 | 2.99 | |
| Mediterranean | ||||
| Income group | ||||
| Low income | 213,982 | 2.5 | 0.51 | 0.3 |
| Lower middle income | 1,991,612 | 7.9 | 1.08 | 1.6 |
| Upper middle income | 3,755,703 | 16.1 | 3.84 | 4.8 |
| High income | 3,186,223 | 28.7 | – | – |
Role of socio-demographic factors
The availability and/or utilization of CAPD in several LMICs is often dependent on prevailing socio-demographic factors, including level of education, availability of electricity and water supply, availability of an efficient sanitation system, distance, and availability of transportation to the nephrology center. Often, CAPD provision may be accessible only through one provincial or regional hospital with several patients who need to access care dwelling very far away from such hospitals. In one study from South Africa, the mean distance patients needed to travel to the hospital was 122.9±78.2 km.41 In reality, this should be a reason to increase utilization of CAPD since patients will not have to travel frequently to hospital. However, the unavailability of other essential infrastructure (such as good road networks and adequate transport services – both of which are necessary for the delivery of CAPD consumables to patients and for patients to visit the hospital in emergencies) makes long distance from a dialysis center (or hospital) a negative factor when considering selection of patients for dialysis in LMICs.35 One study from Canada found that, although remote-dwelling patients were more likely than urban dwellers to commence CAPD in distances ranging from 50 to >300 km than those residing within 50 km, the adjusted rates of death and the adjusted hazard ratio (HR) among patients initiating CAPD were significantly higher in those living further from nephrologists than those living within 50 km.42 Mortality and poor CAPD outcomes in LMICs could be much worse.
Availability of other social services such as housing, number of occupants at home, water supply, and availability of electricity often limits the use of CAPD due to associated high rates of peritonitis. Zent et al had questioned if CAPD should be used in the African context given that outcomes were poorer in communities where social services were unavailable. Their study investigated the relationship between episodes of peritonitis and exit site infection, and predetermined biomedical, socioeconomic, and psychosocial factors in 132 CAPD patients commencing the dialysis program in Cape Town, South Africa between 1987 and 1991.43 They reported a strong association between high peritonitis rate and poor socioeconomic conditions when factors such as unemployment, low-level education, and informal housing with high occupant-to-bedroom ratio, no electricity or running tap water were considered.43 In South Africa where dialysis rationing is still practiced,44,45 socio-demographic factors continue to play a role in determining modality selection for those patients lucky enough to be accepted.
Patients with poor health literacy often lack the knowledge needed to manage their treatment. A study from Taiwan used time-dependent statistical methods to retrospectively analyze factors related to peritonitis in 404 CAPD patients from a single center.46 They reported that patients who had never received compulsory education showed a statistically higher incidence of CAPD-related peritonitis in the univariate analysis (p=0.04) and a proportional hazards model identified education level (less than elementary school vs any higher education level) as having an independent association with CAPD-related peritonitis (HR: 1.45, 95% confidence interval [CI]: 1.01–2.06; p=0.045).46 Low level of education often reduces the ability to assimilate health choices and negotiate access to appropriate providers. For instance, a patient on CAPD may not understand why they have to carry out multiple exchanges everyday while other ESRD patients on HD only need to go for dialysis 3 times a week or why they have to restrict fluid or diets high in potassium or phosphates. A study that examined self-reported adherence to therapeutic regimen for CAPD patients in Hong Kong found significant positive correlations between education level and non-adherence to dietary guidelines (p=0.001), fluid restriction (p=0.001), and dialysis regimen (p=0.009).47 Other studies have shown an opposite trend in correlation.48,49 A low level of literacy and education in many LMICs may therefore contribute to reduced patient selection for this modality.
Other socio-demographic factors have also been reported to affect CAPD utilization in many countries. One study in Northern India showed that financial constraints (100%), lack of patient enthusiasm (100%), doubtful patient compliance (83.2%), and lack of an organized CAPD program (79.2%) were the main factors limiting more widespread use of CAPD.50 It was also reported that none of the interviewed nephrologists as part of the study routinely discussed CAPD as a modality of therapy in their pre-dialysis care of CKD patients.50 In many places, nephrologists perceive CAPD to be burdensome and not lucrative in terms of reimbursement given the fewer patient visits associated with care.35,51 In Canada, following a change from a fee-for-service physician reimbursement system to a gross-revenue-neutral, modality-independent, weekly capitation fee, it was reported that new incentives caused by the altered physician reimbursement increased CAPD and nonhospital-based HD utilization.52
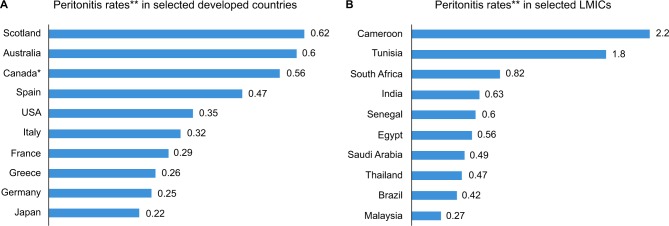
In some centers, their experience with high peritonitis rates may discourage or limit further utilization of CAPD, especially if this is associated with increased mortality. Several LMICs have high peritonitis rates often leading to poor outcome (Figure 1A and B).41,53–71 Youmbissi et al71 concluded that high peritonitis rates will continue to limit the extension of CAPD in the developing world, particularly in tropical Africa, following their experience in Cameroon which showed that there were 40 episodes of peritonitis over 18 patient years, representing 2.2 episodes of peritonitis every patient year. However, other centers have not increased the use of CAPD despite seeing a reduction in peritonitis rates over time due to other factors.33,43
Figure 1.
Peritonitis rates in selected developed (A)53–62 and developing (B)41,63–71 countries.
Notes: *Canadian data mainly from population of First Nations people. **Peritonitis rates expressed as episodes/patient years.
Abbreviation: LMICs, low- to middle-income countries.
Role of comorbid diseases
ESRD patients initiating RRT usually have preexisting comorbidities which increase the risk for poor outcomes.72,73 The presence of single or multiple comorbid conditions makes the option of CAPD difficult against the backdrop of increased cost to their care in addition to an increased risk of mortality.74 Estimates show that the prevalence of NCDs such as diabetes, hypertension, coronary artery disease, heart failure, obesity, chronic respiratory diseases, and various cancers continues to increase globally. The increase has been projected to be higher in LMICs where NCDs will account for 69% of disease burden in 2020, up from 47% in 1990.75
Close to 1 billion people were estimated to be malnourished in 2000–2002 with >90% living in LMICs.76 Nutritional indices have been shown to significantly predict the outcomes in CAPD,77 and malnutrition has been reported to be present in up to 72% of prevalent CAPD patients in India assessed by the subjective global assessment (SGA).78 In one study, various nutritional indices were found to be predictive of peritonitis in CAPD patients including SGA (p=0.009), serum albumin level (p=0.005), and calorie intake (p=0.006).79
Coexistence of diabetes mellitus has been shown to be a negative prognostic factor for patient and technique survival in CAPD.41,80,81 Glucose control often becomes erratic after initiation of CAPD necessitating increased dosing of antidiabetic agents and increasing cost of care for patients paying out of pocket. In a study of 373 CAPD patients with 197 diabetic patients comparing patient survival between diabetic and nondiabetic patients, the relative risk (RR) of mortality in nondiabetics was less than that in diabetic patients (odds ratio [OR]: 0.43, 95% CI: 0.26–0.68; p=0.001) and diabetes was a strong predictor of mortality in multivariate analysis (OR: 1.95, 95% CI: 1.23–3.07; p=0.004).80 Our study from rural Limpopo in South Africa has shown that mortality risk according to dialysis modality was significantly modified by diabetes mellitus status in both univariate and multivariable analyses as mortality risk was ~5 times higher among diabetics on CAPD relative to nondiabetics on HD (HR: 4.99, CI: 2.13–11.71).81
For ESRD patients with concomitant heart failure, CAPD has been reported to be associated with better outcomes than HD, especially for those with contraindications for a heart transplant.82–84 However, patients with significant cardiac disease will be deemed ineligible for chronic RRT in places where dialysis is rationed44,45 and access to treatment for heart failure and other cardiovascular disease may already be proving difficult.85 Nonetheless, CAPD has been shown to be associated with fluid and sodium removal through the peritoneal membrane, and this leads to a significant plasma volume reduction, normalization of serum sodium, and restoration of diuretic responsiveness, as well as an improvement in New York Heart Association (NYHA) functional class, and reduction of hospitalization and readmission rates in patients with heart failure.82–84 In a single center, prospective, nonrandomized study involving patients showing symptoms and signs of congestive heart failure refractory to maximum tolerable drug treatment, CAPD treatment was associated with improved NYHA functional class (p<0.001), raised life expectancy after 12 months, and a better perception of the state of health.83
Obesity has been demonstrated in several studies in both high-income countries and LMICs to confer a survival advantage on patients who are on CAPD.86–88 Despite this, physicians are less likely to prescribe CAPD for obese patients owing to concerns about inadequate ultrafiltration, solute clearance, exit site infections, and peritonitis.89 In LMICs, obese people are likely to be excluded from publicly funded programs mainly due to the policy of rationing and also due to the bias for several complications and poor outcome of kidney transplantation of obese patients (reviewed extensively in Tran et al).90 Well-motivated, appropriately pre-dialysis educated and trained patients may be offered the choice of CAPD.
Effect of transitioning of children and adolescents into adulthood
Worldwide, an increasing number of adolescent and young patients need to be transitioned from pediatric service or present directly to an adult nephrology service. Adolescents and young adults have been recognized to have an increased risk of non-adherence at the time of transfer to adult care.91–93 Thus, kidney outcomes have been shown to be reduced in adolescents compared with other age groups and transitioning from pediatric to adult nephrology services has been identified to be problematic and sometimes traumatic.93,94 The reasons for the adherence issues and poor outcomes observed in adolescents/young adults making transitions to adult care are multifactorial. For many adolescents, leaving the care of their pediatrician may be viewed as a passage from security to uncertainty and many have been known to return to their pediatric caregivers. Also, although the most frequent etiology of adolescent-onset CKD is glomerular diseases, congenital diseases such as renal dysplasia and other hereditary conditions leading to CKD are much more prevalent in younger children and adult nephrologists may be unfamiliar with the treatment of such conditions.93 Finally, the management of chronic diseases in adolescents/young adults alongside other psychological, physical, developmental, and sexual changes associated with this age group can be challenging for patients.
The process of transitioning young adults, as outlined by the International Society of Nephrology and International Pediatric Nephrology Association consensus statement,95 requires the availability of health care professionals working collaboratively in a coordinated health care system. Several LMICs lack the workforce, coordination, and necessary structures to allow for an effective transitioning program and thus adolescents/young adults in these countries often enter adult renal service unprepared for the challenges often leading to poor outcomes. The challenges of treating this group are compounded by the fact that there is paucity of data comparing dialysis outcomes in this specific age group, especially from LMICs.
CAPD should, however, be the ideal form of RRT in adolescents/young adults given that, in the short term, CAPD outcomes have been reported to be better than HD,6,96 allows for preservation of vascular access (important for young patients facing a lifetime of RRT),97 preserves residual renal function,5,98 may have a lower cumulative risk of coronary calcifications than HD,99 and allows children to attend school (if they perform night time CAPD or automated CAPD). One Australian study found school attendance to be significantly higher in adolescents on peritoneal dialysis (PD) than those on HD (p=0.001) and did not report any association between modality and dialysis outcomes.100 In Cape Town, because of the high proportion of young patients in our CAPD service, policies have been implemented which aim to assist with treatment and outcomes. First, all those <25 years old receive automated PD. CAPD enables young adults to achieve greater normalcy in their lives, including the freedom to complete school and studies. This method avoids the need for bag exchanges at school/college. It not only increases the patients comfort with the modality but also facilitates parent involvement and supervision at night. However, it has been noted that adolescents may find the prolonged nocturnal PD treatment to be too intrusive into their social time, leading to skipped or shortened treatments and poor outcome from non-adherence.101 Moreover, the resources and home setting to support CAPD may be unavailable for those from very poor communities without adequate amenities.
PD first policy: is this feasible in LMICs?
PD is an important but underutilized RRT modality for ESRD patients initiating dialysis worldwide (Table 1). It has been suggested that underutilization of CAPD may be related to lack of expertise and absence of pragmatic strategies102 as well as with government policy, economics, provider or health care professional education, and patient-related factors.103 USRDS data show that 93% of all incident dialysis patients start in-center HD even though there is lack of evidence for the discrepancy.104 CAPD has been shown to improve patient survival,6–9 retain residual kidney function,5,98 as well as reduce the financial burden of RRT.105 These should be reasons to embrace CAPD as the initial RRT modality of choice in many LMICs although this is not the case. Liu et al have shown that a CAPD first policy is practiced only in Hong Kong and Thailand, whereas Mexico, Guatemala, USA, Canada, India, China, and Spain have a CAPD-favored policy.103 The advantage of patient survival in CAPD has often been reported within the first few years of RRT initiation. Studies that have not shown superior benefit have reported comparable outcomes between CAPD and HD. Unfortunately, data on improved survival benefit of CAPD are lacking from LMICs, especially from Africa. The Dialysis Outcomes in Colombia showed that there was a higher adjusted mortality risk (12.7%) associated with HD when compared to CAPD, despite CAPD patients being poorer, more likely to be diabetic, and having higher comorbidity scores than HD patients.106 The only available published study in Africa comparing the outcomes of adult patients treated with HD and CAPD, however, reported an increased RR of death in patients receiving CAPD compared to HD.81
Also, a CAPD first policy should be attractive for patients initiating RRT given that CAPD preserves residual renal function and observational studies have reported an association between residual renal function and improved patient survival and quality of life.107 The CANUSA study demonstrated that every 250 mL increment in urine volume in CAPD patients was associated with a 36% decrease in the RR of death (RR: 0.64, 95% CI: 0.51–0.80),5 and other studies have now shown that CAPD protects residual function longer than HD, and it can be preserved for up to 3 years on CAPD.97
Despite the advantages of a CAPD first policy, this initiative can only be possible in an environment with accumulated CAPD experience, government support, and locally produced PD fluid. Li and Chow have suggested that in order to achieve a successful CAPD first policy, inherent patient factors, patient selection strategies, and improving technique-related factors (such as training of physicians, nurses, patients, and caregivers) need to be given careful consideration.102 The presence of several of these factors already creates a barrier to successful implementation of CAPD first policies in several LMICs. If these barriers could be overcome, then CAPD first should be the correct patient choice; however, there is insufficient data to support this approach from LMICs.
Conclusion and future perspectives
There are no easy solutions for improving CAPD uptake as a modality of RRT in LMICs given the enormous health challenges that several of these countries continue to experience. Fundamentally, the cost of CAPD fluids and lack of trained workforce and necessary expertise in carrying out CAPD may underscore the real reasons why CAPD is underutilized. New ideas for making CAPD fluids available should be looked for including ways to commence local (or regional) production of fluids. This is likely to reduce the cost of CAPD and improve utilization. There is also need for training of nephrology workforce in LMICs to improve their uptake and increase their experience in CAPD use. Workforce training and retraining will be necessary to ensure that there is coordination of CAPD programs and increase the use of protocols designed to improve CAPD outcomes such as insertion (and removal) of catheters, treatment of peritonitis, treatment of anemia and bone disease, and timing of transfer to HD. Training of workforce will also improve the quality of patient education through their involvement in the process of care as well as improving patient understanding of various aspects of CAPD.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 4.Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385(9981):1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 5.Bargman JM, Thorpe KE, Churchill DN, Group CPDS. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12(10):2158–2162. doi: 10.1681/ASN.V12102158. [DOI] [PubMed] [Google Scholar]
- 6.Heaf JG, Lokkegaard H, Madsen M. Initial survival advantage of peritoneal dialysis relative to haemodialysis. Nephrol Dial Transplant. 2002;17(1):112–117. doi: 10.1093/ndt/17.1.112. [DOI] [PubMed] [Google Scholar]
- 7.Fenton SS, Schaubel DE, Desmeules M, et al. Hemodialysis versus peritoneal dialysis: a comparison of adjusted mortality rates. Am J Kidney Dis. 1997;30(3):334–342. doi: 10.1016/s0272-6386(97)90276-6. [DOI] [PubMed] [Google Scholar]
- 8.Lukowsky LR, Mehrotra R, Kheifets L, Arah OA, Nissenson AR, Kalantar-Zadeh K. Comparing mortality of peritoneal and hemodialysis patients in the first 2 years of dialysis therapy: a marginal structural model analysis. Clin J Am Soc Nephrol. 2013;8(4):619–628. doi: 10.2215/CJN.04810512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.USRDS . 2009 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2009. [Accessed December 20, 2016]. Available from: https://www.usrds.org/atlas09.aspx. [Google Scholar]
- 10.Jain AK, Blake P, Cordy P, Garg AX. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol. 2012;23(3):533–544. doi: 10.1681/ASN.2011060607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw C, Pruthi R, Pitcher D, Fogarty D. UK Renal Registry 15th annual report: Chapter 2 UK RRT prevalence in 2011: national and centre-specific analyses. Nephron Clin Pract. 2013;123(Suppl 1):29–54. doi: 10.1159/000353321. [DOI] [PubMed] [Google Scholar]
- 12.Neil N, Walker DR, Sesso R, et al. Gaining efficiencies: resources and demand for dialysis around the globe. Value Health. 2009;12(1):73–79. doi: 10.1111/j.1524-4733.2008.00414.x. [DOI] [PubMed] [Google Scholar]
- 13.Naicker S. End-stage renal disease in sub-Saharan Africa. Ethn Dis. 2009;19(1 Suppl 1):S1-13–S1-15. [PubMed] [Google Scholar]
- 14.Katz IJ, Gerntholtz T, Naicker S. Africa and nephrology: the forgotten continent. Nephron Clin Pract. 2011;117(4):c320–c327. doi: 10.1159/000321524. [DOI] [PubMed] [Google Scholar]
- 15.Benghanem Gharbi M. Renal replacement therapies for end-stage renal disease in North Africa. Clin Nephrol. 2010;74(Suppl 1):S17–S19. [PubMed] [Google Scholar]
- 16.USRDS . 2014 annual data report: an overview of the epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2014. [Accessed December 20, 2016]. Available from: https://www.usrds.org/2014/view/ [Google Scholar]
- 17.Abu-Aisha H, Elamin S. Peritoneal dialysis in Africa. Perit Dial Int. 2010;30(1):23–28. doi: 10.3747/pdi.2008.00226. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal SK, Srivastava RK. Chronic kidney disease in India: challenges and solutions. Nephron Clin Pract. 2009;111(3):c197–c203. doi: 10.1159/000199460. discussion c203. [DOI] [PubMed] [Google Scholar]
- 19.Baboolal K, McEwan P, Sondhi S, Spiewanowski P, Wechowski J, Wilson K. The cost of renal dialysis in a UK setting – a multicentre study. Nephrol Dial Transplant. 2008;23(6):1982–1989. doi: 10.1093/ndt/gfm870. [DOI] [PubMed] [Google Scholar]
- 20.Harris A. The organization and funding of the treatment of end-stage renal disease in Australia. Int J Health Care Finance Econ. 2007;7(2–3):113–132. doi: 10.1007/s10754-007-9018-7. [DOI] [PubMed] [Google Scholar]
- 21.Hooi LS, Lim TO, Goh A, et al. Economic evaluation of centre haemodialysis and continuous ambulatory peritoneal dialysis in Ministry of Health hospitals, Malaysia. Nephrology (Carlton) 2005;10(1):25–32. doi: 10.1111/j.1440-1797.2005.00360.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee H, Manns B, Taub K, et al. Cost analysis of ongoing care of patients with end-stage renal disease: the impact of dialysis modality and dialysis access. Am J Kidney Dis. 2002;40(3):611–622. doi: 10.1053/ajkd.2002.34924. [DOI] [PubMed] [Google Scholar]
- 23.Li PK, Chow KM. The cost barrier to peritoneal dialysis in the developing world – an Asian perspective. Perit Dial Int. 2001;21(Suppl 3):S307–S313. [PubMed] [Google Scholar]
- 24.Shih YC, Guo A, Just PM, Mujais S. Impact of initial dialysis modality and modality switches on Medicare expenditures of end-stage renal disease patients. Kidney Int. 2005;68(1):319–329. doi: 10.1111/j.1523-1755.2005.00413.x. [DOI] [PubMed] [Google Scholar]
- 25.Wikstrom B, Fored M, Eichleay MA, Jacobson SH. The financing and organization of medical care for patients with end-stage renal disease in Sweden. Int J Health Care Finance Econ. 2007;7(4):269–281. doi: 10.1007/s10754-007-9014-y. [DOI] [PubMed] [Google Scholar]
- 26.Teerawattananon Y, Mugford M, Tangcharoensathien V. Economic evaluation of palliative management versus peritoneal dialysis and hemodialysis for end-stage renal disease: evidence for coverage decisions in Thailand. Value Health. 2007;10(1):61–72. doi: 10.1111/j.1524-4733.2006.00145.x. [DOI] [PubMed] [Google Scholar]
- 27.Dhanakijcharoen P, Sirivongs D, Aruyapitipan S, Chuengsaman P, Lumpaopong A. The “PD First” policy in Thailand: three-years experiences (2008–2011) J Med Assoc Thailand. 2011;94(Suppl 4):S153–S161. [PubMed] [Google Scholar]
- 28.Yu AW, Chau KF, Ho YW, Li PK. Development of the “peritoneal dialysis first” model in Hong Kong. Perit Dial Int. 2007;27(Suppl 2):S53–S55. [PubMed] [Google Scholar]
- 29.Li PK, Szeto CC. Success of the peritoneal dialysis programme in Hong Kong. Nephrol Dial Transplant. 2008;23(5):1475–1478. doi: 10.1093/ndt/gfn068. [DOI] [PubMed] [Google Scholar]
- 30.Karopadi AN, Mason G, Rettore E, Ronco C. Cost of peritoneal dialysis and haemodialysis across the world. Nephrol Dial Transplant. 2013;28(10):2553–2569. doi: 10.1093/ndt/gft214. [DOI] [PubMed] [Google Scholar]
- 31.Sennfalt K, Magnusson M, Carlsson P. Comparison of hemodialysis and peritoneal dialysis – a cost-utility analysis. Perit Dial Int. 2002;22(1):39–47. [PubMed] [Google Scholar]
- 32.Tungsanga K, Kanjanabuch T, Mahatanan N, Praditpornsilp K, Avihingsanon Y, Eiam-Ong S. The status of, and obstacles to, continuous ambulatory peritoneal dialysis in Thailand. Perit Dial Int. 2008;28(Suppl 3):S53–S58. [PubMed] [Google Scholar]
- 33.Okpechi IG, Rayner BL, Swanepoel CR. Peritoneal dialysis in Cape Town, South Africa. Peri Dial Int. 2012;32(3):254–260. doi: 10.3747/pdi.2011.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finkelstein FO, Abdallah TB, Pecoits-Filho R. Peritoneal dialysis in the developing world: lessons from the Sudan. Perit Dial Int. 2007;27(5):529–530. [PubMed] [Google Scholar]
- 35.Swanepoel CR, Wearne N, Okpechi IG. Nephrology in Africa—not yet uhuru. Nat Rev Nephrol. 2013;9(10):610–622. doi: 10.1038/nrneph.2013.168. [DOI] [PubMed] [Google Scholar]
- 36.Rao M, Rao KD, Kumar AK, Chatterjee M, Sundararaman T. Human resources for health in India. Lancet. 2011;377(9765):587–598. doi: 10.1016/S0140-6736(10)61888-0. [DOI] [PubMed] [Google Scholar]
- 37.Mash R, Almeida M, Wong WC, Kumar R, von Pressentin KB. The roles and training of primary care doctors: China, India, Brazil and South Africa. Hum Resour Health. 2015;13:93. doi: 10.1186/s12960-015-0090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naicker S, Eastwood JB, Plange-Rhule J, Tutt RC. Shortage of healthcare workers in sub-Saharan Africa: a nephrological perspective. Clin Nephrol. 2010;74(Suppl 1):S129–S133. doi: 10.5414/cnp74s129. [DOI] [PubMed] [Google Scholar]
- 39.Sharif MU, Elsayed ME, Stack AG. The global nephrology workforce: emerging threats and potential solutions! Clin Kidney J. 2016;9(1):11–22. doi: 10.1093/ckj/sfv111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO Global Health Workforce Statistics 2014 update. [Accessed November 2016]. Available from: http://www.who.int/hrh/statistics/hwfstats/en/
- 41.Isla RAT, Mapiye D, Swanepoel CR, Rozumyk N, Hubahib JE, Okpechi IG. Continuous ambulatory peritoneal dialysis in Limpopo Province, South Africa: predictors of patient and technique survival. Perit Dial Int. 2014;34(5):518–525. doi: 10.3747/pdi.2013.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tonelli M, Hemmelgarn B, Culleton B, et al. Mortality of Canadians treated by peritoneal dialysis in remote locations. Kidney Int. 2007;72(8):1023–1028. doi: 10.1038/sj.ki.5002443. [DOI] [PubMed] [Google Scholar]
- 43.Zent R, Myers JE, Donald D, Rayner BL. Continuous ambulatory peritoneal dialysis: an option in the developing world? Perit Dial Int. 1994;14(1):48–51. [PubMed] [Google Scholar]
- 44.Okpechi IG, Swanepoel CR, Rayner BL. Outcomes of rationing dialysis therapy in biopsy-proven end-stage renal disease in South Africa. J Nephrol. 2012;25(4):551. doi: 10.5301/jn.5000032. [DOI] [PubMed] [Google Scholar]
- 45.Moosa MR, Kidd M. The dangers of rationing dialysis treatment: the dilemma facing a developing country. Kidney Int. 2006;70(6):1107–1114. doi: 10.1038/sj.ki.5001750. [DOI] [PubMed] [Google Scholar]
- 46.Chern YB, Ho PS, Kuo LC, Chen JB. Lower education level is a major risk factor for peritonitis incidence in chronic peritoneal dialysis patients: a retrospective cohort study with 12-year follow-up. Perit Dial Int. 2013;33(5):552–558. doi: 10.3747/pdi.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lam LW, Twinn SF, Chan SW. Self-reported adherence to a therapeutic regimen among patients undergoing continuous ambulatory peritoneal dialysis. J Adv Nurs. 2010;66(4):763–773. doi: 10.1111/j.1365-2648.2009.05235.x. [DOI] [PubMed] [Google Scholar]
- 48.Blake PG, Korbet SM, Blake R, et al. A multicenter study of noncompliance with continuous ambulatory peritoneal dialysis exchanges in US and Canadian patients. Am J Kidney Dis. 2000;35(3):506–514. doi: 10.1016/s0272-6386(00)70205-8. [DOI] [PubMed] [Google Scholar]
- 49.Wazny LD, Stojimirovic BB, Heidenheim P, Blake PG. Factors influencing erythropoietin compliance in peritoneal dialysis patients. Am J Kidney Dis. 2002;40(3):623–628. doi: 10.1053/ajkd.2002.34925. [DOI] [PubMed] [Google Scholar]
- 50.Mahajan S, Tiwari SC, Kalra V, Bhowmik DM, Agarwal SK. Factors affecting the use of peritoneal dialysis among the ESRD population in India: a single-center study. Perit Dial Int. 2004;24(6):538–541. [PubMed] [Google Scholar]
- 51.Nissenson AR, Prichard SS, Cheng IK, et al. Non-medical factors that impact on ESRD modality selection. Kidney Int Suppl. 1993;40:S120–S127. [PubMed] [Google Scholar]
- 52.Mendelssohn DC, Langlois N, Blake PG. Peritoneal dialysis in Ontario: a natural experiment in physician reimbursement methodology. Perit Dial Int. 2004;24(6):531–537. [PubMed] [Google Scholar]
- 53.Ghali JR, Bannister KM, Brown FG, et al. Microbiology and outcomes of peritonitis in Australian peritoneal dialysis patients. Perit Dial Int. 2011;31(6):651–662. doi: 10.3747/pdi.2010.00131. [DOI] [PubMed] [Google Scholar]
- 54.Fourtounas C, Savidaki E, Dousdabanis P, et al. Peritonitis during the first year after commencement of peritoneal dialysis has an impact on technique survival and patient morbidity. Adv Perit Dial. 2006;22:50–54. [PubMed] [Google Scholar]
- 55.Hasegawa T, Nakai S, Moriishi M, et al. Peritoneal dialysis registry with 2012 survey report. Ther Apher Dial. 2015;19(6):529–539. doi: 10.1111/1744-9987.12382. [DOI] [PubMed] [Google Scholar]
- 56.Hildebrand A, Komenda P, Miller L, et al. Peritonitis and exit site infections in First Nations patients on peritoneal dialysis. Clin J Am Soc Nephrol. 2010;5(11):1988–1995. doi: 10.2215/CJN.04170510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kavanagh D, Prescott GJ, Mactier RA. Peritoneal dialysis-associated peritonitis in Scotland (1999–2002) Nephrol Dial Transplant. 2004;19(10):2584–2591. doi: 10.1093/ndt/gfh386. [DOI] [PubMed] [Google Scholar]
- 58.Marinangeli G, Cabiddu G, Neri L, Viglino G, Russo R, Teatini U. Old and new perspectives on peritoneal dialysis in Italy emerging from the Peritoneal Dialysis Study Group Census. Perit Dial Int. 2012;32(5):558–565. doi: 10.3747/pdi.2011.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Munoz de Bustillo E, Borras F, Gomez-Roldan C, et al. Impact of peritonitis on long-term survival of peritoneal dialysis patients. Nefrologia. 2011;31(6):723–732. doi: 10.3265/Nefrologia.pre2011.Oct.10987. [DOI] [PubMed] [Google Scholar]
- 60.Verger C, Ryckelynck JP, Duman M, et al. French peritoneal dialysis registry (RDPLF): outline and main results. Kidney Int Suppl. 2006(103):S12–S20. doi: 10.1038/sj.ki.5001911. [DOI] [PubMed] [Google Scholar]
- 61.Whaley-Connell A, Pavey BS, Satalowich R, et al. Rates of continuous ambulatory peritoneal dialysis-associated peritonitis at the University of Missouri. Adv Perit Dial. 2005;21:72–75. [PubMed] [Google Scholar]
- 62.Woywodt A, Meier M, Kaiser D, Schneider G, Haller H, Hiss M. In-center intermittent peritoneal dialysis: retrospective ten-year single-center experience with thirty consecutive patients. Perit Dial Int. 2008;28(5):518–526. [PubMed] [Google Scholar]
- 63.Alwakeel JS, Alsuwaida A, Askar A, et al. Outcome and complications in peritoneal dialysis patients: a five-year single center experience. Saudi J Kidney Dis Transplant. 2011;22(2):245–251. [PubMed] [Google Scholar]
- 64.el Matri A, Ben Abdallah T, Kechrid C, Ben Maiz H, Ben Ayed H. Continuous ambulatory peritoneal dialysis in Tunisia. Nephrologie. 1990;11(3):153–156. French. [PubMed] [Google Scholar]
- 65.Kanjanabuch T, Chancharoenthana W, Katavetin P, et al. The incidence of peritoneal dialysis-related infection in Thailand: a nationwide survey. J Med Assoc Thailand. 2011;94(Suppl 4):S7–S12. [PubMed] [Google Scholar]
- 66.Lobo JV, Villar KR, de Andrade Junior MP, Bastos Kde A. Predictor factors of peritoneal dialysis-related peritonitis. J Bras Nefrol. 2010;32(2):156–164. [PubMed] [Google Scholar]
- 67.Mahmoud KM, Sheashaa HA, Gheith OA, et al. Continuous ambulatory peritoneal dialysis in Egypt: progression despite handicaps. Perit Dial Int. 2010;30(3):269–273. doi: 10.3747/pdi.2009.00001. [DOI] [PubMed] [Google Scholar]
- 68.Niang A, Cisse MM, Mahmoud SM, Lemrabott AT, Ka el HF, Diouf B. Pilot experience in Senegal with peritoneal dialysis for end-stage renal disease. Perit Dial Int. 2014;34(5):539–543. doi: 10.3747/pdi.2011.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ong LM, Ch’ng CC, Wee HC, et al. Risk of peritoneal dialysis-related peritonitis in a multi-racial Asian population. Perit Dial Int. 2016 May 4; doi: 10.3747/pdi.2015.00141. Epub. [DOI] [PubMed] [Google Scholar]
- 70.Prasad N, Gupta A, Sharma RK, Prasad KN, Gulati S, Sharma AP. Outcome of gram-positive and gram-negative peritonitis in patients on continuous ambulatory peritoneal dialysis: a single-center experience. Perit Dial Int. 2003;23(Suppl 2):S144–S147. [PubMed] [Google Scholar]
- 71.Youmbissi TJ, Lenthe SS, Ngu JL. Peritonitis in a West African CAPD programme. Perit Dial Int. 1989;9(4):357. [PubMed] [Google Scholar]
- 72.Szeto CC, Chow KM, Kwan BC, Chung KY, Leung CB, Li PK. New-onset hyperglycemia in nondiabetic Chinese patients started on peritoneal dialysis. Am J Kidney Dis. 2007;49(4):524–532. doi: 10.1053/j.ajkd.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 73.Wong PN, Mak SK, Lo KY, Tong GM, Wong Y, Wong AK. Adverse prognostic indicators in continuous ambulatory peritoneal dialysis patients without obvious vascular or nutritional comorbidities. Perit Dial Int. 2003;23(Suppl 2):S109–S115. [PubMed] [Google Scholar]
- 74.Prasad N, Gupta A, Sinha A, et al. Confounding effect of comorbidities and malnutrition on survival of peritoneal dialysis patients. J Renal Nutr. 2010;20(6):384–391. doi: 10.1053/j.jrn.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 75.Boutayeb A, Boutayeb S. The burden of non communicable diseases in developing countries. Int J Equity Health. 2005;4(1):2. doi: 10.1186/1475-9276-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Food and Agriculture Organization of the United Nations . The State of Food Insecurity in the World 2004. Rome: 2004. [Accessed December 20, 2016]. Undernourishment around the world. Available from: http://www.fao.org/3/a-y5650e.pdf. [Google Scholar]
- 77.Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol. 1996;7(2):198–207. doi: 10.1681/ASN.V72198. [DOI] [PubMed] [Google Scholar]
- 78.Sinha APN, Gupta A, Sharma RK, Ahmed M, Kumar A. Prevalence of malnutrition and factors affecting nutritional status of ESRD patients on CAPD in India [Abstract] Perit Dial Int. 2005;25(Suppl 2):S11. [Google Scholar]
- 79.Prasad N, Gupta A, Sharma RK, Sinha A, Kumar R. Impact of nutritional status on peritonitis in CAPD patients. Perit Dial Int. 2007;27(1):42–47. [PubMed] [Google Scholar]
- 80.Prasad N, Gupta A, Sinha A, et al. A comparison of outcomes between diabetic and nondiabetic CAPD patients in India. Perit Dial Int. 2008;28(5):468–476. [PubMed] [Google Scholar]
- 81.Tamayo Isla RA, Ameh OI, Mapiye D, et al. Baseline predictors of mortality among predominantly rural-dwelling end-stage renal disease patients on chronic dialysis therapies in Limpopo, South Africa. PLoS One. 2016;11(6):e0156642. doi: 10.1371/journal.pone.0156642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koch M, Haastert B, Kohnle M, et al. Peritoneal dialysis relieves clinical symptoms and is well tolerated in patients with refractory heart failure and chronic kidney disease. Eur J Heart Failure. 2012;14(5):530–539. doi: 10.1093/eurjhf/hfs035. [DOI] [PubMed] [Google Scholar]
- 83.Sanchez JE, Ortega T, Rodriguez C, et al. Efficacy of peritoneal ultrafiltration in the treatment of refractory congestive heart failure. Nephrol Dial Transplant. 2010;25(2):605–610. doi: 10.1093/ndt/gfp484. [DOI] [PubMed] [Google Scholar]
- 84.Gotloib L, Fudin R, Yakubovich M, Vienken J. Peritoneal dialysis in refractory end-stage congestive heart failure: a challenge facing a no-win situation. Nephrol Dial Transplant. 2005;20(Suppl 7):vii32–vii36. doi: 10.1093/ndt/gfh1105. [DOI] [PubMed] [Google Scholar]
- 85.Jingi AM, Noubiap JJ, Ewane Onana A, et al. Access to diagnostic tests and essential medicines for cardiovascular diseases and diabetes care: cost, availability and affordability in the West Region of Cameroon. PLoS One. 2014;9(11):e111812. doi: 10.1371/journal.pone.0111812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnson DW, Herzig KA, Purdie DM, et al. Is obesity a favorable prognostic factor in peritoneal dialysis patients? Perit Dial Int. 2000;20(6):715–721. [PubMed] [Google Scholar]
- 87.Jin H, Shin JY, Lee SH, Song JH, Kim MJ, Lee SW. Abdominal obesity and mortality in continuous ambulatory peritoneal dialysis patients. Electrolyte Blood Press. 2015;13(1):22–29. doi: 10.5049/EBP.2015.13.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim YK, Kim SH, Kim HW, et al. The association between body mass index and mortality on peritoneal dialysis: a prospective cohort study. Perit Dial Int. 2014;34(4):383–389. doi: 10.3747/pdi.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fernandes NM, Bastos MG, Franco MR, et al. Body size and longitudinal body weight changes do not increase mortality in incident peritoneal dialysis patients of the Brazilian peritoneal dialysis multi-center study. Clinics (Sao Paulo, Brazil) 2013;68(1):51–58. doi: 10.6061/clinics/2013(01)OA08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tran MH, Foster CE, Kalantar-Zadeh K, Ichii H. Kidney transplantation in obese patients. World J Transplant. 2016;6(1):135–143. doi: 10.5500/wjt.v6.i1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Watson AR. Non-compliance and transfer from paediatric to adult transplant unit. Pediatr Nephrol. 2000;14(6):469–472. doi: 10.1007/s004670050794. [DOI] [PubMed] [Google Scholar]
- 92.Dobbels F, Ruppar T, De Geest S, Decorte A, Van Damme-Lombaerts R, Fine RN. Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: a systematic review. Pediatr Transplant. 2010;14(5):603–613. doi: 10.1111/j.1399-3046.2010.01299.x. [DOI] [PubMed] [Google Scholar]
- 93.Ferris ME, Mahan JD. Pediatric chronic kidney disease and the process of health care transition. Semin Nephrol. 2009;29(4):435–444. doi: 10.1016/j.semnephrol.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 94.Chaturvedi S, Jones CL, Walker RG, Sawyer SM. The transition of kidney transplant recipients: a work in progress. Pediatr Nephrol. 2009;24(5):1055–1060. doi: 10.1007/s00467-009-1124-y. [DOI] [PubMed] [Google Scholar]
- 95.Watson AR, Harden PN, Ferris ME, Kerr PG, Mahan JD, Ramzy MF. Transition from pediatric to adult renal services: a consensus statement by the International Society of Nephrology (ISN) and the International Pediatric Nephrology Association (IPNA) Kidney Int. 2011;80(7):704–707. doi: 10.1038/ki.2011.209. [DOI] [PubMed] [Google Scholar]
- 96.Collins AJ, Hao W, Xia H, et al. Mortality risks of peritoneal dialysis and hemodialysis. Am J Kidney Dis. 1999;34(6):1065–1074. doi: 10.1016/S0272-6386(99)70012-0. [DOI] [PubMed] [Google Scholar]
- 97.Laxton MK. Peritoneal dialysis: an effective yet underused renal replacement therapy. JAAPA. 2016;29(5):40–46. doi: 10.1097/01.JAA.0000482300.94949.e4. [DOI] [PubMed] [Google Scholar]
- 98.Chang TI, Ryu DR, Yoo TH, et al. Effect of icodextrin solution on the preservation of residual renal function in peritoneal dialysis patients: a randomized controlled study. Medicine. 2016;95(13):e2991. doi: 10.1097/MD.0000000000002991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342(20):1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 100.Krischock L, Kennedy SE, Hayen A. Multicentre study of treatment outcomes in Australian adolescents and young adults commencing dialysis. Nephrology (Carlton) 2016 Aug 30; doi: 10.1111/nep.12914. Epub. [DOI] [PubMed] [Google Scholar]
- 101.Beier UH, Green C, Meyers KE. Caring for adolescent renal patients. Kidney Int. 2010;77(4):285–291. doi: 10.1038/ki.2009.462. [DOI] [PubMed] [Google Scholar]
- 102.Li PK, Chow KM. Peritoneal dialysis-first policy made successful: perspectives and actions. Am J Kidney Dis. 2013;62(5):993–1005. doi: 10.1053/j.ajkd.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 103.Liu FX, Gao X, Inglese G, Chuengsaman P, Pecoits-Filho R, Yu A. A global overview of the impact of peritoneal dialysis first or favored policies: an opinion. Perit Dial Int. 2015;35(4):406–420. doi: 10.3747/pdi.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.USRDS . USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2012. https://www.usrds.org/atlas12.aspx. [Google Scholar]
- 105.Treharne C, Liu FX, Arici M, Crowe L, Farooqui U. Peritoneal dialysis and in-centre haemodialysis: a cost-utility analysis from a UK payer perspective. Appl Health Econ Health Policy. 2014;12(4):409–420. doi: 10.1007/s40258-014-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sanabria M, Munoz J, Trillos C, et al. Dialysis outcomes in Colombia (DOC) study: a comparison of patient survival on peritoneal dialysis vs hemodialysis in Colombia. Kidney Int Suppl. 2008(108):S165–S172. doi: 10.1038/sj.ki.5002619. [DOI] [PubMed] [Google Scholar]
- 107.Szeto CC, Wong TY, Chow KM, Leung CB, Law MC, Li PK. Independent effects of renal and peritoneal clearances on the mortality of peritoneal dialysis patients. Perit Dial Int. 2004;24(1):58–64. [PubMed] [Google Scholar]